The Effect of Pyrolysis Temperature and the Source Biomass on the Properties of Biochar Produced for the Agronomical Applications as the Soil Conditioner
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Pyrolysis of Biomass Feedstocks
2.2.2. Thermogravimetry
2.2.3. Elemental Analysis
2.2.4. Conductivity and pH of Aqueous Extract
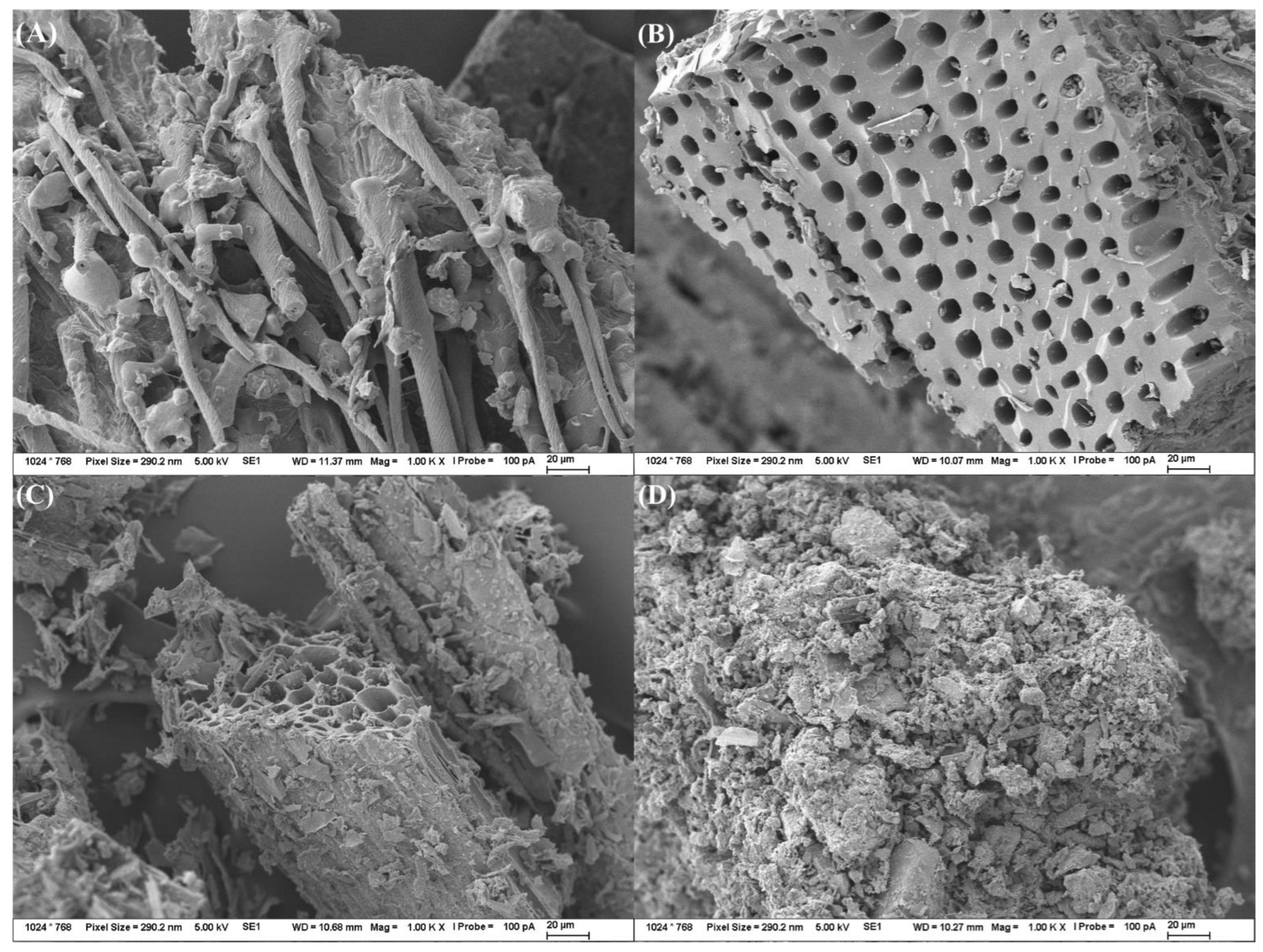
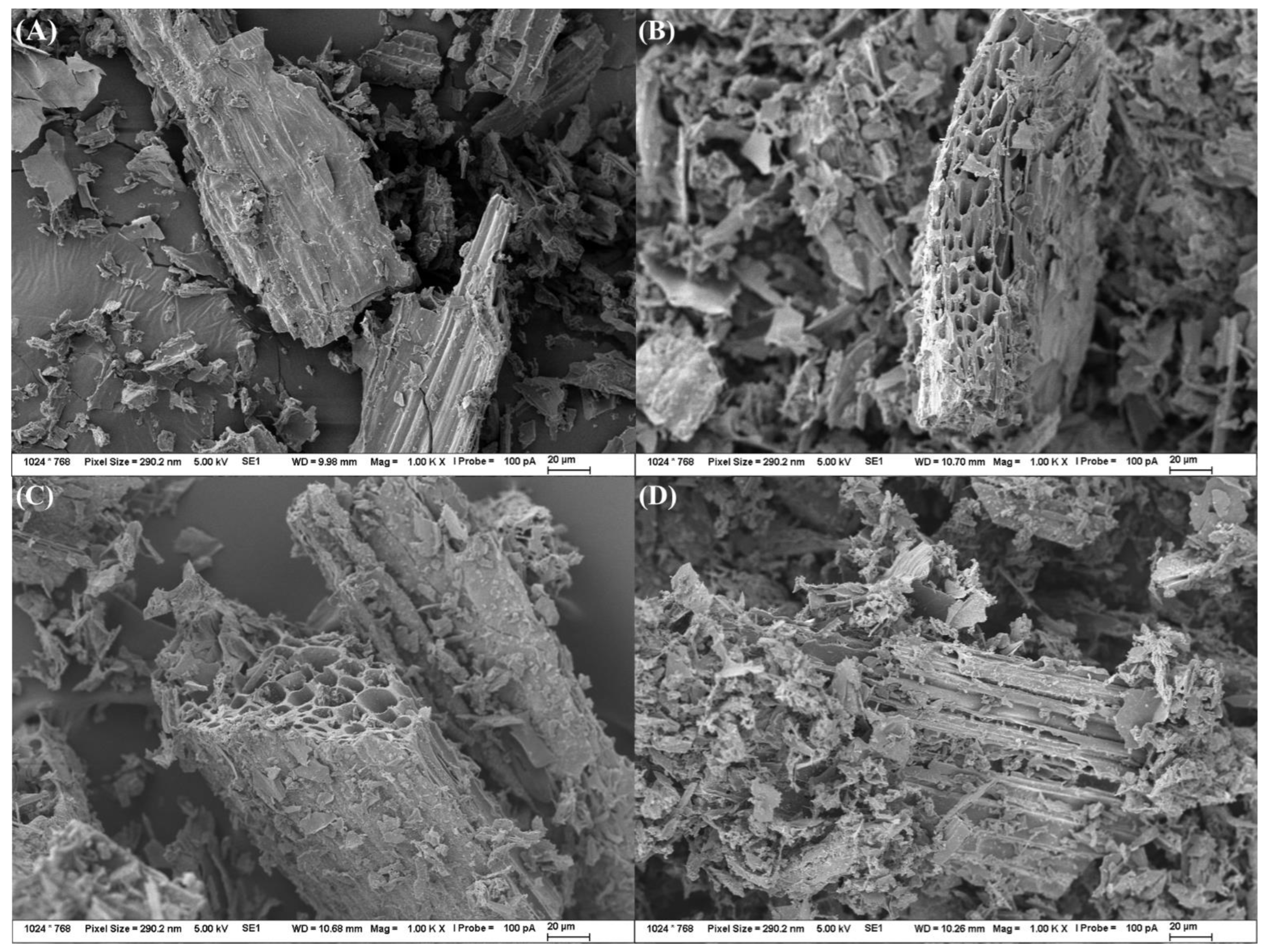
2.2.5. Morphological Characterization
2.2.6. Water Holding Capacity
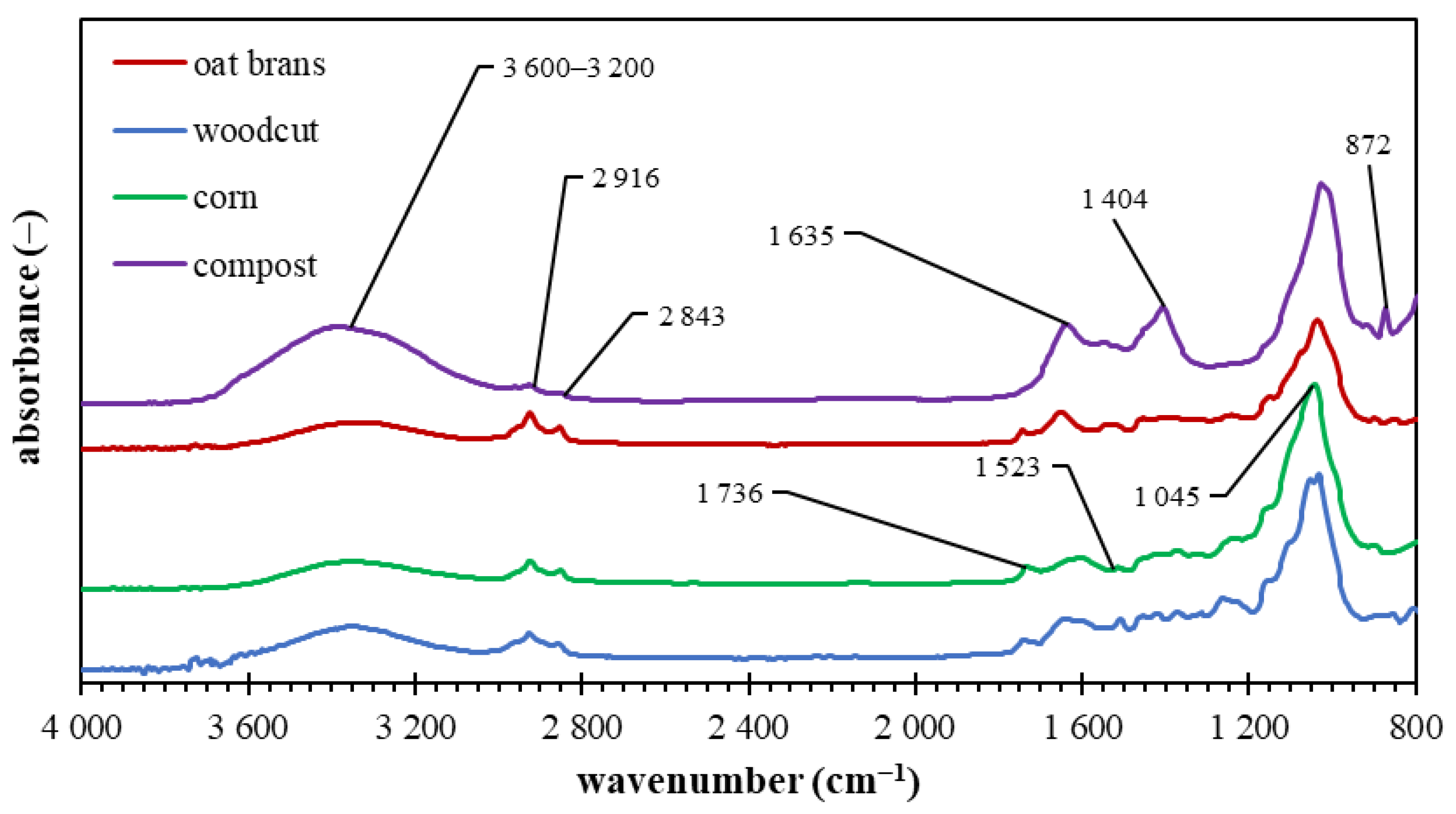
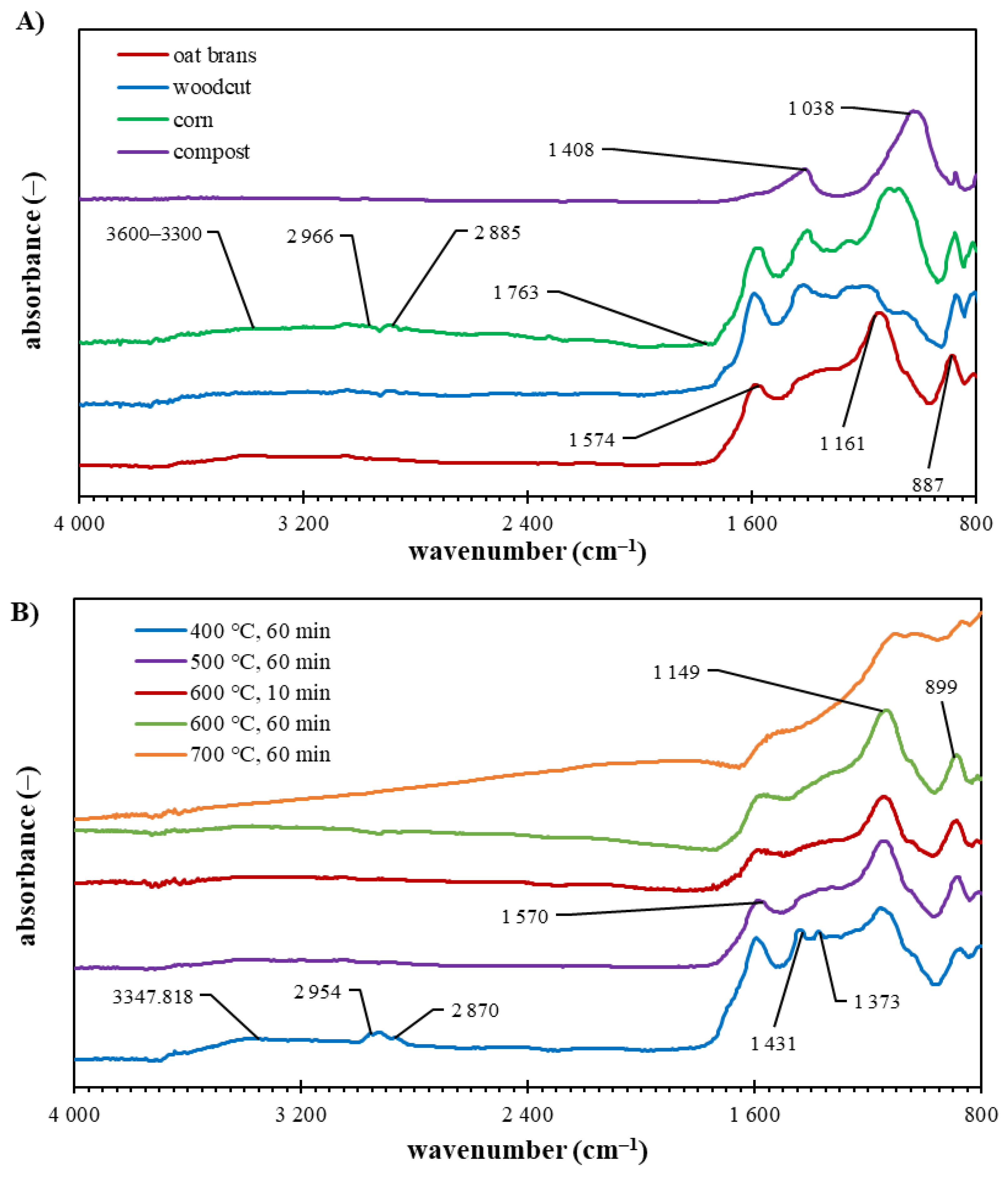
2.2.7. FTIR Spectrometry
2.2.8. Statistical Analysis
3. Results
3.1. Biomass Characterization
3.2. Biochar Yield
3.3. Biochar Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Verheijen, F.; Jeffery, S.; Bastos, A.; Velde, M.; Diafas, I. Biochar Application to Soils—A Critical Scientific Review of Effects on Soil Properties; Processes and Functions; European Commission: Luxembourg, 2010. [Google Scholar]
- EBC. European Biochar Certificate-Guidelines for a Sustainable Production of Biochar (2012–2022); Version 10.0; European Biochar Foundation (EBC): Arbaz, Switzerland; Available online: http://european-biochar.org (accessed on 1 January 2022).
- Supriya, A.; Samantray, R.; Mishra, S.C. Biochar, a substitute for fossil fuels. IOP Conf. Ser. Mater. Sci. Eng. 2017, 178, 012022. [Google Scholar] [CrossRef]
- Kumar, A.; Joseph, S.; Tsechansky, L.; Schreiter, I.J.; Schüth, C.; Taherysoosavi, S.; Mitchell, D.R.G.; Graber, E.R. Mechanistic evaluation of biochar potential for plant growth promotion and alleviation of chromium-induced phytotoxicity in Ficus elastica. Chemosphere 2020, 243, 125332. [Google Scholar] [CrossRef] [PubMed]
- Kalina, M.; Sovova, S.; Hajzler, J.; Kubikova, L.; Trudicova, M.; Smilek, J.; Enev, V. Biochar Texture—A Parameter Influencing Physicochemical Properties, Morphology, and Agronomical Potential. Agronomy 2022, 12, 1768. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sust. Energ. Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Ali, L.; Palamanit, A.; Techato, K.; Ullah, A.; Chowdhury, M.S.; Phoungthong, K. Characteristics of Biochars Derived from the Pyrolysis and Co-Pyrolysis of Rubberwood Sawdust and Sewage Sludge for Further Applications. Sustainability 2022, 14, 3829. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Huang, H.; Xiao, G. Comparison of non-catalytic and catalytic fast pyrolysis of corncob in a fluidized bed reactor. Bioresour. Technol. 2009, 100, 1428–1434. [Google Scholar] [CrossRef]
- Li, J.; Yu, G.; Pan, L.; Li, C.; You, F.; Xie, S.; Wang, Y.; Ma, J.; Shang, X. Study of ciprofloxacin removal by biochar obtained from used tea leaves. J. Environ. Sci. 2018, 73, 20–30. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, M.; Ren, H. Biochar produced from the co-pyrolysis of sewage sludge and walnut shell for ammonium and phosphate adsorption from water. J. Environ. Manage. 2019, 249, 109410. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Qian, T.-T.; Wu, P.; Qin, Q.-Y.; Huang, Y.-N.; Wang, Y.-J.; Zhou, D.-M. Screening of wheat straw biochars for the remediation of soils polluted with Zn (II) and Cd (II). J. Hazard. Mater. 2019, 362, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, T.; Kotroczó, Z.; Kardos, L.; Biró, B. Optimization of increasing biochar doses with soil–plant–microbial functioning and nutrient uptake of maize. Environ. Technol. Innov. 2020, 20, 101191. [Google Scholar] [CrossRef]
- Robb, S.; Joseph, S.; Abdul Aziz, A.; Dargusch, P.; Tisdell, C. Biochar’s cost constraints are overcome in small-scale farming on tropical soils in lower-income countries. Land. Degrad. Dev. 2020, 31, 1713–1726. [Google Scholar] [CrossRef]
- Clare, A.; Shackley, S.; Joseph, S.; Hammond, J.; Pan, G.; Bloom, A. Competing uses for China’s straw: The economic and carbon abatement potential of biochar. GCB Bioenergy 2015, 7, 1272–1282. [Google Scholar] [CrossRef]
- Chew, J.; Zhu, L.; Nielsen, S.; Graber, E.; Mitchell, D.R.G.; Horvat, J.; Mohammed, M.; Liu, M.; van Zwieten, L.; Donne, S.; et al. Biochar-based fertilizer: Supercharging root membrane potential and biomass yield of rice. Sci. Total Environ. 2020, 713, 136431. [Google Scholar] [CrossRef]
- Amalina, F.; Syukor Abd Razak, A.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Advanced techniques in the production of biochar from lignocellulosic biomass and environmental applications. Clean. Mater. 2022, 6, 100137. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Beusch, C. Biochar as a Soil Ameliorant: How Biochar Properties Benefit Soil Fertility—A Review. J. Geosci. Environ. Prot. 2021, 9, 28–46. [Google Scholar] [CrossRef]
- Wijitkosum, S.; Jiwnok, P. Elemental Composition of Biochar Obtained from Agricultural Waste for Soil Amendment and Carbon Sequestration. Appl. Sci. 2019, 9, 3980. [Google Scholar] [CrossRef]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; et al. Characterization of Designer Biochar Produced at Different Temperatures and Their Effects on a Loamy Sand. Ann. Civ. Environ. Eng. 2009, 3, 195–206. [Google Scholar]
- Gezahegn, S.; Sain, M.; Thomas, S. Variation in Feedstock Wood Chemistry Strongly Influences Biochar Liming Potential. Soil Syst. 2019, 3, 26. [Google Scholar] [CrossRef]
- Sovova, S.; Enev, V.; Smilek, J.; Kubikova, L.; Trudicova, M.; Hajzler, J.; Kalina, M. The effect of biochar application on soil properties and growth of the model plant Zea mays. Ecocycles 2021, 7, 46–54. [Google Scholar] [CrossRef]
- Veksha, A.; McLaughlin, H.; Layzell, D.B.; Hill, J.M. Pyrolysis of wood to biochar: Increasing yield while maintaining microporosity. Bioresour. Technol. 2014, 153, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zong, Y. Pore structure and environmental serves of biochars derived from different feedstocks and pyrolysis conditions. Environ. Sci. Pollut. R. 2018, 25, 30401–30409. [Google Scholar] [CrossRef]
- Méndez, A.; Terradillos, M.; Gascó, G. Physicochemical and agronomic properties of biochar from sewage sludge pyrolysed at different temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 124–130. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, B.; Budai, A.; Jeng, A.; Hao, X.; Wei, D.; Zhang, Y.; Rasse, D. Study of Biochar Properties by Scanning Electron Microscope—Energy Dispersive X-Ray Spectroscopy (SEM-EDX). Commun. Soil Sci. Plant Anal. 2016, 47, 593–601. [Google Scholar] [CrossRef]
- Xu, G.; Wei, L.L.; Sun, J.N.; Shao, H.B.; Chang, S.X. What is more important for enhancing nutrient bioavailability with biochar application into a sandy soil: Direct or indirect mechanism? Ecol. Eng. 2013, 52, 119–124. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Chen, W.; Yang, H.; Chen, H. The Structure Evolution of Biochar from Biomass Pyrolysis and Its Correlation with Gas Pollutant Adsorption Performance. Bioresour. Technol. 2017, 246, 101–109. [Google Scholar] [CrossRef]
- Werdin, J.; Fletcher, T.D.; Rayner, J.P.; Williams, N.S.G.; Farrell, C. Biochar made from low density wood has greater plant available water than biochar made from high density wood. Sci. Total Environ. 2020, 705, 135856. [Google Scholar] [CrossRef] [PubMed]
- Suo, F.; You, X.; Yin, S.; Wu, H.; Zhang, C.; Yu, X.; Sun, R.; Li, Y. Preparation and characterization of biochar derived from co-pyrolysis of Enteromorpha prolifera and corn straw and its potential as a soil amendment. Sci. Total Environ. 2021, 798, 149167. [Google Scholar] [CrossRef] [PubMed]
| Biomass Feedstock | Pyrolysis | Yield (wt. %) | Organic Matter (wt. %) | C (wt.%) | H (wt.%) | N (wt.%) | H/C (–) * | |
|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | Time (min) | |||||||
| oat brans | 700 | 60 | 26.6 | 77.2 | 72.7 ± 0.8 | 0.4 ± 0.1 | 3.7 ± 0.2 | 0.057 |
| 600 | 10 | 29.7 | 79.6 | 82.4 ± 0.9 | 2.8 ± 0.2 | 4.4 ± 0.3 | 0.398 | |
| 600 | 60 | 24.7 | 77.2 | 72.8 ± 0.5 | 1.6 ± 0.2 | 3.6 ± 0.3 | 0.257 | |
| 500 | 60 | 31.3 | 76.5 | 70.6 ± 0.4 | 3.0 ± 0.1 | 4.5 ± 0.1 | 0.508 | |
| 400 | 60 | 33.0 | 81.3 | 67.8 ± 0.7 | 5.2 ± 0.2 | 4.7 ± 0.1 | 0.908 | |
| mixed woodcut | 700 | 60 | 25.8 | 94.2 | 88.6 ± 0.3 | 0.6 ± 0.1 | 1.2 ± 0.1 | 0.085 |
| 600 | 10 | 31.4 | 56.4 | 92.5 ± 0.7 | 4.1 ± 0.3 | 1.4 ± 0.2 | 0.533 | |
| 600 | 60 | 30.1 | 89.0 | 87.7 ± 0.1 | 2.6 ± 0.3 | 1.1 ± 0.1 | 0.347 | |
| 500 | 60 | 28.8 | 95.5 | 87.9 ± 0.6 | 4.5 ± 0.3 | 1.3 ± 0.1 | 0.611 | |
| 400 | 60 | 30.8 | 95.2 | 77.3 ± 0.8 | 5.5 ± 0.1 | 1.5 ± 0.1 | 0.851 | |
| corn residues | 700 | 60 | 33.3 | 81.0 | 78.8 ± 0.4 | 0.5 ± 0.1 | 1.1 ± 0.1 | 0.076 |
| 600 | 10 | 34.5 | 83.9 | 75.7 ± 0.4 | 2.6 ± 0.2 | 0.8 ± 0.1 | 0.405 | |
| 600 | 60 | 32.9 | 83.8 | 79.1 ± 0.6 | 0.6 ± 0.2 | 0.4 ± 0.1 | 0.096 | |
| 500 | 60 | 35.6 | 82.9 | 75.2 ± 0.7 | 3.3 ± 0.2 | 1.4 ± 0.1 | 0.520 | |
| 400 | 60 | 37.3 | 79.6 | 62.5 ± 0.8 | 4.7 ± 0.2 | 1.5 ± 0.2 | 0.903 | |
| commercial compost | 700 | 60 | 72.2 | 22.4 | 18.7 ± 0.3 | 1.0 ± 0.3 | 0.9 ± 0.1 | 0.658 |
| 600 | 10 | 76.7 | 16.3 | 14.0 ± 0.4 | 0.8 ± 0.1 | 0.4 ± 0.1 | 0.698 | |
| 600 | 60 | 63.1 | 20.6 | 14.9 ± 0.6 | 0.6 ± 0.3 | 0.4 ± 0.1 | 0.504 | |
| 500 | 60 | 79.0 | 24.7 | 19.9 ± 2.2 | 0.2 ± 0.1 | 1.5 ± 0.1 | 0.108 | |
| 400 | 60 | 81.8 | 26.7 | 17.8 ± 0.7 | 0.3 ± 0.1 | 1.5 ± 0.1 | 0.195 | |
| Feedstock Material | Pyrolysis | pH (–) | Conductivity (mS/m) | SSA (m2/g) | WHC (wt.%) | |
|---|---|---|---|---|---|---|
| Temp. (°C) | Time (min) | |||||
| oat brans | 700 | 60 | 8.62 ± 0.11 | 98 ± 8 | – | – |
| 600 | 10 | 8.08 ± 0.18 | 95 ± 5 | 0.58 ± 0.07 | 32.1 ± 1.3 | |
| 600 | 60 | 8.34 ± 0.09 | 80 ± 8 | 2.05 ± 0.24 | 35.2 ± 0.8 | |
| 500 | 60 | 8.48 ± 0.21 | 102 ± 11 | – | – | |
| 400 | 60 | 8.33 ± 0.12 | 89 ± 6 | – | – | |
| mixed woodcut | 700 | 60 | 8.01 ± 0.08 | 26 ± 2 | 220.41 ± 9.97 | 56.2 ± 1.6 |
| 600 | 10 | 8.20 ± 0.16 | 38 ± 4 | 7.19 ± 2.49 | 42.1 ± 2.1 | |
| 600 | 60 | 7.88 ± 0.04 | 39 ± 7 | 58.30 ± 5.41 | 49.8 ± 0.9 | |
| 500 | 60 | 7.69 ± 0.06 | 38 ± 7 | 14.54 ± 3.00 | 45.6 ± 1.8 | |
| 400 | 60 | 7.59 ± 0.05 | 31 ± 6 | 3.54 ± 0.56 | 38.9 ± 3.2 | |
| corn residues | 700 | 60 | 9.51 ± 0.14 | 596 ± 18 | – | – |
| 600 | 10 | 9.49 ± 0.12 | 702 ± 11 | 8.95 ± 3.73 | 36.4 ± 1.8 | |
| 600 | 60 | 9.66 ± 0.09 | 852 ± 21 | 27.90 ± 3.11 | 42.6 ± 1.7 | |
| 500 | 60 | 9.89 ± 0.11 | 553 ± 13 | – | – | |
| 400 | 60 | 9.72 ± 0.09 | 615 ± 22 | – | – | |
| compost | 700 | 60 | 10.64 ± 0.11 | 196 ± 8 | – | – |
| 600 | 10 | 10.52 ± 0.13 | 198 ± 9 | 3.11 ± 1.13 | 19.0 ± 2.1 | |
| 600 | 60 | 10.11 ± 0.14 | 314 ± 7 | 14.30 ± 2.18 | 22.3 ± 1.5 | |
| 500 | 60 | 10.42 ± 0.16 | 182 ± 13 | – | – | |
| 400 | 60 | 10.22 ± 0.08 | 222 ± 11 | – | – | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalina, M.; Sovova, S.; Svec, J.; Trudicova, M.; Hajzler, J.; Kubikova, L.; Enev, V. The Effect of Pyrolysis Temperature and the Source Biomass on the Properties of Biochar Produced for the Agronomical Applications as the Soil Conditioner. Materials 2022, 15, 8855. https://doi.org/10.3390/ma15248855
Kalina M, Sovova S, Svec J, Trudicova M, Hajzler J, Kubikova L, Enev V. The Effect of Pyrolysis Temperature and the Source Biomass on the Properties of Biochar Produced for the Agronomical Applications as the Soil Conditioner. Materials. 2022; 15(24):8855. https://doi.org/10.3390/ma15248855
Chicago/Turabian StyleKalina, Michal, Sarka Sovova, Jiri Svec, Monika Trudicova, Jan Hajzler, Leona Kubikova, and Vojtech Enev. 2022. "The Effect of Pyrolysis Temperature and the Source Biomass on the Properties of Biochar Produced for the Agronomical Applications as the Soil Conditioner" Materials 15, no. 24: 8855. https://doi.org/10.3390/ma15248855
APA StyleKalina, M., Sovova, S., Svec, J., Trudicova, M., Hajzler, J., Kubikova, L., & Enev, V. (2022). The Effect of Pyrolysis Temperature and the Source Biomass on the Properties of Biochar Produced for the Agronomical Applications as the Soil Conditioner. Materials, 15(24), 8855. https://doi.org/10.3390/ma15248855





