Abstract
To analyze the high-temperature oxidation behavior of TiB2-HfB2-Ni cermet material, TiB2-HfB2-Ni cermets were fabricated by hot-pressing sintering technology. The oxidation resistance and the thermal fracture of TiB2-HfB2-Ni cermet were investigated at 1100 °C for 1, 4, 7, and 10 h, respectively. Before oxidation, TiB2-HfB2-Ni cermet, consisting of TiB2, HfB2, and Ni, had the core-rim structure. The core was TiB2 grain and the rim was composed of Ni and solid solution (Ti, Hf)B2. After oxidation at 1100 °C, the oxides of the TiB2-HfB2-Ni cermet were mainly TiO2, HfO2, B2O3, and NiO, which the oxidation process abided by the parabolic law. With the oxidation time increasing from 1 h to 10 h, the oxidation degree of the TiB2-HfB2-Ni cermet increased, and the oxide layer became thicker. The oxide layer was thin and dense after oxidation at 1100 °C for 1 h. An obvious boundary was discovered between the transition layer and the substrate layer after oxidation at 1100 °C for 7 h. The thermal fracture occurred in the contact regions of different layers at 1100 °C for 10 h. TiB2-HfB2-Ni took place in oxidation at different levels from the outer to the inner, and the components of different oxide layers were certainly distinct.
1. Introduction
TiB2-based cermet demonstrates excellent mechanical, physical, and chemical properties, including high hardness at high temperatures, high melting point, outstanding electrical conductivity, and strong corrosion resistance [1,2,3,4]. TiB2-based cermet materials (TiB2 + BN [4], TiB2 + W [5], TiB2 + HfC [6], TiB2 + Si3N4 [7] and TiB2 + TiC [8]) were used in armored parts, high-temperature components, and cutting tools. Since TiB2-based cermets were usually served in high-temperature oxidation environments [9,10,11,12], it was of great significance to investigate the high-temperature oxidation behavior for long-term use.
Recently, some researchers have investigated the oxidation behavior of TiB2-based cermets at different temperatures. The oxides TiO2 and B2O3 were discovered in the monolithic TiB2 cermet oxidized at 800−1200 °C, and the oxide layer thickness, oxide content, and microstructure were affected by the oxidation temperature and time [13,14,15]. TiB2-WSi2 cermet demonstrated better oxidation resistance than TiB2 cermet in the same high-temperature environment, and oxides TiO2, B2O3, and SiO2 formed on the surface of TiB2-WSi2 cermet [16]. The oxide layer thickness of the TiB2 + EuB6 cermet was about 340 μm during oxidization at 1400 °C for 8 h, and the oxides could enhance the oxidation resistance of the cermet [17]. The glassy B2O3 was discovered in TiB2-MoSi2-CrB2 cermet and TiB2-SiC-B4C cermet oxidized at 1000 °C [18,19].
Moreover, the relationship between oxidation duration and mass gain of TiB2-based cermet was investigated by some researchers. The relationship between the oxidation duration and the mass gain of TiB2 + TiC cermet abided by the linear law at 600 °C, while abided by the parabolic law at 700 °C and 800 °C [20]. TiB2 cermet coating could improve the oxidation temperature by up to 900 °C for molybdenum, and the oxidation behavior abided by the parabolic law [21]. The oxidation kinetics of TiB2 and TiB2 + MoSi2 also abided by the parabolic law at 850 °C [22].
To investigate the oxidation mechanism of the TiB2-HfB2-Ni cermet in high temperatures, TiB2-HfB2-Ni cermets would be fabricated by hot-pressing sintering technology. The microstructures before and after oxidation and the high-temperature oxidation behavior of TiB2-HfB2-Ni cermets would be investigated.
2. Experimental Procedures
Commercially available TiB2, HfB2, and Ni powders (>99.9%, 1 μm) were utilized as raw powders to fabricate TiB2-HfB2-Ni cermet (TBHB). The mixed powders (72 wt.% TiB2, 20 wt.% HfB2, and 8 wt.% Ni), after milling with WC balls and ethanol absolute for 72 h, drying and sieving orderly, were put into the graphite mold and then sintered at 1500 °C for 0.5 h under an axial pressure of 35 MPa in the vacuum sintering furnace (ZT-40-20, Shanghai Chenhua Technology Co., Ltd., Shanghai, China). The sintered ceramics were machined into strip specimens with a dimension of 3 mm × 4 mm × 40 mm and surface roughness of 0.96 μm. More details about the fabricating process can be found in the literature [6,23].
To investigate the oxidation behavior of TBHB, first, the TBHB specimens were cleaned ultrasonically and dried. The lengths, widths, and heights of the TBHB specimens before oxidation were measured using Vernier caliper (100011956107, Shanghai Tool Works Co., Ltd., Shanghai, China, accuracy 0.02 mm) and the surface areas (S) were calculated. The weights (m0) were measured using an electronic balance (BSM-120.4, Shanghai Zhuojing Electronic Technology Co., Ltd., Shanghai, China, accuracy 0.1 mg). Second, the TBHB specimens leaned on the wall of the corundum crucible-arc to reduce the contact area. Then, the corundum crucible-arc was put into the muffle furnace (ZDXS5-2.5-120, Shenzhen Zhongda Strong Electric Furnace Co., Ltd., Shenzhen, China). The TBHB specimens were oxidized at 1100 °C for 1, 4, 7, and 10 h, respectively. The oxidation experiments were completed under static lab air. Finally, the weights (m1) of the TBHB specimens after oxidation were measured. The mass gain per unit area (Δm/S) was calculated as follows:
Δm/S = (m1 − m0)/S
More than 5 specimens were tested to calculate the average. X-ray diffraction (XRD EMPYREAN, PANalytical B.V., Almelo, The Netherlands) and Energy dispersive spectrometer (EDS, ACT-350, Oxford Instruments, Oxford, UK) were employed to measure the compositions of the sintered TBHB and the oxidized TBHB samples. The scanning electron microscope (SEM, Supra-55, Carl Zeiss AG, Jena, Germany) was used to analyze the polished surfaces and fracture morphologies of the TBHB specimens.
3. Results and Discussions
3.1. Microstructure of TBHB before Oxidation
XRD pattern of TBHB before oxidation is shown in Figure 1. TBHB consisted of TiB2, HfB2, and Ni. It demonstrated that no reaction occurred in the sintering process and Ni had good chemical compatibility with TiB2 and HfB2. The literature [5] reported that a small amount of Ni4B3 was discovered in the TiB2-Ni system, and brittle material Ni4B3 was harmful to the mechanical properties of TiB2-based ceramics. However, the Ni4B3 was not discovered in TBHB according to Figure 1.
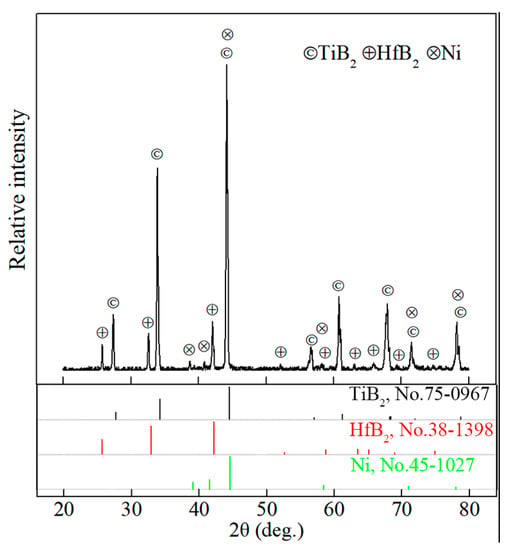
Figure 1.
XRD pattern of TBHB before oxidation.
Figure 2 shows the polished surface and fracture morphology of TBHB before oxidation. There were mainly three phases including a black phase, a gray phase, and a white phase. Figure 3 shows the EDS results of three phases in TBHB before oxidation. Based on the results of XRD and EDS, the black phase was the undissolved TiB2 with abundant Ti, and scant Hf, B, and Ni, as shown in Figure 3a. The gray phase was the solid solution (Ti, Hf)B2 rich in Ti, but poor in Hf, B, and Ni, as shown in Figure 3b. The white phase was the solid solution (Ti, Hf)B2 rich in Ti, Hf, and B, but poor in Ni. In addition, the core-rim structure was discovered in the polished surface and fracture morphology [6]. The core was TiB2 grain and the rim was composed of Ni and the solid solution (Ti, Hf)B2. The elements Ti and Hf, belonging to the same family, have the same valence as B for TiB2 and HfB2. In the liquid sintering process; therefore, some Ti and Hf atoms were probably exchanged to form (Ti, Hf)B2 solid solution and Ni was often distributed among TiB2 grains [24]. The solid solution and Ni around the TiB2 grains formed a skeleton structure in TBHB, which could keep the matrix from fracturing when TBHB undertook high load or was oxidized at a high-temperature environment. In addition, the shapes of TiB2 grains would change from blocky to banded structures, which might result from the mutual replacement of Ti and Hf. The banded structure could enhance the mechanical properties of TiB2-based cermets [6,23].
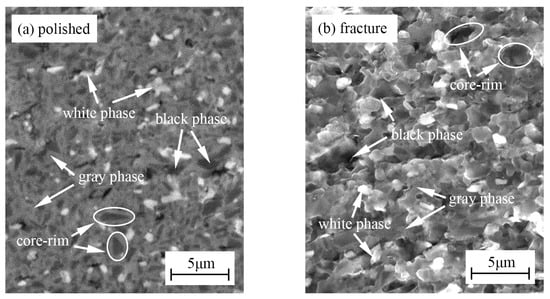
Figure 2.
Polished surface and fracture morphology of TBHB before oxidation: (a) polished surface, (b) fracture morphology.
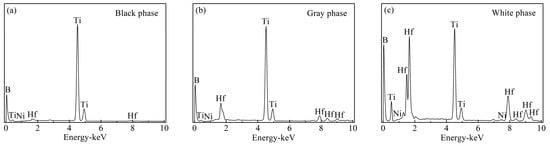
Figure 3.
EDS results of the phases in TBHB before oxidation: (a) black phase, (b) gray phase, (c) white phase.
3.2. Oxides and Mass Gain in TBHB
Figure 4 shows the XRD patterns of the TBHB surface and cross-section after oxidation at 1100 °C for 10 h. There were oxides TiO2, HfO2, B2O3, and NiO on the surface of TBHB, as shown in Figure 4a. However, aside from the above oxides, there was TiB2, HfB2, and Ni on the cross-section of TBHB, as shown in Figure 4b. This indicated that the inside of TBHB were incompletely oxidized.
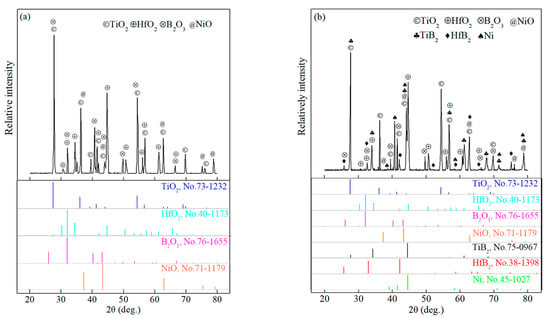
Figure 4.
XRD patterns of TBHB surface and cross-section after oxidation at 1100 °C for 10 h: (a) surface, (b) cross-section.
The following possible reactions occurred during the oxidation process.
Ni(s) + 0.5O2(g) → NiO(s)
TiB2(s) + 2O2(g) → TiO(s) + B2O3(l)
TiB2(s) + 2.5O2(g) → TiO2(s) + B2O3
HfB2(s) + 2.5O2(g) → HfO2(s) + B2O3
2TiB2(s) + 4.5O2(g) → Ti2O3(s) + 2B2O3(l)
B2O3(l) → B2O3(g)
According to reactions (3), (4), and (6), the possible oxides of TiB2 were TiO, Ti2O3, TiO2, and B2O3. However, the obvious peaks of TiO and Ti2O3 were undiscovered in the XRD of the oxidation TBHB. This is because low-valence oxides TiO and Ti2O3 were easily oxidized to TiO2 [25]. Therefore, TiO2 and B2O3 were the main oxides of TiB2 in the TBHB. In the oxidizing process, the oxides NiO, TiO2, and HfO2 were solid because their melting points were far higher than 1100 °C. B2O3 was liquid with a high viscosity because its melting point (450 °C) was lower than 1100 °C. The B2O3 liquid filled the pores among the oxide particles at 1100 °C, which could prevent oxygen from eroding the interior materials and transforming into glassy after cooling [19]. Moreover, the B2O3 liquid evaporated, which would weaken the protective effect of B2O3 on the cermet [16].
Reactions (2)−(6) would result in the mass of TBHB increasing, while reaction (7) would result in the mass decreasing. Figure 5 shows the experimental and fitting curve of oxidation duration and mass gain of TBHB at 1100 °C. As the oxidation time extended, the mass gain increased gradually. When the oxidation times were 1, 4, 7, and 10 h, the values of mass gain of TBHB were 0.56, 1.63, 2.34, and 2.82 mg/cm2, respectively. The fitting curve was derived from the experimental data and the equation was expressed as [20]:
where m and t were the mass gain and the oxidation time, respectively. Equation (8) is a parabolic equation. According to Figure 5, the fitting curve agreed with the experimental curve well, which indicated that the oxidation process of TBHB abided by the parabolic law.
(∆m/S)2 = 0.74t
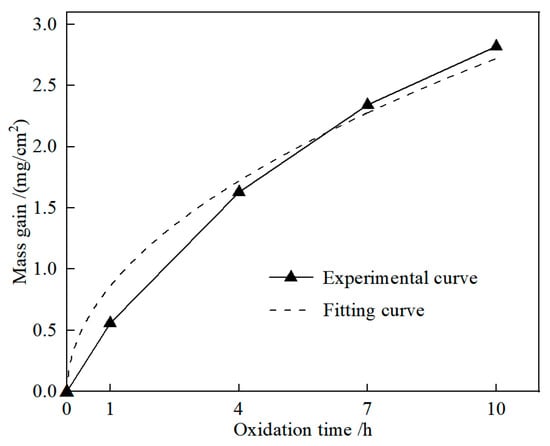
Figure 5.
Experimental and fitting curve of oxidation duration and mass gain of TBHB at 1100 °C.
Figure 6 shows the Gibbs free energy changes of the oxidation reactions of Ni, TiB2, and HfB2 at 0–1100 °C. The Gibbs free energies were calculated using the HSC-chemistry software. Generally, the change of the Gibbs free energy was used to estimate the tendency of the reactions at the same oxidation condition [26,27,28]. The lower the value of the Gibbs free energy, the stronger the reaction tendency. According to Figure 6, the ranking of the reaction tendency was (2) < (3) < (4) < (5) < (6) at 0–1100 °C. Since the outer layer was in direct contact with oxygen, the compounds on the TBHB surface might are oxidized into the oxides such as TiO, Ti2O3, TiO2, HfO2, B2O3, and NiO. However, low-valence titanium ions (Ti2+ and Ti3+) would be further oxidized to high-valence Ti4+ in the atmosphere [25]. Therefore, the final oxides on the surface TBHB surface were TiO2, B2O3, and NiO.
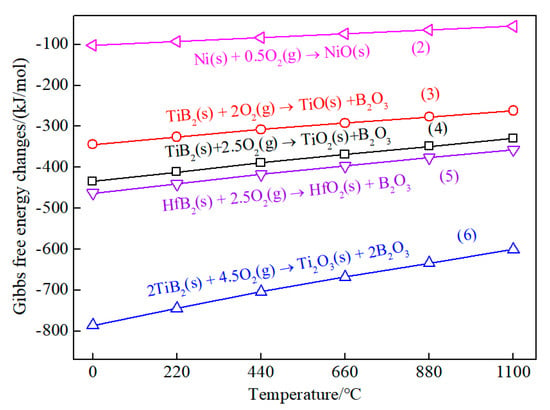
Figure 6.
Variations of the Gibbs free energy for the oxidation reactions of Ni, TiB2, and HfB2 at 0−1100 °C.
The oxidation of the materials in the TBHB inner was controlled by the oxygen diffusion speed. When the oxygen entered into the TBHB inner, TiB2, HfB2, and Ni would be competitive with each other for oxygen to form oxides. According to the reaction tendency, TiB2 firstly reacted with oxygen to form oxides Ti2O3 and B2O3, and low-valence oxide Ti2O3 would be oxidized to TiO2. Then, HfO2 and Ni would be oxidized orderly if there was enough oxygen to maintain the reactions. When the B2O3 liquid formed and filled the pores, the oxygen diffusion would gradually slow down, resulting in oxides decreasing in the TBHB inner. Thus, the oxide content reduced gradually from the outer to the inner. The oxides and the unreacted materials would constitute new composites or mixtures with their physical properties.
3.3. Thermal Fracture of TBHB in the High-Temperature Oxidation Process
Figure 7 presents the fracture morphologies of TBHB oxidized at 1100 °C for 1, 4, 7, and 10 h. The oxidation of TBHB became more severe and the oxide layer became thicker as the oxidation time extended from 1 h to 10 h. In Figure 7a, the oxide layer, consisting of the outer oxide layer (OOL), was about 15 μm at 1100 °C for 1 h. The thin and dense OOL could inhibit the entrance of oxygen into the TBHB inner part. In Figure 7b, the oxide layer, consisting of OOL and the transition layer (TL), was about 30 μm at 1100 °C for 4 h. OOL was composed of oxide particles and pores. The growth of oxide and the evaporation of B2O3 liquid lead to pores. The connected pores in the OOL formed convenient channels for oxygen to enter into the TBHB inner and erode the inner materials, and then the dense TL formed in the TBHB inner. In Figure 7c, the oxide layer, consisting of OOL and TL, was about 50 μm at 1100 °C for 7 h. An apparent boundary between the dense TL and substrate layer (SL) could be observed. In Figure 7d, the oxide layer, consisting of OOL and TL, was about 70 μm at 1100 °C for 10 h. Cracks were observed in TL, which indicated that the thermal fracture took place in the contact layers between the OOL and TL or TL and SL. Moreover, the size of the oxide particles from the outer to the inner gradually decreased as shown in Figure 7. This indicated that TBHB took place in oxidation at different levels from the outer to the inner.
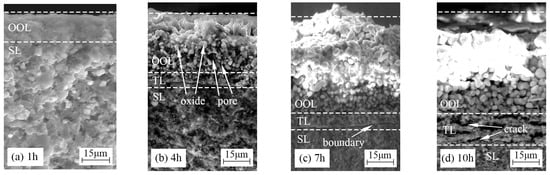
Figure 7.
Fracture morphologies of TBHB at 1100 °C in different oxidation times: (a) 1 h, (b) 4 h, (c) 7 h, (d) 10 h.
Figure 8 shows the BSE patterns and the corresponding EDS map scan results of TBHB cross-sections at 1100 °C in different oxidation times. The scanned area was the gap between two red dotted lines. In Figure 8, the concentrations of O elements in OOL and TL were more than in SL, and B element was less than in SL. This was because the oxidation reactions of TiB2, HfB2, and Ni occurred at 1100 °C, which resulted in an increase in O element. The B2O3 liquid in the reactions (3)–(6) evaporated, which resulted in a decrease in the B element. There were no significant concentration changes in Ti, Hf, and Ni elements.
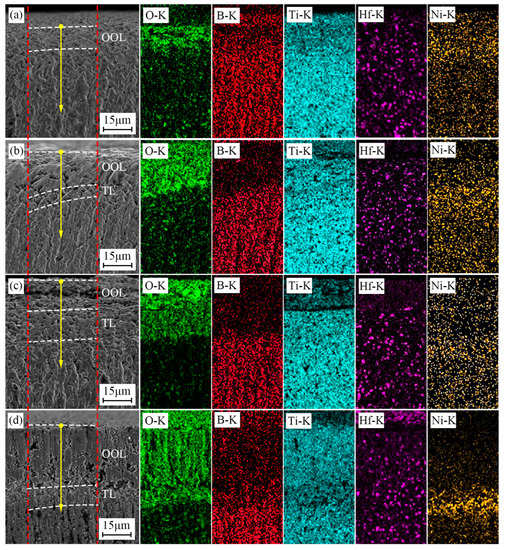
Figure 8.
BSE patterns and the corresponding EDS map scan results of cross-section of TBHB at 1100 °C in different oxidation times: (a) 1 h, (b) 4 h, (c) 7 h, (d) 10 h.
Figure 9 presents the line EDS results of TBHB cross-sections at 1100 °C for 1, 4, 7, and 10 h. The paths of line EDS were shown in the yellow arrows in Figure 8. From the edge to the center of TBHB, the concentrations of O and B elements changed significantly and regularly. When the oxidation times were 1, 4, 7, and 10 h, the concentration of O element increased or B element decreased at a distance of about 15, 30, 50, and 70 μm, respectively. When the oxidation time was 1 h, the content of B element did not change significantly, which was due to the insufficient evaporation of B2O3 in a short time. B2O3 liquid densified the oxide layer after cooling (as shown in Figure 7a). When the oxidation times were 4, 7, and 10 h, the evaporation of B2O3 resulted in obvious pores in OOL. The extreme difference in oxide content resulted in the thermal fracture of OOL and TL or TL and SL, and the cracks were observed in TL (as shown in Figure 7c,d). According to the variations of O and B elements, therefore, TBHB took place in oxidation at different levels from the outer to the inner, and the components of OOL, TL, and SL were certain differences.
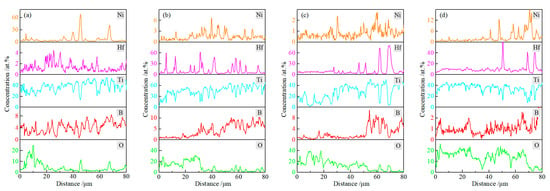
Figure 9.
Line EDS results of cross-section of TBHB at 1100 °C in different oxidation times: (a) 1 h, (b) 4 h, (c) 7 h, (d) 10 h.
4. Conclusions
TiB2-HfB2-Ni cermets were papered by hot-pressing sintering. The microstructures and oxidation mechanism of the TiB2-HfB2-Ni cermets were investigated. The conclusions were as follows:
- (1)
- Before oxidation, TiB2-HfB2-Ni cermets, consisting of TiB2, HfB2, and Ni phases, had the core-rim structure. The core was TiB2 grain and the rim was composed of Ni and the solid solution (Ti, Hf)B2.
- (2)
- After oxidation, the oxides of TiB2-HfB2-Ni cermet surface were TiO2, HfO2, B2O3, and NiO. Aside from the above oxides, there was TiB2, HfB2, and Ni on the cross-section. The values of mass gain at 1100 °C for 1, 4, 7, and 10 h were 0.56, 1.63, 2.34, and 2.82 mg/cm2, respectively. The oxide content reduced gradually from the outer to the inner of TiB2-HfB2-Ni cermet. With the oxidation time increasing from 1 h to 10 h, the oxidation of TiB2-HfB2-Ni cermet surface became more severe and the oxide layer became thicker.
- (3)
- The thicknesses of the oxide layers at 1100 °C for 1, 4, 7, and 10 h were about 15, 30, 50, and 70 μm, respectively. The oxide layer was thinner and dense at 1100 °C for 1 h. An obvious boundary was observed between the transition layer and the substrate layer at 1100 °C for 7 h. The thermal fracture occurred in contact regions of different layers at 1100 °C for 10 h. TBHB took place in oxidation at different levels from the outer to the inner, and there were some differences between the components of the outer oxide layer, transition layer, and substrate layer.
Author Contributions
J.S. and J.G. conceived and designed the experiments; J.G. and Z.W. performed the experiments and analyzed the data; J.S. contributed reagents/materials/analysis tools; J.G. and Z.W. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 51875388 and 52205492) and the Science and Technology Innovation Program of Higher Education Institutions in Shanxi Province (Grant No. 2021L067).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Balcı, Ö.; Burkhardt, U.; Schmidt, M.; Hennicke, J.; Yağcı, M.B.; Somer, M. Densification, microstructure and properties of TiB2 ceramics fabricated by spark plasma sintering. Mater. Charact. 2018, 145, 435–443. [Google Scholar] [CrossRef]
- Shayesteh, F.; Delbari, S.A.; Ahmadi, Z.; Shokouhimehr, M.; Asl, M.S. Influence of TiN dopant on microstructure of TiB2 ceramic sintered by spark plasma. Ceram. Int. 2019, 45, 5306–5311. [Google Scholar] [CrossRef]
- Grigoriev, S.; Pristinskiy, Y.; Soe, T.; Malakhinsky, A.; Mosyanov, M.; Podrabinnik, P.; Smirnov, A.; Pinargote, N.S. Processing and Characterization of Spark Plasma Sintered SiC-TiB2-TiC Powders. Materials 2022, 15, 1946–1961. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Hamidzadeh Mahaseni, Z.; Dashti Germi, M.; Delbari, S.A.; Le, Q.V.; Ahmadi, Z.; Shokouhimehr, M.; Shahedi Asl, M.; Namini, A.S. Densification behavior and microstructure development in TiB2 ceramics doped with h-BN. Ceram. Int. 2020, 46, 18970–18975. [Google Scholar] [CrossRef]
- Song, J.; Huang, C.; Zou, B.; Liu, H.; Liu, L.; Wang, J. Effects of sintering additives on microstructure and mechanical properties of TiB2–WC ceramic–metal composite tool materials. Int. J. Refract. Met. Hard Mater. 2012, 30, 91–95. [Google Scholar] [CrossRef]
- Song, J.; Xie, J.; Lv, M.; Gao, J.; An, J. Microstructure and Mechanical Properties of TiB2-HfC Ceramic Tool Materials. JOM 2018, 70, 2544–2554. [Google Scholar] [CrossRef]
- Ramesh, B.; Showman, E.; Abraar, S.A.M.; Saxena, K.K.; Tharwan, M.Y.; Alsaadi, N.; Al Sofyani, S.; Elsheikh, A.H. Microstructure, Mechanical Characteristics, and Wear Performance of Spark Plasma Sintered TiB2-Si3N4 as Affected by B4N Doping. Materials 2022, 15, 7096–7113. [Google Scholar] [CrossRef]
- Song, J.; Huang, C.; Lv, M.; Zou, B.; Wang, S.; Wang, J.; An, J. Effects of TiC content and melt phase on microstructure and mechanical properties of ternary TiB2-based ceramic cutting tool materials. Mater. Sci. Eng. A 2014, 605, 137–143. [Google Scholar] [CrossRef]
- Vaferi, K.; Nekahi, S.; Vajdi, M.; Sadegh Moghanlou, F.; Shokouhimehr, M.; Motallebzadeh, A.; Sha, J.; Asl, M.S. Heat transfer, thermal stress and failure analyses in a TiB2 gas turbine stator blade. Ceram. Int. 2019, 45, 19331–19339. [Google Scholar] [CrossRef]
- Qiu, X.-L.; He, C.-Y.; Zhao, P.; Liu, B.-H.; Guo, H.-X.; Liu, G.; Gao, X.-H. Reinforcement optical performance and thermal tolerance in a TiB2-HfB2-based double-layer spectral selective absorber via a pre-annealing strategy. Mater. Today Phys. 2022, 24, 100690–100698. [Google Scholar] [CrossRef]
- Vajdi, M.; Sadegh Moghanlou, F.; Ahmadi, Z.; Motallebzadeh, A.; Shahedi Asl, M. Thermal diffusivity and microstructure of spark plasma sintered TiB2-SiC-Ti composite. Ceram. Int. 2019, 45, 8333–8344. [Google Scholar] [CrossRef]
- Jinpeng, S.; Shaowei, L.; Ahmad, R.; Jiaojiao, G.; Ming, L. Tribological behaviour of TiB2 -HfC ceramic tool material under dry sliding condition. Ceram. Int. 2020, 46, 20320–20327. [Google Scholar] [CrossRef]
- Andrievskii, R.A.; Shul’ga, Y.M.; Volkova, L.S.; Korobov, I.I.; Dremova, N.N.; Kabachkov, E.N.; Kalinnikov, G.V.; Shilkin, S.P. Oxidation behavior of TiB2 micro- and nanoparticles. Inorg. Mater. 2016, 52, 686–693. [Google Scholar] [CrossRef]
- Ni, C.; Tang, Y.; Abdellatif, H.R.S.; Huang, X.; Xie, D.; Ni, J. Boron-Doped TiO2 from Anodization of TiB2 for Efficient Photocatalysis. J. Electrochem. Soc. 2020, 167, 126505–126512. [Google Scholar] [CrossRef]
- Yang, Y.; Kang, Y.; Liu, G.; Cheng, H.-M. Homogeneous boron doping in a TiO2 shell supported on a TiB2 core for enhanced photocatalytic water oxidation. Chin. J. Catal. 2018, 39, 431–437. [Google Scholar] [CrossRef]
- Murthy, T.S.R.C.; Sonber, J.K.; Subramanian, C.; Hubli, R.C.; Suri, A.K. Densification, characterization and oxidation studies of TiB2-WSi2 composite. Int. J. Refract. Met. Hard Mater. 2012, 33, 10–21. [Google Scholar] [CrossRef]
- Murthy, T.S.R.C.; Sonber, J.K.; Vishwanadh, B.; Nagaraj, A.; Sairam, K.; Bedse, R.D.; Chakravartty, J.K. Densification, characterization and oxidation studies of novel TiB2+EuB6 compounds. J. Alloys Compd. 2016, 670, 85–95. [Google Scholar] [CrossRef]
- Murthy, T.S.R.C.; Sonber, J.K.; Subramanian, C.; Hubli, R.C.; Krishnamurthy, N.; Suri, A.K. Densification and oxidation behavior of a novel TiB2–MoSi2–CrB2 composite. Int. J. Refract. Met. Hard Mater. 2013, 36, 243–253. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Cheng, X.; Xie, S. Microstructural evolution and oxidation behavior of TiB2-SiC-B4C composite fabricated by reactive spark plasma sintering. J. Alloys Compd. 2018, 765, 158–165. [Google Scholar] [CrossRef]
- Yi, M.; Zhang, X.; Ge, C.; Liu, G.; Xu, S.; Zhong, D.; Qiao, G. Oxidation behavior of in situ synthesized (TiB + TiC)/Ti–6Al–4V composites from Ti–B4C–C and Ti–TiB2–TiC systems. J. Mater. Res. 2019, 34, 1762–1772. [Google Scholar] [CrossRef]
- Huang, X.; Sun, S.; Tu, G. Investigation of mechanical properties and oxidation resistance of CVD TiB2 ceramic coating on molybdenum. J. Mater. Res. Technol. 2020, 9, 282–290. [Google Scholar] [CrossRef]
- Murthy, T.S.R.C.; Balasubramaniam, R.; Basu, B.; Suri, A.K.; Mungole, M.N. Oxidation of monolithic TiB2 and TiB2–20 wt.% MoSi2 composite at 850 °C. J. Eur. Ceram. Soc. 2006, 26, 187–192. [Google Scholar] [CrossRef]
- An, J.; Song, J.; Liang, G.; Gao, J.; Xie, J.; Cao, L.; Wang, S.; Lv, M.; Agrawal, D. Effects of HfB2 and HfN Additions on the Microstructures and Mechanical Properties of TiB2-Based Ceramic Tool Materials. Materials 2017, 10, 461–471. [Google Scholar] [CrossRef]
- Song, J.; Huang, C.; Zou, B.; Liu, H.; Wang, J. Microstructure and mechanical properties of TiB2–TiC–WC composite ceramic tool materials. Mater. Des. 2012, 36, 69–74. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, R.; Sun, J.; Liu, C. Oxidation Mechanism of Biomedical Titanium Alloy Surface and Experiment. Int. J. Corros. 2020, 167, 1678615–1678623. [Google Scholar] [CrossRef]
- Nisar, A.; Ariharan, S.; Venkateswaran, T.; Sreenivas, N.; Balani, K. Oxidation studies on TaC based ultra-high temperature ceramic composites under plasma arc jet exposure. Corros. Sci. 2016, 109, 50–61. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Y.; Wang, S.; Li, M.; Xu, J.; Qian, Y.; Zuo, J.; Zhang, D. Improved both mechanical and anti-oxidation performances of ZrB2-SiC ceramics with molybdenum disilicide addition. Mater. Chem. Phys. 2019, 223, 53–59. [Google Scholar] [CrossRef]
- Astapov, A.N.; Pogozhev, Y.S.; Prokofiev, M.V.; Lifanov, I.P.; Potanin, A.Y.; Levashov, E.A.; Vershinnikov, V.I. Kinetics and mechanism of high-temperature oxidation of the heterophase ZrSi2-MoSi2-ZrB2 ceramics. Ceram. Int. 2019, 45, 6392–6404. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).