Synthesis Method of Unsolvated Organic Derivatives of Metal Borohydrides
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions and Discussion
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grochala, W.; Edwards, P.P. Thermal Decomposition of the Non-Interstitial Hydrides for the Storage and Production of Hydrogen. Chem. Rev. 2004, 104, 1283–1316. [Google Scholar] [CrossRef] [PubMed]
- Bannenberg, L.J.; Heere, M.; Benzidi, H.; Montero, J.; Dematteis, E.M.; Suwarno, S.; Jaroń, T.; Winny, M.; Orłowski, P.A.; Wegner, W.; et al. Metal (boro-) hydrides for high energy density storage and relevant emerging technologies. Int. J. Hydrogen Energy 2020, 45, 33687–33730. [Google Scholar] [CrossRef]
- Churchard, A.J.; Banach, E.; Borgschulte, A.; Caputo, R.; Chen, J.C.; Clary, D.; Fijalkowski, K.J.; Geerlings, H.; Genova, R.V.; Grochala, W.; et al. A multifaceted approach to hydrogen storage. Phys. Chem. Chem. Phys. 2011, 13, 16955–16972. [Google Scholar] [CrossRef] [PubMed]
- Černý, R.; Schouwink, P. The crystal chemistry of inorganic metal boro-hydrides and their relation to metal oxides. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2015, 71, 619–640. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Finholt, A.E.; Gilbreath, J.R.; Hoekstra, H.R.; Hyde, E.K. Sodium Borohydride, Its Hydrolysis and its Use as a Reducing Agent and in the Generation of Hydrogen. J. Am. Chem. Soc. 1953, 75, 215–219. [Google Scholar] [CrossRef]
- Nora de Souza, M.V.; Alves Vasconcelos, T.R. Recent methodologies mediated by sodium borohydride in the reduction of different classes of compounds. Appl. Organomet. Chem. 2006, 20, 798–810. [Google Scholar] [CrossRef]
- Wegner, W.; Jaroń, T.; Grochala, W. Preparation of a series of lanthanide borohydrides and their thermal decomposition to refractory lanthanide borides. J. Alloys Compd. 2018, 744, 57–63. [Google Scholar] [CrossRef]
- Jensen, J.A.; Gozum, J.E.; Pollina, D.M.; Girolami, G.S. Titanium, zirconium, and hafnium tetrahydroborates as “tailored” CVD precursors for metal diboride thin films. J. Am. Chem. Soc. 1988, 110, 1643–1644. [Google Scholar] [CrossRef]
- Gennari, F.C. Mechanochemical synthesis of erbium borohydride: Polymorphism, thermal decomposition and hydrogen storage. J. Alloys Compd. 2013, 581, 192–195. [Google Scholar] [CrossRef]
- Zavorotynska, O.; El-Kharbachi, A.; Deledda, S.; Hauback, B.C. Recent progress in magnesium borohydride Mg(BH4)2: Fundamentals and applications for energy storage. Int. J. Hydrogen Energy 2016, 41, 14387–14403. [Google Scholar] [CrossRef]
- Wegner, W.; Jaroń, T.; Dobrowolski, M.A.; Dobrzycki, Ł.; Cyrański, M.K.; Grochala, W. Organic derivatives of Mg(BH4)2 as precursors towards MgB2 and novel inorganic mixed-cation borohydrides. Dalt. Trans. 2016, 45, 14370–14377. [Google Scholar] [CrossRef] [PubMed]
- Wegner, W.; Fijalkowski, K.J.; Grochala, W. A low temperature pyrolytic route to amorphous quasi-hexagonal boron nitride from hydrogen rich (NH4)3Mg(BH4)5. Dalt. Trans. 2020, 49, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, O.; Borgschulte, A.; Kato, S.; Buchter, F.; Gremaud, R.; Remhof, A.; Züttel, A. Low-Temperature Synthesis of LiBH4 by Gas-Solid Reaction. Chem. A Eur. J. 2009, 15, 5531–5534. [Google Scholar] [CrossRef]
- Arbaoui, A.; Redshaw, C. Metal catalysts for ε-caprolactone polymerisation. Polym. Chem. 2010, 1, 801. [Google Scholar] [CrossRef]
- Lombardo, L.; Ko, Y.; Zhao, K.; Yang, H.; Züttel, A. Direct CO2 Capture and Reduction to High-End Chemicals with Tetraalkylammonium Borohydrides. Angew. Chemie Int. Ed. 2021, 60, 9580–9589. [Google Scholar] [CrossRef]
- Payandeh Gharib Doust, S.; Brighi, M.; Sadikin, Y.; Ravnsbæk, D.B.; Černý, R.; Skibsted, J.; Jensen, T.R. Synthesis, Structure, and Li-Ion Conductivity of LiLa(BH4)3 X, X = Cl, Br, I.J. Phys. Chem. C 2017, 121, 19010–19021. [Google Scholar] [CrossRef]
- Gulino, V.; Brighi, M.; Dematteis, E.M.; Murgia, F.; Nervi, C.; Černý, R.; Baricco, M. Phase Stability and Fast Ion Conductivity in the Hexagonal LiBH4-LiBr-LiCl Solid Solution. Chem. Mater. 2019, 31, 5133–5144. [Google Scholar] [CrossRef]
- Ley, M.B.; Boulineau, S.; Janot, R.; Filinchuk, Y.; Jensen, T.R. New Li Ion conductors and solid state hydrogen storage materials: LiM(BH4)3Cl, M = La, Gd. J. Phys. Chem. C 2012, 116, 21267–21276. [Google Scholar] [CrossRef]
- Yan, Y.; Grinderslev, J.B.; Lee, Y.S.; Jørgensen, M.; Cho, Y.W.; Černý, R.; Jensen, T.R. Ammonia-Assisted fast Li-ion conductivity in a new hemiammine lithium borohydride, LiBH4·1/2NH3. Chem. Commun. 2020, 56, 3971–3974. [Google Scholar] [CrossRef]
- Matsuo, M.; Nakamori, Y.; Orimo, S.I.; Maekawa, H.; Takamura, H. Lithium superionic conduction in lithium borohydride accompanied by structural transition. Appl. Phys. Lett. 2007, 91, 224103. [Google Scholar] [CrossRef]
- Wegner, W.; van Leusen, J.; Majewski, J.; Grochala, W.; Kögerler, P. Borohydride as Magnetic Superexchange Pathway in Late Lanthanide Borohydrides. Eur. J. Inorg. Chem. 2019, 2019, 1776–1783. [Google Scholar] [CrossRef]
- Wegner, W.; Zakrzewski, J.J.; Zychowicz, M.; Chorazy, S. Incorporation of expanded organic cations in dysprosium(III) borohydrides for achieving luminescent molecular nanomagnets. Sci. Rep. 2021, 11, 11354. [Google Scholar] [CrossRef]
- Jaroń, T.; Orłowski, P.A.; Wegner, W.; Fijałkowski, K.J.; Leszczyński, P.J.; Grochala, W. Hydrogen Storage Materials: Room-Temperature Wet-Chemistry Approach toward Mixed-Metal Borohydrides. Angew. Chem. Int. Ed. 2015, 54, 1236–1239. [Google Scholar] [CrossRef]
- Jaroń, T.; Wegner, W.; Fijałkowski, K.J.; Leszczyński, P.J.; Grochala, W. Facile Formation of Thermodynamically Unstable Novel Borohydride Materials by a Wet Chemistry Route. Chem. A Eur. J. 2015, 21, 5689–5692. [Google Scholar] [CrossRef]
- Starobrat, A.; Tyszkiewicz, M.J.; Wegner, W.; Pancerz, D.; Orłowski, P.A.; Leszczyński, P.J.; Fijalkowski, K.J.; Jaroń, T.; Grochala, W. Salts of highly fluorinated weakly coordinating anions as versatile precursors towards hydrogen storage materials. Dalt. Trans. 2015, 44, 19469–19477. [Google Scholar] [CrossRef]
- Wietelmann, U.; Felderhoff, M.; Rittmeyer, P. Hydrides. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 1–39. [Google Scholar]
- Hagemann, H.; Černý, R. Synthetic approaches to inorganic borohydrides. Dalt. Trans. 2010, 39, 6006. [Google Scholar] [CrossRef]
- Jaroń, T.; Wegner, W.; Grochala, W. M[Y(BH4)4] and M2Li[Y(BH4)6−xClx] (M = Rb, Cs): New borohydride derivatives of yttrium and their hydrogen storage properties. Dalt. Trans. 2013, 42, 6886. [Google Scholar] [CrossRef]
- Malinowski, P.J.; Jaroń, T.; Domańska, M.; Slattery, J.M.; Schmitt, M.; Krossing, I. Building blocks for the chemistry of perfluorinated alkoxyaluminates [Al{OC(CF3)3}4]−: Simplified preparation and characterization of Li+-Cs+, Ag+, NH4+, N2H5+ and N2H7+ salts. Dalt. Trans. 2020, 49, 7766–7773. [Google Scholar] [CrossRef]
- Malinowski, P.J.; Zhuravlev, V.; Jaroń, T.; Santiso-Quinones, G.; Krossing, I. Extending the chemistry of weakly basic ligands: Solvates of Ag+ and Cu+ stabilized by [Al{OC(CF3)3}4]− anion as model examples in the screening for useful weakly-interacting solvents. Dalt. Trans. 2021, 50, 2050–2056. [Google Scholar] [CrossRef]
- Jaroń, T.; Grochala, W. Probing Lewis acidity of Y(BH4)3 via its reactions with MBH4 (M = Li, Na, K, NMe4). Dalt. Trans. 2011, 40, 12808. [Google Scholar] [CrossRef]
- Jaroń, T.; Wegner, W.; Cyrański, M.K.; Dobrzycki; Grochala, W. Tetrabutylammonium cation in a homoleptic environment of borohydride ligands: [(n-Bu)4N][BH4] and [(n-Bu)4N][Y(BH4)4]. J. Solid State Chem. 2012, 191, 279–282. [Google Scholar] [CrossRef]
- Starobrat, A.; Jaroń, T.; Grochala, W. Synthesis and characterization of a series of mixed-cation borohydrides of scandium: [Cat][Sc(BH4)4], [Cat] = [Me4N], [n-Bu4N], and [Ph4P]. Inorg. Chim. Acta 2015, 437, 70–73. [Google Scholar] [CrossRef]
- Wegner, W.; Jaroń, T. Synthesis, Polymorphism and Thermal Decomposition Process of (n-C4H9)4NRE(BH4)4 for RE = Ho, Tm and Yb. Materials 2021, 14, 1329. [Google Scholar] [CrossRef]
- Olsen, J.E.; Frommen, C.; Jensen, T.R.; Riktor, M.D.; Sørby, M.H.; Hauback, B.C. Structure and thermal properties of composites with RE-borohydrides (RE = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Er, Yb or Lu) and LiBH4. RSC Adv. 2014, 4, 1570–1582. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Krist. 2014, 229, 345–352. [Google Scholar]
- Antsyshkina, A.S.; Sadikov, G.G.; Borisov, P.; Makhaev, V.D. Complexes of Yttrium, Thulium, and Lutetium Tetrahydridoborates with Tetraphenylphosphonium Tetrahydridoborate (Ph4P)[M(BH4)4] (M = Y, Tm, Lu): Crystal Structure of (Ph4P)[Tm(BH4)4]. Russ. J. Inorg. Chem. 2001, 46, 1141–1146. [Google Scholar]
- Wegner, W. Method of Synthesis of Unsolvated Organic Derivatives of Metal Borohydrides; Polish Patent Application: Warsaw, Poland, 2022; p. 442137. [Google Scholar]
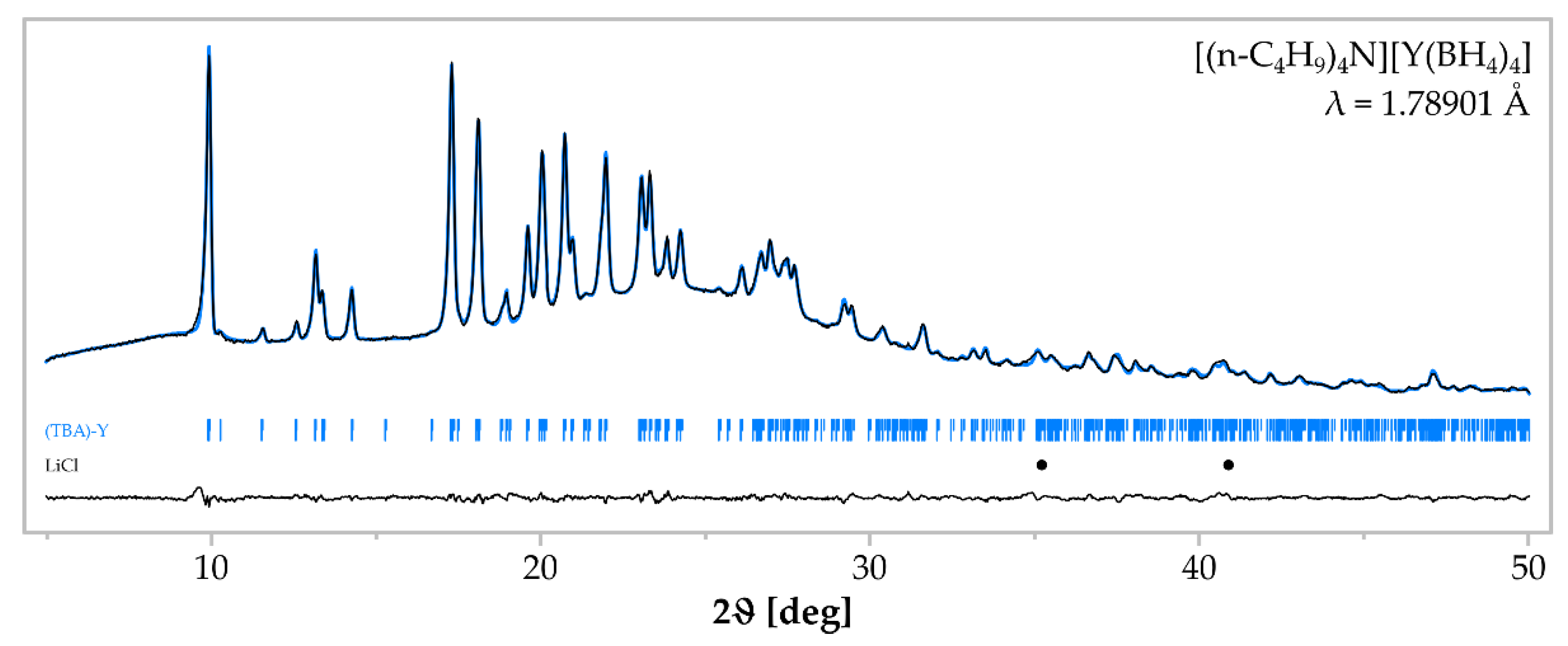


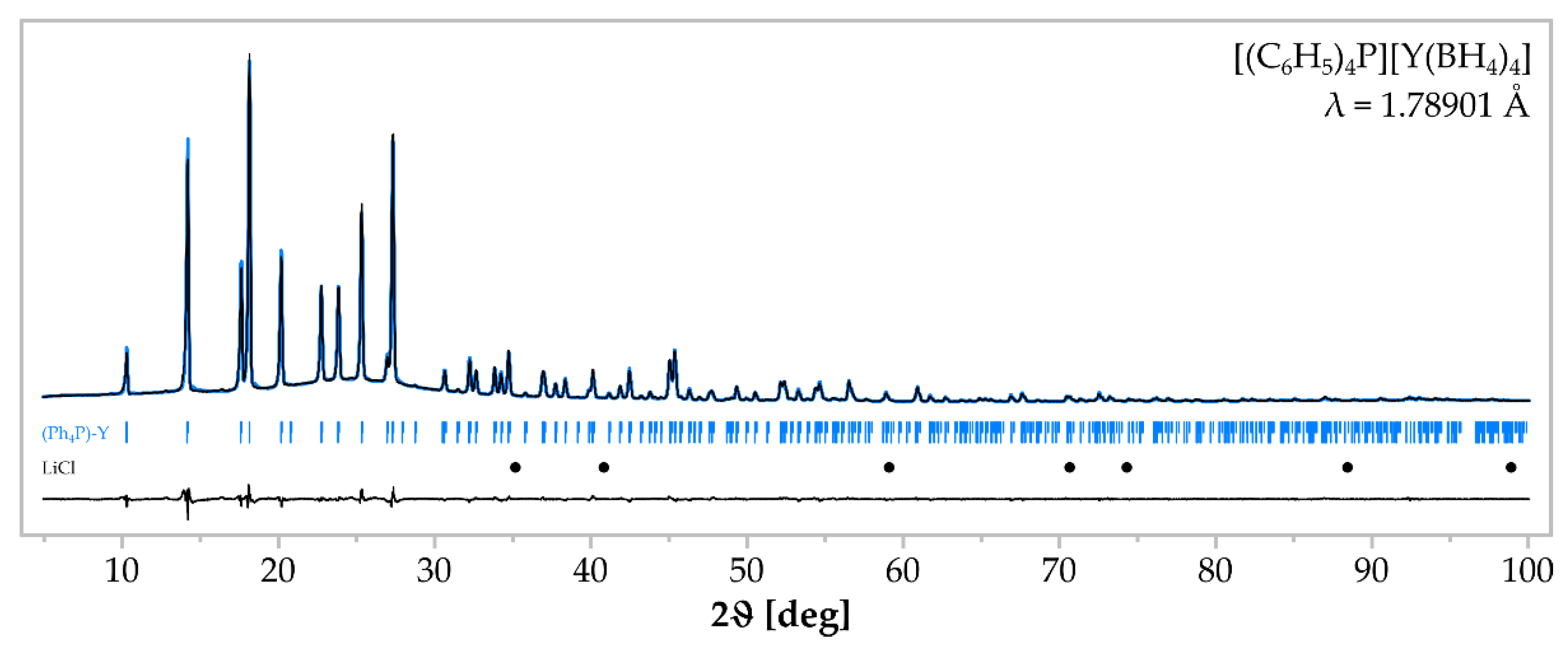
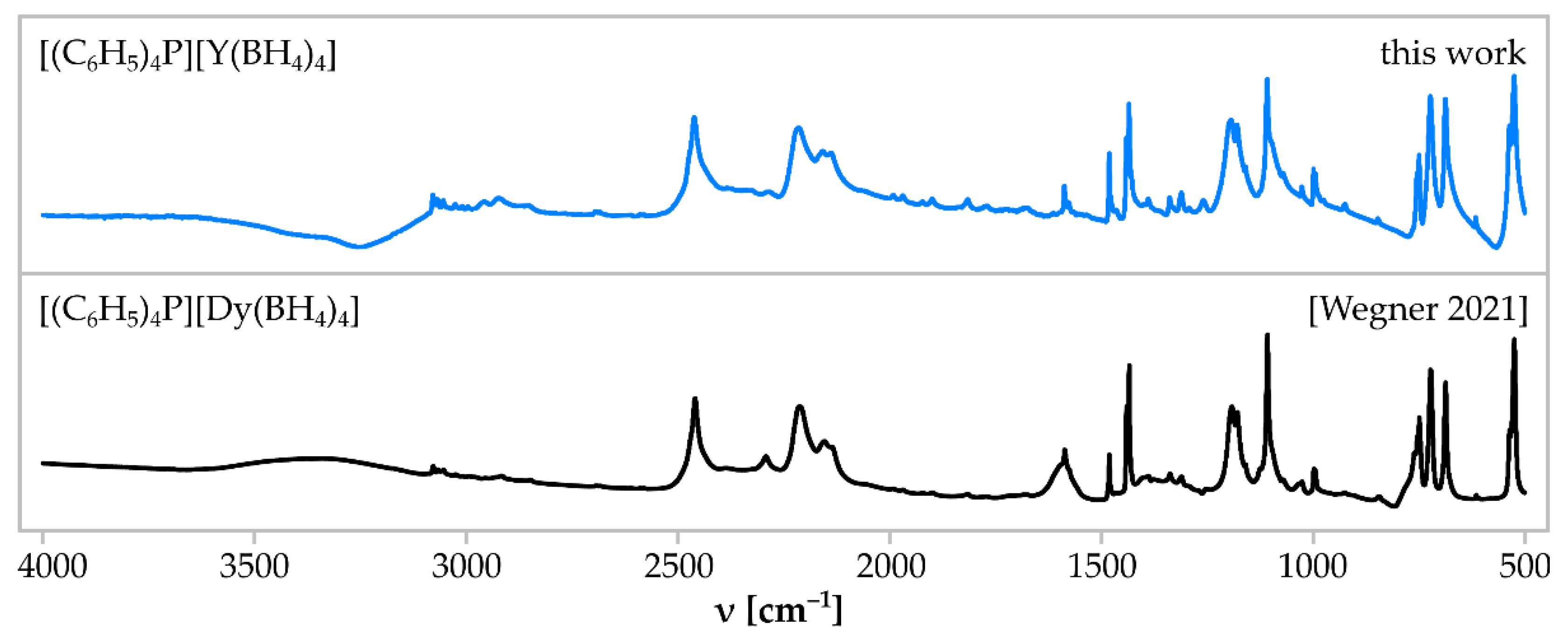
| Sample | Reactants (mmol) | Reaction Condition | Crystalline Products |
|---|---|---|---|
| 1 | [(n-C4H9)4N]BH4, YCl3, 3 LiBH4 | Reaction (8), stirring 12 h in 40 mL DCM, filtration, evaporation of the solvent | [(n-C4H9)4N][Y(BH4)4] |
| 2 | [Ph4P]Cl, YCl3, 4 LiBH4 | Reaction (9), stirring 12 h in 40 mL DCM, filtration, evaporation of the solvent | [Ph4P][Y(BH4)4] |
| Parameter | [Ph4P][Y(BH4)4] | [Ph4P][Tm(BH4)4] [37] | [Ph4P][Dy(BH4)4] [22] |
|---|---|---|---|
| Space group | I 41/a | I 41/a | I 41/a |
| a (Å) | 14.3628 (6) | 14.344 (2) | 14.373 (2) |
| c (Å) | 13.5567 (7) | 13.517 (3) | 13.560 (3) |
| α = β = γ (°) | 90 | 90 | 90 |
| V (Å3) | 2796.6 (2) | 2781.13 | 2801.3 (10) |
| Z | 4 | 4 | 4 |
| Element | Theoretical Value (%) | Experimental Value (%) |
|---|---|---|
| C | 59.1 | 58.3 |
| Y | 18.2 | – |
| B | 8.9 | – |
| H | 7.4 | 7.3 |
| P | 6.4 | – |
| N | – | 0 |
| Cl | – | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wegner, W.; Fijalkowski, K.J. Synthesis Method of Unsolvated Organic Derivatives of Metal Borohydrides. Materials 2022, 15, 8653. https://doi.org/10.3390/ma15238653
Wegner W, Fijalkowski KJ. Synthesis Method of Unsolvated Organic Derivatives of Metal Borohydrides. Materials. 2022; 15(23):8653. https://doi.org/10.3390/ma15238653
Chicago/Turabian StyleWegner, Wojciech, and Karol J. Fijalkowski. 2022. "Synthesis Method of Unsolvated Organic Derivatives of Metal Borohydrides" Materials 15, no. 23: 8653. https://doi.org/10.3390/ma15238653
APA StyleWegner, W., & Fijalkowski, K. J. (2022). Synthesis Method of Unsolvated Organic Derivatives of Metal Borohydrides. Materials, 15(23), 8653. https://doi.org/10.3390/ma15238653







