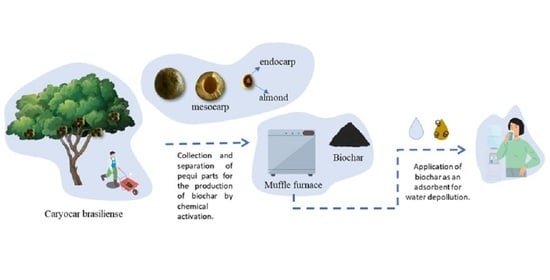
Biochar Obtained from Caryocar brasiliense Endocarp for Removal of Dyes from the Aqueous Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Biochar Preparation
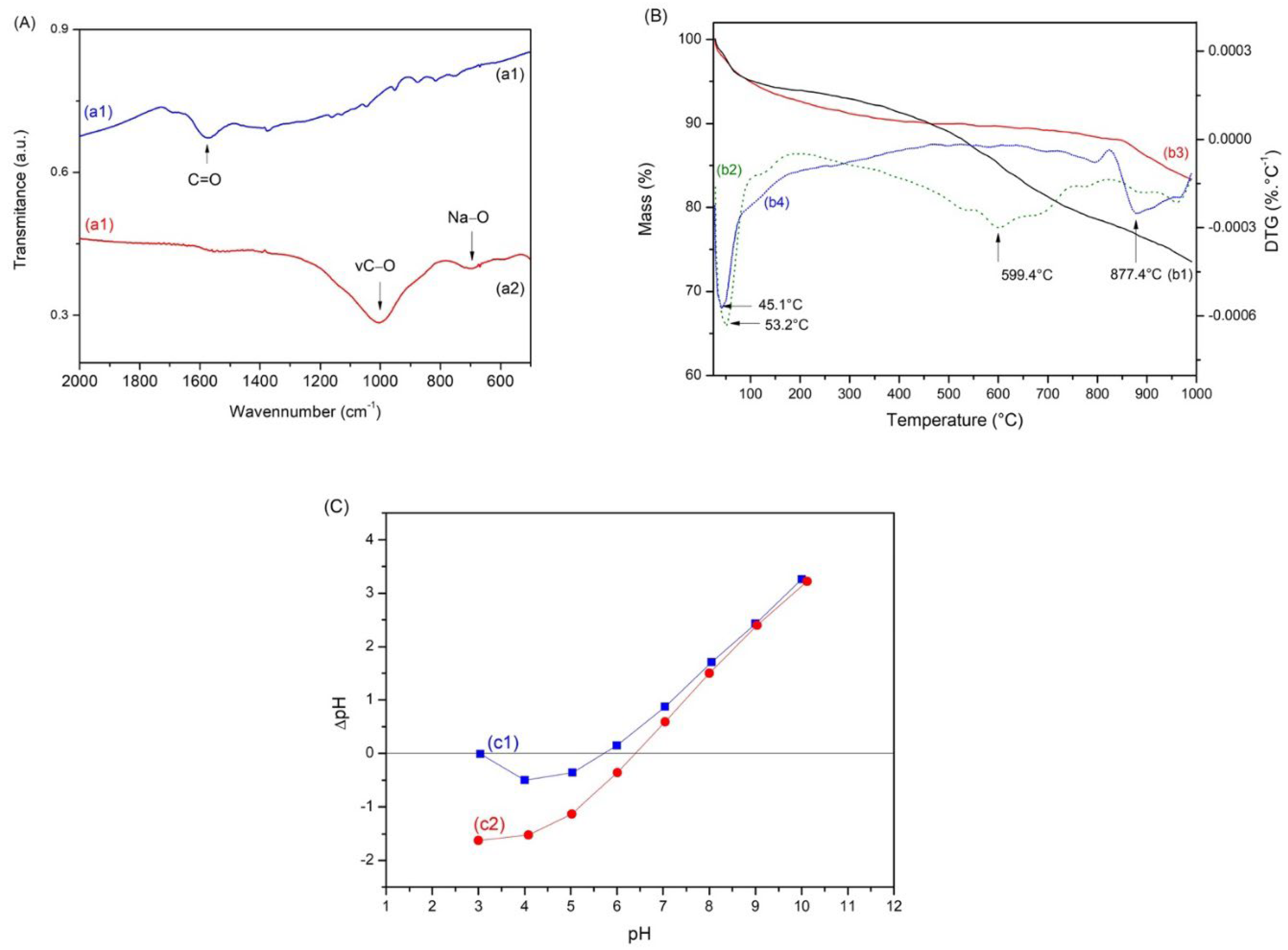
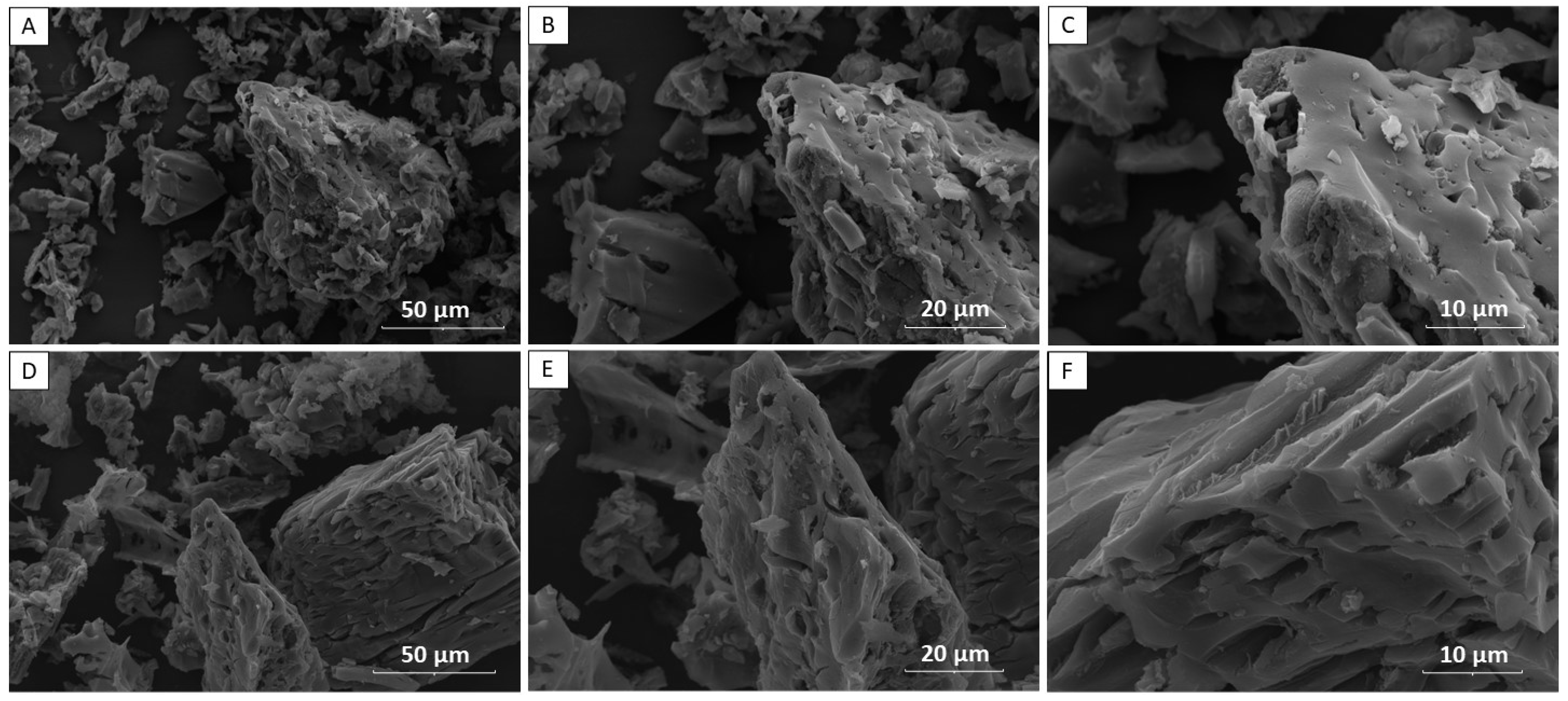
2.3. Biochar Characterizations
2.4. Point of Zero Charge (pHPZC)
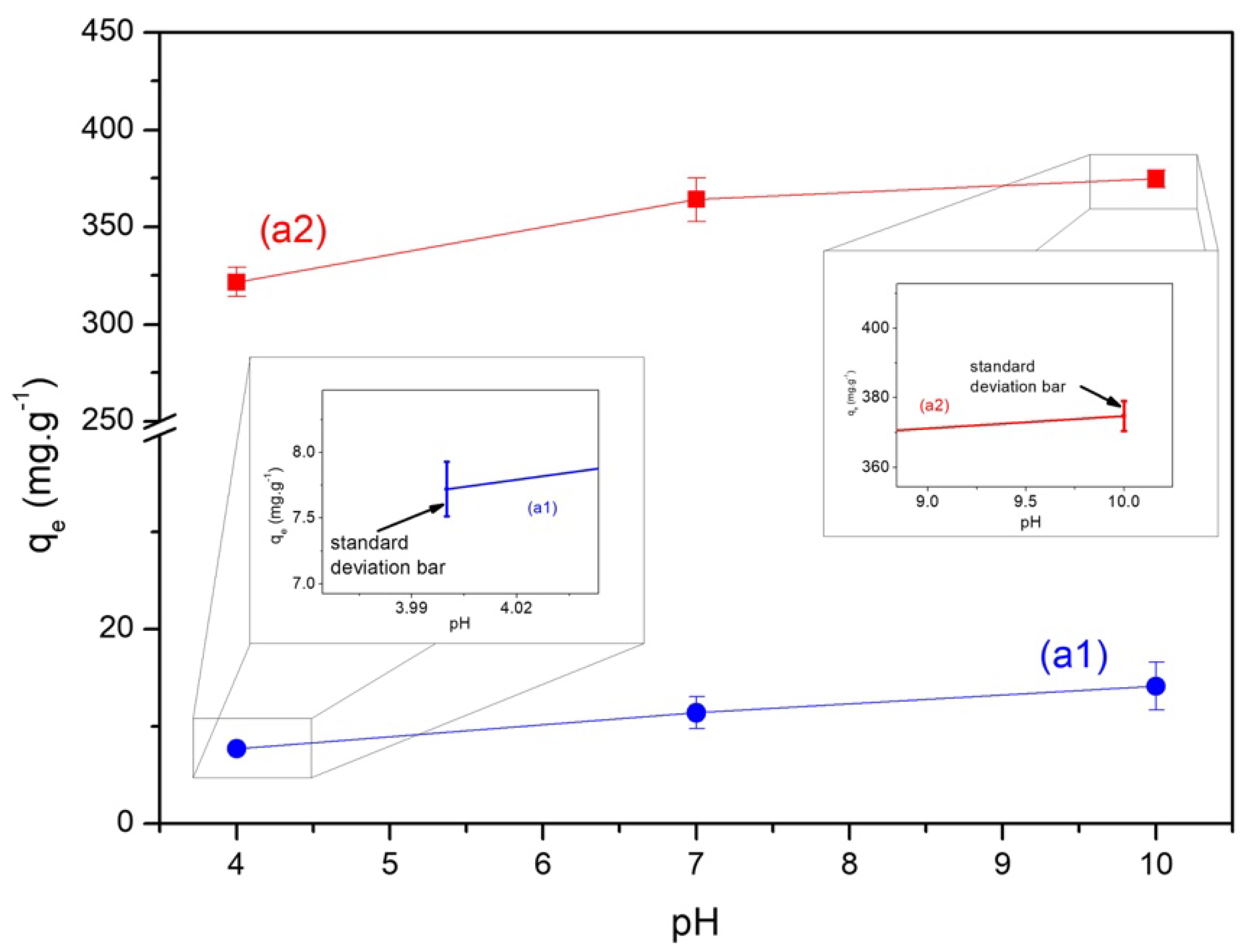
2.5. Influence of pH
2.6. Adsorption Kinetics
2.7. Adsorption Isotherm
3. Results and Discussion
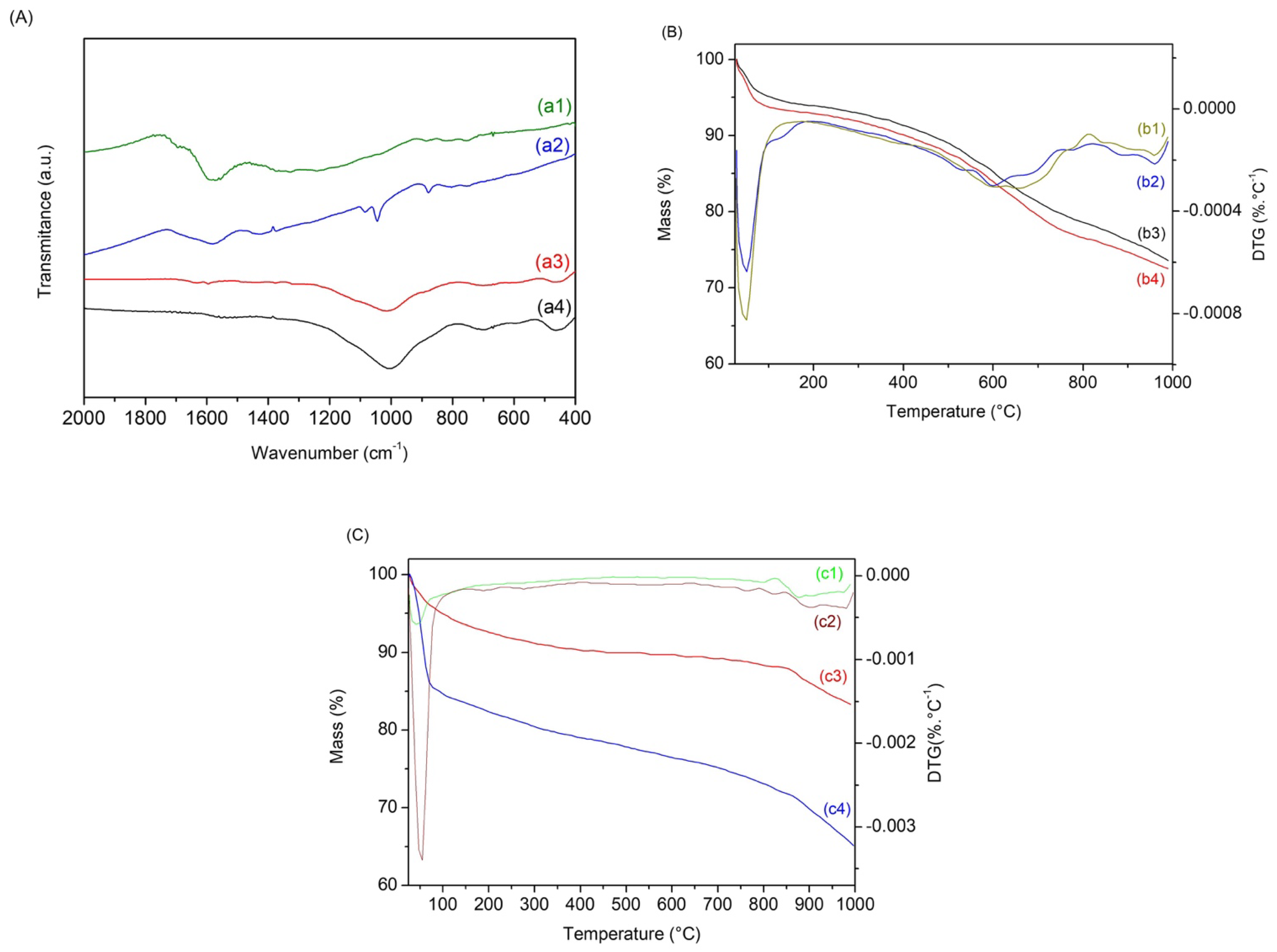
3.1. Characterization of Biochars
3.2. Characterization of Biochars after Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, A.B.C.; Andrade, R.M.D.F.; Freire, F.B.; Nagalli, A.; Carvalho, K.Q.D.; Passig, F.H.; Kreutz, C. Application of red clay as adsorbent in the removal of textile dye Direct Blue of an aqueous solution. Mater.-Rio Jan. 2017, 22, 983–999. [Google Scholar]
- Mehra, S.; Singh, M.; Chadha, P. Adverse impact of textile dyes on the aquatic environment as well as on human beings. Toxicol. Int. 2021, 28, 165–176. [Google Scholar]
- Castro, A.S.; Franco, C.R.; Cidade, M.J.A. Adsorção de Corantes Azul Indosol, Laranja Indosol e Vermelho Drimaren em Solução Aquosa por Argila Branca Adsorption of Dyes Indosol Blue, Indosol Orange and Drimarene Red in Aqueous Solution by White Clay. Rev. Virtual Quim. 2018, 10, 1502–1515. [Google Scholar] [CrossRef]
- Silva, L.S.; Ferreira, F.J.; Silva, M.S.; Citó, A.M.; Meneguin, A.; Sábio, R.; Barud, H.S.; Bezerra, R.D.; Osajima, J.A.; Filho, E.C.S. Potential of amino-functionalized cellulose as an alternative sorbent intended to remove anionic dyes from aqueous solutions. Int. J. Biol. Macromol. 2018, 116, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, J.; Xu, S.; Wang, M.; Zhang, Y.; Xue, X. A modified method for enhancing adsorption capability of banana pseudostem biochar towards methylene blue at low temperature. Bioresour. Technol. 2019, 282, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.T.; Barros, A.Z.B.; Morais, A.I.S.; Melo, A.L.F.C.; Bezerra, R.D.S.; Osajima, J.A.; Silva-Filho, E.C. Application of Water Hyacinth Biomass (Eichhornia crassipes) as an Adsorbent for Methylene Blue Dye from Aqueous Medium: Kinetic and Isothermal Study. Polymers 2022, 14, 2732. [Google Scholar] [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2020, 12, 70–87. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Liu, S.; Wei, W.; Wu, S.; Zhang, F. Preparation of hierarchical porous activated carbons from different industrial lignin for highly efficient adsorption performance. J. Porous Mater. 2020, 27, 1523–1533. [Google Scholar] [CrossRef]
- Ghosh, A.; Razzino, C.D.A.; Dasgupta, A.; Fujisawa, K.; Vieira, L.H.S.; Subramanian, S.; Costa, R.S.; Lobo, A.O.; Ferreira, O.P.; Robinson, J.; et al. Structural and electrochemical properties of babassu coconut mesocarp-generated activated carbon and few-layer graphene. Carbon 2019, 145, 175–186. [Google Scholar] [CrossRef]
- Heylmann KK, A.; Lopes, B.V.; Afonso, T.F.; Demarco, C.F.; Cadaval Junior, T.R.; Quadro, M.S.; Andreazza, R. Production, characterization, and application of activated charcoal from peach kernel in textile effluent treatment. Eng. Sanit. E Ambient. 2021, 26, 485–494. [Google Scholar]
- Ferreira, A.A.; Guimarães, E.R.; Contador, J.C. Patente como instrumento competitivo e como fonte de informação tecnológica. Gest. Prod. 2009, 16, 209–221. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2020, 763, 144204. [Google Scholar] [CrossRef] [PubMed]
- Ghesti, G.F.; Silveira, E.A.; Guimarães, M.G.; Evaristo, R.B.; Costa, M. Towards a sustainable waste-to-energy pathway to pequi biomass residues: Biochar, syngas, and biodiesel analysis. Waste Manag. 2022, 143, 144–156. [Google Scholar] [CrossRef]
- Scapin, E.; Rambo, M.K.D.; Viana, G.C.C.; Borges, M.S.; Rambo, M.C.; Carneiro, C. Production of Furanic Compounds and Organic Acids from Brazilian Pequi (Caryocar brasiliensis Camb.) Residues Using Green Chemistry. J. Braz. Chem. Soc. 2020, 31, 1383–1391. [Google Scholar] [CrossRef]
- da Luz, G.R.; Rodrigues, P.M.S.; Menino, G.C.O.; Coutinho, E.S.; Nunes, Y.R.S. Caracterização física de frutos e putâmens e taxa de ataque por carmenta sp. a pequizeiros (Ccaryocar brasiliense camb.) no norte de Minas Gerais. Rev. Bras. Frutic. 2011, 33, 746–756. [Google Scholar] [CrossRef]
- Fábio, F.B.F.; Wanessa, F.A.; Mônica, R.F.M.; Ariel, E.S.; Liliane, N.; Sandra, A.B.-R.; Frederico, A.G.G.; Andréia, V.C.D.A. Therapeutic applications of Caryocar brasiliense: Systematic review. J. Med. Plants Res. 2021, 15, 380–389. [Google Scholar] [CrossRef]
- Bailão, E.F.L.C.; de Oliveira, M.G.; de Almeida, L.M.; Amaral, V.C.S.; Chen, L.C.; Caramori, S.S.; de Paula, J.A.M.; de Melo Cruvinel, W.; Borges, L.L. Food Composition Data: Edible Plants in Cerrado. In Local Food Plants of Brazil; Springer: Cham, Switzerland, 2021; pp. 179–224. [Google Scholar]
- Qiu, R.; Lin, M.; Qin, B.; Xu, Z.; Ruan, J. Environmental-friendly recovery of non-metallic resources from waste printed circuit boards: A review. J. Clean. Prod. 2021, 279, 123738. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Gonçalves, L.E.N.; Coutinho, M.P.; Peixoto, N.; Gatto, A. Aspectos Socioambientais da Comercialização de Pequi em Goiás. Floresta E Ambient. 2017, 24, e00058213. [Google Scholar] [CrossRef]
- Pai, S.; Kini, M.S.; Selvaraj, R. A review on adsorptive removal of dyes from wastewater by hydroxyapatite nanocomposites. Environ. Sci. Pollut. Res. 2021, 28, 11835–11849. [Google Scholar] [CrossRef]
- Roy, H.; Shahinoor Islam, M.d.; Tanvir Arifin, M.; Firoz, S.H. Chitosan-ZnO decorated Moringa oleifera seed biochar for sequestration of methylene blue: Isotherms, kinetics, and response surface analysis. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100752. [Google Scholar] [CrossRef]
- Sahu, S.; Pahi, S.; Tripathy, S.; Singh, S.K.; Behera, A.; Sahu, U.K.; Patel, R.K. Adsorption of methylene blue on chemically modified lychee seed biochar: Dynamic, equilibrium, and thermodynamic study. J. Mol. Liq. 2020, 315, 113743. [Google Scholar] [CrossRef]
- Borba, L.L.; Cuba, R.M.F.; Terán, F.J.C.; Castro, M.N.; Mendes, T.A. Use of Adsorbent Biochar from Pequi (Caryocar brasiliense) Husks for the Removal of Commercial Formulation of Glyphosate from Aqueous Media. Braz. Arch. Biol. Technol. 2019, 62, e19180450. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into activated carbon from different kinds of chemical activating agents: A review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef] [PubMed]
- Cazetta, A.L.; Vargas, A.M.M.; Nogami, E.M.; Kunita, M.H.; Guilherme, M.R.; Martins, A.C.; Silva, T.L.; Moraes, J.C.G.; Almeida, V.C. NaOH-activated carbon of high surface area produced from coconut shell: Kinetics and equilibrium studies from the methylene blue adsorption. Chem. Eng. J. 2011, 174, 117–125. [Google Scholar] [CrossRef]
- Amoim, F.A.C. Estudo de Remoção de Cu(II) em Meio Aquoso Utilizando Carvão Preparado a partir da Casca do Licurí (Syagrus coronata). Rev. Virtual Quim. 2017, 9, 2121–2134. [Google Scholar]
- Costa, L.F.; de Oliveira, D.G.; Moreira, F.M.S.; de Urzedo, A.P.F.M.; Cestarolli, D.T.; Bernardes-Silva, A.C. Use of Biochar and Advanced Oxidative Processes for the Removal of Propranolol from Simulated Aqueous Effluents. Rev. Virtual Quim. 2018, 10, 295–312. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, J.; Liu, W.; Li, Z.; He, K. Efficient removal of dyes from dyeing wastewater by powder activated charcoal/titanate nanotube nanocomposites: Adsorption and photoregeneration. Environ. Sci. Pollut. Res. 2019, 26, 10263–10273. [Google Scholar] [CrossRef]
- Zhang, X.; Mao, X.; Pi, L.; Wu, T.; Hu, Y. Adsorptive and capacitive properties of the activated carbons derived from pig manure residues. J. Environ. Chem. Eng. 2019, 7, 103066. [Google Scholar] [CrossRef]
- Ferreira, F.J.; Silva, L.S.; da Silva, M.S.; Osajima, J.A.; Meneguin, A.B.; Santagneli, S.H.; Barud, H.S.; Bezerra, R.D.; Silva-Filho, E.C. Understanding kinetics and thermodynamics of the interactions between amitriptyline or eosin yellow and aminosilane-modified cellulose. Carbohydr. Polym. 2019, 225, 115246. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in lösungen. Z. Für Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Mokhtar, M. Application of Synthetic Layered Sodium Silicate Magadiite Nanosheets for Environmental Remediation of Methylene Blue Dye in Water. Materials 2017, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Zapién, K.V.; Torres-Pérez, J.; Reyes-López, S.Y. Environmental application of quartz-based construction waste: Tartrazine removal from aqueous media. Int. J. Environ. Sci. Technol. 2021, 19, 10381–10392. [Google Scholar] [CrossRef]
- Khalit, W.N.A.W.; Marliza, T.S.; Taufiq-Yap, Y.H. Synthesis and Characterizations of Nickel Supported on Activated Charcoal and its Application for Green Fuel Production. Mater. Sci. Forum 2020, 1010, 424–430. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- Doğan, M.; Sabaz, P.; Bicil, Z.; Kizilduman, B.K.; Turhan, Y. Activated carbon synthesis from tangerine peel and its use in hydrogen storage. J. Energy Inst. 2020, 93, 2176–2185. [Google Scholar] [CrossRef]
- Ouhammou, M.; Lahnine, L.; Mghazli, S.; Hidar, N.; Bouchdoug, M.; Jaouad, A.; Mandi, L.; Mahrouz, M. Valorisation of cellulosic waste basic cactus to prepare activated carbon. J. Saudi Soc. Agric. Sci. 2019, 18, 133–140. [Google Scholar] [CrossRef]
- Sanou, Y.; Alfa-Sika, M.S.L.; Vunain, E.; Pare, S. Preparation and Characterization of Husk Based Carbons: Effect of the Temperature. J. Environ. Treat. Tech. 2019, 7, 150–157. [Google Scholar]
- Bezerra, R.D.S.; Morais, A.I.S.; Osajima, J.A.; Nunes, L.C.C.; Silva Filho, E.C. Development of new phosphated cellulose for application as an efficient biomaterial for the incorporation/release of amitriptyline. Int. J. Biol. Macromol. 2016, 86, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Li, J.; Cai, Z. Preparation and characterization of chars and activated carbons from wood wastes. Carbon Lett. 2021, 31, 941–956. [Google Scholar] [CrossRef]
- Gaydukova, A.M.; Nenasheva, A.S.; Kolesnikov, V.A.; Vetlugin, N.A. The Extraction of Activated Carbon from Aqueous Solutions in the Presence of Organic and Inorganic Compounds by the Electroflotation Method. J. Water Chem. Technol. 2021, 43, 116–122. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Mastuli, M.S. Mesoporous Crosslinked Chitosan-Activated Charcoal Composite for the Removal of Thionine Cationic Dye: Comprehensive Adsorption and Mechanism Study. J. Polym. Environ. 2020, 28, 1095–1105. [Google Scholar] [CrossRef]
- Yin, Y.; Zhou, T.; Luo, H.; Geng, J.; Yu, W.; Jiang, Z. Adsorption of arsenic by activated charcoal coated zirconium-manganese nanocomposite: Performance and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 318–328. [Google Scholar] [CrossRef]
- del Hombre Bueno, B.R.; Boluda-Botella, N.; Rico, D.P. Removal of emerging pollutants in water treatment plants: Adsorption of methyl and propylparaben onto powdered activated carbon. Adsorption 2019, 25, 983–999. [Google Scholar] [CrossRef]

| Biochar | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| qe (mg.g−1) | K1 (min−1) | R2 | qe (mg.g−1) | K2 (mg.g−1.min−1) | R2 | |
| BE | 11.20 | −0.00982 | 0.07518 | 7.78 | 0.13992 | 0.9892 |
| ABE | 13.46 | −0.00700 | 0.91000 | 476.19 | 1.61538 | 0.9999 |
| Biochar | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qo | KL | R2 | n | KF | R2 | |
| BE | 70.92 | 0.003995 | 0.8784 | 3.057 | 5.93 | 0.8749 |
| ABE | 500.0 | 0.009809 | 0.9962 | 4.091 | 2.53 | 0.9105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, A.L.F.C.; Carneiro, M.T.; Nascimento, A.M.S.S.; Morais, A.I.S.; Bezerra, R.D.S.; Viana, B.C.; Osajima, J.A.; Silva-Filho, E.C. Biochar Obtained from Caryocar brasiliense Endocarp for Removal of Dyes from the Aqueous Medium. Materials 2022, 15, 9076. https://doi.org/10.3390/ma15249076
Melo ALFC, Carneiro MT, Nascimento AMSS, Morais AIS, Bezerra RDS, Viana BC, Osajima JA, Silva-Filho EC. Biochar Obtained from Caryocar brasiliense Endocarp for Removal of Dyes from the Aqueous Medium. Materials. 2022; 15(24):9076. https://doi.org/10.3390/ma15249076
Chicago/Turabian StyleMelo, André L. F. C., Marcelo T. Carneiro, Ariane M. S. S. Nascimento, Alan I. S. Morais, Roosevelt D. S. Bezerra, Bartolomeu C. Viana, Josy A. Osajima, and Edson C. Silva-Filho. 2022. "Biochar Obtained from Caryocar brasiliense Endocarp for Removal of Dyes from the Aqueous Medium" Materials 15, no. 24: 9076. https://doi.org/10.3390/ma15249076
APA StyleMelo, A. L. F. C., Carneiro, M. T., Nascimento, A. M. S. S., Morais, A. I. S., Bezerra, R. D. S., Viana, B. C., Osajima, J. A., & Silva-Filho, E. C. (2022). Biochar Obtained from Caryocar brasiliense Endocarp for Removal of Dyes from the Aqueous Medium. Materials, 15(24), 9076. https://doi.org/10.3390/ma15249076