Glutathione Fluorescence Sensing Based on a Co-Doped Carbon Dot/Manganese Dioxide Nanocoral Composite
Abstract
1. Introduction
2. Methods
2.1. Chemicals
2.2. Instruments
2.3. Preparation of NPCDs
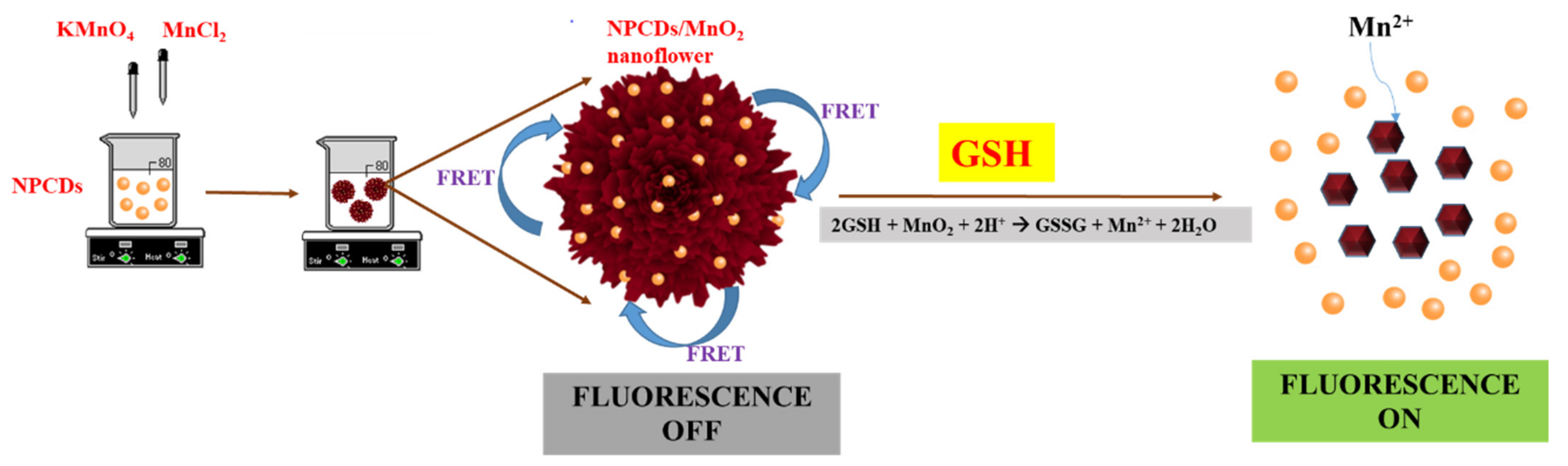
2.4. NPCD-MnO2 Nanocoral Composite Synthesis
2.5. GSH Detection
2.6. GSH Detection in Human Serum
2.7. Selectivity
3. Results and Discussion
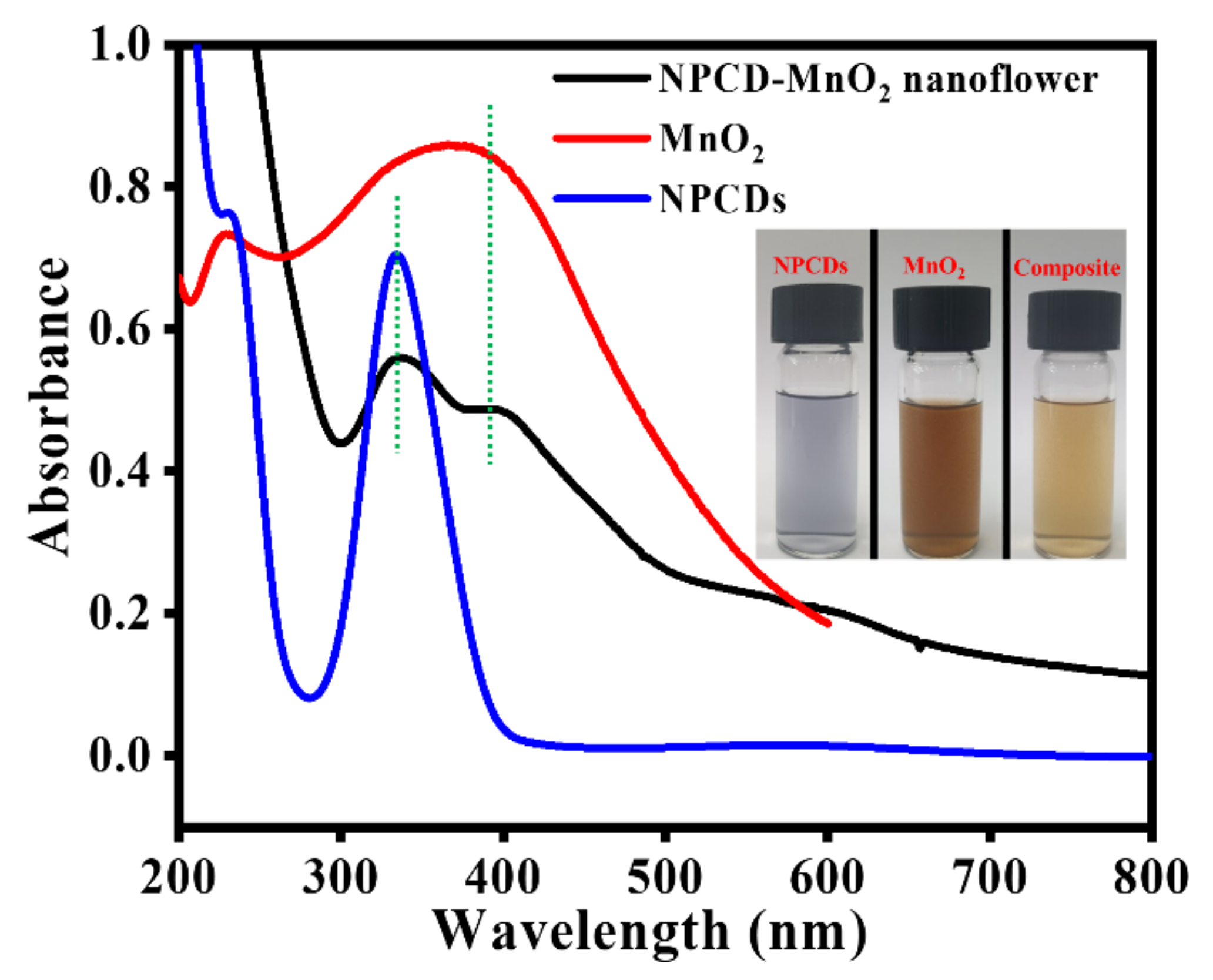
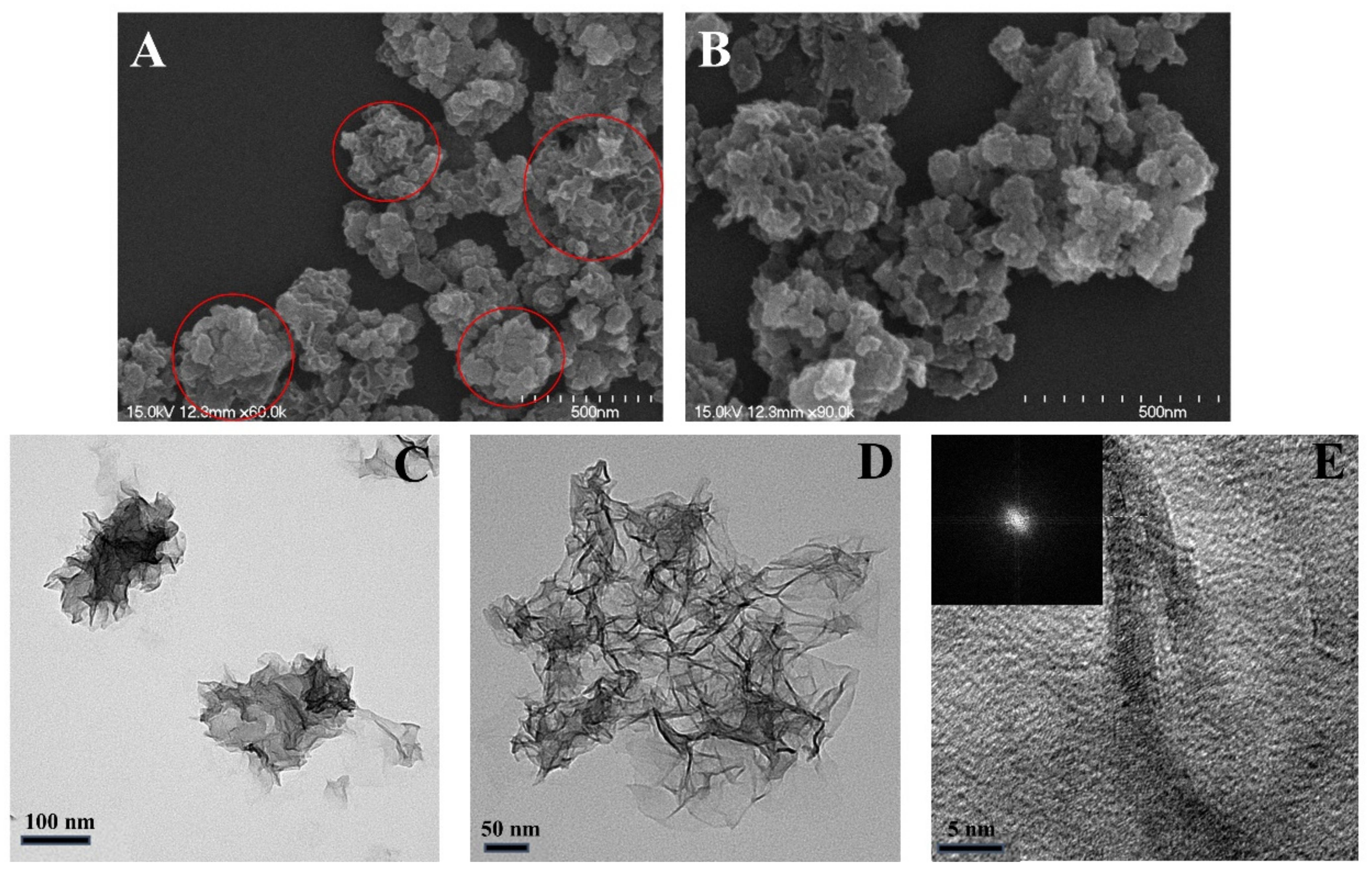
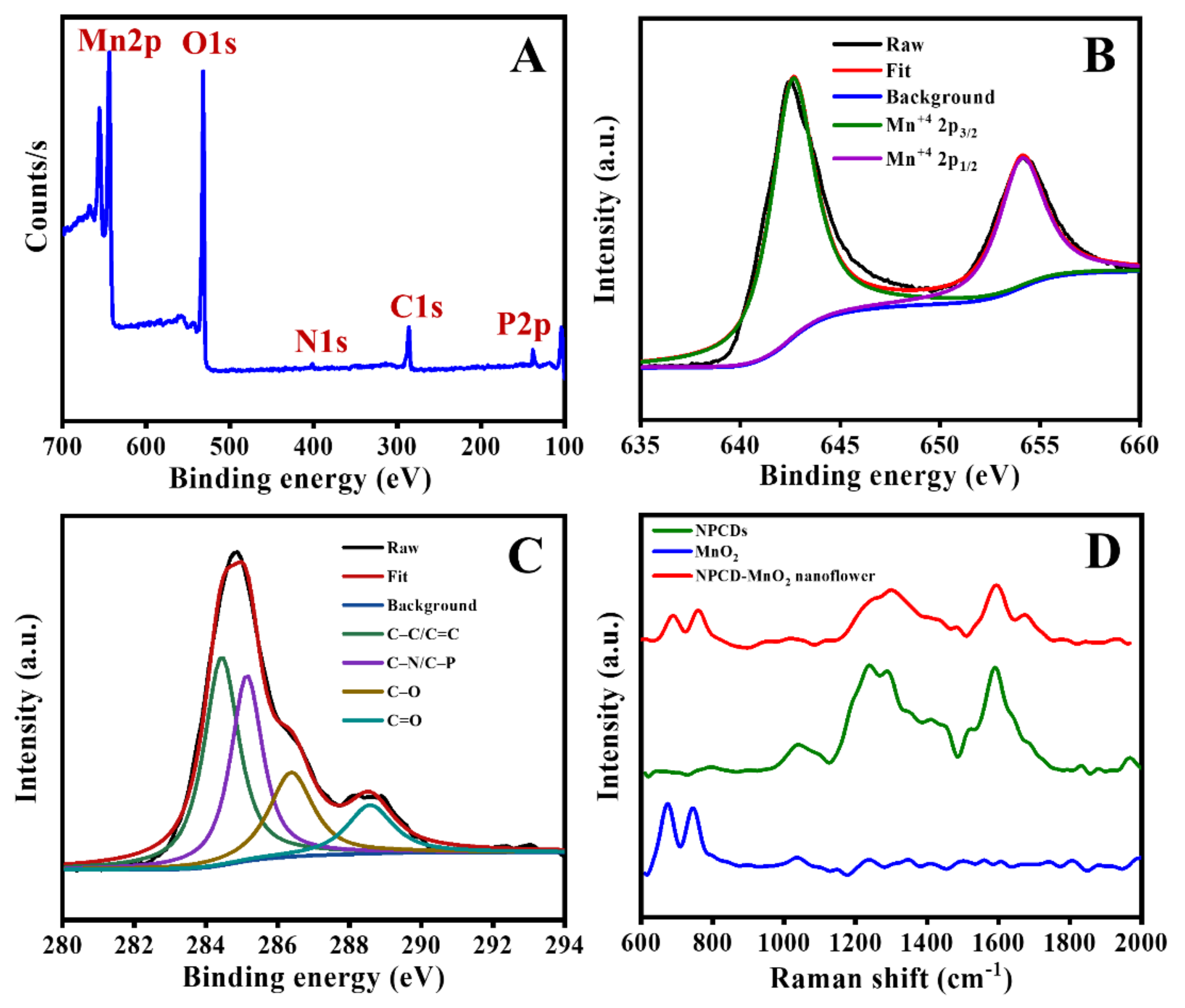
3.1. NPCD-MnO2 Nanocoral Composite Characteristics
3.2. Sensing of GSH
3.2.1. Sensing Mechanism
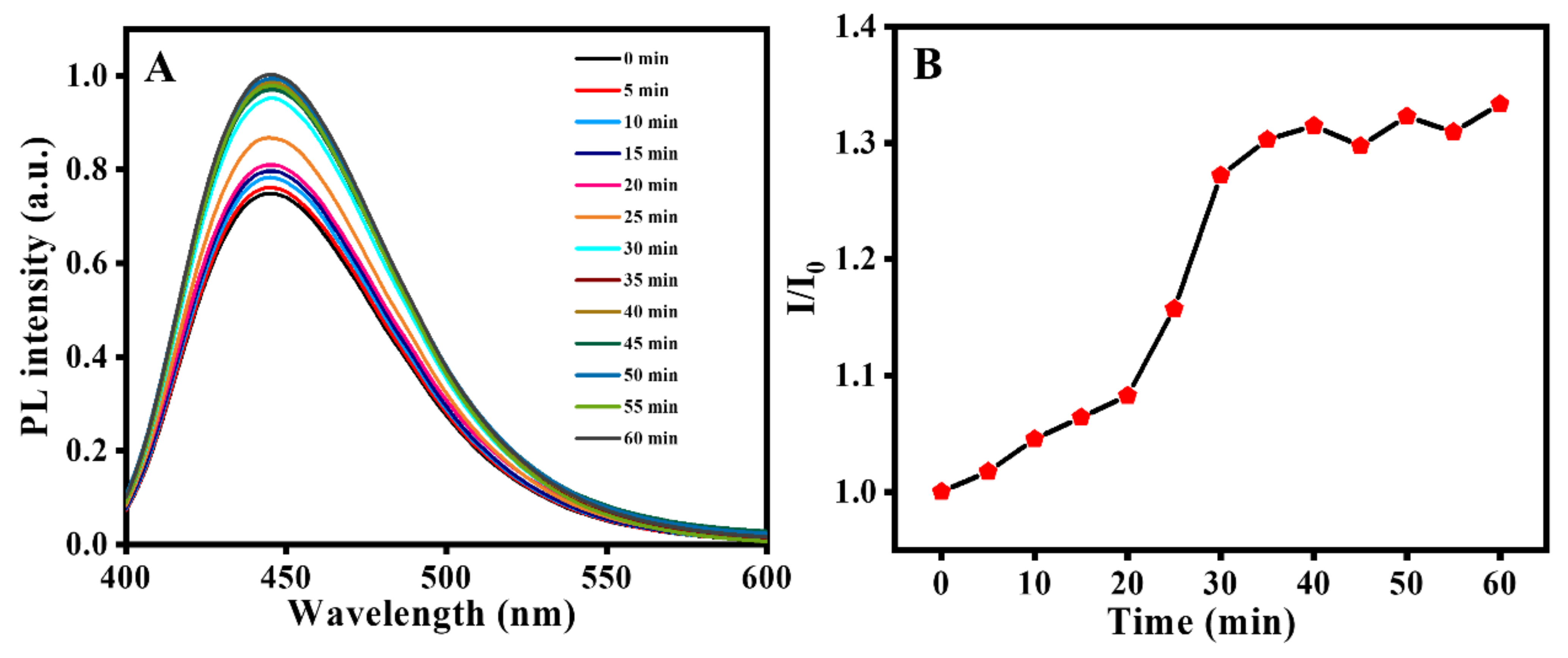
3.2.2. Optimization
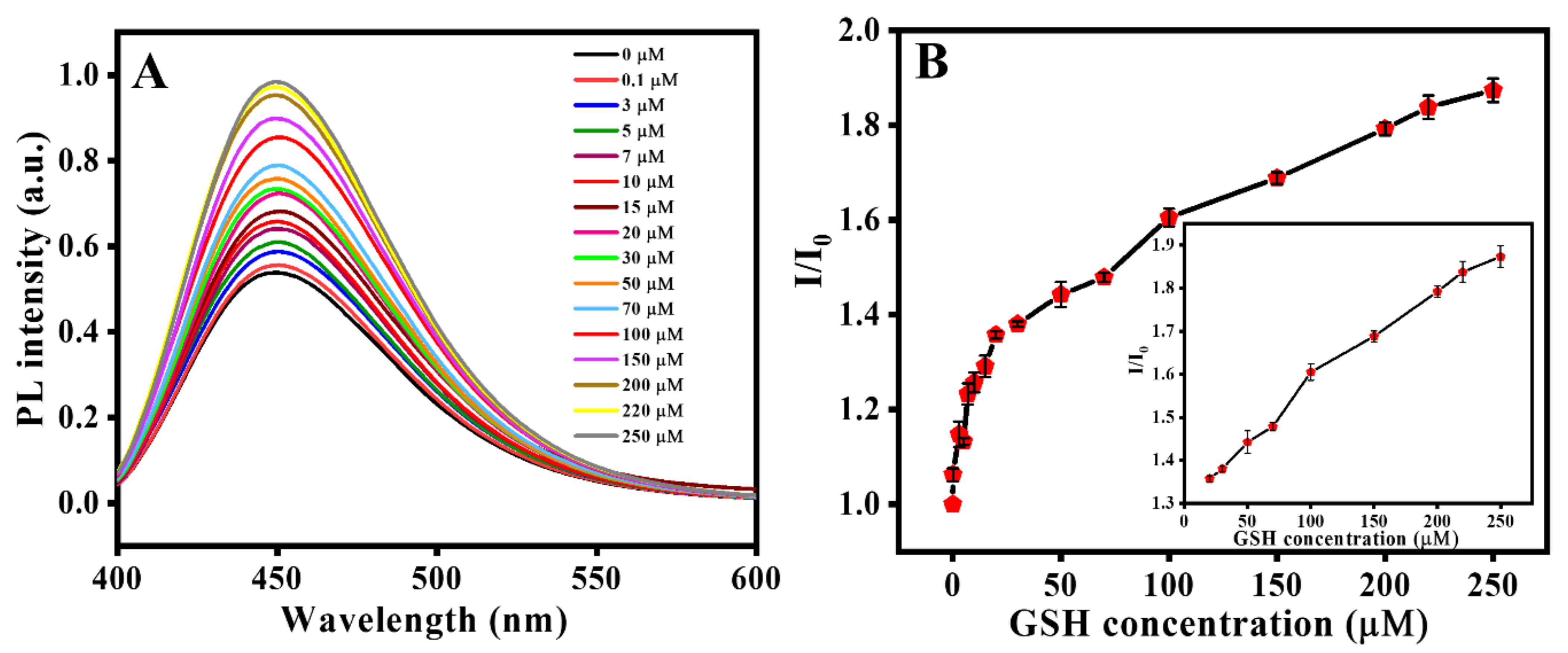
3.2.3. GSH Sensing
3.2.4. GSH Sensing in Human Serum
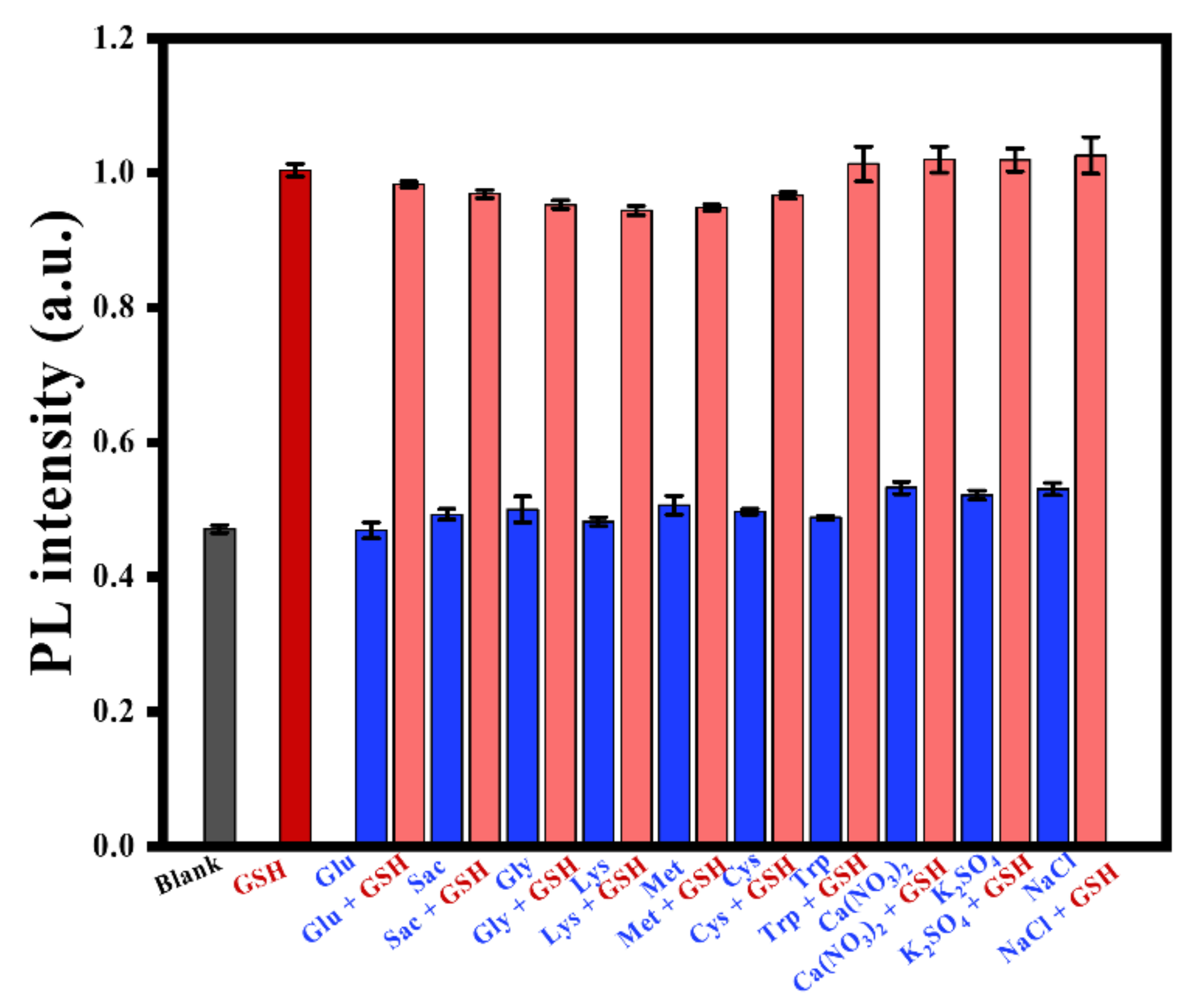
3.2.5. Selective Investigation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saydam, N.; Kirb, A.; Demir, O.; Hazan, E.; Oto, O.; Saydam, O.; Guner, G. Determination of glutathione, glutathione reductase, glutathione peroxidase and glutathione S-transferase levels in human lung cancer tissues. Cancer Lett. 1997, 119, 13–19. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Tsiasioti, A.; Tzanavaras, P.D. Determination of glutathione and glutathione disulfide using zone fluidics and fluorimetric detection. Talanta 2021, 222, 121559. [Google Scholar] [CrossRef]
- Sen, C.K. Nutritional biochemistry of cellular glutathione. J. Nutr. Biochem. 1997, 8, 660–672. [Google Scholar] [CrossRef]
- Torres, S.; Matías, N.; Baulies, A.; Nuñez, S.; Alarcon-Vila, C.; Martinez, L.; Nuño, N.; Fernandez, A.; Caballeria, J.; Levade, T.; et al. Mitochondrial GSH replenishment as a potential therapeutic approach for Niemann Pick type C disease. Redox Biol. 2017, 11, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Liang, Z.; Luan, D.; Liu, X.; Xu, K.; Tang, B. A Glutathione (GSH)-Responsive Near-Infrared (NIR) Theranostic Prodrug for Cancer Therapy and Imaging. Anal. Chem. 2016, 88, 6450–6456. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.-B.; Chandra, P.; Moon, J.O.; Shim, Y.-B. In vivo detection of glutathione disulfide and oxidative stress monitoring using a biosensor. Biomaterials 2012, 33, 2600–2607. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.-J.; Zhu, R.-H.; Cosnier, S.; Zhang, X.-J.; Shan, D. Ferrocyanide-Ferricyanide Redox Couple Induced Electrochemiluminescence Amplification of Carbon Dots for Ultrasensitive Sensing of Glutathione. Anal. Chem. 2015, 87, 11150–11156. [Google Scholar] [CrossRef]
- Saha, A.; Jana, N.R. Detection of Cellular Glutathione and Oxidized Glutathione Using Magnetic–Plasmonic Nanocomposite-Based “Turn-Off” Surface Enhanced Raman Scattering. Anal. Chem. 2013, 85, 9221–9228. [Google Scholar] [CrossRef]
- Yang, C.; Deng, W.; Liu, H.; Ge, S.; Yan, M. Turn-on fluorescence sensor for glutathione in aqueous solutions using carbon dots–MnO2 nanocomposites. Sens. Actuators B Chem. 2015, 216, 286–292. [Google Scholar] [CrossRef]
- Giustarini, D.; Dalle-Donne, I.; Colombo, R.; Milzani, A.; Rossi, R. An improved HPLC measurement for GSH and GSSG in human blood. Free Radic. Biol. Med. 2003, 35, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Balkrishna, S.J.; Hodage, A.S.; Kumar, S.; Panini, P.; Kumar, S. Sensitive and regenerable organochalcogen probes for the colorimetric detection of thiols. RSC Adv. 2014, 4, 11535–11538. [Google Scholar] [CrossRef]
- Le, T.H.; Kim, J.H.; Park, S.J. ”Turn on” Fluorescence Sensor of Glutathione Based on Inner Filter Effect of Co-Doped Carbon Dot/Gold Nanoparticle Composites. Int. J. Mol. Sci. 2022, 23, 190. [Google Scholar] [CrossRef] [PubMed]
- Burratti, L.; Ciotta, E.; De Matteis, F.; Prosposito, P. Metal Nanostructures for Environmental Pollutant Detection Based on Fluorescence. Nanomaterials 2021, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Ming, F.L.; Hou, J.Z.; Hou, C.J.; Yang, M.; Wang, X.F.; Li, J.W.; Huo, D.Q.; He, Q. One-step synthesized fluorescent nitrogen doped carbon dots from thymidine for Cr (VI) detection in water. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 8. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.R.; Wu, L.N.; Cao, X.Z.; Li, Y.; Liu, A.R.; Liu, S.Q. Nitrogen-doped carbon quantum dots for fluorescence detection of Cu2+ and electrochemical monitoring of bisphenol A. Rsc Adv. 2018, 8, 20000–20006. [Google Scholar] [CrossRef]
- Le, T.H.; Lee, H.J.; Kim, J.H.; Park, S.J. Detection of Ferric Ions and Catecholamine Neurotransmitters via Highly Fluorescent Heteroatom Co-Doped Carbon Dots. Sensors 2020, 20, 3470. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Arora, R.; Dhiman, H.; Pahwa, R. Carbon Quantum Dots: Synthesis, Characterization and Biomedical Applications. Turk. J. Pharm. Sci. 2018, 15, 219–230. [Google Scholar] [CrossRef]
- Li, M.X.; Chen, T.; Gooding, J.J.; Liu, J.Q. Review of Carbon and Graphene Quantum Dots for Sensing. Acs Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Ma, J.L.; Yin, B.C.; Wu, X.; Ye, B.C. Simple and Cost-Effective Glucose Detection Based on Carbon Nanodots Supported on Silver Nanoparticles. Anal. Chem. 2017, 89, 1323–1328. [Google Scholar] [CrossRef]
- Halawa, M.I.; Wu, F.X.; Zafar, M.N.; Mostafa, I.M.; Abdussalam, A.; Han, S.; Xu, G.B. Turn-on fluorescent glutathione detection based on lucigenin and MnO2 nanosheets. J. Mater. Chem. B 2020, 8, 3542–3549. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, J.; Liu, S.; Liu, X.; Yang, S. Synthesis of hydrothermally reduced graphene/MnO2 composites and their electrochemical properties as supercapacitors. J. Power Sources 2011, 196, 8160–8165. [Google Scholar] [CrossRef]
- Deng, R.R.; Xie, X.J.; Vendrell, M.; Chang, Y.T.; Liu, X.G. Intracellular Glutathione Detection Using MnO2-Nanosheet-Modified Upconversion Nanoparticles. J. Am. Chem. Soc. 2011, 133, 20168–20171. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zheng, C.; Guo, S.S.; Li, J.; Yang, H.H.; Chen, G.N. Turn-On Fluorescence Sensor for Intracellular Imaging of Glutathione Using g-C3N4 Nanosheet-MnO2 Sandwich Nanocomposite. Anal. Chem. 2014, 86, 3426–3434. [Google Scholar] [CrossRef] [PubMed]
- Vobornikova, I.; Pohanka, M. Smartphone-based colorimetric detection of glutathione. Neuroendocrinol. Lett. 2016, 37, 139–143. [Google Scholar]
- Wang, Q.; Li, L.F.; Wang, X.D.; Dong, C.; Shuang, S.M. Graphene quantum dots wrapped square-plate-like MnO2 nanocomposite as a fluorescent turn-on sensor for glutathione. Talanta 2020, 219, 121180. [Google Scholar] [CrossRef]
- Zheng, C.; Ding, L.; Wu, Y.N.; Tan, X.H.; Zeng, Y.Y.; Zhang, X.L.; Liu, X.L.; Liu, J.F. A near-infrared turn-on fluorescence probe for glutathione detection based on nanocomposites of semiconducting polymer dots and MnO2 nanosheets. Anal. Bioanal. Chem. 2020, 412, 8167–8176. [Google Scholar] [CrossRef]
- Chu, S.Y.; Wang, H.Q.; Du, Y.X.; Yang, F.; Yang, L.; Jiang, C.L. Portable Smartphone Platform Integrated with a Nanoprobe-Based Fluorescent Paper Strip: Visual Monitoring of Glutathione in Human Serum for Health Prognosis. ACS Sustain. Chem. Eng. 2020, 8, 8175–8183. [Google Scholar] [CrossRef]
- Anik, U.; Cubukcu, M.; Ertas, F.N. An effective electrochemical biosensing platform for the detection of reduced glutathione. Artif. Cells Nanomed. Biotechnol. 2016, 44, 971–977. [Google Scholar] [CrossRef]
- Rawat, B.; Mishra, K.K.; Barman, U.; Arora, L.; Pal, D.; Paily, R.P. Two-Dimensional MoS2-Based Electrochemical Biosensor for Highly Selective Detection of Glutathione. IEEE Sens. J. 2020, 20, 6937–6944. [Google Scholar] [CrossRef]
- Barman, U.; Mukhopadhyay, G.; Goswami, N.; Ghosh, S.S.; Paily, R.P. Detection of Glutathione by Glutathione-S-Transferase-Nanoconjugate Ensemble Electrochemical Device. IEEE Trans. Nanobiosci. 2017, 16, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhao, P.; Zhang, S.S.; Huo, G.A.; Suo, Z.C.; Yue, Z.; Zhang, S.M.; Huang, W.P.; Zhu, B.L. Platinum and Iridium Oxide Co-modified TiO2 Nanotubes Array Based Photoelectrochemical Sensors for Glutathione. Nanomaterials 2020, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cruz, M.S.; Mendieta, J.; Tauler, R.; Esteban, M. Cadmium-binding properties of glutathione: A chemometrical analysis of voltammetric data. J. Inorg. Biochem. 1997, 66, 29–36. [Google Scholar] [CrossRef]



| Technique | Linear Range (nM) | LOD (nM) | Reference |
|---|---|---|---|
| Colorimetry | [25] | ||
| Fluorescence | 48 | [26] | |
| Fluorescence | 260 | [27] | |
| Fluorescence | 190 | [28] | |
| Electrochemistry | [29] | ||
| Electrochemistry | 703 | [30] | |
| Electrochemistry | 41.9 | [31] | |
| Photoelectrochemsitry | [32] | ||
| Fluorescence | Our approach |
| Sample | Added (µM) | Detected (µM) | Recovery (%) | RSD (n = 3) | |
|---|---|---|---|---|---|
| 1 | 30 | 30.3 | 101.1 | 3.6 | 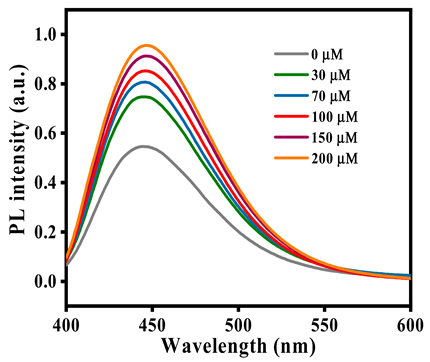 |
| 2 | 70 | 66.1 | 94.5 | 2.4 | |
| 3 | 100 | 108.4 | 108.4 | 2.7 | |
| 4 | 150 | 154.6 | 103.0 | 1.5 | |
| 5 | 200 | 190.5 | 95.3 | 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.-H.; Lee, H.-J.; Tran, Q.-N. Glutathione Fluorescence Sensing Based on a Co-Doped Carbon Dot/Manganese Dioxide Nanocoral Composite. Materials 2022, 15, 8677. https://doi.org/10.3390/ma15238677
Le T-H, Lee H-J, Tran Q-N. Glutathione Fluorescence Sensing Based on a Co-Doped Carbon Dot/Manganese Dioxide Nanocoral Composite. Materials. 2022; 15(23):8677. https://doi.org/10.3390/ma15238677
Chicago/Turabian StyleLe, Thi-Hoa, Hyun-Jong Lee, and Quang-Nhat Tran. 2022. "Glutathione Fluorescence Sensing Based on a Co-Doped Carbon Dot/Manganese Dioxide Nanocoral Composite" Materials 15, no. 23: 8677. https://doi.org/10.3390/ma15238677
APA StyleLe, T.-H., Lee, H.-J., & Tran, Q.-N. (2022). Glutathione Fluorescence Sensing Based on a Co-Doped Carbon Dot/Manganese Dioxide Nanocoral Composite. Materials, 15(23), 8677. https://doi.org/10.3390/ma15238677








