Water Kefir Grains—Microbial Biomass Source for Carbonaceous Materials Used as Sulfur-Host Cathode in Li-S Batteries
Abstract
1. Introduction
2. Materials and Methods
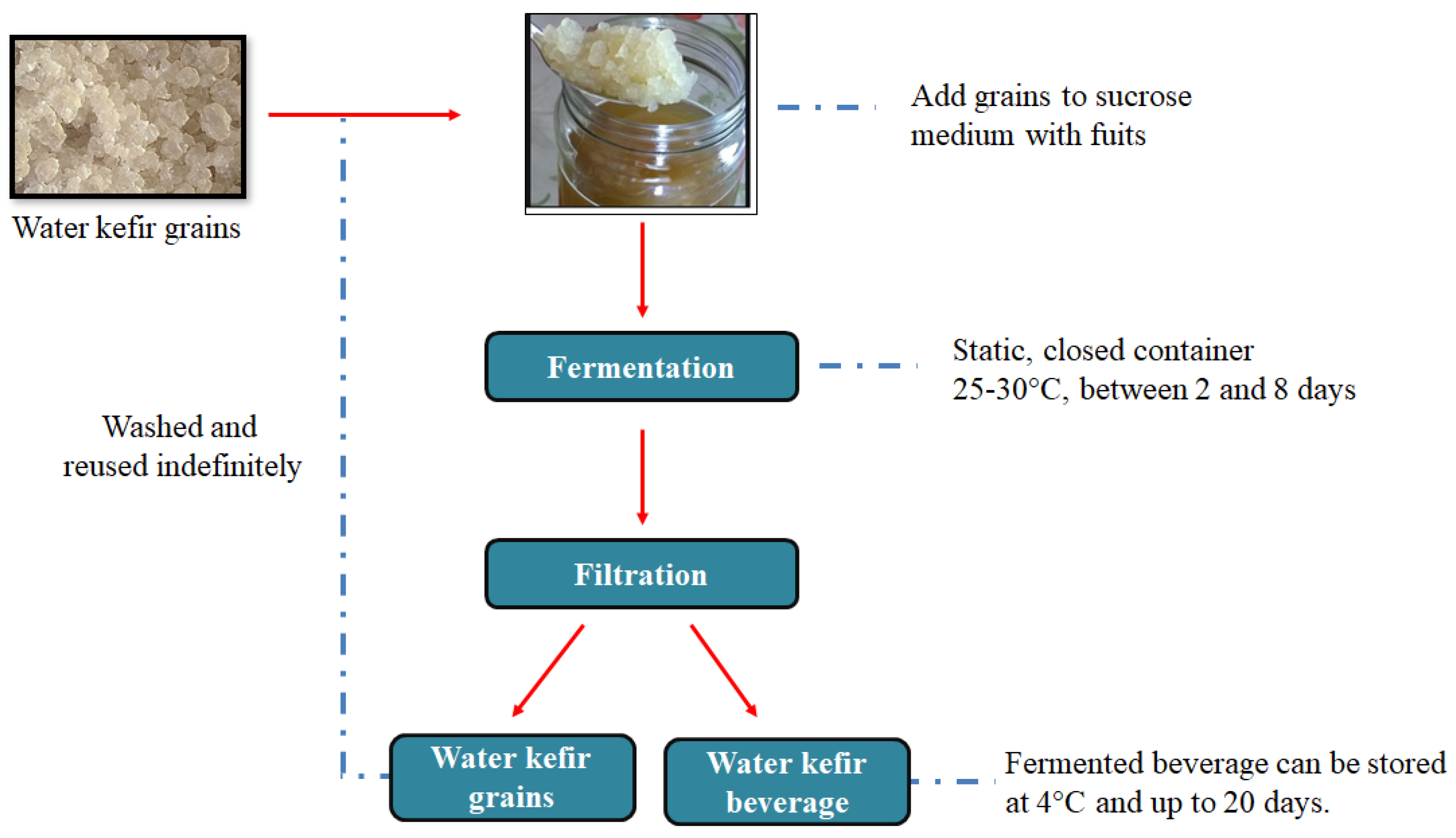
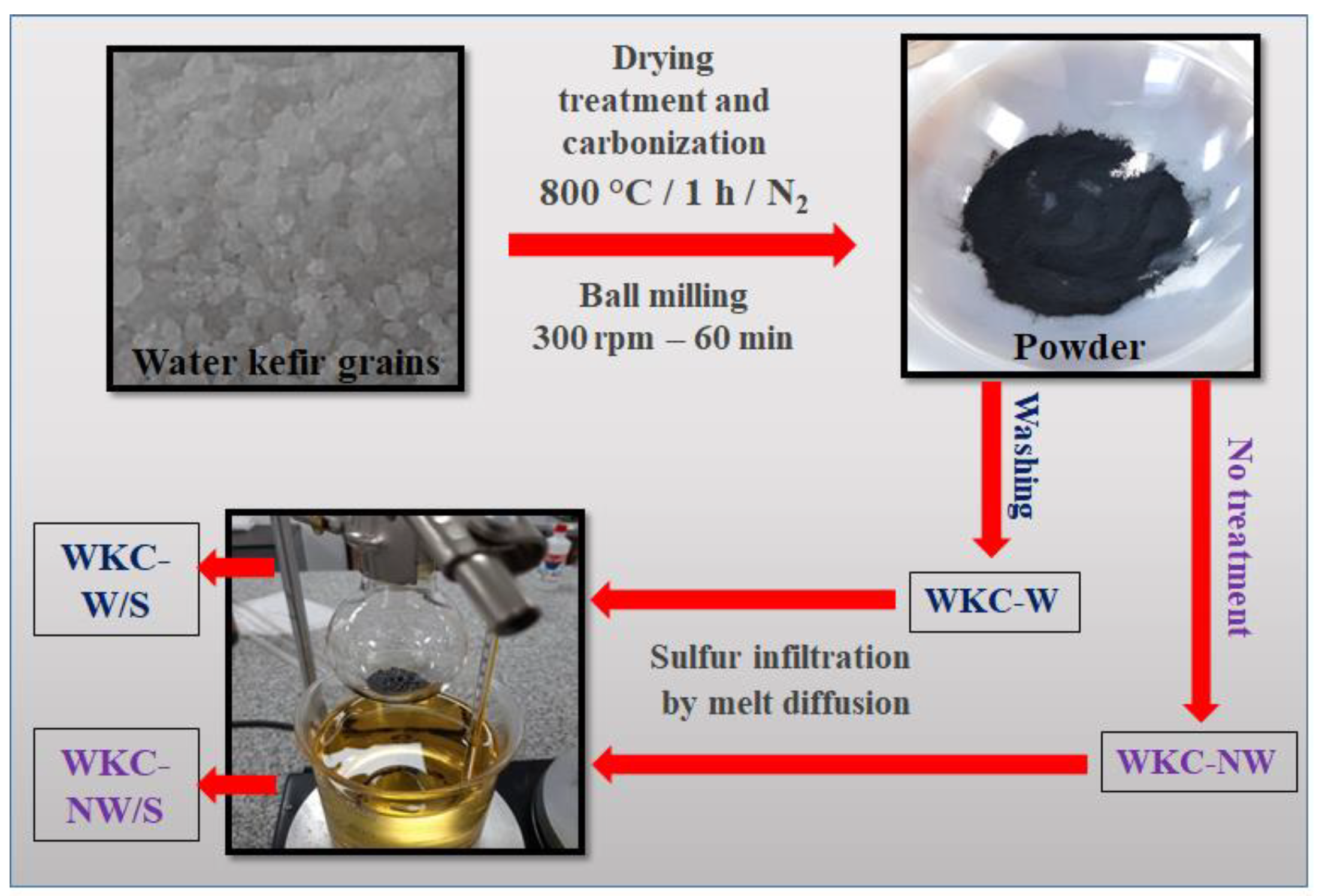
2.1. Preparation of Carbonaceous Material
2.2. Preparation of Carbon/Sulfur Composite
2.3. Water Kefir Carbon and Composite Material Characterization
2.4. Cathode Preparation
2.5. Electrochemical Characterization
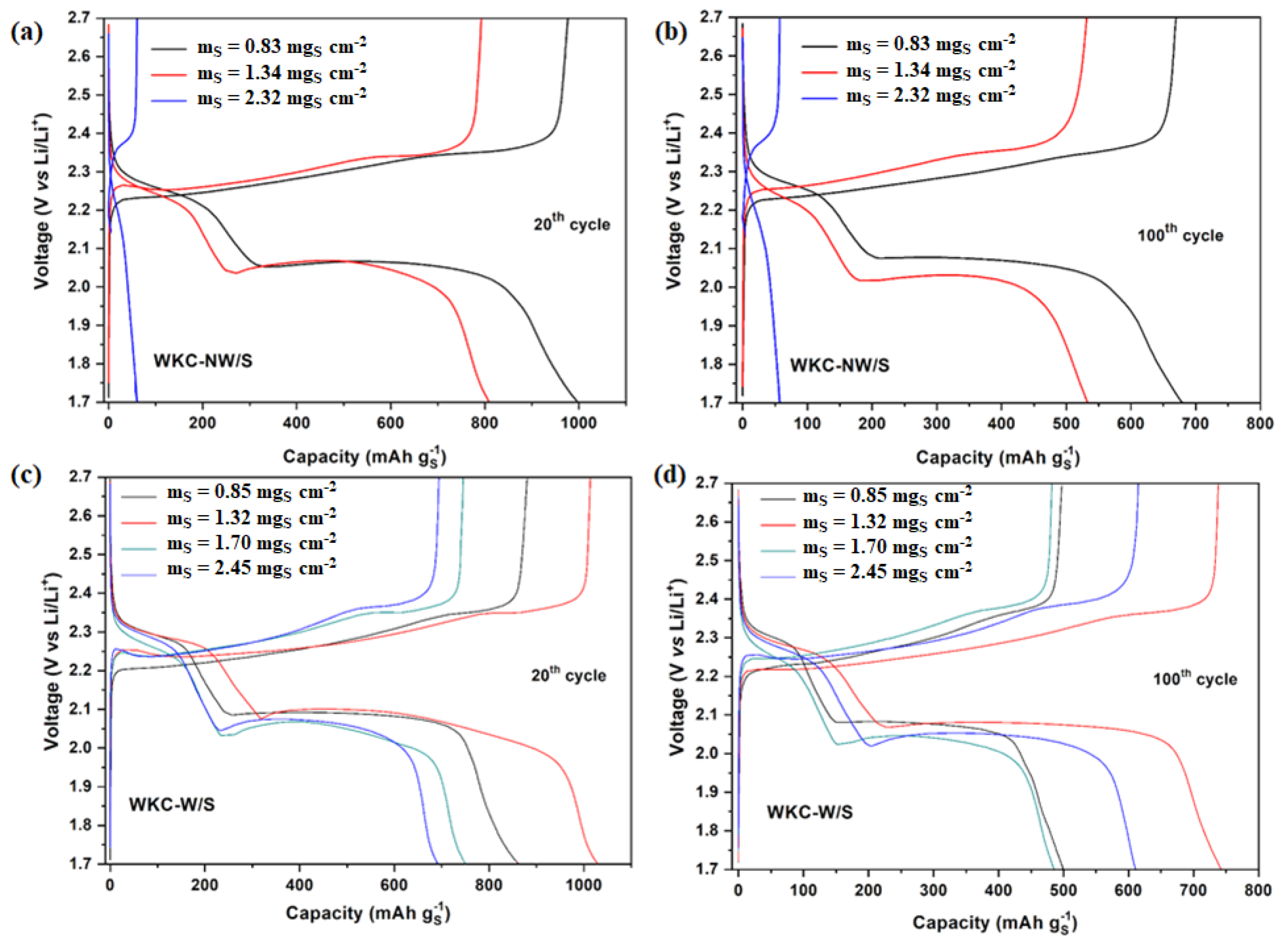
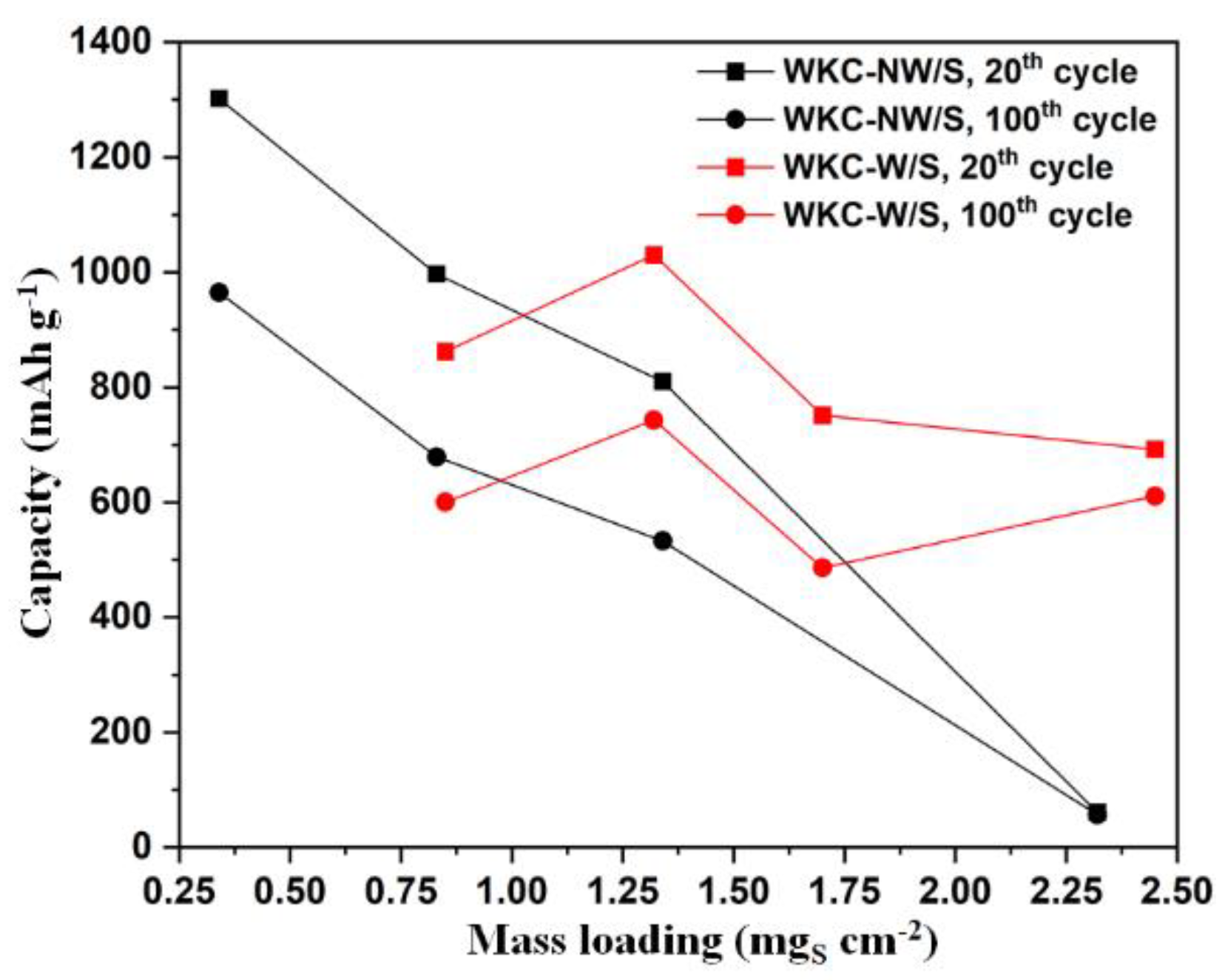
3. Results and Discussion
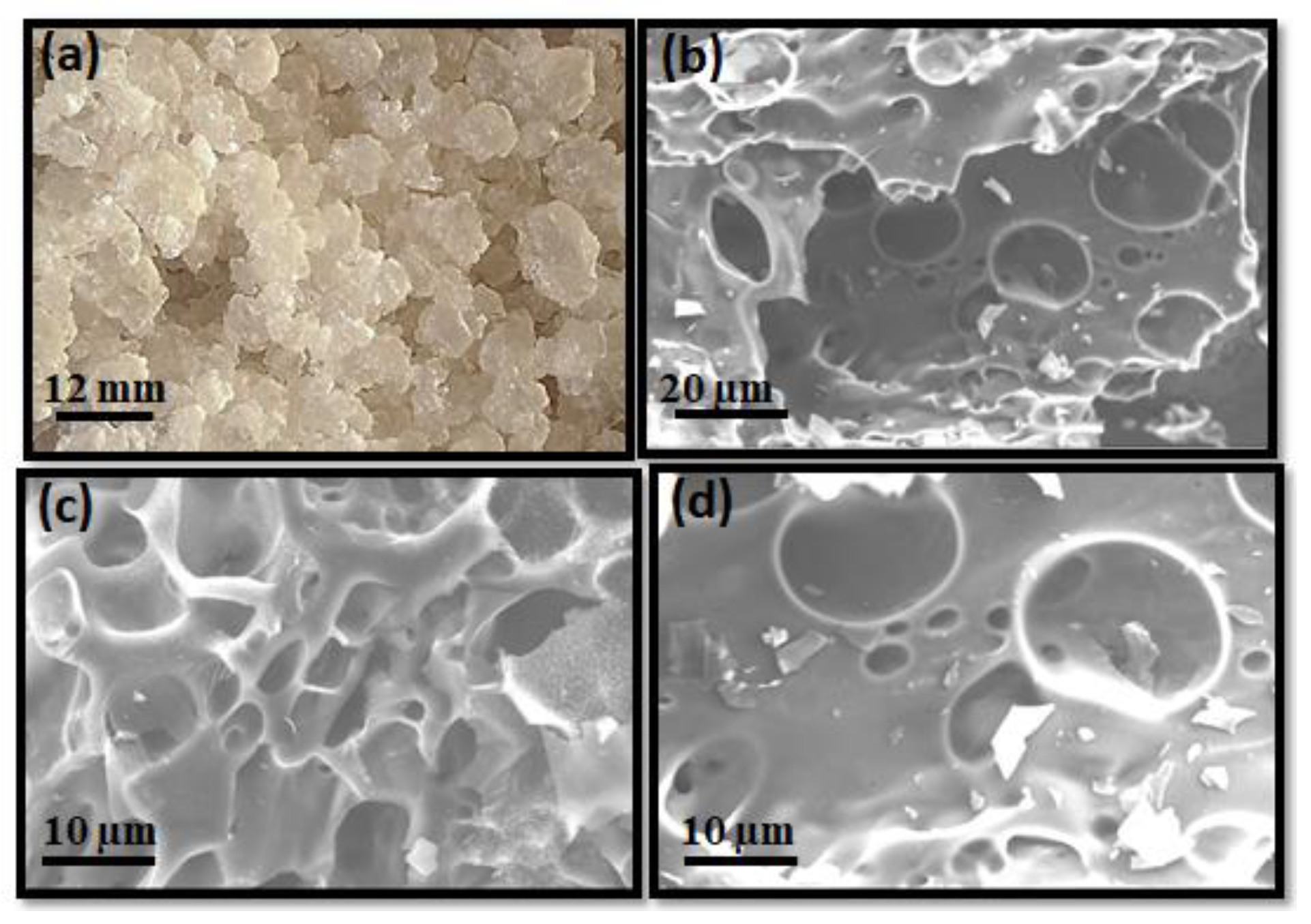
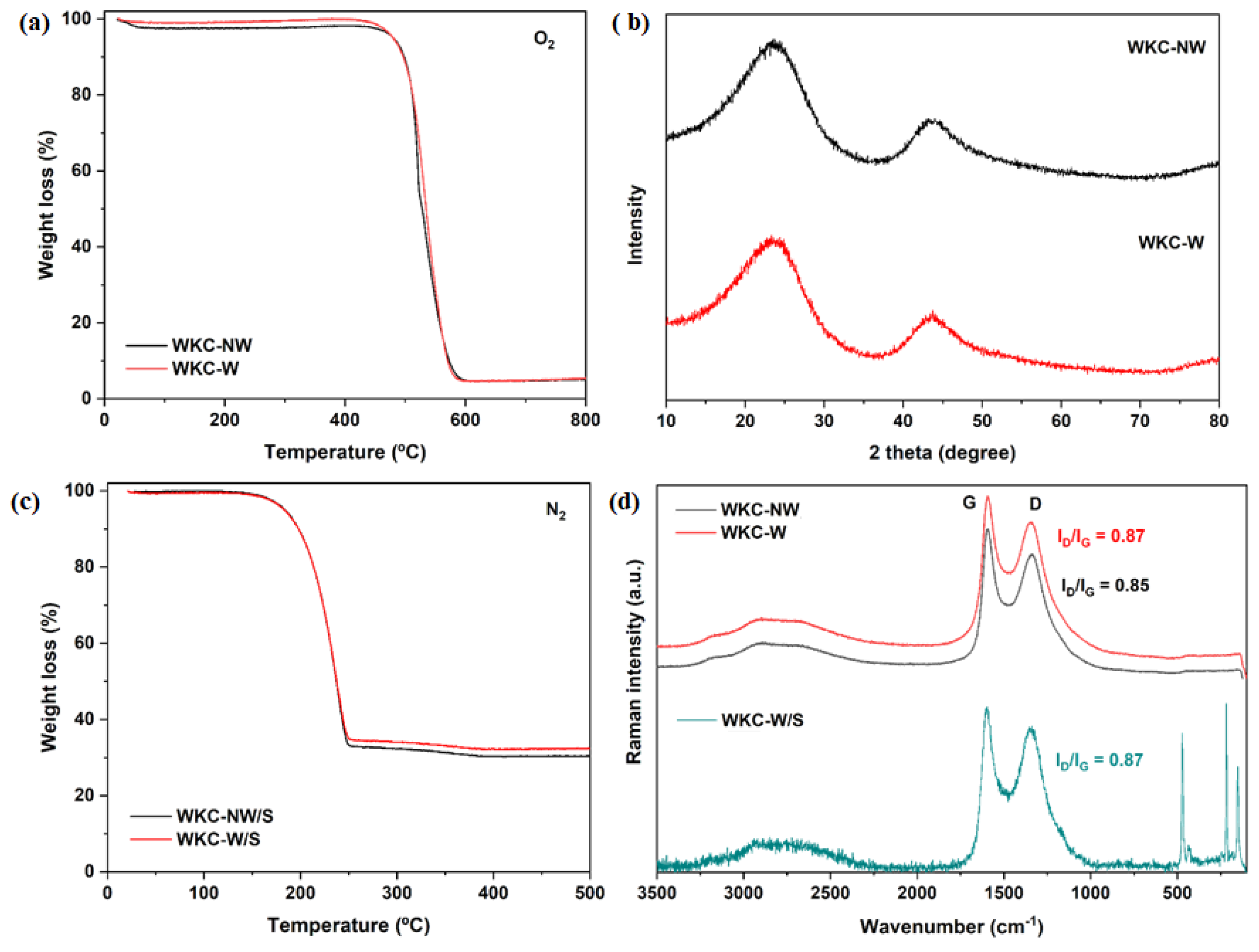
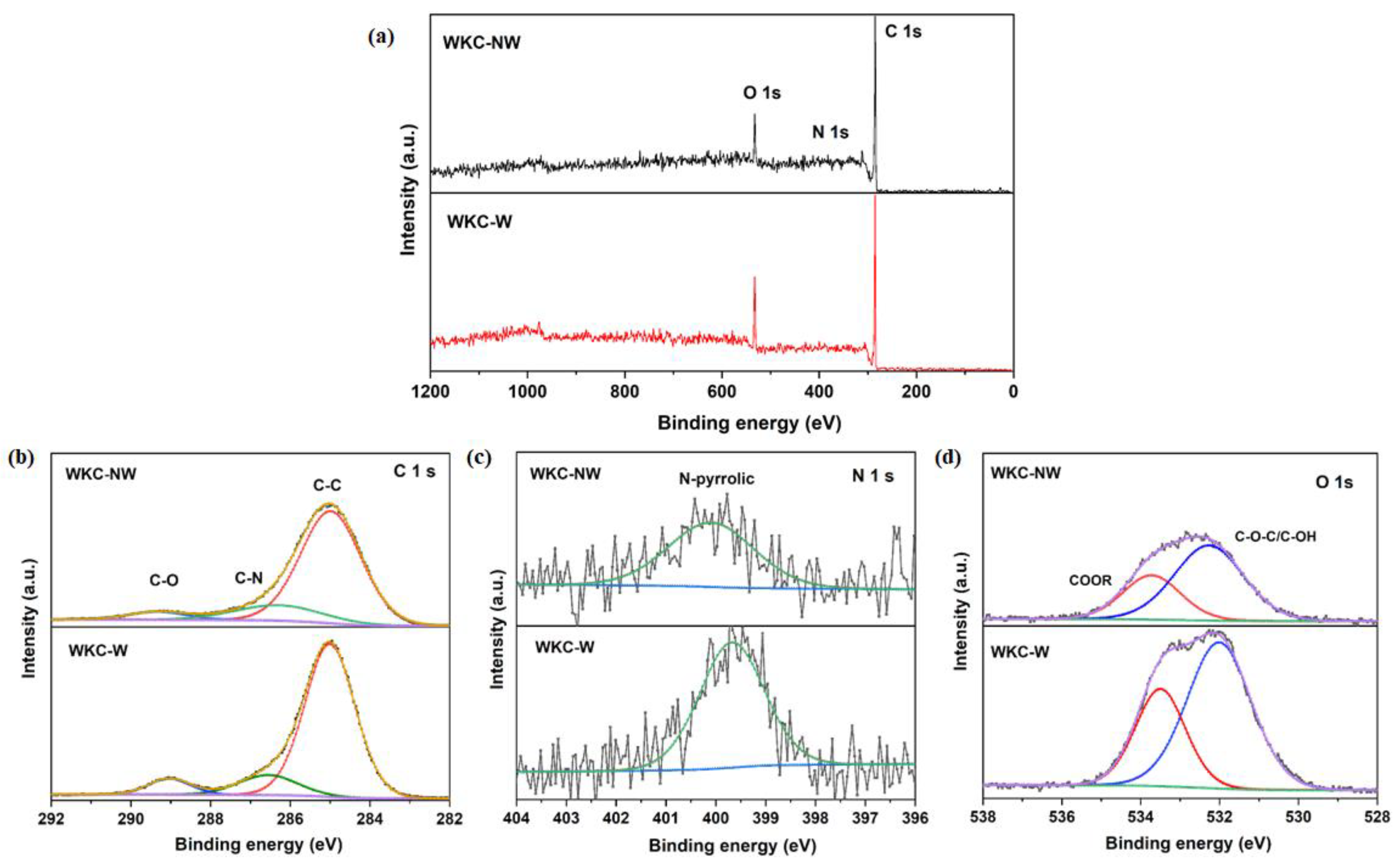
3.1. Structural, Morphological and Chemical Properties
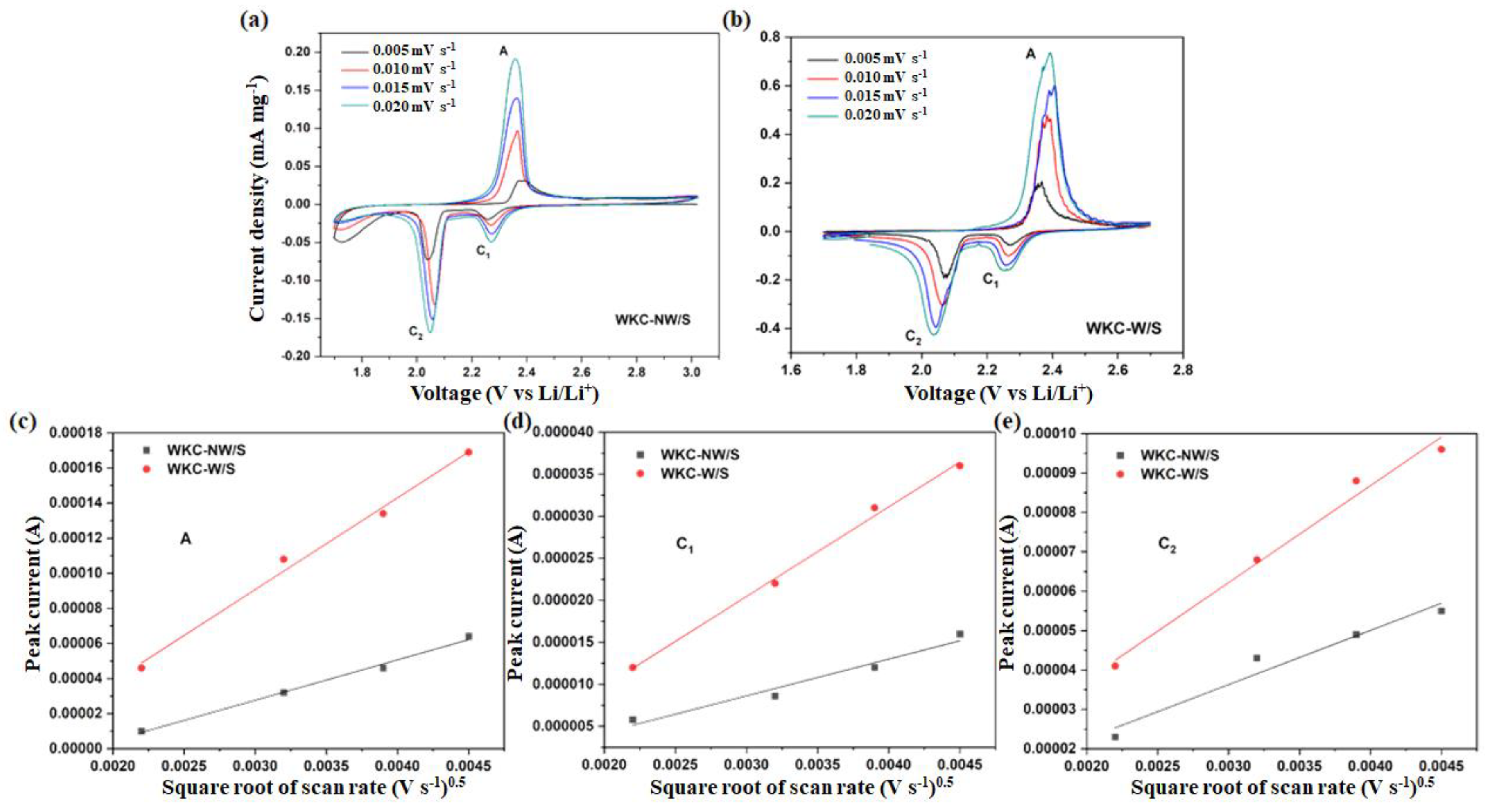
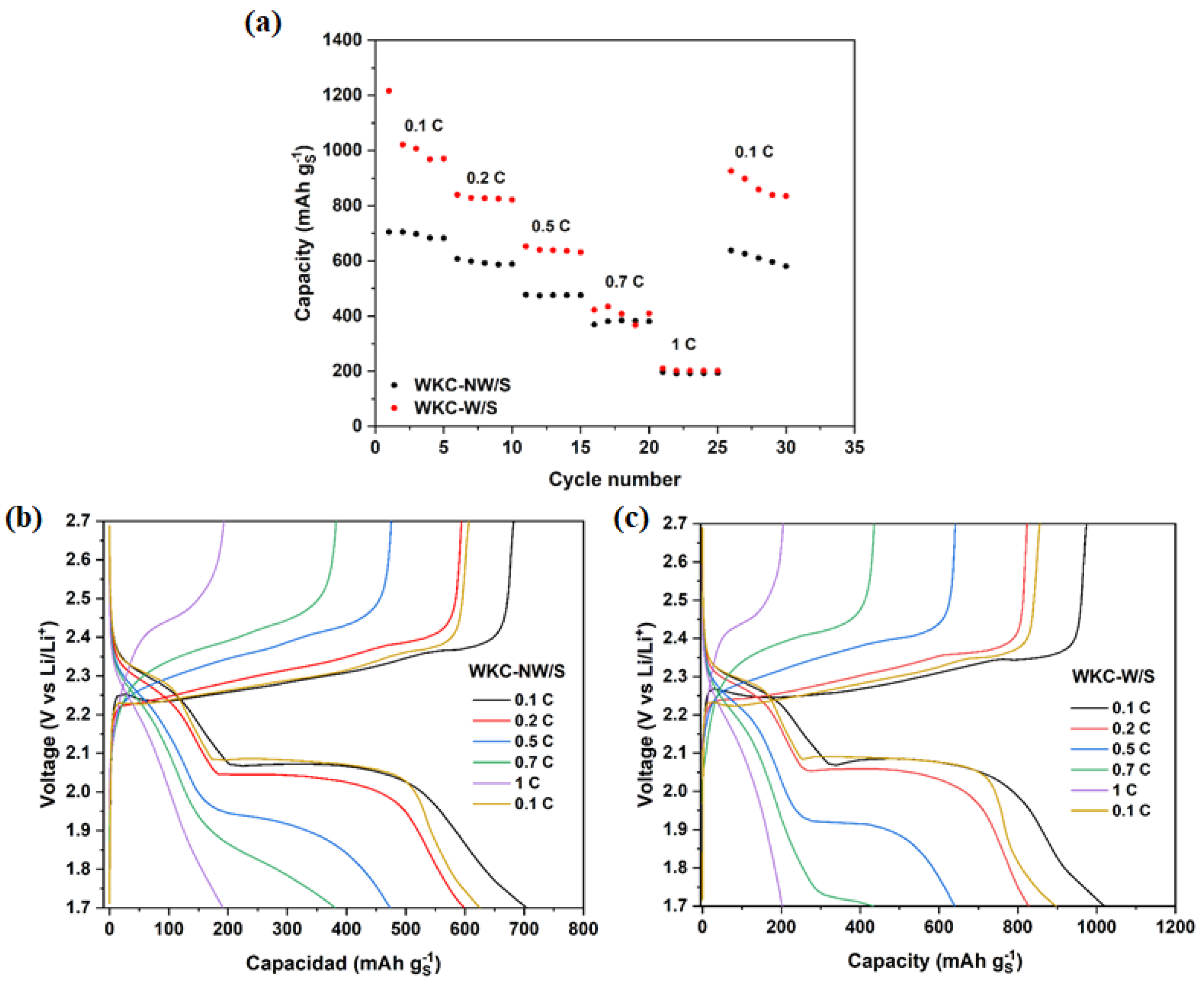
3.2. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abas, N.; Kalair, A.; Khan, N. Review of fossil fuels and future energy technologies. Futures 2015, 69, 31–49. [Google Scholar] [CrossRef]
- Canals Casals, L.; Martinez-Laserna, E.; Amante García, B.; Nieto, N. Sustainability analysis of the electric vehicle use in Europe for CO2 emissions reduction. J. Clean. Prod. 2016, 127, 425–437. [Google Scholar] [CrossRef]
- Yuan, J.; Dorn-Gomba, L.; Callegaro, A.D.; Reimers, J.; Emadi, A. A review of bidirectional on-board chargers for electric vehicles. IEEE Access 2021, 9, 51501–51518. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, B.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Lv, X.; Xie, Y.; Chen, B.; Hu, W.; et al. Battery Technologies for Grid-Level Large-Scale Electrical Energy Storage. Trans. Tianjin Univ. 2020, 26, 92–103. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Goutam, S.; Oliveira, L.M.; Timmermans, J.M.; Omar, N.; Messagie, M.; Van Den Bossche, P.; Van Mierlo, J. A comprehensive study on rechargeable energy storage technologies. J. Electrochem. Energy Convers. Storage 2016, 13, 1–107. [Google Scholar] [CrossRef]
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G.J. Lithium sulfur batteries, a mechanistic review. Energy Environ. Sci. 2015, 8, 3477–3494. [Google Scholar] [CrossRef]
- Shen, X.; Liu, H.; Cheng, X.B.; Yan, C.; Huang, J.Q. Beyond lithium ion batteries: Higher energy density battery systems based on lithium metal anodes. Energy Storage Mater. 2018, 12, 161–175. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Silva SR, P.; Ding, B.; Zhang, P.; Shao, G. Lithium–Sulfur Batteries Meet Electrospinning: Recent Advances and the Key Parameters for High Gravimetric and Volume Energy Density. Adv. Sci. 2022, 9, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Siczek, K.J. Introduction to Lithium-Sulfur Batteries. In Next-Generation Batteries with Sulfur Cathodes; Academic Press: Cambridge, MA, USA, 2019; pp. 5–13. [Google Scholar]
- Paez Jerez, A.L.; Davies, L.; Tesio, A.Y.; Flexer, V. Synergistic Combination of TiO2 and S-PAN for Li-S Batteries with Long-Term Cyclability at High C-Rates. J. Electrochem. Soc. 2021, 168, 120536. [Google Scholar] [CrossRef]
- Páez Jerez, A.L.; Chemes, D.M.; Sham, E.L.; Davies, L.E.; Tesio, A.Y.; Flexer, V. Low-Temperature Synthesis of a Sulfur-Polyacrylonitrile Composite Cathode for Lithium-Sulfur Batteries. ChemistrySelect 2020, 5, 5465–5472. [Google Scholar] [CrossRef]
- Arias, A.N.; Tesio, A.Y.; Flexer, V. Review—Non-Carbonaceous Materials as Cathodes for Lithium-Sulfur Batteries. J. Electrochem. Soc. 2018, 165, A6119-35. [Google Scholar] [CrossRef]
- Hou, R.; Zhang, S.; Zhang, Y.; Li, N.; Wang, S.; Ding, B.; Shao, G.; Zhang, P.A. “Three-Region” Configuration for Enhanced Electrochemical Kinetics and High-Areal Capacity Lithium–Sulfur Batteries. Adv. Funct. Mater. 2022, 32, 2200302. [Google Scholar] [CrossRef]
- Shan, Y.H.; Li, L.B.; Du, J.T.; Zhai, M. High performance lithium-sulfur batteries using three-dimensional hierarchical porous carbons to host the sulfur. Xinxing Tan Cailiao/New Carbon Mater. 2021, 36, 1094–1102. [Google Scholar] [CrossRef]
- Yu, C.H.; Yen, Y.J.; Chung, S.H. Nanoporosity of carbon–sulfur nanocomposites toward the lithium–sulfur battery electrochemistry. Nanomaterials 2021, 11, 1518. [Google Scholar] [CrossRef]
- Yen, Y.J.; Chung, S.H. Lean-electrolyte lithium-sulfur electrochemical cells with high-loading carbon nanotube/nanofiber-polysulfide cathodes. Chem. Commun. 2021, 57, 2009–2012. [Google Scholar] [CrossRef]
- Houghton, R.A.; Hole, W. Quantities of Biomass Importance. Gen. Ecol. 2008, 448–453. [Google Scholar]
- Wei, S.; Zhang, H.; Huang, Y.; Wang, W.; Xia, Y.; Yu, Z. Pig bone derived hierarchical porous carbon and its enhanced cycling performance of lithium-sulfur batteries. Energy Environ. Sci. 2011, 4, 736–740. [Google Scholar] [CrossRef]
- Benítez, A.; Amaro-Gahete, J.; Chien, Y.-C.; Caballero, Á.; Morales, J.; Brandell, D. Recent advances in lithium-sulfur batteries using biomass-derived carbons as sulfur host. Renew. Sustain. Energy Rev. 2022, 154, 111783. [Google Scholar] [CrossRef]
- Xia, L.; Zhou, Y.; Ren, J.; Wu, H.; Lin, D.; Xie, F.; Jie, W.; Lam, K.H.; Xu, C.; Zheng, Q. An Eco-friendly Microorganism Method to Activate Biomass for Cathode Materials for High-Performance Lithium-Sulfur Batteries. Energy Fuels 2018, 32, 9997–10007. [Google Scholar] [CrossRef]
- Wu, H.; Lin, J.; Mou, J.; Zheng, Q.; Lin, D. A sustainable hierarchical carbon derived from cultivated fibroid fungus for high performance lithium-sulfur batteries. RSC Adv. 2017, 7, 47407–47415. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; He, Y.; Liu, N.; Tan, T.; Liang, C. Biomass derived nitrogen-doped highly porous carbon material with a hierarchical porous structure for high-performance lithium/sulfur batteries. Materials 2017, 10, 1158. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cheng, Z.; Xie, T.; Dong, Z.; Liu, G. Cellulose-Derived Carbon Microfiber Mesh for Binder-Free Lithium—Sulfur Batteries. J. Nanosci. Nanotechnol. 2020, 20, 5629–5635. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhu, H.; Luo, W.; Shen, F.; Fan, X.; Dai, J.; Liang, Y.; Wang, C.; Hu, L. Atomic-Layer-Deposition Functionalized Carbonized Mesoporous Wood Fiber for High Sulfur Loading Lithium Sulfur Batteries. ACS Appl. Mater. Interfaces 2017, 9, 14801–14807. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Peng, H.J.; Tang, C.; Lieder, M.; Zhang, Q.; Titirici, M.M. Porous carbon derived from rice husks as sustainable bioresources: Insights into the role of micro-/mesoporous hierarchy in hosting active species for lithium-sulphur batteries. Green Chem. 2016, 18, 5169–5179. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, K.; Chen, F.; Chen, W.; Wei, X.; Liu, X.W.; Liu, J. Natural Integrated Carbon Architecture for Rechargeable Lithium-Sulfur Batteries. ACS Sustain. Chem. Eng. 2016, 4, 666–670. [Google Scholar] [CrossRef]
- Lynch, K.M.; Wilkinson, S.; Daenen, L.; Arendt, E. An update on water kefir: Microbiology, composition and production. Int. J. Food Microbiol. 2021, 345, 109128. [Google Scholar] [CrossRef]
- Egea, M.B.; Santos, D.C.D.; Oliveira Filho, J.G.; Ores, J.D.C.; Takeuchi, K.P.; Lemes, A.C. A review of nondairy kefir products: Their characteristics and potential human health benefits. Crit. Rev. Food Sci. Nutr. 2020, 62, 1–17. [Google Scholar] [CrossRef]
- Coma, M.E.; Peltzer, M.A.; Delgado, J.F.; Salvay, A.G. Water kefir grains as an innovative source of materials: Study of plasticiser content on film properties. Eur. Polym. J. 2019, 120, 109234. [Google Scholar] [CrossRef]
- Cottet, C.; Ramirez-Tapias, Y.A.; Delgado, J.F.; De la Osa, O.; Salvay, A.G.; Peltzer, M.A. Biobased Materials from Microbial Biomass and Its Derivatives. Materials 2020, 13, 1263. [Google Scholar] [CrossRef]
- Ferrari, A.; Vinderola, G.; Weill, R. (Eds.) Alimentos Fermentados: Microbiologia, Nutricion, Salud y Cultura; Instituto Danone del Cono Sur: Ciudad Autonoma de Buenos Airesed, Argentina, 2020. [Google Scholar]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, X.; Liang, J.; Hu, L.; Zhu, Y.; Qian, Y. A simple melting-diffusing-reacting strategy to fabricate S/NiS2-C for lithium-sulfur batteries. Nanoscale 2016, 8, 17616–17622. [Google Scholar] [CrossRef]
- Tesio, A.Y.; Gómez-Cámer, J.L.; Morales, J.; Caballero, A. Simple and Sustainable Preparation of Nonactivated Porous Carbon from Brewing Waste for High-Performance Lithium–Sulfur Batteries. ChemSusChem 2020, 13, 3439–3446. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A. Films Delgados Mesoporosos Híbridos Conteniendo el Grupo Amino: Una Plataforma Para el Diseño y Producción de Membranas Permeoselectivas; Universidad Nacional de General San Martín Instituto de Investigación e Ingeniería Ambiental: Villa Lynch, Argentina, 2010. [Google Scholar]
- Liu, P.; Wang, Y.; Liu, J. Biomass-derived porous carbon materials for advanced lithium sulfur batteries. J. Energy Chem. 2019, 34, 171–185. [Google Scholar] [CrossRef]
- Kong, L.; Tian, S.; Luo, R.; Liu, W.; Tu, Y.; Xiong, Y. Demineralization of sludge-based adsorbent by post-washing for development of porosity and removal of dyes. J. Chem. Technol. Biotechnol. 2013, 88, 1473–1480. [Google Scholar] [CrossRef]
- Kwon, G.; Bhatnagar, A.; Wang, H.; Kwon, E.E.; Song, H. A review of recent advancements in utilization of biomass and industrial wastes into engineered biochar. J. Hazard. Mater. 2020, 400, 123242. [Google Scholar] [CrossRef]
- Ahmedna, M.; Marshall, W.E.; Rao, R.M. Surface properties of granular activated carbons from agricultural by-products and their effects on raw sugar decolorization. Bioresour. Technol. 2000, 71, 103–112. [Google Scholar] [CrossRef]
- Popova, A.N. Crystallographic analysis of graphite by X-ray diffraction. Coke Chem. 2017, 60, 361–365. [Google Scholar] [CrossRef]
- Moreno, N.; Caballero, A.; Hernán, L.; Morales, J. Lithium-sulfur batteries with activated carbons derived from olive stones. Carbon N. Y. 2014, 70, 241–248. [Google Scholar] [CrossRef]
- Steudel, R.; Eckert, B. Elemental Sulfur and Sulfur-Rich Compounds ed R Steudel; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–80. [Google Scholar]
- Arias, A.N.; Villarroel-Rocha, J.; Sapag, K.; Mori, M.F.; Planes, G.A.; Tesio, A.Y.; Flexer, V. High nitrogen content carbons: Morphological and chemical changes with synthesis temperature and application in lithium–sulfur batteries. Electrochim. Acta 2020, 359, 136942. [Google Scholar] [CrossRef]
- Zhong, M.e.; Guan, J.; Sun, J.; Guo, H.; Xiao, Z.; Zhou, N.; Gui, Q.; Gong, D. Carbon nanodot-decorated alveolate N, O, S tridoped hierarchical porous carbon as efficient electrocatalysis of polysulfide conversion for lithium-sulfur batteries. Electrochim. Acta 2019, 299, 600–609. [Google Scholar] [CrossRef]
- Ren, J.; Zhou, Y.; Wu, H.; Xie, F.; Xu, C.; Lin, D. Sulfur-encapsulated in heteroatom-doped hierarchical porous carbon derived from goat hair for high performance lithium–sulfur batteries. J. Energy Chem. 2019, 30, 121–131. [Google Scholar] [CrossRef]
- Chen, F.; Yang, J.; Bai, T.; Long, B.; Zhou, X. Biomass waste-derived honeycomb-like nitrogen and oxygen dual-doped porous carbon for high performance lithium-sulfur batteries. Electrochim. Acta 2016, 192, 99–109. [Google Scholar] [CrossRef]
- Zhang, S.S. Sulfurized carbon: A class of cathode materials for high performance lithium/sulfur batteries. Front. Energy Res. 2013, 1, 1–9. [Google Scholar] [CrossRef]
- Luna-Lama, F.; Caballero, A.; Morales, J. Synergistic effect between PPy:PSS copolymers and biomass-derived activated carbons: A simple strategy for designing sustainable high-performance Li–S batteries. Sustain. Energy Fuels 2022, 6, 1568–1586. [Google Scholar] [CrossRef]
- Marangon, V.; Di Lecce, D.; Orsatti, F.; Brett DJ, L.; Shearing, P.R.; Hassoun, J. Investigating high-performance sulfur-metal nanocomposites for lithium batteries. Sustain. Energy Fuels 2020, 4, 2907–2923. [Google Scholar] [CrossRef]
- Pu, J.; Wang, Z.; Xue, P.; Zhu, K.; Li, J.; Yao, Y. The effect of NiO-Ni3N interfaces in in-situ formed heterostructure ultrafine nanoparticles on enhanced polysulfide regulation in lithium-sulfur batteries. J. Energy Chem. 2021, 68, 762–770. [Google Scholar] [CrossRef]
- Li, W.; Qian, J.; Zhao, T.; Ye, Y.; Xing, Y.; Huang, Y.; Wei, L.; Zhang, N.; Chen, N.; Li, L.; et al. Boosting High-Rate Li–S Batteries by an MOF-Derived Catalytic Electrode with a Layer-by-Layer Structure. Adv. Sci. 2019, 6, 1802362. [Google Scholar] [CrossRef]
- Ma, Z.; Sui, W.; Liu, J.; Wang, W.; Li, S.; Chen, T.; Yang, G.; Zhu, K.; Li, Z. Pomelo peel-derived porous carbon as excellent LiPS anchor in lithium-sulfur batteries. J. Solid State Electrochem. 2022, 26, 973–984. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, B.; Guo, P.; Shang, X.; Lv, M.; Liu, D.; He, D. Enhanced electrochemical kinetics in lithium-sulfur batteries by using carbon nanofibers/manganese dioxide composite as a bifunctional coating on sulfur cathode. Electrochim. Acta 2018, 269, 180–187. [Google Scholar] [CrossRef]
- Long, J.; Zhang, H.; Ren, J.; Li, J.; Zhu, M.; Han, T.; Sun, B.; Zhu, S.; Zhang, H.; Liu, J. A metal organic foam-derived multi-layered and porous copper sulfide scaffold as sulfur host with multiple shields for preventing shuttle effect in lithium-sulfur batteries. Electrochim. Acta 2020, 356, 136853. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Z.; Zhang, Y.; Wang, B.; Peng, H. The recent progress of nitrogen-doped carbon nanomaterials for electrochemical batteries. J. Mater. Chem. A 2018, 6, 12932–12944. [Google Scholar] [CrossRef]
- Rana, M.; Luo, B.; Kaiser, M.R.; Gentle, I.; Knibbe, R. The role of functional materials to produce high areal capacity lithium sulfur battery. J. Energy Chem. 2020, 42, 195–209. [Google Scholar] [CrossRef]
- Hippauf, F.; Nickel, W.; Hao, G.P.; Schwedtmann, K.; Giebeler, L.; Oswald, S.; Borchardt, L.; Doerfler, S.; Weigand, J.J.; Kaskel, S. The Importance of Pore Size and Surface Polarity for Polysulfide Adsorption in Lithium Sulfur Batteries. Adv. Mater. Interfaces 2016, 3, 1–9. [Google Scholar] [CrossRef]
- Xie, Y.; Fang, L.; Cheng, H.; Hu, C.; Zhao, H.; Xu, J.; Fang, J.; Lu, X.; Zhang, J. Biological cell derived N-doped hollow porous carbon microspheres for lithium-sulfur batteries. J. Mater. Chem. A 2016, 4, 15612–15620. [Google Scholar] [CrossRef]
- Xia, Y.; Fang, R.; Xiao, Z.; Huang, H.; Gan, Y.; Yan, R.; Lu, X.; Liang, C.; Zhang, J.; Tao, X.; et al. Confining Sulfur in N-Doped Porous Carbon Microspheres Derived from Microalgaes for Advanced Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2017, 9, 23782–23791. [Google Scholar] [CrossRef]
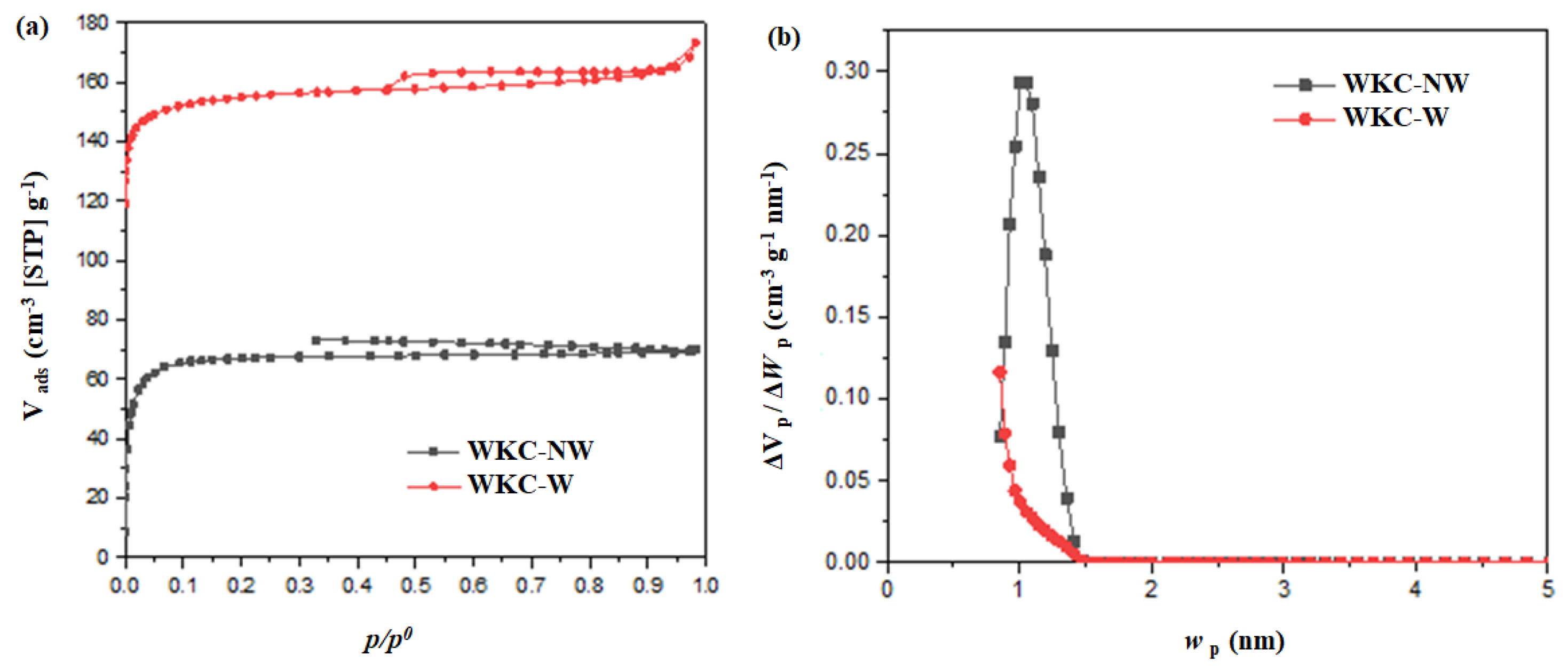

| Sample | SBET (m2 g−1) | VµP (cm3 g−1) | VTP (cm3 g−1) |
|---|---|---|---|
| WKC-NW | 270 | 0.10 | 0.11 |
| WKC-W | 630 | 0.23 | 0.27 |
| Li+ Diffusion Coefficient D (cm2 s−1) | ||
|---|---|---|
| WKC-NW/S | WKC-W/S | |
| Peak A | 5.30 × 10−9 | 2.20 × 10−8 |
| Peak C1 | 1.53 × 10−9 | 7.50 × 10−9 |
| Peak C2 | 1.51 × 10−8 | 3.96 × 10−8 |
| Sulfur Host | SBET (m2 g−1) | Sulfur Content (%) | Mass Loading (mgS cm2) | Discharge Capacity (mAh g−1) | Current Density/Cycle Number | Ref. |
|---|---|---|---|---|---|---|
| Banana peels with yeast | 112 | 74 | 1.3 | 700 | 0.1 C/100 | [21] |
| Green algae | 101 | 63 | 3.5 | 757 | 0.1 C/100 | [23] |
| Fungus * | 509 | 52 | 1.5–1.6 | 663 | 0.1 C/100 | [22] |
| Microalgaes | 671 | 65 | 1.7–2.0 | 1031 | 0.06 C/100 | [60] |
| Yeast | 721 | 65 | Not specified | 725 | 0.1 C/400 | [59] |
| Rice husk | 525 | 56 | 1.0 | 600 | 0.1 C/500 | [26] |
| Wood microfiber | 586 | 70 | 1.3 | 859 | 0.2 C/450 | [25] |
| Bark of plane trees | 528 | 48 | 3.2–4.2 | 608 | 0.1 C/60 | [27] |
| Filter paper | 581 | Not specified | 1.9 | 500 | 0.2 C/100 | [24] |
| Water kefir carbon | 630 | 68 | 1.3 2.5 | 743 611 | 0.1 C/100 0.1 C/100 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Páez Jerez, A.L.; Mori, M.F.; Flexer, V.; Tesio, A.Y. Water Kefir Grains—Microbial Biomass Source for Carbonaceous Materials Used as Sulfur-Host Cathode in Li-S Batteries. Materials 2022, 15, 8856. https://doi.org/10.3390/ma15248856
Páez Jerez AL, Mori MF, Flexer V, Tesio AY. Water Kefir Grains—Microbial Biomass Source for Carbonaceous Materials Used as Sulfur-Host Cathode in Li-S Batteries. Materials. 2022; 15(24):8856. https://doi.org/10.3390/ma15248856
Chicago/Turabian StylePáez Jerez, Ana L., M. Fernanda Mori, Victoria Flexer, and Alvaro Y. Tesio. 2022. "Water Kefir Grains—Microbial Biomass Source for Carbonaceous Materials Used as Sulfur-Host Cathode in Li-S Batteries" Materials 15, no. 24: 8856. https://doi.org/10.3390/ma15248856
APA StylePáez Jerez, A. L., Mori, M. F., Flexer, V., & Tesio, A. Y. (2022). Water Kefir Grains—Microbial Biomass Source for Carbonaceous Materials Used as Sulfur-Host Cathode in Li-S Batteries. Materials, 15(24), 8856. https://doi.org/10.3390/ma15248856








