Hydrogen Generation by Hydrolysis of MgH2-LiH Composite
Abstract
:1. Introduction
2. Experimental Details
2.1. Sample Preparation
2.2. Hydrolysis Experiment
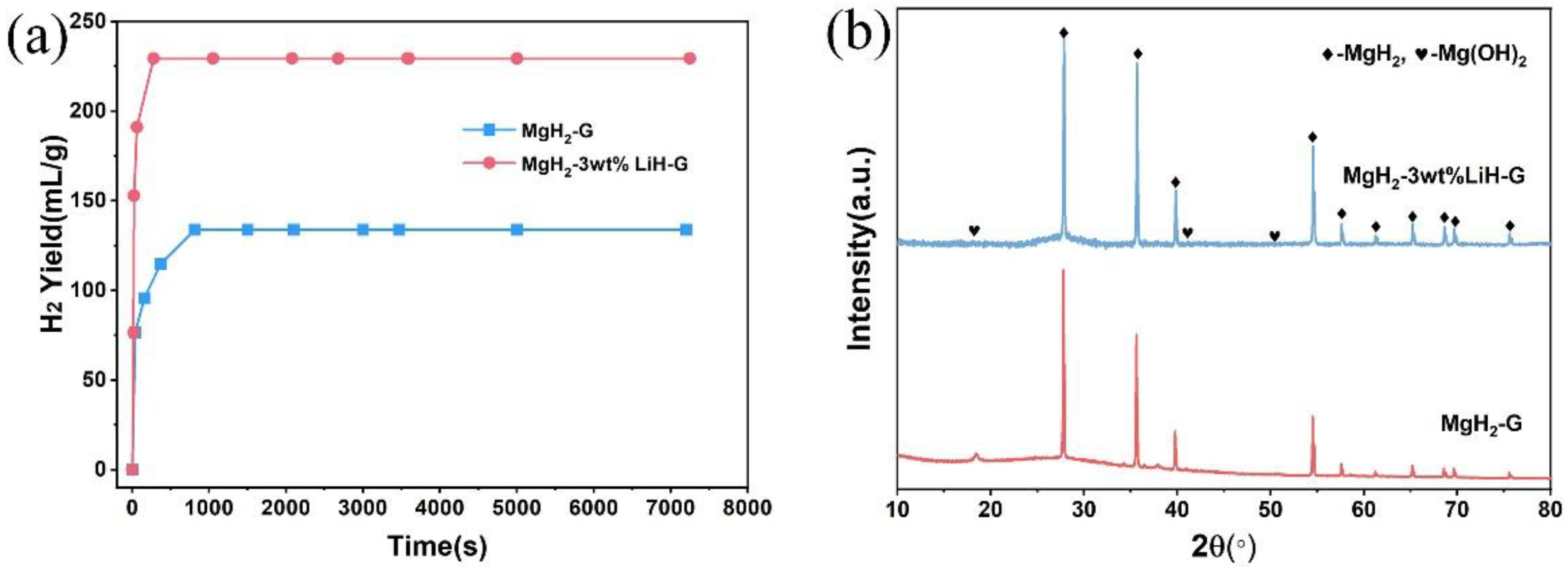
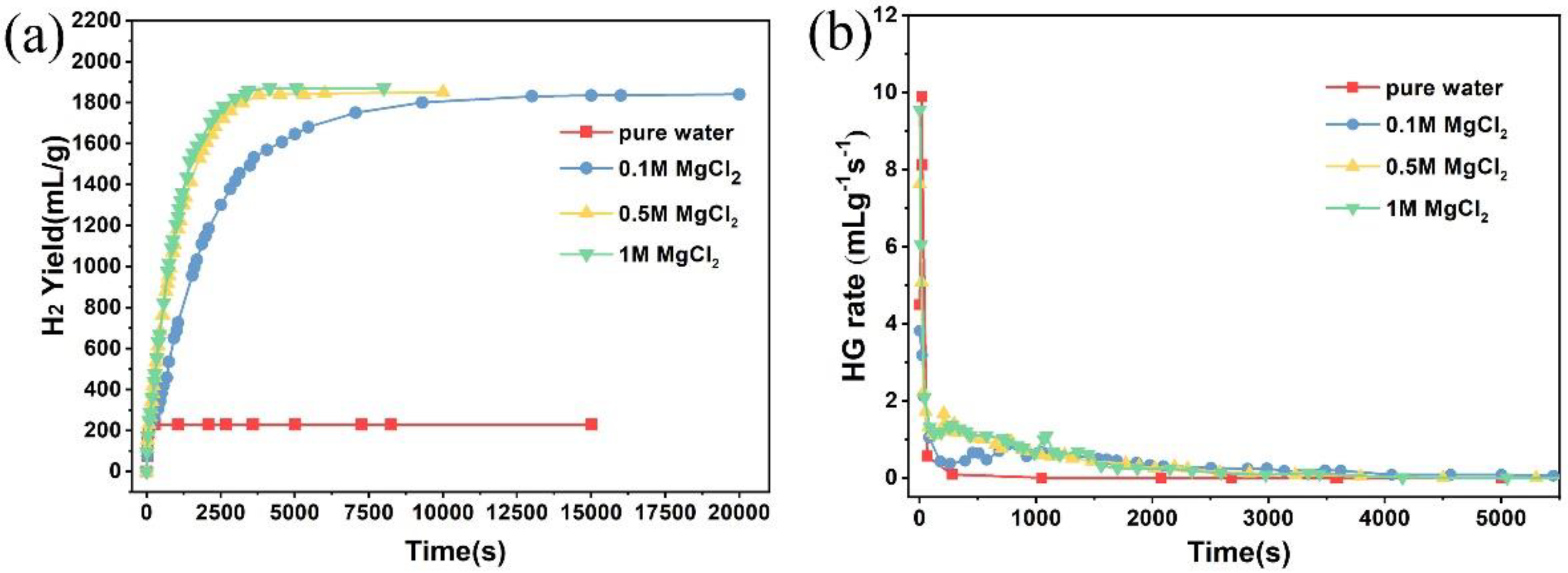
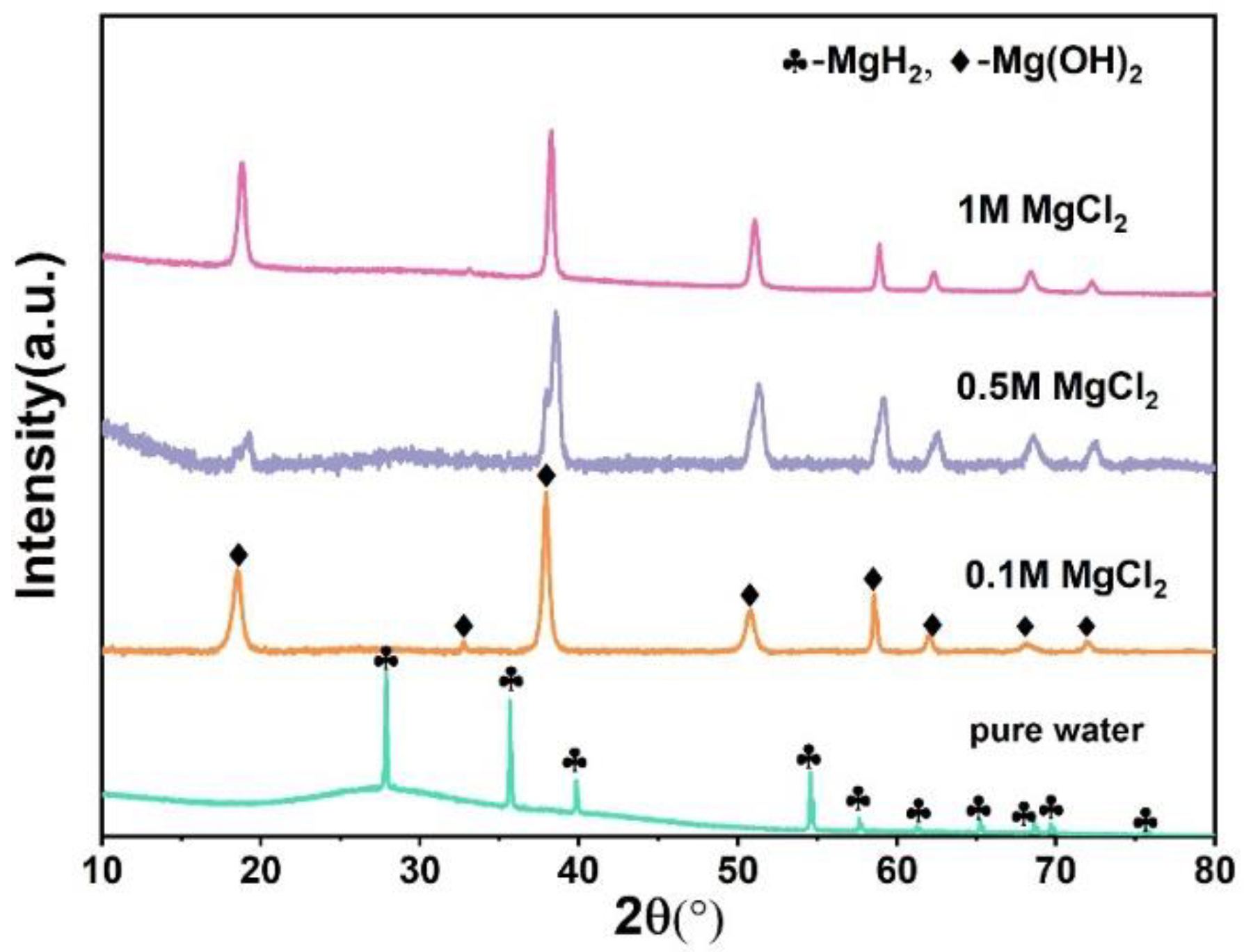
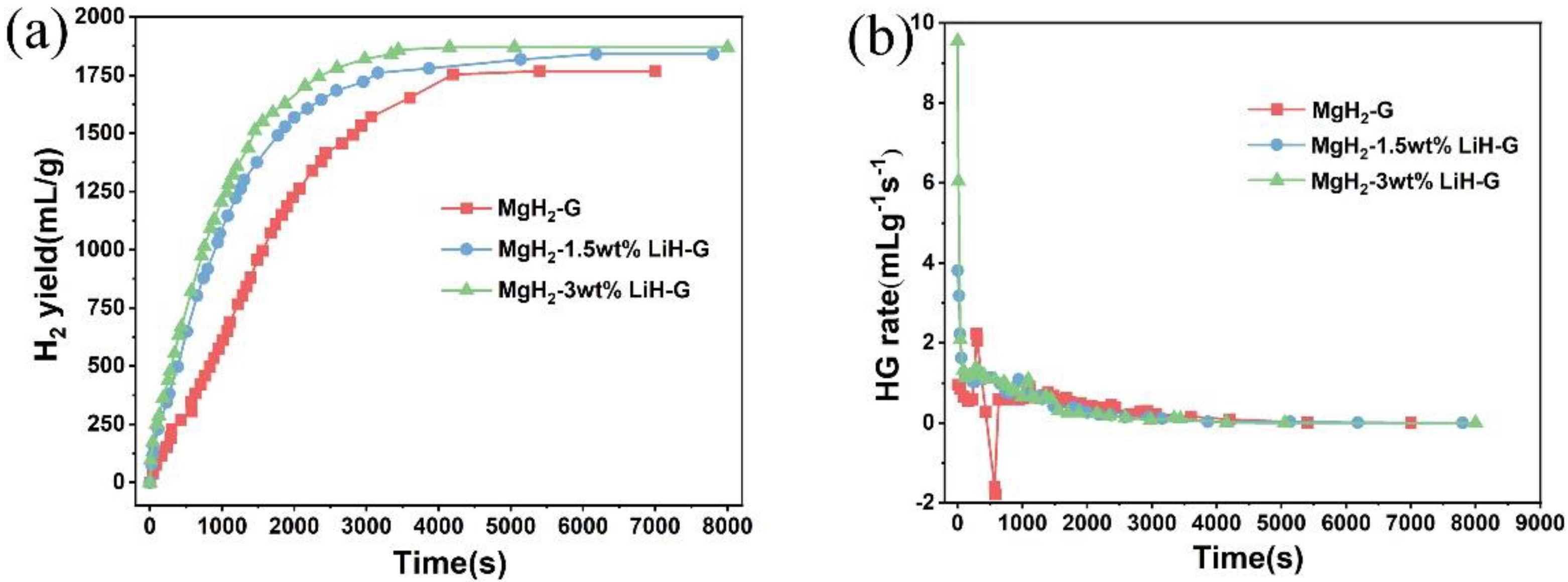
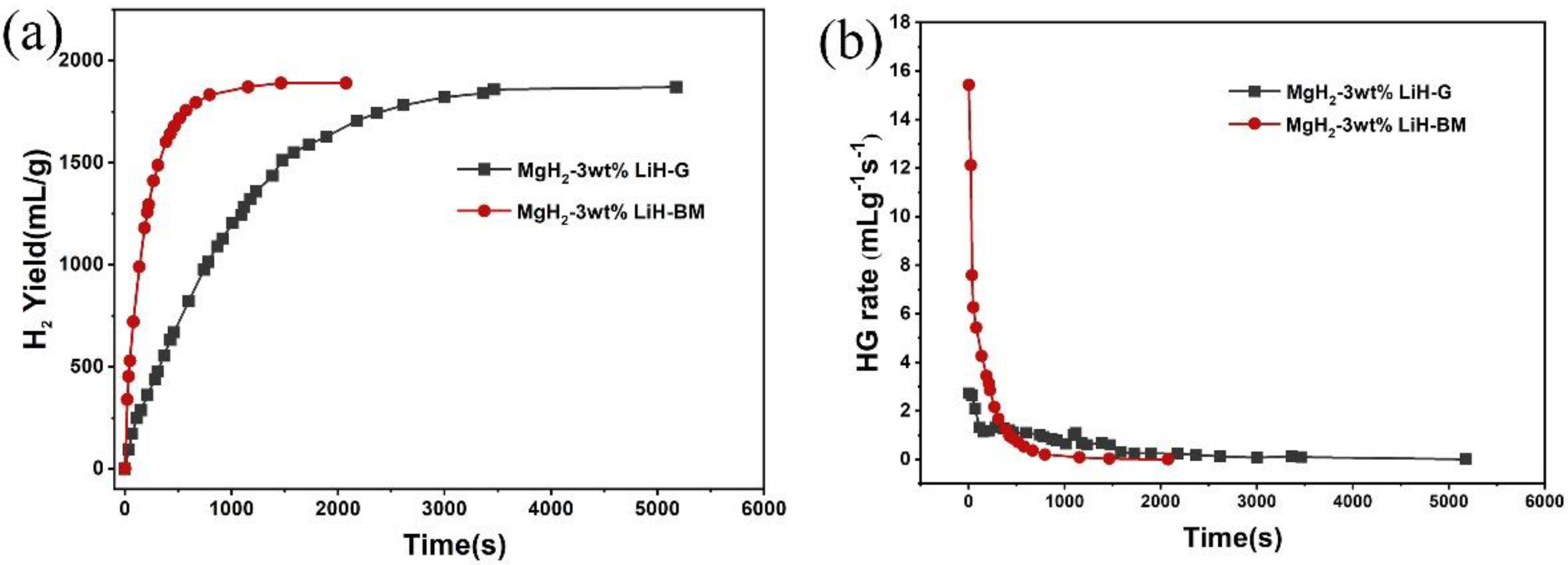
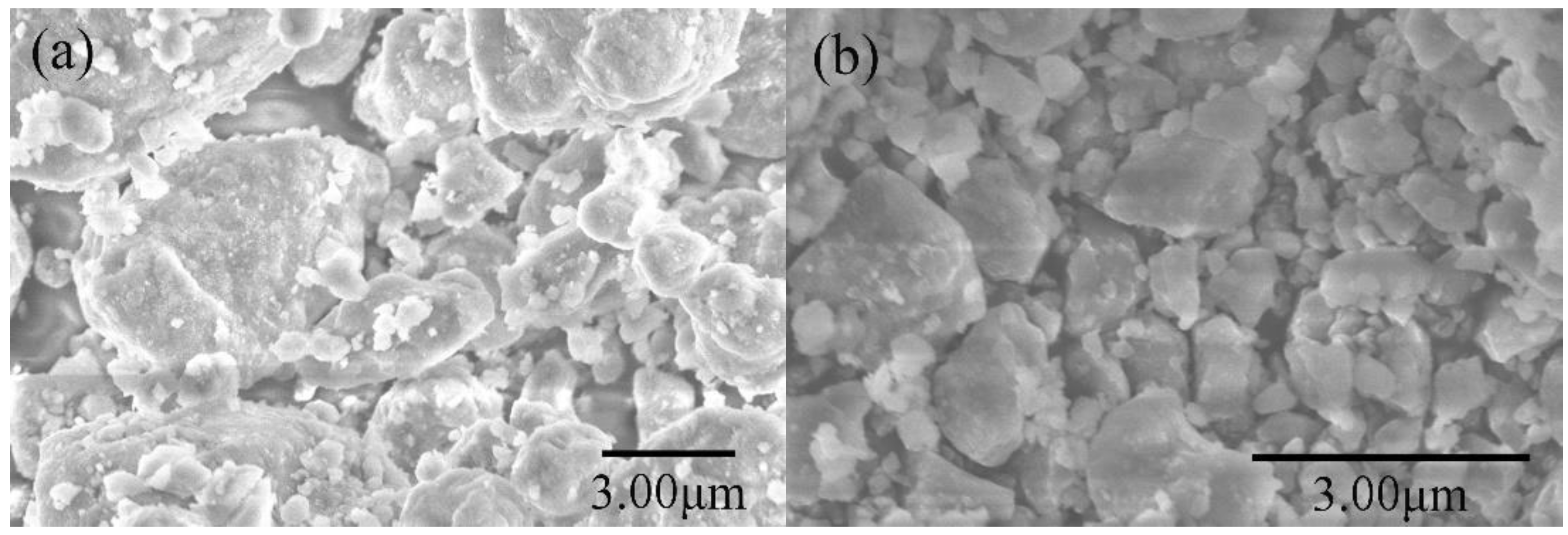
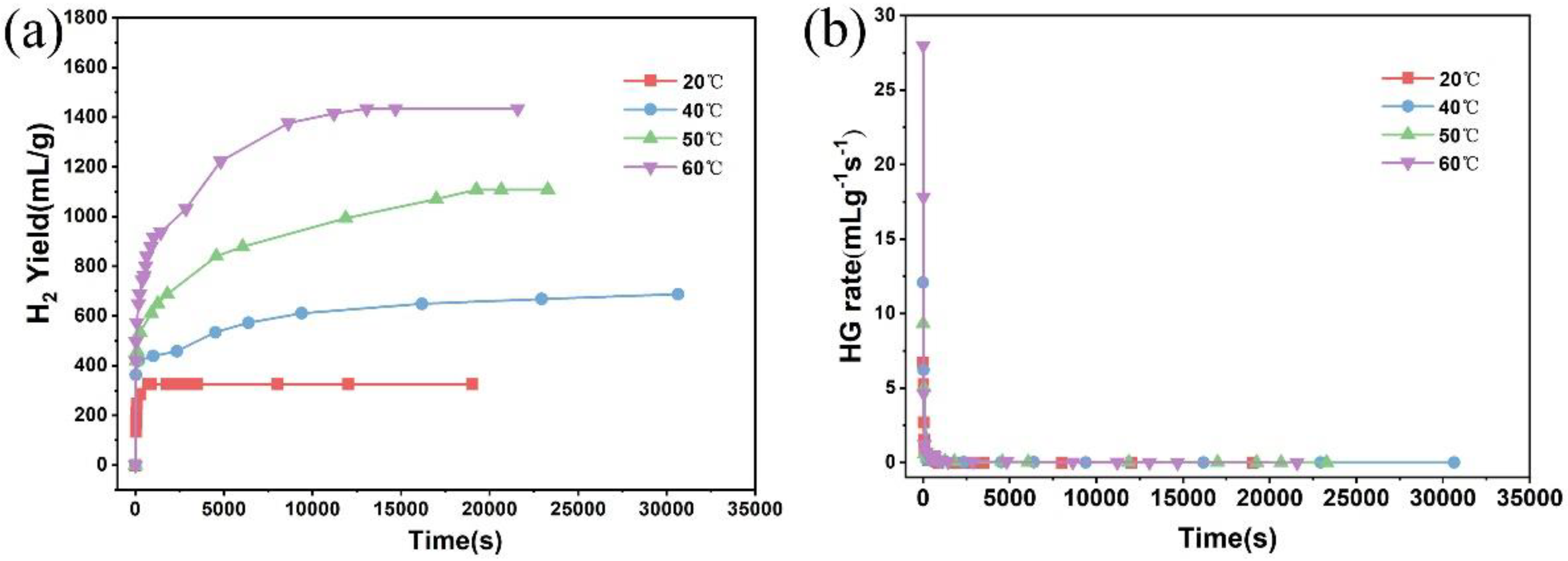
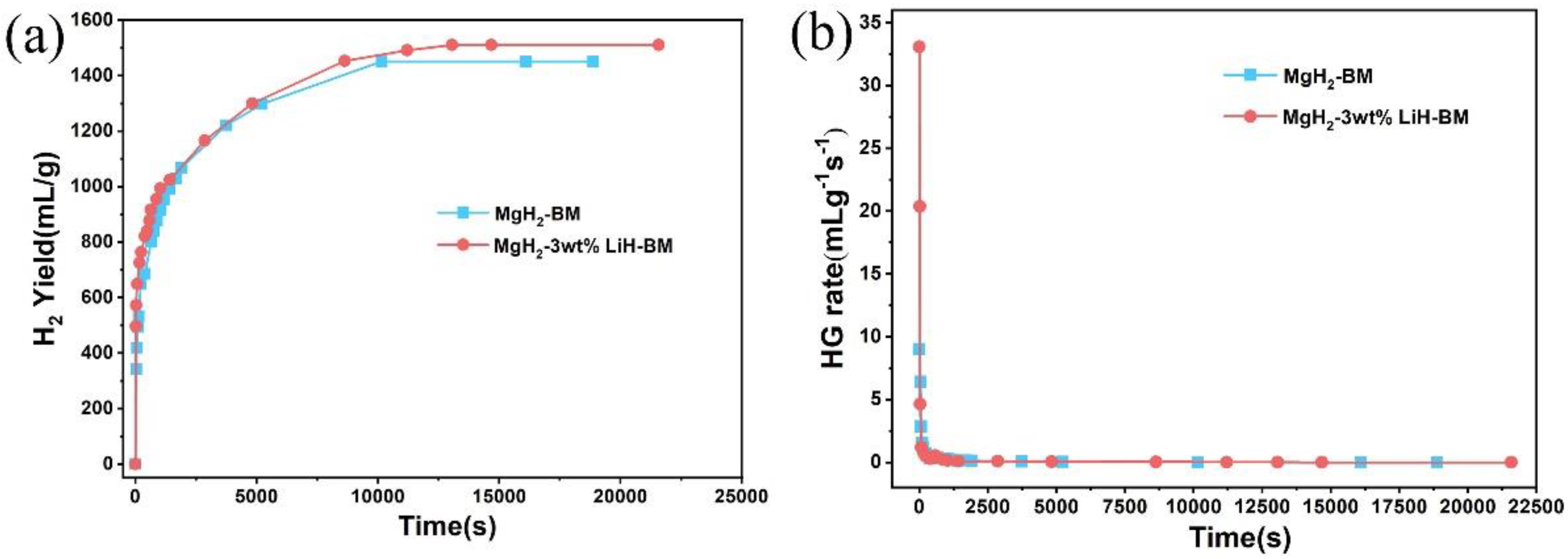
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, H.; He, L.; Lin, H.; Li, H.W. Progress and trends in magnesium-based materials for energy-storage research: A review. Energy Technol. 2018, 6, 445–458. [Google Scholar] [CrossRef] [Green Version]
- Rusman, N.A.A.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrog. Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamaridarkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- He, T.; Pachfule, P.; Wu, H.; Xu, Q.; Chen, P. Hydrogen carriers. Nat. Rev. Mater. 2016, 1, 16059. [Google Scholar] [CrossRef]
- Badea, N.I. Hydrogen as energy sources-basic concepts. Energies 2021, 14, 5783. [Google Scholar] [CrossRef]
- Deng, J.F.; Chen, S.P.; Wu, X.J.; Zheng, J.; Li, X.G. Recent progress on materials for hydrogen generation via hydrolysis. J. Inorg. Mater. 2021, 36, 1–8. [Google Scholar]
- Olabi, A.G.; Bahri, A.S.; Abdelghafar, A.A.; Baroutaji, A.; Sayed, E.T.; Alami, A.H.; Rezk, H.; Abdelkareem, M.A. Large-vscale hydrogen production and storage technologies: Current status and future directions. Int. J. Hydrog. Energy 2021, 46, 23498–23528. [Google Scholar] [CrossRef]
- Ma, M.; Ouyang, L.; Liu, J.; Wang, H.; Shao, H.; Zhu, M. Air-stable hydrogen generation materials and enhanced hydrolysis performance of MgH2-LiNH2 composites. J. Power Sources 2017, 359, 427–434. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, M.; Fu, W.; Wei, L.; Zhao, X.; Zhou, X.; Ni, M.; Wang, H. Highly active and durable catalyst for hydrogen generation by the NaBH4 hydrolysis reaction: CoWB/NF nanodendrite with an acicular array structure. J. Alloys Compd. 2020, 836, 155429. [Google Scholar] [CrossRef]
- Huang, C.; Yu, Y.; Tang, X.; Liu, Z.; Zhang, J.; Ye, C.; Ye, Y.; Zhang, R. Hydrogen generation by ammonia decomposition over Co/CeO2 catalyst: Influence of support morphologies. Appl. Surf. Sci. 2020, 532, 147335. [Google Scholar] [CrossRef]
- Lei, W.; Jin, H.; Gao, J.; Chen, Y. Efficient hydrogen generation from the NaBH4 hydrolysis by amorphous Co-Mo-B alloy supported on reduced graphene oxide. J. Mater. Res. 2021, 36, 4154–4168. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Y.; Guo, Q.; Li, Y.; Ma, X.; Li, B.; Zhu, H.; Yuan, C.G. Preparation of Ag@PNCMs nanocomposite as an effective catalyst for hydrogen generation from hydrolysis of sodium borohydride. Mater. Lett. 2021, 297, 129828. [Google Scholar] [CrossRef]
- Deng, J.; Sun, B.; Xu, J.; Shi, Y.; Xie, L.; Zheng, J.; Li, X. A monolithic sponge catalyst for hydrogen generation from sodium borohydride solution for portable fuel cells. Inorg. Chem. Front. 2021, 8, 35–40. [Google Scholar] [CrossRef]
- Figen, A.K.; Taşçı, K. Hydrolysis characteristics of calcium hydride (CaH2) powder in the presence of ethylene glycol, methanol, and ethanol for controllable hydrogen production. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 37–42. [Google Scholar] [CrossRef]
- Khzouz, M.; Gkanas, E.I.; Girella, A.; Statheros, T.; Milanese, C. Sustainable hydrogen production via LiH hydrolysis for unmanned air vehicle (UAV) applications. Int. J. Hydrog. Energy 2020, 45, 5384–5394. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Y.; Yang, Y.; Hu, R.; Yang, G.; Feng, L.; Suo, G.; Ye, X.; Zhang, L.; Shi, H.; et al. Enhanced hydrogen generation behaviors and hydrolysis thermodynamics of as-cast Mg-Ni-Ce magnesium-rich alloys in simulate seawater. Int. J. Hydrog. Energy 2019, 44, 24086–24097. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, J.; Liu, J.; Shi, R.; Zhu, Y.; Liu, Y.; Li, L. Enhanced hydrogen generation via hydrolysis of Mg-Mg2NiH4 system. J. Power Sources 2020, 476, 228499. [Google Scholar] [CrossRef]
- Hou, X.; Shi, H.; Yang, L.; Hou, K.; Wang, Y.; Feng, L.; Suo, G.; Ye, X.; Zhang, L.; Yang, Y. H2 generation kinetics/thermodynamics and hydrolysis mechanism of high-performance La-doped Mg-Ni alloys in NaCl solution-A large-scale and quick strategy to get hydrogen. J. Magnes. Alloy. 2021, 9, 1068–1083. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, D.; Li, W.; Fu, H.; Li, X. Promoting H2 generation from the reaction of Mg nanoparticles and water using cations. Chem. Commun. 2013, 49, 9437–9439. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Chen, W.; Yang, Z.; Huang, Z.; Yu, X. The effect of various cations/anions for MgH2 hydrolysis reaction. J. Mater. Sci. Technol. 2021, 73, 186–192. [Google Scholar] [CrossRef]
- Chen, J.; Fu, H.; Xiong, Y.; Xu, J.; Zheng, J.; Li, X. MgCl2 promoted hydrolysis of MgH2 nanoparticles for highly efficient H2 generation. Nano Energy 2014, 10, 337–343. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Y.; Hu, R.; Shi, H.; Feng, L.; Suo, G.; Ye, X.; Zhang, L.; Yang, Y. Catalytic effect of EG and MoS2 on hydrolysis hydrogen generation behavior of high-energy ball-milled Mg-10wt.%Ni alloys in NaCl solution-A powerful strategy for superior hydrogen generation performance. Int. J. Energy Res. 2019, 43, 8426–8438. [Google Scholar]
- Ma, M.; Yang, L.; Ouyang, L.; Shao, H.; Zhu, M. Promoting hydrogen generation from the hydrolysis of Mg-Graphite composites by plasma-assisted milling. Energy 2019, 167, 1205–1211. [Google Scholar] [CrossRef]
- Al Bacha, S.; Pighin, S.A.; Urretavizcaya, G.; Zakhour, M.; Castro, F.J.; Nakhl, M.; Bobet, J.L. Hydrogen generation from ball milled Mg alloy waste by hydrolysis reaction. J. Power Sources 2020, 479, 228711. [Google Scholar] [CrossRef]
- Xie, L.; Ren, J.; Qin, Y.; Wang, X.; Chen, F.; Ba, Z. Microstructure and modified hydrogen generation performance via hydrolysis of Mg-Nd-Ni alloys. Int. J. Hydrog. Energy 2021, 46, 15288–15297. [Google Scholar] [CrossRef]
- Jiang, J.; Ouyang, L.; Wang, H.; Liu, J.; Shao, H.; Zhu, M. Controllable Hydrolysis Performance of MgLi Alloys and Their Hydrides. ChemPhysChem 2019, 20, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Yang, L.; Hou, K.; Shu, Q.; Cao, Q.; Liu, Y.; Feng, L.; Suo, G.; Ye, X.; Zhang, L.; et al. Hydrolysis hydrogen generation medium regulated by alkali metal cations for Mg-based alloy-Green seawater modification strategy. J. Power Sources 2021, 509, 230364. [Google Scholar] [CrossRef]
- Pighin, S.A.; Urretavizcaya, G.; Bobet, J.L.; Castro, F.J. Nanostructured Mg for hydrogen production by hydrolysis obtained by MgH2 milling and dehydriding. J. Alloys Compd. 2020, 827, 154000. [Google Scholar] [CrossRef]
- Shao, H.; Wang, Y.; Xu, H.; Li, X. Hydrogen storage properties of magnesium ultrafine particles prepared by hydrogen plasma-metal reaction. Mater. Sci. Eng. B 2004, 110, 221–226. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Dong, Z.; Liu, H.; Li, S.; Ge, H.; Yan, M. Hydrogen generation from the hydrolysis of Mg powder ball-milled with AlCl3. Energy 2013, 53, 147–152. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Xue, H.; Peng, Y.; Deng, J.; Xie, Z.; Zheng, J.; Li, X.; Li, S. Hydrogen Generation by Hydrolysis of MgH2-LiH Composite. Materials 2022, 15, 1593. https://doi.org/10.3390/ma15041593
Wu X, Xue H, Peng Y, Deng J, Xie Z, Zheng J, Li X, Li S. Hydrogen Generation by Hydrolysis of MgH2-LiH Composite. Materials. 2022; 15(4):1593. https://doi.org/10.3390/ma15041593
Chicago/Turabian StyleWu, Xiaojuan, Huaqing Xue, Yong Peng, Jifeng Deng, Zewei Xie, Jie Zheng, Xingguo Li, and Shuan Li. 2022. "Hydrogen Generation by Hydrolysis of MgH2-LiH Composite" Materials 15, no. 4: 1593. https://doi.org/10.3390/ma15041593
APA StyleWu, X., Xue, H., Peng, Y., Deng, J., Xie, Z., Zheng, J., Li, X., & Li, S. (2022). Hydrogen Generation by Hydrolysis of MgH2-LiH Composite. Materials, 15(4), 1593. https://doi.org/10.3390/ma15041593




