Abstract
This paper reports a detailed study of crystal structure and dielectric properties of ruthenium-substituted calcium-copper titanates (CaCu3Ti4−xRuxO12, CCTRO). A series of three samples with different stoichiometry was prepared: CaCu3Ti4−xRuxO12, x = 0, 1 and 4, abbreviated as CCTO, CCT3RO and CCRO, respectively. A detailed structural analysis of CCTRO samples was done by the Rietveld refinement of XRPD data. The results show that, regardless of whether Ti4+ or Ru4+ ions are placed in B crystallographic position in AA’3B4O12 (CaCu3Ti4−xRuxO12) unit cell, the crystal structure remains cubic with symmetry. Slight increases in the unit cell parameters, cell volume and interatomic distances indicate that Ru4+ ions with larger ionic radii (0.62 Å) than Ti4+ (0.605 Å) are incorporated in the CaCu3Ti4−xRuxO12 crystal lattice. The structural investigations were confirmed using TEM, HRTEM and ADF/STEM analyses, including EDXS elemental mapping. The effect of Ru atoms share in CaCu3Ti4−xRuxO12 samples on their electrical properties was determined by impedance and dielectric measurements. Results of dielectric measurements indicate that one atom of ruthenium per CaCu3Ti4−xRuxO12 unit cell transforms dielectric CCTO into conductive CCT3RO while preserving cubic crystal structure. Our findings about CCTO and CCT3RO ceramics promote them as ideal tandem to overcome the problem of stress on dielectric-electrode interfaces in capacitors.
1. Introduction
The CaCu3Ti4O12 (CCTO) materials belong to a large family of the AA’3B4O12 A-site ordered perovskites. During the last two decades, CCTO materials have been extensively investigated due to very high dielectric permittivity (up to 105), which is practically constant in a broad temperature (100 and 600 K) and frequency (1 kHz–1 MHz) range [1,2,3,4]. Due to such characteristics, CCTO ceramics attract the attention of researchers as very promising materials for applications in microelectronics and microwave devices [5]. The giant dielectric permittivity of CCTO materials promotes their utilization for the fabrication of capacitors with improved capacitive performance for portable electronic devices [6]. Though, it was observed that the nature of the ceramic-electrode interface could affect the decrease of the dielectric permittivity. Significant differences in the crystal structure and electrical properties of the CaCu3Ti4O12 as a dielectric material and a metallic electrode cause an energy barrier and the occurrence of stress on the ceramic-electrode interface, which reduces the dielectric permittivity. Therefore, for the fabrication of capacitors with good capacitive performance, it is important to combine dielectric ceramic and electrodes with similar crystal structures and unit cell parameters [7]. The reduction of the stress on the ceramic-electrode interfaces can be achieved by using commercially available materials as an interlayer having a close lattice parameter match with both dielectric and electrode. It was noticed that the incorporation of Ru4+ ions in the CCTO crystal structure dramatically increases the conductivity of these materials [8,9,10,11]. The CaCu3Ru4O12 (CCRO) materials are isostructural with CaCu3Ti4O12 materials with cubic space group showing the Pauli-paramagnetic and metallic character [12]. With the CCRO material as an interface between CCTO ceramic and metallic electrodes, it is possible to reduce the stress on the dielectric-electrode interfaces.
A cubic perovskite-structure oxide with the general chemical formula AA’3B4O12 is derived from that of a simple perovskite, ABO3 type, structure. The crystal structure of ABO3 perovskite is very flexible, enabling a wide range of ionic substitutions into both A and B crystallographic positions giving the possibility of creating new compounds with new functional properties. However, the AA’3B4O12 structural type is more rigid than the parent ABO3. This structure was induced by a+a+a+ (Glazer’s nomenclature), an octahedral tilting distortion of BO6 octahedra resulting in the B–O–B angle varying considerably away from 180o, which is typical for the ideal cubic perovskite structure [13,14,15,16]. Such octahedral distortion produces a structure where three-fourths of the A-site cations have square planar coordination (A’-cation site), while the remaining one-fourth of the A-site cations retain 12-fold coordination. The lattice parameters are twice as long as the ideal perovskite cubic unit cell (V = 2ap·2ap·2ap). The A’- cation site is occupied by Cu2+ or Mn3+ ions, while the crystallographic A position is usually occupied by larger ions, such as alkaline-earth ions or lanthanides. The B cation determines the electronic and magnetic properties of the materials [17,18]. Titanium ions in the B position (in the CaCu3Ti4O12 compounds) contribute to the high dielectric permittivity and better semiconductor properties. Over the years, different preparation techniques have been used to improve the dielectric properties of CCTO ceramics as well as metal ion substitution, both single and co-doped. It has been reported that metal ions such as Mg [19], Sn [20], Ge [21], Zr [22], and a combination of Zn and Mn [23], Y and Zr [24], or Mg with Ge [25] can noticeably reduce the dielectric loss (tgδ) and lead to the retention of a very high value for the dielectric constant (ε’).
On the contrary, our interest was to synthesize CaCu3Ti4−xRuxO12 as a compound isostructural with CaCu3Ti4O12 but with much larger conductivity which can be successfully used as an interlayer between dielectric ceramic and metallic electrode with the aim to reduce interlayer stress. Even though Ru4+ ions in the B position dramatically increase the conductivity of AA’3B4O12 materials, ruthenium is not acceptable for commercial application due to its highcost.
The aim of our work was to optimize the amount of ruthenium in the CaCu3Ti4−xRuxO12 crystal structure in order to obtain inexpensive and commercially acceptable materials with the desired functional properties. A series of CaCu3Ti4−xRuxO12 (x = 0, 1, and 4) ceramics was synthesized by the semi-wet precipitation method. A detailed structural analysis was done by the Rietveld refinement of X-ray powder diffraction data. In order to confirm the results of the structural investigations, TEM, HRTEM, and ADF/STEM analysis with EDXS elemental mapping were performed. To comprehend the influence of ruthenium amount in the CaCu3Ti4−xRuxO12 crystal structure on its electrical properties, we employed impedance spectroscopy and dielectric measurements.
2. Materials and Methods
Semi-wet precipitation method was used for the synthesis of CCTRO with three different stoichiometries: CaCu3Ti4−xRuxO12; x = 0, 1 and 4, abbreviated as CCTO, CCT3RO, and CCRO, respectively. Semi-wet precipitation method was chosen for the preparation of the materials since, compared with the solid-state technique, it enables better homogenization of the starting reagents. In a typical synthesis, a weighed amount of Ca(CH3COO)2 × H2O (99%, Alfa Aesar) and Cu(NO3)2 × 2.5H2O (99.99%, Aldrich, St. Louis, MO, USA) were dissolved in 20 mL of distilled water. A source of Ti4+ was Ti[OCH(CH3)2]4 (97%, Aldrich Chemical Company Inc.), which was dissolved in 10 mL of acidified water. The stable solutions were mixed, and, in this solution, a RuO2 (99.9%, Aldrich) was dispersed. The prepared dispersion was evaporated in a silicon oil bath while mixing with a magnetic stirrer. Obtained dry powder was homogenized with an agate mortar and pestle and consecutively calcined at 700 °C. The resulting powder was milled in planetary YTZ ball-mil for 0.5 h in ethanol and fired in the air at 900 °C for 20 h.
The structural investigations were performed by the Rietveld refinement [19] of the XRD data recorded at room temperature on a Philips PW 1050 diffractometer with CuKα1,2 (λ = 1.54178 Å) Ni-filtrated radiation. The diffraction intensity was measured from 10 to 130° 2θ, using a step size of 0.02° with a counting time of 12 s per step. The working conditions were 40 kV and 20 mA. The Rietveld refinement of the CaCu3Ti4−xRuxO12 crystal structure was performed using the FullProf computing program in the WinPLOTR environment [26,27,28,29]. The unit cell parameters were calculated using the program LSUCRI (least squares unit cell refinement with indexing) [30]. The Rietveld refinement was started from the calculated unit cell parameters and previously reported atomic positions [15]. A TCH-pseudo-Voigt function was chosen for the line shape of the diffraction peaks, whereas a 6-coefficient polynomial function was used for the background description. Transmission electron microscopy (TEM) and ADF/STEM with EDXS elemental mapping were performed using Jeol JEM 210 TEM and Titan G2 80-200 with ChemiSTEM technology. The fast Fourier transformation (FFT) analysis was done in the Digital Micrograph computer program.
The synthesized CCTRO powders were uniaxially pressed in a die (∅ 10 mm) under the pressure of about 400 MPa. Each compact has a thickness of circa 3 mm and 60 ± 2% of theoretical density. Sintering of the green compacts was carried out in a Protherm tube furnace in an air atmosphere, with a heating rate of 5°/min up to 1050 °C and with a dwell time of 12 h. The electrical measurements were done on the sintered CCTRO pellets. The capacitance and conductivity measurements were performed in function of temperature at a frequency of 1 kHz (internal frequency of the instrument) at the Wayne Kerr Universal Bridge B224. All measurements were done in the cooling mode from 150 to 21.3 °C in an air atmosphere. In order to improve the contact between the silver electrodes and the surface of the samples, both sample bases are coated with a suspension of silver powder and ethyl acetate. Impedance measurements were performed on the Potentiostat-Galvanostat PAR 273A coupled with Dual Phaslock-in Amplifier 5210 brand. The measurements were done in the frequency interval from 0.01 Hz to 100 kHz, and the temperature range from 150 °C to room temperature. Mathematical modeling of experimentally obtained data was done by Z-View2 (version 2.6 demo) computer program.
3. Results and Discussion
The structural refinement of room temperature XRD data of three investigated powders: CaCu3Ti4O12 (CCTO), CaCu3Ti3RuO12 (CCT3RO) and CaCu3Ru4O12 (CCRO), were performed in the space group . The Rietveld analysis was done by using the FullProf computing program. A pseudo-Voigt function was chosen to generate the line shape of the diffraction maximums. The scale factor, peak-shape parameters, lattice parameters, variable fractional atomic coordinates, and displacement parameters were refined. The unit cell parameters of the CCTO materials are doubled in all three crystallographic axes (V = 2ap·2ap·2ap) compared to the ideal (ABO3) cubic perovskite lattice (ap). The Rietveld refinements were started from the previously reported atomic positions: Ca atoms were situated in 2a Wyckoff sites at the (0 0 0) position, Cu atoms occupied the 6b at (0 ½ ½), Ti in 8c (0 ¼ ¼), and O in 24g (x y 0) sites. The literature data [15] of the positional coordinates were used as starting values for structural refinement. Figure 1 illustrates the good agreement between calculated and observed structural models. The obtained results confirm that the powders are a single-phase perovskite oxide without a secondary phase.
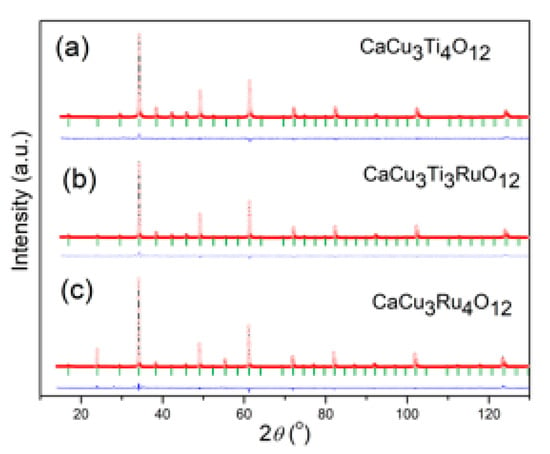
Figure 1.
The Rietveld refinement plot of (a) CaCu3Ti4O12 (CCTO), (b) CaCu3Ti3RuO12 (CCT3RO), and (c) CaCu3Ru4O12 (CCRO) samples.
The structural refinement was started by allowing varying the values of the zero point, the scale factor, and the background coefficients. Then, lattice parameters, asymmetry, and half-width parameters (U, V, W) were refined. When satisfactory values of profile parameters were obtained, the structural parameters, i.e., atomic positional parameters and isotropic displacement parameters, were allowed to vary. The overall displacement parameter was refined for every atom; the obtained value was set as starting isotropic displacement parameter for each of the atoms. The isotropic displacement parameters for Ca, Cu, Ti and Ru atoms were refined independently, while for the O atoms, the displacement parameters were constrained at the same value. The titanium and ruthenium atoms in the CaCu3Ti4−xRuxO12 crystal structure are situated in the same crystallographic positions, so their fractional atomic coordinates were set to be equal during refinement. Their occupational factors are interrelated, and only the Ti/Ru ratio was allowed to vary, keeping the overall occupancy of the respective sites constant according to the appropriate stoichiometric ratio. The refined values of the unit cell parameters, atomic position parameters, and displacement parameters are listed in Table 1.

Table 1.
The refined values of the fractional coordinates and isotropic displacement parameters of the CaCu3Ti4−xRuxO12 powders.
The refined values of the unit cell parameters and unit cell volume increase with increases in ruthenium ions content in the crystal structure of the investigated powders (Table 1, Figure 2). The increases in unit cell parameters are provoked by partial (1 of 4) or complete (4 of 4) replacement of Ti4+ ions (0.605 Å ionic radii for six coordination) by larger Ru4+ ions (0.62 Å ionic radii for six coordination) in the CaCu3Ti4−xRuxO12 crystal structures.
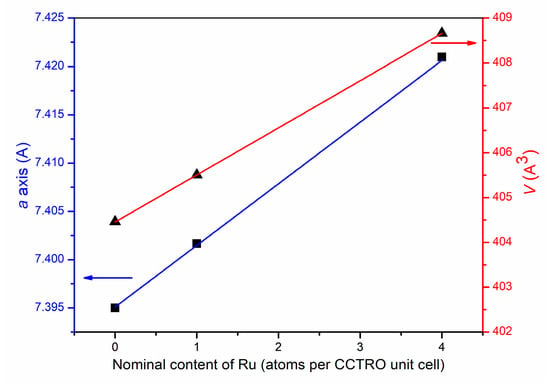
Figure 2.
Relationship between the unit cell parameters and cell volume with a nominal content of Ru atoms per CCTRO unit cell.
The interatomic distances in the CaCu3Ti4O12, CaCu3Ti3RuO12 and CaCu3Ru4O12 powders were calculated from the structural results obtained by Rietveld refinement and listed in Table 2. The increase of the average value of the interatomic Ti/Ru-O distance from 1.953 Å (CCTO) to 1.967 Å (CCRO) indicates the substitution of smaller Ti4+ by larger Ru4+ cation in the CaCu3Ti4−xRuxO12 crystal structure.

Table 2.
The interatomic distances (Å) and reliability factors of CaCu3Ti4−xRuxO12 powders.
The results of the structural investigation were confirmed by TEM and SAED analyses. TEM and SAED analyses of CCTO and CCRO powders are presented in Figure 3. Figure 3a reveals significant agglomeration of CCTO single-crystalline particles whose size is about several tens of nanometers (inset in Figure 3a). SAED pattern, presented in Figure 3b, confirms crystallographic information obtained through Rietveld refinement. Interplanar distances of 3.701, 2.597, 1.837, 1.516, and 1.253 Å correspond well too (200), (220), (400), (422), and (433) planes of CaCu3Ti4O12 phase, JCPDS 75-2188. Similarly, many randomly oriented CCRO particles are presented in Figure 3c. Their single-crystalline nature is exposed in Figure 3d, which shows the overlapping of crystals with 2 different zone axes with low mismatching. Observed interplanar distances of 3.740, 1.860, 2.630, and 1.320 Å are assigned to (200), (400), (220), and (440) planes of CaCu3Ru4O12 phase, JCPDS 41-0821.
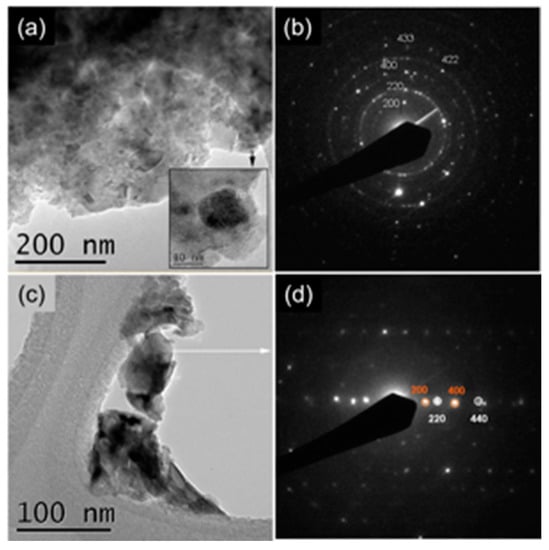
Figure 3.
TEM and SAED of (a,b) CCTO and (c,d) CCRO samples.
The TEM, HRTEM, and FFT analysis of the CCT3RO sample presented in Figure 4 confirm the cubic crystal symmetry with space group. Crystallite viewed in the [−111] zone axis as well as two sets of crystallographic planes (400) and (220) with interplanar distances of 1.8 and 2.6 Å, respectively, agree with a literature data for cubic CaCu3Ti4O12 (JCPDS 75-2188).
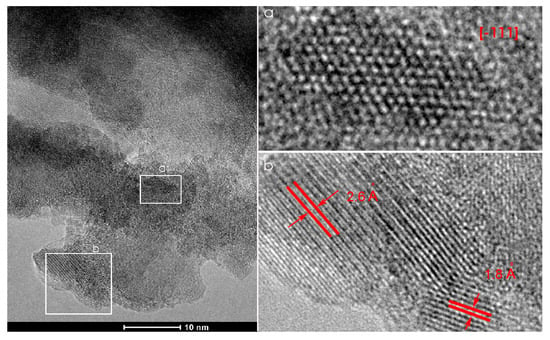
Figure 4.
TEM (left) and HRTEM (a,b—right) of CCT3RO sample.
High-angle annular dark-field STEM and corresponding EDXS elemental mapping presented in Figure 5 implies the homogeneous distribution of Ca, Cu, Ti, Ru and O in CCT3RO particles, independently of their size.
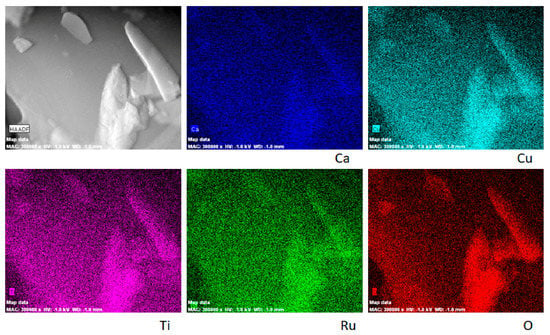
Figure 5.
ADF/STEM analysis and EDXS elemental mapping of CCT3RO powder.
Complex impedance spectroscopy (IS) was used to determine the electrical properties of sintered CCTRO ceramics. Recorded Nyquist plots are presented in Figure 6.
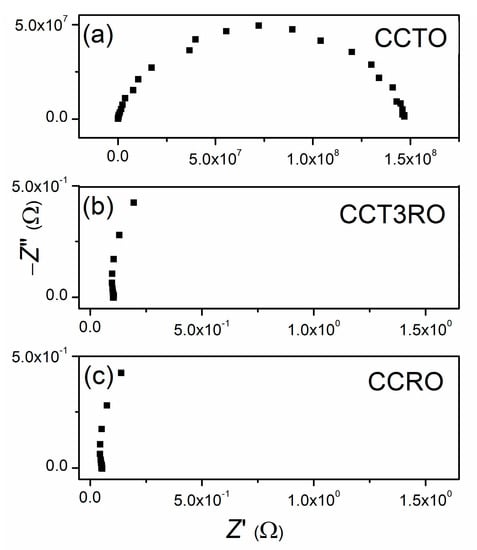
Figure 6.
Complex impedance spectra recorded at room temperature for sintered ceramics: (a) CCTO, (b) CCT3RO, and (c) CCRO.
The recorded complex impedance spectra of CCTRO sintered ceramics were fitted using the Z-View2 computing program according to the equivalent circuit. The equivalent circuit consisting of one resistor (R) and one capacitor (constant phase element, CPE) connected in parallel best describes the impedance spectra of CCTRO materials. The calculated resistance values are in the range of 1 to 150 MΩ. Relatively high values of resistance indicate good dielectric properties of CCTO material. On the contrary, the impedance spectra of sintered ceramics with ruthenium content show an accumulation of points for higher and middle-frequency values of about 0.1 Ω and 0.05 Ω for CCT3R and CCRO, respectively. Only at very low-frequency values, from 0.1 to 0.01 Hz, the impedance spectra of these ceramics can be described by an equivalent circuit of one resistor (R) and one capacitor (CPE) connected in parallel. However, the difference in the obtained resistivity values for CCTRO materials with and without ruthenium content is more than eight orders of magnitude in the direct current circuit. Such behavior indicates good conductivity of CCT3RO and CCRO ceramics. The electrical conductivity of investigated ceramics at four characteristic frequency points is presented in Table 3.

Table 3.
Dependence of the electrical conductivity and the frequency value at room temperature.
The difference in electrical conductivity can be explained by different electron configurations of Ti4+ and Ru4+ ions [14,31,32]. Titanium is a 3d element with an electronic configuration:
Ti: 1s2 2s2 2p6 3s2 3p6 4s2 3d2
However, the electronic configuration of Ti4+ cation in the CCTO crystal structure turns into:
with closed external 3p6 shells, which explains the relatively low conductivity of these materials. The isomorphic replacement of the Ti4+ ions by Ru4+ leads to different electronic configurations. Explicitly, Ru4+ has the following electronic configuration:
with uncompleted d subshells. These four electrons in the octahedral crystalline environment are situated in tg crystalline orbitals containing two unpaired d electrons, i.e., Ru4 + represents a magnetic ion in the CCRO crystal. The CCRO conductivity can be explained by the Kondo mechanism. Namely, CCRO materials are d-electron heavy-fermion system, which behaves similarly to an f-electron heavy-fermion system. In CCRO structures, the Cu 3d electrons are localized, while 4d electrons of magnetic Ru4+ ions contribute to the conductivity [14,32,33].
Ti4+: 1s2 2s2 2p6 3s2 3p6
Ru4+: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s0 4d4,
Figure 7 displays the complex impedance spectra (Nyquist plot) of CCTO ceramic measured in an air atmosphere, in the temperature range from 150 to 23 °C (room temperature) and frequency interval from 100 kHz to 0.01 Hz. The obtained impedance diagrams represent the sum of the grain-interior and grain boundary resistance, which is impossible to separate. Thus, the fitting of summary data was done. The fitting was achieved based on the corresponding equivalent circuit (inset in Figure 7) and the Z-View2 computing program.
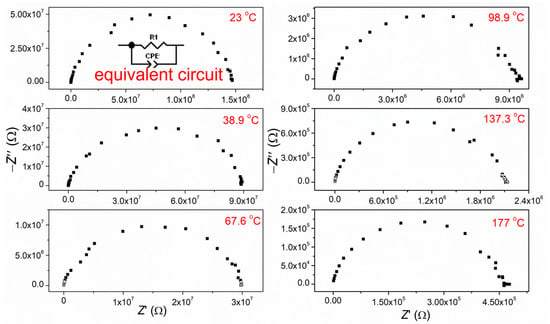
Figure 7.
Complex impedance spectra for CCTO ceramics at selected temperature; as an inset: scheme of an equivalent circuit model used for fitting impedance spectra of CCTO ceramic.
The values of electric resistivity determined by fitting with an appropriate equivalent circuit were used to calculate the values of electrical resistivity ρ [Ω⋅cm] and specific electrical conductivity σ (S·cm−1). The calculated values are presented in Table 4. The calculated values of electrical conductivity (Table 4) were used to determine the activation energies Ea based on the Arrhenius equation, Equation (1) [34,35]:
where Ea is the activation energy, kB is the Boltzmann’s constant (8.8617343 × 10−5 eV/K), and σo is a constant. The obtained relationship is almost linear (Figure 8). The calculated value of activation energy of 0.49 eV is in accordance with literature data [1,2,3].

Table 4.
Change in the value of specific electrical resistance and specific electrical conductivity with temperature change for CCTO.
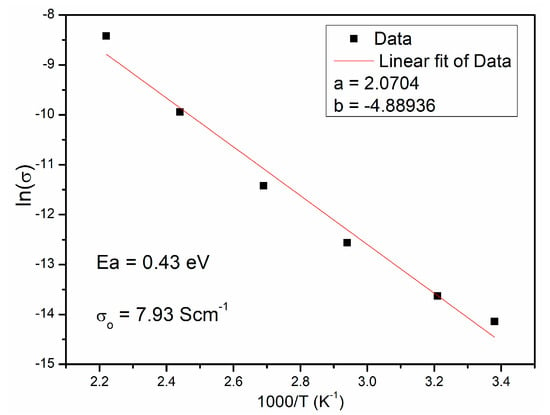
Figure 8.
The Arrhenius plot of conductivity ln(σ) versus 1000/T of CCTO ceramic.
Figure 9 represents the influence of both the temperature and the frequency on the dielectric properties of the CCTO ceramic. The measurements were done in the frequency interval from 100 kHz to 0.01 Hz and in the temperature range between 177 and 23 °C.
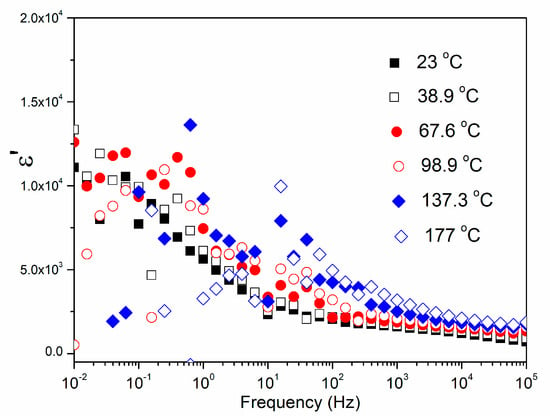
Figure 9.
Frequency−dependent dielectric constant ε’ of the CCTO ceramic at different temperatures.
It is obvious that CCTO materials possess a high dielectric permittivity ε’ (>103) which significantly depends on temperature and frequency (Table 5).

Table 5.
The frequency and temperature dependence of the dielectric constant (ε’) and the dielectric loss (tgδ) for CCTO ceramic.
The influence of temperature and frequency on the dielectric loss (tgδ) of the CCTO ceramic is shown in Figure 10. For the three highest temperatures, the dielectric loss monotonically decreases with increasing frequency. For the three lowest temperatures, the dielectric losses slightly increase in the frequency range from 10 to 100 kHz.
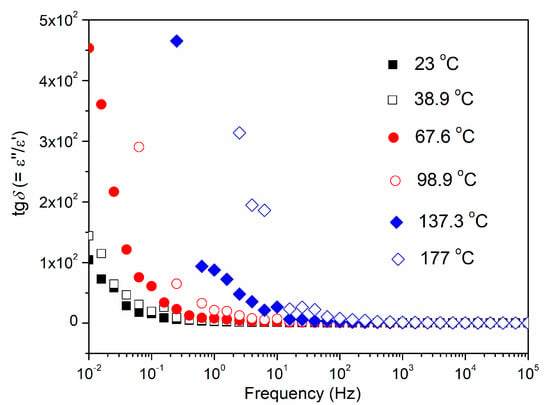
Figure 10.
Frequency−dependent dielectric loss (tgδ) of the CCTO ceramic at different temperatures.
The relative dielectric constant εr for CCTO material was measured in cooling mode from 150 °C to room temperature at a constant frequency of 1 kHz. The relative dielectric constant increases with increasing temperature from about 1460 at room temperature to 2460 at 150 °C (Figure 11a). It can be seen that these measurements are in good agreement with the values obtained from the impedance measurements at the same frequency of 1 kHz (Table 5).
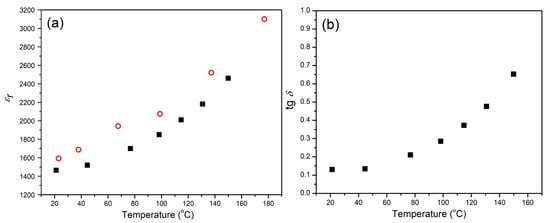
Figure 11.
The temperature dependence of (a) relative dielectric constant was calculated based on impedance measurements (circle) and based on measured capacitance (square), and (b) dielectric loss for CCTO ceramic.
The dependence of dielectric loss (tgδ) on temperature is represented in Figure 11b. It can be seen that the tgδ increases with an increase in the temperature. These values are in the range between 0.13 at room temperature and 0.65 at 150 °C, which is in good agreement with the values obtained at 1 kHz (Figure 10).
The results of electrical measurements clearly show that the stoichiometry of CaCu3Ti4−xRuxO12 significantly affects their electrical properties. It is known that CaCu3Ti4O12 material exhibits excellent dielectric properties with a dielectric constant in excess of 104. Quite the contrary, the CaCu3Ru4O12 material exhibits conductive behavior [7,36,37,38]. The results presented in this paper revealed that the substitution of one Ti4+ ion by Ru4+ per the CaCu3Ti4O12 unit cell changes the electrical characteristics of these materials from dielectric to conductive. Such electrical properties of these materials are very important for their potential application in the electronics industry for capacitors production. However, in these components, the problem of stress on the dielectric-electrode interfaces occurs due to the different structural characteristics of the metal (electrode) and ceramic materials. This problem can be overcome by the use of materials that would have the same or similar structural characteristics both with dielectric and with an electrode, which would be used as an interface. Potential materials that could be used for the production of a contact or interlayer are CCT3RO and CCRO, owing to the same crystal structure and close lattice parameters (Table 1).
4. Conclusions
In this paper, we presented a detailed analysis of crystal structure and dielectric properties of three CaCu3Ti4−xRuxO12 (x = 0, 1 and 4) samples. The detailed analysis of the crystal structure was determined by Rietveld’s refinement of XRD data. The results of the structural analysis show that regardless of whether Ti4+ or Ru4+ ions are placed in B crystallographic position in the CaCu3Ti4−xRuxO12 unit cell, the crystal structure remains cubic with space group. Slight increases in the unit cell parameters, cell volume and interatomic distances indicate that Ru4+ ions with larger ionic radii (0.62 Å) than Ti4+ (0.605 Å) are incorporated in the CaCu3Ti4−xRuxO12 crystal lattice. Results of TEM, HRTEM and ADF/STEM analyses with EDXS elemental mapping confirmed the results of structural investigations. The electrical properties of the CaCu3Ti4−xRuxO12 materials were investigated based on impedance spectroscopy and dielectric measurements. Values of electrical resistance measured at room temperature indicate that the ruthenium ions in the CaCu3Ti4O12 structure change the electrical properties of these materials from dielectric to conductive. What is more, just one atom of ruthenium per unit cell is enough to drastically improve the conductive properties of these materials. With the addition of ruthenium ions, conductivity increases for more than five orders of magnitude for the frequency of 100 kHz and for almost eight orders of magnitude for the frequency of 100 Hz.
Our findings about CCTO and CCT3RO ceramics, as both an inexpensive and ideal tandem to overcome the problem of stress on dielectric-electrode interfaces in capacitors, promote their commercial application in the electronic industry.
Author Contributions
L.V.: Investigation, Methodology, Writing-original draft; M.M.: Investigation, Supervision; L.M.: Writing—Reviewing and Editing; P.M.J.: Investigation; S.D.Š.: Investigation, Writing—Reviewing; N.C.: Conceptualization, Supervision, Writing—Reviewing and Editing; M.D.M.: Investigation; S.M.: Conceptualization, Methodology, Writing—Reviewing and Editing, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia through the agreement related to the realization and funding of scientific research work at the Institute of Technical Sciences of SASA in 2022, Contract number 451-03-68/2022-14/200175 and Faculty of Physical Chemistry, University of Belgrade, contract number 451-03-68/2022-14/200146. Miloš Milović acknowledges the support of the Science Fund of the Republic of Serbia under the project PROMIS #6062667 (HISUPERBAT). Paula Jardim acknowledges the Multiuser Microscopy Nucleus of COPPE at UFRJ (Brazil) for their support related to the use of electron microscopes.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, R.; Zulfequar, M.; Sharma, L.; Singh, V.N.; Senguttuvan, T.D. Growth of Nanocrystalline CaCu3Ti4O12 Ceramic by the Microwave Flash Combustion Method: Structural and Impedance Spectroscopic Studies. Cryst. Growth Des. 2015, 15, 1374–1379. [Google Scholar] [CrossRef]
- Pal, K.; Dey, A.; Jana, R.; Ray, P.P.; Bera, P.; Kumar, L.; Mandal, T.K.; Mohanty, P.; Seikh, M.M.; Gayen, A. Citrate Combustion Synthesized Al-Doped CaCu3Ti4O12 Quadruple Perovskite: Synthesis, Characterization and Multifunctional Properties. Phys. Chem. Chem. Phys. 2020, 22, 3499–3511. [Google Scholar] [CrossRef] [PubMed]
- Zhuk, N.A.; Sekushin, N.A.; Krzhizhanovskaya, M.G.; Belyy, V.A.; Korolev, R.I. Electrical Properties of Ni-Doped CaCu3Ti4O12 Ceramics. Solid State Ion. 2021, 364, 115633. [Google Scholar] [CrossRef]
- Thongbai, P.; Jumpatam, J.; Putasaeng, B.; Yamwong, T.; Maensiri, S. The Origin of Giant Dielectric Relaxation and Electrical Responses of Grains and Grain Boundaries of W-Doped CaCu3Ti4O12 Ceramics. J. Appl. Phys. 2012, 112, 114115. [Google Scholar] [CrossRef]
- Marković, S.; Lukić, M.; Jovalekić, Č.; Škapin, S.D.; Suvorov, D.; Uskoković, D. Sintering Effect on Microstructure and Electrical Properties of CaCu3Ti4O12 ceramics. Ceram. Trans. 2013, 240, 337–348. [Google Scholar] [CrossRef]
- Fernández, J.F.; Leret, P.; Romero, J.J.; De Frutos, J.; De La Rubia, M.Á.; Martín-González, M.S.; Costa-Krämer, J.L.; Fierro, J.L.G.; Quesada, A.; García, M.Á. Proofs of the Coexistence of Two Magnetic Contributions in Pure and Doped CaCu3Ti4O12 Giant Dielectric Constant Ceramics. J. Am. Ceram. Soc. 2009, 92, 2311–2318. [Google Scholar] [CrossRef]
- Brizé, V.; Autret-Lambert, C.; Wolfman, J.; Gervais, M.; Gervais, F. Synthesis and Microstructural TEM Investigation of CaCu3Ru4O12 Ceramic and Thin Film. J. Solid State Chem. 2011, 184, 2719–2723. [Google Scholar] [CrossRef]
- Ebbinghaus, S.G.; Weidenkaff, A.; Cava, R.J. Structural Investigations of ACu3Ru4O12 (A = Na, Ca, Sr, La, Nd)—A Comparison between XRD-Rietveld and EXAFS Results. J. Solid State Chem. 2002, 167, 126–136. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Sleight, A.W. ACu3Ti4O12 and ACu3Ru4O12 Perovskites: High Dielectric Constants and Valence Degeneracy. Solid State Sci. 2002, 4, 347–351. [Google Scholar] [CrossRef]
- Shimakawa, Y. A-Site-Ordered Perovskites with Intriguing Physical Properties. Inorg. Chem. 2008, 47, 8562–8570. [Google Scholar] [CrossRef]
- Yanchevskii, O.Z.; V’yunov, O.I.; Belous, A.G.; Kovalenko, L.L. Dielectric Properties of CaCu3Ti4O12 Ceramics Doped with Aluminium and Fluorine. J. Alloys Compd. 2021, 874, 159861. [Google Scholar] [CrossRef]
- Tran, T.T.; Takubo, K.; Mizokawa, T.; Kobayashi, W.; Terasaki, I. Electronic Structure of CaCu3Ru4O12 Studied by X-Ray Photoemission Spectroscopy. Phys. Rev. B-Condens. Matter Mater. Phys. 2006, 73, 8–11. [Google Scholar] [CrossRef]
- Boonlakhorn, J.; Chanlek, N.; Manyam, J.; Srepusharawoot, P.; Krongsuk, S.; Thongbai, P. Enhanced Giant Dielectric Properties and Improved Nonlinear Electrical Response in Acceptor-Donor (Al3+, Ta5+)-Substituted CaCu3Ti4O12 Ceramics. J. Adv. Ceram. 2021, 10, 1243–1255. [Google Scholar] [CrossRef]
- Kobayashi, W.; Terasaki, I.; Takeya, J.I.; Tsukada, I.; Ando, Y. A Novel Heavy-Fermion State in CaCu3Ru4O12. J. Phys. Soc. Jpn. 2004, 73, 2373–2376. [Google Scholar] [CrossRef]
- Manik, S.K.; Pradhan, S.K. Microstructure Characterization of Ball-Mill-Prepared Nanocrystalline CaCu3Ti4O12 by Rietveld Method. Phys. E Low-Dimens. Syst. Nanostruct. 2006, 33, 160–168. [Google Scholar] [CrossRef]
- Miao, X.; Zhang, L.; Wu, L.; Hu, Z.; Shi, L.; Zhou, S. Quadruple Perovskite Ruthenate as a Highly Efficient Catalyst for Acidic Water Oxidation. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Deng, J.; Han, F.; Schwarz, B.; Knapp, M.; Ehrenberg, H.; Hua, W.; Hinterstein, M.; Li, G.; He, Y.; Wang, J.; et al. Dielectric Relaxation and Magnetic Structure of A-Site-Ordered Perovskite Oxide Semiconductor CaCu3Fe2Ta2O12. Inorg. Chem. 2021, 60, 6999–7007. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Q.; Li, Y.; Rehman, M.U.; Khan, W.Q. Sr and Zr Co-Doped CaCu3Ti4O12 Ceramics with Improved Dielectric Properties. Materials 2022, 15, 4243. [Google Scholar] [CrossRef]
- Li, M.; Cai, G.; Zhang, D.F.; Wang, W.Y.; Wang, W.J.; Chen, X.L. Enhanced dielectric responses in Mg-doped CaCu3Ti4O12. J. Appl. Phys. 2008, 104, 074107. [Google Scholar] [CrossRef]
- Boonlakhorn, J.; Chanlek, N.; Srepusharawoot, P.; Thongbai, P. Improved dielectric properties of CaCu3-xSnxTi4O12 ceramics with high permittivity and reduced loss tangent. J. Mater. Sci. Mater. Electron. 2020, 31, 15599–15607. [Google Scholar] [CrossRef]
- Boonlakhorn, J.; Thongbai, P.; Putasaeng, B.; Kidkhunthod, P.; Maensiri, S.; Chindaprasirt, P. Microstructural evolution, non-Ohmic properties, and giant dielectric response in CaCu3Ti4-xGexO12 ceramics. J. Am. Ceram. Soc. 2017, 100, 3478–3487. [Google Scholar] [CrossRef]
- Xu, D.; Zhu, Y.; Zhang, B.; Yue, X.; Jiao, L.; Song, J.; Zhong, S.; Ma, J.; Bao, L.; Zhang, L. Excellent dielectric performance and nonlinear electrical behaviors of Zr-doped CaCu3Ti4O12 thin films. J. Mater. Sci. Mater. Electron. 2018, 29, 5116–5123. [Google Scholar] [CrossRef]
- Xu, Z.; Qiang, H. Enhanced dielectric properties of Zn and Mn co-doped CaCu3Ti4O12 ceramics. J. Mater. Sci. Mater. Electron. 2017, 28, 376–380. [Google Scholar] [CrossRef]
- Xu, Z.; Qiang, H.; Chen, Y.; Chen, Z. Microstructure and enhanced dielectric properties of yttrium and zirconium co-doped CaCu3Ti4O12 ceramics. Mater. Chem. Phys. 2017, 191, 1–5. [Google Scholar] [CrossRef]
- Boonlakhorn, J.; Chanlek, N.; Manyam, J.; Srepusharawoot, P.; Thongbai, P. Simultaneous two-step enhanced permittivity and reduced loss tangent in Mg/Ge-Doped CaCu3Ti4O12 ceramics. J. Alloys Compd. 2021, 877, 160322. [Google Scholar] [CrossRef]
- Young, R.A. Rietveld Refinement; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Rodriguez-Carvajal, J. FullProf2k, Version 2.40-May 2005; LLB JRC Laboratory, Léon Brillouin (CEA–CNRS), CEA–Sarclay: Gif-sur-Yvette, France, 2005.
- Rodríguez-Carvajal, J. FULLPROF: A Program for Rietveld Refinement and Pattern Matching Analysis. In Proceedings of the Satellite Meeting on Powder Diffraction of the XV Congress of the IUCr, Toulouse, France, 16–19 July 1990; p. 127. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodríquez-Carvajal, J. WinPLOTR: A Windows Tool for Powder Diffraction Pattern Analysis. Mater. Sci. Forum 2001, 378–381, 118–123. [Google Scholar] [CrossRef]
- Garvey, R.G. LSUCRI Least Squares Unit Cell Refinement for the Personal Computer. Powder Diffr. 1986, 1, 114–116. [Google Scholar]
- Wang, Y.; Klenk, M.; Page, K.; Lai, W. Local Structure and Dynamics of Lithium Garnet Ionic Conductors: A Model Material Li5La3Ta2O12. Chem. Mater. 2014, 26, 5613–5624. [Google Scholar] [CrossRef]
- Takegami, D.; Kuo, C.Y.; Kasebayashi, K.; Kim, J.G.; Chang, C.F.; Liu, C.E.; Wu, C.N.; Kasinathan, D.; Altendorf, S.G.; Hoefer, K.; et al. CaCu3Ru4O12: A High-Kondo-Temperature Transition-Metal Oxide. Phys. Rev. X 2022, 12, 11017. [Google Scholar] [CrossRef]
- Liu, H.; Cao, Y.; Xu, Y.; Gawryluk, D.J.; Pomjakushina, E.; Gao, S.Y.; Dudin, P.; Shi, M.; Yan, L.; Yang, Y.F.; et al. Observation of Flat Bands Due to Band Hybridization in the 3d -Electron Heavy-Fermion Compound CaCu3Ru4O12. Phys. Rev. B 2020, 102, 1–7. [Google Scholar] [CrossRef]
- Sangwong, N.; Somphan, W.; Thongbai, P.; Yamwong, T.; Meansiri, S. Electrical Responses and Dielectric Relaxations in Giant Permittivity NaCu3Ti3TaO12 Ceramics. Appl. Phys. A Mater. Sci. Process. 2012, 108, 385–392. [Google Scholar] [CrossRef]
- Dulian, P.; Bąk, W.; Piz, M.; Garbarz-Glos, B.; Sachuk, O.V.; Wieczorek-Ciurowa, K.; Lisińska-Czekaj, A.; Czekaj, D. Mg2+ Doping Effects on the Structural and Dielectric Properties of CaCu3Ti4O12 Ceramics Obtained by Mech anochemical Synthesis. Materials 2021, 14, 1187. [Google Scholar] [CrossRef] [PubMed]
- Gunther, A.; Riegg, S.; Kraetschmer, W.; Wehrmeister, S.; Buttgen, N.; Scheidt, E.W.; Von Nidda, H.A.K.; Eremin, M.V.; Arkhipova, E.A.; Eremina, R.M.; et al. Electronic Correlations and Crystal-Field Effects in RCu3Ru4O12 (R=La, Pr, Nd) electronic correlations and crystal-field. Phys. Rev. B 2020, 102, 1–18. [Google Scholar] [CrossRef]
- Krohns, S.; Lu, J.; Lunkenheimer, P.; Brizé, V.; Autret-Lambert, C.; Gervais, M.; Gervais, F.; Bourée, F.; Porcher, F.; Loidl, A. Correlations of Structural, Magnetic, and Dielectric Properties of Undoped and Doped CaCu3Ti4O12. Eur. Phys. J. B 2009, 72, 173–182. [Google Scholar] [CrossRef]
- Marković, S.; Jovalekić, C.; Veselinović, L.; Mentus, S.; Uskoković, D. Electrical Properties of Barium Titanate Stannate Functionally Graded Materials. J. Eur. Ceram. Soc. 2010, 30, 1427–1435. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).