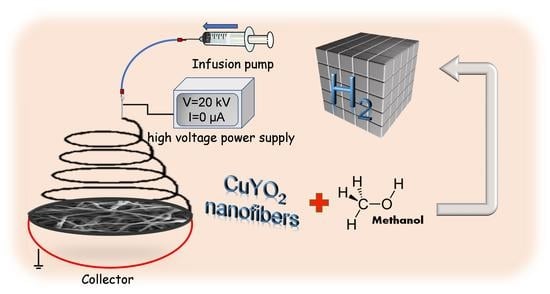
Fabrication of CuYO2 Nanofibers by Electrospinning and Applied to Hydrogen Harvest
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Preparation of Cu2Y2O5 Nanofibers
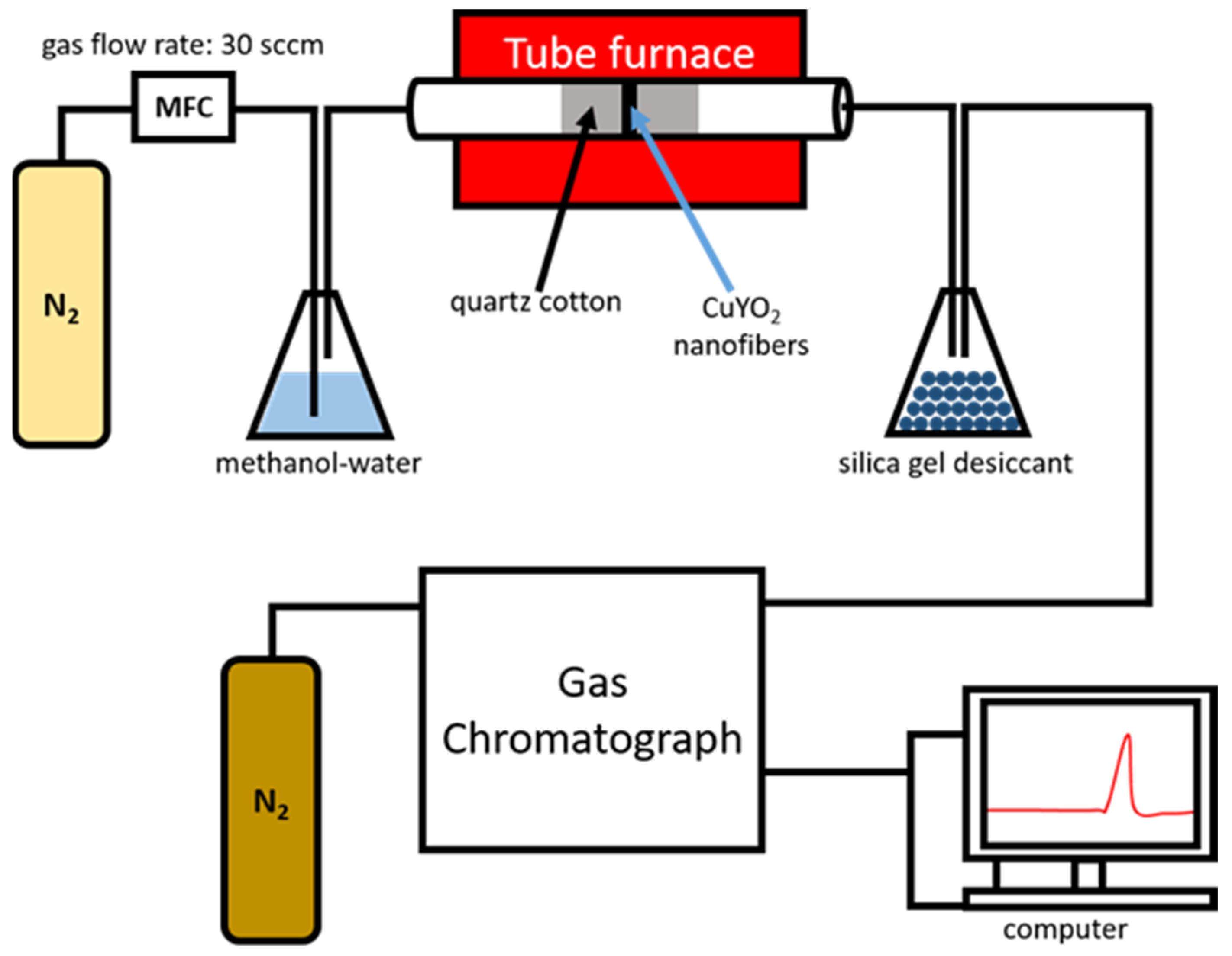
2.3. Methanol Steam Reforming
3. Results and Analysis
3.1. TGA Analysis of Cu2Y2O5 Nanofibers
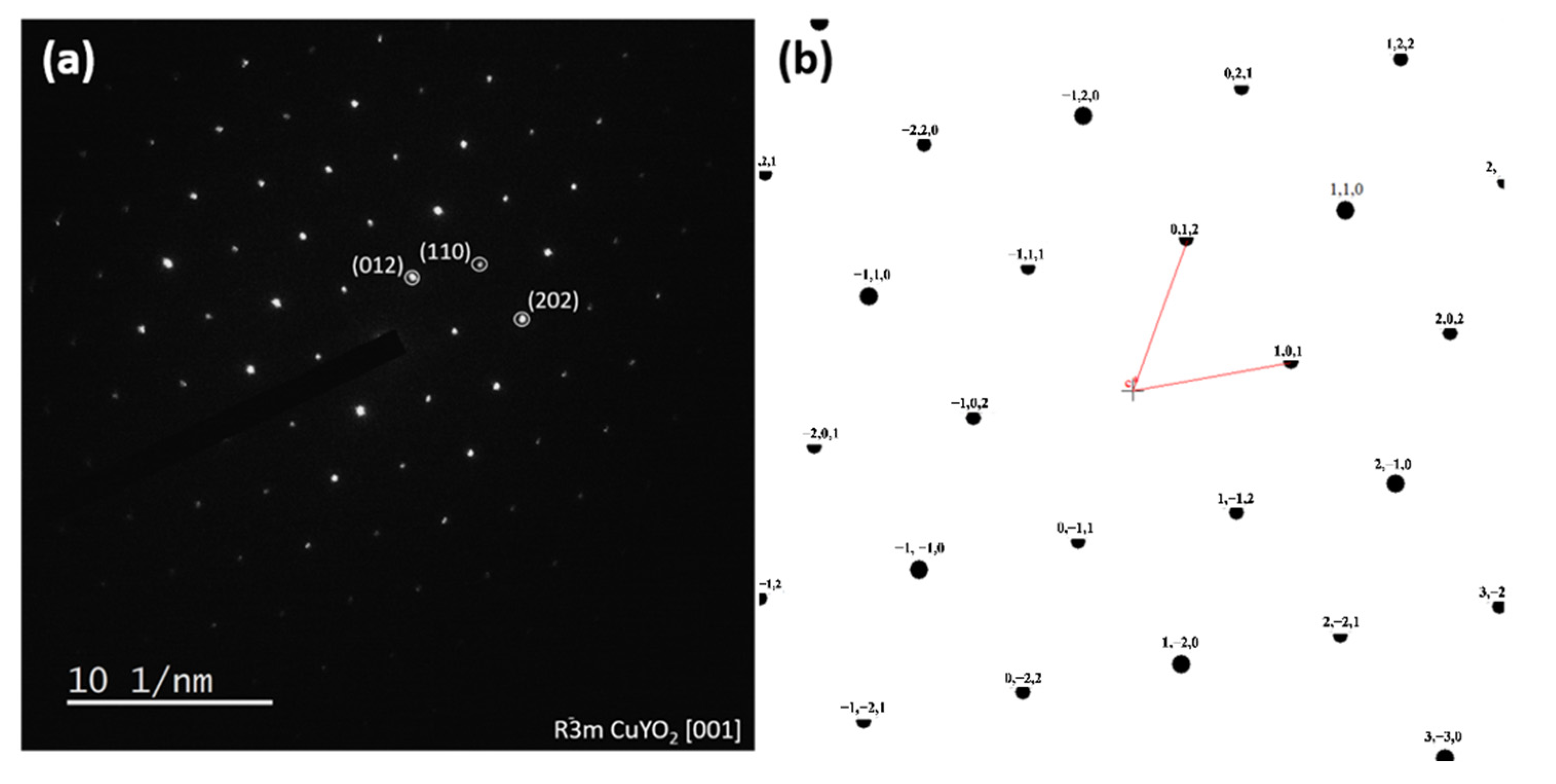
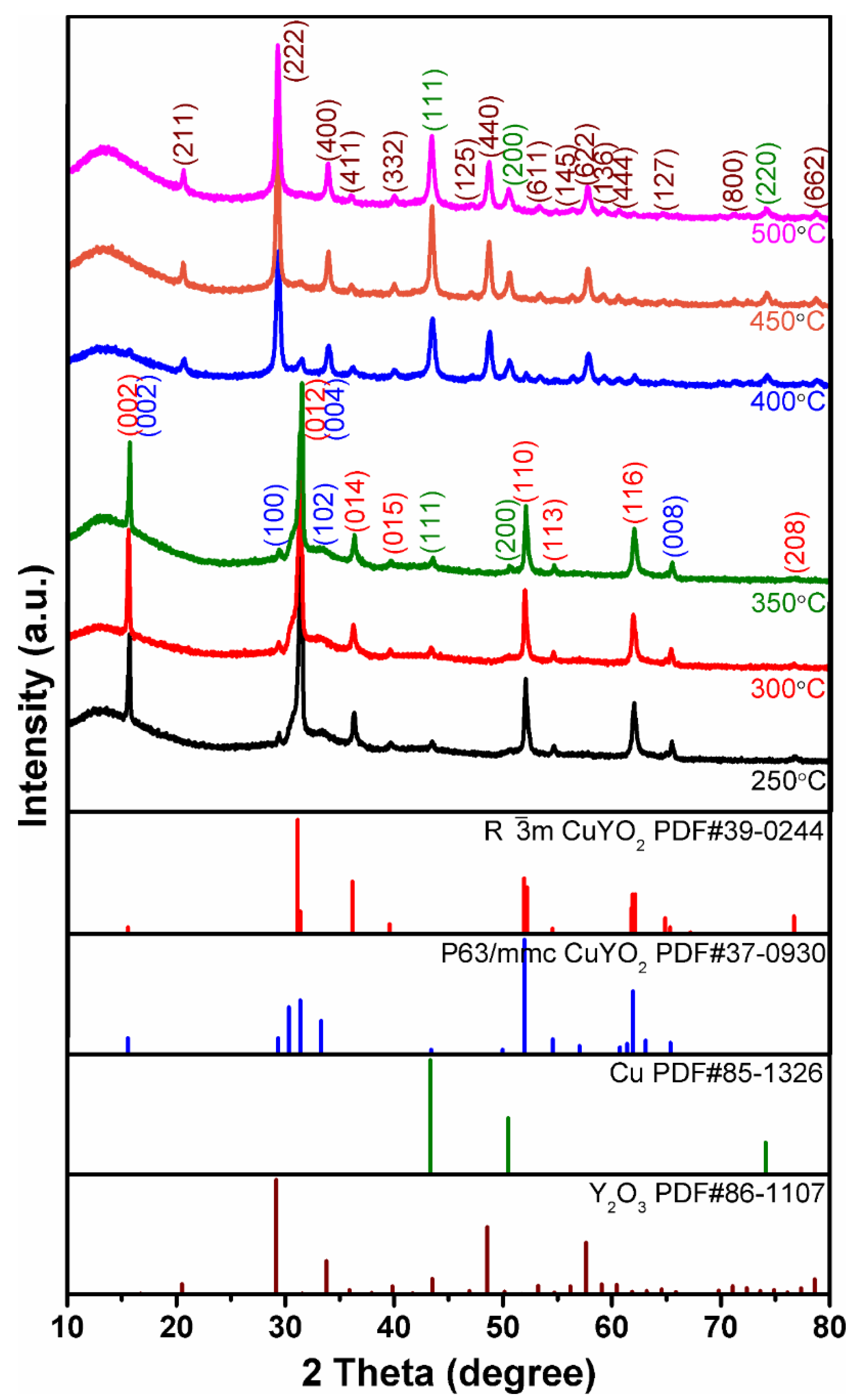
3.2. XRD Analysis of Cu2Y2O5 Nanofibers and CuYO2 Nanofibers
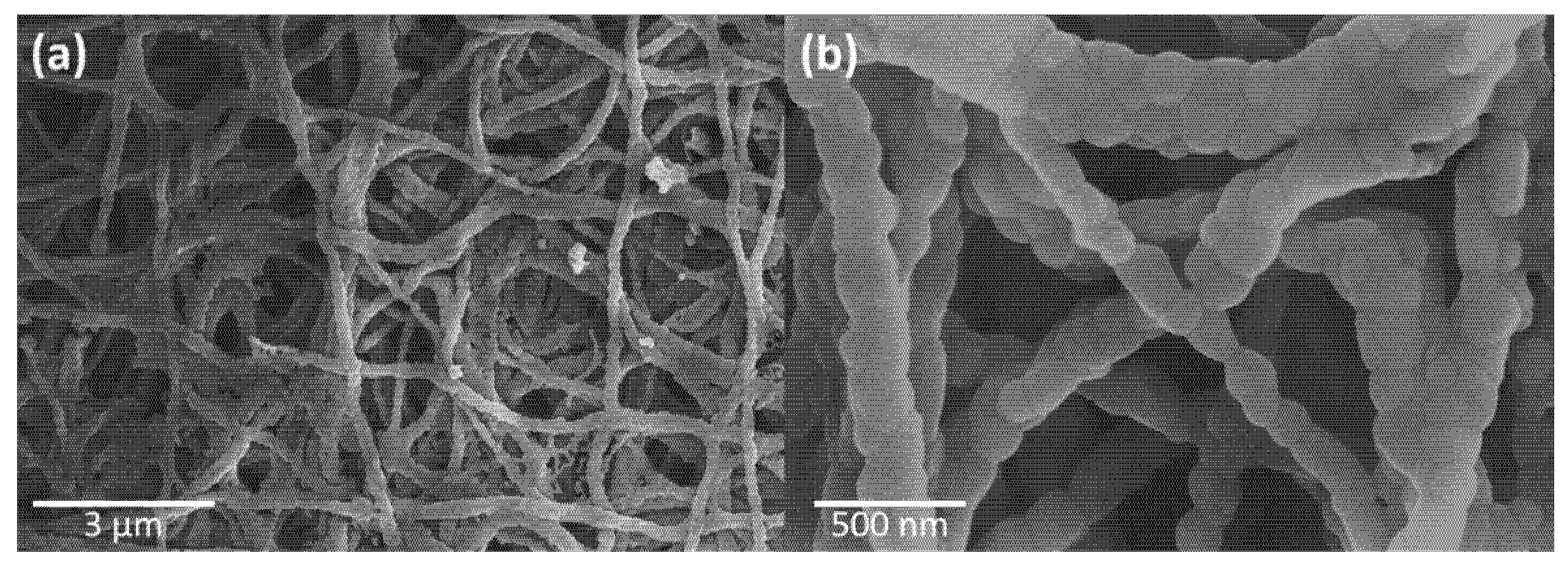
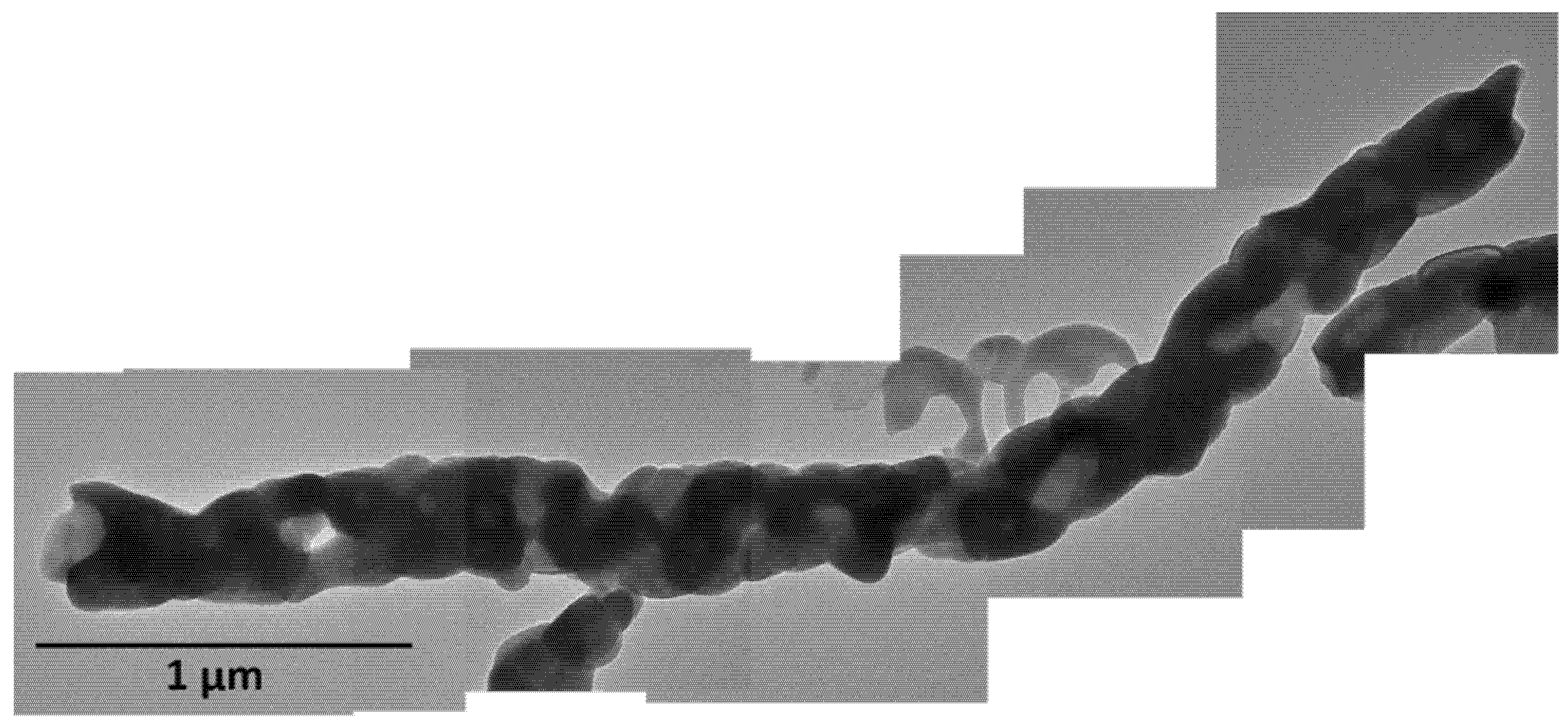
3.3. FESEM and TEM Analyses of Cu2Y2O5 Nanofibers
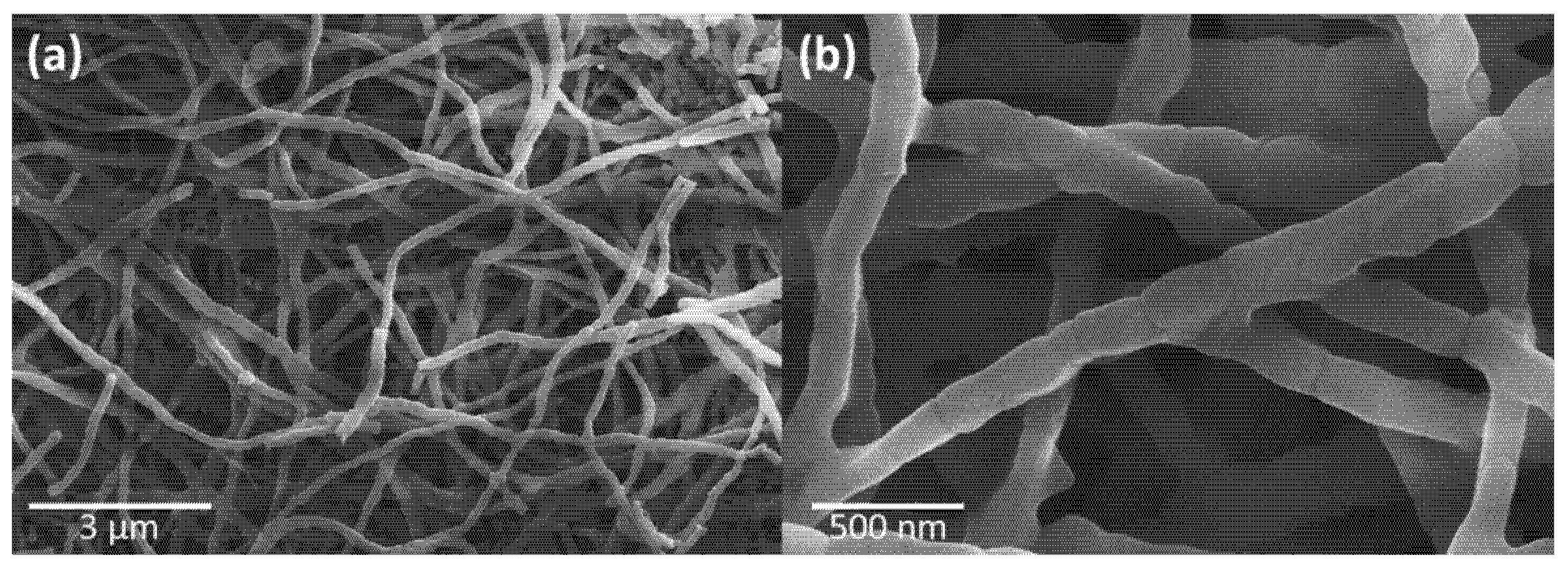
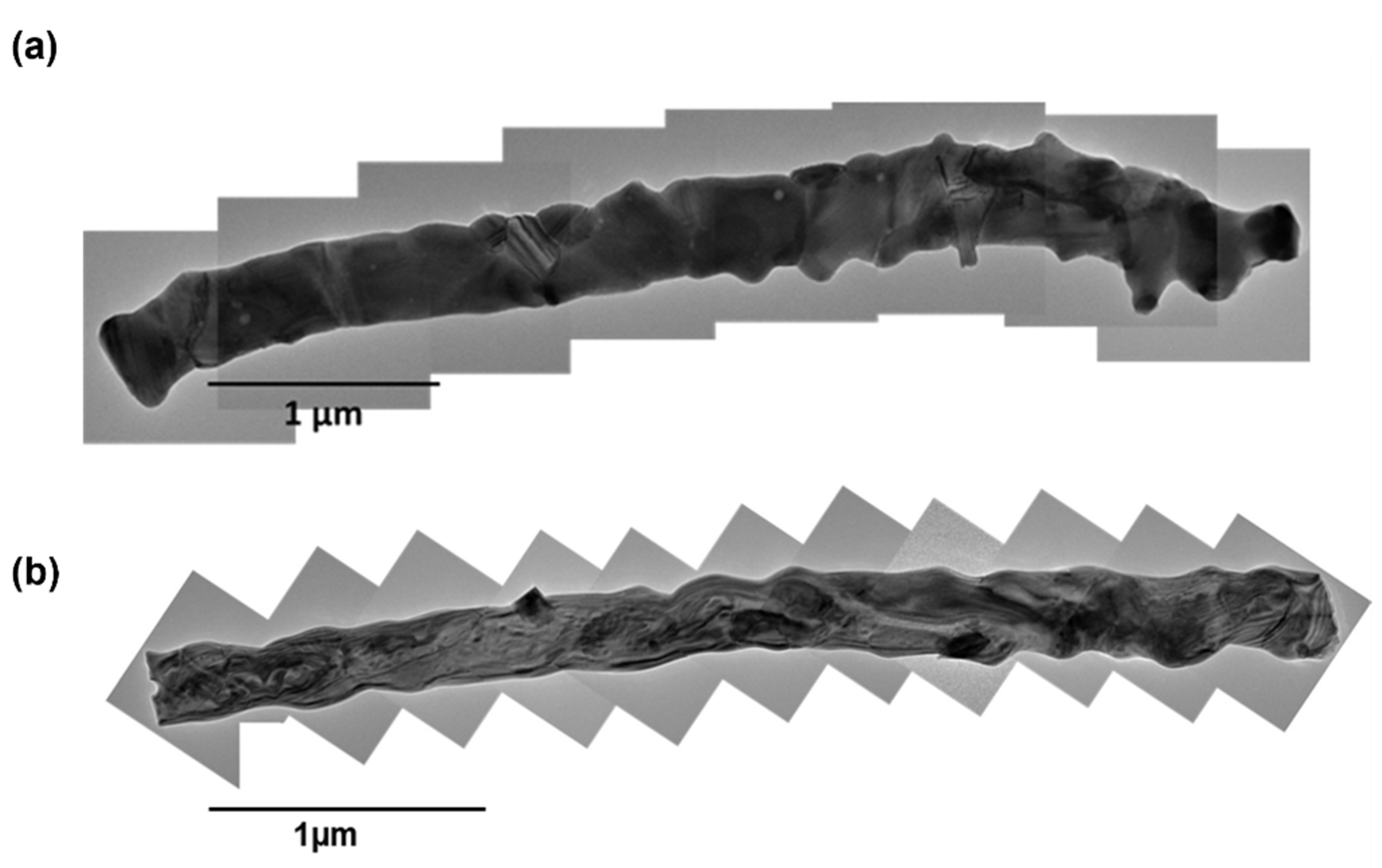
3.4. FESEM and TEM Analysis of CuYO2 Nanofibers
3.5. Specific Surface Area Analysis of CuYO2 Nanofibers
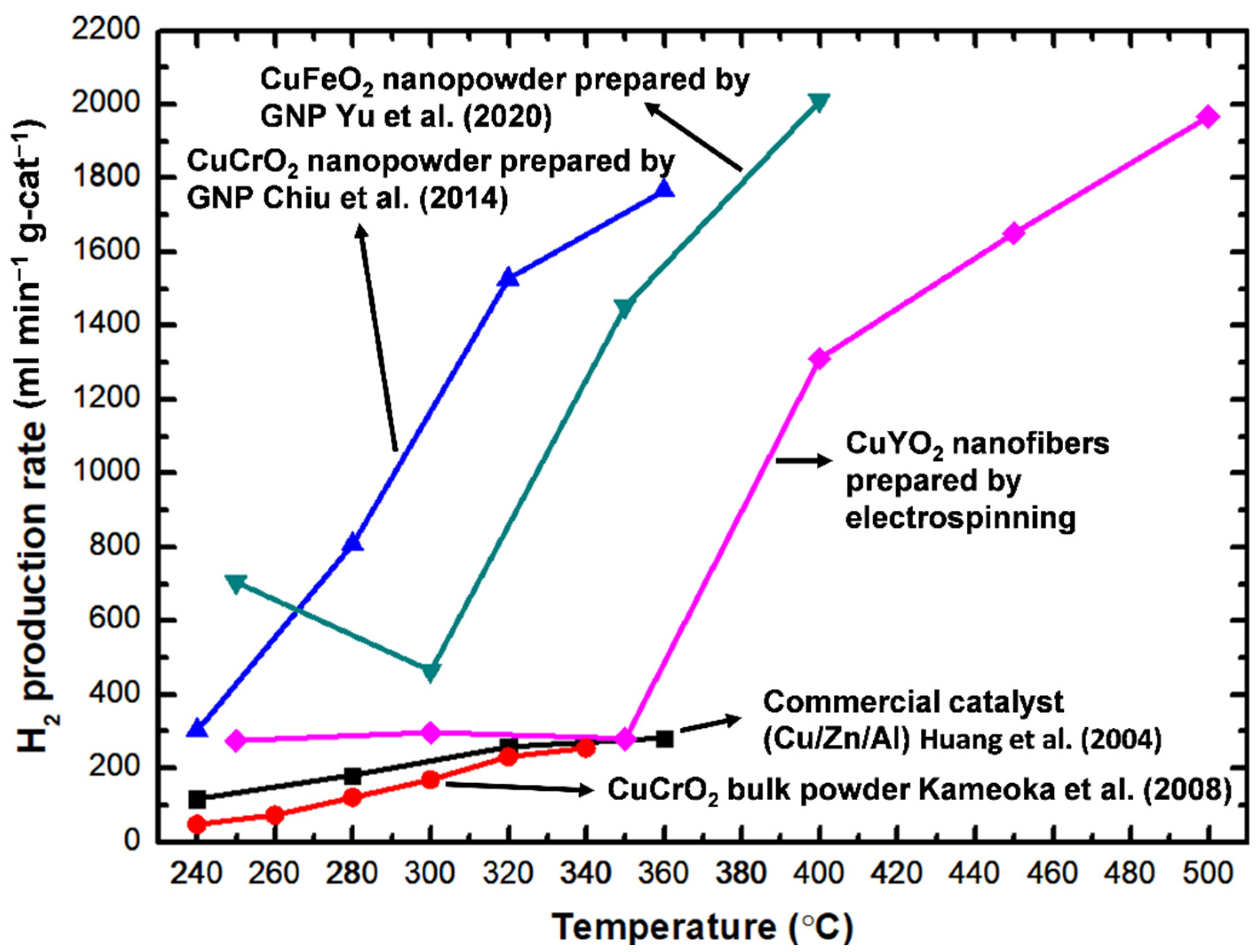
3.6. Methanol Steam Reforming Performance
3.7. XRD and SEM Analysis of CuYO2 Nanofibers after MSR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishio, K.; Okada, T.; Kikuchi, N.; Mikusu, S.; Iida, T.; Tokiwa, K.; Watanabe, T.; Kineri, T. Preparation of Delafossite CuYO2 by Metal-citric Acid Complex Decomposition Method. MRS Proc. 2009, 1166, 1166-N03-13. [Google Scholar] [CrossRef]
- Sinnarasa, I.; Thimont, Y.; Presmanes, L.; Barnabe, A.; Tailhades, P. Thermoelectric and Transport Properties of Delafossite CuCrO2:Mg Thin Films Prepared by RF Magnetron Sputtering. Nanomaterials 2017, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Sanam, P.J.; Shah, M.; Pradyumnan, P. Structure induced modification on thermoelectric and optical properties by Mg doping in CuCrO2 nanocrystals. Solid State Commun. 2022, 353, 114855. [Google Scholar] [CrossRef]
- Barnabé, A.; Thimont, Y.; Lalanne, M.; Presmanes, L.; Tailhades, P. p-Type conducting transparent characteristics of delafossite Mg-doped CuCrO2 thin films prepared by RF-sputtering. J. Mater. Chem. C 2015, 3, 6012–6024. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Rajakumaran, R.; Keyan, A.K.; Yu, C.-L.; Wu, C.-F.; Vinothini, S.; Chen, S.-M.; Chiu, T.-W. Novel construction of carbon nanofiber/CuCrO2 composite for selective determination of 4-nitrophenol in environmental samples and for supercapacitor application. RSC Adv. 2021, 11, 15856–15870. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Rajakumaran, R.; Keyan, A.K.; Vasu, D.; Chen, S.M.; Chiu, T.W. Synthesis of B-RGO-MWCNT/CuFeO2 Composite for Efficient Hydrogen Evolution Reaction. ECS J. Solid State Sci. Technol. 2021, 10, 111001. [Google Scholar] [CrossRef]
- Manoj, R.; Nisha, M.; Vanaja, K.A.; Jayaraj, M.K. Effect of oxygen intercalation on properties of sputtered CuYO2 for potential use as p-type transparent conducting films. Bull. Mater. Sci. 2008, 31, 49–53. [Google Scholar] [CrossRef]
- Trari, M.; Bouguelia, A.; Bessekhouad, Y. p-Type CuYO2 as hydrogen photocathode. Sol. Energy Mater. Sol. Cells 2006, 90, 190–202. [Google Scholar] [CrossRef]
- Ehara, T. Preparation of CuYO2 Thin Films by Sol-Gel Method Using Copper Acetate and Yttrium Acetate as Metal Sources. J. Mater. Sci. Chem. Eng. 2016, 4, 24–28. [Google Scholar]
- Tsuboi, N.; Ohara, H.; Hoshino, T.; Kobayashi, S.; Kato, K.; Kaneko, F. Luminescence Properties of Delafossite-Type CuYO2 Doped with Calcium, Oxygen or Rare Earth Tb. Jpn. J. Appl. Phys. 2005, 44, 765–768. [Google Scholar] [CrossRef]
- Yu, C.-L.; Sakthinathan, S.; Chen, S.-Y.; Yu, B.-S.; Chiu, T.-W.; Dong, C. Hydrogen generation by methanol steam reforming process by delafossite-type CuYO2 nanopowder catalyst. Microporous Mesoporous Mater. 2021, 324, 111305. [Google Scholar] [CrossRef]
- Sun, J.; Qiu, X.-P.; Wu, F.; Zhu, W.-T. H2 from steam reforming of ethanol at low temperature over Ni/Y2O3, Ni/La2O3 and Ni/Al2O3 catalysts for fuel-cell application. Int. J. Hydrogen Energy 2005, 30, 437–445. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, Q.; Peng, S.; Ramakrishna, S.; Zhang, D.; Zhou, K. Electrospun Inorganic Nanofibers for Oxygen Electrocatalysis: Design, Fabrication, and Progress. Adv. Energy Mater. 2020, 10, 1902115. [Google Scholar] [CrossRef]
- Locarno, S.; Eleta-Lopez, A.; Lupo, M.G.; Gelmi, M.L.; Clerici, F.; Bittner, A.M. Electrospinning of pyrazole-isothiazole derivatives: Nanofibers from small molecules. RSC Adv. 2019, 9, 20565–20572. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.-C.; Sakthinathan, S.; Chiu, T.-W.; Fu, Y. Anisotropic delafossite-type CuFeO2 thin films deposited by electrospinning with rotating collector. J. Ceram. Soc. Jpn. 2019, 127, 498–503. [Google Scholar] [CrossRef]
- Yu, C.-L.; Lai, G.-T.; Sakthinathan, S.; Lin, C.-C.; Chiu, T.-W.; Liu, M.-C. Hydrogen generation from methanol steam reforming process of CuCrO2-CeO2 nanopowders catalyst. Mater. Sci. Eng. B 2022, 286, 115989. [Google Scholar] [CrossRef]
- Huang, R.J.; Sakthinathan, S.; Chiu, T.W.; Dong, C. Hydrothermal synthesis of high surface area CuCrO2 for H2 production by methanol steam reforming. RSC Adv. 2021, 11, 12607–12613. [Google Scholar] [CrossRef]
- Yu, C.L.; Sakthinathan, S.; Lai, G.T.; Lin, C.C.; Chiu, T.W.; Liu, M.C. ZnO-ZnCr2O4 composite prepared by a glycine nitrate process method and applied for hydrogen production by steam reforming of methanol. RSC Adv. 2022, 12, 22097–22107. [Google Scholar] [CrossRef]
- Abdi, A.; Bagtache, R.; Trari, M. Physical and photo-electrochemical properties of oxygen-rich delafossite CuYO2. J. Solid State Electrochem. 2018, 22, 3191–3196. [Google Scholar] [CrossRef]
- Younsi, M.; Saadi, S.; Bouguelia, A.; Aider, A.; Trari, M. Synthesis and characterization of oxygen-rich delafossite CuYO2+x—Application to H2-photo production. Sol. Energy Mater. Sol. Cells 2007, 91, 1102–1109. [Google Scholar] [CrossRef]
- Schneller, T.; Waser, R.; Kosec, M.; Payne, D. Chemical Solution Deposition of Functional Oxide Thin Films; Springer: Vienna, Austria, 2013. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Production of hydrogen via combined steam reforming of methanol over CuO-CeO2 catalysts. Catal. Commun. 2004, 5, 231–235. [Google Scholar] [CrossRef]
- Brown, J.; Gulari, E. Hydrogen production from methanol decomposition over Pt/Al2O3 and ceria promoted Pt/Al2O3 catalysts. Catal. Commun. 2004, 5, 431–436. [Google Scholar] [CrossRef]
- Poirier, M.G.; Sapundzhiev, C. Catalytic decomposition of natural gas to hydrogen for fuel cell applications. Int. J. Hydrogen Energy 1997, 22, 429–433. [Google Scholar] [CrossRef]
- Agrell, J.; Hasselbo, K.; Jansson, K.; Järås, S.G.; Boutonnet, M. Production of hydrogen by partial oxidation of methanol over Cu/ZnO catalysts prepared by microemulsion technique. Appl. Catal. A Gen. 2001, 211, 239–250. [Google Scholar] [CrossRef]
- Kameoka, S.; Tanabe, T.; Tsai, A.P. Self-assembled porous nano-composite with high catalytic performance by reduction of tetragonal spinel CuFe2O4. Appl. Catal. A Gen. 2010, 375, 163–171. [Google Scholar] [CrossRef]
- Pajaie, H.S. Hydrogen Production from Methanol Steam Reforming over Cu/ZnO/Al2O3/CeO2/ZrO2 Nanocatalyst in an Adiabatic Fixed-Bed Reactor. Iran. J. Energy Environ. 2012, 3, 307–313. [Google Scholar]
- Chiu, T.-W.; Hong, R.-T.; Yu, B.-S.; Huang, Y.-H.; Kameoka, S.; Tsai, A.-P. Improving steam-reforming performance by nanopowdering CuCrO2. Int. J. Hydrogen Energy 2014, 39, 14222–14226. [Google Scholar] [CrossRef]
- Chiu, T.-W.; Lu, Y.-S. Preparation of CuCr1-xFexO2 Delafossite Solid Solution Powder Via a Self-Combustion Glycine Nitrate Process. Ferroelectrics 2016, 491, 149–154. [Google Scholar] [CrossRef]
- Chiu, T.-W.; Huang, P.-S. Preparation of delafossite CuFeO2 coral-like powder using a self-combustion glycine nitrate process. Ceram. Int. 2013, 39, S575–S578. [Google Scholar] [CrossRef]
- Lalanne, M.; Barnabee, A.; Mathieu, F.; Tailhades, P. Synthesis and Thermostructural Studies of a CuFe1−xCrxO2 Delafossite Solid Solution with 0 ≤ x ≤ 1. Inorg. Chem. 2009, 48, 6065–6071. [Google Scholar] [CrossRef] [PubMed]
- Çetin, C.; Akyıldız, H. Production, and characterization of CuCrO2 nanofibers. Mater. Chem. Phys. 2016, 170, 138–144. [Google Scholar] [CrossRef]
- Chao, T.-C.; Chiu, T.-W.; Fu, Y. Fabrication and characteristic of delafossite-type CuFeO2 nanofibers by electrospinning method. Ceram. Int. 2018, 44, S80–S83. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Akhoundzadeh, H.; Rezayan, A.; Sadeghian, M. Excellent catalytic performance of 3D-mesoporous KIT-6 supported Cu and Ce nanoparticles in methanol steam reforming. Int. J. Hydrogen Energy 2018, 43, 10926–10937. [Google Scholar] [CrossRef]
- Iulianelli, A.; Longo, T.; Basile, A. Methanol steam reforming reaction in a Pd–Ag membrane reactor for CO-free hydrogen production. Int. J. Hydrogen Energy 2008, 33, 5583–5588. [Google Scholar] [CrossRef]
- Yu, C.-L.; Sakthinathan, S.; Hwang, B.-Y.; Lin, S.-Y.; Chiu, T.-W.; Yu, B.-S.; Fan, Y.-J.; Chuang, C. CuFeO2–CeO2 nanopowder catalyst prepared by self-combustion glycine nitrate process and applied for hydrogen production from methanol steam reforming. Int. J. Hydrogen Energy 2020, 45, 15752–15762. [Google Scholar] [CrossRef]
- Kameoka, S.; Okada, M.; Tsai, A.P. Preparation of a Novel Copper Catalyst in Terms of the Immiscible Interaction Between Copper and Chromium. Catal. Lett. 2007, 120, 252–256. [Google Scholar] [CrossRef]






| Processes | Specific Surface Area (m2/g) | Reference |
|---|---|---|
| GNP method (CuCrO2) | 25.70 | [30] |
| GNP method (CuFeO2) | 11.38 | [31] |
| Solid-state (CuCrO2) | 1.94 | [32] |
| Solid-state (CuFeO2) | 0.57 | [32] |
| Electrospinning (CuCrO2) | 7.85 | [33] |
| Electrospinning (CuFeO2) | 4.33 | [34] |
| Electrospinning (CuYO2) | 10.22 | This study |
| Temperature (°C) | Hydrogen Production Rate (mL min−1 g-cat−1) |
|---|---|
| 250 | 275.61 |
| 300 | 297.77 |
| 350 | 281.17 |
| 400 | 1311.86 |
| 450 | 1651.84 |
| 500 | 1967.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, K.-C.; Keyan, A.K.; Hung, C.-W.; Sakthinathan, S.; Yu, C.-L.; Chiu, T.-W.; Nagaraj, K.; Fan, F.-Y.; Shan, Y.-K.; Chen, P.-C. Fabrication of CuYO2 Nanofibers by Electrospinning and Applied to Hydrogen Harvest. Materials 2022, 15, 8957. https://doi.org/10.3390/ma15248957
Hsu K-C, Keyan AK, Hung C-W, Sakthinathan S, Yu C-L, Chiu T-W, Nagaraj K, Fan F-Y, Shan Y-K, Chen P-C. Fabrication of CuYO2 Nanofibers by Electrospinning and Applied to Hydrogen Harvest. Materials. 2022; 15(24):8957. https://doi.org/10.3390/ma15248957
Chicago/Turabian StyleHsu, Kai-Chun, Arjunan Karthi Keyan, Chin-Wei Hung, Subramanian Sakthinathan, Chung-Lun Yu, Te-Wei Chiu, Karuppiah Nagaraj, Fang-Yu Fan, Yung-Kang Shan, and Po-Chou Chen. 2022. "Fabrication of CuYO2 Nanofibers by Electrospinning and Applied to Hydrogen Harvest" Materials 15, no. 24: 8957. https://doi.org/10.3390/ma15248957
APA StyleHsu, K.-C., Keyan, A. K., Hung, C.-W., Sakthinathan, S., Yu, C.-L., Chiu, T.-W., Nagaraj, K., Fan, F.-Y., Shan, Y.-K., & Chen, P.-C. (2022). Fabrication of CuYO2 Nanofibers by Electrospinning and Applied to Hydrogen Harvest. Materials, 15(24), 8957. https://doi.org/10.3390/ma15248957