Carbon Nanomaterials (CNMs) and Enzymes: From Nanozymes to CNM-Enzyme Conjugates and Biodegradation
Abstract
:1. Introduction
1.1. Enzyme Classification
- The first component refers to the general type of reaction being catalyzed. For instance, EC 1 indicates oxidoreductases that catalyze redox reactions, and EC 3 identifies hydrolases that catalyze hydrolytic reactions (Figure 1).
- The second number indicates the subclass based on the type of compound or functional group involved in the reaction. For example, EC 1.13 refers to oxygenases that insert oxygen on the substrate, and EC 2.3 indicates acyl-transferases that transfer acyl groups, etc.).
- The third component denotes the sub-subclass, by further specifying the reaction being catalyzed, for instance in terms of acceptors, or specific groups being transferred. As an example, EC 2.1.1 indicates methyl transferases.
- The fourth component is simply a serial number that refers to the specific enzyme.
1.2. Carbon Nanomaterials (CNMs)
1.2.1. Fullerenes
1.2.2. Carbon Nano-Onions (CNOs)
1.2.3. Carbon Nanohorns (CNHs)
1.2.4. Carbon Nanodots (CNDs)
1.2.5. Nanodiamonds (NDs)
1.2.6. Carbon Nanotubes (CNTs)
1.2.7. Graphene (G) and Graphene-Based Materials
1.3. Bibliometric Analysis of CNMs and Enzymes
2. CNMs for Enzyme Mimicry, Inhibition, or Monitoring
2.1. CNMs for Enzyme Mimicry
2.2. CNMs as Enzyme Inhibitors
2.3. CNMs for Enzyme Monitoring
3. Applications of CNM-Enzyme Conjugates
3.1. Biosensing
3.2. Biofuel Cells
3.3. Biocatalysis
3.4. Water Remediation and Environmental Monitoring
3.5. Innovative Therapy and Theranostics
4. Enzymatic Biodegradation of CNMs
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calvaresi, M.; Zerbetto, F. The Devil and Holy Water: Protein and Carbon Nanotube Hybrids. Acc. Chem. Res. 2013, 46, 2454–2463. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Prato, M. Under the lens: Carbon nanotube and protein interaction at the nanoscale. Chem. Commun. 2015, 51, 4347–4359. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, Y.; Chen, M.; Yan, M.; Zeng, G.; Huang, D. How do proteins ‘response’ to common carbon nanomaterials? Adv. Colloid Interface Sci. 2019, 270, 101–107. [Google Scholar] [CrossRef]
- Chaudhary, K.; Kumar, K.; Venkatesu, P.; Masram, D.T. Protein immobilization on graphene oxide or reduced graphene oxide surface and their applications: Influence over activity, structural and thermal stability of protein. Adv. Colloid Interface Sci. 2021, 289, 102367. [Google Scholar] [CrossRef]
- Botta, L.; Bizzarri, B.M.; Crucianelli, M.; Saladino, R. Advances in biotechnological synthetic applications of carbon nanostructured systems. J. Mater. Chem. B 2017, 5, 6490–6510. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wan, B.; Yang, Y.; Ren, X.-M.; Guo, L.-H.; Liu, J.-F. Biodegradation of single-walled carbon nanotubes in macrophages through respiratory burst modulation. Int. J. Mol. Sci. 2016, 17, 409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Yang, M.; Bussy, C.; Iijima, S.; Kostarelos, K.; Yudasaka, M. Biodegradation of carbon nanohorns in macrophage cells. Nanoscale 2015, 7, 2834–2840. [Google Scholar] [CrossRef]
- Srivastava, I.; Sar, D.; Mukherjee, P.; Schwartz-Duval, A.S.; Huang, Z.; Jaramillo, C.; Civantos, A.; Tripathi, I.; Allain, J.P.; Bhargava, R.; et al. Enzyme-catalyzed biodegradation of carbon dots follows sequential oxidation in a time dependent manner. Nanoscale 2019, 11, 8226–8236. [Google Scholar] [CrossRef]
- Czarnecka, J.; Kwiatkowski, M.; Wiśniewski, M.; Roszek, K. Protein corona hinders N-CQDs oxidative potential and favors their application as nanobiocatalytic system. Int. J. Mol. Sci. 2021, 22, 8136. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomedicine 2016, 12, 333–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinals, R.L.; Yang, D.; Lui, A.; Cao, W.; Landry, M.P. Corona exchange dynamics on carbon nanotubes by multiplexed fluorescence monitoring. J. Am. Chem. Soc. 2020, 142, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Kostarelos, K.; Bianco, A.; Prato, M. The winding road for carbon nanotubes in nanomedicine. Mater. Today 2015, 18, 12–19. [Google Scholar] [CrossRef]
- Wang, W.; Hou, Y.; Martinez, D.; Kurniawan, D.; Chiang, W.-H.; Bartolo, P. Carbon nanomaterials for electro-active structures: A review. Polymers 2020, 12, 2946. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Nyein, H.Y.Y.; Gao, W.; Javey, A. Flexible electrochemical bioelectronics: The rise of in situ bioanalysis. Adv. Mater. 2020, 32, e1902083. [Google Scholar] [CrossRef] [PubMed]
- Speranza, G. Carbon nanomaterials: Synthesis, functionalization and sensing applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- ExplorEnz—The Enzyme Database. Available online: https://www.enzyme-database.org/ (accessed on 19 January 2022).
- McDonald, A.G.; Tipton, K.F. Enzyme nomenclature and classification: The state of the art. FEBS J. 2021; in press. [Google Scholar] [CrossRef] [PubMed]
- KEGG PATHWAY Database. Available online: https://www.genome.jp/kegg/pathway.html (accessed on 19 January 2022).
- Institute of Biochemistry and Bioinformatics at the Technical University of Braunschweig. BRENDA The Comprehensive Enzyme Information System. Available online: https://www.brenda-enzymes.org/ (accessed on 19 January 2022).
- NIST National Institute of Science and Technology. Thermodynamics Research Center. Available online: https://trc.nist.gov/ (accessed on 19 January 2022).
- The European Society of Human Genetics. ESHG Home. Available online: https://www.eshg.org/index.php?id=home (accessed on 19 January 2022).
- NCBI. Gene Search Database. Available online: https://www.ncbi.nlm.nih.gov/gene (accessed on 19 January 2022).
- Rawlings, N.D.; Waller, M.; Barrett, A.J.; Bateman, A. MEROPS: The database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2014, 42, D503–D509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swiss Insitute of Bioinformatics. Expasy Swiss Bioinformatics Resource Portal. Available online: https://www.expasy.org/ (accessed on 19 January 2022).
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad family of carbon nanoallotropes: Classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Adorinni, S.; Cringoli, M.C.; Perathoner, S.; Fornasiero, P.; Marchesan, S. Green approaches to carbon nanostructure-based biomaterials. Appl. Sci. 2021, 11, 2490. [Google Scholar] [CrossRef]
- Ugarte, D. Onion-like graphitic particles. Carbon 1995, 33, 989–993. [Google Scholar] [CrossRef]
- Marchesan, S.; Melchionna, M.; Prato, M. Carbon nanostructures for nanomedicine: Opportunities and challenges. Fuller. Nanotub. Carbon Nanostructures 2014, 22, 190–195. [Google Scholar] [CrossRef]
- Oliveira, S.F.; Bisker, G.; Bakh, N.A.; Gibbs, S.L.; Landry, M.P.; Strano, M.S. Protein functionalized carbon nanomaterials for biomedical applications. Carbon 2015, 95, 767–779. [Google Scholar] [CrossRef] [Green Version]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Basso, L.; Cazzanelli, M.; Orlandi, M.; Miotello, A. Nanodiamonds: Synthesis and application in sensing, catalysis, and the possible connection with some processes occurring in space. Appl. Sci. 2020, 10, 4094. [Google Scholar] [CrossRef]
- Tonellato, M.; Piccione, M.; Gasparotto, M.; Bellet, P.; Tibaudo, L.; Vicentini, N.; Bergantino, E.; Menna, E.; Vitiello, L.; Di Liddo, R.; et al. Commitment of autologous human multipotent stem cells on biomimetic poly-L-lactic acid-based scaffolds is strongly influenced by structure and concentration of carbon nanomaterial. Nanomaterials 2020, 10, 415. [Google Scholar] [CrossRef] [Green Version]
- Piovesana, S.; Iglesias, D.; Melle-Franco, M.; Kralj, S.; Cavaliere, C.; Melchionna, M.; Laganà, A.; Capriotti, A.L.; Marchesan, S. Carbon nanostructure morphology templates nanocomposites for phosphoproteomics. Nano Res. 2020, 13, 380–388. [Google Scholar] [CrossRef]
- Vicentini, N.; Gatti, T.; Salerno, M.; Gomez, Y.S.H.; Bellon, M.; Gallio, S.; Marega, C.; Filippini, F.; Menna, E. Effect of different functionalized carbon nanostructures as fillers on the physical properties of biocompatible poly (l-lactic acid) composites. Mater. Chem. Phys. 2018, 214, 265–276. [Google Scholar] [CrossRef]
- Iglesias, D.; Melle-Franco, M.; Kurbasic, M.; Melchionna, M.; Abrami, M.; Grassi, M.; Prato, M.; Marchesan, S. Oxidized nanocarbons-tripeptide supramolecular hydrogels: Shape matters! ACS Nano 2018, 12, 5530–5538. [Google Scholar] [CrossRef]
- Eissa, S.; Alshehri, N.; Rahman, A.M.A.; Dasouki, M.; Abu-Salah, K.M.; Zourob, M. Electrochemical immunosensors for the detection of survival motor neuron (SMN) protein using different carbon nanomaterials-modified electrodes. Biosens. Bioelectron. 2018, 101, 282–289. [Google Scholar] [CrossRef]
- Iglesias, D.; Bosi, S.; Melchionna, M.; Da Ros, T.; Marchesan, S. The glitter of carbon nanostructures in hybrid/composite hydrogels for medicinal use. Curr. Top. Med. Chem. 2016, 16, 1976–1989. [Google Scholar] [CrossRef] [Green Version]
- Maziukiewicz, D.; Maciejewska, B.M.; Litowczenko, J.; Kościński, M.; Warowicka, A.; Wychowaniec, J.K.; Jurga, S. Designing biocompatible spin-coated multiwall carbon nanotubes-polymer composite coatings. Surf. Coat. Technol. 2020, 385, 125199. [Google Scholar] [CrossRef]
- Tadyszak, K.; Wychowaniec, J.K.; Litowczenko, J. Biomedical applications of graphene-based structures. Nanomaterials 2018, 8, 944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tofail, S.A.M.; Koumoulos, E.P.; Bandyopadhyay, A.; Bose, S.; O’Donoghue, L.; Charitidis, C. Additive manufacturing: Scientific and technological challenges, market uptake and opportunities. Mater. Today 2018, 21, 22–37. [Google Scholar] [CrossRef]
- Marchesan, S.; Ballerini, L.; Prato, M. Nanomaterials for stimulating nerve growth. Science 2017, 356, 1010–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydin, T.; Gurcan, C.; Taheri, H.; Yilmazer, A. Graphene based materials in neural tissue regeneration. Adv. Exp. Med. Biol. 2018, 1107, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Bei, H.P.; Yang, Y.; Zhang, Q.; Tian, Y.; Luo, X.; Yang, M.; Zhao, X. Graphene-based nanocomposites for neural tissue engineering. Molecules 2019, 24, 658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farokhi, M.; Mottaghitalab, F.; Saeb, M.R.; Shojaei, S.; Zarrin, N.K.; Thomas, S.; Ramakrishna, S. Conductive biomaterials as substrates for neural stem cells differentiation towards neuronal lineage cells. Macromol. Biosci. 2021, 21, e2000123. [Google Scholar] [CrossRef] [PubMed]
- Monaco, A.M.; Giugliano, M. Carbon-based smart nanomaterials in biomedicine and neuroengineering. Beilstein J. Nanotechnol. 2014, 5, 1849–1863. [Google Scholar] [CrossRef]
- Amin, D.R.; Sink, E.; Narayan, S.P.; Abdel-Hafiz, M.; Mestroni, L.; Peña, B. Nanomaterials for cardiac tissue engineering. Molecules 2020, 25, 5189. [Google Scholar] [CrossRef]
- Marchesan, S.; Bosi, S.; Alshatwi, A.; Prato, M. Carbon nanotubes for organ regeneration: An electrifying performance. Nano Today 2016, 11, 398–401. [Google Scholar] [CrossRef]
- Ashtari, K.; Nazari, H.; Ko, H.; Tebon, P.; Akhshik, M.; Akbari, M.; Alhosseini, S.N.; Mozafari, M.; Mehravi, B.; Soleimani, M.; et al. Electrically conductive nanomaterials for cardiac tissue engineering. Adv. Drug Deliv. Rev. 2019, 144, 162–179. [Google Scholar] [CrossRef]
- Ghai, P.; Mayerhofer, T.; Jha, R.K. Exploring the effectiveness of incorporating carbon nanotubes into bioengineered scaffolds to improve cardiomyocyte function. Expert Rev. Clin. Pharmacol. 2020, 13, 1347–1366. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, A.; Vahdat, S.; Baheiraei, N.; Razavi, M.; Norahan, M.H.; Baharvand, H. Multifunctional conductive biomaterials as promising platforms for cardiac tissue engineering. ACS Biomater. Sci. Eng. 2021, 7, 55–82. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, H.; Patino-Guerrero, A.; Hasany, M.; Ansari, M.O.; Memic, A.; Dolatshahi-Pirouz, A.; Nikkhah, M. Electroconductive biomaterials for cardiac tissue engineering. Acta Biomater. 2021; in press. [Google Scholar] [CrossRef] [PubMed]
- Sainio, S.; Leppänen, E.; Mynttinen, E.; Palomäki, T.; Wester, N.; Etula, J.; Isoaho, N.; Peltola, E.; Koehne, J.; Meyyappan, M.; et al. Integrating carbon nanomaterials with metals for bio-sensing applications. Mol. Neurobiol. 2020, 57, 179–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melchionna, M.; Prato, M.; Fornasiero, P. Mix and match metal oxides and nanocarbons for new photocatalytic frontiers. Catal. Today 2016, 277, 202–213. [Google Scholar] [CrossRef]
- Rosso, C.; Filippini, G.; Criado, A.; Melchionna, M.; Fornasiero, P.; Prato, M. Metal-free photocatalysis: Two-dimensional nanomaterial connection toward advanced organic synthesis. ACS Nano 2021, 15, 3621–3630. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P.; Prato, M. Into the carbon: A matter of core and shell in advanced electrocatalysis. APL Mater. 2020, 8, 020905. [Google Scholar] [CrossRef] [Green Version]
- Antonietti, M.; Bandosz, T.; Centi, G.; Costa, R.; Cruz-Silva, R.; Di, J.; Feng, X.; Frank, B.; Gebhardt, P.; Guldi, D.M.; et al. Nanocarbon-Inorganic Hybrids: Next Generation Composites for Sustainable Energy Applications; Eder, D., Schlogl, R., Eds.; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2014. [Google Scholar]
- Lu, H.; Tournet, J.; Dastafkan, K.; Liu, Y.; Ng, Y.H.; Karuturi, S.K.; Zhao, C.; Yin, Z. Noble-metal-free multicomponent nanointegration for sustainable energy conversion. Chem. Rev. 2021, 121, 10271–10366. [Google Scholar] [CrossRef]
- Chen, S.; Qiu, L.; Cheng, H.-M. Carbon-based fibers for advanced electrochemical energy storage devices. Chem. Rev. 2020, 120, 2811–2878. [Google Scholar] [CrossRef]
- Liu, H.; Wu, S.; Tian, N.; Yan, F.; You, C.; Yang, Y. Carbon foams: 3D porous carbon materials holding immense potential. J. Mater. Chem. A 2020, 8, 23699–23723. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.; Duan, X.; Sun, H.; Shao, G.; Wang, S. Porous carbons: Structure-oriented design and versatile applications. Adv. Funct. Mater. 2020, 30, 1909265. [Google Scholar] [CrossRef]
- Perovic, M.; Qin, Q.; Oschatz, M. From molecular precursors to nanoparticles—Tailoring the adsorption properties of porous carbon materials by controlled chemical functionalization. Adv. Funct. Mater. 2020, 30, 1908371. [Google Scholar] [CrossRef]
- Wang, T.; Okejiri, F.; Qiao, Z.-A.; Dai, S. Tailoring polymer colloids derived porous carbon spheres based on specific chemical reactions. Adv. Mater. 2020, 32, e2002475. [Google Scholar] [CrossRef]
- Niu, J.; Shao, R.; Liu, M.; Zan, Y.; Dou, M.; Liu, J.; Zhang, Z.; Huang, Y.; Wang, F. Porous carbons derived from collagen-enriched biomass: Tailored design, synthesis, and application in electrochemical energy storage and conversion. Adv. Funct. Mater. 2019, 29, 1905095. [Google Scholar] [CrossRef]
- Zhang, Z.; Cano, Z.P.; Luo, D.; Dou, H.; Yu, A.; Chen, Z. Rational design of tailored porous carbon-based materials for CO2 capture. J. Mater. Chem. A 2019, 7, 20985–21003. [Google Scholar] [CrossRef]
- Singh, G.; Lakhi, K.S.; Sil, S.; Bhosale, S.V.; Kim, I.; Albahily, K.; Vinu, A. Biomass derived porous carbon for CO2 capture. Carbon 2019, 148, 164–186. [Google Scholar] [CrossRef]
- Kumar, K.V.; Preuss, K.; Titirici, M.-M.; Rodriguez-Reinoso, F. Nanoporous materials for the onboard storage of natural gas. Chem. Rev. 2017, 117, 1796–1825. [Google Scholar] [CrossRef]
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C.-M. Biomass-derived porous carbon materials with different dimensions for supercapacitor electrodes: A review. J. Mater. Chem. A 2019, 7, 16028–16045. [Google Scholar] [CrossRef]
- Liu, T.; Liu, G. Block copolymer-based porous carbons for supercapacitors. J. Mater. Chem. A 2019, 7, 23476–23488. [Google Scholar] [CrossRef]
- Tian, H.; Wang, T.; Zhang, F.; Zhao, S.; Wan, S.; He, F.; Wang, G. Tunable porous carbon spheres for high-performance rechargeable batteries. J. Mater. Chem. A 2018, 6, 12816–12841. [Google Scholar] [CrossRef]
- Xu, M.; Yu, Q.; Liu, Z.; Lv, J.; Lian, S.; Hu, B.; Mai, L.; Zhou, L. Tailoring porous carbon spheres for supercapacitors. Nanoscale 2018, 10, 21604–21616. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhuang, X.; Lei, C.; Lei, L.; Hou, Y.; Mai, Y.; Feng, X. Porous carbon nanosheets: Synthetic strategies and electrochemical energy related applications. Nano Today 2019, 24, 103–119. [Google Scholar] [CrossRef]
- Matos, I.; Bernardo, M.; Fonseca, I. Porous carbon: A versatile material for catalysis. Catal. Today 2017, 285, 194–203. [Google Scholar] [CrossRef]
- Cao, Y.; Mao, S.; Li, M.; Chen, Y.; Wang, Y. Metal/porous carbon composites for heterogeneous catalysis: Old catalysts with improved performance promoted by N-doping. ACS Catal. 2017, 7, 8090–8112. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Talapaneni, S.N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent advances in functionalized micro and mesoporous carbon materials: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 2680–2721. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Diederich, F.; Ettl, R.; Rubin, Y.; Whetten, R.L.; Beck, R.; Alvarez, M.; Anz, S.; Sensharma, D.; Wudl, F.; Khemani, K.C.; et al. The higher fullerenes: Isolation and characterization of C76, C84, C90, C94, and C70O, an oxide of D5h-C70. Science 1991, 252, 548–551. [Google Scholar] [CrossRef]
- Howard, J.B.; McKinnon, J.T.; Makarovsky, Y.; Lafleur, A.L.; Johnson, M.E. Fullerenes C60 and C70 in flames. Nature 1991, 352, 139–141. [Google Scholar] [CrossRef]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Guldi, D.M.; Martin, N. 1.07—Functionalized fullerenes: Synthesis and functions. In Comprehensive Nanoscience and Nanotechnology, 2nd ed.; Andrews, D.L., Lipson, R.H., Nann, T., Eds.; Academic Press: Oxford, UK, 2011; pp. 187–204. [Google Scholar]
- Von Delius, M.; Hirsch, A. Heterofullerenes: Doped buckyballs. In Chemical Synthesis and Applications of Graphene and Carbon Materials; Antonietti, M., Müllen, K., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 191–216. [Google Scholar]
- Ceron, M.R.; Maffeis, V.; Stevenson, S.; Echegoyen, L. Endohedral fullerenes: Synthesis, isolation, mono- and bis-functionalization. Inorg. Chim. Acta 2017, 468, 16–27. [Google Scholar] [CrossRef]
- Jalife, S.; Arcudia, J.; Pan, S.; Merino, G. Noble gas endohedral fullerenes. Chem. Sci. 2020, 11, 6642–6652. [Google Scholar] [CrossRef] [PubMed]
- Candian, A. A fresh mechanism for how buckyballs form in space. Nature 2019, 574, 490–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazemzadeh, H.; Mozafari, M. Fullerene-based delivery systems. Drug Discov. Today 2019, 24, 898–905. [Google Scholar] [CrossRef]
- Illescas, B.M.; Rojo, J.; Delgado, R.; Martin, N. Multivalent glycosylated nanostructures to inhibit ebola virus infection. J. Am. Chem. Soc. 2017, 139, 6018–6025. [Google Scholar] [CrossRef] [Green Version]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, L.; Su, H.; Yan, L.; Gu, Z.; Chen, Z.; Zhang, A.; Zhao, F.; Zhao, Y. The pharmaceutical multi-activity of metallofullerenol invigorates cancer therapy. Nanoscale 2019, 11, 14528–14539. [Google Scholar] [CrossRef]
- Collavini, S.; Delgado, J.L. Fullerenes: The stars of photovoltaics. Sustain. Energy Fuels 2018, 2, 2480–2493. [Google Scholar] [CrossRef]
- Deng, L.-L.; Xie, S.-Y.; Gao, F. Fullerene-based materials for photovoltaic applications: Toward efficient, hysteresis-free, and stable perovskite solar cells. Adv. Electron. Mater. 2018, 4, 1700435. [Google Scholar] [CrossRef] [Green Version]
- Camisasca, A.; Giordani, S. Carbon nano-onions for bioimaging and cancer therapy applications. In Nanooncology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 417–455. [Google Scholar] [CrossRef]
- Palkar, A.; Melin, F.; Cardona, C.M.; Elliott, B.; Naskar, A.K.; Edie, D.D.; Kumbhar, A.; Echegoyen, L. Reactivity differences between carbon nano onions (CNOs) prepared by different methods. Chem.-Asian J. 2007, 2, 625–633. [Google Scholar] [CrossRef]
- Camisasca, A.; Giordani, S. Carbon nano-onions in biomedical applications: Promising theranostic agents. Inorg. Chim. Acta 2017, 468, 67–76. [Google Scholar] [CrossRef]
- Lettieri, S.; Camisasca, A.; d’Amora, M.; Diaspro, A.; Uchida, T.; Nakajima, Y.; Yanagisawa, K.; Maekawa, T.; Giordani, S. Far-red fluorescent carbon nano-onions as a biocompatible platform for cellular imaging. RSC Adv. 2017, 7, 45676–45681. [Google Scholar] [CrossRef] [Green Version]
- Zeiger, M.; Jäckel, N.; Mochalin, V.N.; Presser, V. Review: Carbon onions for electrochemical energy storage. J. Mater. Chem. A 2016, 4, 3172–3196. [Google Scholar] [CrossRef] [Green Version]
- Camisasca, A.; Sacco, A.; Brescia, R.; Giordani, S. Boron/nitrogen-codoped carbon nano-onion electrocatalysts for the oxygen reduction reaction. ACS Appl. Nano Mater. 2018, 1, 5763–5773. [Google Scholar] [CrossRef]
- Marchesano, V.; Ambrosone, A.; Bartelmess, J.; Strisciante, F.; Tino, A.; Echegoyen, L.; Tortiglione, C.; Giordani, S. Impact of carbon nano-onions on Hydra vulgaris as a model organism for nanoecotoxicology. Nanomaterials 2015, 5, 1331–1350. [Google Scholar] [CrossRef] [Green Version]
- D’Amora, M.; Maffeis, V.; Brescia, R.; Barnes, D.; Scanlan, E.; Giordani, S. Carbon nano-onions as non-cytotoxic carriers for cellular uptake of glycopeptides and proteins. Nanomaterials 2019, 9, 1069. [Google Scholar] [CrossRef] [Green Version]
- Lettieri, S.; d’Amora, M.; Camisasca, A.; Diaspro, A.; Giordani, S. Carbon nano-onions as fluorescent on/off modulated nanoprobes for diagnostics. Beilstein J. Nanotechnol. 2017, 8, 1878–1888. [Google Scholar] [CrossRef]
- D’Amora, M.; Camisasca, A.; Boarino, A.; Arpicco, S.; Giordani, S. Supramolecular functionalization of carbon nano-onions with hyaluronic acid-phospholipid conjugates for selective targeting of cancer cells. Colloids Surf. B 2020, 188, 110779. [Google Scholar] [CrossRef]
- Bartelmess, J.; Giordani, S. Carbon nano-onions (multi-layer fullerenes): Chemistry and applications. Beilstein J. Nanotechnol. 2014, 5, 1980–1998. [Google Scholar] [CrossRef]
- Flavin, K.; Chaur, M.N.; Echegoyen, L.; Giordani, S. Functionalization of multilayer fullerenes (carbon nano-onions) using diazonium compounds and “click” chemistry. Org. Lett. 2010, 12, 840–843. [Google Scholar] [CrossRef]
- Bartelmess, J.; Frasconi, M.; Balakrishnan, P.B.; Signorelli, A.; Echegoyen, L.; Pellegrino, T.; Giordani, S. Non-covalent functionalization of carbon nano-onions with pyrene–BODIPY dyads for biological imaging. RSC Adv. 2015, 5, 50253–50258. [Google Scholar] [CrossRef]
- Frasconi, M.; Maffeis, V.; Bartelmess, J.; Echegoyen, L.; Giordani, S. Highly surface functionalized carbon nano-onions for bright light bioimaging. Methods Appl. Fluoresc. 2015, 3, 044005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revuri, V.; Cherukula, K.; Nafiujjaman, M.; Cho, K.J.; Park, I.-K.; Lee, Y.-K. White-light-emitting carbon nano-onions: A tunable multichannel fluorescent nanoprobe for glutathione-responsive bioimaging. ACS Appl. Nano Mater. 2018, 1, 662–674. [Google Scholar] [CrossRef]
- Lin, C.-T.; Lee, C.-Y.; Chiu, H.-T.; Chin, T.-S. Graphene structure in carbon nanocones and nanodiscs. Langmuir 2007, 23, 12806–12810. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S.; Yudasaka, M.; Yamada, R.; Bandow, S.; Suenaga, K.; Kokai, F.; Takahashi, K. Nano-aggregates of single-walled graphitic carbon nano-horns. Chem. Phys. Lett. 1999, 309, 165–170. [Google Scholar] [CrossRef]
- Yuge, R.; Yudasaka, M.; Toyama, K.; Yamaguchi, T.; Iijima, S.; Manako, T. Buffer gas optimization in CO2 laser ablation for structure control of single-wall carbon nanohorn aggregates. Carbon 2012, 50, 1925–1933. [Google Scholar] [CrossRef]
- Liu, X.; Ying, Y.; Ping, J. Structure, synthesis, and sensing applications of single-walled carbon nanohorns. Biosens. Bioelectron. 2020, 167, 112495. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Lanceta, A.; Medrano-Bosch, M.; Melgar-Lesmes, P. Single-walled carbon nanohorns as promising nanotube-derived delivery systems to treat cancer. Pharmaceutics 2020, 12, 850. [Google Scholar] [CrossRef] [PubMed]
- Kagkoura, A.; Tagmatarchis, N. Carbon nanohorn-based electrocatalysts for energy conversion. Nanomaterials 2020, 10, 1407. [Google Scholar] [CrossRef]
- Iglesias, D.; Giuliani, A.; Melchionna, M.; Marchesan, S.; Criado, A.; Nasi, L.; Bevilacqua, M.; Tavagnacco, C.; Vizza, F.; Prato, M.; et al. N-doped graphitized carbon nanohorns as a forefront electrocatalyst in highly selective O2 reduction to H2O2. Chem 2018, 4, 106–123. [Google Scholar] [CrossRef]
- Melchionna, M.; Beltram, A.; Montini, T.; Monai, M.; Nasi, L.; Fornasiero, P.; Prato, M. Highly efficient hydrogen production through ethanol photoreforming by a carbon nanocone/Pd@TiO2 hybrid catalyst. Chem. Commun. 2016, 52, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Melchionna, M.; Bracamonte, M.V.; Giuliani, A.; Nasi, L.; Montini, T.; Tavagnacco, C.; Bonchio, M.; Fornasiero, P.; Prato, M. Pd@TiO2/carbon nanohorn electrocatalysts: Reversible CO2 hydrogenation to formic acid. Energy Environ. Sci. 2018, 11, 1571–1580. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Xu, N.; Fan, J.; Sun, W.; Peng, X. Carbon dots for in vivo bioimaging and theranostics. Small 2019, 15, e1805087. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Anwar, S.; Ding, H.; Xu, M.; Hu, X.; Li, Z.; Wang, J.; Liu, L.; Jiang, L.; Wang, D.; Dong, C.; et al. Recent advances in synthesis, optical properties, and biomedical applications of carbon dots. ACS Appl. Bio Mater. 2019, 2, 2317–2338. [Google Scholar] [CrossRef]
- Zhao, P.; Zhu, L. Dispersibility of carbon dots in aqueous and/or organic solvents. Chem. Commun. 2018, 54, 5401–5406. [Google Scholar] [CrossRef]
- Sharma, V.; Tiwari, P.; Mobin, S.M. Sustainable carbon-dots: Recent advances in green carbon dots for sensing and bioimaging. J. Mater. Chem. B 2017, 5, 8904–8924. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, M.; Niu, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Natural-product-derived carbon dots: From natural products to functional materials. ChemSusChem 2018, 11, 11–24. [Google Scholar] [CrossRef]
- Bag, P.; Maurya, R.K.; Dadwal, A.; Sarkar, M.; Chawla, P.A.; Narang, R.K.; Kumar, B. Recent development in synthesis of carbon dots from natural resources and their applications in biomedicine and multi-sensing platform. ChemistrySelect 2021, 6, 2774–2789. [Google Scholar] [CrossRef]
- Kang, C.; Huang, Y.; Yan, X.F.; Yang, H.; Chen, Z.P. A review of carbon dots produced from biomass wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Barati, A.; Karami, S. Long-wavelength, multicolor, and white-light emitting carbon-based dots: Achievements made, challenges remaining, and applications. Carbon 2017, 124, 429–472. [Google Scholar] [CrossRef]
- Xiong, Y.; Schneider, J.; Ushakova, E.V.; Rogach, A.L. Influence of molecular fluorophores on the research field of chemically synthesized carbon dots. Nano Today 2018, 23, 124–139. [Google Scholar] [CrossRef]
- Yao, B.; Huang, H.; Liu, Y.; Kang, Z. Carbon Dots: A small conundrum. Trends Chem. 2019, 1, 235–246. [Google Scholar] [CrossRef]
- Righetto, M.; Carraro, F.; Privitera, A.; Marafon, G.; Moretto, A.; Ferrante, C. The elusive nature of carbon nanodot fluorescence: An unconventional perspective. J. Phys. Chem. C 2020, 124, 22314–22320. [Google Scholar] [CrossRef]
- He, C.; Xu, P.; Zhang, X.; Long, W. The synthetic strategies, photoluminescence mechanisms and promising applications of carbon dots: Current state and future perspective. Carbon 2022, 186, 91–127. [Google Scholar] [CrossRef]
- Rosso, C.; Filippini, G.; Prato, M. Carbon dots as nano-organocatalysts for synthetic applications. ACS Catal. 2020, 10, 8090–8105. [Google Scholar] [CrossRef]
- Hutton, G.A.M.; Martindale, B.C.M.; Reisner, E. Carbon dots as photosensitisers for solar-driven catalysis. Chem. Soc. Rev. 2017, 46, 6111–6123. [Google Scholar] [CrossRef] [Green Version]
- Akbar, K.; Moretti, E.; Vomiero, A. Carbon dots for photocatalytic degradation of aqueous pollutants: Recent advancements. Adv. Opt. Mater. 2021, 9, 2100532. [Google Scholar] [CrossRef]
- Hu, C.; Li, M.; Qiu, J.; Sun, Y.-P. Design and fabrication of carbon dots for energy conversion and storage. Chem. Soc. Rev. 2019, 48, 2315–2337. [Google Scholar] [CrossRef]
- Wang, C.; Strauss, V.; Kaner, R.B. Carbon nanodots for capacitor electrodes. Trends Chem. 2019, 1, 858–868. [Google Scholar] [CrossRef]
- Reina, G.; Zhao, L.; Bianco, A.; Komatsu, N. Chemical functionalization of nanodiamonds: Opportunities and challenges ahead. Angew. Chem. Int. Ed. 2019, 58, 17918–17929. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Guo, Q.; Zhi, J. Nanodiamond-based theranostic platform for drug delivery and bioimaging. Small 2019, 15, e1902238. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.; Rosenholm, J.M. Nanodiamonds for advanced optical bioimaging and beyond. Curr. Opin. Colloid Interface Sci. 2019, 39, 220–231. [Google Scholar] [CrossRef]
- Torelli, M.D.; Nunn, N.A.; Shenderova, O.A. A perspective on fluorescent nanodiamond bioimaging. Small 2019, 15, e1902151. [Google Scholar] [CrossRef] [PubMed]
- Whitlow, J.; Pacelli, S.; Paul, A. Multifunctional nanodiamonds in regenerative medicine: Recent advances and future directions. J. Control. Release 2017, 261, 62–86. [Google Scholar] [CrossRef] [PubMed]
- Namdar, R.; Nafisi, S. Nanodiamond applications in skin preparations. Drug Discov. Today 2018, 23, 1152–1158. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Kedia, D.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Nanodiamonds: Emerging face of future nanotechnology. Carbon 2019, 143, 678–699. [Google Scholar] [CrossRef]
- Saraf, J.; Kalia, K.; Bhattacharya, P.; Tekade, R.K. Growing synergy of nanodiamonds in neurodegenerative interventions. Drug Discov. Today 2019, 24, 584–594. [Google Scholar] [CrossRef]
- Chipaux, M.; van der Laan, K.J.; Hemelaar, S.R.; Hasani, M.; Zheng, T.; Schirhagl, R. Nanodiamonds and their applications in cells. Small 2018, 14, e1704263. [Google Scholar] [CrossRef]
- Hui, Y.Y.; Hsiao, W.W.-W.; Haziza, S.; Simonneau, M.; Treussart, F.; Chang, H.-C. Single particle tracking of fluorescent nanodiamonds in cells and organisms. Curr. Opin. Solid State Mater. Sci. 2017, 21, 35–42. [Google Scholar] [CrossRef]
- Van der Laan, K.J.; Hasani, M.; Zheng, T.; Schirhagl, R. Nanodiamonds for in vivo applications. Small 2018, 14, e1703838. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Sun, X.; Su, D.S.; Centi, G.; Perathoner, S. Catalysis by hybrid sp2/sp3 nanodiamonds and their role in the design of advanced nanocarbon materials. Chem. Soc. Rev. 2018, 47, 8438–8473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Malik, S.; Marchesan, S. Growth, properties, and applications of branched carbon nanostructures. Nanomaterials 2021, 11, 2728. [Google Scholar] [CrossRef]
- Qiu, L.; Ding, F. Understanding single-walled carbon nanotube growth for chirality controllable synthesis. Acc. Mater. Res. 2021, 2, 828–841. [Google Scholar] [CrossRef]
- Melchionna, M.; Prato, M. 3—Functionalization of carbon nanotubes. In Nanocarbon-Inorganic Hybrids: Next Generation Composites for Sustainable Energy Applications; Eder, D., Schlögl, R., Eds.; De Gruyter: Berlin, Germany, 2014; pp. 43–70. [Google Scholar] [CrossRef]
- Deline, A.R.; Frank, B.P.; Smith, C.L.; Sigmon, L.R.; Wallace, A.N.; Gallagher, M.J.; Goodwin, D.G., Jr.; Durkin, D.P.; Fairbrother, D.H. Influence of oxygen-containing functional groups on the environmental properties, transformations, and toxicity of carbon nanotubes. Chem. Rev. 2020, 120, 11651–11697. [Google Scholar] [CrossRef]
- Yang, F.; Wang, M.; Zhang, D.; Yang, J.; Zheng, M.; Li, Y. Chirality pure carbon nanotubes: Growth, sorting, and characterization. Chem. Rev. 2020, 120, 2693–2758. [Google Scholar] [CrossRef]
- Qian, L.; Xie, Y.; Zou, M.; Zhang, J. Building a bridge for carbon nanotubes from nanoscale structure to macroscopic application. J. Am. Chem. Soc. 2021, 143, 18805–18819. [Google Scholar] [CrossRef]
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B.; et al. Carbon nanotubes and related nanomaterials: Critical advances and challenges for synthesis toward mainstream commercial applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Zhao, X.; Shang, Y.; Chang, S.; Dai, L.; Cao, A. Application-driven carbon nanotube functional materials. ACS Nano 2021, 15, 7946–7974. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, A.; Donati, D.M.; Choong, P.; Lucarelli, E.; Wallace, G. Dielectric elastomer actuators, neuromuscular interfaces, and foreign body response in artificial neuromuscular prostheses: A review of the literature for an in vivo application. Adv. Healthc. Mater. 2021, 10, 2100041. [Google Scholar] [CrossRef] [PubMed]
- Mirvakili, S.M.; Hunter, I.W. Artificial muscles: Mechanisms, applications, and challenges. Adv. Mater. 2018, 30, 1704407. [Google Scholar] [CrossRef] [PubMed]
- Gibney, S.; Hicks, J.M.; Robinson, A.; Jain, A.; Sanjuan-Alberte, P.; Rawson, F.J. Toward nanobioelectronic medicine: Unlocking new applications using nanotechnology. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1693. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced carbon for flexible and wearable electronics. Adv. Mater. 2019, 31, e1801072. [Google Scholar] [CrossRef]
- Farrera, C.; Torres, F.A.; Feliu, N. Carbon nanotubes as optical sensors in biomedicine. ACS Nano 2017, 11, 10637–10643. [Google Scholar] [CrossRef]
- Pan, J.; Li, F.; Choi, J.H. Single-walled carbon nanotubes as optical probes for bio-sensing and imaging. J. Mater. Chem. B 2017, 5, 6511–6522. [Google Scholar] [CrossRef]
- Aoki, K.; Ogihara, N.; Tanaka, M.; Haniu, H.; Saito, N. Carbon nanotube-based biomaterials for orthopaedic applications. J. Mater. Chem. B 2020, 8, 9227–9238. [Google Scholar] [CrossRef]
- Vashist, A.; Kaushik, A.; Sagar, V.; Nair, M.; Vashist, A.; Ghosal, A.; Gupta, Y.K.; Ahmad, S. Advances in carbon nanotubes-hydrogel hybrids in nanomedicine for therapeutics. Adv. Healthc. Mater. 2018, 7, e1701213. [Google Scholar] [CrossRef]
- Simon, J.; Flahaut, E.; Simon, J.; Golzio, M. Overview of carbon nanotubes for biomedical applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negri, V.; Pacheco-Torres, J.; Calle, D.; Lopez-Larrubia, P. Carbon nanotubes in biomedicine. Top. Curr. Chem. 2020, 378, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, G.; Vecitis, C.D. Prospects of an electroactive carbon nanotube membrane toward environmental applications. Acc. Chem. Res. 2020, 53, 2892–2902. [Google Scholar] [CrossRef]
- Thamaraiselvan, C.; Wang, J.; James, D.K.; Narkhede, P.; Singh, S.P.; Jassby, D.; Tour, J.M.; Arnusch, C.J. Laser-induced graphene and carbon nanotubes as conductive carbon-based materials in environmental technology. Mater. Today 2020, 34, 115–131. [Google Scholar] [CrossRef]
- Bati, A.S.R.; Yu, L.; Batmunkh, M.; Shapter, J.G. Recent Advances in Applications of Sorted Single-Walled Carbon nanotubes. Adv. Funct. Mater. 2019, 29, 1902273. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube- and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Boncel, S.; Kolanowska, A.; Kuziel, A.W.; Krzyzewska, I. Carbon nanotube wind turbine blades: How Far are we today from laboratory tests to industrial implementation? ACS Appl. Nano Mater. 2018, 1, 6542–6555. [Google Scholar] [CrossRef]
- Jeon, I.; Xiang, R.; Shawky, A.; Matsuo, Y.; Maruyama, S. Single-walled carbon nanotubes in emerging solar cells: Synthesis and electrode applications. Adv. Energy Mater. 2019, 9, 1801312. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Wang, Y.; Zhang, Q. Roles of carbon nanotubes in novel energy storage devices. Carbon 2017, 122, 462–474. [Google Scholar] [CrossRef]
- He, M.; Zhang, S.; Zhang, J. Horizontal single-walled carbon nanotube arrays: Controlled synthesis, characterizations, and applications. Chem. Rev. 2020, 120, 12592–12684. [Google Scholar] [CrossRef]
- Gaviria Rojas, W.A.; Hersam, M.C. Chirality-enriched carbon nanotubes for next-generation computing. Adv. Mater. 2020, 32, 1905654. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Quan, X. Carbon-based materials for electrochemical reduction of CO2 to C2+ oxygenates: Recent progress and remaining challenges. ACS Catal. 2021, 11, 2076–2097. [Google Scholar] [CrossRef]
- Sheng, J.; Li, Y. Applications of carbon nanotubes in oxygen electrocatalytic reactions. ACS Appl. Mater. Interfaces, 2021; in press. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Zhu, L.; Chen, H.; Qu, J.; Dai, L. Recent advances in carbon-based metal-free electrocatalysts. Adv. Mater. 2019, 31, e1806403. [Google Scholar] [CrossRef]
- Iglesias, D.; Melchionna, M. Enter the tubes: Carbon nanotube endohedral catalysis. Catalysts 2019, 9, 128. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Ganesan, P.; Ishihara, A.; Nakashima, N. Carbon Nanotube-based non-precious metal electrode catalysts for fuel cells, water splitting and zinc-air batteries. ChemCatChem 2019, 11, 5929–5944. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, J.; Duyar, M.S.; Ordomsky, V.V.; Khodakov, A.Y.; Liu, J. Carbon-based catalysts for Fischer-Tropsch synthesis. Chem. Soc. Rev. 2021, 50, 2337–2366. [Google Scholar] [CrossRef]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vázquez, E.; Ballerini, L.; et al. Classification framework for graphene-based materials. Angew. Chem. Int. Ed. 2014, 53, 7714–7718. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Wu, H.; Chen, H.; Xu, G.; Li, C. Graphene oxide in aqueous and nonaqueous media: Dispersion behaviour and solution chemistry. Carbon 2020, 158, 568–579. [Google Scholar] [CrossRef]
- Nishina, Y.; Eigler, S. Chemical and electrochemical synthesis of graphene oxide—A generalized view. Nanoscale 2020, 12, 12731–12740. [Google Scholar] [CrossRef]
- Jakhar, R.; Yap, J.E.; Joshi, R. Microwave reduction of graphene oxide. Carbon 2020, 170, 277–293. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.-H.; Joshi, R.; Yoshimura, M. Restoration of the graphitic structure by defect repair during the thermal reduction of graphene oxide. Carbon 2020, 166, 74–90. [Google Scholar] [CrossRef]
- Liu, J.; Feng, X. Synthetic tailoring of graphene nanostructures with zigzag-edged topologies: Progress and perspectives. Angew. Chem. Int. Ed. 2020, 59, 23386–23401. [Google Scholar] [CrossRef] [PubMed]
- Jolly, A.; Miao, D.; Daigle, M.; Morin, J.-F. Emerging bottom-up strategies for the synthesis of graphene nanoribbons and related structures. Angew. Chem. Int. Ed. 2020, 59, 4624–4633. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, D.G.; Li, Z.; Liu, M.; Kinloch, I.A.; Young, R.J. Mechanisms of mechanical reinforcement by graphene and carbon nanotubes in polymer nanocomposites. Nanoscale 2020, 12, 2228–2267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Lee, Y.; Kang, M.; Hu, L.; Zhao, S.; Ahn, J.-H. 2D Materials for skin-mountable electronic devices. Adv. Mater. 2021, 33, 2005858. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shehzad, K.; Gao, L.; Long, M.; Guo, H.; Qin, S.; Wang, X.; Wang, F.; Shi, Y.; Hu, W.; et al. Graphene hybrid structures for integrated and flexible optoelectronics. Adv. Mater. 2020, 32, e1902039. [Google Scholar] [CrossRef] [PubMed]
- You, R.; Liu, Y.-Q.; Hao, Y.-L.; Han, D.-D.; Zhang, Y.-L.; You, Z. Laser fabrication of graphene-based flexible electronics. Adv. Mater. 2020, 32, e1901981. [Google Scholar] [CrossRef] [PubMed]
- Sattari-Esfahlan, S.M.; Kim, C.-H. Flexible graphene-channel memory devices: A review. ACS Appl. Nano Mater. 2021, 4, 6542–6556. [Google Scholar] [CrossRef]
- Kim, S.D.; Sarkar, A.; Ahn, J.-H. Graphene-based nanomaterials for flexible and stretchable batteries. Small 2021, 17, 2006262. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Muni, M.; Strauss, V.; Borenstein, A.; Chang, X.; Huang, A.; Qu, S.; Sung, K.; Gilham, T.; Kaner, R.B. Graphene’s role in emerging trends of capacitive energy storage. Small 2021, 17, 2006875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, D.; Lau, A.; Ma, T.; Lin, H.; Jia, B. Hybridized graphene for supercapacitors: Beyond the limitation of pure graphene. Small 2021, 17, 2007311. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, H.; Yang, Q.-H. Compact energy storage enabled by graphenes: Challenges, strategies and progress. Mater. Today 2021, 51, 552–565. [Google Scholar] [CrossRef]
- Lu, B.; Jin, X.; Han, Q.; Qu, L. Planar graphene-based microsupercapacitors. Small 2021, 17, 2006827. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Maegawa, K.; Tan, W.K.; Kawamura, G.; Kar, K.K.; Matsuda, A. Heteroatom doped graphene engineering for energy storage and conversion. Mater. Today 2020, 39, 47–65. [Google Scholar] [CrossRef]
- Bhol, P.; Yadav, S.; Altaee, A.; Saxena, M.; Misra, P.K.; Samal, A.K. Graphene-based membranes for water and wastewater treatment: A review. ACS Appl. Nano Mater. 2021, 4, 3274–3293. [Google Scholar] [CrossRef]
- Zhao, G.; Zhu, H. Cation-π interactions in graphene-containing systems for water treatment and beyond. Adv. Mater. 2020, 32, 1905756. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Nishina, Y. Graphene-based carbocatalysts for carbon-carbon bond formation. Nanoscale 2020, 12, 12210–12227. [Google Scholar] [CrossRef]
- Cui, H.; Guo, Y.; Zhou, Z. Three-dimensional graphene-based macrostructures for electrocatalysis. Small 2021, 17, 2005255. [Google Scholar] [CrossRef]
- Bie, C.; Yu, H.; Cheng, B.; Ho, W.; Fan, J.; Yu, J. Design, fabrication, and mechanism of nitrogen-doped graphene-based photocatalyst. Adv. Mater. 2021, 33, e2003521. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, H.-Y.; Zhang, X.; Liang, J.-X.; Yu, Q.; Xiao, H.; Li, J. Theoretical understandings of graphene-based metal single-atom catalysts: Stability and catalytic performance. Chem. Rev. 2020, 120, 12315–12341. [Google Scholar] [CrossRef] [PubMed]
- Reina, G.; Iglesias, D.; Samori, P.; Bianco, A. Graphene: A disruptive opportunity for COVID-19 and future pandemics? Adv. Mater. 2021, 33, 2007847. [Google Scholar] [CrossRef] [PubMed]
- Bellet, P.; Gasparotto, M.; Pressi, S.; Fortunato, A.; Scapin, G.; Mba, M.; Menna, E.; Filippini, F. Graphene-based scaffolds for regenerative medicine. Nanomaterials 2021, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jing, Q.; Ao, S.; Schneider, G.F.; Kireev, D.; Zhang, Z.; Fu, W. Ultrasensitive Field-Effect Biosensors Enabled by the Unique Electronic Properties of Graphene. Small 2020, 16, e1902820. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Lee, J.-H.; Shen, X.; Chen, X.; Kim, J.-K. Graphene-based wearable piezoresistive physical sensors. Mater. Today 2020, 36, 158–179. [Google Scholar] [CrossRef]
- Wyss, K.M.; Luong, D.X.; Tour, J.M. Large-scale syntheses of 2-D Materials: Flash joule heating and other methods. Adv. Mater. 2021, 2106970. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Hu, Y.H. Ultrafast, low-cost, and mass production of high-quality graphene. Angew. Chem. Int. Ed. 2020, 59, 9232–9234. [Google Scholar] [CrossRef]
- Zhu, Y.; Qu, B.; Andreeva, D.V.; Ye, C.; Novoselov, K.S. Graphene standardization: The lesson from the East. Mater. Today 2021, 47, 9–15. [Google Scholar] [CrossRef]
- Adorinni, S.; Rozhin, P.; Marchesan, S. Smart hydrogels meet carbon nanomaterials for new frontiers in medicine. Biomedicines 2021, 9, 570. [Google Scholar] [CrossRef] [PubMed]
- Rahamathulla, M.; Bhosale, R.R.; Osmani, R.A.M.; Mahima, K.C.; Johnson, A.P.; Hani, U.; Ghazwani, M.; Begum, M.Y.; Alshehri, S.; Ghoneim, M.M.; et al. Carbon nanotubes: Current perspectives on diverse applications in targeted drug delivery and therapies. Materials 2021, 14, 6707. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Saito, N. Biocompatibility and carcinogenicity of carbon nanotubes as biomaterials. Nanomaterials 2020, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Saleemi, M.A.; Fouladi, M.H.; Yong, P.V.C.; Chinna, K.; Palanisamy, N.K.; Wong, E.H. Toxicity of carbon nanotubes: Molecular mechanisms, signaling cascades, and remedies in biomedical applications. Chem. Res. Toxicol. 2021, 34, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.A.; Jena, P.V.; Pasquali, M.; Kostarelos, K.; Delogu, L.G.; Meidl, R.E.; Rotkin, S.V.; Scheinberg, D.A.; Schwartz, R.E.; Terrones, M.; et al. Banning carbon nanotubes would be scientifically unjustified and damaging to innovation. Nat. Nanotechnol. 2020, 15, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Zygouri, P.; Spyrou, K.; Mitsari, E.; Barrio, M.; Macovez, R.; Patila, M.; Stamatis, H.; Verginadis, I.I.; Velalopoulou, A.P.; Evangelou, A.M.; et al. A facile approach to hydrophilic oxidized fullerenes and their derivatives as cytotoxic agents and supports for nanobiocatalytic systems. Sci. Rep. 2020, 10, 8244. [Google Scholar] [CrossRef] [PubMed]
- Beilinson, R.M.; Yavisheva, A.A.; Medyantseva, E.P.; Budnikov, H.C. Amperometric tyrosinase biosensors modified by nanomaterials of different nature for determining diclofenac. J. Anal. Chem. 2021, 76, 653–659. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, S.; Zhao, M.; Zhang, P.; Xin, Z.; Tao, J.; Bai, L. Amperometric DNA biosensor for Mycobacterium tuberculosis detection using flower-like carbon nanotubes-polyaniline nanohybrid and enzyme-assisted signal amplification strategy. Biosens. Bioelectron. 2018, 119, 215–220. [Google Scholar] [CrossRef]
- Sharma, S.K.; Micic, M.; Li, S.; Hoar, B.; Paudyal, S.; Zahran, E.M.; Leblanc, R.M. Conjugation of carbon dots with β-galactosidase enzyme: Surface chemistry and use in biosensing. Molecules 2019, 24, 3275. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Kashayap, S.; Singh, V.; Kayastha, A.M.; Mishra, H.; Saxena, P.S.; Srivastava, A.; Singh, R.K. QPRTase modified N-doped carbon quantum dots: A fluorescent bioprobe for selective detection of neurotoxin quinolinic acid in human serum. Biosens. Bioelectron. 2018, 101, 103–109. [Google Scholar] [CrossRef]
- Hetmann, A.; Wujak, M.; Bolibok, P.; Zięba, W.; Wiśniewski, M.; Roszek, K. Novel biocatalytic systems for maintaining the nucleotide balance based on adenylate kinase immobilized on carbon nanostructures. Mater. Sci. Eng. C 2018, 88, 130–139. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, C.; Liu, X.-P.; Wei, Y.-P.; Mao, C.-J.; Zhu, J.-J. A novel electrochemiluminescence biosensor for the detection of microRNAs based on a DNA functionalized nitrogen doped carbon quantum dots as signal enhancers. Biosens. Bioelectron. 2017, 92, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, Y.; Wang, H.; Wang, B.; Zhou, Y.; Liu, Y.; Shao, M.; Huang, H.; Lu, F.; Kang, Z. Chiral control of carbon dots via surface modification for tuning the enzymatic activity of glucose oxidase. ACS Appl. Mater. Interfaces 2021, 13, 5877–5886. [Google Scholar] [CrossRef] [PubMed]
- Bravo, I.; Gutierrez-Sanchez, C.; Garcia-Mendiola, T.; Revenga-Parra, M.; Pariente, F.; Lorenzo, E. Enhanced performance of reagent-less carbon nanodots based enzyme electrochemical biosensors. Sensors 2019, 19, 5576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Wang, W.; Wu, F.; Zheng, T.; Ashley, J.; Mohammadniaei, M.; Zhang, Q.; Wang, M.; Li, L.; Shen, J.; et al. Biodegradable poly(γ-glutamic acid)@glucose oxidase@carbon dot nanoparticles for simultaneous multimodal imaging and synergetic cancer therapy. Biomaterials 2020, 252, 120106. [Google Scholar] [CrossRef]
- Nashruddin, S.N.A.; Abdullah, J.; Haniff, M.A.S.M.; Zaid, M.H.M.; Choon, O.P.; Wee, M.F.M.R. Label free glucose electrochemical biosensor based on poly(3,4-ethylenedioxy thiophene): Polystyrene sulfonate/titanium carbide/graphene quantum dots. Biosensors 2021, 11, 267. [Google Scholar] [CrossRef]
- Cho, M.-J.; Park, S.-Y. Carbon-dot-based ratiometric fluorescence glucose biosensor. Sens. Actuators B 2019, 282, 719–729. [Google Scholar] [CrossRef]
- Wu, G.; Gao, Y.; Zhao, D.; Ling, P.; Gao, F. Methanol/oxygen enzymatic biofuel cell using laccase and NAD+-dependent dehydrogenase cascades as biocatalysts on carbon nanodots electrodes. ACS Appl. Mater. Interfaces 2017, 9, 40978–40986. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Wang, B.; Ma, Y.; Huang, H.; Liu, Y.; Shao, M.; Yao, B.; Kang, Z. Maltase decorated by chiral carbon dots with inhibited enzyme activity for glucose level control. Small 2019, 15, e1901512. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.H.; Tieves, F.; Choi, D.S.; Hollmann, F.; Paul, C.E.; Park, C.B. Biocatalytic C=C bond reduction through carbon nanodot-sensitized regeneration of NADH analogues. Angew. Chem. Int. Ed. 2018, 57, 13825–13828. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Development of a novel electrochemical biosensor based on carbon nanofibers-cobalt phthalocyanine-laccase for the detection of p-coumaric acid in phytoproducts. Int. J. Mol. Sci. 2021, 22, 9302. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Development of a novel electrochemical biosensor based on carbon nanofibers-gold nanoparticles-tyrosinase for the detection of ferulic acid in cosmetics. Sensors 2020, 20, 6724. [Google Scholar] [CrossRef] [PubMed]
- Ford, R.; Devereux, S.J.; Quinn, S.J.; O’Neill, R.D. Carbon nanohorn modified platinum electrodes for improved immobilisation of enzyme in the design of glutamate biosensors. Analyst 2019, 144, 5299–5307. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Li, X.; Zhang, L.; Zhong, W.; Wang, J. Highly sensitive electrochemiluminescent immunoassay for neuron-specific enolase amplified by single-walled carbon nanohorns and enzymatic biocatalytic precipitation. J. Electroanal. Chem. 2018, 818, 257–264. [Google Scholar] [CrossRef]
- Sok, V.; Fragoso, A. Preparation and characterization of alkaline phosphatase, horseradish peroxidase, and glucose oxidase conjugates with carboxylated carbon nano-onions. Prep. Biochem. Biotechnol. 2018, 48, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, J.; Ananthoju, B.; Nair, V.; Mitra, A.; Bahadur, D.; Medhekar, N.V.; Aslam, M. Enzymatic and non-enzymatic electrochemical glucose sensor based on carbon nano-onions. Appl. Surf. Sci. 2018, 442, 332–341. [Google Scholar] [CrossRef]
- Sok, V.; Fragoso, A. Carbon nano-onion peroxidase composite biosensor for electrochemical detection of 2,4-D and 2,4,5-T. Appl. Sci. 2021, 11, 6889. [Google Scholar] [CrossRef]
- Povedano, E.; Cincotto, F.H.; Parrado, C.; Diez, P.; Sanchez, A.; Canevari, T.C.; Machado, S.A.S.; Pingarron, J.M.; Villalonga, R. Decoration of reduced graphene oxide with rhodium nanoparticles for the design of a sensitive electrochemical enzyme biosensor for 17β-estradiol. Biosens. Bioelectron. 2017, 89, 343–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albishri, H.M.; El-Hady, D.A. Hyphenation of enzyme/graphene oxide-ionic liquid/glassy carbon biosensors with anodic differential pulse stripping voltammetry for reliable determination of choline and acetylcholine in human serum. Talanta 2019, 200, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Anton-Millan, N.; Garcia-Tojal, J.; Marty-Roda, M.; Garroni, S.; Cuesta-Lopez, S.; Tamayo-Ramos, J.A. Influence of three commercial graphene derivatives on the catalytic properties of a lactobacillus plantarum α-L-rhamnosidase when used as immobilization matrices. ACS Appl. Mater. Interfaces 2018, 10, 18170–18182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Z.; Jiao, K.; Xu, X.; Peng, R.; Jiao, S.; Hu, Z. Graphene oxide-supported carbon nanofiber-like network derived from polyaniline: A novel composite for enhanced glucose oxidase bioelectrode performance. Biosens. Bioelectron. 2017, 96, 367–372. [Google Scholar] [CrossRef]
- Fotiadou, R.; Patila, M.; Hammami, M.A.; Enotiadis, A.; Moschovas, D.; Tsirka, K.; Spyrou, K.; Giannelis, E.P.; Avgeropoulos, A.; Paipetis, A.; et al. Development of effective lipase-hybrid nanoflowers enriched with carbon and magnetic nanomaterials for biocatalytic transformations. Nanomaterials 2019, 9, 808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakchin, P.S.; Ghanbari, H.; Saber, R.; Omidi, Y. Electrochemical immunosensor based on chitosan-gold nanoparticle/carbon nanotube as a platform and lactate oxidase as a label for detection of CA125 oncomarker. Biosens. Bioelectron. 2018, 122, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Z.; Xie, Q.; Li, Y.; Ying, Y.; Fu, Y. Bioinspired assembly of reduced graphene oxide by fibrin fiber to prepare multi-functional conductive bionanocomposites as versatile electrochemical platforms. Carbon 2019, 153, 504–512. [Google Scholar] [CrossRef]
- Magar, H.S.; Ghica, M.E.; Abbas, M.N.; Brett, C.M.A. A novel sensitive amperometric choline biosensor based on multiwalled carbon nanotubes and gold nanoparticles. Talanta 2017, 167, 462–469. [Google Scholar] [CrossRef]
- Hyun, K.; Kang, S.; Kim, J.; Kwon, Y. New biocatalyst including a 4-nitrobenzoic acid mediator embedded by the cross-linking of chitosan and genipin and its use in an energy device. ACS Appl. Mater. Interfaces 2020, 12, 23635–23643. [Google Scholar] [CrossRef]
- Kim, B.C.; Lee, I.; Kwon, S.-J.; Wee, Y.; Kwon, K.Y.; Jeon, C.; An, H.J.; Jung, H.-T.; Ha, S.; Dordick, J.S.; et al. Fabrication of enzyme-based coatings on intact multi-walled carbon nanotubes as highly effective electrodes in biofuel cells. Sci. Rep. 2017, 7, 40202. [Google Scholar] [CrossRef]
- Luo, X.; Shi, W.; Liu, Y.; Sha, P.; Chu, Y.; Cui, Y. A smart tongue depressor-based biosensor for glucose. Sensors 2019, 19, 3864. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Li, T.; Jiang, M.; Wei, C.; Ma, S.; Chen, D.; Tong, W.; Huang, X. Construction of flexible enzymatic electrode based on gradient hollow fiber membrane and multi-wall carbon tubes meshes. Biosens. Bioelectron. 2020, 152, 112001. [Google Scholar] [CrossRef]
- Inamuddin; Shakeel, N.; Ahamed, M.I.; Kanchi, S.; Kashmery, H.A. Green synthesis of ZnO nanoparticles decorated on polyindole functionalized-MCNTs and used as anode material for enzymatic biofuel cell applications. Sci. Rep. 2020, 10, 5052. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Liu, Y.; Wang, J.; Wei, T.; Dai, Z. Mild hyperthermia-enhanced enzyme-mediated tumor cell chemodynamic therapy. ACS Appl. Mater. Interfaces 2019, 11, 23065–23071. [Google Scholar] [CrossRef]
- Gentil, S.; Rousselot-Pailley, P.; Sancho, F.; Robert, V.; Mekmouche, Y.; Guallar, V.; Tron, T.; Le Goff, A. Efficiency of Site-specific clicked laccase-carbon nanotubes biocathodes towards O2 reduction. Chem. Eur. J. 2020, 26, 4798–4804. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, T.; Ostric, A.; Andreola, F.; Filocamo, M.; Pietrogrande, M.; Corsolini, F.; Stroppiano, M.; Bruni, S.; Serafino, A.; Fiorito, S. Carbon nanotubes as nanovectors for intracellular delivery of laronidase in Mucopolysaccharidosis type I. Nanoscale 2018, 10, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Su, F.; Li, K.; Ke, C.; Yan, Y. Carbon nanotube filled with magnetic iron oxide and modified with polyamidoamine dendrimers for immobilizing lipase toward application in biodiesel production. Sci. Rep. 2017, 7, 45643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, L.Y.; Mubarak, N.M.; Yon, L.S.; Bing, C.H.; Khalid, M.; Jagadish, P.; Abdullah, E.C. Immobilization of peroxidase on functionalized MWCNTs-buckypaper/polyvinyl alcohol nanocomposite membrane. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezaei, B.; Shoushtari, A.M.; Rabiee, M.; Uzun, L.; Mak, W.C.; Turner, A.P.F. An electrochemical immunosensor for cardiac Troponin I using electrospun carboxylated multi-walled carbon nanotube-whiskered nanofibres. Talanta 2018, 182, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hong, S.-G.; Wee, Y.; Hu, S.; Kwon, Y.; Ha, S.; Kim, J. Enzyme precipitate coating of pyranose oxidase on carbon nanotubes and their electrochemical applications. Biosens. Bioelectron. 2017, 87, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Wongnate, T.; Chuaboon, L.; Chaiyen, P.; Yu, E.H. Enzymatic fuel cells with an oxygen resistant variant of pyranose-2-oxidase as anode biocatalyst. Biosens. Bioelectron. 2018, 107, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wee, Y.; Park, S.; Kwon, Y.H.; Ju, Y.; Yeon, K.-M.; Kim, J. Tyrosinase-immobilized CNT based biosensor for highly-sensitive detection of phenolic compounds. Biosens. Bioelectron. 2019, 132, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, H.; Liu, P.; Cheng, J. A 3D electrochemical biosensor based on Super-Aligned Carbon NanoTube array for point-of-care uric acid monitoring. Biosens. Bioelectron. 2021, 179, 113082. [Google Scholar] [CrossRef]
- Dalkiran, B.; Erden, P.E.; Kilic, E. Amperometric biosensors based on carboxylated multiwalled carbon nanotubes-metal oxide nanoparticles-7,7,8,8-tetracyanoquinodimethane composite for the determination of xanthine. Talanta 2017, 167, 286–295. [Google Scholar] [CrossRef]
- Zappi, D.; Caminiti, R.; Ingo, G.M.; Sadun, C.; Tortolini, C.; Antonelli, M.L. Biologically friendly room temperature ionic liquids and nanomaterials for the development of innovative enzymatic biosensors. Talanta 2017, 175, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Zappi, D.; Gabriele, S.; Gontrani, L.; Dini, D.; Sadun, C.; Marini, F.; Antonelli, M.L. Biologically friendly room temperature ionic liquids and nanomaterials for the development of innovative enzymatic biosensors: Part II. Talanta 2019, 194, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Blazek, T.; Gorski, W. Oxidases, carbon nanotubes, and direct electron transfer: A cautionary tale. Biosens. Bioelectron. 2020, 163, 112260. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Patnode, K.; Li, H.; Baryeh, K.; Liu, G.; Hu, J.; Chen, B.; Pan, Y.; Yang, Z. Enhancing enzyme immobilization on carbon nanotubes via metal-organic frameworks for large-substrate biocatalysis. ACS Appl. Mater. Interfaces 2019, 11, 12133–12141. [Google Scholar] [CrossRef] [PubMed]
- Gentil, S.; Lalaoui, N.; Dutta, A.; Nedellec, Y.; Cosnier, S.; Shaw, W.J.; Artero, V.; Le Goff, A. Carbon nanotube-supported bio-inspired nickel catalyst and its integration in hybrid hydrogen/air fuel cells. Angew. Chem. Int. Ed. 2017, 56, 1845–1849. [Google Scholar] [CrossRef] [PubMed]
- Zelechowska, K.; Trawinski, B.; Draminska, S.; Majdecka, D.; Bilewicz, R.; Kusz, B. Oxygen biosensor based on carbon nanotubes directly grown on graphitic substrate. Sens. Actuators B Chem. 2017, 240, 1308–1313. [Google Scholar] [CrossRef]
- Boussema, F.; Gross, A.J.; Hmida, F.; Ayed, B.; Majdoub, H.; Cosnier, S.; Maaref, A.; Holzinger, M. Dawson-type polyoxometalate nanoclusters confined in a carbon nanotube matrix as efficient redox mediators for enzymatic glucose biofuel cell anodes and glucose biosensors. Biosens. Bioelectron. 2018, 109, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Zhao, H. Bimetallic Fe/Mn metal-organic-frameworks and Au nanoparticles anchored carbon nanotubes as a peroxidase-like detection platform with increased active sites and enhanced electron transfer. Talanta 2020, 210, 120678. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Zhu, Y.; Wu, J.; Li, K.; Lin, G.; Li, X.; Liu, R.; Liu, X.; Wong, C.-P. Facile one-step fabrication of glucose oxidase loaded polymeric nanoparticles decorating MWCNTs for constructing glucose biosensing platform: Structure matters. Biosens. Bioelectron. 2019, 135, 153–159. [Google Scholar] [CrossRef]
- Amatatongchai, M.; Sroysee, W.; Chairam, S.; Nacapricha, D. Amperometric flow injection analysis of glucose using immobilized glucose oxidase on nano-composite carbon nanotubes-platinum nanoparticles carbon paste electrode. Talanta 2017, 166, 420–427. [Google Scholar] [CrossRef]
- Chen, C.; Ran, R.; Yang, Z.; Lv, R.; Shen, W.; Kang, F.; Huang, Z.-H. An efficient flexible electrochemical glucose sensor based on carbon nanotubes/carbonized silk fabrics decorated with Pt microspheres. Sens. Actuators B Chem. 2018, 256, 63–70. [Google Scholar] [CrossRef]
- Da Silva, W.; Ghica, M.E.; Brett, C.M.A. Biotoxic trace metal ion detection by enzymatic inhibition of a glucose biosensor based on a poly(brilliant green)-deep eutectic solvent/carbon nanotube modified electrode. Talanta 2020, 208, 120427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, L.; Han, Y.; Yu, Y.; Xu, M.; Zhang, X.; Huang, L.; Dong, S. RGO/Au NPs/N-doped CNTs supported on nickel foam as an anode for enzymatic biofuel cells. Biosens. Bioelectron. 2017, 97, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Christwardana, M.; Chung, Y.; Kwon, Y. Co-immobilization of glucose oxidase and catalase for enhancing the performance of a membraneless glucose biofuel cell operated under physiological conditions. Nanoscale 2017, 9, 1993–2002. [Google Scholar] [CrossRef]
- Kang, Z.; Jiao, K.; Cheng, J.; Peng, R.; Jiao, S.; Hu, Z. A novel three-dimensional carbonized PANI1600@CNTs network for enhanced enzymatic biofuel cell. Biosens. Bioelectron. 2018, 101, 60–65. [Google Scholar] [CrossRef]
- Maity, D.; Kumar, R.T.R. Highly sensitive amperometric detection of glutamate by glutamic oxidase immobilized Pt nanoparticle decorated multiwalled carbon nanotubes(MWCNTs)/polypyrrole composite. Biosens. Bioelectron. 2019, 130, 307–314. [Google Scholar] [CrossRef]
- Coelho, J.H.; Eisele, A.P.P.; Valezi, C.F.; Mattos, G.J.; Schirmann, J.G.; Dekker, R.F.H.; Barbosa-Dekker, A.M.; Sartori, E.R. Exploring the exocellular fungal biopolymer botryosphaeran for laccase-biosensor architecture and application to determine dopamine and spironolactone. Talanta 2019, 204, 475–483. [Google Scholar] [CrossRef]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Microneedle-based biosensor for minimally-invasive lactate detection. Biosens. Bioelectron. 2019, 123, 152–159. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Xiang, X.; Tang, S.; Yu, D.; Huang, H.; Hu, Y. Immobilization of Candida antarctic lipase B on MWNTs modified by ionic liquids with different functional groups. Colloids Surf. B 2017, 160, 416–422. [Google Scholar] [CrossRef]
- Di Tocco, A.; Robledo, S.N.; Osuna, Y.; Sandoval-Cortez, J.; Granero, A.M.; Vettorazzi, N.R.; Martinez, J.L.; Segura, E.P.; Ilina, A.; Zon, M.A.; et al. Development of an electrochemical biosensor for the determination of triglycerides in serum samples based on a lipase/magnetite-chitosan/copper oxide nanoparticles/multiwalled carbon nanotubes/pectin composite. Talanta 2018, 190, 30–37. [Google Scholar] [CrossRef]
- Markiton, M.; Boncel, S.; Janas, D.; Chrobok, A. Highly active nanobiocatalyst from lipase noncovalently immobilized on multiwalled carbon nanotubes for baeyer-villiger synthesis of lactones. ACS Sustain. Chem. Eng. 2017, 5, 1685–1691. [Google Scholar] [CrossRef]
- Gentil, S.; Mansor, S.M.C.; Jamet, H.; Cosnier, S.; Cavazza, C.; Le Goff, A. Oriented immobilization of [NiFeSe] hydrogenases on covalently and noncovalently functionalized carbon nanotubes for H2/air enzymatic fuel cells. ACS Catal. 2018, 8, 3957–3964. [Google Scholar] [CrossRef]
- Franco, J.H.; Klunder, K.J.; Lee, J.; Russell, V.; de Andrade, A.R.; Minteer, S.D. Enhanced electrochemical oxidation of ethanol using a hybrid catalyst cascade architecture containing pyrene-TEMPO, oxalate decarboxylase and carboxylated multi-walled carbon nanotube. Biosens. Bioelectron. 2020, 154, 112077. [Google Scholar] [CrossRef]
- Bocanegra-Rodriguez, S.; Molins-Legua, C.; Campins-Falco, P.; Giroud, F.; Gross, A.J.; Cosnier, S. Monofunctional pyrenes at carbon nanotube electrodes for direct electron transfer H2O2 reduction with HRP and HRP-bacterial nanocellulose. Biosens. Bioelectron. 2021, 187, 113304. [Google Scholar] [CrossRef] [PubMed]
- Chokkareddy, R.; Bhajanthri, N.K.; Redhi, G.G. An enzyme-induced novel biosensor for the sensitive electrochemical determination of isoniazid. Biosensors 2017, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Yaari, Z.; Cheung, J.M.; Baker, H.A.; Frederiksen, R.S.; Jena, P.V.; Horoszko, C.P.; Jiao, F.; Scheuring, S.; Luo, M.; Heller, D.A. Nanoreporter of an enzymatic suicide inactivation pathway. Nano Lett. 2020, 20, 7819–7827. [Google Scholar] [CrossRef]
- Ding, H.; Hu, B.; Zhang, B.; Zhang, H.; Yan, X.; Nie, G.; Liang, M. Carbon-based nanozymes for biomedical applications. Nano Res. 2021, 14, 570–583. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Shang, Y.; Liu, F.; Wang, Y.; Li, N.; Ding, B. Enzyme mimic nanomaterials and their biomedical applications. ChemBioChem 2020, 21, 2408–2418. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Chandrawati, R.; Hosta-Rigau, L. Therapeutic applications of multifunctional nanozymes. Nanoscale 2019, 11, 21046–21060. [Google Scholar] [CrossRef]
- Wu, Y.; Darland, D.C.; Zhao, J.X. Nanozymes—Hitting the biosensing “target”. Sensors 2021, 21, 5201. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhao, B.; Wang, C.; Ozaki, Y.; Lu, X. Functional nanomaterials with unique enzyme-like characteristics for sensing applications. J. Mater. Chem. B 2019, 7, 850–875. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yang, F.; Nie, C.; Ma, L.; Cheng, C.; Haag, R. Biocatalytic nanomaterials: A new pathway for bacterial disinfection. Adv. Mater. 2021, 33, 2100637. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon nanozymes: Enzymatic Properties, catalytic mechanism, and applications. Angew. Chem. Int. Ed. 2018, 57, 9224–9237. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hojamberdiev, M.; Geng, D. Recent advances in enzyme-free electrochemical hydrogen peroxide sensors based on carbon hybrid nanocomposites. J. Mater. Chem. C 2021, 9, 6970–6990. [Google Scholar] [CrossRef]
- Liu, X.; Huang, D.; Lai, C.; Qin, L.; Zeng, G.; Xu, P.; Li, B.; Yi, H.; Zhang, M. Peroxidase-Like activity of smart nanomaterials and their advanced application in colorimetric glucose biosensors. Small 2019, 15, e1900133. [Google Scholar] [CrossRef] [PubMed]
- Nothling, M.D.; Xiao, Z.; Bhaskaran, A.; Blyth, M.T.; Bennett, C.W.; Coote, M.L.; Connal, L.A. Synthetic catalysts inspired by hydrolytic enzymes. ACS Catal. 2019, 9, 168–187. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Zhou, S. Progress and perspective on carbon-based nanozymes for peroxidase-like applications. J. Phys. Chem. Lett. 2021, 12, 11751–11760. [Google Scholar] [CrossRef]
- Li, Y.; Ma, W.; Sun, J.; Lin, M.; Niu, Y.; Yang, X.; Xu, Y. Electrochemical generation of Fe3C/N-doped graphitic carbon nanozyme for efficient wound healing in vivo. Carbon 2020, 159, 149–160. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, M.; Huang, Y.; Xia, Y. Carbon dot nanozymes: How to be close to natural enzymes. Chem. Eur. J. 2019, 25, 954–960. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, X.; Tang, D.; Wu, S.; Hou, X.; Liu, J.; Wu, P. Phosphorescent carbon dots for highly efficient oxygen photosensitization and as photo-oxidative nanozymes. ACS Appl. Mater. Interfaces 2018, 10, 40808–40814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, S.; Ma, L.; Wu, P.; Liu, J. Graphene oxide as a photocatalytic nuclease mimicking nanozyme for DNA cleavage. Nano Res. 2020, 13, 455–460. [Google Scholar] [CrossRef]
- Zhan, Y.; Yang, S.; Luo, F.; Guo, L.; Zeng, Y.; Qiu, B.; Lin, Z. Emission wavelength switchable carbon dots combined with biomimetic inorganic nanozymes for a two-photon fluorescence immunoassay. ACS Appl. Mater. Interfaces 2020, 12, 30085–30094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, S.; Lu, X.; Wu, P.; Liu, J. Manganese as a catalytic mediator for photooxidation and breaking the pH limitation of nanozymes. Nano Lett. 2019, 19, 3214–3220. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, S.; Guo, X.; Dong, Y.; Yao, C.; Liu, Y.; Song, Y.; Tan, X.; Gao, L.; Yang, D. Chiral carbon dots mimicking topoisomerase I to mediate the topological rearrangement of supercoiled DNA enantioselectively. Angew. Chem. Int. Ed. 2020, 59, 11087–11092. [Google Scholar] [CrossRef]
- Dehvari, K.; Chiu, S.-H.; Lin, J.-S.; Girma, W.M.; Ling, Y.-C.; Chang, J.-Y. Heteroatom doped carbon dots with nanoenzyme like properties as theranostic platforms for free radical scavenging, imaging, and chemotherapy. Acta Biomater. 2020, 114, 343–357. [Google Scholar] [CrossRef]
- Chen, J.; Yan, J.; Feng, Q.; Miao, X.; Dou, B.; Wang, P. Label-free and enzyme-free fluorescence detection of microRNA based on sulfydryl-functionalized carbon dots via target-initiated hemin/G-quadruplex-catalyzed oxidation. Biosens. Bioelectron. 2021, 176, 112955. [Google Scholar] [CrossRef]
- Gan, H.; Han, W.; Fu, Z.; Wang, L. The chain-like Au/carbon dots nanocomposites with peroxidase-like activity and their application for glucose detection. Colloids Surf. B 2021, 199, 111553. [Google Scholar] [CrossRef]
- Xue, T.; Sheng, Y.; Xu, J.; Li, Y.; Lu, X.; Zhu, Y.; Duan, X.; Wen, Y. In-situ reduction of Ag+ on black phosphorene and its NH2-MWCNT nanohybrid with high stability and dispersibility as nanozyme sensor for three ATP metabolites. Biosens. Bioelectron. 2019, 145, 111716. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, Z.; Liu, G.; Lu, H.; Gao, Y.; Liu, F.; Wang, C.; Cui, J.; Lu, G. High-activity Mo, S co-doped carbon quantum dot nanozyme-based cascade colorimetric biosensor for sensitive detection of cholesterol. J. Mater. Chem. B 2019, 7, 7042–7051. [Google Scholar] [CrossRef]
- Tripathi, K.M.; Ahn, H.T.; Chung, M.; Le, X.A.; Saini, D.; Bhati, A.; Sonkar, S.K.; Kim, M.I.; Kim, T. N, S, and P-co-doped carbon quantum dots: Intrinsic peroxidase activity in a wide pH range and its antibacterial applications. ACS Biomater. Sci. Eng. 2020, 6, 5527–5537. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Zhang, T.; Wang, X.; Zhang, Q.; Huang, S.; Zhang, L.; Zhang, L.; Zhu, W.; Wang, Y.; Wu, M.; et al. Cu, Zn dopants boost electron transfer of carbon dots for antioxidation. Small 2021, 17, 2102178. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.S.; Gadhari, N.S.; Srivastava, A.K. Biomimetic sensor for ethambutol employing β-cyclodextrin mediated chiral copper metal organic framework and carbon nanofibers modified glassy carbon electrode. Biosens. Bioelectron. 2020, 165, 112397. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Li, Y.; Zhang, K.; Lin, Z.; Tang, D. Carbon dots/g-C3N4 nanoheterostructures-based signal-generation tags for photoelectrochemical immunoassay of cancer biomarkers coupling with copper nanoclusters. ACS Appl. Mater. Interfaces 2017, 9, 38336–38343. [Google Scholar] [CrossRef]
- Luo, N.; Yang, Z.; Tang, F.; Wang, D.; Feng, M.; Liao, X.; Yang, X. Fe3O4/carbon nanodot hybrid nanoparticles for the indirect colorimetric detection of glutathione. ACS Appl. Nano Mater. 2019, 2, 3951–3959. [Google Scholar] [CrossRef]
- Wang, D.; Song, X.; Li, P.; Gao, X.J.; Gao, X. Origins of the peroxidase mimicking activities of graphene oxide from first principles. J. Mater. Chem. B 2020, 8, 9028–9034. [Google Scholar] [CrossRef]
- Kim, M.S.; Cho, S.; Joo, S.H.; Lee, J.; Kwak, S.K.; Kim, M.I.; Lee, J. N- and B-codoped graphene: A strong candidate to replace natural peroxidase in sensitive and selective bioassays. ACS Nano 2019, 13, 4312–4321. [Google Scholar] [CrossRef]
- Wang, H.; Li, P.; Yu, D.; Zhang, Y.; Wang, Z.; Liu, C.; Qiu, H.; Liu, Z.; Ren, J.; Qu, X. Unraveling the enzymatic activity of oxygenated carbon nanotubes and their application in the treatment of bacterial infections. Nano Lett. 2018, 18, 3344–3351. [Google Scholar] [CrossRef]
- Cheng, N.; Li, J.-C.; Liu, D.; Lin, Y.; Du, D. Single-atom nanozyme based on nanoengineered Fe-N-C catalyst with superior peroxidase-like activity for ultrasensitive bioassays. Small 2019, 15, e1901485. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Q.; Chen, J.; Huang, L.; Zhang, H.; Rong, K.; Dong, S. Biomimetic design for enhancing the peroxidase mimicking activity of hemin. Nanoscale 2019, 11, 12603–12609. [Google Scholar] [CrossRef]
- Xu, M.; Xing, S.; Zhao, Y.; Zhao, C. Peptide nucleic acid-assisted colorimetric detection of single-nucleotide polymorphisms based on the intrinsic peroxidase-like activity of hemin-carbon nanotube nanocomposites. Talanta 2021, 232, 122420. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, X.-Y.; Sha, J.-Q.; Han, T.; Du, C.-J.; Sun, Y.-J.; Lan, Y.-Q. POMOF/SWNT nanocomposites with prominent peroxidase-mimicking activity for L-cysteine “on-off switch” colorimetric biosensing. ACS Appl. Mater. Interfaces 2019, 11, 16896–16904. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Y.; Dong, W.; Zhou, J.; Han, B.; Jiao, J.; Lan, L.; Miao, P.; Chen, Q. Copper (II)-ploy-L-histidine functionalized multi walled carbon nanotubes as efficient mimetic enzyme for sensitive electrochemical detection of salvianic acid A. Biosens. Bioelectron. 2018, 121, 257–264. [Google Scholar] [CrossRef]
- Wu, L.; Lu, Z.; Ye, J. Enzyme-free glucose sensor based on layer-by-layer electrodeposition of multilayer films of multi-walled carbon nanotubes and Cu-based metal framework modified glassy carbon electrode. Biosens. Bioelectron. 2019, 135, 45–49. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Cai, Y.; Yang, Z.; Li, P.; Lei, H.; Liu, W.; Liu, Y. A dual-signal readout enzyme-free immunosensor based on hybridization chain reaction-assisted formation of copper nanoparticles for the detection of microcystin-LR. Biosens. Bioelectron. 2019, 126, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Gallay, P.; Eguilaz, M.; Rivas, G. Designing electrochemical interfaces based on nanohybrids of avidin functionalized-carbon nanotubes and ruthenium nanoparticles as peroxidase-like nanozyme with supramolecular recognition properties for site-specific anchoring of biotinylated residues. Biosens. Bioelectron. 2020, 148, 111764. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hou, B.; Qian, L.; Zhang, X.; Liu, G. Non-enzymatic electrochemical sensor based on sliver nanoparticle-decorated carbon nanotubes. Molecules 2019, 24, 3411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Ma, C.; Wang, L.; Zhu, Z. Platinum nanoparticle-deposited multi-walled carbon nanotubes as a NADH oxidase mimic: Characterization and applications. Nanoscale 2020, 12, 19284–19292. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Lee, M.A.; Rajan, A.G.; Rahaman, I.; Sun, J.H.; Park, M.; Salem, D.P.; Strano, M.S. A synthetic mimic of phosphodiesterase type 5 based on corona phase molecular recognition of single-walled carbon nanotubes. Proc. Natl. Acad. Sci. USA 2020, 117, 26616–26625. [Google Scholar] [CrossRef] [PubMed]
- Mikhalchan, A.; Vilatela, J.J. A perspective on high-performance CNT fibres for structural composites. Carbon 2019, 150, 191–215. [Google Scholar] [CrossRef]
- Konstantopoulos, G.; Semitekolos, D.; Koumoulos, E.P.; Charitidis, C. Carbon fiber reinforced composites: Study of modification effect on weathering-induced ageing via nanoindentation and deep learning. Nanomaterials 2021, 11, 2631. [Google Scholar] [CrossRef] [PubMed]
- Semitekolos, D.; Trompeta, A.-F.; Husarova, I.; Man’ko, T.; Potapov, A.; Romenskaya, O.; Liang, Y.; Li, X.; Giorcelli, M.; Dong, H.; et al. Comparative physical-mechanical properties assessment of tailored surface-treated carbon fibres. Materials 2020, 13, 3136. [Google Scholar] [CrossRef]
- Mikhalchan, A.; Vila, M.; Arévalo, L.; Vilatela, J.J. Simultaneous improvements in conversion and properties of molecularly controlled CNT fibres. Carbon 2021, 179, 417–424. [Google Scholar] [CrossRef]
- Senokos, E.; Rana, M.; Vila, M.; Fernandez-Cestau, J.; Costa, R.D.; Marcilla, R.; Vilatela, J.J. Transparent and flexible high-power supercapacitors based on carbon nanotube fibre aerogels. Nanoscale 2020, 12, 16980–16986. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Ma, F.; Zhu, Y.; Chen, S.; Wang, C.; Lu, X. Fe3C/nitrogen-doped carbon nanofibers as highly efficient biocatalyst with oxidase-mimicking activity for colorimetric sensing. ACS Sustain. Chem. Eng. 2018, 6, 16766–16776. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Zhong, M.; Yu, D.; Wang, C.; Lu, X. Fe(III)-tannic acid complex derived Fe3C decorated carbon nanofibers for triple-enzyme mimetic activity and their biosensing application. ACS Biomater. Sci. Eng. 2019, 5, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, D.; Senokos, E.; Alemán, B.; Cabana, L.; Navío, C.; Marcilla, R.; Prato, M.; Vilatela, J.J.; Marchesan, S. Gas-Phase functionalization of macroscopic carbon nanotube fiber assemblies: Reaction control, electrochemical properties, and use for flexible supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 5760–5770. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wu, X.; Hu, X.; Wang, K.; Zhang, C.; Gyimah, E.; Yakubu, S.; Zhang, Z. Electrochemical immunosensor based on Ag+-dependent CTAB-AuNPs for ultrasensitive detection of sulfamethazine. Biosens. Bioelectron. 2019, 144, 111643. [Google Scholar] [CrossRef]
- Bracamonte, M.V.; Melchionna, M.; Giuliani, A.; Nasi, L.; Tavagnacco, C.; Prato, M.; Fornasiero, P. H2O2 sensing enhancement by mutual integration of single walled carbon nanohorns with metal oxide catalysts: The CeO2 case. Sens. Actuators B 2017, 239, 923–932. [Google Scholar] [CrossRef]
- Mykhailiv, O.; Zubyk, H.; Brzezinski, K.; Gras, M.; Lota, G.; Gniadek, M.; Romero, E.; Echegoyen, L.; Plonska-Brzezinska, M.E. Improvement of the structural and chemical properties of carbon nano-onions for electrocatalysis. ChemNanoMat 2017, 3, 583–590. [Google Scholar] [CrossRef]
- Chen, T.M.; Tian, X.M.; Huang, L.; Xiao, J.; Yang, G.W. Nanodiamonds as pH-switchable oxidation and reduction catalysts with enzyme-like activities for immunoassay and antioxidant applications. Nanoscale 2017, 9, 15673–15684. [Google Scholar] [CrossRef] [PubMed]
- Gülseren, G.; Saylam, A.; Marion, A.; Özçubukçu, S. Fullerene-based mimics of biocatalysts show remarkable activity and modularity. ACS Appl. Mater. Interfaces 2021, 13, 45854–45863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Z.; Kong, J.; Liang, Y.; Chen, K.; Chang, Y.; Yuan, H.; Wang, Y.; Liang, H.; Li, J.; et al. Fullerenol nanoparticles eradicate Helicobacter pylori via pH-responsive peroxidase activity. ACS Appl. Mater. Interfaces, 2020; in press. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, A.; Naghizadeh, E.; Zakariazadeh, M.; Azamat, J. Molecular dynamics simulation study of the HIV-1 protease inhibit ion using fullerene and new fullerene derivatives of carbon nanostructures. Mini-Rev. Med. Chem. 2017, 17, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Bag, S.; Chakraborty, D.; Dasgupta, S. Exploring the inhibitory and antioxidant effects of Fullerene and Fullerenol on Ribonuclease A. ACS Omega 2018, 3, 12270–12283. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Kazansky, Y.; Wathieu, D.; Fushman, D. Hydrophobic patch of Ubiquitin is important for its optimal activation by Ubiquitin activating enzyme E1. Anal. Chem. 2017, 89, 7852–7860. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yan, B.; Winkler, D.A.; Fu, J.; Zhang, A. Competitive inhibition mechanism of acetylcholinesterase without catalytic active site interaction: Study on functionalized C60 nanoparticles via in vitro and in silico assays. ACS Appl. Mater. Interfaces 2017, 9, 18626–18638. [Google Scholar] [CrossRef]
- Barros, M.R.; da Silva, L.P.; Menezes, T.M.; Garcia, Y.S.; Neves, J.L. Efficient tyrosinase nano-inhibitor based on carbon dots behaving as a gathering of hydrophobic cores and key chemical group. Colloids Surf. B 2021, 207, 112006. [Google Scholar] [CrossRef]
- Di Giosia, M.; Marforio, T.D.; Cantelli, A.; Valle, F.; Zerbetto, F.; Su, Q.; Wang, H.; Calvaresi, M. Inhibition of α-chymotrypsin by pristine single-wall carbon nanotubes: Clogging up the active site. J. Colloid Interface Sci. 2020, 571, 174–184. [Google Scholar] [CrossRef]
- Liu, J.J.; Tang, D.; Chen, Z.; Yan, X.; Zhong, Z.; Kang, L.; Yao, J. Chemical redox modulated fluorescence of nitrogen-doped graphene quantum dots for probing the activity of alkaline phosphatase. Biosens. Bioelectron. 2017, 94, 271–277. [Google Scholar] [CrossRef]
- Li, H.; Yan, X.; Qiao, S.; Lu, G.; Su, X. Yellow-emissive carbon dot-based optical sensing platforms: Cell imaging and analytical applications for biocatalytic reactions. ACS Appl. Mater. Interfaces 2018, 10, 7737–7744. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhou, J.; Qian, Z.; Ma, Y.; Huang, Y.; Feng, H. A universal fluorometric assay strategy for glycosidases based on functional carbon quantum dots: β-galactosidase activity detection in vitro and in living cells. J. Mater. Chem. B 2017, 5, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Ao, H.; Feng, H.; Huang, X.; Zhao, M.; Qian, Z. A reversible fluorescence nanoswitch based on dynamic covalent B-O bonds using functional carbon quantum dots and its application for α-glucosidase activity monitoring. J. Mater. Chem. C 2017, 5, 2826–2832. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Singh, A.; Garg, N.; Singh, N. Carbon dot based, Naphthalimide coupled FRET pair for highly selective ratiometric detection of thioredoxin reductase and cancer screening. ACS Appl. Mater. Interfaces 2017, 9, 25847–25856. [Google Scholar] [CrossRef] [PubMed]
- Shumeiko, V.; Paltiel, Y.; Bisker, G.; Hayouka, Z.; Shoseyov, O. A paper-based near-infrared optical biosensor for quantitative detection of protease activity using peptide-encapsulated SWCNTs. Sensors 2020, 20, 5247. [Google Scholar] [CrossRef] [PubMed]
- Palomar, Q.; Xu, X.; Selegaard, R.; Aili, D.; Zhang, Z. Peptide decorated gold nanoparticle/carbon nanotube electrochemical sensor for ultrasensitive detection of matrix metalloproteinase-7. Sens. Actuators B 2020, 325, 128789. [Google Scholar] [CrossRef]
- Del Barrio, M.; Rana, M.; Vilatela, J.J.; Lorenzo, E.; De Lacey, A.L.; Pita, M. Photoelectrocatalytic detection of NADH on n-type silicon semiconductors facilitated by carbon nanotube fibers. Electrochim. Acta 2021, 377, 138071. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized enzymes in biosensor applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef] [Green Version]
- Amine, A.; Arduini, F.; Moscone, D.; Palleschi, G. Recent advances in biosensors based on enzyme inhibition. Biosens. Bioelectron. 2016, 76, 180–194. [Google Scholar] [CrossRef]
- Maciá-Agulló, J.A.; Corma, A.; Garcia, H. Photobiocatalysis: The power of combining photocatalysis and enzymes. Chem. Eur. J. 2015, 21, 10940–10959. [Google Scholar] [CrossRef] [Green Version]
- Kucherenko, I.S.; Soldatkin, O.O.; Kucherenko, D.Y.; Soldatkina, O.V.; Dzyadevych, S.V. Advances in nanomaterial application in enzyme-based electrochemical biosensors: A review. Nanoscale Adv. 2019, 1, 4560–4577. [Google Scholar] [CrossRef] [Green Version]
- Lisdat, F. Trends in the layer-by-layer assembly of redox proteins and enzymes in bioelectrochemistry. Curr. Opin. Electrochem. 2017, 5, 165–172. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Developments in support materials for immobilization of oxidoreductases: A comprehensive review. Adv. Colloid Interface Sci. 2018, 258, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Yanez-Sedeno, P.; Pingarro, J.M. Carbon dots and graphene quantum dots in electrochemical biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cajigas, S.; Orozco, J. Nanobioconjugates for signal amplification in electrochemical biosensing. Molecules 2020, 25, 3542. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Pang, J.; Li, Y.; Sun, B.; Ibarlucea, B.; Liu, X.; Gemming, T.; Cheng, Q.; Zhang, S.; Liu, H.; et al. Graphene Biodevices for Early Disease Diagnosis Based on Biomarker Detection. ACS Sens. 2021, 6, 3841–3881. [Google Scholar] [CrossRef]
- Cumba, L.R.; Camisasca, A.; Giordani, S.; Forster, R.J. Electrochemical properties of screen-printed carbon nano-onion electrodes. Molecules 2020, 25, 3884. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Rawat, K. Au@carbon dot nanoconjugates as a dual mode enzyme-free sensing platform for cholesterol. J. Mater. Chem. B 2017, 5, 5425–5432. [Google Scholar] [CrossRef]
- Hayat, A.; Haider, W.; Raza, Y.; Marty, J.L. Colorimetric cholesterol sensor based on peroxidase like activity of zinc oxide nanoparticles incorporated carbon nanotubes. Talanta 2015, 143, 157–161. [Google Scholar] [CrossRef]
- Isoaho, N.; Peltola, E.; Sainio, S.; Wester, N.; Protopopova, V.; Wilson, B.P.; Koskinen, J.; Laurila, T. Carbon Nanostructure Based Platform for Enzymatic Glutamate Biosensors. J. Phys. Chem. C 2017, 121, 4618–4626. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Voltamperometric sensors and biosensors based on carbon nanomaterials used for detecting caffeic acid—A review. Int. J. Mol. Sci. 2020, 21, 9275. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Xia, H.-Q.; Wu, R.; Bai, L.; Yan, L.; Magner, E.; Cosnier, S.; Lojou, E.; Zhu, Z.; Liu, A. Tackling the challenges of enzymatic (bio)fuel cells. Chem. Rev. 2019, 119, 9509–9558. [Google Scholar] [CrossRef] [PubMed]
- Ruth, J.C.; Spormann, A.M. Enzyme electrochemistry for industrial energy applications—A perspective on future areas of focus. ACS Catal. 2021, 11, 5951–5967. [Google Scholar] [CrossRef]
- Yin, S.; Jin, Z.; Miyake, T. Wearable high-powered biofuel cells using enzyme/carbon nanotube composite fibers on textile cloth. Biosens. Bioelectron. 2019, 141, 111471. [Google Scholar] [CrossRef] [PubMed]
- Pyser, J.B.; Chakrabarty, S.; Romero, E.O.; Narayan, A.R.H. State-of-the-art biocatalysis. ACS Cent. Sci. 2021, 7, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Arnold, F.H. Navigating the unnatural reaction space: Directed evolution of heme proteins for selective Carbene and Nitrene transfer. Acc. Chem. Res. 2021, 54, 1209–1225. [Google Scholar] [CrossRef]
- Ramakrishna, T.R.B.; Nalder, T.D.; Yang, W.; Marshall, S.N.; Barrow, C.J. Controlling enzyme function through immobilization on graphene, graphene derivatives and other two dimensional nanomaterials. J. Mater. Chem. B 2018, 6, 3200–3218. [Google Scholar] [CrossRef]
- Zor, C.; Reeve, H.A.; Quinson, J.; Thompson, L.A.; Lonsdale, T.H.; Dillon, F.; Grobert, N.; Vincent, K.A. H2-Driven biocatalytic hydrogenation in continuous flow using enzyme-modified carbon nanotube columns. Chem. Commun. 2017, 53, 9839–9841. [Google Scholar] [CrossRef] [Green Version]
- Xia, H.; Zeng, J. Rational surface modification of carbon nanomaterials for improved direct electron transfer-type bioelectrocatalysis of redox enzymes. Catalysts 2020, 10, 1447. [Google Scholar] [CrossRef]
- Wang, M.; Mohanty, S.K.; Mahendra, S. Nanomaterial-supported enzymes for water purification and monitoring in point-of-use water supply systems. Acc. Chem. Res. 2019, 52, 876–885. [Google Scholar] [CrossRef]
- Hilali, N.; Mohammadi, H.; Amine, A.; Zine, N.; Errachid, A. Recent advances in electrochemical monitoring of chromium. Sensors 2020, 20, 5153. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, X.; Lu, G.; Su, X. Carbon dot-based bioplatform for dual colorimetric and fluorometric sensing of organophosphate pesticides. Sens. Actuators B Chem. 2018, 260, 563–570. [Google Scholar] [CrossRef]
- Kaspar, C.; Ravoo, B.J.; van der Wiel, W.G.; Wegner, S.V.; Pernice, W.H.P. The rise of intelligent matter. Nature 2021, 594, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.B.; Decuzzi, P. Harnessing endogenous stimuli for responsive materials in theranostics. ACS Nano 2021, 15, 2068–2098. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Lin, J.; Huang, P.; Chen, X. Development of endogenous enzyme-responsive nanomaterials for theranostics. Chem. Soc. Rev. 2018, 47, 5554–5573. [Google Scholar] [CrossRef]
- Du, B.; Tung, C.-H. Enzyme-assisted photodynamic therapy based on nanomaterials. ACS Biomater. Sci. Eng. 2020, 6, 2506–2517. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sorescu, D.C.; Star, A. Composition and structure of fluorescent graphene quantum dots generated by enzymatic degradation of graphene oxide. J. Phys. Chem. C 2021, 125, 13361–13369. [Google Scholar] [CrossRef]
- Parks, A.N.; Chandler, G.T.; Ho, K.T.; Burgess, R.M.; Ferguson, P.L. Environmental biodegradability of [14C] single-walled carbon nanotubes by Trametes versicolor and natural microbial cultures found in New Bedford Harbor sediment and aerated wastewater treatment plant sludge. Environ. Toxicol. Chem. 2015, 34, 247–251. [Google Scholar] [CrossRef]
- Zhang, L.; Petersen, E.J.; Habteselassie, M.Y.; Mao, L.; Huang, Q. Degradation of multiwall carbon nanotubes by bacteria. Environ. Pollut. 2013, 181, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Qin, X.; Zeng, G. Biodegradation of carbon nanotubes, graphene, and their derivatives. Trends Biotechnol. 2017, 35, 836–846. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Zeng, G.; Shao, B.; Chen, M.; Li, Z.; Jiang, Y.; Liu, Y.; Zhang, Y.; Zhong, H. Application of molecular docking for the degradation of organic pollutants in the environmental remediation: A review. Chemosphere 2018, 203, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Lavado, E.; Iturrioz-Rodriguez, N.; Padin-Gonzalez, E.; Gonzalez, J.; Garcia-Hevia, L.; Heuts, J.; Pesquera, C.; Gonzalez, F.; Villegas, J.C.; Valiente, R.; et al. Biodegradable multi-walled carbon nanotubes trigger anti-tumoral effects. Nanoscale 2018, 10, 11013–11020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, C.; Jun, G.; Schurhammer, R.; Reina, G.; Chen, P.; Bianco, A.; Menard-Moyon, C. Enzymatic degradation of graphene quantum dots by human peroxidases. Small 2019, 15, 1905405. [Google Scholar] [CrossRef]
- Piotrovskiy, L.B.; Litasova, E.V.; Sokolov, A.V.; Iljin, V.V.; Utsal, V.A.; Zhurkovich, I.K. Degradation of fullerene C60 by human myeloperoxidase and some reaction products. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 196–201. [Google Scholar] [CrossRef]
- Kurapati, R.; Bianco, A. Peroxidase mimicking DNAzymes degrade graphene oxide. Nanoscale 2018, 10, 19316–19321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotchey, G.P.; Allen, B.L.; Vedala, H.; Yanamala, N.; Kapralov, A.A.; Tyurina, Y.Y.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. The enzymatic oxidation of graphene oxide. ACS Nano 2011, 5, 2098–2108. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, L.; Shi, X.; Wang, X.; Yang, Y.; Yang, K.; Liu, T.; Yang, G.; Liu, Z. Surface coating-dependent cytotoxicity and degradation of graphene derivatives: Towards the design of non-toxic, degradable nano-graphene. Small 2014, 10, 1544–1554. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, C.; Fan, M.; Chen, C.; Huang, Y.; Hao, Q.; Yang, J.; Wang, H.; Sun, D. Oxidation and degradation of graphitic materials by naphthalene-degrading bacteria. Nanoscale 2015, 7, 13619–13628. [Google Scholar] [CrossRef]
- Salas, E.C.; Sun, Z.; Lüttge, A.; Tour, J.M. Reduction of graphene oxide via bacterial respiration. ACS Nano 2010, 4, 4852–4856. [Google Scholar] [CrossRef]
- Allen, B.L.; Kotchey, G.P.; Chen, Y.; Yanamala, N.V.K.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. Mechanistic investigations of horseradish peroxidase-catalyzed degradation of single-walled carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 17194–17205. [Google Scholar] [CrossRef]
- Avanasi, R.; Jackson, W.A.; Sherwin, B.; Mudge, J.F.; Anderson, T.A. C60 fullerene soil sorption, biodegradation, and plant uptake. Environ. Sci. Technol. 2014, 48, 2792–2797. [Google Scholar] [CrossRef] [PubMed]
- Berry, T.D.; Filley, T.R.; Clavijo, A.P.; Gray, M.B.; Turco, R. Degradation and microbial uptake of C60 fullerols in contrasting agricultural soils. Environ. Sci. Technol. 2017, 51, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.S.; Li, Q. Characterizing photochemical transformation of aqueous nC60 under environmentally relevant Conditions. Environ. Sci. Technol. 2010, 44, 3008–3013. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.R.; Hunt, D.E.; Ikuma, K.; Yang, S.; Cho, J.; Gunsch, C.K.; Liu, J.; Wiesner, M.R. Aging of fullerene C60 nanoparticle suspensions in the presence of microbes. Water Res. 2014, 65, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Musil, M.; Konegger, H.; Hon, J.; Bednar, D.; Damborsky, J. Computational design of stable and soluble biocatalysts. ACS Catal. 2019, 9, 1033–1054. [Google Scholar] [CrossRef] [Green Version]
- Mazurenko, S.; Prokop, Z.; Damborsky, J. Machine learning in enzyme engineering. ACS Catal. 2020, 10, 1210–1223. [Google Scholar] [CrossRef]
- Chen, K.; Arnold, F.H. Engineering new catalytic activities in enzymes. Nat. Catal. 2020, 3, 203–213. [Google Scholar] [CrossRef]
- Nelson, A. Catalytic machinery of enzymes expanded. Nature 2019, 570, 172–173. [Google Scholar] [CrossRef] [Green Version]
- Drienovska, I.; Roelfes, G. Expanding the enzyme universe with genetically encoded unnatural amino acids. Nat. Catal. 2020, 3, 193–202. [Google Scholar] [CrossRef]
- Hwang, E.T.; Lee, S. Multienzymatic cascade reactions via enzyme complex by immobilization. ACS Catal. 2019, 9, 4402–4425. [Google Scholar] [CrossRef]
- Vazquez-Gonzalez, M.; Wang, C.; Willner, I. Biocatalytic cascades operating on macromolecular scaffolds and in confined environments. Nat. Catal. 2020, 3, 256–273. [Google Scholar] [CrossRef]
- Kornienko, N.; Ly, K.H.; Robinson, W.E.; Heidary, N.; Zhang, J.Z.; Reisner, E. Advancing techniques for investigating the enzyme-electrode interface. Acc. Chem. Res. 2019, 52, 1439–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeerapan, I.; Sempionatto, J.R.; Wang, J. On-body bioelectronics: Wearable biofuel cells for bioenergy harvesting and self-powered biosensing. Adv. Funct. Mater. 2020, 30, 1906243. [Google Scholar] [CrossRef]
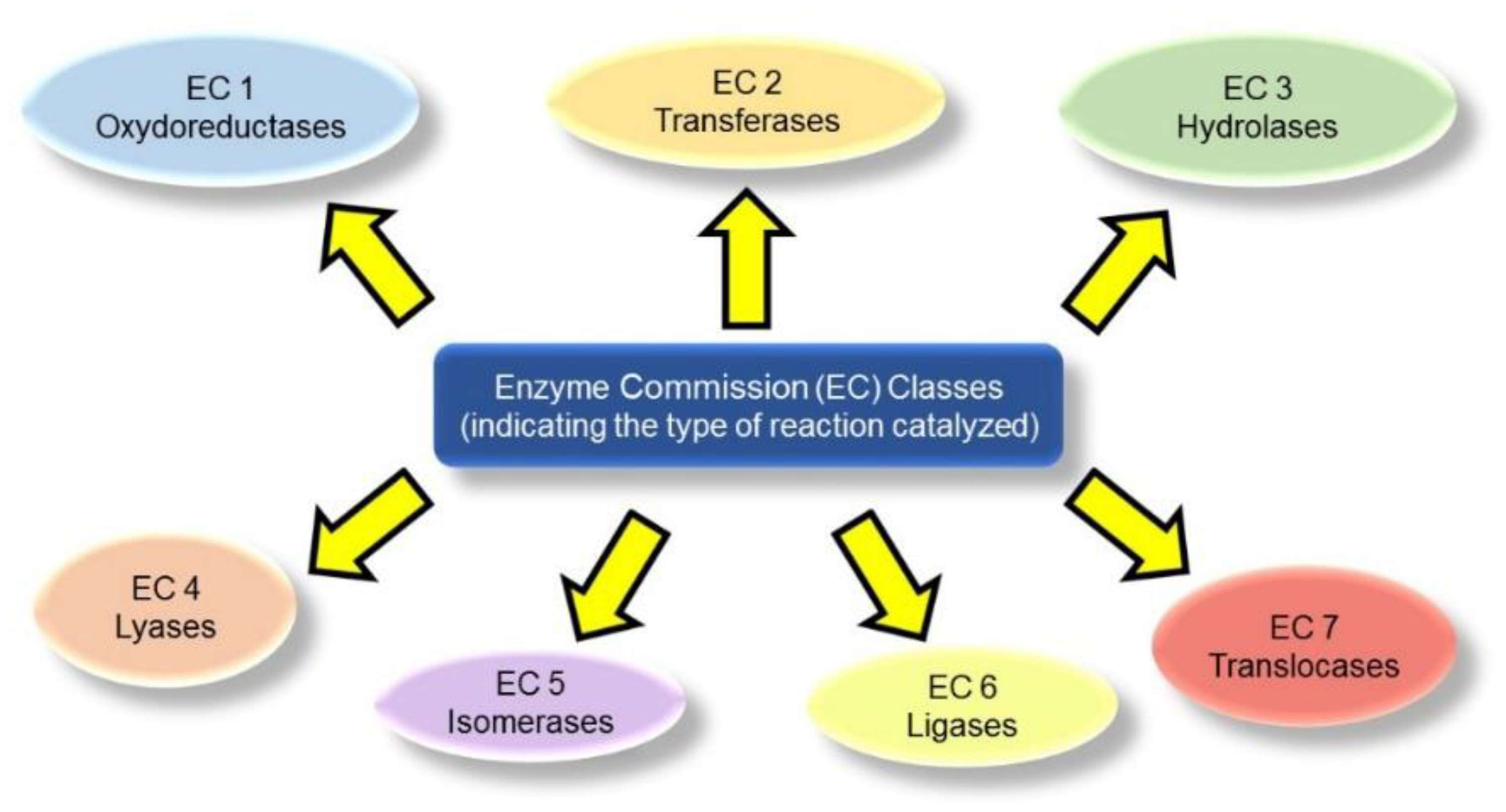
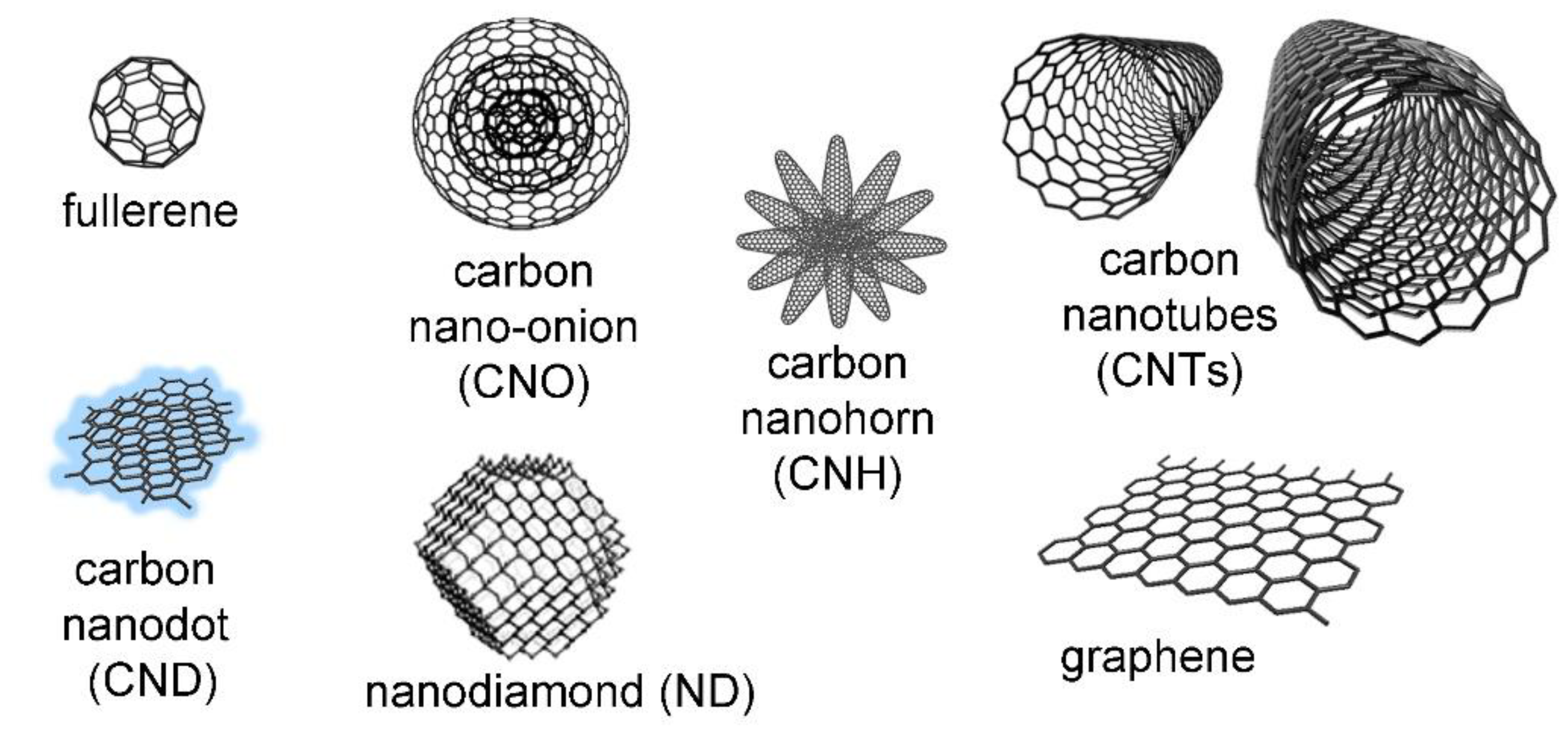
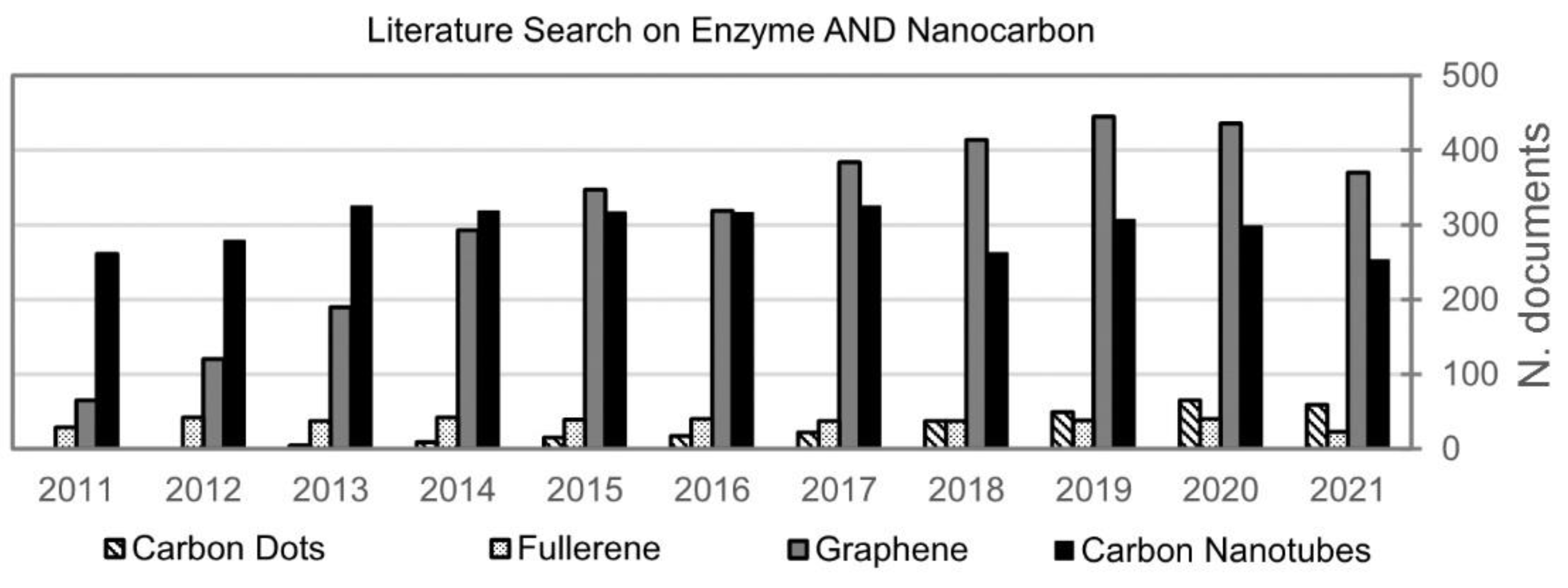
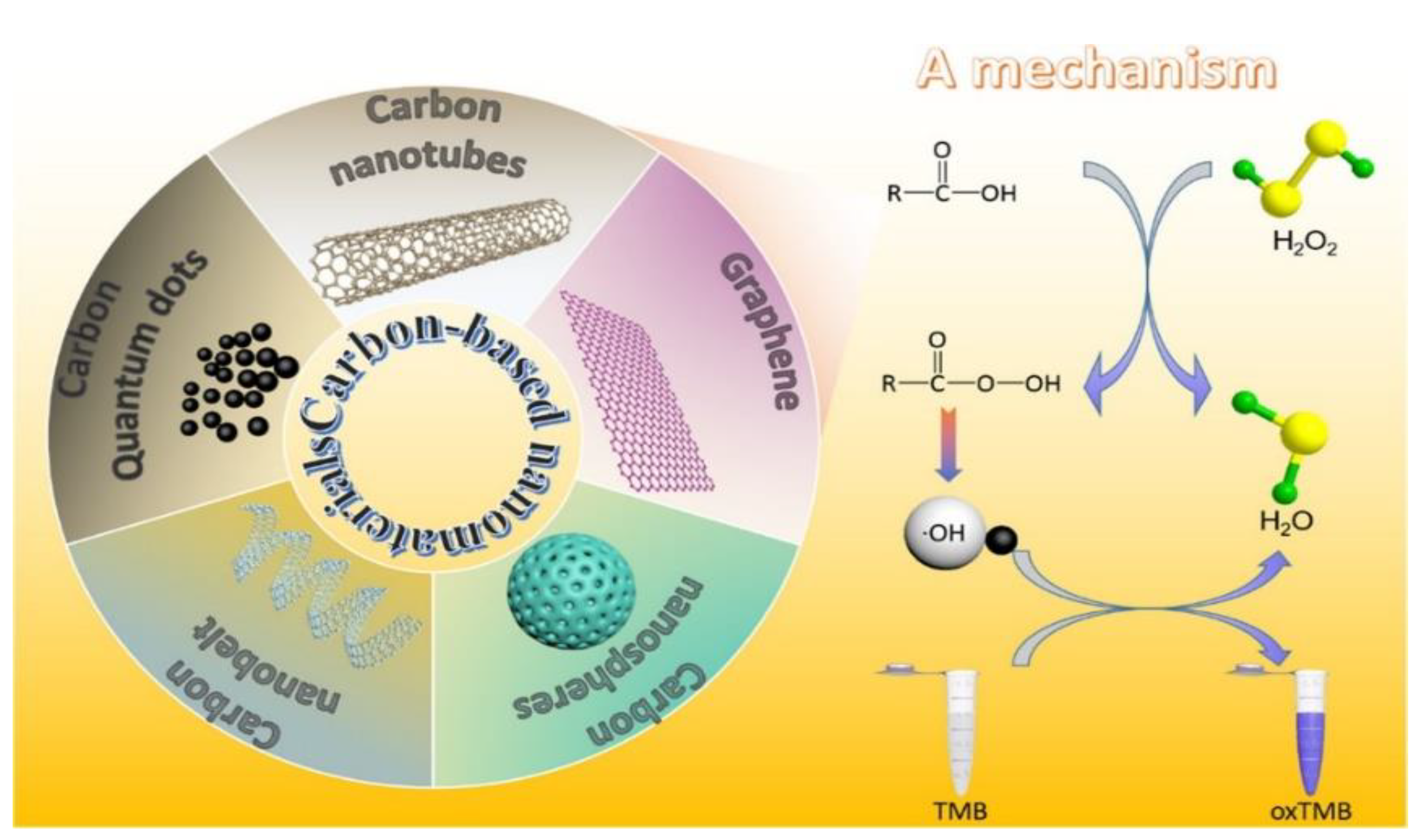

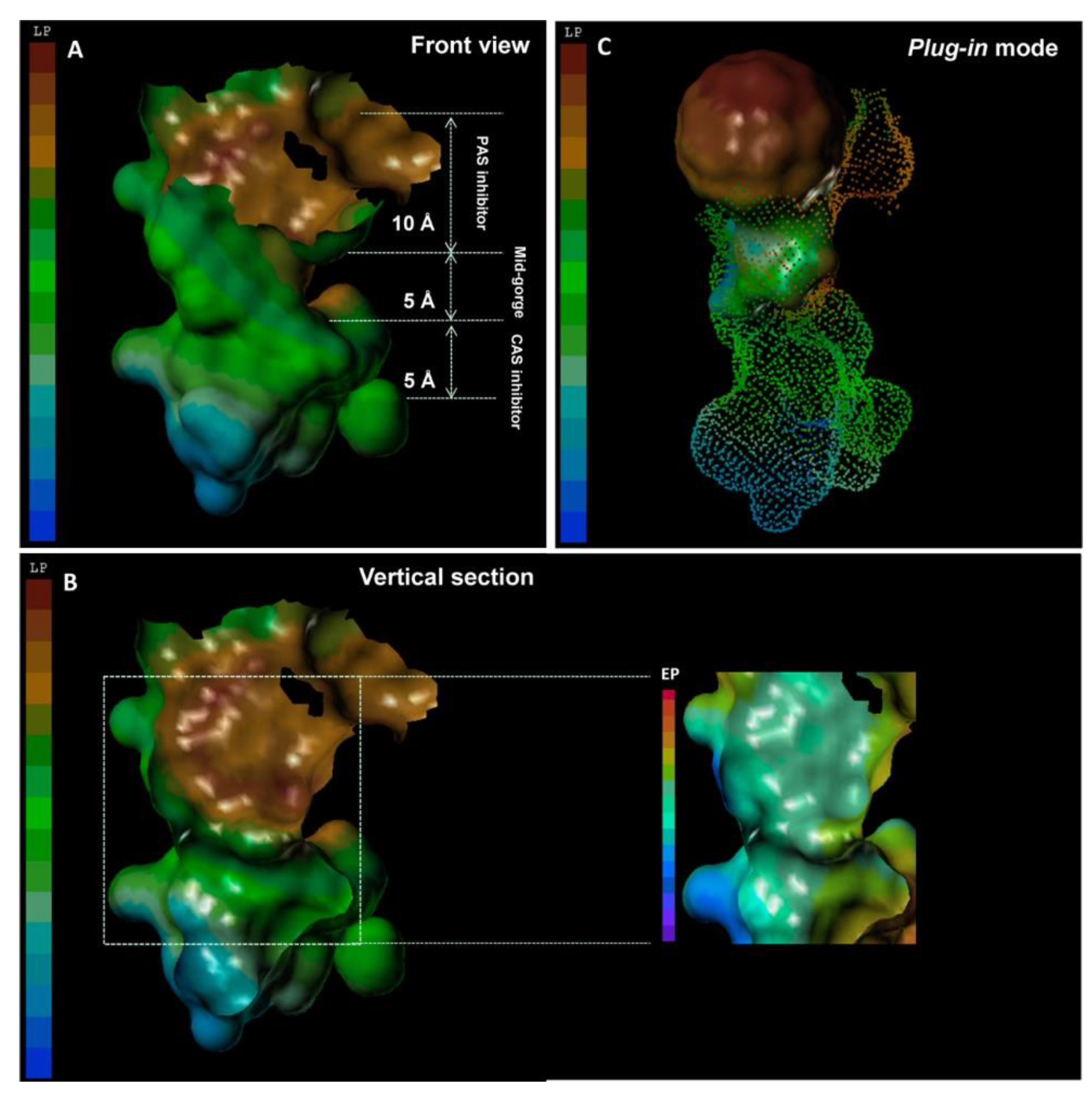
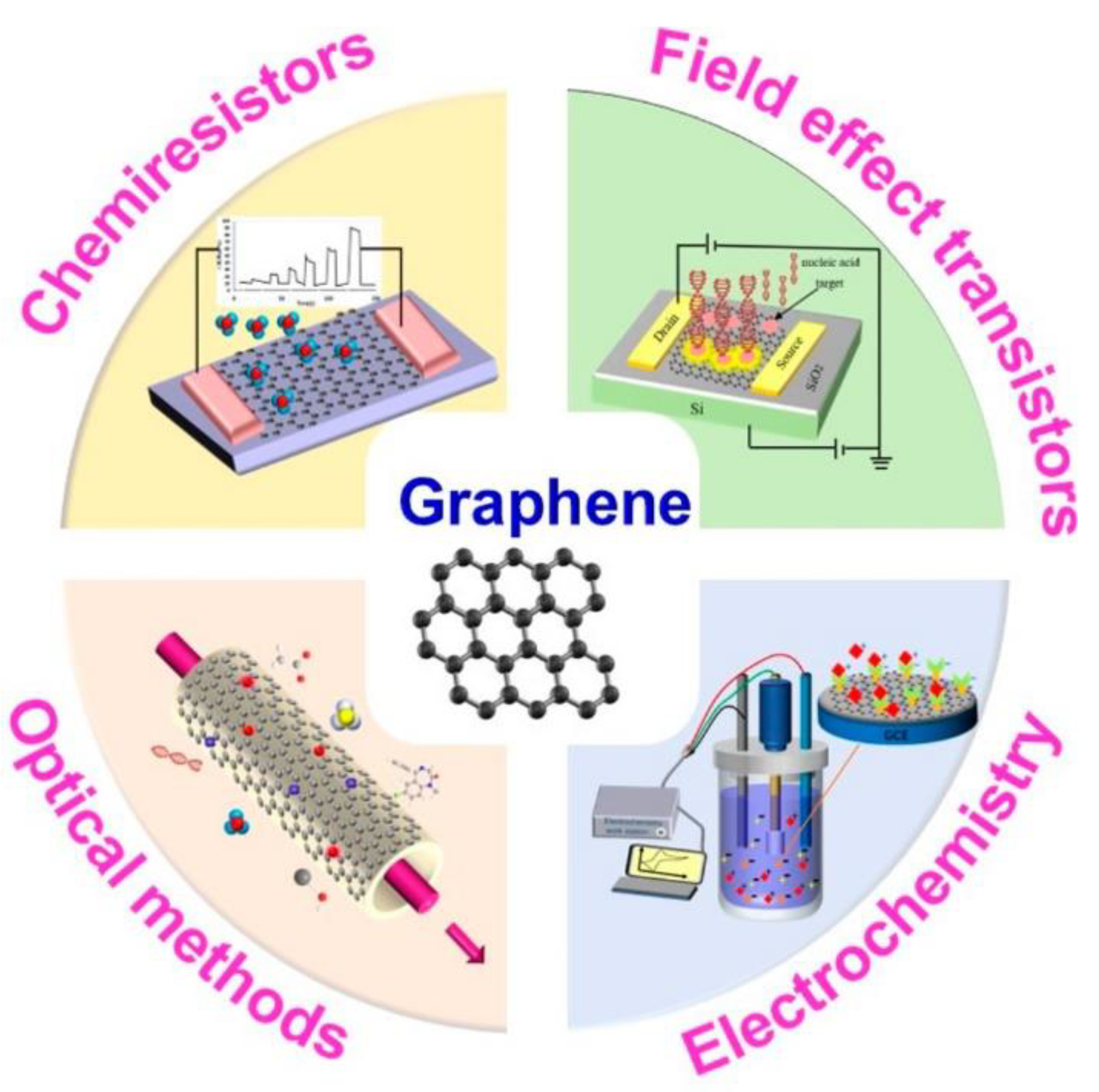
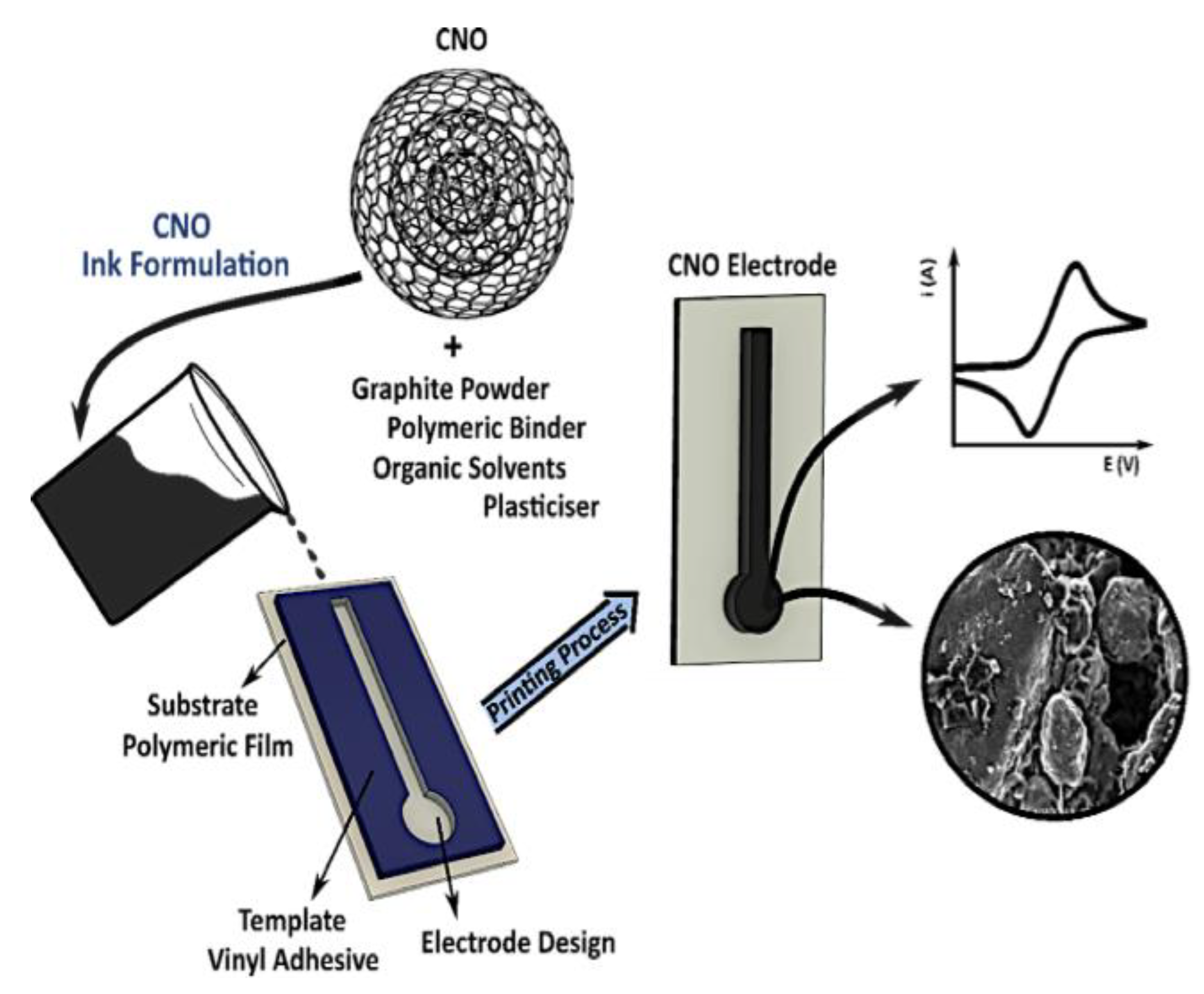
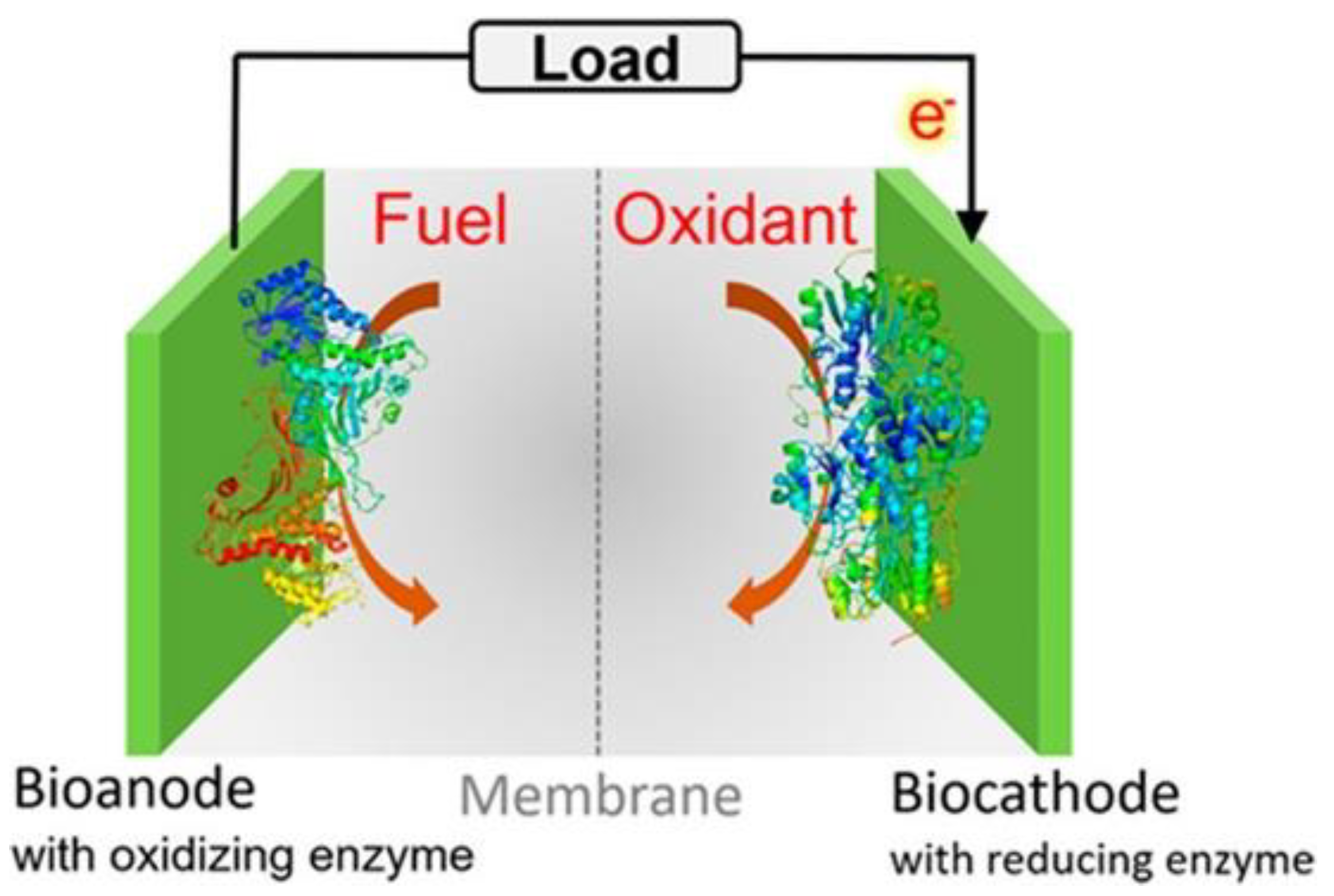
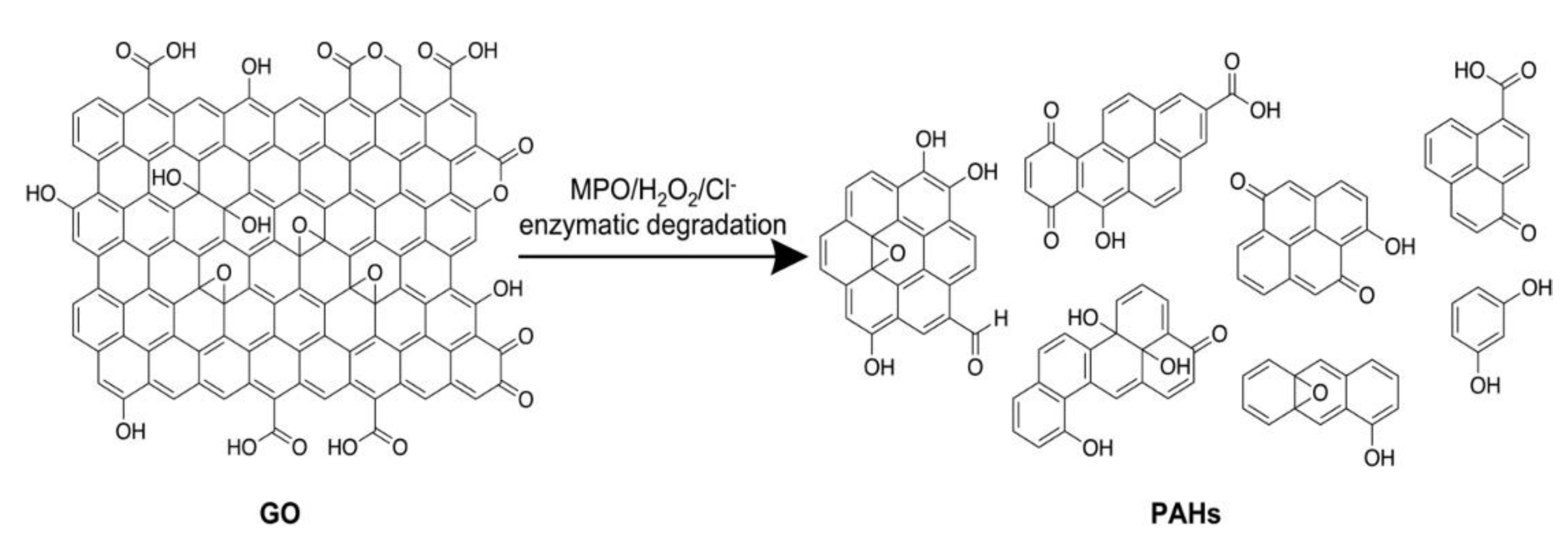
| CNM Type | Conjugation | Enzyme | EC Class | Application | Ref. |
|---|---|---|---|---|---|
| C60 | Non-covalent | Laccase | 1 | Water purification | [217] |
| Covalent | Laccase | 1 | Water purification | [217] | |
| Tyrosinase | 1 | Biosensing | [218] | ||
| C60, MWCNTs | Non-covalent | Endonuclease | 3 | Biosensing | [219] |
| CNDs | Covalent | Galactosidase | 3 | Biosensing | [220] |
| Quinolinate phosphoribosyl transferase | 2 | Biosensing | [221] | ||
| Non-covalent | Adenylate kinase | 2 | Biocatalysis | [222] | |
| Endonucleases | 3 | Biosensing | [223] | ||
| Glucose oxidase | 1 | Biosensing/therapy | [224,225,226,227] | ||
| Glucose oxidase, peroxidase | 1 | Biosensing | [228] | ||
| Laccase, NAD+-dependent DHase, alcohol DHase, aldehyde DHase, formate DHase | 1 | Biofuel cell | [229] | ||
| Lactate oxidase | 1 | Biosensing | [225] | ||
| Maltase | 3 | Therapy | [230] | ||
| Old yellow enzyme | 1 | Biocatalysis | [231] | ||
| Uricase | 1 | Biosensing | [225] | ||
| CNFs | Covalent | Laccase | 1 | Biosensing | [232] |
| Non-covalent | Tyrosinase | 3 | Biosensing | [233] | |
| CNHs | Covalent | Glutamate oxidase | 1 | Biosensing | [234] |
| Non-covalent | Peroxidase | 1 | Biosensing | [235] | |
| CNOs | Covalent | Alkaline phosphatase | 3 | Biosensing | [236] |
| Glucose oxidase | 1 | Biosensing | [236,237] | ||
| Peroxidase | 1 | Biosensing | [236,238] | ||
| Non-covalent | Adenylate kinase | 2 | Biocatalysis | [222] | |
| GO | Covalent | Laccase | 1 | Biosensing | [239] |
| Non-covalent | Choline oxidase/acetylcholine esterase | 1/3 | Biosensing | [240] | |
| Rhamnosidase | 3 | Biocatalysis | [241] | ||
| Adenylate kinase | 2 | Biocatalysis | [222] | ||
| Glucose Oxidase | 1 | Biosensing/fuel cells | [242] | ||
| Lipase | 3 | Biocatalysis | [243] | ||
| GO, MWCNTs | Non-covalent | Lactate oxidase | 1 | Biosensing | [244] |
| rGO | Non-covalent | Acetylcholine esterase | 3 | Biosensing | [245] |
| Thrombin | 1 | Biosensing | [245] | ||
| MWCNTs | Covalent | Choline Oxidase | 1 | Biosensing | [246] |
| Glucose oxidase | 1 | Biosensing/fuel cells | [247,248,249,250,251] | ||
| Glucose oxidase | 1 | Cancer therapy | [252] | ||
| Laccase | 1 | Biofuel cells | [253] | ||
| Laronidase | 3 | Therapy | [254] | ||
| Lipase | 3 | Biofuel cells | [255] | ||
| Peroxidase | 1 | Sensing/membranes | [256,257] | ||
| Pyranose oxidase | 1 | Biosensing/fuel cells | [258,259] | ||
| Tyrosinase | 3 | Biosensing | [218,260] | ||
| Uricase | 1 | Biosensing | [261] | ||
| Xanthine oxidase | 1 | Biosensing | [262] | ||
| Non-covalent | Alcohol dehydrogenase | 1 | Biosensing | [263,264] | |
| Alcohol oxidase | 1 | Biosensing | [265] | ||
| Amino acid oxidase | 1 | Biosensing | [265] | ||
| Amylase, lysozyme | 3 | Biocatalysis | [266] | ||
| Bilirubin oxidase | 1 | Biosensing/fuel cells | [267,268] | ||
| Choline oxidase | 1 | Biosensing | [265] | ||
| Glucose dehydrogenase | 1 | Biosensing/fuel cells | [269] | ||
| Glucose oxidase | 1 | Biosensing/fuel cells | [247,270,271,272,273,274,275] | ||
| Glucose oxidase/catalase | 1 | Biofuel cells | [276] | ||
| Glucose oxidase, laccase | 1 | Biosensing/fuel cells | [277] | ||
| Glutamate oxidase | 1 | Biosensing | [278] | ||
| Laccase | 1 | Biosensing | [279] | ||
| Lactate oxidase | 1 | Biosensing | [280] | ||
| Lipase | 3 | Biosensing/biocatalysis | [263,264,281,282,283] | ||
| [NiFeSe]-hydrogenase | 1 | Biofuel cells | [284] | ||
| Oxalate decarboxylase | 4 | Biofuel cells | [285] | ||
| Peroxidase | 1 | Biosensing/fuel cells | [286,287] | ||
| Pyranose oxidase | 1 | Biosensing/fuel cells | [258,265] | ||
| SWCNTs | Covalent | Tyrosinase | 1 | Biosensing | [288] |
| Non-covalent | Choline oxidase | 1 | Biosensing | [265] |
| CNM Type | Conjugation | Linear Range | Detection Limit | Sensitivity | Ref. |
|---|---|---|---|---|---|
| CNDs | Non-covalent | 250–3000 μM | n.a. | n.a. | [224] |
| 0.1–500 μM | 65 µM | 21.6 µA·mM−1·cm−2 | [227] | ||
| Covalent | 0.1–500 μM | 0.04 μM | n.a. | [228] | |
| CNOs | Covalent | 1000–10,000 μM | 210 μM | 26.5 µA·mM−1·cm−2 | [237] |
| CNTs | Covalent | 100–6000 μM | 9.01 μM | n.a. | [249] |
| n.a. | n.a. | 0.27–3.7 µA·mM−1·cm−2 | [258] | ||
| Non-covalent | 1000–20,000 μM | n.a. | 0.198 µA·mM−1·cm−2 | [269] | |
| 1–5000 μM | 0.36 μM | n.a. | [271] | ||
| 0–5000 μM | 50 μM | 289 μA·mM−1·cm−2 | [273] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozhin, P.; Abdel Monem Gamal, J.; Giordani, S.; Marchesan, S. Carbon Nanomaterials (CNMs) and Enzymes: From Nanozymes to CNM-Enzyme Conjugates and Biodegradation. Materials 2022, 15, 1037. https://doi.org/10.3390/ma15031037
Rozhin P, Abdel Monem Gamal J, Giordani S, Marchesan S. Carbon Nanomaterials (CNMs) and Enzymes: From Nanozymes to CNM-Enzyme Conjugates and Biodegradation. Materials. 2022; 15(3):1037. https://doi.org/10.3390/ma15031037
Chicago/Turabian StyleRozhin, Petr, Jada Abdel Monem Gamal, Silvia Giordani, and Silvia Marchesan. 2022. "Carbon Nanomaterials (CNMs) and Enzymes: From Nanozymes to CNM-Enzyme Conjugates and Biodegradation" Materials 15, no. 3: 1037. https://doi.org/10.3390/ma15031037
APA StyleRozhin, P., Abdel Monem Gamal, J., Giordani, S., & Marchesan, S. (2022). Carbon Nanomaterials (CNMs) and Enzymes: From Nanozymes to CNM-Enzyme Conjugates and Biodegradation. Materials, 15(3), 1037. https://doi.org/10.3390/ma15031037










