Abstract
Borogermanate glasses singly doped with Dy3+ ions were synthesized and then studied using the absorption and luminescence spectra. Spectroscopic changes of Dy3+ ions have been examined for compositional-dependent glasses with various molar ratios GeO2:B2O3. In this work, several spectroscopic parameters of Dy3+ ions were obtained experimentally and compared to the calculated values from the Judd–Ofelt theory. Luminescence spectra measured for borogermanate glasses consist of blue, yellow and red bands, which correspond to 4F9/2 → 6H15/2, 4F9/2 → 6H13/2 and 4F9/2 → 6H11/2 transitions of Dy3+, respectively. Luminescence lifetimes for the 4F9/2 excited state are reduced, whereas the stimulated emission cross-sections for the most intense 4F9/2 → 6H13/2 yellow transition of Dy3+ increase with increasing GeO2 and decreasing B2O3 concentrations in glass-hosts. Quantum efficiency of the 4F9/2 (Dy3+) excited state is nearly independent on molar ratios GeO2:B2O3. Attractive spectroscopic properties related to the 4F9/2 → 6H13/2 transition of Dy3+ ions are found for borogermanate glasses implying their potential utility for yellow laser action and solid-state lighting technology.
1. Introduction
Judd [1] and Ofelt [2] published their pioneering scientific works concerning the absorption intensities of rare earths 60 years ago. Based on the Judd–Ofelt (J-O) framework, several spectroscopic parameters for the optically active ions from the lanthanide series can be determined, which are really important from the optical and laser points of view. The most important of which are the radiative transition probability, the luminescence branching ratio and the radiative lifetime for the upper laser state of rare earth ions. The radiative transition probability and spectral linewidth for luminescent transition of rare earth ions can be used to calculate the peak stimulated emission cross-section, whereas measured emission lifetime and radiative lifetime calculated from the J-O framework are usually applied to estimate the quantum efficiency of the excited state. As a result, many glass matrices doped with rare earths can be quite well evaluated for a variety of possible applications such as for optical components and devices. Therefore, the J–O theory made a huge contribution to the development of optical and laser glasses in modern photonics. Since then, numerous published papers have been devoted to the study of glasses, glass-ceramics and other inorganic compounds singly or doubly doped with rare earth ions [3,4,5,6,7,8] using the theory on the intensities of 4f-4f electronic transitions introduced by Judd and Ofelt in 1962. In particular, the Judd–Ofelt analysis was performed for trivalent Nd3+ [9,10,11,12,13,14,15,16,17], Er3+ [18,19,20,21,22,23,24,25,26,27,28,29,30], Sm3+ [31,32,33,34,35], Pr3+ [36,37,38,39,40], Tm3+ [41,42,43,44], Ho3+ [45,46,47,48,49,50] and Dy3+ [51,52,53,54,55,56,57,58] ions in various inorganic glasses. The later trivalent rare earth ions, i.e., Dy3+ ions, were successfully used as an optical probe to study the luminescence behavior of inorganic glasses [59]. Recently, systematic investigations indicate that dysprosium doped glasses are excellent candidates for solid-state yellow lasers, white LEDs and other photonic device applications [60,61,62,63]. Their luminescence properties depend strongly on glass-network-modifiers [64], excitation wavelength and activator concentration [65].
In this work, the influence of glass-network-formers on the spectroscopic properties of dysprosium ions in borogermanate glasses has been examined in detail. Previous studies revealed that borogermanate glass belongs to amorphous systems with extremely different glass-network-formers B2O3 and GeO2, influencing the spectroscopic properties of Cr3+ and Eu3+ [66]. Further investigations revealed that borogermanate glasses are suitable to fabricate CsPbBr3−xIx quantum dots with tunable visible emissions ranging from 577 nm to 672 nm [67]. In addition, they are able to accommodate rare earth ions. Thus, borogermanate glasses singly [68] and doubly [69] doped with rare earths are promising for luminescence applications. In recent years, borogermanate glass doped with Dy3+ has also been studied, but luminescence properties were analyzed as a function of activator content. Gökçe and Koçyiğit [70] suggest that Dy3+ doped gadolinium borogermanate glass matrix with the following composition 30B2O3-40GeO2-(30-x)Gd2O3-xDy2O3 (where x = 0.25, 0.5, 1) presents excellent luminescence properties and can be used for laser and white LED applications. Numerous works published in recent years are concerned with glasses and their spectroscopic properties varying with activator (Dy3+) content [71,72,73,74,75,76,77,78,79,80,81]. These aspects have been discussed along with the reported Dy3+ doped glass systems in an excellent paper published last year [82]. However, Dy3+-doped glasses have not been examined often as a function of chemical composition and the spectroscopic results are less documented in the literature. Previous studies for compositional-dependent germanosilicate glasses demonstrate that the intensities of emission bands are the largest for glass samples with the molar ratio GeO2:SiO2 = 3:1 and the optimal concentration of Dy3+ ions equal to 0.5 mol% [83]. It was also confirmed for zinc aluminoborosilicate glasses, where luminescence quenching is observed beyond 0.5 mol% Dy3+ ions suggesting the presence of an energy-transfer process through cross-relaxation channels [84].
This paper is concerned with Dy3+-doped borogermanate glasses and their emission properties varying with glass-host composition. Absorption and emission properties have been analyzed for glass samples, where the molar ratios of glass-network-formers GeO2:B2O3 are changed significantly and the activator concentration is equal to 0.5 mol% Dy3+. In particular, several spectroscopic parameters for Dy3+ ions were obtained experimentally and compared with theoretical values calculated from the J–O theory.
2. Materials and Methods
Borogermanate glasses doped with Dy3+ ions with the following composition given in molar%: (60-x)GeO2-xB2O3-30BaO-9.5Ga2O3-0.5Dy2O3 (x = 0, 5, 10, 20, 30, 40, 50, 60) were prepared previously and details are given in Ref. [85]. For better clarity, glass codes and chemical compositions of the studied samples varying with glass formers GeO2 and B2O3 marked in bold are shown in Table 1.

Table 1.
Glass codes and chemical compositions for glass samples doped with Dy3+ ions.
Glass samples were synthesized using a melt-quenching technique. Starting components of high purity 99.99% (Aldrich Chemical Co., St. Louis, MO, USA) were used for glass synthesis. All oxide components were mixed in an agate mortar for homogenization. Then, glass batches were placed in an Al2O3 crucible and melted at temperature T = 1250 °C for t = 45 min in an electric furnace. The received glass samples were cooled to room temperature and then polished for optical measurements. Photographs of the Dy3+ doped glass samples are shown in Figure 1.

Figure 1.
Photographs of the Dy3+ doped glass samples: GeO2-BaO-Ga2O3 (1), GeO2:B2O3 = 11:1 (2), GeO2:B2O3 = 5:1 (3), GeO2:B2O3 = 2:1 (4), GeO2:B2O3 = 1:1 (5), GeO2:B2O3 = 1:2 (6), GeO2:B2O3 = 1:5 (7) and B2O3-BaO-Ga2O3 (8).
The refractive indices of glass series were determined using the Metricon 2010 prism coupler at a wavelength of 632.8 nm. The optical absorption spectra measurements were performed using the UV-VIS-NIR spectrophotometer (Cary 5000, Agilent Technology, Santa Clara, CA, USA). The emission spectra and decays were recorded using laser equipment, which consists of a Photon Technology International (PTI) Quanta-Master 40 (QM40) UV/VIS Steady State Spectrofluorometer (Photon Technology International, Birmingham, NJ, USA) with a xenon lamp as an excitation source, Nd:YAG laser (Opotek Opolette 355 LD, OPOTEK, Carlsband, CA, USA) with a tunable pulsed optical parametric oscillator, double 200 mm monochromator and multimode UVVIS PMT R928 detector (PTI Model 914). Resolution for emission spectra measurements was 0.1 nm, whereas decays were measured with an accuracy of 1 µs.
3. Theoretical Background
The measured oscillator strengths of transitions were obtained from the absorption bands of Dy3+ ions. They were estimated by measuring the areas under the absorption bands of Dy3+ ions using the equation:
where: ∫ε(ν) represents the area under the absorption line and ε(ν) = A/(c × l), A indicates the absorbance, c is the concentration of the Dy3+ ion (in mol × l−1) and l denotes the optical path length. The theoretical oscillator strengths for each absorption transition of Dy3+ ions were calculated using the Judd–Ofelt theory [1,2]. The theoretical oscillator strength is defined as follows:
where λ denotes the mean wavelength of each transition, whereas m, c, h and n are the mass of the electron, the velocity of light, the Planck constant and the refractive index of the medium, respectively. In this relation, ║Ut║2 represents the square of the matrix elements of the unit tensor operator Ut. The values of ║Ut║2 used for Dy3+ were adopted from Ref. [86]. The theoretical oscillator strengths were compared to the experimental values obtained from the optical absorption spectra of Dy3+ ions in borogermanate glasses and the phenomenological intensity parameters Ωt (where t = 2, 4, 6) were determined. The fit quality was expressed by the magnitude of the root-mean-square deviation. It was defined by rms = Σ (Pmeas − Pcalc)2. These three Judd–Ofelt intensity parameters Ωt (t = 2, 4, 6) were used to calculate the radiative transition probabilities, the luminescence branching ratios and the radiative lifetimes. The radiative transition probabilities AJ for the excited states of Dy3+ ions were calculated using the relation given below:
The luminescence branching ratio β is related to the relative intensities of transitions from the excited state to all terminal states of Dy3+ ions.
The radiative lifetime τrad is the inverse of the total radiative transition probability (the sum of the AJ terms). Its value was compared to the experimental lifetime received from the luminescence decay curve. Both calculated and measured lifetimes were applied to determine the quantum efficiency of an excited state η. The appropriate relations are given below:
Finally, the emission linewidth Δλ referred as full width at half maximum (FWHM) and the radiative transition probability AJ were successfully used to calculate the peak stimulated emission cross-section σem using the following expression:
where λp is the peak emission wavelength for the electronic transition of Dy3+.
All theoretical and experimental spectroscopic parameters for Dy3+ ions in the studied borogermanate glasses are summarized in Table 2.

Table 2.
Theoretical and experimental spectroscopic parameters for Dy3+ in borogermanate glasses.
4. Results and Discussion
Judd–Ofelt analysis of Dy3+ ions in mixed borogermanate glasses with various GeO2:B2O3 molar ratios equal to 11:1, 5:1, 2:1, 1:1, 1:2 and 1:5 was carried out. Theoretical and experimental results were compared to GeO2-BaO-Ga2O3 and B2O3-BaO-Ga2O3 glasses. Absorption and emission properties have been examined for glass samples, where the concentration of Dy3+ ions was the same (0.5 mol%). Firstly, the absorption spectra measurements for Dy3+ ions in borogermanate glasses were carried out at room temperature. The absorption spectra of borogermanate glasses doped with Dy3+ ions were measured in the UV-visible and near-infrared spectral ranges, respectively. The spectra consist of inhomogeneously broadened absorption bands characteristic for 4f9-4f9 electronic transitions of Dy3+. The absorption bands correspond to transitions originating from the 6H15/2 ground state to the following excited states: 6H11/2, 6F11/2, 6F9/2, 6F7/2, 6F3/2, 4F9/2, 4I15/2, 4G11/2, 4I13/2, 4F7/2, (4M19/2+4D3/2+6P5/2), 6P7/2 and 6P3/2. The later transition, i.e., 6H15/2 → 6P3/2 transition, is clearly visible for GeO2-BaO-Ga2O3 glass contrary to B2O3-BaO-Ga2O3 glass. For mixed borogermanate glasses, the 6H15/2 → 6P3/2 transition of Dy3+ lies on the absorption edge. This indicates that the absorption edge is shifted to longer wavelengths from GeO2-BaO-Ga2O3 glass via mixed B2O3-GeO2-BaO-Ga2O3 compositions to B2O3-BaO-Ga2O3 glass, respectively. The absorption spectra are presented in Figure 2.
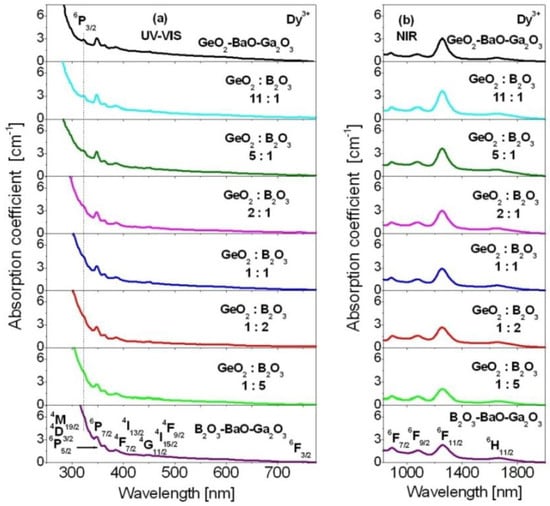
Figure 2.
Absorption spectra for Dy3+ ions in borogermanate glasses with various molar ratios GeO2:B2O3 compared to GeO2-BaO-Ga2O3 and B2O3-BaO-Ga2O3 glasses. The spectra were measured in the UV-visible (a) and near-infrared (b) spectral ranges.
From the optical absorption spectra, the experimental oscillator strengths for Dy3+ ions have been determined. Owing to the standard procedure, the x-axes of absorption spectra were converted to wavenumbers (given in cm−1). In the next step, the baseline was fitted individually to each absorption band. The integrated areas of absorption bands were calculated. The intensities of absorption lines of Dy3+ ions presented in Figure 2 were estimated by measuring the areas under the bands, and then applied to determine the experimental oscillator strengths using relation (1). The commercially available software OriginPro was used during the calculation procedure.
The theoretical oscillator strengths for each transition of Dy3+ ions were calculated from the J–O framework (Part 3) using relation (2). In order to perform the analysis, the refractive index of the medium was used for calculations. The refractive index is changed from 1.736 for GeO2-BaO-Ga2O3 glass to 1.605 for B2O3-BaO-Ga2O3 glass. The refractive indices for the studied glass samples are schematized in Figure 3.
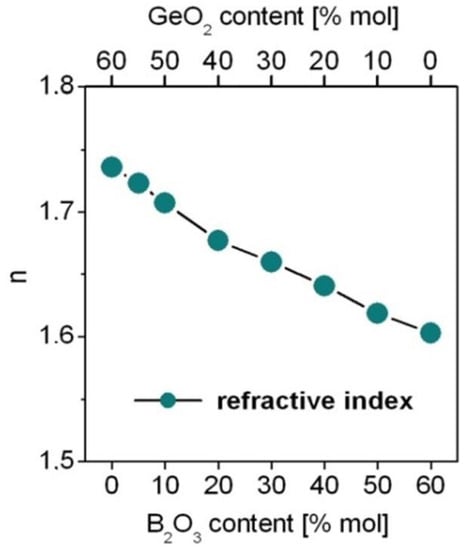
Figure 3.
The refractive indices for borogermanate glasses.
The experimental oscillator strengths from the absorption spectra and theoretical oscillator strengths were compared. They are shown in Table 3 and Table 4.

Table 3.
Measured and calculated oscillator strengths (P × 10−6) for Dy3+ ions in GeO2-BaO-Ga2O3 glass and mixed borogermanate glasses with GeO2:B2O3 = 11:1, 5:1 and 2:1.

Table 4.
Measured and calculated oscillator strengths (P × 10−6) for Dy3+ ions in B2O3-BaO-Ga2O3 glass and mixed borogermanate glasses with GeO2:B2O3 = 1:1, 1:2 and 1:5.
The main calculation process is related to three phenomenological Judd–Ofelt intensity parameters Ωt (t = 2, 4, 6), which were obtained by comparison of the experimental oscillator strengths from the absorption spectra with the theoretical oscillator strengths from Equation (2) of the Judd–Ofelt framework (Part 3) using the fitting procedure. The quality of the fit shown in Table 3 and Table 4 expressed by the rms deviation defined by Σ(Pmeas − Pcalc)2 (see Part 3) is quite good. The rms deviations for the studied glass systems varying with GeO2/B2O3 molar ratios are in the range 0.23–0.58 (×10−6). The error is within the acceptable range compared to similar glass doped with Dy3+ [70], which was studied using the Judd–Ofelt framework. The three Judd–Ofelt intensity parameters Ωt (t = 2, 4, 6) are necessary to calculate some spectroscopic parameters such as the radiative transition probabilities and the luminescence branching ratios, and then the radiative lifetimes, the quantum efficiencies of excited states and the peak stimulated emission cross-sections for electronic transitions of Dy3+ ions. The three Judd–Ofelt intensity parameters Ωt (t = 2, 4, 6) for Dy3+ ions in borogermanate glasses are given in Table 5.

Table 5.
Judd–Ofelt intensity parameters for Dy3+ ions in the studied glass systems.
It is generally accepted that the phenomenological Judd–Ofelt intensity parameter Ω2 reflects the asymmetry of the environment of trivalent dysprosium ions. In other words, the values of Ω2 exhibit the degree of covalency between Dy3+ ions and their nearest surroundings. For the studied glass systems, the Judd–Ofelt parameter Ω2 is reduced from 8.73 × 10−20 cm2 for GeO2-BaO-Ga2O3 glass to 5.92 × 10−20 cm2 for B2O3-BaO-Ga2O3 glass suggesting more ionic bonding between Dy3+ ions and ligands with increasing B2O3 concentration. The results are in good agreement with values of Ω2 calculated for similar germanate or germanate-tellurite glasses based on Na2O-MgO-Al2O3-GeO2 composition (Ω2 = 8.62 × 10−20 cm2) referred to as NMAG [87] and Na2O-ZnO-PbO-GeO2-TeO2 composition (Ω2 = 7.34 × 10−20 cm2) referred to as NZPGT [88] as well as obtained for similar borate glasses based on the B2O3-CaF2-CaO-BaO-Al2O3 system (Ω2 = 5.98 × 10−20 cm2) referred to as CFB [89] and B2O3-ZnO-Al2O3-Bi2O3 (Ω2 = 6.20 × 10−20 cm2) referred to as ZnAlBiB [90]. Following that, the Judd–Ofelt intensity parameters Ω4 and Ω6 are structure-dependent, i.e., the parameter Ω4 describes the viscosity of the glass medium while the parameter Ω6 is connected with the rigidity of the glass medium. Interestingly, GeO2-BaO-Ga2O3 glass and borogermanate glasses with lower B2O3 content (GeO2:B2O3 from 11:1 to 2:1) exhibit Ω4 > Ω6, whereas B2O3-BaO-Ga2O3 glass and borogermanate glasses with relatively higher B2O3 content (GeO2:B2O3 = 1:2 and 1:5) possess Ω4 < Ω6 (Table 3). The same situation was observed earlier for germanate, germanate-tellurite and tellurite glasses [87,87,91], where Ω4 > Ω6 contrary to borate or phosphate glasses [90,92,93], where Ω4 < Ω6. However, further investigations for borate-based glasses suggest that Ω4 < Ω6 can be changed to Ω4 > Ω6 with decreasing Dy3+ concentration [94]. For glass with GeO2:B2O3 = 1:1 both the Judd–Ofelt parameters Ω4 and Ω6 are nearly the same as Dy3+ doped silicate glass based on SiO2–Al2O3–PbF2–AlF3–YbF3–DyF3 composition [95].
It was concluded that the intensity parameters Ω4 and Ω6 depend not only on the viscosity and rigidity, but they are also affected by the acidity and basicity of the glass-host, i.e., the highest Ω4 and Ω6 indicates the lowest basicity of the glass and the highest hardness [83]. In particular, the parameter Ω6 is reduced systematically with increasing basicity and decreasing rigidity of the glass [96]. The calculation results given in Table 5 clearly indicate that the intensity parameter Ω6 increases from GeO2-BaO-Ga2O3 glass to B2O3-BaO-Ga2O3 glass. The values of Ω6 are larger for glass samples containing higher B2O3 concentrations suggesting their lower basicity and higher rigidity. Further studies suggest that the Judd–Ofelt intensity parameters Ω4 and Ω6 not only influence the physicochemical properties of glasses but also strongly affect the radiative transition probabilities as a result of the interaction between trivalent dysprosium ions and their nearest environments [97].
Following that, the spectroscopic quality parameter χ referred to as the magnitude of Ω4/Ω6 belongs to important factors characterizing the optical potential of the currently prepared glass. It was presented and discussed in detail for several glass systems doped with Dy3+ ions [91]. For borogermanate glass systems, the values of χ are relatively large, which demonstrates quite well the intense luminescent transitions of Dy3+ ions. Luminescence studies for multicomponent glass based on B2O3–Bi2O3–SrO–Al2O3–PbO–Dy2O3 revealed that the spectroscopic quality factor χ ≥ 0.50, can be suggested as a good optical candidate for the lasing action of dysprosium ions [80].
The three phenomenological J–O intensity parameters Ωt (t = 2, 4, 6) were applied to calculate the radiative transition probabilities and the luminescence branching ratios using the appropriate Relations (3) and (4) given in Part 3. The results are summarized in Table 6 and Table 7. The total radiative transition probability ATOTAL referred to as the sum of the AJ terms from the 4F9/2 excited state of dysprosium ions increases with increasing GeO2 concentration. The value of ATOTAL changed from 904 s−1 for B2O3-BaO-Ga2O3 glass to 933 s−1 (GeO2:B2O3 = 1:5), 1065 s−1 (GeO2:B2O3 = 1:2), 1095 s−1 (GeO2:B2O3 = 1:1), 1124 s−1 (GeO2:B2O3 = 2:1), 1278 s−1 (GeO2:B2O3 = 5:1), 1311 s−1 (GeO2:B2O3 = 11:1) and 1495 s−1 for GeO2-BaO-Ga2O3 glass, respectively. In all cases, the luminescence branching ratio is the highest for the 4F9/2 → 6H13/2 electronic transition of Dy3+ ions at 573 nm. Its value changed from 69.1% to 74.5% depending on the chemical composition of the glass-host. The calculation results suggest that the studied borogermanate glass systems are promising for yellow emission independently on molar ratios GeO2:B2O3. The luminescence spectra measurements confirm this hypothesis.

Table 6.
The radiative transition probabilities and luminescence branching ratios for Dy3+ ions in GeO2-BaO-Ga2O3 glass and mixed borogermanate glasses with GeO2:B2O3 = 11:1, 5:1 and 2:1.

Table 7.
The radiative transition probabilities and luminescence branching ratios for Dy3+ ions in B2O3-BaO-Ga2O3 glass and mixed borogermanate glasses with GeO2:B2O3 = 1:1, 1:2 and 1:5.
Figure 4 presents the luminescence spectra of Dy3+ ions in borogermanate glasses. The spectra for glasses based on B2O3-BaO-Ga2O3 and GeO2-BaO-Ga2O3 are also indicated. The emission spectra show three characteristic bands of Dy3+ ions located at blue, yellow and red spectral range. These luminescence bands are attributed to 4F9/2 → 6H15/2 (blue), 4F9/2 → 6H13/2 (yellow) and 4F9/2 → 6H11/2 transitions of trivalent dysprosium. In previous work [85], the influence of glass former (GeO2), oxide (CaO/SrO/BaO) and fluoride (CaF2/SrF2/BaF2) glass modifiers on spectral properties, the yellow-to-blue luminescence intensity ratios and CIE coordinates of Dy3+ in borate-based glasses have been examined in detail. The studies revealed that the CIE chromaticity coordinates (x, y) are changed significantly with molar ratios GeO2:B2O3 in glass composition. The CIE coordinates are changed from (x = 0.405, y = 0.452) to (x = 0.430, y = 0.472) with increasing GeO2 content, which contributes to color modification of the borogermanate glass system from greenish to yellowish. These experimental results are presented and discussed in the previously published work [85]. The luminescent transitions of Dy3+ ions are indicated in the energy level diagram shown in Figure 5.
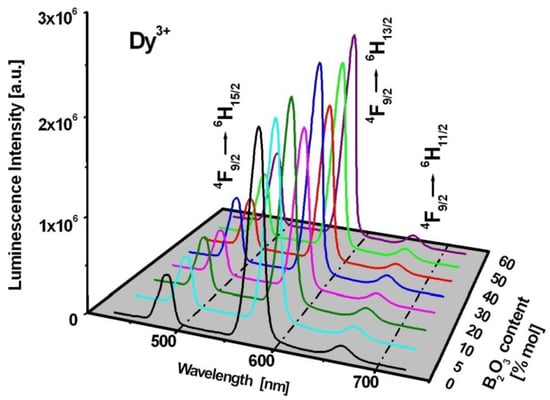
Figure 4.
Luminescence spectra for borogermanate glasses doped with Dy3+.
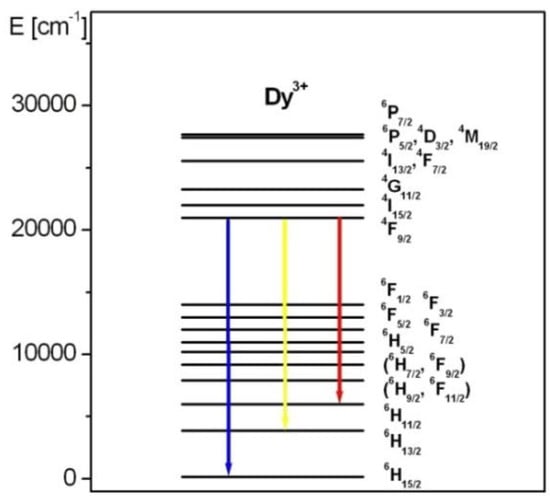
Figure 5.
Energy level diagram for Dy3+ ions. Luminescent transitions are also indicated.
The luminescent results presented in Figure 4 indicate that the intensities are the highest for yellow bands related to the 4F9/2 → 6H13/2 transition of Dy3+, independently on GeO2:B2O3 ratios. In addition, the 4F9/2 → 6H13/2 transition of Dy3+ ions is so-called hypersensitive transition, which follows the selection rules ⏐S⏐ = 0, ⏐ΔL⏐ ≤ 2 and ⏐ΔJ⏐ ≤ 2. The emission intensities as well as the spectral profiles and positions are very sensitive to even small changes of the nearest environment around dysprosium ions. The same situation is observed for the absorption band centered near 1250 nm due to transition originating from the 6H15/2 ground state to the 6F11/2 state. Figure 6 shows hypersensitive absorption and emission transitions of Dy3+ varying with GeO2:B2O3 molar ratios. In order to compare the spectral profile and position of hypersensitive transitions, the spectra were normalized. Spectroscopic analysis indicates that the spectra are broader with increasing B2O3 content. These effects are significantly stronger for absorption than emission bands.
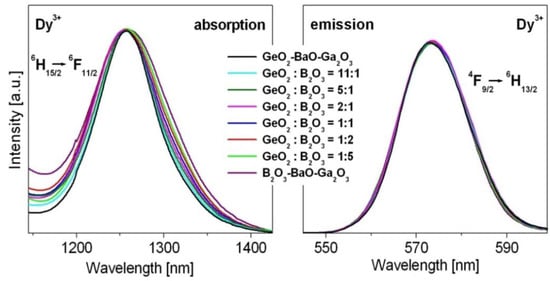
Figure 6.
Hypersensitive absorption and emission transitions Dy3+ ions.
Further luminescent studies suggest that yellow-to-blue factor Y/B (Dy3+) due to the ratio of the integrated emission intensities (4F9/2 → 6H13/2)/(4F9/2 → 6H15/2) is changed significantly with molar ratios GeO2:B2O3 in glass composition. The values of Y/B (Dy3+) are reduced from 4.22 for GeO2-BaO-Ga2O3 glass to 2.80 for B2O3-BaO-Ga2O3 glass with increasing B2O3 concentration suggesting more ionic bonding between Dy3+ ions and surrounding ligands. The results are in a good agreement with the calculated values of the intensity parameters Ω2, which decrease from 8.73 for GeO2-BaO-Ga2O3 glass to 5.92 for B2O3-BaO-Ga2O3 glass indicating more ionic bonding in character. It was schematized on Figure 7.
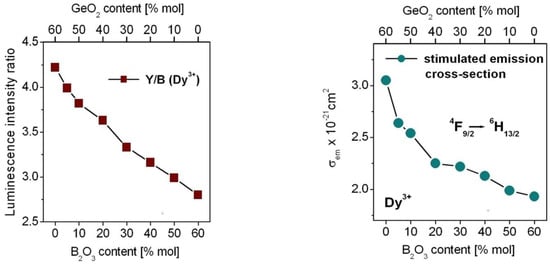
Figure 7.
Luminescence intensity ratio Y/B (on left) and the peak stimulated emission cross-section for the 4F9/2 → 6H13/2 transition of Dy3+ ions in borogermanate glasses (on right).
The same situation is also observed for the peak stimulated emission cross-section calculated from Equation (7) in Part 3 for the 4F9/2 → 6H13/2 transition of Dy3+ at 573 nm, which is decreased with increasing B2O3 content (Figure 7). The values of σem given in 10−21 cm2 are changed from 3.05 for GeO2-BaO-Ga2O3 glass to 2.63 (GeO2:B2O3 = 11:1), 2.54 (GeO2:B2O3 = 5:1), 2.25 (GeO2:B2O3 = 2:1), 2.22 (GeO2:B2O3 = 1:1), 2.13 (GeO2:B2O3 = 1:2), 1.99 (GeO2:B2O3 = 1:5) and 1.93 for B2O3-BaO-Ga2O3 glass, respectively.
From the series of the studied glass samples, the stimulated emission cross section is the highest (σem = 3.05 × 10−21 cm2) for GeO2-BaO-Ga2O3 glass. Its value is comparable to the one obtained for the 4F9/2 → 6H13/2 transition of Dy3+ ions in germanate-tellurite glasses based on GeO2-TeO2-SrF2 composition (σem = 3.1 × 10−21 cm2) referred as GTS [98] and Na2O-ZnO-PbO-GeO2-TeO2 composition (σem = 3.66 × 10−21 cm2) known as NZPGT [88].
Finally, luminescence decays from the 4F9/2 state of Dy3+ ions have been analyzed in detail. Decay curves for the 4F9/2 (Dy3+) state in borogermanate glasses were measured under excitation 454 nm and monitoring emission wavelength 573 nm. The luminescence decay curves for Dy3+ are presented in Figure 8. The obtained results clearly demonstrated that decays are longer with increasing B2O3 concentration in glass composition.
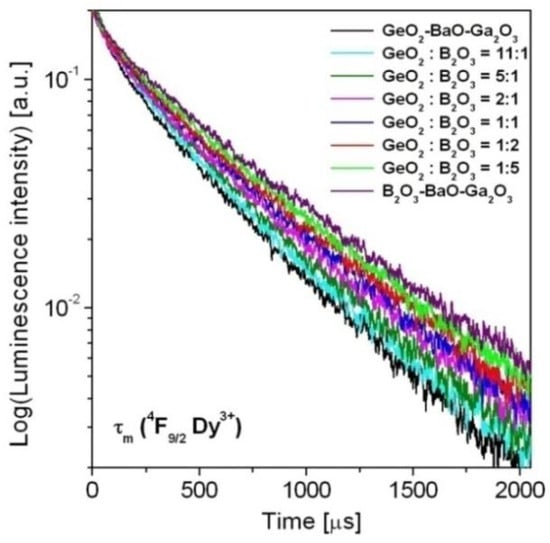
Figure 8.
Luminescence decay curves for the 4F9/2 state of Dy3+ ions in borogermanate glasses.
Based on decay curve measurements, luminescence lifetimes for the 4F9/2 state of Dy3+ ions were determined. Next, measured lifetimes were compared to the radiative lifetimes (Equation (5), Part 3) calculated from the J–O theory. Both measured τm and radiative τrad lifetimes were used to calculate quantum efficiency (Equation (6), Part 3). The measured lifetimes and quantum efficiencies for the 4F9/2 state of Dy3+ varying with GeO2:B2O3 molar ratios are schematically shown in Figure 9.
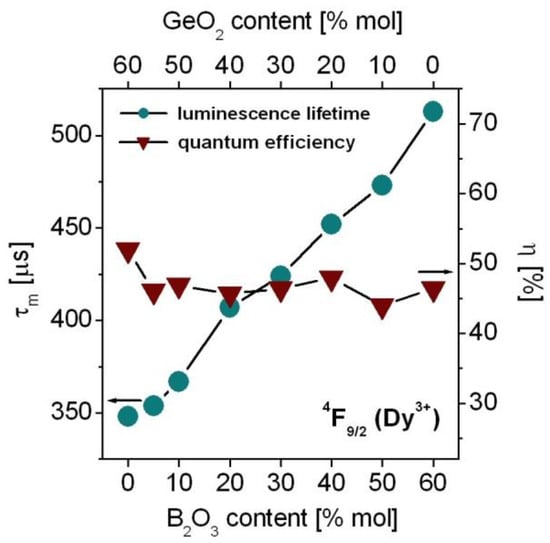
Figure 9.
Luminescence lifetimes and quantum efficiencies for the 4F9/2 state of Dy3+ ions in borogermanate glasses.
The 4F9/2 lifetime of Dy3+ ions in GeO2-BaO-Ga2O3 glass is close to 348 µs and its value is comparable to the results (τm = 356 µs) obtained for lead germanate glass based on PbO-Ga2O3-GeO2 [99]. The experimental values of τm for mixed borogermanate glasses are equal to 352 µs (GeO2:B2O3 = 11:1), 367 µs (GeO2:B2O3 = 5:1), 407 µs (GeO2:B2O3 = 2:1), 424 µs (GeO2:B2O3 = 1:1), 452 µs (GeO2:B2O3 = 1:2), and 473 µs (GeO2:B2O3 = 1:5). The 4F9/2 lifetime of Dy3+ ions is the highest (τm = 513 µs) for B2O3-BaO-Ga2O3 glass. In contrast to the dependences of luminescence intensity ratio Y/B and the peak stimulated emission cross-section for the 4F9/2® 6H13/2 transition (Figure 7), the luminescence lifetime for the 4F9/2 state of Dy3+ increases with increasing B2O3 content. It is experimentally evidenced that the non-radiative multiphonon relaxation probabilities of rare earth ions are increased significantly with increasing phonon energy from GeO2 to B2O3. Glass based on GeO2-BaO-Ga2O3 (~800 cm−1) has relatively smaller phonon energy than B2O3-BaO-Ga2O3 glass (~1400 cm−1). Thus, the measured lifetimes of rare earth ions are reduced from GeO2 to B2O3 because multiphonon relaxation probabilities become higher with increasing B2O3 content. For example, this situation is observed for Er3+ ions, where the energy separation between the excited state 4I13/2 and next lower-lying ground state 4I15/2 is relatively small and non-radiative multiphonon relaxation provides an important contribution to the total relaxation process. The opposite effects are observed for other rare earth ions such as Tb3+, Eu3+ or Dy3+, where the energy gaps between the interacting levels are relatively large and non-radiative relaxation probabilities are negligibly small. Thus, luminescence lifetimes 4F9/2 (Dy3+) are nearly equal to radiative lifetimes calculated from the J–O theory and their experimental values increase from GeO2-BaO-Ga2O3 glass to B2O3-BaO-Ga2O3 glass. It was also confirmed earlier by the measurements of luminescence decay curves for rare earth ions in heavy metal oxide glasses referred to as HMOG. Previously published work clearly demonstrated that the dependence of experimental luminescence lifetimes on the phonon energies of HMOG glass systems is completely different for the 5D0 state of Eu3+ than for the 4I13/2 state of Er3+ [100].
Further studies indicate that the quantum efficiency of excited state 4F9/2 (Dy3+) is almost unchanged with increasing B2O3 concentration. The quantum efficiency for the 4F9/2 state of Dy3+ in mixed borogermanate glasses seems to be 47 ± 1%, independently of GeO2:B2O3 molar ratios. For GeO2-BaO-Ga2O3 glass (η = 52%) the quantum efficiency is above 50%. The results obtained for borogermanate glasses singly doped with Dy3+ ions suggest their potential luminescent applications in the yellow spectral range [71,101].
5. Conclusions
Borogermanate glasses doped with Dy3+ have been studied experimentally and theoretically using the Judd–Ofelt framework. Based on absorption and emission spectra measurements, several spectroscopic parameters for Dy3+ ions were determined, such as the measured and calculated oscillator strengths, the Judd–Ofelt intensity parameters, the radiative transition probabilities, the luminescence branching ratios, the peak stimulated emission cross-sections, the measured and radiative (calculated) luminescence lifetimes and the quantum efficiencies of excited state. They have been examined as a function of GeO2:B2O3 molar ratios in glass composition. The systematic investigations demonstrated that the peak stimulated emission cross-sections for the most intense 4F9/2 → 6H13/2 yellow transition of Dy3+ ions decrease, whereas the 4F9/2 luminescence lifetimes are enhanced with increasing B2O3 concentration. The quantum efficiencies for the 4F9/2 state of Dy3+ ions are close to η = 47 ± 1% and their values are nearly independent of GeO2:B2O3 ratios. It was suggested that the results for borogermanate glasses doped with Dy3+ are attractive for yellow luminescence, providing an important contribution to the development of optical glasses and celebrating the 60th anniversary of the Judd–Ofelt theory.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the author.
Acknowledgments
The author thanks Marta Kuwik for luminescence spectra measurements. The research activities were co-financed by the funds granted under the Research Excellence Initiative of the University of Silesia in Katowice.
Conflicts of Interest
The author declares no conflict of interest.
References
- Judd, B.R. Optical absorption intensities of rare-earth ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of crystal spectra of rare-earth ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Hehlen, M.P.; Brik, M.G.; Krämer, K.W. 50th anniversary of the Judd–Ofelt theory: An experimentalist’s view of the formalism and its application. J. Lumin. 2013, 136, 221–239. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, Y.; Hu, L.; Chen, D. Judd–Ofelt analysis and energy transfer processes of Er3+ and Nd3+ doped fluoroaluminate glasses with low phosphate content. Opt. Mater. 2014, 38, 167–173. [Google Scholar] [CrossRef]
- Lalla, E.A.; Konstantinidis, M.; De Souza, I.; Daly, M.G.; Martín, I.R.; Lavín, V.; Rodríguez-Mendoza, U.R. Judd-Ofelt parameters of RE3+-doped fluorotellurite glass (RE3+ = Pr3+, Nd3+, Sm3+, Tb3+, Dy3+, Ho3+, Er3+, and Tm3+). J. Alloys Compd. 2020, 845, 156028. [Google Scholar] [CrossRef]
- Hu, Y.; Shao, X.; Tan, L.; Shen, Y.; Liu, S.; Yue, Y. Spectroscopic properties of Er3+-doped oxyfluoro-germanate glass ceramics: A Judd-Ofelt theory analysis. J. Non-Cryst. Solids 2021, 574, 121167. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.-H.; Xu, Q.; Zhang, D.-L.; Zhang, P.; Wong, W.-H. Judd-Ofelt spectroscopic properties of Er3+-doped NaLa(WO4)2 polycrystalline powder. Spectrochim. Acta A 2021, 249, 119335. [Google Scholar] [CrossRef]
- Liang, H.; Liu, S.; Zhang, P.; Lei, W.; Luo, Z.; Lu, A. Er3+/Yb3+ co-doped SiO2-Al2O3-CaO-CaF2 glass: Structure, J-O analysis and fluorescent properties. Mater. Sci. Eng. B 2021, 264, 114919. [Google Scholar] [CrossRef]
- Choi, J.H.; Margaryan, A.; Margaryan, A.; Shi, F.G. Judd–Ofelt analysis of spectroscopic properties of Nd3+-doped novel fluorophosphate glass. J. Lumin. 2005, 114, 167–177. [Google Scholar] [CrossRef]
- Kumar, G.N.H.; Rao, J.L.; Prasad, K.R.; Ratnakaram, Y.C. Fluorescence and Judd–Ofelt analysis of Nd3+ doped P2O5–Na2O–K2O glass. J. Alloys Compd. 2009, 480, 208–215. [Google Scholar] [CrossRef]
- Raju, C.N.; Reddy, C.A.; Sailaja, S.; Seo, H.J.; Reddy, B.S. Judd–Ofelt theory: Optical absorption and NIR emission spectral studies of Nd3+:CdO–Bi2O3–B2O3 glasses for laser applications. J. Mater. Sci. 2012, 47, 772–778. [Google Scholar] [CrossRef]
- Chanthima, N.; Kaewkhao, J.; Tariwong, Y.; Sangwaranatee, N.; Sangwaranatee, N.W. Luminescence study and Judd-Ofelt analysis of CaO-BaO-P2O5 glasses doped with Nd3+ ions. Mater. Today Proc. 2017, 4, 6091–6098. [Google Scholar] [CrossRef]
- Zaman, F.; Srisittipokakun, N.; Rooh, G.; Khattak, S.A.; Singkiburin, N.; Kim, H.J.; Sangwaranatee, N.; Kaewkhao, J. Investigation of Li2O–Gd2O3–MO–B2O3–Nd2O3 (MO=Ba/Bi) glasses for laser applications by Judd–Ofelt (J–O) theory. J. Lumin. 2019, 215, 116639. [Google Scholar] [CrossRef]
- Mahraz, Z.A.S.; Sazali, E.S.; Sahar, M.R.; Amran, N.U.; Yaacob, S.N.S.; Aziz, S.M.; Mawlud, S.Q.; Noor, F.M.; Harun, A.N. Spectroscopic investigations of near-infrared emission from Nd3+-doped zinc-phosphate glasses: Judd-Ofelt evaluation. J. Non-Cryst. Solids 2019, 509, 106–114. [Google Scholar] [CrossRef]
- Ahmad, A.U.; Hashim, S.; Goshal, S.K. Optical traits of neodymium-doped new types of borate glasses: Judd-Ofelt analysis. Optik 2019, 199, 163515. [Google Scholar] [CrossRef]
- Nasser, K.; Aseev, V.; Ivanov, S.; Ignatiev, A.; Nikonorov, N. Optical, spectroscopic properties and Judd–Ofelt analysis of Nd3+-doped photo-thermo-refractive glass. J. Lumin. 2019, 213, 255–262. [Google Scholar] [CrossRef]
- Lin, X.; Liang, H.; Jiang, X.; Liu, L.; Wang, Z.; Luo, Y.; Liu, T.; Ning, T.; Lu, A. Thermal and fluorescence properties of Nd2O3-doped Gd2O3-Ga2O3-GeO2 glass based on the Judd-Ofelt theory. J. Non-Cryst. Solids 2022, 594, 121810. [Google Scholar] [CrossRef]
- Tanabe, S.; Ohyagi, T.; Soga, N.; Hanada, T. Compositional dependence of Judd-Ofelt parameters of Er3+ ions in alkali-metal borate glasses. Phys. Rev. B 1992, 46, 3305–3310. [Google Scholar] [CrossRef] [PubMed]
- Jlassi, I.; Elhouichet, H.; Ferid, M.; Barthou, C. Judd–Ofelt analysis and improvement of thermal and optical properties of tellurite glasses by adding P2O5. J. Lumin. 2010, 130, 2394–2401. [Google Scholar] [CrossRef]
- Awang, A.; Ghoshal, S.K.; Sahar, M.R.; Dousti, M.R.; Amjad, R.J.; Nawaz, F. Enhanced spectroscopic properties and Judd-Ofelt parameters of Er-doped tellurite glass: Effect of gold nanoparticles. Curr. Appl. Phys. 2013, 13, 1813–1818. [Google Scholar] [CrossRef]
- Amjad, R.J.; Dousti, M.R.; Sahar, M.R. Spectroscopic investigation and Judd-Ofelt analysis of silver nanoparticles embedded Er3+-doped tellurite glass. Curr. Appl. Phys. 2015, 15, 1–7. [Google Scholar] [CrossRef]
- Gaafar, M.S.; Marzouk, S.Y. Judd-Ofelt analysis of spectroscopic properties of Er3+ doped TeO2-BaO-ZnO glasses. J. Alloys Compd. 2017, 723, 1070–1078. [Google Scholar] [CrossRef]
- Yusof, N.N.; Ghoshal, S.K.; Azlan, M.N. Optical properties of titania nanoparticles embedded Er3+-doped tellurite glass: Judd-Ofelt analysis. J. Alloys Compd. 2017, 724, 1083–1092. [Google Scholar] [CrossRef]
- Moustafa, S.Y.; Sahar, M.R.; Ghoshal, S.K. Spectroscopic attributes of Er3+ ions in antimony phosphate glass incorporated with Ag nanoparticles: Judd-Ofelt analysis. J. Alloys Compd. 2017, 712, 781–794. [Google Scholar] [CrossRef]
- Lachheb, R.; Herrmann, A.; Assadi, A.A.; Reiter, J.; Körner, J.; Hein, J.; Rüssel, C.; Maâlej, R.; Damak, K. Judd–Ofelt analysis and experimental spectroscopic study of erbium doped phosphate glasses. J. Lumin. 2018, 201, 245–254. [Google Scholar] [CrossRef]
- Rodin, N.L.A.; Sahar, M.R. Erbium doped sodium magnesium boro-tellurite glass: Stability and Judd-Ofelt analysis. Mater. Chem. Phys. 2018, 216, 177–185. [Google Scholar] [CrossRef]
- Mariyappan, M.; Arunkumar, S.; Marimuthu, K. Judd-Ofelt analysis and NIR luminescence investigations on Er3+ ions doped B2O3–Bi2O3–Li2O–K2O glasses for photonic applications. Phys. B 2019, 572, 27–35. [Google Scholar] [CrossRef]
- Mandal, P.; Aditya, S.; Ghosh, S. Optimization of rare earth (Er3+) doping level in lead zinc phosphate glass through Judd-Ofelt analysis. Mater. Chem. Phys. 2020, 246, 122802. [Google Scholar] [CrossRef]
- Zanane, H.; Velazquez, M.; Denux, D.; Duclere, J.-R.; Cornette, J.; Kermaoui, A.; Kellou, H.; Lahaye, M.; Buffiere, S. Judd-Ofelt analysis and crystal field calculations of Er3+ ions in new oxyfluorogermanotellurite glasses and glass-ceramics. Opt. Mater. 2020, 100, 109640. [Google Scholar] [CrossRef]
- Iezid, M.; Goumeidane, F.; Abidi, A.; Poulain, M.; Legouera, M.; Prasad, P.S.; Środa, M.; Rao, P.V. Judd-Ofelt analysis and luminescence studies of Er3+ doped halogeno-antimonate glasses. Opt. Mater. 2021, 120, 111422. [Google Scholar] [CrossRef]
- Jayasimhadri, M.; Cho, E.-J.; Jang, K.-W.; Lee, H.S.; Kim, S.I. Spectroscopic properties and Judd–Ofelt analysis of Sm3+ doped lead–germanate–tellurite glasses. J. Phys. D Appl. Phys. 2008, 41, 175101. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Shukla, R.; Sanghi, S.; Agarwal, A.; Pal, I. Spectroscopic properties of Sm3+ doped lead bismosilicate glasses using Judd–Ofelt theory. Spectrochim. Acta A 2014, 117, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Herrmann, A.; Rüssel, C. Judd–Ofelt analysis of Sm3+-doped lanthanum-aluminosilicate glasses. J. Lumin. 2015, 157, 390–397. [Google Scholar] [CrossRef]
- Mawlud, S.Q.; Ameen, M.M.; Sahar, M.R.; Mahraz, Z.A.S.; Ahmed, K.F. Spectroscopic properties of Sm3+ doped sodium-tellurite glasses: Judd-Ofelt analysis. Opt. Mater. 2017, 69, 318–327. [Google Scholar] [CrossRef]
- Ravina; Naveen; Sheetal; Kumar, V.; Dahiya, S.; Deopa, N.; Punia, R.; Rao, A.S. Judd-Ofelt itemization and influence of energy transfer on Sm3+ ions activated B2O3–ZnF2–SrO–SiO2 glasses for orange-red emitting devices. J. Lumin. 2021, 229, 117651. [Google Scholar] [CrossRef]
- Olivier, M.; Doualan, J.-L.; Nazabal, V.; Camy, P.; Adam, J.-L. Spectroscopic study and Judd–Ofelt analysis of Pr3+-doped Zr–Ba–La–Al glasses in visible spectral range. J. Opt. Soc. Amer. B 2013, 30, 2032–2042. [Google Scholar] [CrossRef]
- Kumar, M.V.V.; Gopal, K.R.; Reddy, R.R.; Reddy, G.V.L.; Hussain, N.S.; Jamalaiah, B.C. Application of modified Judd–Ofelt theory and the evaluation of radiative properties of Pr3+-doped lead telluroborate glasses for laser applications. J. Non-Cryst. Solids 2013, 364, 20–27. [Google Scholar] [CrossRef]
- Zhang, F.; Bi, Z.; Huang, A.; Xiao, Z. Luminescence and Judd–Ofelt analysis of the Pr3+ doped fluorotellurite glass. J. Lumin. 2015, 160, 85–89. [Google Scholar] [CrossRef]
- Flizikowski, G.A.S.; Zanuto, V.S.; Nunes, L.A.O.; Baesso, M.L.; Malacarne, L.C.; Astrath, N.G.C. Standard and modified Judd-Ofelt theories in Pr3+-doped calcium aluminosilicate glasses: A comparative analysis. J. Alloys Compd. 2019, 780, 705–710. [Google Scholar] [CrossRef]
- Shoaib, M.; Khan, I.; Rooh, G.; Wabaidur, S.M.; Islam, M.A.; Chanthima, N.; Kothan, S.; Ullah, I.; Ahad, A.; Kaewkhao, J. Judd-Ofelt and luminescence properties of Pr3+ doped ZnO-Gd2O3/GdF3-BaO-P2O5 glasses for visible and NIR applications. J. Lumin. 2022, 247, 118884. [Google Scholar] [CrossRef]
- Moorthy, L.R.; Rao, T.S.; Janardhanam, K.; Rao, A.S.; Subramanyam, Y. Judd-Ofelt parametrization and radiative transitions analysis of Tm3+ doped alkali chloroborophosphate glasses. Opt. Mater. 1999, 12, 459–465. [Google Scholar] [CrossRef]
- Florez, A.; Florez, M.; Messaddeq, Y.; Aegerter, M.A.; Porcher, P. Application of standard and modified Judd-Ofelt theories to thulium doped fluoroindate glass. J. Non-Cryst. Solids 1999, 247, 215–221. [Google Scholar] [CrossRef]
- Kadono, K.; Yazawa, T.; Shojiya, M.; Kawamoto, Y. Judd-Ofelt analysis and luminescence property of Tm3+ in Ga2S3-GeS2-La2S3 glasses. J. Non-Cryst. Solids 2000, 274, 75–80. [Google Scholar] [CrossRef]
- Yu, S.; Yang, Z.; Xu, S. Judd–Ofelt and laser parameterization of Tm3+-doped barium gallo-germanate glass fabricated with efficient dehydration methods. Opt. Mater. 2009, 31, 1723–1728. [Google Scholar] [CrossRef]
- Shojiya, M.; Kawamoto, Y.; Kadono, K. Judd–Ofelt parameters and multiphonon relaxation of Ho3+ ions in ZnCl2-based glass. J. Appl. Phys. 2001, 89, 4944–4950. [Google Scholar] [CrossRef]
- Zhou, B.; Pun, E.Y.B.; Lin, H.; Yang, D.; Huang, L. Judd–Ofelt analysis, frequency upconversion, and infrared photoluminescence of Ho3+-doped and Ho3+/Yb3+-codoped lead bismuth gallate oxide glasses. J. Appl. Phys. 2009, 106, 103105. [Google Scholar] [CrossRef]
- Balaji, S.; Sontakke, A.D.; Sen, R.; Kalyandurg, A. Efficient ~2.0 μm emission from Ho3+ doped tellurite glass sensitized by Yb3+ ions: Judd-Ofelt analysis and energy transfer mechanism. Opt. Mater. Exp. 2011, 1, 138–150. [Google Scholar] [CrossRef]
- Azam, M.; Rai, V.K. Ho3+-Yb3+ codoped tellurite based glasses in visible lasers and optical devices: Judd-Ofelt analysis and frequency upconversion. Solid State Sci. 2017, 66, 7–15. [Google Scholar] [CrossRef]
- Alqarni, A.S.; Hussin, R.; Ghoshal, S.K.; Alamri, S.N.; Yamusa, Y.A.; Jupri, S.A. Intense red and green luminescence from holmium activated zincsulfo-boro-phosphate glass: Judd-Ofelt evaluation. J. Alloys Compd. 2019, 808, 151706. [Google Scholar] [CrossRef]
- Azam, M.; Mohanty, D.K.; Rai, V.K.; Singh, K. Luminescence and Judd-Ofelt study of Ho3+/Ho3+-Yb3+ doped/ codoped lead tellurite glasses for multifunctional applications. J. Lumin. 2021, 239, 118319. [Google Scholar] [CrossRef]
- Ravi, O.; Reddy, C.M.; Reddy, B.S.; Raju, B.D.P. Judd–Ofelt analysis and spectral properties of Dy3+ ions doped niobium containing tellurium calcium zinc borate glasses. Opt. Commun. 2014, 312, 263–268. [Google Scholar] [CrossRef]
- El-Maaref, A.A.; Shaaban, K.H.S.; Abdelawwad, M.; Saddeek, Y.B. Optical characterizations and Judd-Ofelt analysis of Dy3+ doped borosilicate glasses. Opt. Mater. 2017, 72, 169–176. [Google Scholar] [CrossRef]
- Tuyen, V.P.; Quang, V.X.; Do, P.V.; Thanh, L.D.; Ca, N.X.; Hoa, V.X.; van Tuat, L.; Thi, L.A.; Nogami, M. An in-depth study of the Judd-Ofelt analysis, spectroscopic properties and energy transfer of Dy3+ in alumino-lithium-telluroborate glasses. J. Lumin. 2019, 210, 435–443. [Google Scholar] [CrossRef]
- George, H.; Deopa, N.; Kaur, S.; Prasad, A.; Sreenivasulu, M.; Jayasimhadri, M.; Rao, A.S. Judd-Ofelt parametrization and radiative analysis of Dy3+ ions doped Sodium Bismuth Strontium Phosphate glasses. J. Lumin. 2019, 215, 116693. [Google Scholar] [CrossRef]
- Ichoja, A.; Hashim, S.; Ghoshal, S.K. Judd−Ofelt calculations for spectroscopic characteristics of Dy3+-activated strontium magnesium borate glass. Optik 2020, 218, 165001. [Google Scholar] [CrossRef]
- Poonam; Shivani; Anu; Kumar, A.; Sahu, M.K.; Rani, P.R.; Deopa, N.; Punia, R.; Rao, A.S. Judd-Ofelt Parameterization and Luminescence Characterization of Dy3+ Doped Oxyfluoride Lithium Zinc Borosilicate Glasses for Lasers and w-LEDs. J. Non-Cryst. Solids 2020, 544, 120187. [Google Scholar] [CrossRef]
- Lakshminarayana, G.; Wagh, A.; Lira, A.; Kityk, I.V.; Lee, D.-E.; Yoon, J.; Park, T. Dy3+: B2O3–Al2O3–ZnO–Bi2O3–BaO–M2O (M = Li; Na; and K) glasses: Judd–Ofelt analysis and photoluminescence investigation for WLED applications. J. Mater. Sci. Mater. Electron. 2020, 31, 2481–2496. [Google Scholar] [CrossRef]
- Okasha, A.; Marzouk, S.Y. Linear and nonlinear optical properties and luminescence of Dy+3-doped aluminoborate glasses: Judd–Ofelt investigation. J. Mater. Sci. Mater. Electron. 2021, 32, 20431–20444. [Google Scholar] [CrossRef]
- Divina, R.; Teresa, P.E.; Marimuthu, K. Dy3+ ion as optical probe to study the luminescence behavior of Alkali lead bismuth borate glasses for w-LED application. J. Alloys Compd. 2021, 883, 160845. [Google Scholar] [CrossRef]
- Mahamuda, S.K.; Syed, F.; Devi, C.B.A.; Swapna, K.; Prasad, M.V.V.K.S.; Venkateswarlu, M.; Rao, A.S. Spectral characterization of Dy3+ ions doped phosphate glasses for yellow laser applications. J. Non-Cryst. Solids 2021, 555, 120538. [Google Scholar] [CrossRef]
- Vidhi; Ankita; Anu; Rao, A. S. Spectroscopic characterizations of Dy3+ ions doped phosphate glasses for epoxy-free white LED applications. Opt. Mater. 2022, 132, 112863. [Google Scholar] [CrossRef]
- Chandrappa, V.; Basavapoornima, C.; Kesavulu, C.R.; Babu, A.M.; Depuru, S.R.; Jayasankar, C.K. Spectral studies of Dy3+:zincphosphate glasses for white light source emission applications: A comparative study. J. Non-Cryst. Solids 2022, 583, 121466. [Google Scholar] [CrossRef]
- Anu; Deopa, N.; Rao, A.S. Structural and luminescence characteristics of thermally stable Dy3+ doped oxyfluoride strontium zinc borosilicate glasses for photonic device applications. Opt. Laser Technol. 2022, 154, 108328. [Google Scholar] [CrossRef]
- Poojha, M.K.K.; Vijayakumar, M.; Matheswaran, P.; Yousef, E.S.; Marimuthu, K. Modifier’s influence on spectral properties of dysprosium ions doped lead boro-telluro-phosphate glasses for white light applications. Opt. Laser Technol. 2022, 156, 108585. [Google Scholar] [CrossRef]
- Ravi, N.; Neelima, G.; Nallabala, N.K.R.; Kummara, V.K.; Ravanamma, R.; Reddy, V.J.; Prasanth, M.; Suresh, K.; Babu, P.; Venkatramu, V. Role of excitation wavelength and dopant concentration on white light tunability of dysprosium doped titania-fluorophosphate glasses. Opt. Mater. 2021, 111, 110593. [Google Scholar] [CrossRef]
- Kowalska, K.; Kuwik, M.; Polak, J.; Pisarska, J.; Pisarski, W.A. Transition Metals (Cr3+) and Lanthanides (Eu3+) in Inorganic Glasses with Extremely Different Glass-Formers B2O3 and GeO2. Materials 2021, 14, 7156. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Han, J.; Liu, C.; Ruan, J. Ultrafast charge carrier dynamics and photoluminescence of CsPbBr3−xIx quantum dots in boro-germanate glasses. J. Am. Ceram. Soc. 2022, 105, 7228–7237. [Google Scholar] [CrossRef]
- Gökçe, M.; Şentürk, U.; Uslu, D.K.; Burgaz, G.; Şahin, Y.; Gökçe, A.G. Investigation of europium concentration dependence on luminescent properties of borogermanate glasses. J. Lumin. 2017, 192, 263–268. [Google Scholar] [CrossRef]
- Malashkevich, G.E.; Sigaev, V.N.; Golubev, N.V.; Savinkov, V.I.; Sarkisov, P.D.; Khodasevich, I.A.; Dashkevich, V.I.; Mudryi, A.V. Luminescence of borogermanate glasses activated by Er3+ and Yb3+ ions. J. Non-Cryst. Solids 2011, 357, 67–72. [Google Scholar] [CrossRef]
- Gökçe, M.; Kocyiğit, D. Spectroscopic investigations of Dy3+ doped borogermanate glasses for laser and wLED applications. Opt. Mater. 2019, 89, 568–575. [Google Scholar] [CrossRef]
- Manasa, P.; Jayasankar, C.K. Spectroscopic assessment of Dy3+ ions in lead fluorosilicate glass as a prospective material for solid state yellow laser. Spectrochim. Acta A 2019, 212, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.R.; Venkateswarlu, M.; Mahamuda, S.; Swapna, K.; Deopa, N.; Rao, A.S. Spectroscopic studies of Dy3+ ions doped barium lead alumino fluoro borate glasses. J. Alloys Compd. 2019, 787, 503–518. [Google Scholar] [CrossRef]
- Lodi, T.A.; Dantas, N.F.; Goncalves, T.S.; de Camargo, A.S.S.; Pedrochi, F.; Steimacher, A. Dy3+ doped calcium boroaluminate glasses and Blue Led for smart white light generation. J. Lumin. 2019, 207, 378–385. [Google Scholar] [CrossRef]
- Kıbrıslı, O.; Ersundu, A.E.; Ersundu, M.C. Dy3+ doped tellurite glasses for solid-state lighting: An investigation through physical, thermal, structural and optical spectroscopy studies. J. Non-Cryst. Solids 2019, 513, 125–136. [Google Scholar] [CrossRef]
- Shasmal, N.; Karmakar, B. White light-emitting Dy3+-doped transparent chloroborosilicate glass: Synthesis and optical properties. J. Asian Ceram. Soc. 2019, 7, 42–52. [Google Scholar] [CrossRef]
- Shoaib, M.; Rajaramakrishna, R.; Rooh, G.; Chanthima, N.; Kim, H.J.; Saiyasombat, C.; Botta, R.; Nuntawong, N.; Kothan, S.; Kaewkhao, J. Structural and luminescence study of Dy3+ doped phosphate glasses for solid state lighting applications. Opt. Mater. 2020, 109, 110322. [Google Scholar] [CrossRef]
- Zaman, F.; Srisittipokakun, N.; Rooh, G.; Khattak, S.A.; Kaewkhao, J.; Rani, M.; Kim, H.J. Comparative study of Dy3+ doped borate glasses on the basis of luminescence and lasing properties for white-light generation. Opt. Mater. 2021, 119, 111308. [Google Scholar] [CrossRef]
- Amjad, R.J.; Sales, T.O.; Sattar, A.; Jacinto, C.; Dousti, M.R. Spectral studies of highly Dy3+ doped PbO–ZnO–B2O3–P2O5 glasses. J. Lumin. 2021, 231, 117839. [Google Scholar] [CrossRef]
- Roopa, B.; Eraiah, B. Experimental and theoretical approach on the physical, structural and optical properties of ZrO2-Na2O-B2O3 glasses doped with Dy2O3. J. Non-Cryst. Solids 2021, 551, 120394. [Google Scholar] [CrossRef]
- Prakash, A.H.D.; Mahamuda, S.; Alzahrani, J.S.; Sailaja, P.; Swapna, K.; Venkateswarlu, M.; Rao, A.S.; Alrowaili, Z.A.; Olarinoye, I.O.; Al-Buriahi, M.S. Synthesis and characterization of B2O3–Bi2O3–SrO–Al2O3–PbO–Dy2O3 glass system: The role of Bi2O3/Dy2O3 on the optical, structural, and radiation absorption parameters. Mater. Res. Bull. 2022, 155, 111952. [Google Scholar] [CrossRef]
- Jayasimhadri, V.M.; Divi Haranath, D. Spectroscopic investigations of Dy3+-doped tungstate–tellurite glasses for solid-state lighting applications. Int. J. Appl. Glass Sci. 2022, 13, 645–654. [Google Scholar] [CrossRef]
- Chandrappa, V.; Basavapoornima, C.; Venkatramu, V.; Depuru, S.R.; Kaewkhao, J.; Pecharapa, W.; Jayasankar, C.K. A critical review and future prospects of Dy3+-doped glasses for white light emission applications. Optik 2022, 266, 169583. [Google Scholar] [CrossRef]
- Liu, R.; Chen, M.; Zhu, X.; Zhou, Y.; Zeng, F.; Su, Z. Luminescent properties and structure of Dy3+ doped germanosilicate glass. J. Lumin. 2020, 226, 117378. [Google Scholar] [CrossRef]
- Monisha, M.; Mazumder, N.; Lakshminarayana, G.; Mandal, S.; Kamath, S.D. Energy transfer and luminescence study of Dy3+ doped zincaluminoborosilicate glasses for white light emission. Ceram. Int. 2021, 47, 598–610. [Google Scholar] [CrossRef]
- Kuwik, M.; Górny, A.; Pisarski, W.A.; Pisarska, J. Influence of glass formers and glass modifiers on spectral properties and CIE coordinates of Dy3+ ions in lead-free borate glasses. Spectrochim. Acta A 2022, 268, 120693. [Google Scholar] [CrossRef]
- Jayasankar, C.K.; Rukmini, E. Spectroscopic investigations of Dy3+ ions in borosulphate glasses. Phys. B 1997, 240, 273–288. [Google Scholar] [CrossRef]
- Li, H.Y.; Shen, L.F.; Pun, E.Y.B.; Lin, H. Dy3+-doped germanate glasses for waveguide-typed irradiation light sources. J. Alloys Compd. 2015, 646, 586–591. [Google Scholar] [CrossRef]
- Li, Y.H.; Chen, B.J.; Pun, E.Y.B.; Lin, H. Multichannel transition emissions of Dy3+ in fiber-adaptive germanium tellurite glasses. J. Appl. Phys. 2013, 113, 123507. [Google Scholar] [CrossRef]
- Kumar, J.S.; Pavani, K.; Babu, A.M.; Giri, N.K.; Rai, S.B.; Moorthy, L.R. Fluorescence characteristics of Dy3+ ions in calcium fluoroborate glasses. J. Lumin. 2010, 130, 1916–1923. [Google Scholar] [CrossRef]
- Swapna, K.; Mahamuda, S.; Rao, A.S.; Jayasimhadri, M.; Sasikala, T.; Moorthy, L.R. Visible fluorescence characteristics of Dy3+ doped zinc alumino bismuth borate glasses for optoelectronic devices. Ceram. Int. 2013, 39, 8459–8465. [Google Scholar] [CrossRef]
- Mohd Saidi, M.S.A.; Ghoshal, S.K.; Arifin, R.; Roslan, M.K.; Muhammad, R.; Shamsuri, W.N.W.; Abdullah, M.; Shaharin, M.S. Spectroscopic properties of Dy3+ doped tellurite glass with Ag/TiO2 nanoparticles inclusion: Judd-Ofelt analysis. J. Alloys Compd. 2018, 754, 171–183. [Google Scholar] [CrossRef]
- Huy, B.T.; Seo, M.-H.; Lim, J.-M.; Lee, Y.-I.; Thanh, N.T.; Quang, V.X.; Hoai, T.T.; Hong, N.A. Application of the Judd—Ofelt Theory to Dy3+-Doped Fluoroborate/Sulphate Glasses. J. Korean Phys. Soc. 2011, 59, 3300–3307. [Google Scholar] [CrossRef]
- Naick, B.N.; Damodaraiah, S.; Prasad, V.R.; Vijaya Lakshmi, R.P.; Ratnakaram, Y.C. Judd-Ofelt analysis and luminescence studies on Dy3+ -doped different phosphate glasses for white light emitting material applications. Optik 2019, 192, 162980. [Google Scholar] [CrossRef]
- Ahmad, A.U.; Hashim, S.; Ghoshal, S.K. Spectroscopic characteristics of Dy3+ impurities–doped borate-based glasses: Judd–Ofelt calculation. Mater. Chem. Phys. 2020, 253, 123386. [Google Scholar] [CrossRef]
- Duan, Z.; Zhang, J.; Hu, L. Spectroscopic properties and Judd-Ofelt theory analysis of Dy3+ doped oxyfluoride silicate glass. J. Appl. Phys. 2007, 101, 043110. [Google Scholar] [CrossRef]
- Zekri, M.; Hermann, A.; Turki, R.; Rüssel, C.; Maâlej, R.; Damak, K. Experimental and theoretical studies of Dy3+ doped alkaline earth aluminosilicate glasses. J. Lumin. 2019, 212, 354–360. [Google Scholar] [CrossRef]
- Krishna, V.M.; Mahamuda, S.; Talewar, R.A.; Swapna, K.; Venkateswarlu, M.; Rao, A.S. Dy3+ ions doped oxy-fluoro boro tellurite glasses for the prospective optoelectronic device applications. J. Alloys Compd. 2018, 762, 814–826. [Google Scholar] [CrossRef]
- Lisiecki, R. Oxyfluoride germanatetellurite glasses doped with dysprosium—Spectroscopic characteristic and luminescence thermometry qualities. J. Non-Cryst. Solids 2022, 597, 121922. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Pisarska, J.; Żur, L.; Goryczka, T. Structural and optical aspects for Eu3+ and Dy3+ ions in heavy metal glasses based on PbO–Ga2O3–XO2 (X = Te, Ge, Si). Opt. Mater. 2013, 35, 1051–1056. [Google Scholar] [CrossRef]
- Pisarska, J.; Pisarski, W.A.; Lisiecki, R.; Ryba-Romanowski, W. Phonon sideband analysis and near-infrared emission in heavy metal oxide glasses. Materials 2021, 14, 121. [Google Scholar] [CrossRef]
- Linganna, K.; Haritha, P.; Krishnaiah, K.V.; Venkatramu, V.; Jayasankar, C.K. Optical and luminescence properties of Dy3+ ions in K–Sr–Al phosphate glasses for yellow laser applications. Appl. Phys. B 2014, 117, 75–84. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).