Hollow Silica Nano and Micro Spheres with Polystyrene Templating: A Mini-Review
Abstract
1. Introduction
1.1. Hollow Silica Sphere
1.2. Comparison of Synthesis Strategy
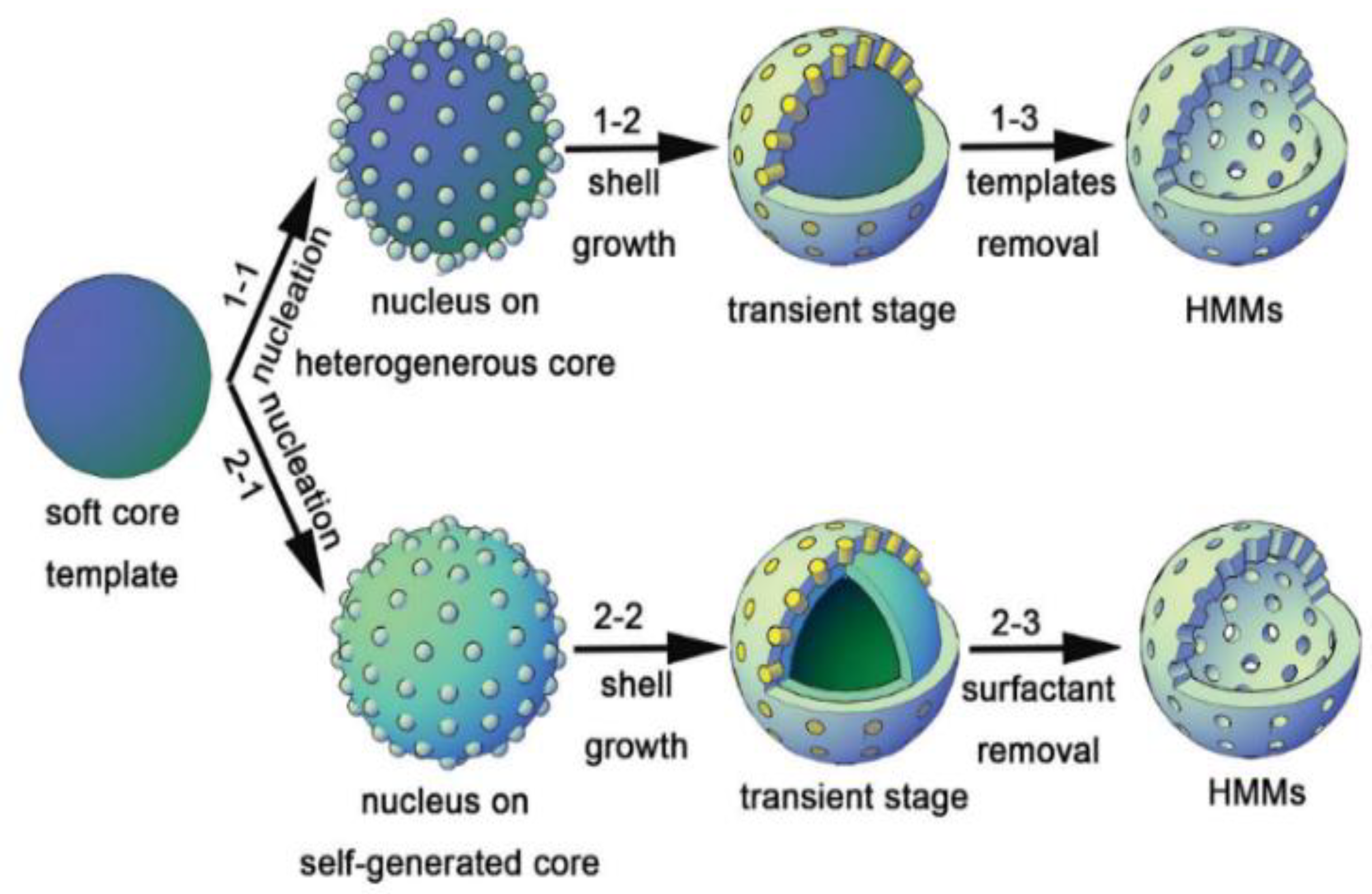

2. Sol–Gel
- Sol: Stable suspension of colloidal solid particles in a liquid. The repulsive force allows the small particles to overcome the attractive force and not aggregate. Gravity forces are negligible due to the small size of particles.
- Gel: A porous three-dimensionally interconnected solid network in a liquid. Based on the solvent nature and drying method, a gel can be converted to a hydrogel, aerogel, alcogel, or xerogel.
Sol–Gel Chemistry
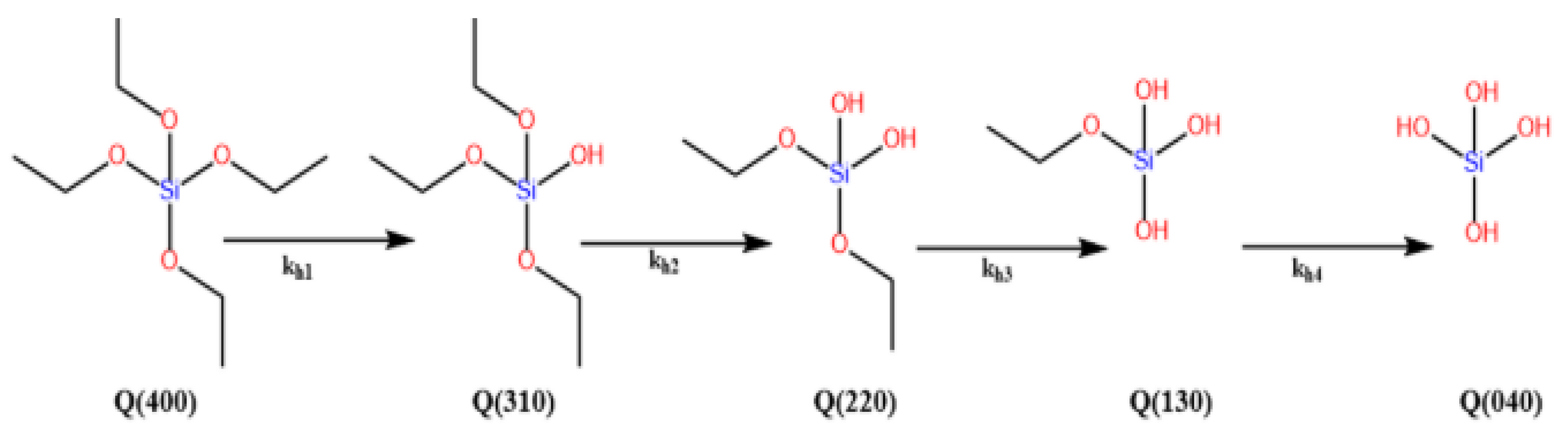
3. Other Chemicals
3.1. Initiator and Stabilisers for PS Template
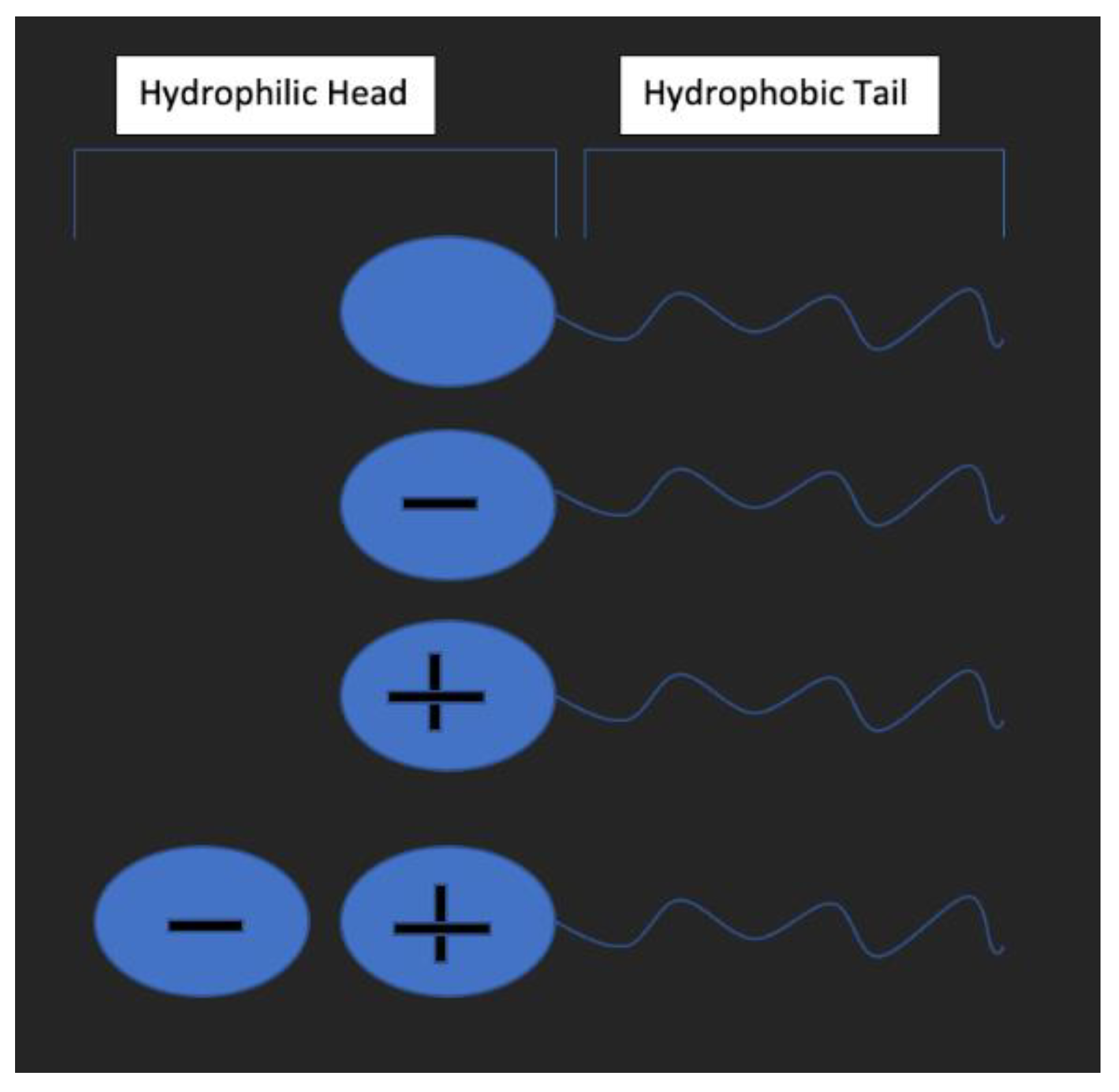
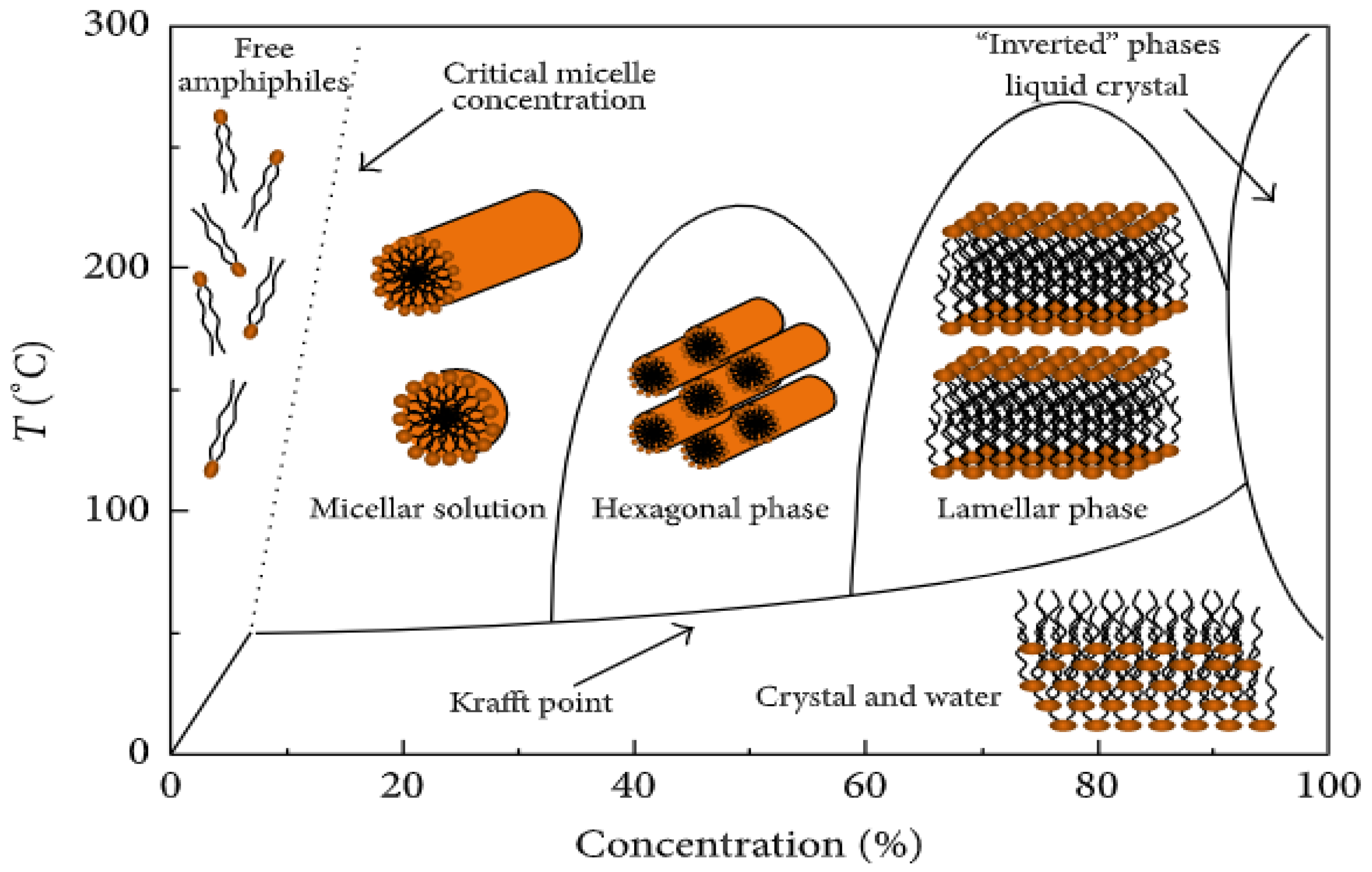
3.2. Surfactant
4. Research Gap
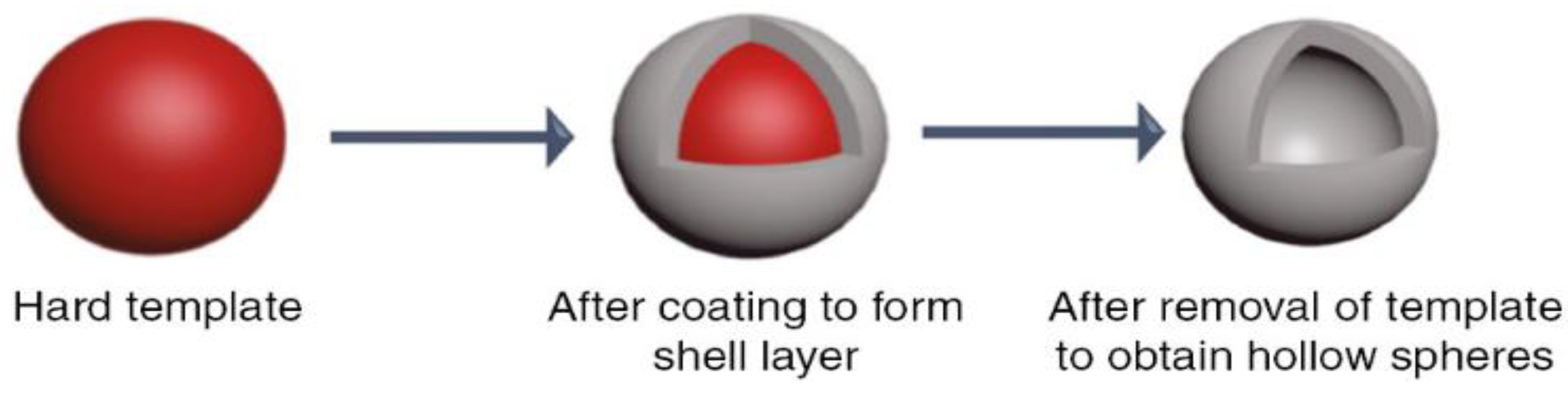
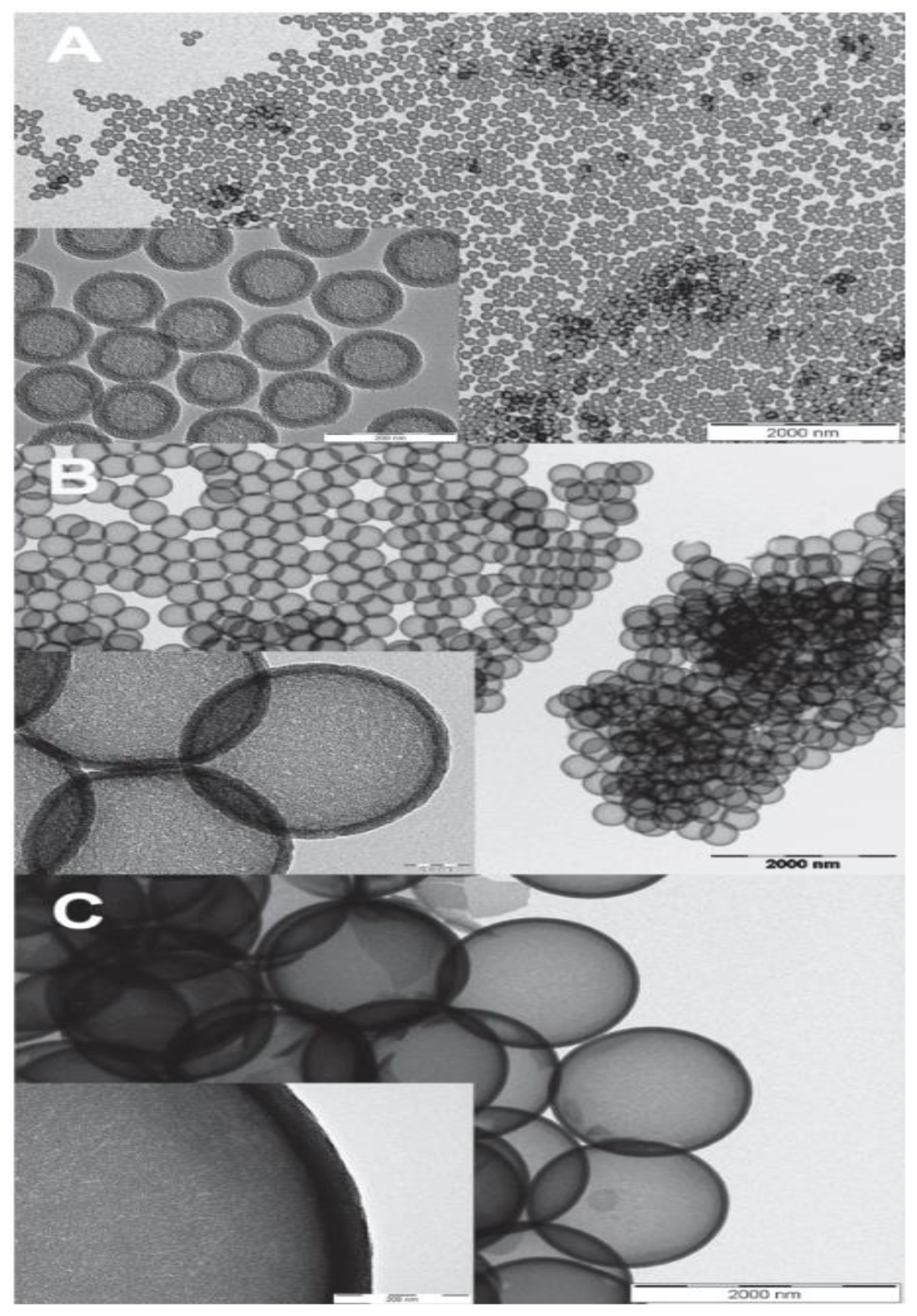
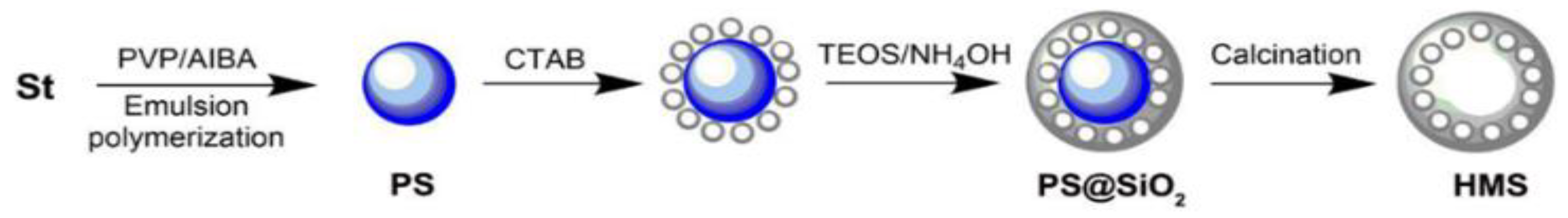
5. Polystyrene (Organic Hard Template Route)
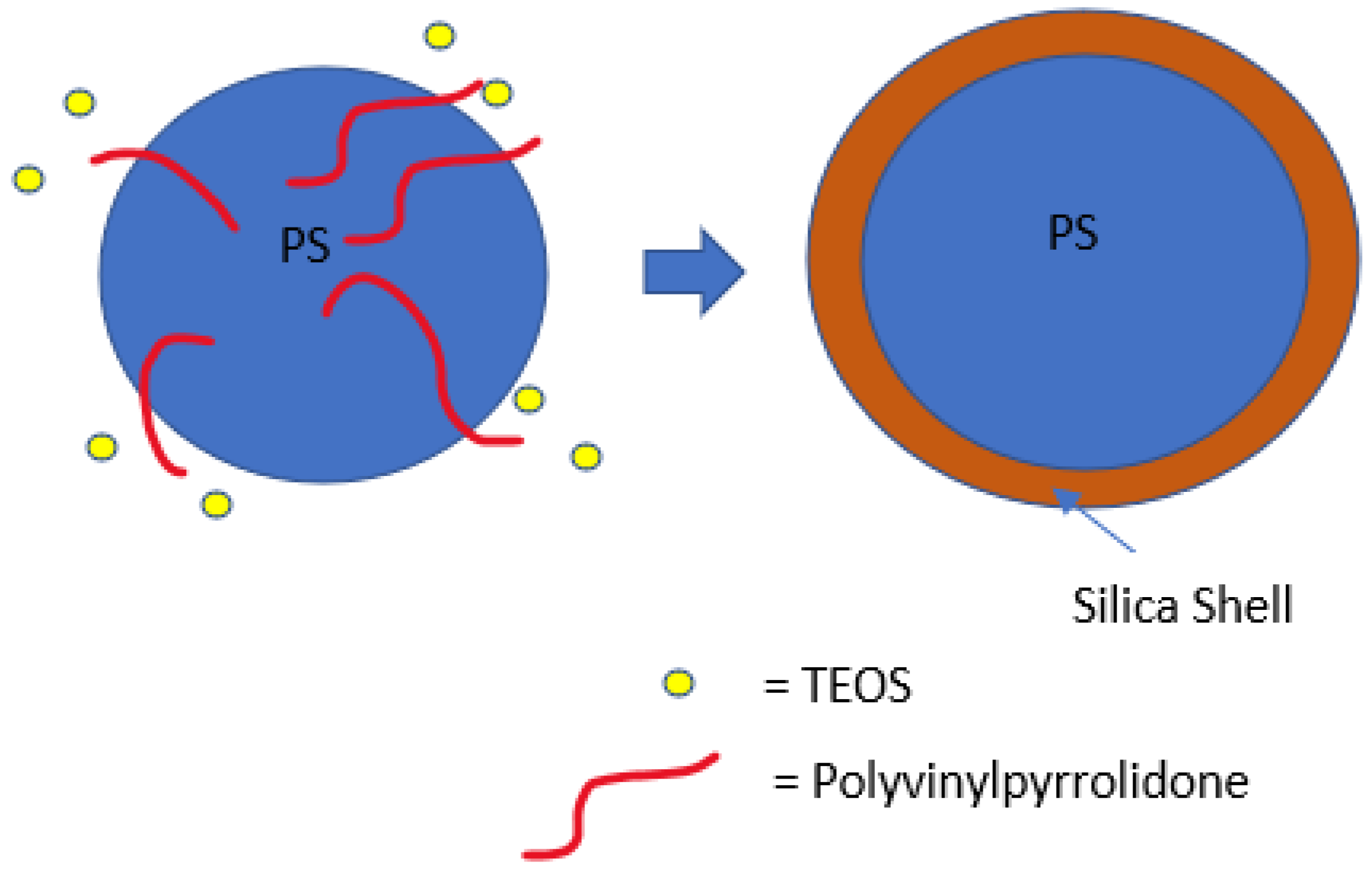
6. Polystyrene Coating
7. Surface Modification
8. Effect of Surfactant
9. Supercritical Fluid
10. Other Factors
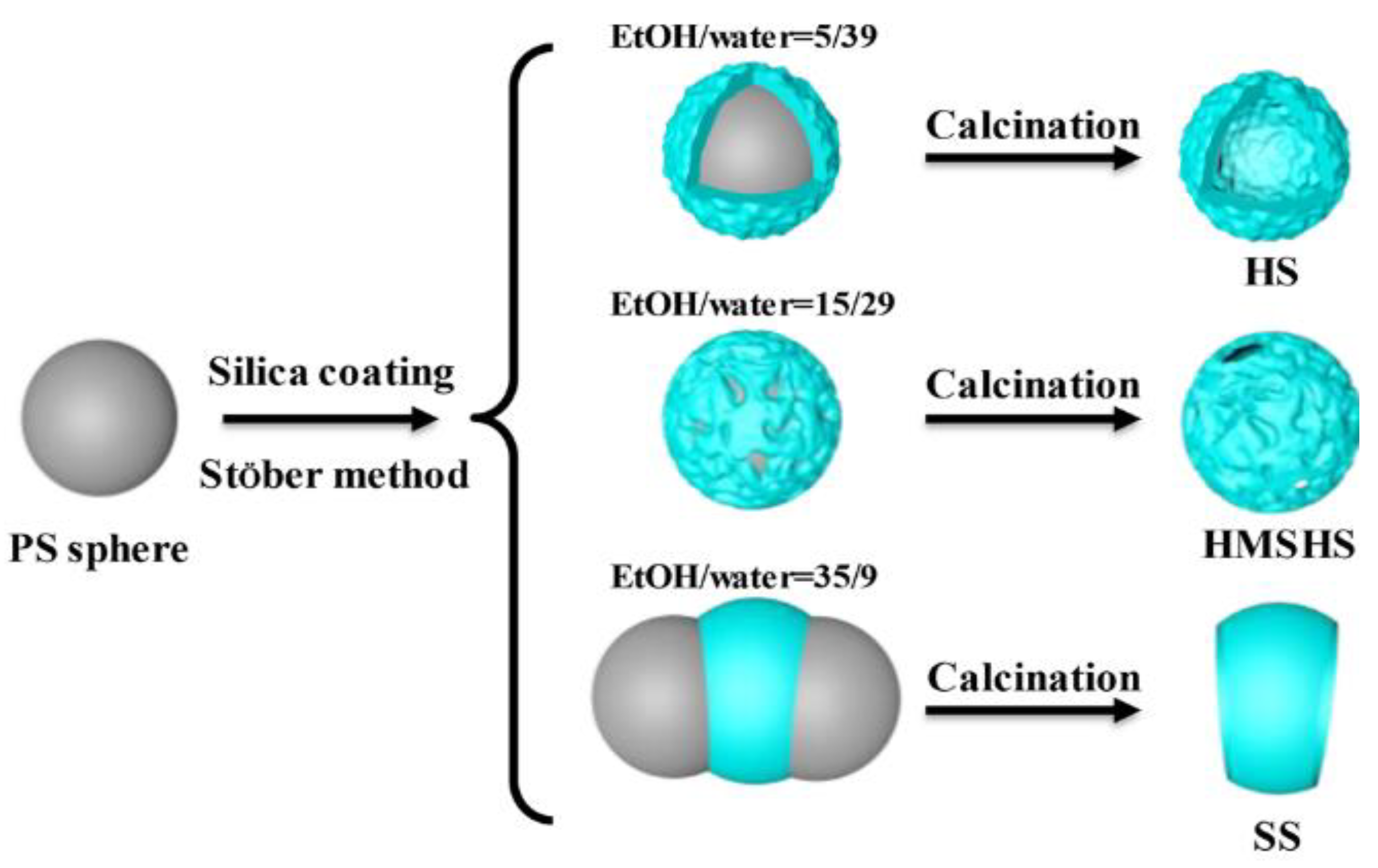
10.1. Effect of Type of Solution
10.2. Effect of Temperature in Calcination and Aging
10.3. Effect of pH
11. Applications
12. Summary
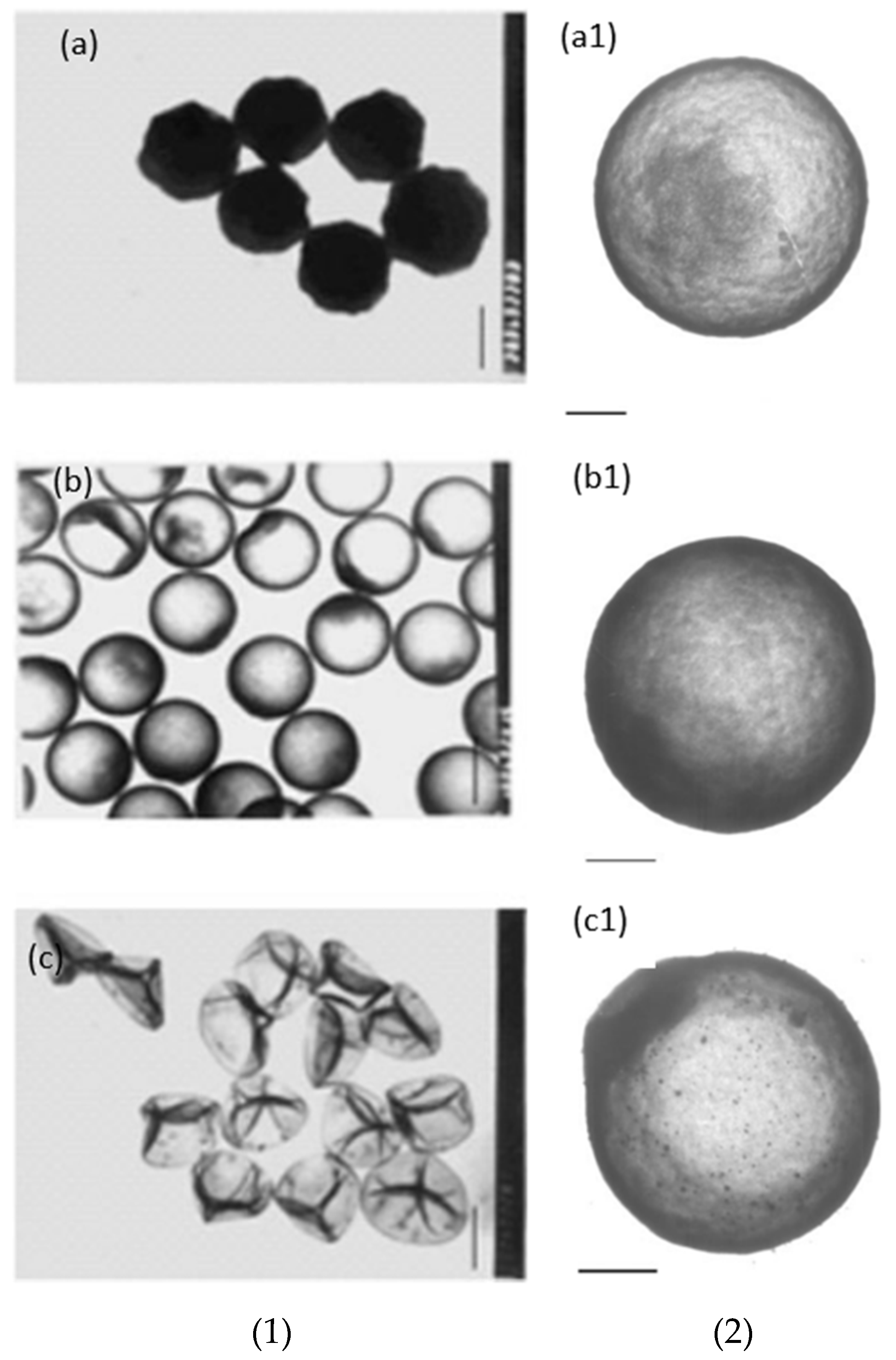
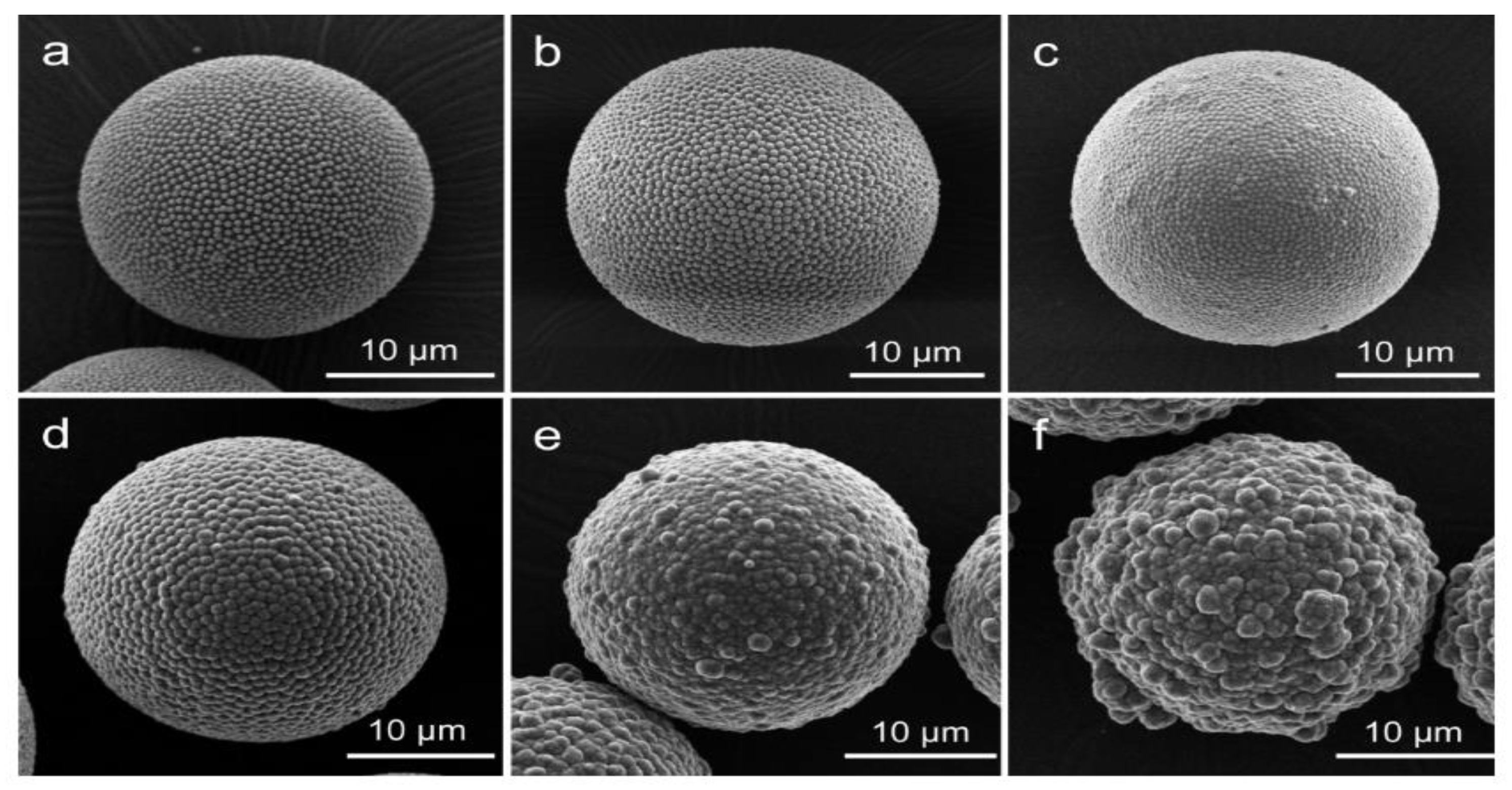
- Controlling the TEOS concentration is the best way to control the reaction kinetics and have high efficiency in the coating. TEOS concentration should be as low as possible to suppress the secondary nucleation by starving the system from the precursor, so the amount of free solid particles would be low. Most of the TEOS will be consumed by the PS surface as there will be less competition. However, if too low, a uniform coating cannot be achieved as there is insufficient TEOS to cover the surface, while a high TEOS concentration results in an increased number of free solid silica particles.
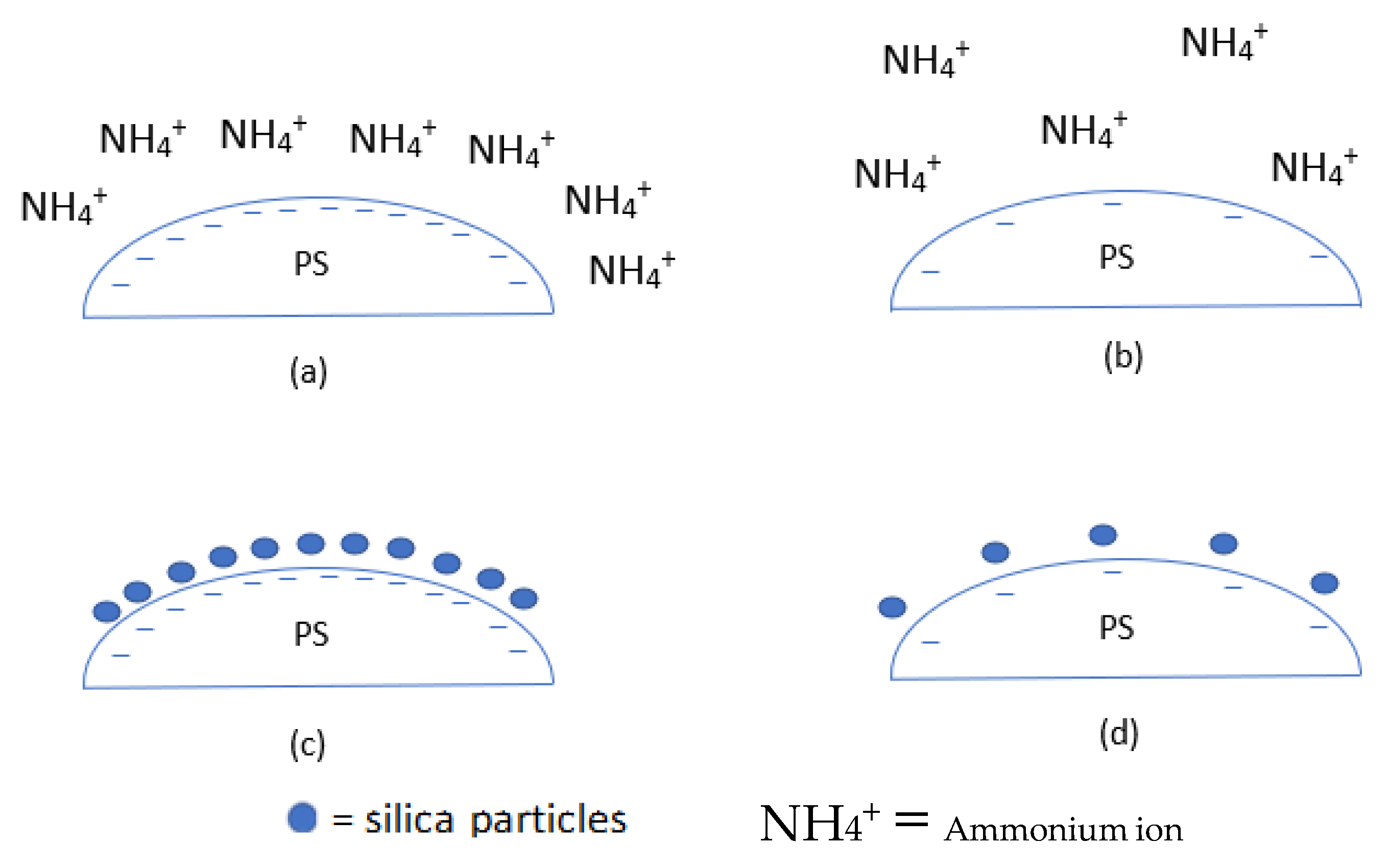
- The catalyst has been pointed out as a primary factor in controlling the sol–gel reaction and affecting the coating of PS. The concentration and strength of the catalyst play a vital role in controlling pH as well as the reaction kinetics of the sol–gel and has been shown to affect the structure by influencing the degree of hydrolysis during the induction period. Hollow silica spheres have been produced in low and high pH, depending on the other chosen parameters.
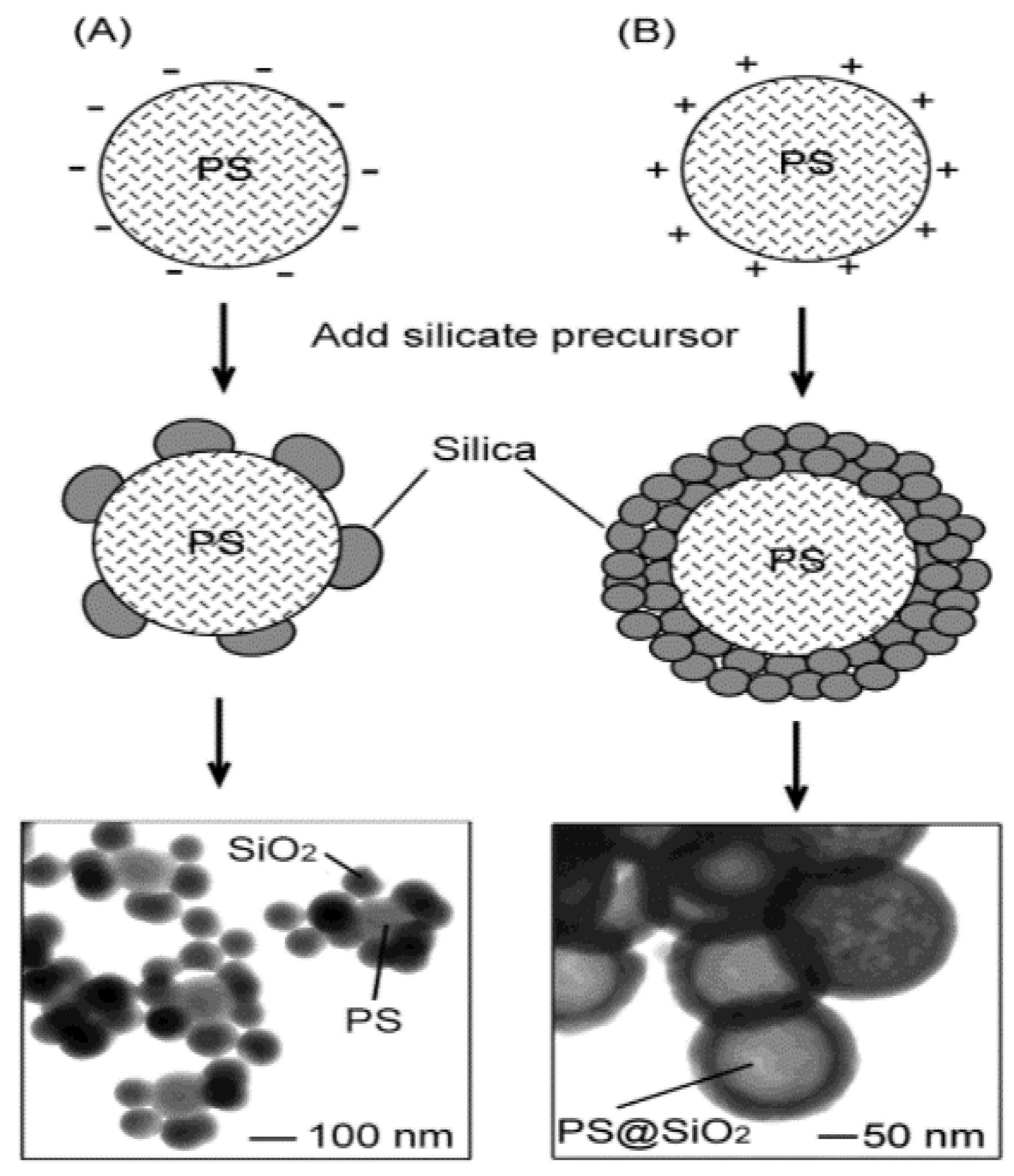
- Initiators and stabilisers used during the synthesis of PS have been shown to significantly affect the coating by providing electrostatic attraction and nucleation sites, respectively. Functional groups can be introduced during template synthesis or later via coating or chemical treatment to produce the desired surface charge. Both negatively and positively charged PS has been used to make hollow silica spheres. It has been suggested that positively charged PS would be better for coating as the coating’s negatively charged surface is greatly affected by the charge density.
- Surfactants have been shown to improve the coating significantly and it has been suggested that they act as a linker between the template surface and silica. It also has been recommended to act as a pore structuring agent. Surfactant concentration affects the pore distribution but not the pore size. This can be used to control the porosity. The morphology, shell thickness, and pore distribution can be tuned by controlling the surfactant concentration. The surfactant concentration was shown to affect the stability of hollow silica, and this effect could also be tuned by varying the ethanol:water ratio in the solution.
- Chemicals such as polymers, silica particles, and other reactive chemicals can be introduced onto the surface of PS during or after the synthesis of spheres to improve the coating. After the synthesis of PS spheres, reactive chemicals can be introduced by coating. Subjecting it to supercritical CO2 can make the PS template vulnerable to penetration by TEOS. The overall aim of these methods was to have more reactive chemicals on the surface, which will have better interaction with silica in the solution than PS.
Author Contributions
Funding
Conflicts of Interest
References
- Rosace, G.; Guido, E.; Colleoni, C.; Barigozzi, G. Influence of Textile Structure and Silica Based Finishing on Thermal Insulation Properties of Cotton Fabrics. Int. J. Polym. Sci. 2016, 2016, 1726475. [Google Scholar] [CrossRef]
- Jurkić, L.M.; Cepanec, I.; Pavelić, S.K.; Pavelić, K. Biological and Therapeutic Effects of Ortho-Silicic Acid and Some Ortho-Silicic Acid-Releasing Compounds: New Perspectives for Therapy. Nutr. Metab. 2013, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-Evaluation of Silicon Dioxide (E 551) as a Food Additive. EFSA J. 2018, 16, e05088. [Google Scholar] [CrossRef] [PubMed]
- Fruijtier-Pölloth, C. The Safety of Nanostructured Synthetic Amorphous Silica (SAS) as a Food Additive (E 551). Arch. Toxicol. 2016, 90, 2885–2916. [Google Scholar] [CrossRef] [PubMed]
- Lecloux, A.J.; Bronckart, J.; Noville, F.; Dodet, C.; Marchot, P.; Pirard, J.P. Study of the Texture of Monodisperse Silica Sphere Samples in the Nanometer Size Range. Colloids Surf. 1986, 19, 359–374. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Ren, J.; Liu, Y.; Rahman, M.M.; Shon, H.K.; Naidu, R. Hollow Porous Silica Nanosphere with Single Large Pore Opening for Pesticide Loading and Delivery. ACS Appl. Nano Mater. 2020, 3, 105–113. [Google Scholar] [CrossRef]
- Li, X.; Shi, Z.; Cui, Z.; Zhu, S. Silver Chloride Loaded Hollow Mesoporous Silica Particles and Their Application in the Antibacterial Coatings on Denture Base. Chem. Res. Chin. Univ. 2018, 34, 495–499. [Google Scholar] [CrossRef]
- Stober, W.; Fink, A. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Hong, Z.; Wei, D.; Yong, F.; Hao, C.; Yang, Y.; Yu, J.; Jin, L. Dielectric Properties of Polyimide/SiO2 Hollow Spheres Composite Films with Ultralow Dielectric Constant. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2016, 203, 13–18. [Google Scholar] [CrossRef]
- Ernawati, L.; Ogi, T.; Balgis, R.; Okuyama, K.; Stucki, M.; Hess, S.C.; Stark, W.J. Hollow Silica as an Optically Transparent and Thermally Insulating Polymer Additive. Langmuir 2016, 32, 338–345. [Google Scholar] [CrossRef]
- Specialty Silica Market Size, Share & Trends Analysis Report by Product (Precipitated, Silica Gel, Colloidal, Fumed), by Application (Rubber, Paints & Coatings, Food), And Segment Forecasts, 2019–2025. Available online: https://www.grandviewresearch.com/industry-analysis/specialty-silica-market (accessed on 9 November 2021).
- Ng, S.; Jelle, B.P.; Sandberg, L.I.; Gao, T.; Alex Mofid, S. Hollow Silica Nanospheres as Thermal Insulation Materials for Construction: Impact of Their Morphologies as a Function of Synthesis Pathways and Starting Materials. Constr. Build Mater. 2018, 166, 72–80. [Google Scholar] [CrossRef]
- Li, Z.Z.; Xu, S.A.; Wen, L.X.; Liu, F.; Liu, A.Q.; Wang, Q.; Sun, H.Y.; Yu, W.; Chen, J.F. Controlled Release of Avermectin from Porous Hollow Silica Nanoparticles: Influence of Shell Thickness on Loading Efficiency, UV-Shielding Property and Release. J. Control. Release 2006, 111, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Huang, W.; Yeh, C.S. Shell-by-Shell Synthesis of Multi-Shelled Mesoporous Silica Nanospheres for Optical Imaging and Drug Delivery. Biomaterials 2011, 32, 556–564. [Google Scholar] [CrossRef]
- Sharma, J.; Polizos, G. Hollow Silica Particles: Recent Progress and Future Perspectives. Nanomaterials 2020, 10, 1599. [Google Scholar] [CrossRef]
- Zhong, K.; Song, K.; Clays, K. Hollow Spheres: Crucial Building Blocks for Novel Nanostructures and Nanophotonics. Nanophotonics 2018, 7, 693–713. [Google Scholar] [CrossRef]
- Jafelicci, M., Jr.; Davolos, M.R.; Jos, F.; Jos, S. Hollow Silica Particles from Microemulsion. J. Non-Cryst. Solids 1999, 247, 98–102. [Google Scholar] [CrossRef]
- Hah, H.J.; Kim, J.S.; Jeon, B.J.; Koo, S.M.; Lee, Y.E. Simple Preparation of Monodisperse Hollow Silica Particles without Using Templates. Chem. Commun. 2003, 3, 1712–1713. [Google Scholar] [CrossRef]
- Yoon, H.; Hong, J.; Park, C.W.; Park, D.W.; Shim, S.E. An Inexpensive Route to Prepare Mesoporous Hollow Silica Microspheres Using W/O Ethanol/Edible Soybean Oil Macroemulsion as the Template. Mater. Lett. 2009, 63, 2047–2050. [Google Scholar] [CrossRef]
- Le, Y.; Chen, J.F.; Wang, J.X.; Shao, L.; Wang, W.C. A Novel Pathway for Synthesis of Silica Hollow Spheres with Mesostructured Walls. Mater. Lett. 2004, 58, 2105–2108. [Google Scholar] [CrossRef]
- Fuji, M.; Shin, T.; Watanabe, H.; Takei, T. Shape-Controlled Hollow Silica Nanoparticles Synthesized by an Inorganic Particle Template Method. Adv. Powder Technol. 2012, 23, 562–565. [Google Scholar] [CrossRef]
- Nakashima, Y.; Takai, C.; Razavi-Khosroshahi, H.; Suthabanditpong, W.; Fuji, M. Synthesis of Ultra-Small Hollow Silica Nanoparticles Using the Prepared Amorphous Calcium Carbonate in One-Pot Process. Adv. Powder Technol. 2018, 29, 904–908. [Google Scholar] [CrossRef]
- Williamson, P.A.; Blower, P.J.; Green, M.A. Synthesis of Porous Hollow Silica Nanostructures Using Hydroxyapatite Nanoparticle Templates. Chem. Commun. 2011, 47, 1568–1570. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, J. Hollow-Structured Mesoporous Materials: Chemical Synthesis, Functionalization and Applications. Adv. Mater. 2014, 26, 3176–3205. [Google Scholar] [CrossRef]
- Brinker, J.C.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Li, B.; Wang, X.; Yan, M.; Li, L. Preparation and Characterization of Nano-TiO2 Powder. Mater. Chem. Phys. 2002, 78, 184–188. [Google Scholar] [CrossRef]
- Vijayalakshmi, R.; Rajendran, V. Synthesis and Characterization of Nano-TiO2 via Different Methods. Arch. Appl. Sci. Res. 2012, 4, 1183–1190. Available online: www.scholarsresearchlibrary.com (accessed on 8 September 2021).
- Jaroenworaluck, A.; Sunsaneeyametha, W.; Kosachan, N.; Stevens, R. Characteristics of Silica-Coated TiO2 and Its UV Absorption for Sunscreen Cosmetic Applications. Surf. Interface Anal. 2006, 38, 473–477. [Google Scholar] [CrossRef]
- Verma, R.; Mantri, B.; Ramphal; Srivastava, A.K. Shape Control Synthesis, Characterizations, Mechanisms and Optical Properties of Large Scaled Metal Oxide Nanostructures of ZnO and TiO. Adv. Mater. Lett. 2015, 6, 324–333. [Google Scholar] [CrossRef]
- Verma, R.; Awasthi, A.; Singh, P.; Srivastava, R.; Sheng, H.; Wen, J.; Miller, D.J.; Srivastava, A.K. Interactions of Titania Based Nanoparticles with Silica and Green-Tea: Photo-Degradation and -Luminescence. J. Colloid Interface Sci. 2016, 475, 82–95. [Google Scholar] [CrossRef]
- Li, H.; Bian, Z.; Zhu, J.; Zhang, D.; Li, G.; Huo, Y.; Li, H.; Lu, Y. Mesoporous Titania Spheres with Tunable Chamber Stucture and Enhanced Photocatalytic Activity. J. Am. Chem. Soc. 2007, 129, 8406–8407. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Robben, L.; Alkaim, A.; Bahnemann, D. Brookite versus Anatase TiO2 Photocatalysts: Phase Transformations and Photocatalytic Activities. In Photochemical and Photobiological Sciences; Royal Society of Chemistry: London, UK, 2013; Volume 12, pp. 602–609. [Google Scholar] [CrossRef]
- Zhang, L.; Menendez-Flores, V.M.; Murakami, N.; Ohno, T. Improvement of Photocatalytic Activity of Brookite Titanium Dioxide Nanorods by Surface Modification Using Chemical Etching. Appl. Surf. Sci. 2012, 258, 5803–5809. [Google Scholar] [CrossRef]
- Righini, G.C.; Chiappini, A. Glass Optical Waveguides: A Review of Fabrication Techniques. Opt. Eng. 2014, 53, 071819. [Google Scholar] [CrossRef]
- Meier, M.; Ungerer, J.; Klinge, M.; Nirschl, H. Synthesis of Nanometric Silica Particles via a Modified Stöber Synthesis Route. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 559–564. [Google Scholar] [CrossRef]
- Yokoi, T.; Wakabayashi, J.; Otsuka, Y.; Fan, W.; Iwama, M.; Watanabe, R.; Aramaki, K.; Shimojima, A.; Tatsumi, T.; Okubo, T. Mechanism of Formation of Uniform-Sized Silica Nanospheres Catalyzed by Basic Amino Acids. Chem. Mater. 2009, 21, 3719–3729. [Google Scholar] [CrossRef]
- Masalov, V.M.; Sukhinina, N.S.; Emel’chenko, G.A. Synthesis of Monodisperse Silica Nanoparticles via Heterogeneous Tetraethoxysilane Hydrolysis Using L-Arginine as a Catalyst. Inorg. Mater. 2018, 54, 156–162. [Google Scholar] [CrossRef]
- Bernards, T.N.M.; van Bommel, M.J.; Boonstra, A.H. Hydrolysis-Condensation Processes of the Tetra-Alkoxysilanes TPOS, TEOS and TMOS in Some Alcoholic Solvents. J. Non Cryst. Solids 1991, 134, 1–13. [Google Scholar] [CrossRef]
- LaMer, V.K.; Dinegar, R.H. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Chiang, Y.D.; Lian, H.Y.; Leo, S.Y.; Wang, S.G.; Yamauchi, Y.; Wu, K.C.W. Controlling Particle Size and Structural Properties of Mesoporous Silica Nanoparticles Using the Taguchi Method. J. Phys. Chem. C 2011, 115, 13158–13165. [Google Scholar] [CrossRef]
- Qi, D.; Lin, C.; Zhao, H.; Liu, H.; Lü, T. Size Regulation and Prediction of the SiO2 Nanoparticles Prepared via Stöber Process. J. Dispers. Sci. Technol. 2017, 38, 70–74. [Google Scholar] [CrossRef]
- Han, Y.; Lu, Z.; Teng, Z.; Liang, J.; Guo, Z.; Wang, D.; Han, M.Y.; Yang, W. Unraveling the Growth Mechanism of Silica Particles in the Stöber Method: In Situ Seeded Growth Model. Langmuir 2017, 33, 5879–5890. [Google Scholar] [CrossRef]
- Rao, K.S.; El-Hami, K.; Kodaki, T.; Matsushige, K.; Makino, K. A Novel Method for Synthesis of Silica Nanoparticles. J. Colloid Interface Sci. 2005, 289, 125–131. [Google Scholar] [CrossRef]
- Wang, X.D.; Shen, Z.X.; Sang, T.; Cheng, X.B.; Li, M.F.; Chen, L.Y.; Wang, Z.S. Preparation of Spherical Silica Particles by Stöber Process with High Concentration of Tetra-Ethyl-Orthosilicate. J. Colloid Interface Sci. 2010, 341, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Wu, C.Y.; Chung, C.C.; Lai, M.H.; Chung, T.W. Analysis of Parameters and Interaction between Parameters in Preparation of Uniform Silicon Dioxide Nanoparticles Using Response Surface Methodology. Ind. Eng. Chem. Res. 2006, 45, 8043–8048. [Google Scholar] [CrossRef]
- Satoh, T.; Akitaya, M.; Konno, M.; Saito, S. Particle Size Distributions Produced by Hydrolysis and Condensation of Tetraethylorthosilicate. J. Chem. Eng. Jpn. 1997, 30, 759–762. [Google Scholar] [CrossRef]
- van Helden, A.K.; Jansen, J.W.; Vrij, A. Preparation and Characterization of Spherical Monodisperse Silica Dispersions in Nonaqueous Solvents. J. Colloid Interface Sci. 1981, 81, 354–368. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, K.D.; Kim, H.T. Preparation of Silica Nanoparticles: Determination of the Optimal Synthesis Conditions for Small and Uniform Particles. Colloids Surf. A Physicochem. Eng. Asp. 2002, 197, 7–17. [Google Scholar] [CrossRef]
- Bogush, G.H.; Tracy, M.A.; Zukoski Iv, C.F. Preparation of Monodisperse Silica Particles: Control of Size and Mass Fraction. J. Non-Cryst. Solids 1988, 104, 95–106. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, D.; Ma, H.; Sun, Z.; Guan, J. Controllable Preparation and Formation Mechanism of Monodispersed Silica Particles with Binary Sizes. J. Colloid Interface Sci. 2012, 388, 40–46. [Google Scholar] [CrossRef]
- Milea, C.A.; Bogatu, C.; Duta, A. The Influence of Parameters in Silica Sol-Gel Process. Eng. Sci. 2011, 4, 59–66. [Google Scholar]
- Fernandes, R.S.; Raimundo, I.M.; Pimentel, M.F. Revising the Synthesis of Stöber Silica Nanoparticles: A Multivariate Assessment Study on the Effects of Reaction Parameters on the Particle Size. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 1–7. [Google Scholar] [CrossRef]
- Chen, S.L.; Dong, P.; Yang, G.H.; Yang, J.J. Characteristic Aspects of Formation of New Particles during the Growth of Monosize Silica Seeds. J. Colloid Interface Sci. 1996, 180, 237–241. [Google Scholar] [CrossRef]
- Sang, M.C.; Lee, M.; Kim, W.S. Preparation of Large Monodispersed Spherical Silica Particles Using Seed Particle Growth. J. Colloid Interface Sci. 2005, 286, 536–542. [Google Scholar] [CrossRef]
- Spanhel, L.; Anderson, M.A. Semiconductor Clusters in the Sol-Gel Process: Quantized Aggregation, Gelation, and Crystal Growth in Concentrated ZnO Colloids. J. Am. Chem. Soc. 1991, 113, 2826–2833. [Google Scholar] [CrossRef]
- Issa, A.A.; Luyt, A.S. Kinetics of Alkoxysilanes and Organoalkoxysilanes Polymerization: A Review. Polymers 2019, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhou, S.; Wu, L.; Xie, S.; Chen, Y. Preparation of Silica-Coated Polystyrene Hybrid Spherical Colloids. Macromol. Chem. Phys. 2005, 206, 1896–1902. [Google Scholar] [CrossRef]
- Sandberg, L.I.C.; Gao, T.; Jelle, B.P.; Gustavsen, A. Synthesis of Hollow Silica Nanospheres by Sacrificial Polystyrene Templates for Thermal Insulation Applications. Adv. Mater. Sci. Eng. 2016, 2013, 483651. [Google Scholar] [CrossRef]
- Tan, B.; Rankin, S.E. Dual Latex/Surfactant Templating of Hollow Spherical Silica Particles with Ordered Mesoporous Shells. Langmuir 2005, 21, 8180–8187. [Google Scholar] [CrossRef]
- Khoeini, M.; Najafi, A.; Rastegar, H.; Amani, M. Improvement of Hollow Mesoporous Silica Nanoparticles Synthesis by Hard-Templating Method via CTAB Surfactant. Ceram. Int. 2019, 45, 12700–12707. [Google Scholar] [CrossRef]
- Wu, X.; Tian, Y.; Cui, Y.; Wei, L.; Wang, Q.; Chen, Y. Raspberry-like Silica Hollow Spheres: Hierarchical Structures by Dual Latex-Surfactant Templating Route. J. Phys. Chem. C 2007, 111, 9704–9708. [Google Scholar] [CrossRef]
- Yin, L.; Tian, Q.; Boyjoo, Y.; Hou, G.; Shi, X.; Liu, J. Synthesis of Colloidal Mesoporous Silica Spheres with Large Through-Holes on the Shell. Langmuir 2020, 36, 6984–6993. [Google Scholar] [CrossRef]
- Rivera-Virtudazo, R.V.; Fuji, M.; Takai, C.; Shirai, T. Fabrication of Unique Hollow Silicate Nanoparticles with Hierarchically Micro/Mesoporous Shell Structure by a Simple Double Template Approach. Nanotechnology 2012, 23. [Google Scholar] [CrossRef]
- Vu, K.B.; Khoa, T.; Tran, T.T.T.; Mugemana, C.; Giang, H.N.; Le, T.; Nhi, P. Polystyrene Nanoparticles Prepared by Nanoprecipitation: A Recyclable Template for Fabricating Hollow Silica. J. Ind. Eng. Chem. 2021, 97, 307–315. [Google Scholar] [CrossRef]
- Peng, W.; Zhang, Z.; Rong, M.; Zhang, M. Core-Shell Structure Design of Hollow Mesoporous Silica Nanospheres Based on Thermo-Sensitive PNIPAM and PH-Responsive Catechol-Fe3+ Complex. Polymers 2019, 11, 1832. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A.; Magazù, S.; Calandra, P. Amphiphiles Self-Assembly: Basic Concepts and Future Perspectives of Supramolecular Approaches. Adv. Condens. Matter Phys. 2015, 2015, 151683. [Google Scholar] [CrossRef]
- Li, D.; Guan, Z.; Zhang, W.; Zhou, X.; Zhang, W.Y.; Zhuang, Z.; Wang, X.; Yang, C.J. Synthesis of Uniform-Size Hollow Silica Microspheres through Interfacial Polymerization in Monodisperse Water-in-Oil Droplets. ACS Appl. Mater. Interfaces 2010, 2, 2711–2714. [Google Scholar] [CrossRef]
- Jeong, W.C.; Choi, M.; Lim, C.H.; Yang, S.M. Microfluidic Synthesis of Atto-Liter Scale Double Emulsions toward Ultrafine Hollow Silica Spheres with Hierarchical Pore Networks. Lab Chip 2012, 12, 5262–5271. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Ma, C.; Yan, S.; Inglis, D.; Feng, S. A Review of Capillary Pressure Control Valves in Microfluidics. Biosensors 2021, 11, 405. [Google Scholar] [CrossRef]
- Lima, R.; Oliveira, M.S.N.; Ishikawa, T.; Kaji, H.; Tanaka, S.; Nishizawa, M.; Yamaguchi, T. Axisymmetric Polydimethysiloxane Microchannels for in Vitro Hemodynamic Studies. Biofabrication 2009, 1, 035005. [Google Scholar] [CrossRef]
- Olanrewaju, A.; Beaugrand, M.; Yafia, M.; Juncker, D. Capillary Microfluidics in Microchannels: From Microfluidic Networks to Capillaric Circuits. Lab Chip 2018, 18, 2323–2347. [Google Scholar] [CrossRef]
- Hassan, S.U.; Tariq, A.; Noreen, Z.; Donia, A.; Zaidi, S.Z.J.; Bokhari, H.; Zhang, X. Capillary-Driven Flow Microfluidics Combined with Smartphone Detection: An Emerging Tool for Point-of-Care Diagnostics. Diagnostics 2020, 10, 509. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and Applications of Microfluidic Devices: A Review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef]
- Marre, S.; Jensen, K.F. Synthesis of Micro and Nanostructures in Microfluidic Systems. Chem. Soc. Rev. 2010, 39, 1183–1202. [Google Scholar] [CrossRef] [PubMed]
- Krishna, K.S.; Li, Y.; Li, S.; Kumar, C.S.S.R. Lab-on-a-Chip Synthesis of Inorganic Nanomaterials and Quantum Dots for Biomedical Applications. Adv. Drug Deliv. Rev. 2013, 65, 1470–1495. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Qui, S.; Osamu, T.; Wei, Y. Polystyrene Bead-Assisted Self-Assembly of Microstructured Silica Hollow Spheres in Highly Alkaline Media. J. Am. Chem. Soc. 2001, 123, 7723–7724. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, L.; Zhou, S.; You, B. A Method for the Fabrication of Monodisperse Hollow Silica Spheres. Adv. Mater. 2006, 18, 801–806. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, M.; Zhou, S.; You, B.; Wu, L. A Novel Method for the Fabrication of Monodisperse Hollow Silica Spheres. Langmuir 2006, 22, 6403–6407. [Google Scholar] [CrossRef]
- Blas, H.; Save, M.; Pasetto, P.; Boissière, C.; Sanchez, C.; Charleux, B. Elaboration of Monodisperse Spherical Hollow Particles with Ordered Mesoporous Silica Shells via Dual Latex/Surfactant Templating: Radial Orientation of Mesopore Channels. Langmuir 2008, 24, 13132–13137. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Ran, Q.; Shen, J. A Simple and Low-Cost Method for the Preparation of Monodisperse Hollow Silica Spheres. J. Phys. Chem. C 2008, 112, 11623–11629. [Google Scholar] [CrossRef]
- Ge, C.; Zhang, D.; Wang, A.; Yin, H.; Ren, M.; Liu, Y.; Jiang, T.; Yu, L. Synthesis of Porous Hollow Silica Spheres Using Polystyrene-Methyl Acrylic Acid Latex Template at Different Temperatures. J. Phys. Chem. Solids 2009, 70, 1432–1437. [Google Scholar] [CrossRef]
- Zhang, L.; D’Acunzi, M.; Kappl, M.; Auernhammer, G.K.; Vollmer, D.; van Kats, C.M.; van Blaaderen, A. Hollow Silica Spheres: Synthesis and Mechanical Properties. Langmuir 2009, 25, 2711–2717. [Google Scholar] [CrossRef]
- Qi, G.; Wang, Y.; Estevez, L.; Switzer, A.K.; Duan, X.; Yang, X.; Giannelis, E.P. Facile and Scalable Synthesis of Monodispersed Spherical Capsules with a Mesoporous Shell. Chem. Mater. 2010, 22, 2693–2695. [Google Scholar] [CrossRef]
- Kato, N.; Ishii, T.; Koumoto, S. Synthesis of Monodisperse Mesoporous Silica Hollow Microcapsules and Their Release of Loaded Materials. Langmuir 2010, 26, 14334–14344. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, M.; Wang, D.; Zhang, W.; Liu, S. Synthesis of Hollow Mesoporous Silica Microspheres through Surface Sol–Gel Process on Polystyrene-Co-Poly(4-Vinylpyridine) Core–Shell Microspheres. Microporous Mesoporous Mater. 2011, 139, 1–7. [Google Scholar] [CrossRef]
- Wei, M.; van Oers, C.J.; Hao, X.; Qiu, Q.; Cool, P.; Liu, S. Influence of Silica Forming Media on the Synthesis of Hollow Silica Microspheres. Microporous Mesoporous Mater. 2011, 138, 17–21. [Google Scholar] [CrossRef]
- Fan, H.; Lei, Z.; Pan, J.H.; Zhao, X.S. Sol-Gel Synthesis, Microstructure and Adsorption Properties of Hollow Silica Spheres. Mater. Lett. 2011, 65, 1811–1814. [Google Scholar] [CrossRef]
- Sun, G.; Chen, Z.; Wang, S.; Li, L.; Fu, J.; Chen, J.; Xu, Q. Preparation of Hollow Silica Microspheres with Controlled Shell Thickness in Supercritical Fluids. Colloid Polym. Sci. 2011, 289, 1397–1406. [Google Scholar] [CrossRef]
- Qu, Q.; Si, Y.; Xuan, H.; Zhang, K.; Chen, X.; Ding, Y.; Feng, S.; Yu, H.Q. Synthesis of Core-Shell Silica Spheres with Tunable Pore Diameters for HPLC. Mater. Lett. 2018, 211, 40–42. [Google Scholar] [CrossRef]
- Li, J.; Dong, F.; Lu, L.; Li, H.; Xiong, Y.; Ha, C.S. Raspberry-Like Polysilsesquioxane Particles with Hollow-Spheres-on-Sphere Structure: Rational Design, Controllable Synthesis, and Catalytic Application. Polymers 2019, 11, 1350. [Google Scholar] [CrossRef]
- Sharma, J.; Cullen, D.A.; Polizos, G.; Nawaz, K.; Wang, H.; Muralidharan, N.; Smith, D.B. Hybrid Hollow Silica Particles: Synthesis and Comparison of Properties with Pristine Particles. RSC Adv. 2020, 10, 22331–22334. [Google Scholar] [CrossRef]
- Hong, J.; Han, H.; Hong, C.K.; Shim, S.E. A Direct Preparation of Silica Shell on Polystyrene Microspheres Prepared by Dispersion Polymerization with Polyvinylpyrrolidone. Rapid Commun. 2008, 46, 2884–2890. [Google Scholar] [CrossRef]
- Etching, D.S.; Chen, Y.; Chen, H.; Guo, L.; He, Q.; Chen, F.; Zhou, J.; Feng, J.; Shi, J. Hollow/Rattle-Type Mesoporous Nanostructures by a Structural Difference-Based Selective Etching Strategy. ACS Nano 2010, 4, 529–539. [Google Scholar]
- Zhang, T.; Zhang, Q.; Ge, J.; Goebl, J.; Sun, M.; Yan, Y.; Liu, Y.S.; Chang, C.; Guo, J.; Yin, Y. A Self-Templated Route to Hollow Silica Microspheres. J. Phys. Chem. C 2009, 113, 3168–3175. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, T.; Ge, J.; Yin, Y. Permeable Silica Shell through Surface-Protected Etching. Nano Lett. 2008, 8, 2867–2871. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.N.; Park, Y.S. Synthesis of Stable Hollow Silica Nanospheres. J. Ind. Eng. Chem. 2009, 15, 365–369. [Google Scholar] [CrossRef]
- Belostozky, A.; Bretler, S.; Kolitz-Domb, M.; Grinberg, I.; Margel, S. Solidification of Oil Liquids by Encapsulation within Porous Hollow Silica Microspheres of Narrow Size Distribution for Pharmaceutical and Cosmetic Applications. Mater. Sci. Eng. C 2019, 97, 760–767. [Google Scholar] [CrossRef]
- Lu, Y.; McLellan, J.; Xia, Y. Synthesis and Crystallization of Hybrid Spherical Colloids Composed of Polystyrene Cores and Silica Shells. Langmuir 2004, 20, 3464–3470. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Takai, C.; Khosroshahi, H.R.; Fuji, M.; Shirai, T. Surfactant-Free Fabrication of SiO2-Coated Negatively Charged Polymer Beads and Monodisperse Hollow SiO2 Particles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 375–383. [Google Scholar] [CrossRef]
- Shang, Q.; Zhou, Y. Facile Fabrication of Hollow Mesoporous Silica Microspheres with Hierarchical Shell Structure via a Sol–Gel Process. J. Solgel Sci. Technol. 2015, 75, 206–214. [Google Scholar] [CrossRef]
- Liu, C.; Ge, C.; Wang, A.; Yin, H.; Ren, M.; Zhang, Y.; Yu, L.; Jiang, T. Synthesis of Porous Hollow Silica Spheres Using Functionalized Polystyrene Latex Spheres as Templates. Korean J. Chem. Eng. 2011, 28, 1458–1463. [Google Scholar] [CrossRef]
- Wei, X.; Gong, C.; Chen, X.; Fan, G.; Xu, X. Preparation of Porous Hollow Silica Spheres via a Layer-by-Layer Process and the Chromatographic Performance. Front. Mater. Sci. 2017, 11, 33–41. [Google Scholar] [CrossRef]
- Guo, Y.; Andrew Davidson, R.; Peck, K.A.; Guo, T. Encapsulation of Multiple Large Spherical Silica Nanoparticles in Hollow Spherical Silica Shells. J. Colloid Interface Sci. 2015, 445, 112–118. [Google Scholar] [CrossRef]
- Gorsd, M.N.; Pizzio, L.R.; Blanco, M.N. Synthesis and Characterization of Hollow Silica Spheres. Procedia Mater. Sci. 2015, 8, 567–576. [Google Scholar] [CrossRef]
- Zhang, L.; D’Acunzi, M.; Kappl, M.; Imhof, A.; van Blaaderen, A.; Butt, H.J.; Graf, R.; Vollmer, D. Tuning the Mechanical Properties of Silica Microcapsules. Phys. Chem. Chem. Phys. 2010, 12, 15392–15398. [Google Scholar] [CrossRef] [PubMed]
- Ruckdeschel, P.; Kemnitzer, T.W.; Nutz, F.A.; Senker, J.; Retsch, M. Hollow Silica Sphere Colloidal Crystals: Insights into Calcination Dependent Thermal Transport. Nanoscale 2015, 7, 10059–10070. [Google Scholar] [CrossRef] [PubMed]
- Takafuji, M.; Hano, N.; Alam, M.A.; Ihara, H. Fabrication of Hollow Silica Microspheres with Orderly Hemispherical Protrusions and Capability for Heat-Induced Controlled Cracking. Langmuir 2017, 33, 10679–10689. [Google Scholar] [CrossRef]
- Chen, Z.; Li, S.; Xue, F.; Sun, G.; Luo, C.; Chen, J.; Xu, Q. A Simple and Efficient Route to Prepare Inorganic Hollow Microspheres Using Polymer Particles as Template in Supercritical Fluids. Colloids Surf. A Physicochem. Eng. Asp. 2010, 355, 45–52. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Xu, B.; Dou, T. Synthesis and Characterization of Carbon Sphere-Silica Core–Shell Structure and Hollow Silica Spheres. Colloids Surf. A Physicochem. Eng. Asp. 2009, 345, 141–146. [Google Scholar] [CrossRef]
- Zhang, Q.; Ge, J.; Goebl, J.; Hu, Y.; Lu, Z.; Yin, Y. Rattle-Type Silica Colloidal Particles Prepared by a Surface-Protected Etching Process. Nano Res. 2009, 2, 583–591. [Google Scholar] [CrossRef]
- Zhang, T.; Ge, J.; Hu, Y.; Zhang, Q.; Aloni, S.; Yin, Y. Formation of Hollow Silica Colloids through a Spontaneous Dissolution-Regrowth Process. Angew. Chem. Int. Ed. 2008, 47, 5806–5811. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, L.; Liu, H.; Zhao, Y.; Zhang, Y.; Wang, Q.; Yu, Z.; Liu, Z. A Dual Template Method for Synthesizing Hollow Silica Spheres with Mesoporous Shells. Mater. Lett. 2009, 63, 258–259. [Google Scholar] [CrossRef]
- Matsoukas, T.; Gulari, E. Dynamics of Growth of Silica Particles from Ammonia-Catalyzed Hydrolysis of Tetra-Ethyl-Orthosilicate. J. Colloid Interface Sci. 1988, 124, 252–261. [Google Scholar] [CrossRef]
- Bogush, G.H.; Zukoski IV, C.F. Studies of the Kinetics of the Precipitation of Uniform Silica Particles through the Hydrolysis and Condensation of Silicon Alkoxides. J. Colloid Interface Sci. 1991, 142, 1–18. [Google Scholar] [CrossRef]
- Verwey, E.J.W.; Laboratorium, N.; Waals, D. Theory of the Stability of Lyophobic Colloids. J. Colloid Sci. 1946, 10, 631–636. [Google Scholar]
- Kim, S.; Zukoski, C.F. A Model of Growth by Hetero-Coagulation in Seeded Colloidal Dispersions. J. Colloid Interface Sci. 1990, 139, 198–212. [Google Scholar] [CrossRef]
- Ilgaz, S.; Sat, I.G.; Polat, A. Effects of Processing Parameters on the Caffeine Extraction Yield during Decaffeination of Black Tea Using Pilot-Scale Supercritical Carbon Dioxide Extraction Technique. J. Food Sci. Technol. 2018, 55, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Bourebrab, M.A.; Oben, D.T.; Durand, G.G.; Taylor, P.G.; Bruce, J.I.; Bassindale, A.R.; Taylor, A. Influence of the Initial Chemical Conditions on the Rational Design of Silica Particles. J. Solgel Sci. Technol. 2018, 88, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sugawara-Narutaki, A.; Fukao, M.; Yokoi, T.; Shimojima, A.; Okubo, T. Two-Phase Synthesis of Monodisperse Silica Nanospheres with Amines or Ammonia Catalyst and Their Controlled Self-Assembly. ACS Appl. Mater. Interfaces 2011, 3, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Takai-Yamashita, C.; Ando, M.; Noritake, M.; Khosroshahi, H.R.; Fuji, M. Emulsion Templating of Poly (Acrylic Acid) by Ammonium Hydroxide/Sodium Hydroxide Aqueous Mixture for High-Dispersed Hollow Silica Nanoparticles. Adv. Powder Technol. 2017, 28, 398–405. [Google Scholar] [CrossRef]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and Surface Functionalization of Silica Nanoparticles for Nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef]
- Yong, W.Y.D.; Zhang, Z.; Cristobal, G.; Chin, W.S. One-Pot Synthesis of Surface Functionalized Spherical Silica Particles. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 151–157. [Google Scholar] [CrossRef]
- Wang, J.G.; Li, F.; Zhou, H.J.; Sun, P.C.; Ding, D.T.; Chen, T.H. Silica Hollow Spheres with Ordered and Radially Oriented Amino-Functionalized Mesochannels. Chem. Mater. 2009, 21, 612–620. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, C.; Ma, J.; Huang, Y.; Cao, K.; Chang, G.; Yang, J. Ultra-Low Dielectric Closed Porous Materials via Incorporating Surface-Functionalized Hollow Silica Microspheres: Preparation, Interface Property and Low Dielectric Performance. RSC Adv. 2016, 6, 1870–1876. [Google Scholar] [CrossRef]
- Hanif, A.; Lu, Z.; Cheng, Y.; Diao, S.; Li, Z. Effects of Different Lightweight Functional Fillers for Use in Cementitious Composites. Int. J. Concr. Struct. Mater. 2017, 11, 99–113. [Google Scholar] [CrossRef]
- Chen, J.F.; Ding, H.M.; Wang, J.X.; Shao, L. Preparation and Characterization of Porous Hollow Silica Nanoparticles for Drug Delivery Application. Biomaterials 2004, 25, 723–727. [Google Scholar] [CrossRef]
- Ai, C.; Gong, G.; Zhao, X.; Liu, P. Macroporous Hollow Silica Microspheres-Supported Palladium Catalyst for Selective Hydrogenation of Nitrile Butadiene Rubber. J. Taiwan Inst. Chem. Eng. 2017, 77, 250–256. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Akane, Y.; Ogi, T.; Okuyama, K. Mesopore-Free Hollow Silica Particles with Controllable Diameter and Shell Thickness via Additive-Free Synthesis. Langmuir 2012, 28, 8616–8624. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Jelle, B.P.; Sandberg, L.I.C.; Gustavsen, A. Monodisperse Hollow Silica Nanospheres for Nano Insulation Materials: Synthesis, Characterization, and Life Cycle Assessment. ACS Appl. Mater. Interfaces 2013, 5, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Barbé, C.; Bartlett, J.; Kong, L.; Finnie, K.; Lin, H.Q.; Larkin, M.; Calleja, S.; Bush, A.; Calleja, G. Silica Particles: A Novel Drug-Delivery System. Adv. Mater. 2004, 16, 1959–1966. [Google Scholar] [CrossRef]
- Yang, K.N.; Zhang, C.Q.; Wang, W.; Wang, P.C.; Zhou, J.P.; Liang, X.J. PH-Responsive Mesoporous Silica Nanoparticles Employed in Controlled Drug Delivery Systems for Cancer Treatment. Cancer Biol. Med. 2014, 11, 34–43. [Google Scholar] [CrossRef]
- Wang, A.; Edwards, B.J. Modeling Controlled Release from Hollow Porous Nanospheres. Int. J. Heat Mass Transf. 2016, 103, 997–1007. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, Q.; Ji, X.; Zhang, S.; Chen, H.; Zheng, Y.; Sun, Y.; Qu, H.; Wang, Z.; Li, Y.; et al. Manganese Oxide-Based Multifunctionalized Mesoporous Silica Nanoparticles for PH-Responsive MRI, Ultrasonography and Circumvention of MDR in Cancer Cells. Biomaterials 2012, 33, 7126–7137. [Google Scholar] [CrossRef]
- Guo, H.C.; Feng, X.M.; Sun, S.Q.; Wei, Y.Q.; Sun, D.H.; Liu, X.T.; Liu, Z.X.; Luo, J.X.; Yin, H. Immunization of Mice by Hollow Mesoporous Silica Nanoparticles as Carriers of Porcine Circovirus Type 2 ORF2 Protein. Virol. J. 2012, 9, 108. [Google Scholar] [CrossRef]
- Weigum, S.E.; Xiang, L.; Osta, E.; Li, L.; López, G.P. Hollow Silica Microspheres for Buoyancy-Assisted Separation of Infectious Pathogens from Stool. J. Chromatogr. A 2016, 1466, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Kuwahara, Y.; Sumida, Y.; Yamashita, H. Synthesis of Ag Nanoparticles Encapsulated in Hollow Silica Spheres for Efficient and Selective Removal of Low-Concentrated Sulfur Compounds. J. Mater. Chem. A Mater. 2017, 5, 25431–25437. [Google Scholar] [CrossRef]
- Yang, Q.; Li, L.; Zhao, F.; Wang, Y.; Ye, Z.; Hua, C.; Liu, Z.; Bohinc, K.; Guo, X. Spherical Polyelectrolyte Brushes as Templates to Prepare Hollow Silica Spheres Encapsulating Metal Nanoparticles. Nanomaterials 2020, 10, 799. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Chen, J.S.; Qi, G.; Duan, X.; Wang, Z.; Giannelis, E.P.; Archer, L.A.; Lou, X.W. Formation of SnO2 Hollow Nanospheres inside Mesoporous Silica Nanoreactors. J. Am. Chem. Soc. 2011, 133, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Cevik, E.; Gunday, S.T.; Akhtar, S.; Yamani, Z.H.; Bozkurt, A. Sulfonated Hollow Silica Spheres as Electrolyte Store/Release Agents: High-Performance Supercapacitor Applications. Energy Technol. 2019, 7, 1900511. [Google Scholar] [CrossRef]



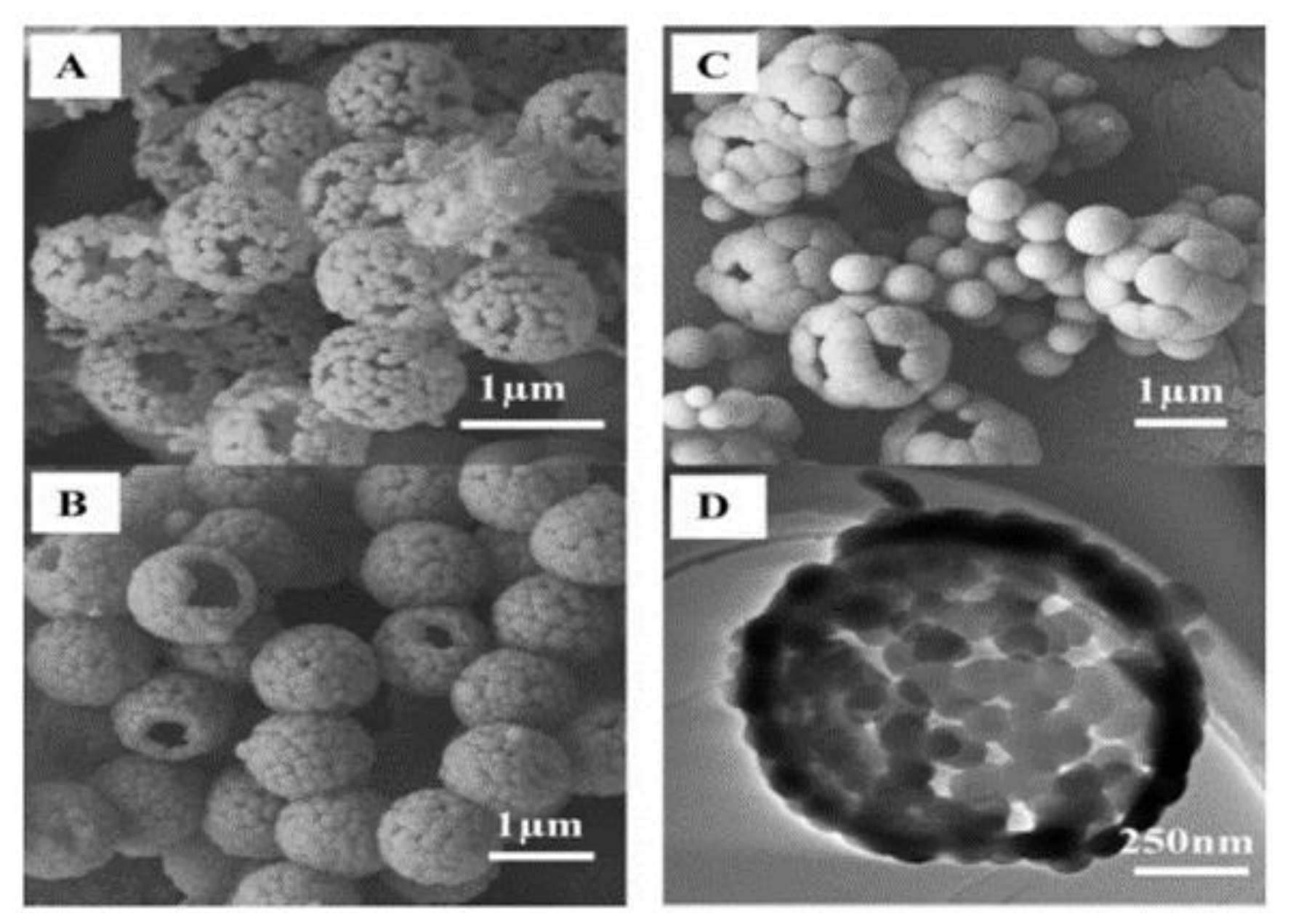

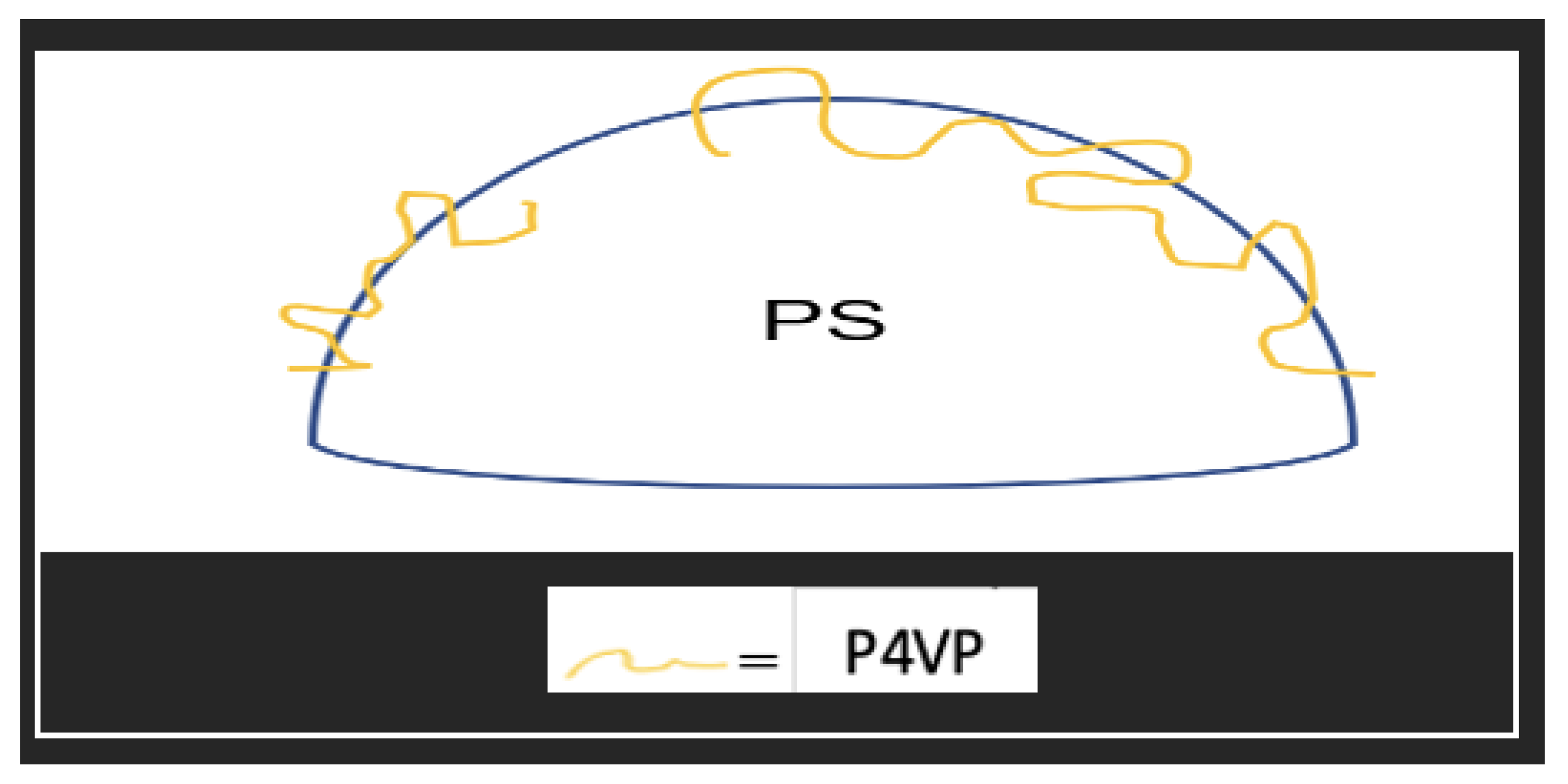
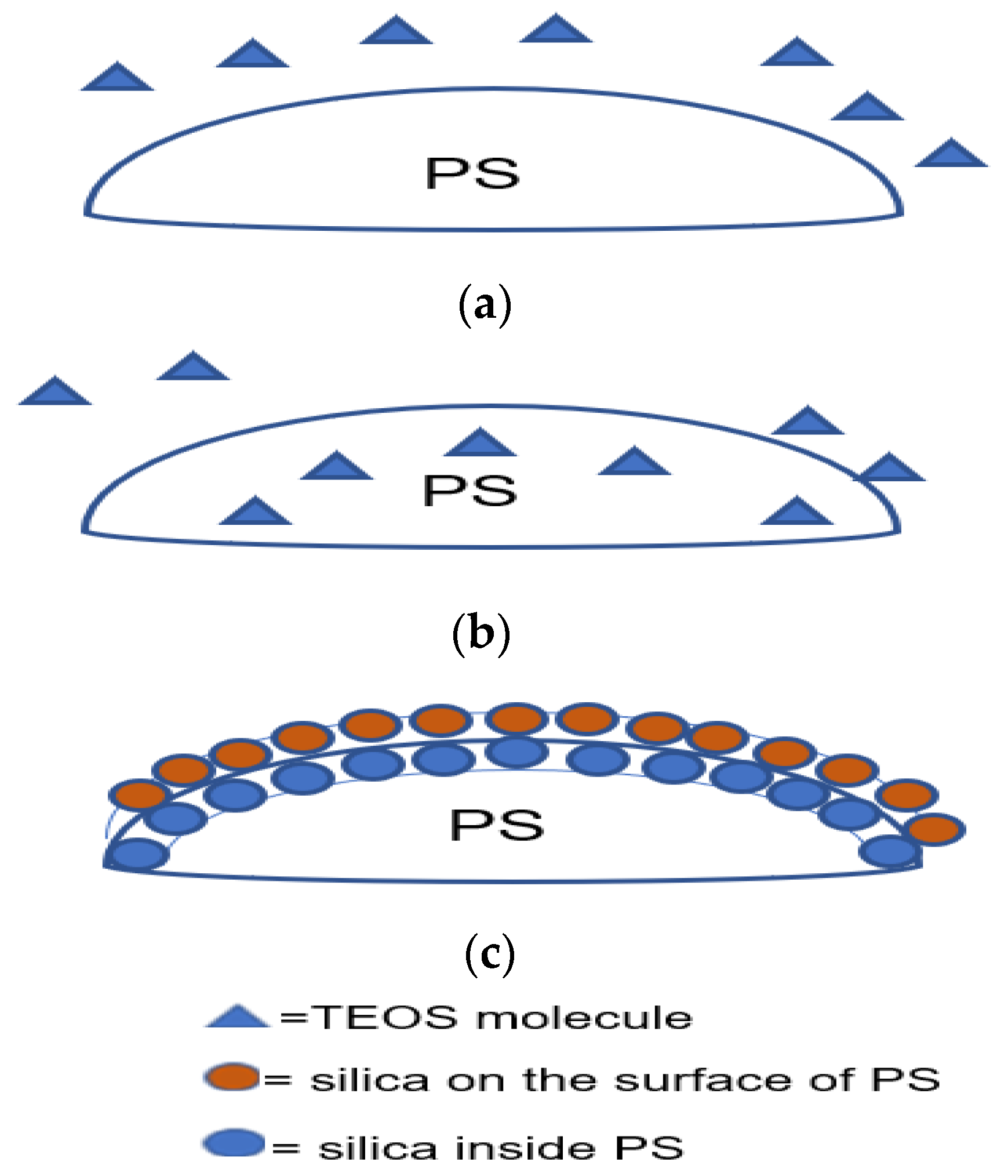
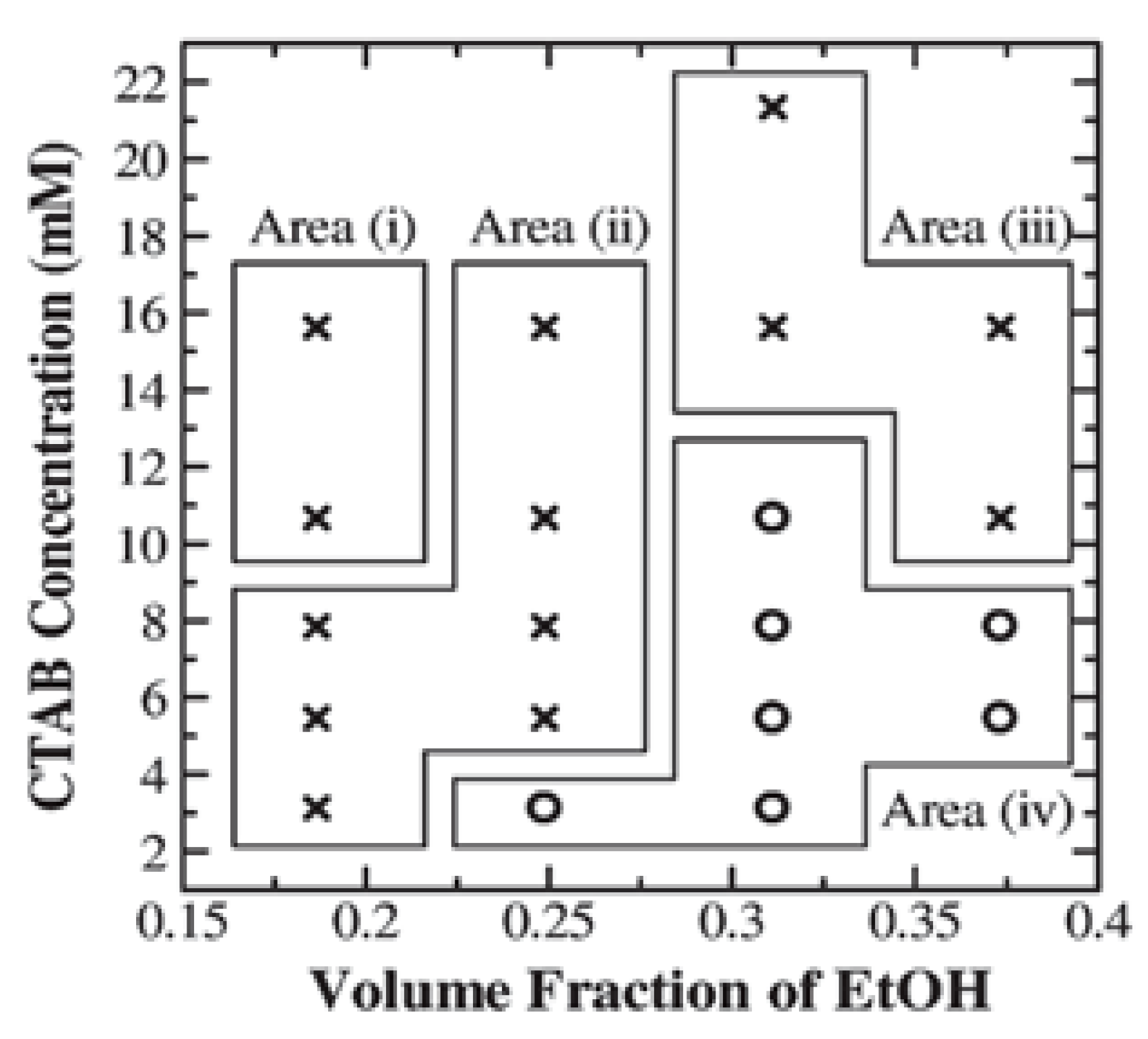
| No. | Strategy | Advantages | Disadvantages |
|---|---|---|---|
| 1 | Polymer micelles/emulsions Surfactant examples: CTAB |
|
|
| 2 | Inorganic template Example: Carbon |
|
|
| 3 | Organic template Example: Polystyrene |
|
|
| 4 | Solid silica particle etching |
|
|
| 5 | Spray drying method |
|
|
| 6 | Spray pyrolysis |
|
|
| 7 | Bacteria/virus templates |
|
|
| No. | Template | Precursor | Catalyst | Average Particle Size (nm) | Shell Thickness (nm) | Pore Size (nm) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | PS | TEOS, TMOS | NaOH | NA | NA | 1.2 | [76] |
| 2 | PS/CTAB | TEOS | NH4OH | NA | 62, 24 | 2.7 | [59] |
| 3 | Positive PS | TEOS | NH4OH | NA | 50, 100 | NA | [77] |
| 4 | Positive PS | TEOS | NH4OH | NA | 20, 30, 45 | 24.3, 37.4 | [78] |
| 5 | PS/CTAB | TEOS | NH4OH | NA | NA | 2.7 | [61] |
| 6 | PS/CTAB | TEOS | NH4OH | 90,200 | 5 40 | 2.3, 1.12 | [79] |
| 7 | PS | TEOS | NH4OH | NA | 15, 40 | NA | [80] |
| 8 | PS-co-PMMA, CTAB | TEOS | NH4OH | 350 | 30 | 1.8, 2.71 | [81] |
| 9 | PS(PAH/PVP) | TEOS | NH4OH | NA | 15, 70 | NA | [82] |
| 10 | PS/CTAB | TEOS | NH4OH | NA | 12, 24, 51 | 3, 3.6 | [83] |
| 11 | PS/CTAB | TEOS | NH4OH | 425, 767 | 57, 142 | NA | [84] |
| 12 | PS-co-P4VP/CTAB | TEOS | P4VP | 375 | 12, 26, 39 | 2.7, 5.4 | [85] |
| 13 | PS, CTAB | TEOS | NH4OH | 45, 90 | NA | 2.27, 2.19 | [86] |
| 14 | PS/CTAB | TEOS | NH4OH | NA | 15–95 | NA | [87] |
| 15 | PS-EGDMA, PS-DVB/trapped TEOS | TEOS | NH4OH | 240, 420 | 15–60 | NA | [88] |
| 16 | CTAB/ODA | TEOS | NH4OH | 300 | NA | 1.9, 2.0 | [89] |
| 17 | PS | TEOS | NH4OH | 2500 | 70 | 2.8,4.1 | [90] |
| 18 | PS/CTAB | MTM/MPTMS | TMAOH | 2400 | 25 | macropores | [60] |
| 19 | PS/CTAB | TEOS | NH4OH | 157, 245 | 14.1, 19 | 2.2, 2.3 | [65] |
| 20 | PS | TEOS | NH4OH | 152, 228 | 14, 19 | 2.3 | [91] |
| 21 | PS/CTAB | TEOS | NH4OH | NA | NA | 7.1, 22.3 | [62] |
| 22 | PS-PVP/PAA | TEOS | NH4OH | 1000–2320 | 50–70 | NA | [92] |
| 23 | SiO2 | TEOS | NH4OH | 45–450 | 30 | 2.5, 3.2, 3.4 | [93] |
| 24 | SiO2 | TEOS | NH4OH | 73–100, 330, 333, 418, 2600 | 5, 14–18, 41, 66, 88 | 3.6, 5.5 | [94] |
| 25 | SiO2 | TEOS | NH4OH | 430 | NA | NA | [95] |
| 26 | PS | TEOS | NH4OH | 350–450 | NA | NA | [96] |
| 27 | PS | TEOS | NH4OH | 2500 | 70 | NA | [97] |
| 28 | PS | TEOS | NH4OH | NA | 50–150 | NA | [98] |
| 29 | PS- AA | TEOS | NH4OH | NA | 15, 20, 25 | NA | [99] |
| 30 | PS/CTAB | TEOS | NH4OH | 320 | 5, 32.8, 58.4 | 5.04 | [100] |
| 31 | PSS-MAA/CTAB | Na2SiO3 | NH4OH | 362, 421, 750 | 18, 51 | 1.78, 5.57 | [101] |
| 32 | PS/CTAB | TEOS | NH4OH | 1500 | 150 | 8.36 | [102] |
| 33 | PS-AA | TEOS | NH4OH | 540, 1700 | 10–20, 40 | NA | [103] |
| 34 | PS | TEOS | NH4OH | NA | 100 | NA | [104] |
| 35 | PS-PAH-PVP | TEOS | NH4OH | 1520–1810 | 1590–1830 | NA | [105] |
| 36 | PS | TEOS | NH4OH | 310–316 | 43–44 | NA | [106] |
| 37 | PS + MSiN | TEOS | NH4OH | 20,000 | 100–1250 | NA | [107] |
| 38 | PS/trapped TEOS | TEOS | NH4OH | 325 | 60 | 7.03 | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurung, S.; Gucci, F.; Cairns, G.; Chianella, I.; Leighton, G.J.T. Hollow Silica Nano and Micro Spheres with Polystyrene Templating: A Mini-Review. Materials 2022, 15, 8578. https://doi.org/10.3390/ma15238578
Gurung S, Gucci F, Cairns G, Chianella I, Leighton GJT. Hollow Silica Nano and Micro Spheres with Polystyrene Templating: A Mini-Review. Materials. 2022; 15(23):8578. https://doi.org/10.3390/ma15238578
Chicago/Turabian StyleGurung, Siddharth, Francesco Gucci, Gareth Cairns, Iva Chianella, and Glenn J. T. Leighton. 2022. "Hollow Silica Nano and Micro Spheres with Polystyrene Templating: A Mini-Review" Materials 15, no. 23: 8578. https://doi.org/10.3390/ma15238578
APA StyleGurung, S., Gucci, F., Cairns, G., Chianella, I., & Leighton, G. J. T. (2022). Hollow Silica Nano and Micro Spheres with Polystyrene Templating: A Mini-Review. Materials, 15(23), 8578. https://doi.org/10.3390/ma15238578






