Crystal Structure and Concentration-Driven Phase Transitions in Lu(1−x)ScxFeO3 (0 ≤ x ≤ 1) Prepared by the Sol–Gel Method
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Crystal Structure of the Compounds by Diffraction Measurements
3.2. Raman Spectroscopy Analysis
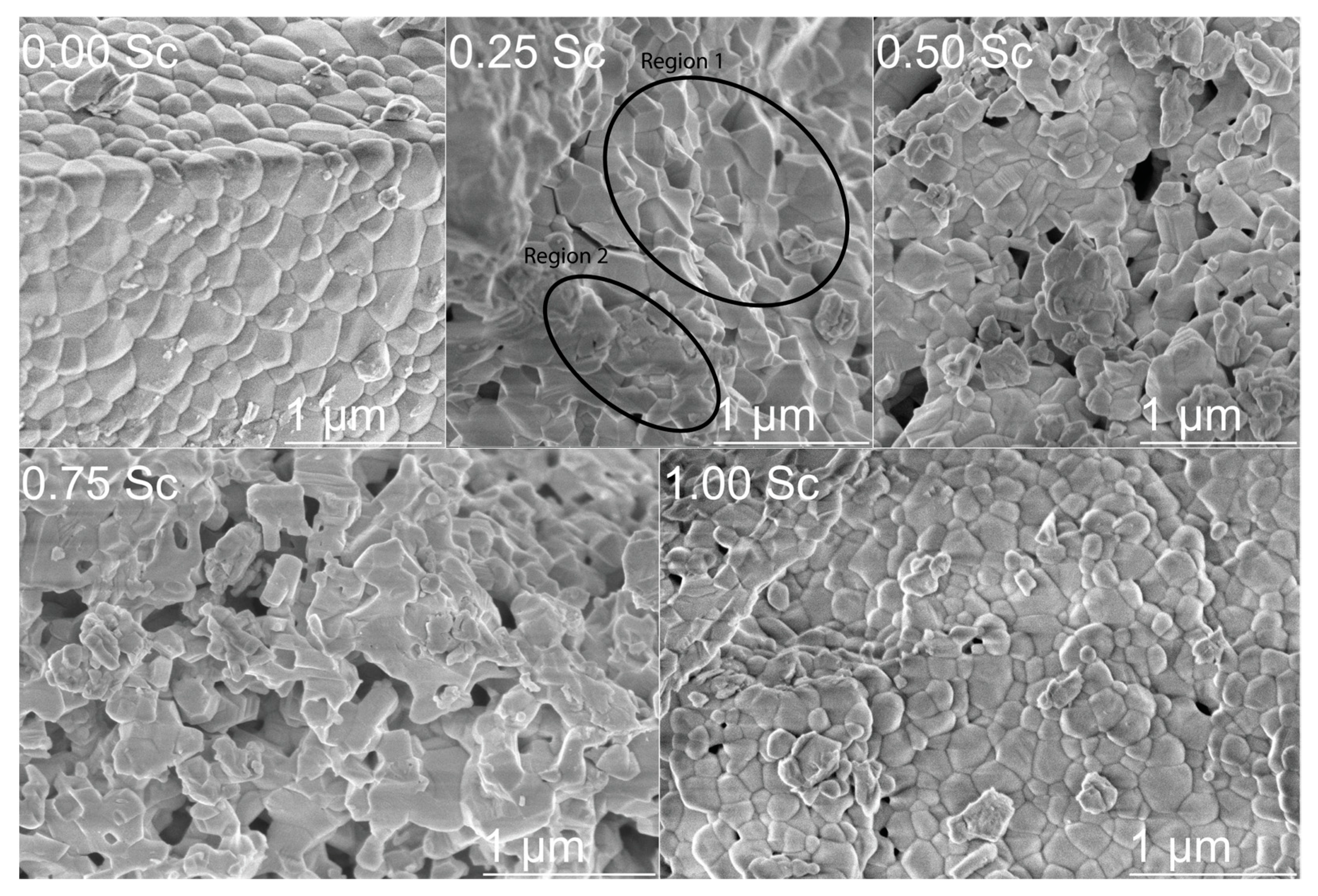
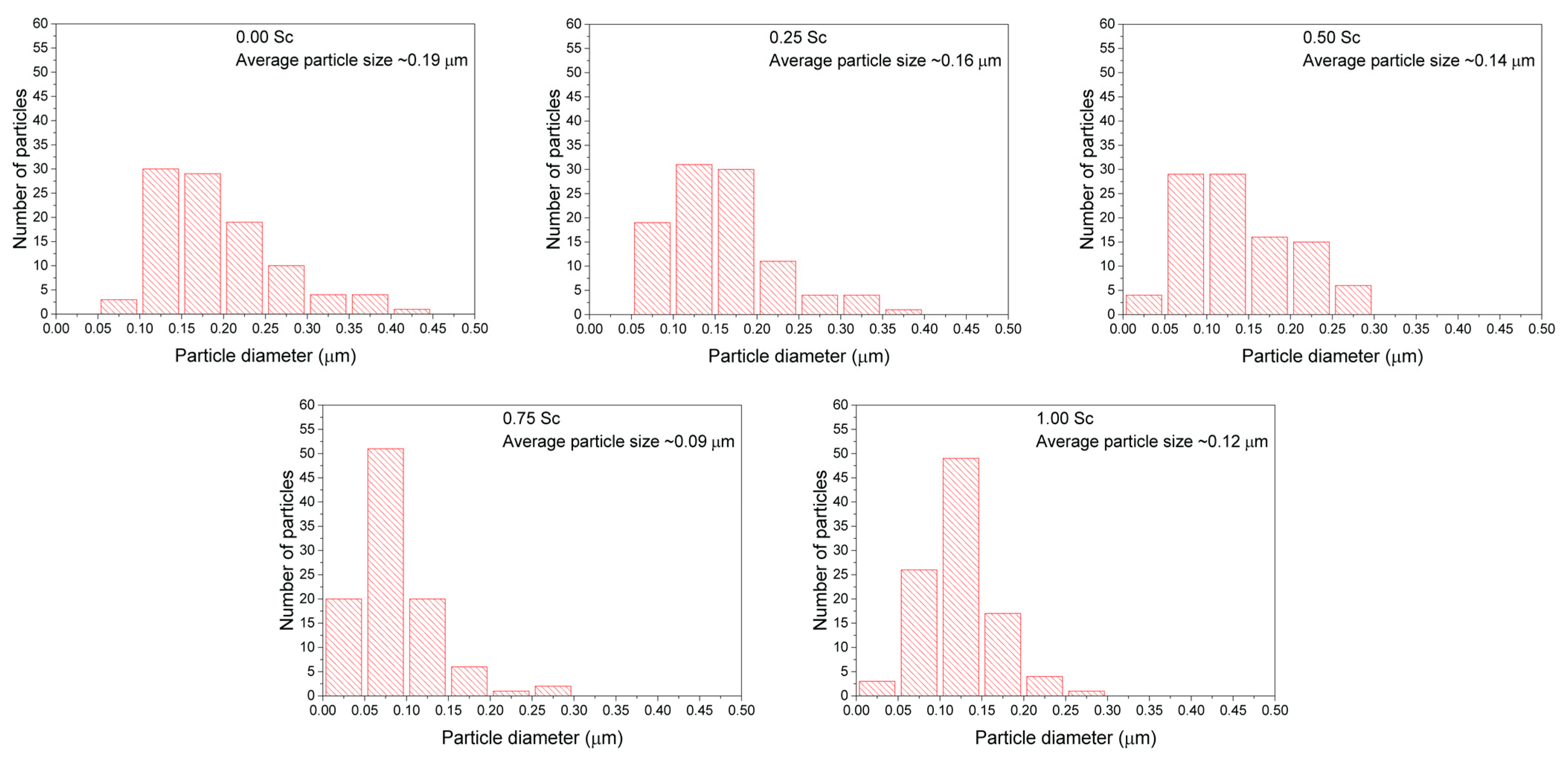
3.3. Scanning Electron Microscopy and EDX Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ishihara, T. Inorganic perovskite oxides. In Springer Handbooks; Springer: Berlin/Heidelberg, Germany, 2017; p. 1. [Google Scholar]
- Song, Z.; Zhao, J.; Liu, Q. Luminescent perovskites: Recent advances in theory and experiments. Inorg. Chem. Front. 2019, 6, 2969–3011. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X. A brief review on perovskite multiferroics. Ferroelectrics 2017, 507, 69–85. [Google Scholar] [CrossRef]
- Lone, I.H.; Aslam, J.; Radwan, N.R.E.; Bashal, A.H.; Ajlouni, A.F.A.; Akhter, A. Multiferroic ABO3 Transition Metal Oxides: A Rare Interaction of Ferroelectricity and Magnetism. Nanoscale Res. Lett. 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Shojaei, F.; Yin, W.J. Stability Trend of Tilted Perovskites. J. Phys. Chem. C 2018, 122, 15214–15219. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Takagi, S.; Deledda, S.; Hauback, B.C.; Orimo, S.I. Extending the applicability of the Goldschmidt tolerance factor to arbitrary ionic compounds. Sci. Rep. 2016, 6, 23592. [Google Scholar] [CrossRef] [Green Version]
- Bartel, C.J.; Sutton, C.; Goldsmith, B.R.; Ouyang, R.; Musgrave, C.B.; Ghiringhelli, L.M.; Scheffler, M. New tolerance factor to predict the stability of perovskite oxides and halides. Sci. Adv. 2019, 5, eaav0693. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Kumar, A.; Anshul, A.; Sharma, S. Advances and future challenges in multifunctional nanostructures for their role in fast, energy efficient memory devices. Mater. Lett. 2020, 277, 128369. [Google Scholar] [CrossRef]
- Hill, N.A. Why are there so few magnetic ferroelectrics? J. Phys. Chem. B 2000, 104, 6694–6709. [Google Scholar] [CrossRef]
- Fiebig, M.; Lottermoser, T.; Meier, D.; Trassin, M. The evolution of multiferroics. Nat. Rev. Mater. 2016, 1, 16046. [Google Scholar] [CrossRef]
- Spaldin, N.A.; Ramesh, R. Advances in magnetoelectric multiferroics. Nat. Mater. 2019, 18, 203–212. [Google Scholar] [CrossRef]
- Kalinkin, A.N.; Kozhbakhteev, E.M.; Polyakov, A.E.; Skorikov, V.M. Application of BiFeO3 and Bi4Ti3O12 in ferroelectric memory, phase shifters of a phased array, and microwave HEMTs. Inorg. Mater. 2013, 49, 1031–1043. [Google Scholar] [CrossRef]
- Lu, C.; Wu, M.; Lin, L.; Liu, J.M. Single-phase multiferroics: New materials, phenomena, and physics. Natl. Sci. Rev. 2019, 6, 653–668. [Google Scholar] [CrossRef] [Green Version]
- Čebela, M.; Zagorac, D.; Batalović, K.; Radaković, J.; Stojadinović, B.; Spasojević, V.; Hercigonja, R. BiFeO3 perovskites: A multidisciplinary approach to multiferroics. Ceram. Int. 2017, 43, 1256–1264. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, H.M.; Liu, M.F.; Shen, S.; Zhou, S.; Li, D.; Wang, X.; Yan, Z.B.; Zhang, Z.D.; Zhao, J.; et al. Hexagonal phase stabilization and magnetic orders of multiferroic Lu1-xScxFeO3. Phys. Rev. B 2016, 93, 075146. [Google Scholar] [CrossRef] [Green Version]
- Moyer, J.A.; Misra, R.; Mundy, J.A.; Brooks, C.M.; Heron, J.T.; Muller, D.A.; Schlom, D.G.; Schiffer, P. Intrinsic magnetic properties of hexagonal LuFeO3 and the effects of nonstoichiometry. APL Mater. 2014, 2, 012106. [Google Scholar] [CrossRef]
- Jeong, Y.K.; Lee, J.-H.; Ahn, S.-J.; Jang, H.M. Epitaxially Constrained Hexagonal Ferroelectricity and Canted Triangular Spin Order in LuFeO3 Thin Films. Chem. Mater. 2012, 24, 2426–2428. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, J.; Wang, W.; Gai, Z.; Balke, N.; Chi, M.; Lee, H.N.; Tian, W.; Zhu, L.; Cheng, X.; et al. Room-temperature multiferroic hexagonal LuFeO3 films. Phys. Rev. Lett. 2013, 110, 237601. [Google Scholar] [CrossRef] [Green Version]
- Akbashev, A.R.; Semisalova, A.S.; Perov, N.S.; Kaul, A.R. Weak ferromagnetism in hexagonal orthoferrites RFeO3 (R = Lu, Er-Tb). Appl. Phys. Lett. 2011, 99, 122502. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, X.; Paudel, T.R.; Sinha, K.; Wang, X.; Jiang, X.; Wang, W.; Brutsche, S.; Wang, J.; Ryan, P.J.; et al. On the structural origin of the single-ion magnetic anisotropy in LuFeO3. J. Phys. Condens. Matter 2016, 28, 156001. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, A.S.; Knight, K.S.; Lightfoot, P. High-temperature phase transitions of hexagonal YMnO3. Phys. Rev. B Condens. Matter Mater. Phys. 2011, 83, 094111. [Google Scholar] [CrossRef] [Green Version]
- Luxová, J.; Šulcová, P. The effect of partial substitution of Bi on colour properties and thermal stability of BixPr1−xFeO3 pigments. J. Therm. Anal. Calorim. 2019, 138, 4303–4312. [Google Scholar] [CrossRef]
- Wang, W.; Sun, W.; Zhang, G.; Cheng, Z.; Wang, Y. Magnetic domain-wall induced ferroelectric polarization in rare-earth orthoferrites AFeO3 (A = Lu, Y, Gd): First-principles calculations. J. Mater. Chem. C 2019, 7, 10059–10065. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Lan, Y.S.; Zeng, Z.Y.; Chen, X.R.; Chen, Q.F. Magnetic structures and optical properties of rare-earth orthoferrites RFeO3 (R = Ho, Er, Tm and Lu). Solid State Commun. 2019, 288, 10–17. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.M. Dielectric relaxation in LuFeO3 ceramics. Solid State Commun. 2009, 149, 1317–1321. [Google Scholar] [CrossRef]
- Deng, S.; Li, J.; Småbråten, D.R.; Shen, S.; Wang, W.; Zhao, J.; Tao, J.; Aschauer, U.; Chen, J.; Zhu, Y.; et al. Critical Role of Sc Substitution in Modulating Ferroelectricity in Multiferroic LuFeO3. Nano Lett. 2021, 21, 6648–6655. [Google Scholar] [CrossRef]
- White, J.; Sinha, K.; Xu, X. Structural phase diagram and magnetic properties of Sc-substituted rare earth ferrites R1−xScxFeO3 (R = Lu, Yb, Er, and Ho). J. Appl. Phys. 2019, 125, 244101. [Google Scholar] [CrossRef]
- Suresh, P.; Vijaya Laxmi, K.; Anil Kumar, P.S. Enhanced room temperature multiferroic characteristics in hexagonal LuFe1−xNixO3 (x = 0 − 0.3) nanoparticles. J. Magn. Magn. Mater. 2018, 448, 117–122. [Google Scholar] [CrossRef]
- Disseler, S.M.; Luo, X.; Gao, B.; Oh, Y.S.; Hu, R.; Wang, Y.; Quintana, D.; Zhang, A.; Huang, Q.; Lau, J.; et al. Multiferroicity in doped hexagonal LuFeO3. Phys. Rev. B 2015, 92, 54435. [Google Scholar] [CrossRef] [Green Version]
- Pęczkowski, P.; Zachariasz, P.; Kowalik, M.; Zalecki, R.; Jastrzębski, C. Characterization of the superconductor-multiferroic type materials based on YBa2Cu3O7−δ–YMnO3 composites. Ceram. Int. 2019, 45, 18189–18204. [Google Scholar] [CrossRef]
- Pęczkowski, P.; Zachariasz, P.; Kowalik, M.; Tokarz, W.; Kumar Naik, S.P.; Żukrowski, J.; Jastrzębski, C.; Dadiel, L.J.; Tabiś, W.; Gondek, Ł. Iron diffusivity into superconducting YBa2Cu3O7−δ at oxygen-assisted sintering: Structural, magnetic, and transport properties. J. Eur. Ceram. Soc. 2021, 41, 7085–7097. [Google Scholar] [CrossRef]
- Du, K.; Gao, B.; Wang, Y.; Xu, X.; Kim, J.; Hu, R.; Huang, F.T.; Cheong, S.W. Vortex ferroelectric domains, large-loop weak ferromagnetic domains, and their decoupling in hexagonal (Lu, Sc)FeO3. Npj Quantum Mater. 2018, 3, 33. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W. Multiferroic hexagonal ferrites (h-RFeO3, R = Y, Dy-Lu): A brief experimental review. Mod. Phys. Lett. B 2014, 28, 1430008. [Google Scholar] [CrossRef] [Green Version]
- Jo, W.; Kim, T.H.; Kim, D.Y.; Pabi, S.K. Effects of grain size on the dielectric properties of Pb (Mg1/3Nb2/3) O3 -30 mol % PbTiO3 ceramics. J. Appl. Phys. 2007, 102, 074116. [Google Scholar] [CrossRef]
- Behera, C.; Choudhary, R.N.P.; Das, P.R. Structural, electrical and multiferroic characteristics of thermo-mechanically fabricated BiFeO3-(BaSr)TiO3 solid solutions. Mater. Res. Express 2018, 5, 056301. [Google Scholar] [CrossRef]
- Pakalniškis, A.; Lukowiak, A.; Niaura, G.; Głuchowski, P.; Karpinsky, D.V.; Alikin, D.O.; Abramov, A.S.; Zhaludkevich, A.; Silibin, M.; Kholkin, A.L.; et al. Nanoscale ferroelectricity in pseudo-cubic sol-gel derived barium titanate—Bismuth ferrite (BaTiO3– BiFeO3) solid solutions. J. Alloy. Compd. 2020, 830, 154632. [Google Scholar] [CrossRef]
- Karoblis, D.; Zarkov, A.; Mazeika, K.; Baltrunas, D.; Niaura, G.; Beganskiene, A.; Kareiva, A. Sol-gel synthesis, structural, morphological and magnetic properties of BaTiO3–BiMnO3 solid solutions. Ceram. Int. 2020, 46, 16459–16464. [Google Scholar] [CrossRef]
- Trusovas, R.; Račiukaitis, G.; Niaura, G.; Barkauskas, J.; Valušis, G.; Pauliukaite, R. Recent Advances in Laser Utilization in the Chemical Modification of Graphene Oxide and Its Applications. Adv. Opt. Mater. 2016, 4, 37–65. [Google Scholar] [CrossRef]
- Venugopalan, S.; Becker, M.M. Raman scattering study of LuFeO3. J. Chem. Phys. 1998, 93, 3833. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, W.; Zhang, J.; Huang, J.; Dong, M.; Yuan, H.; Xu, T.; Shi, L.; Dai, Y.; Liu, Q.; et al. Effects of mechanochemical activation on the structural and electrical properties of orthorhombic LuFeO3 ceramics. J. Am. Ceram. Soc. 2021, 104, 3019–3029. [Google Scholar] [CrossRef]
- Ahmad Mir, F.; Ikram, M.; Kumar, R. Temperature-dependent Raman study of PrFeO3 thin film. J. Raman Spectrosc. 2011, 42, 201–208. [Google Scholar] [CrossRef]
- Ibáñez, J.; Blázquez, O.; Hernández, S.; Garrido, B.; Rodríguez-Hernández, P.; Muñoz, A.; Velázquez, M.; Veber, P.; Manjón, F.J. Lattice dynamics study of cubic Tb2O3. J. Raman Spectrosc. 2018, 49, 2021–2027. [Google Scholar] [CrossRef]
- Abrashev, M.V.; Todorov, N.D.; Geshev, J. Raman spectra of R2O3 (R—Rare earth) sesquioxides with C-type bixbyite crystal structure: A comparative study. J. Appl. Phys. 2014, 116, 103508. [Google Scholar] [CrossRef] [Green Version]
- Disseler, S.M.; Borchers, J.A.; Brooks, C.M.; Mundy, J.A.; Moyer, J.A.; Hillsberry, D.A.; Thies, E.L.; Tenne, D.A.; Heron, J.; Holtz, M.E.; et al. Magnetic structure and ordering of multiferroic hexagonal LuFeO3. Phys. Rev. Lett. 2015, 114, 217602. [Google Scholar] [CrossRef] [Green Version]
- Suresh, P.; Vijaya Laxmi, K.; Bera, A.K.; Yusuf, S.M.; Chittari, B.L.; Jung, J.; Anil Kumar, P.S. Magnetic ground state of the multiferroic hexagonal LuFeO3. Phys. Rev. B 2018, 97, 184419. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Shyam, P.; Shirolkar, M.M.; Krishna, S.S.; Sinha, B.; Caliebe, W.; Kalinko, A.; Srinivasan, G.; Ogale, S. Unusual magnetic ordering transitions in nanoscale biphasic LuFeO3: The role of the ortho-hexa phase ratio and the local structure. J. Mater. Chem. C 2020, 8, 17000–17008. [Google Scholar] [CrossRef]
- Smith, K.A.; Ramkumar, S.P.; Harms, N.C.; Clune, A.J.; Cheong, S.W.; Liu, Z.; Nowadnick, E.A.; Musfeldt, J.L. Pressure-induced phase transition and phonon softening in h-Lu0.6Sc0.4FeO3. Phys. Rev. B 2021, 104, 094109. [Google Scholar] [CrossRef]
- Sarkar, T.; Manna, K.; Elizabeth, S.; Anil Kumar, P.S. Investigation of multiferroicity, spin-phonon coupling, and unusual magnetic ordering close to room temperature in LuMn0.5Fe0.5O3. J. Appl. Phys. 2017, 121, 084102. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.K. First principle study of crystal growth morphology: An application to crystalline urea. arXiv 2006, arXiv:cond-mat/0602385. [Google Scholar]
- Popa, M.; Van Hong, L.; Kakihana, M. Particle morphology characterization and magnetic properties of LaMnO3+d perovskites. Phys. B Condens. Matter 2003, 327, 237–240. [Google Scholar] [CrossRef]
- Bruncková, H.; Medvecký, Ľ.; Ďurišin, J.; Girman, V. Phase transformation and particle morphology of perovskite La1/3TaO3 precursors prepared by polymeric tartrate complex sol–gel method. Mater. Lett. 2014, 115, 184–186. [Google Scholar] [CrossRef]
- Inkrataite, G.; Kemere, M.; Sarakovskis, A.; Skaudzius, R. Influence of boron on the essential properties for new generation scintillators. J. Alloy. Compd. 2021, 875, 160002. [Google Scholar] [CrossRef]
- Cho, S.; Yun, C.; Kim, Y.S.; Wang, H.; Jian, J.; Zhang, W.; Huang, J.; Wang, X.; Wang, H.; MacManus-Driscoll, J.L. Strongly enhanced dielectric and energy storage properties in lead-free perovskite titanate thin films by alloying. Nano Energy 2018, 45, 398–406. [Google Scholar] [CrossRef]
- Kitanaka, Y.; Miyayama, M.; Noguchi, Y. Ferrielectric-mediated morphotropic phase boundaries in Bi-based polar perovskites. Sci. Rep. 2019, 9, 4087. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.-B.M.A.; Murgan, R.; Rahman, M.K.A.; Osman, J. Morphotropic Phase Boundary in Ferroelectric Materials. In Ferroelectrics—Physical Effects; Mickaël, L., Ed.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef] [Green Version]
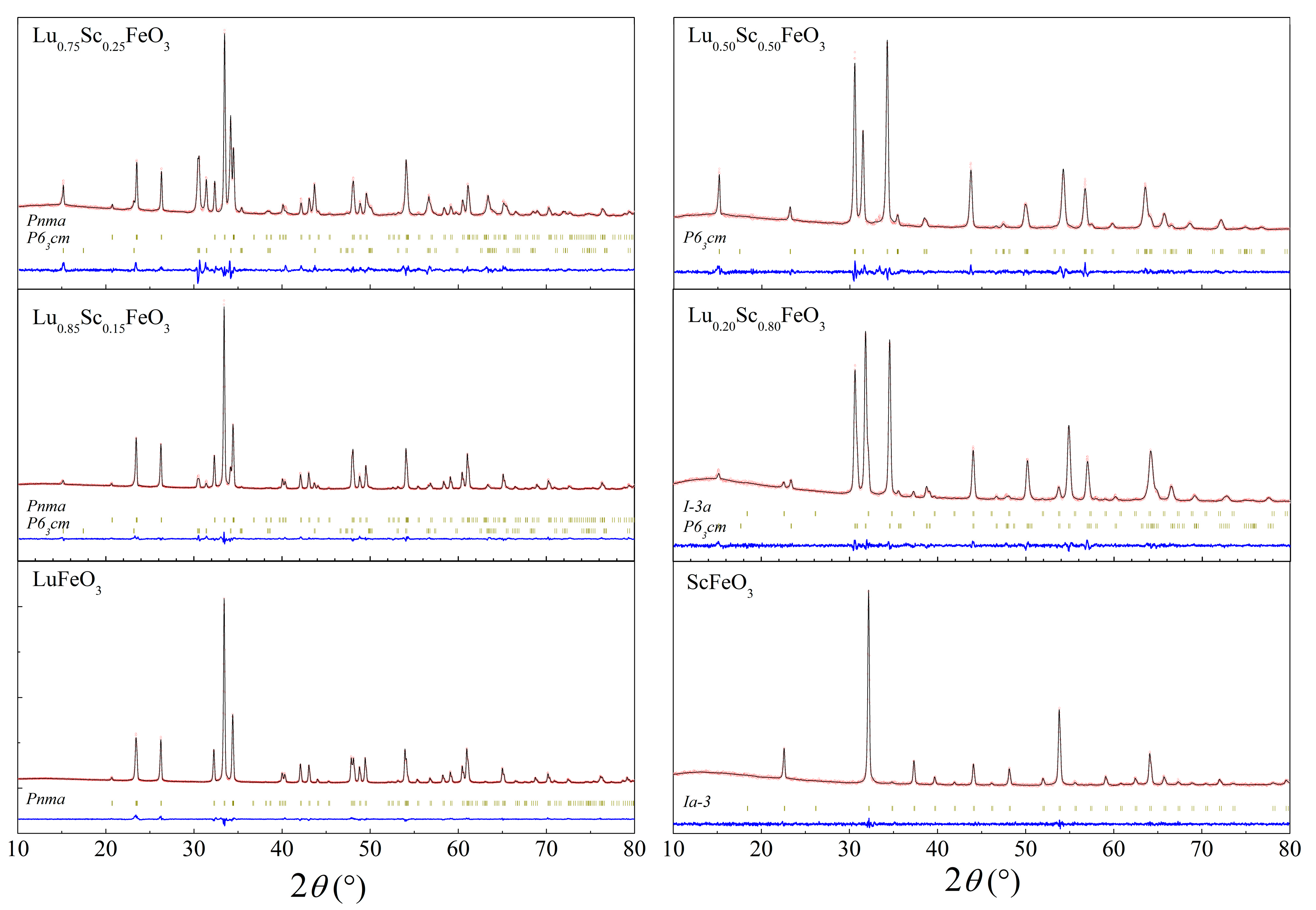
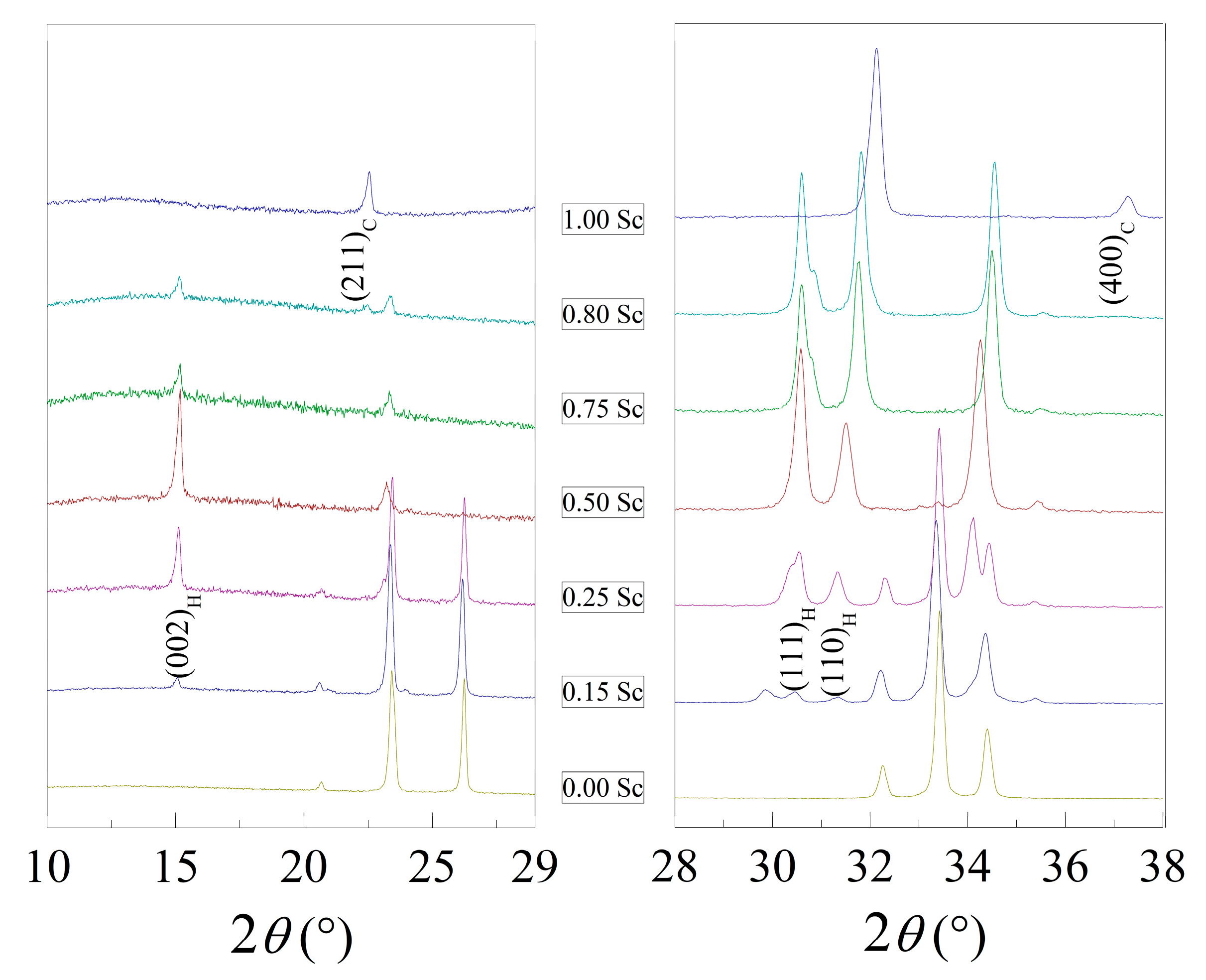
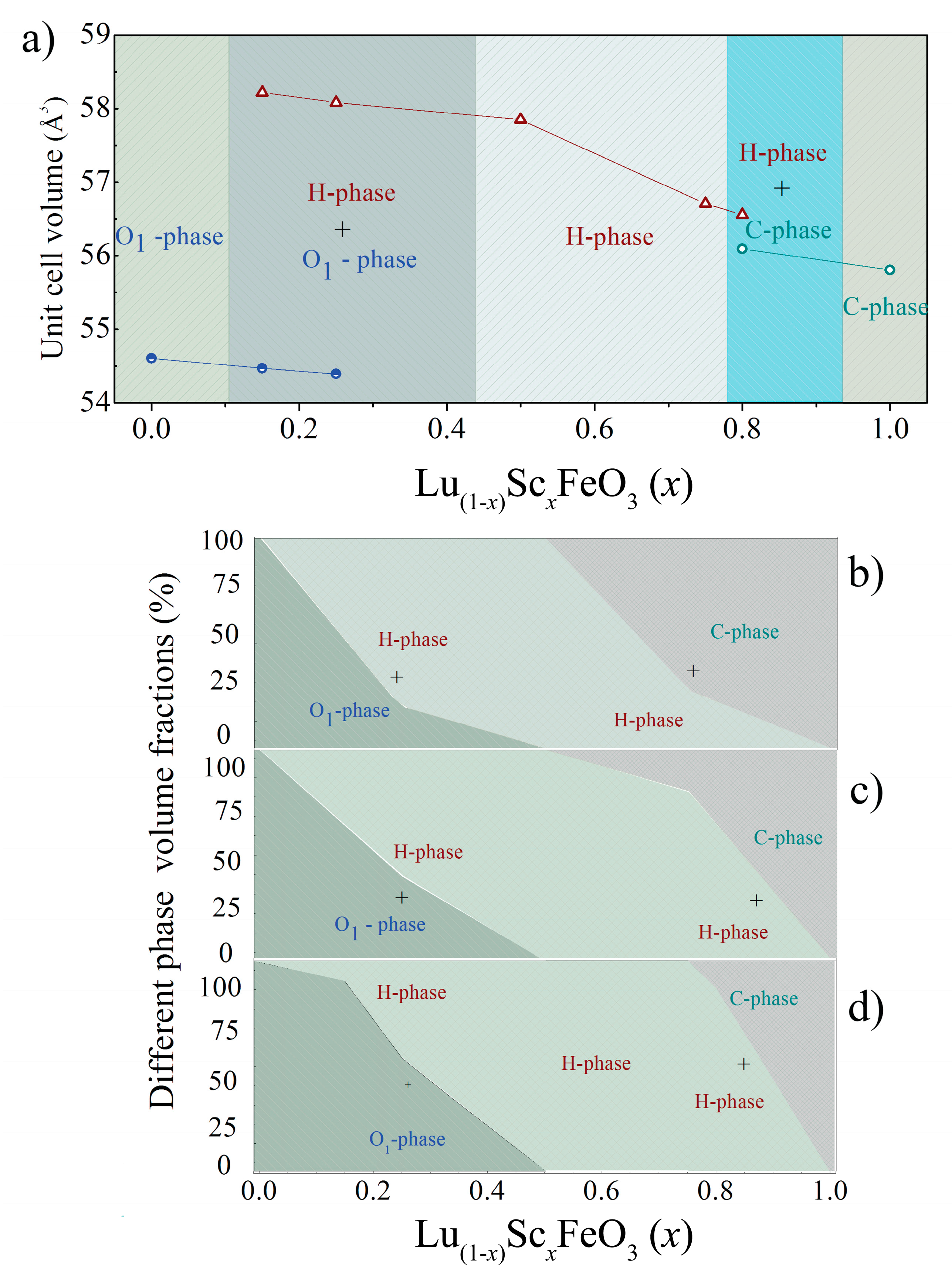
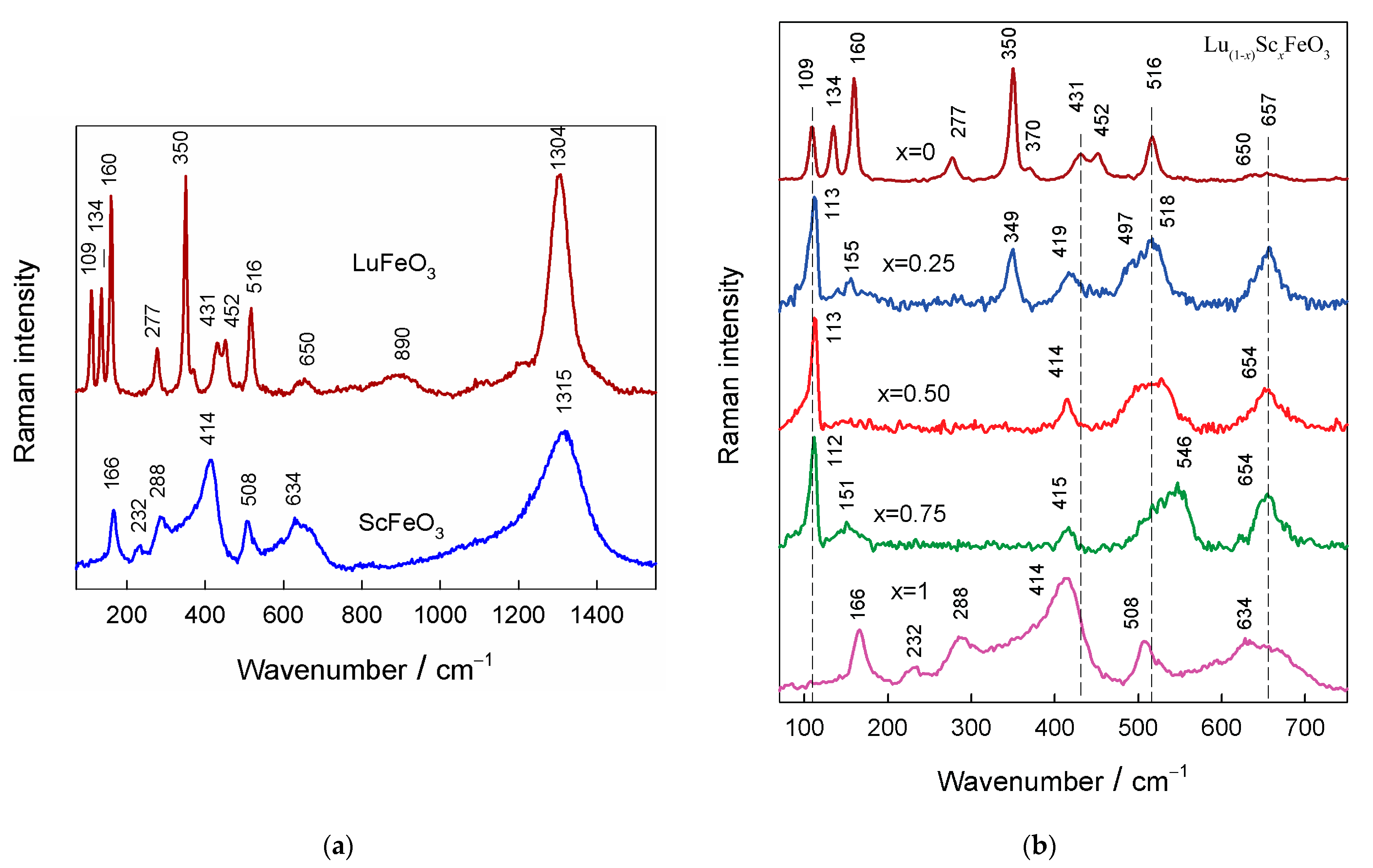
| Sample | Phase | a, Å | b, Å | c, Å | Volume Å 3 (Per Reduced Cell) | Sc/(Sc + Lu) Ratio | (Sc + Lu)/Fe Ratio |
|---|---|---|---|---|---|---|---|
| LuFeO3 | Pnma | 5.546(4) | 7.557(5) | 5.211(3) | 54.60(6) | 0.000 | 0.935 |
| Lu0.85Sc0.15FeO3 | Pnma (91%) | 5.533(4) | 7.564(2) | 5.205(1) | 54.46(2) | — | — |
| P63cm (9%) | 5.875(8) | 5.875(8) | 11.688(7) | 58.22(5) | |||
| Lu0.75Sc0.25FeO3 | Pnma (54%) | 5.532(1) | 7.560(5) | 5.201(8) | 54.39(2) | 0.197 | 0.994 |
| P63cm (46%) | 5.869(9) | 5.869(9) | 11.682(2) | 58.08(2) | |||
| Lu0.50Sc0.50FeO3 | P63cm | 5.856(1) | 5.856(1) | 11.690(1) | 57.84(8) | 0.447 | 0.932 |
| Lu0.25Sc0.75FeO3 | P63cm | 5.800(9) | 5.800(9) | 11.678(8) | 56.70(7) | 0.713 | 0.914 |
| Lu0.20Sc0.80FeO3 | P63cm (88%) | 5.793(9) | 5.793(9) | 11.676(1) | 56.55(8) | — | — |
| Ia-3 (12%) | 9.646(1) | 9.646(1) | 9.646(1) | 56.09(5) | |||
| ScFeO3 | Ia-3 | 9.629(4) | 9.629(4) | 9.629(4) | 55.80(6) | 1.00 | 1.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakalniškis, A.; Alikin, D.O.; Turygin, A.P.; Zhaludkevich, A.L.; Silibin, M.V.; Zhaludkevich, D.V.; Niaura, G.; Zarkov, A.; Skaudžius, R.; Karpinsky, D.V.; et al. Crystal Structure and Concentration-Driven Phase Transitions in Lu(1−x)ScxFeO3 (0 ≤ x ≤ 1) Prepared by the Sol–Gel Method. Materials 2022, 15, 1048. https://doi.org/10.3390/ma15031048
Pakalniškis A, Alikin DO, Turygin AP, Zhaludkevich AL, Silibin MV, Zhaludkevich DV, Niaura G, Zarkov A, Skaudžius R, Karpinsky DV, et al. Crystal Structure and Concentration-Driven Phase Transitions in Lu(1−x)ScxFeO3 (0 ≤ x ≤ 1) Prepared by the Sol–Gel Method. Materials. 2022; 15(3):1048. https://doi.org/10.3390/ma15031048
Chicago/Turabian StylePakalniškis, Andrius, Denis O. Alikin, Anton P. Turygin, Alexander L. Zhaludkevich, Maxim V. Silibin, Dmitry V. Zhaludkevich, Gediminas Niaura, Aleksej Zarkov, Ramūnas Skaudžius, Dmitry V. Karpinsky, and et al. 2022. "Crystal Structure and Concentration-Driven Phase Transitions in Lu(1−x)ScxFeO3 (0 ≤ x ≤ 1) Prepared by the Sol–Gel Method" Materials 15, no. 3: 1048. https://doi.org/10.3390/ma15031048
APA StylePakalniškis, A., Alikin, D. O., Turygin, A. P., Zhaludkevich, A. L., Silibin, M. V., Zhaludkevich, D. V., Niaura, G., Zarkov, A., Skaudžius, R., Karpinsky, D. V., & Kareiva, A. (2022). Crystal Structure and Concentration-Driven Phase Transitions in Lu(1−x)ScxFeO3 (0 ≤ x ≤ 1) Prepared by the Sol–Gel Method. Materials, 15(3), 1048. https://doi.org/10.3390/ma15031048












