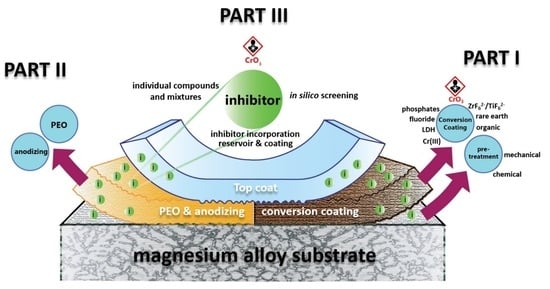
Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: Part III—Corrosion Inhibitors and Combining Them with Other Protection Strategies
Abstract
1. Foreword
2. Inorganic Inhibitors
2.1. Chromate Based Inhibition
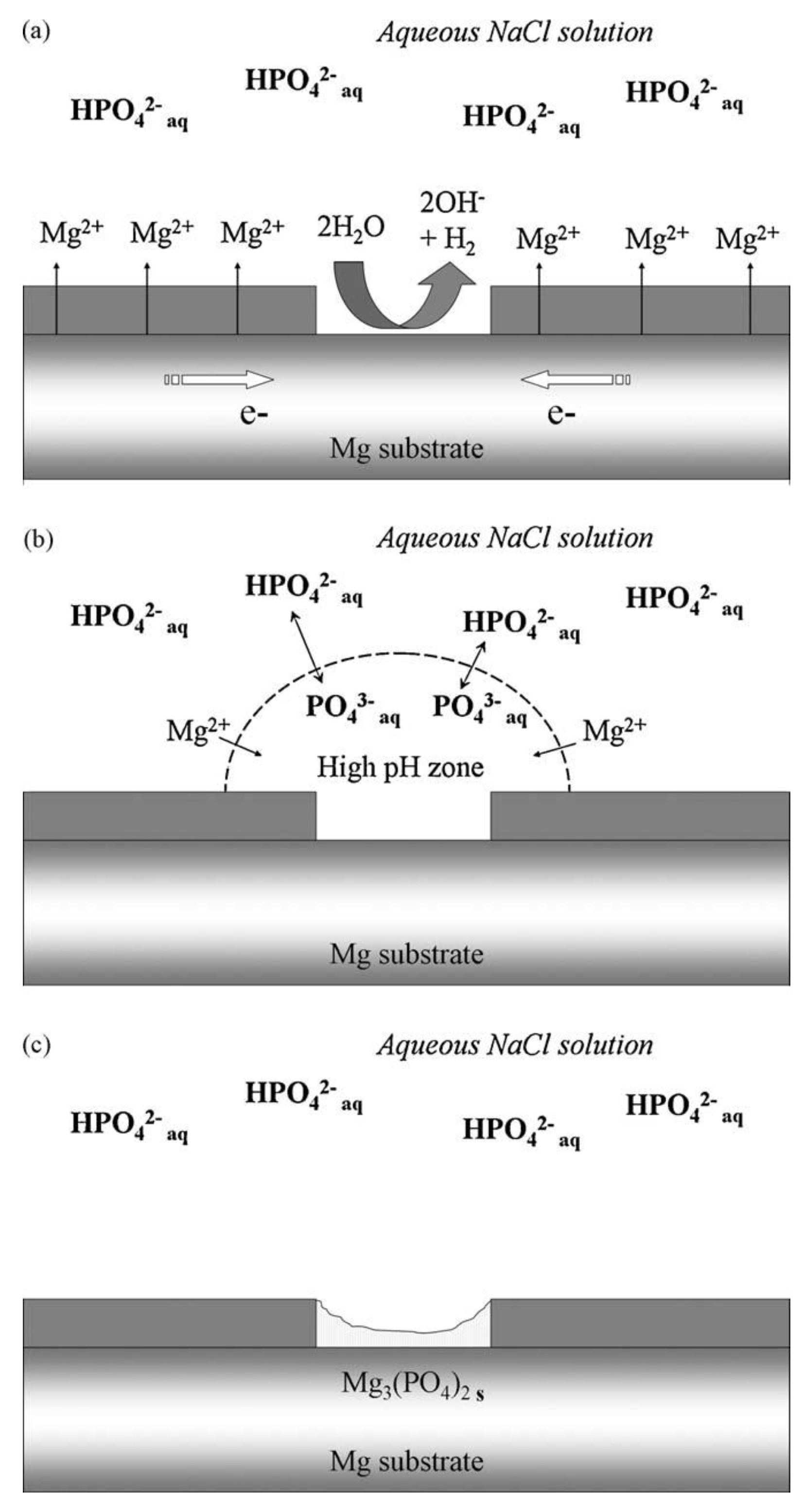
2.2. Phosphates
2.3. Rare Earth Salts
2.4. Inhibition Preventing Hydrogen Re-Combination
2.5. Other Inorganic Inhibitors
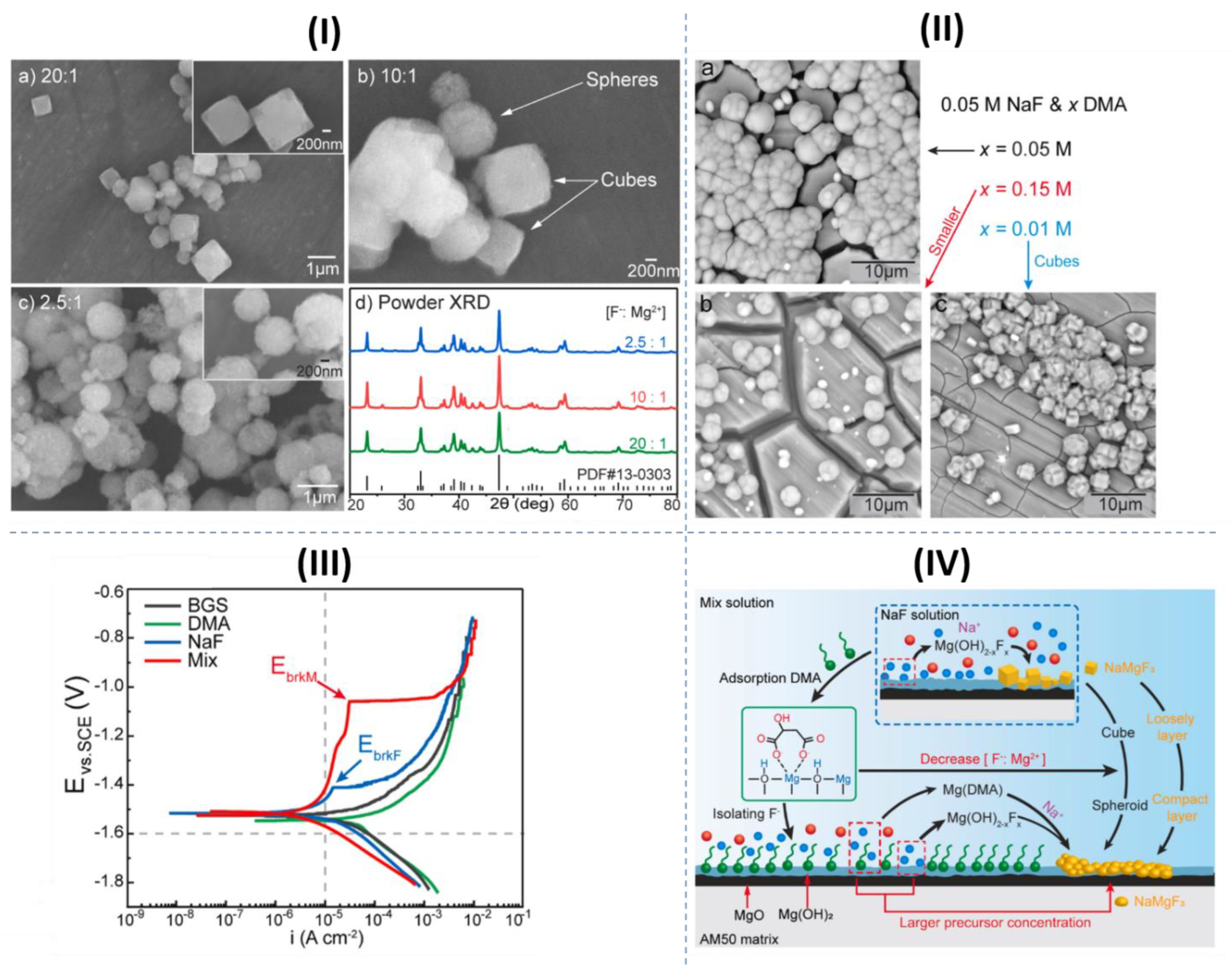
- Fluorides
- Bi/Carbonate
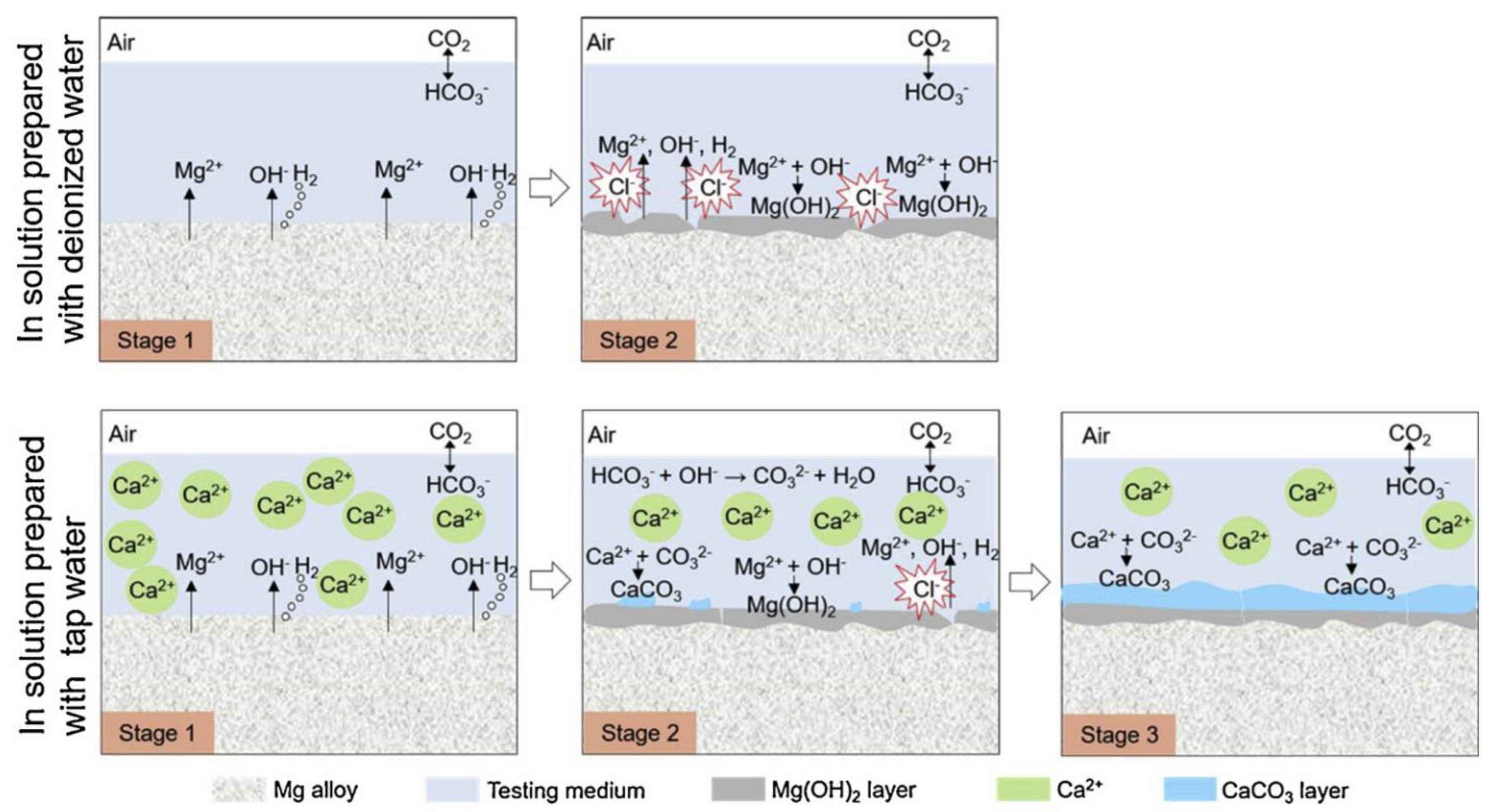
- Calcium
- Vanadates
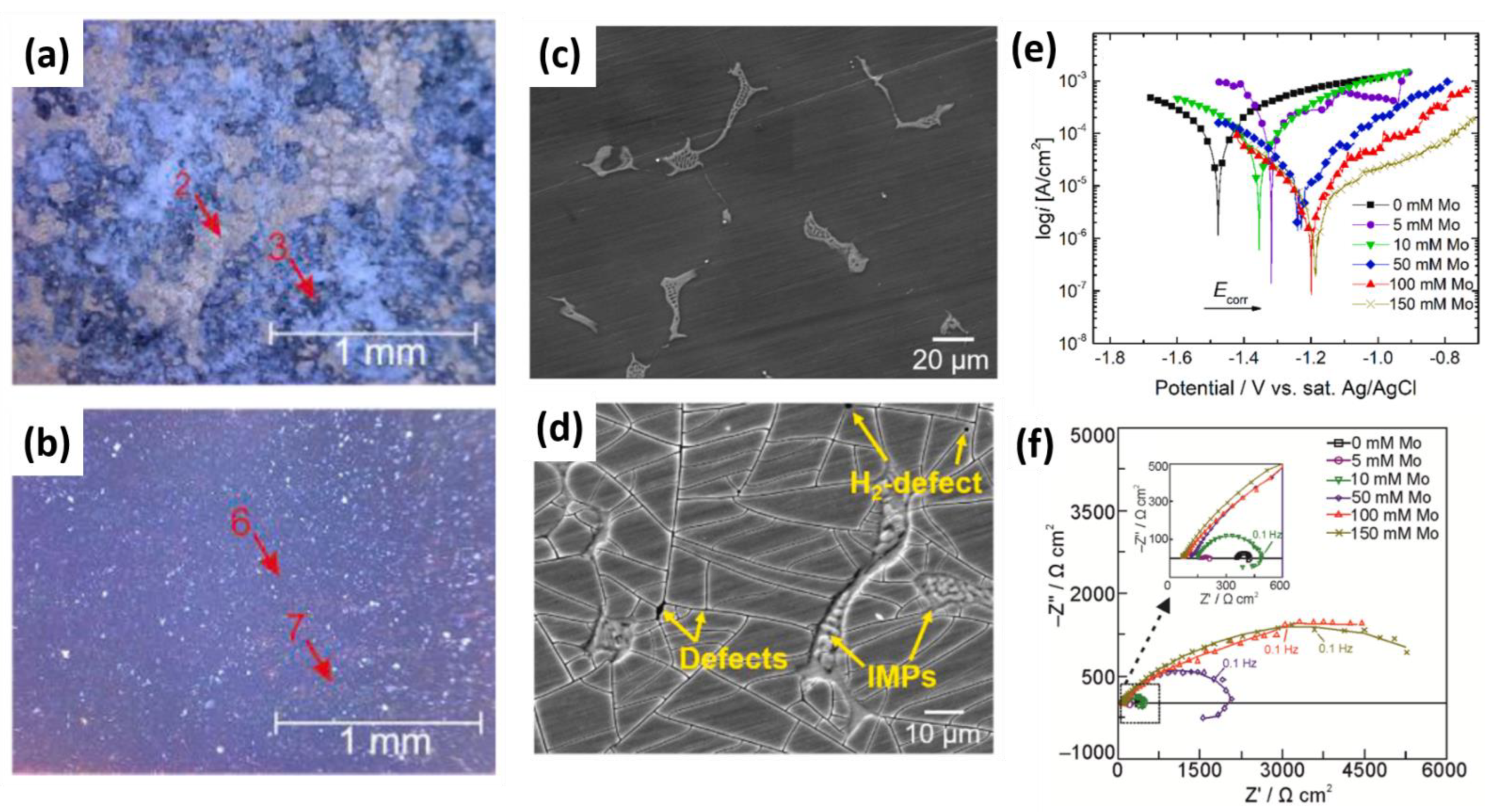
- Molybdates
- Selenite
- Nitrates
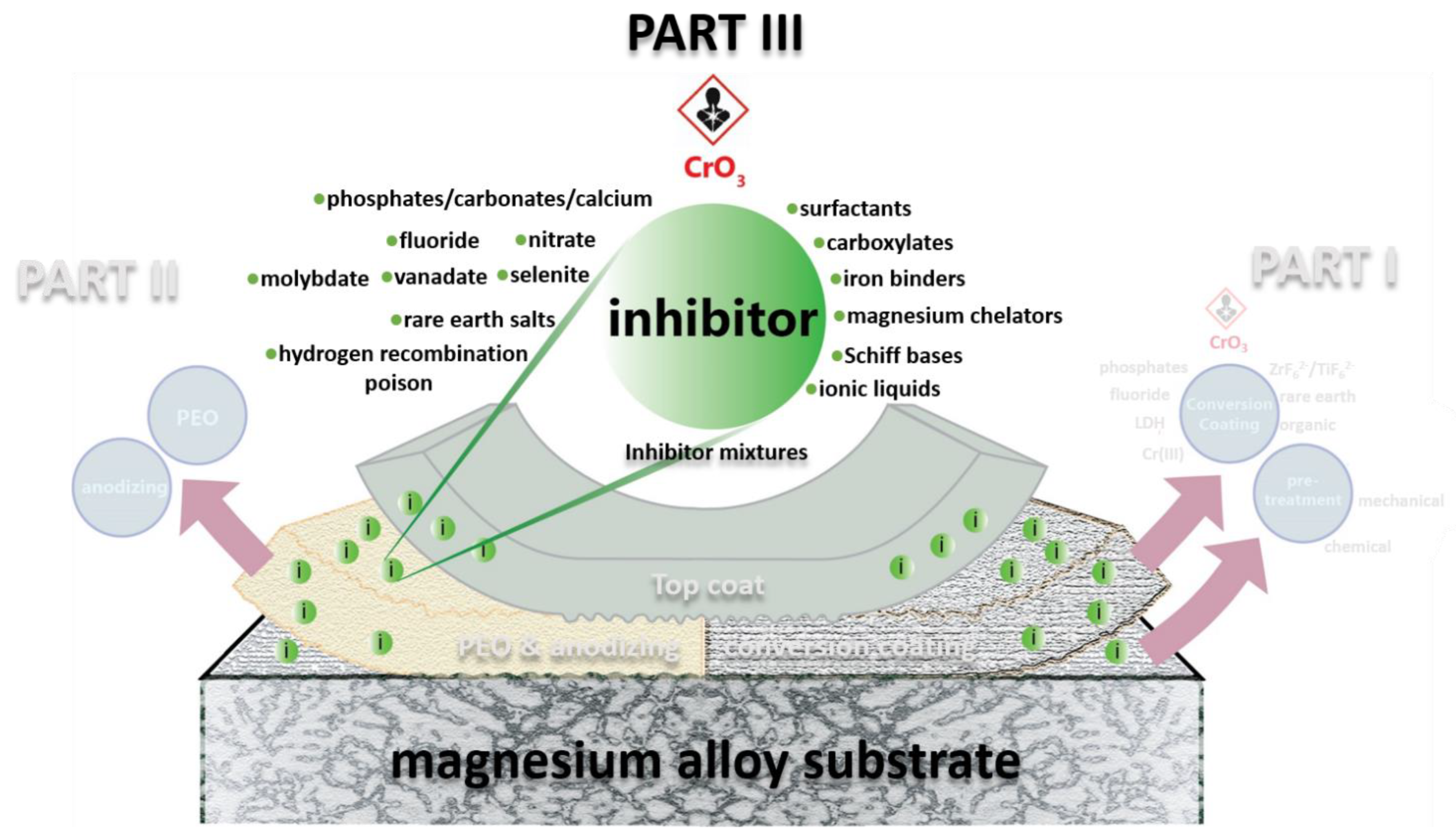
3. Organic Inhibitors
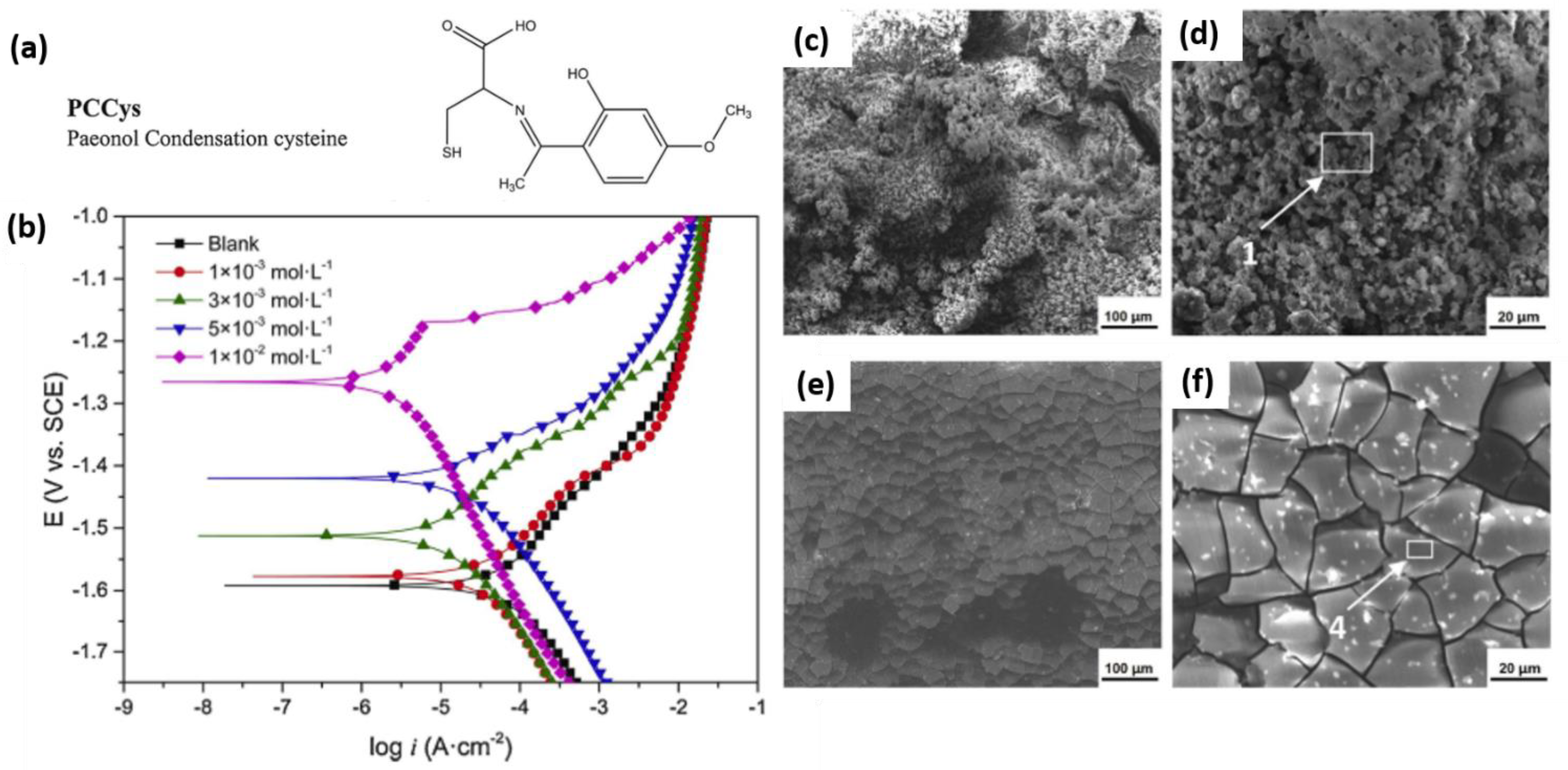
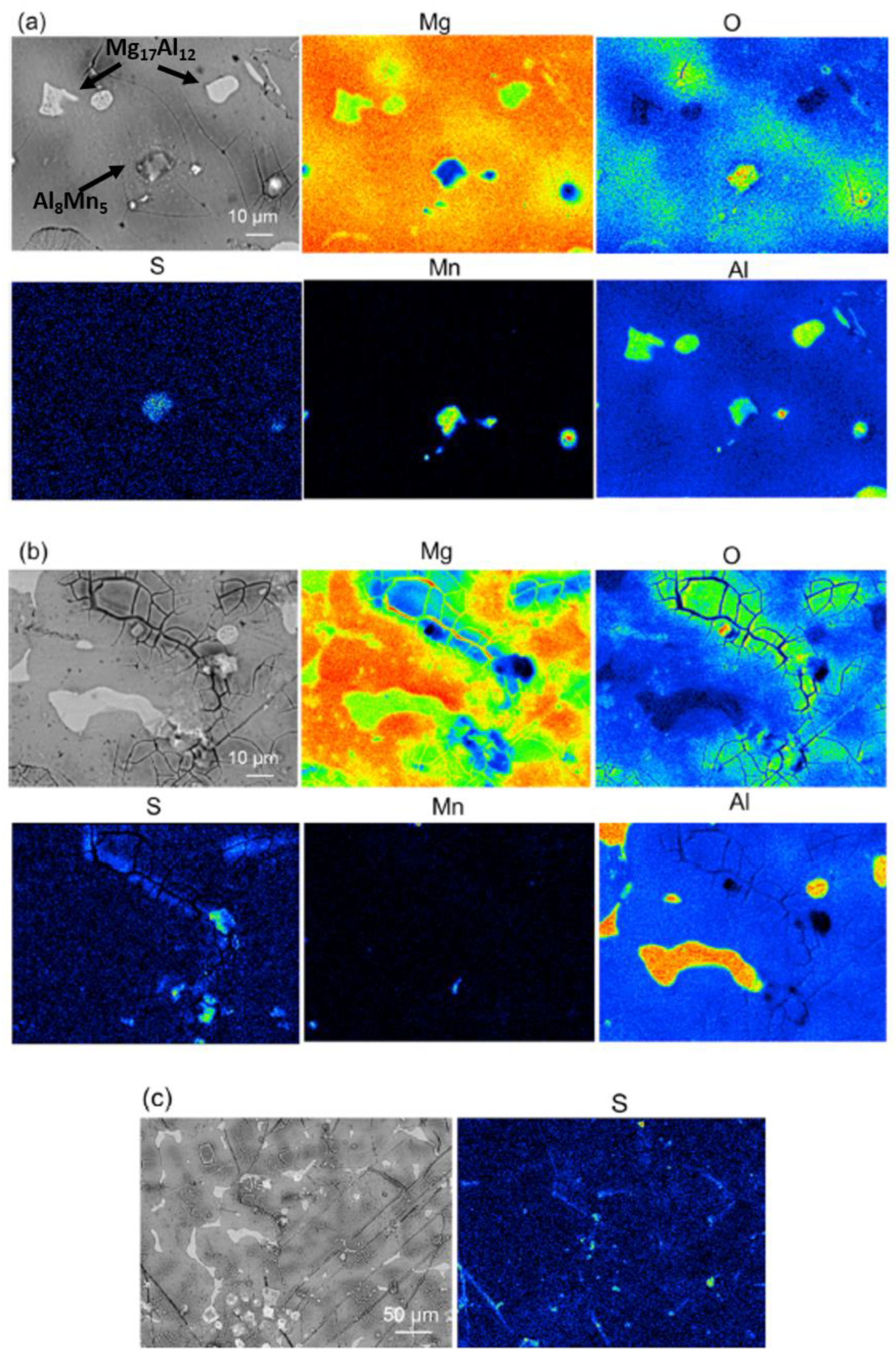
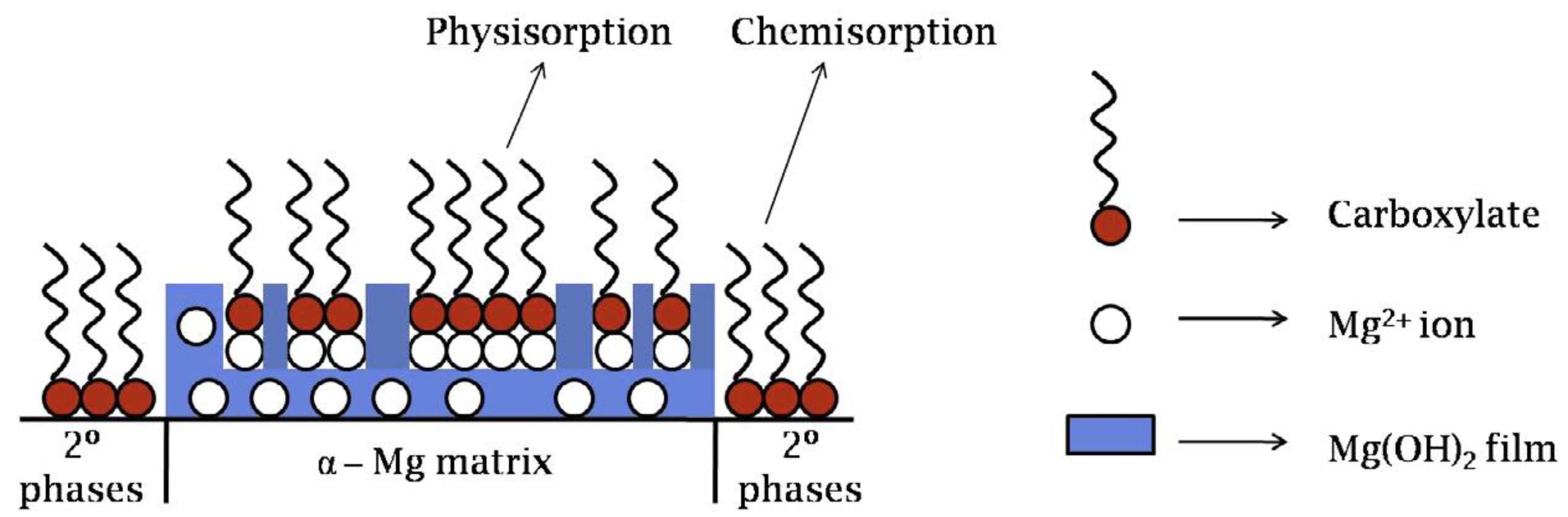
3.1. Adsorption and Precipitation Inhibitors
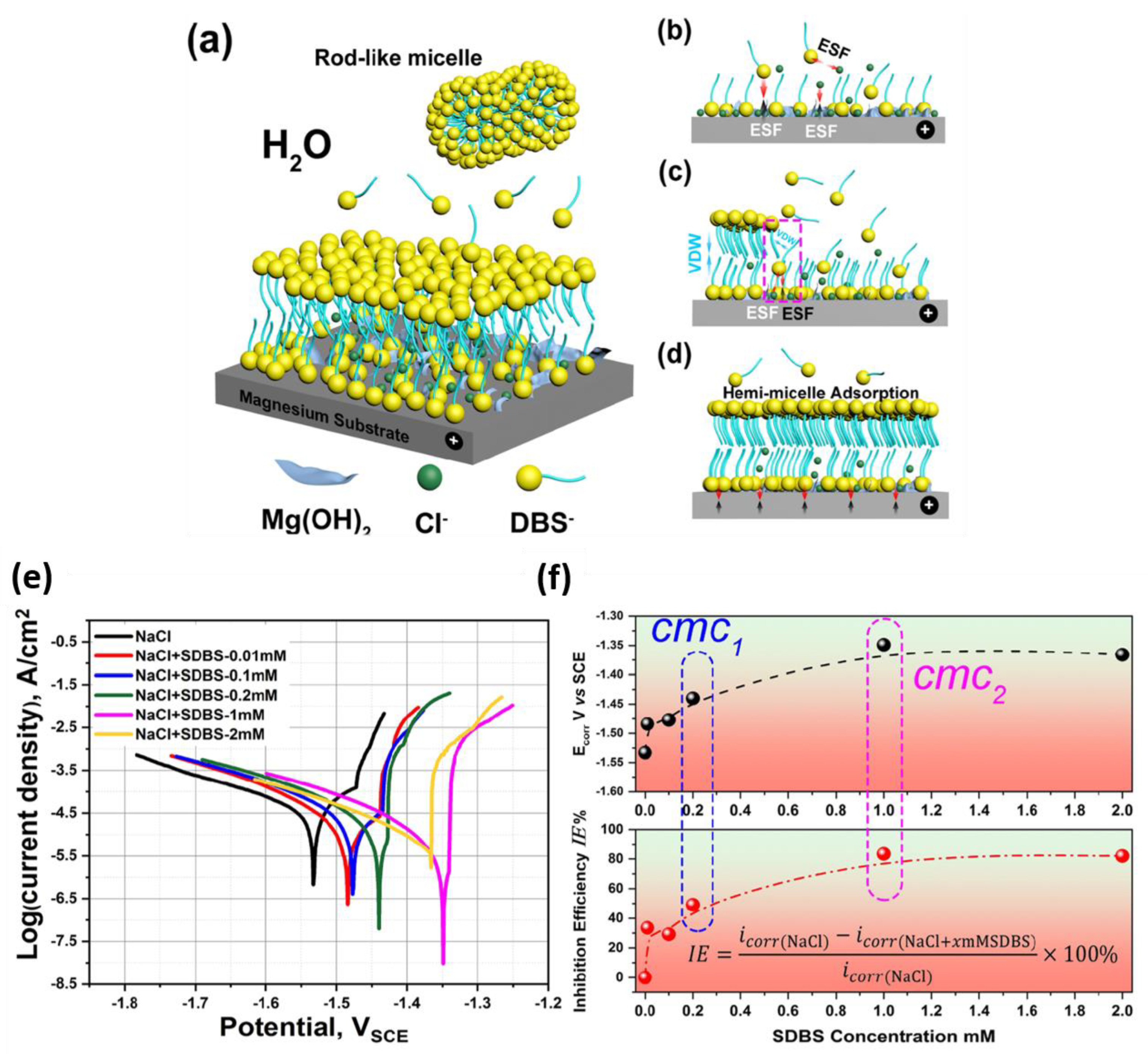
3.2. Surfactants
- Due to the hydrophobic properties of the tail of surfactants in aqueous environments, they can effectively keep corrosive media from coming into contact with the magnesium surface.
- Due to the bulky tail of surfactants, they are highly effective at covering surfaces, even in small concentrations.
3.3. Ionic Liquids
3.4. Iron Binding Inhibitors
3.5. In Silico Screening of Corrosion Inhibitors
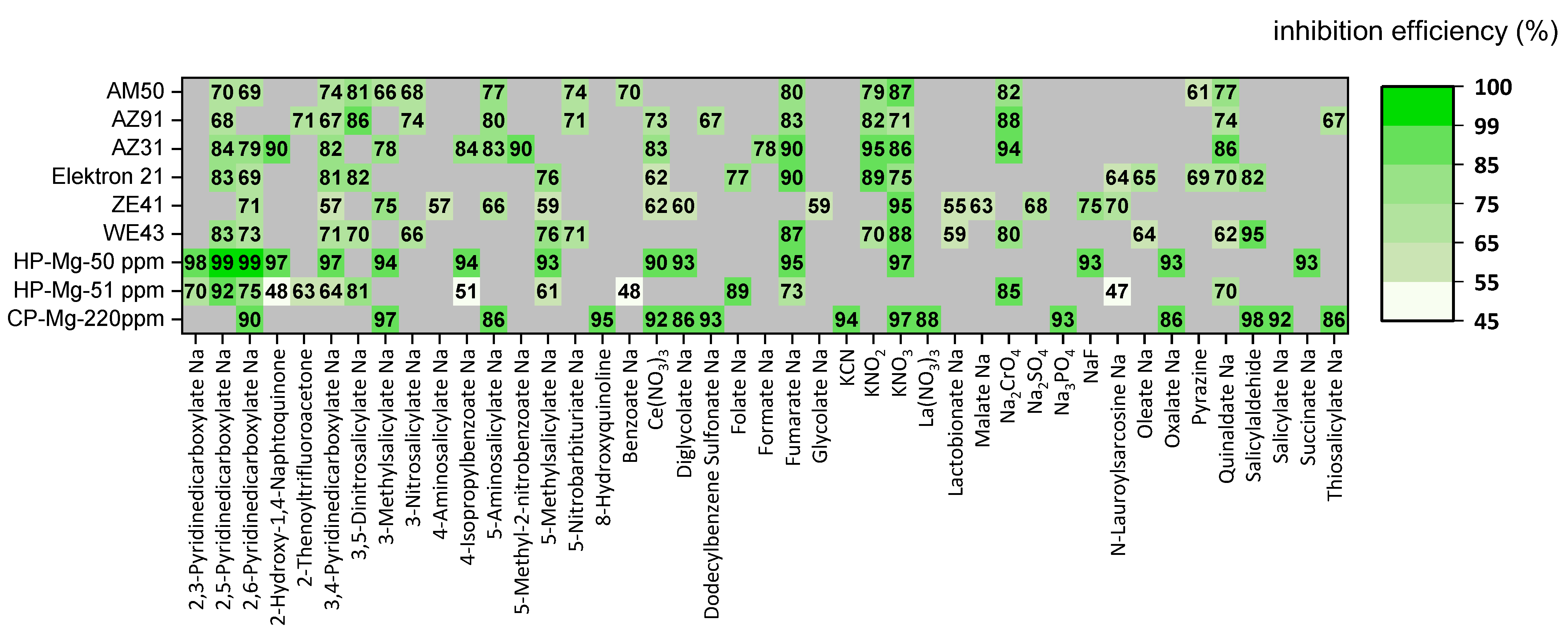
4. Inhibitor Mixtures: Synergistic Inhibiting Effect
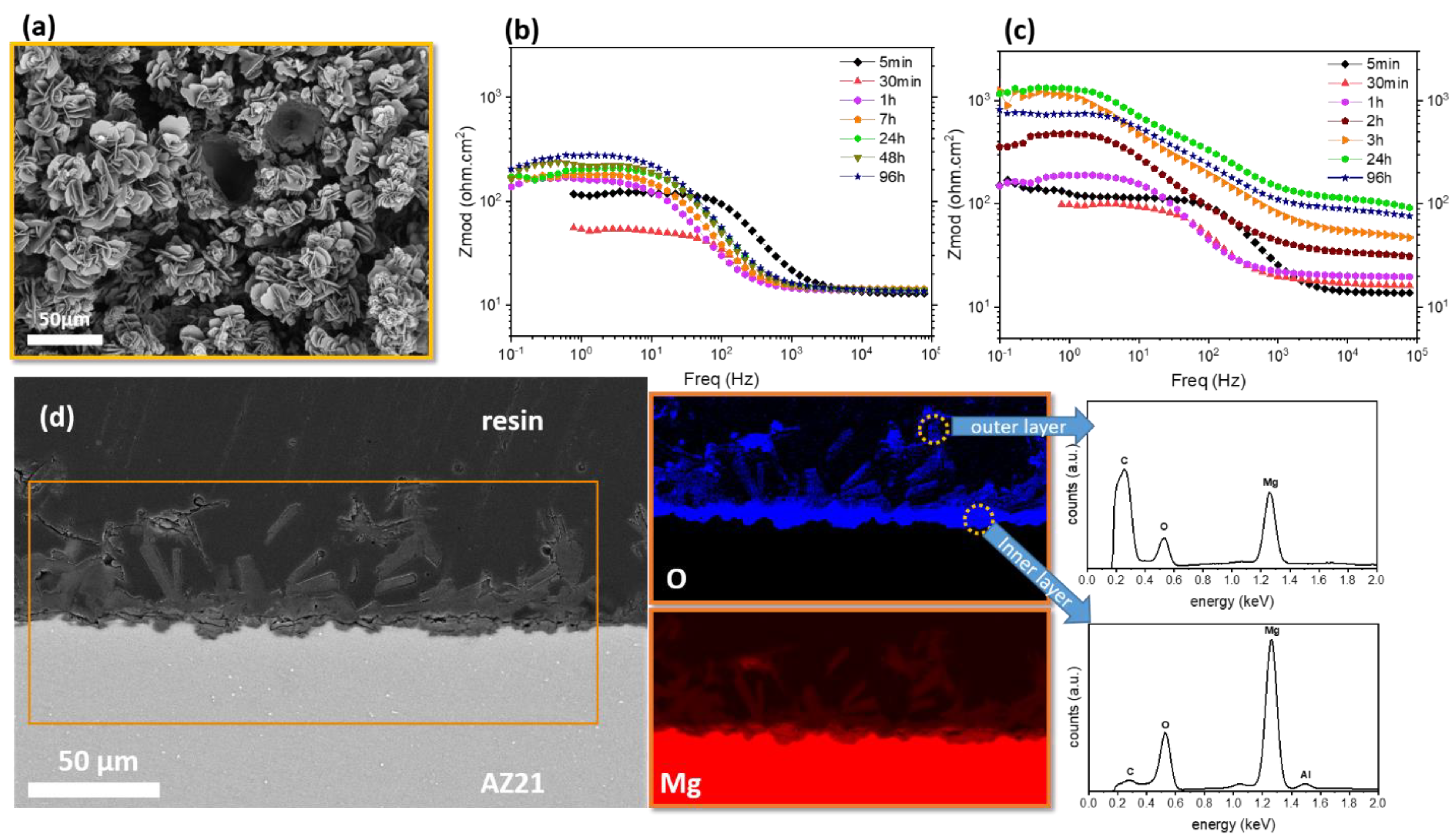
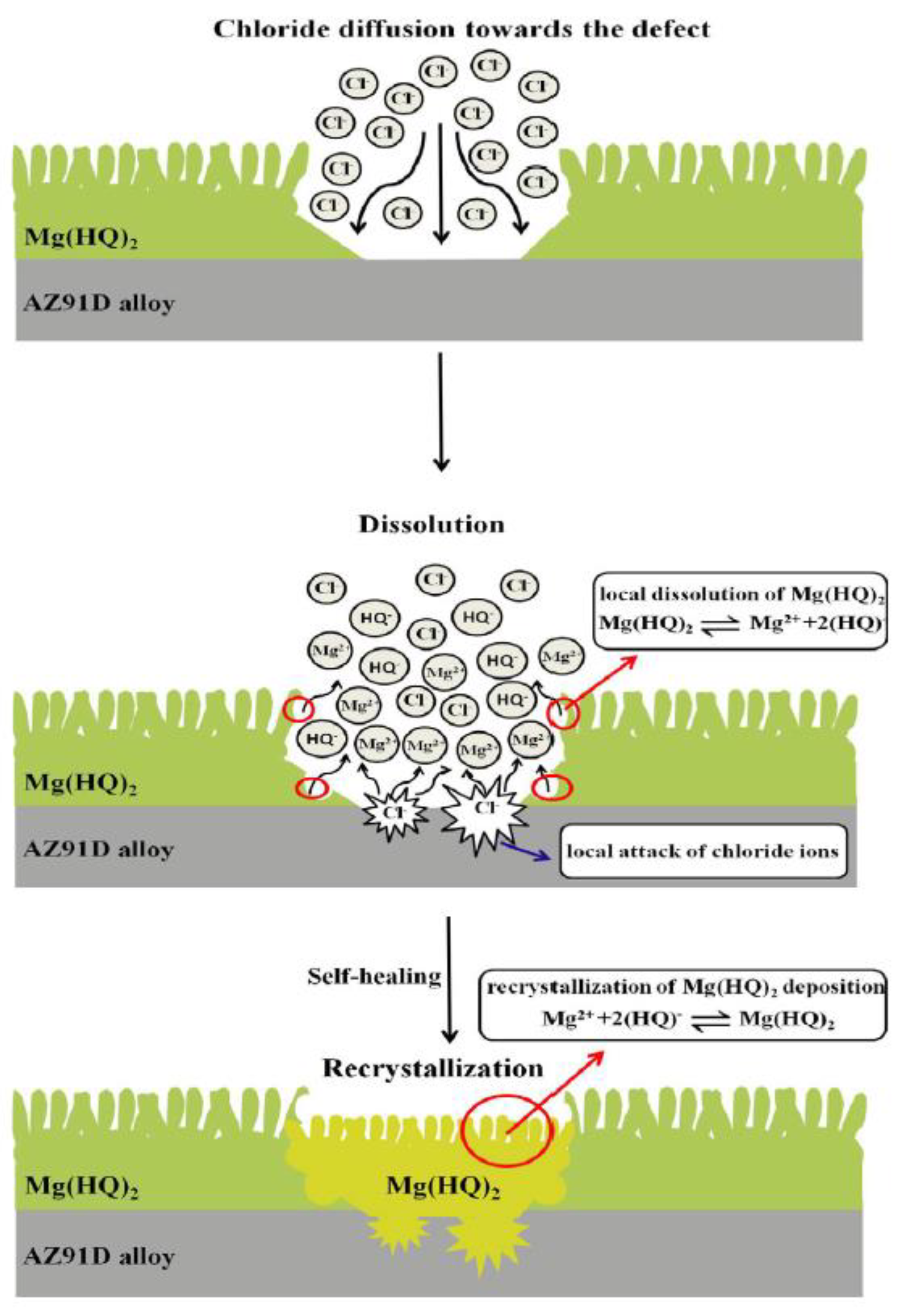
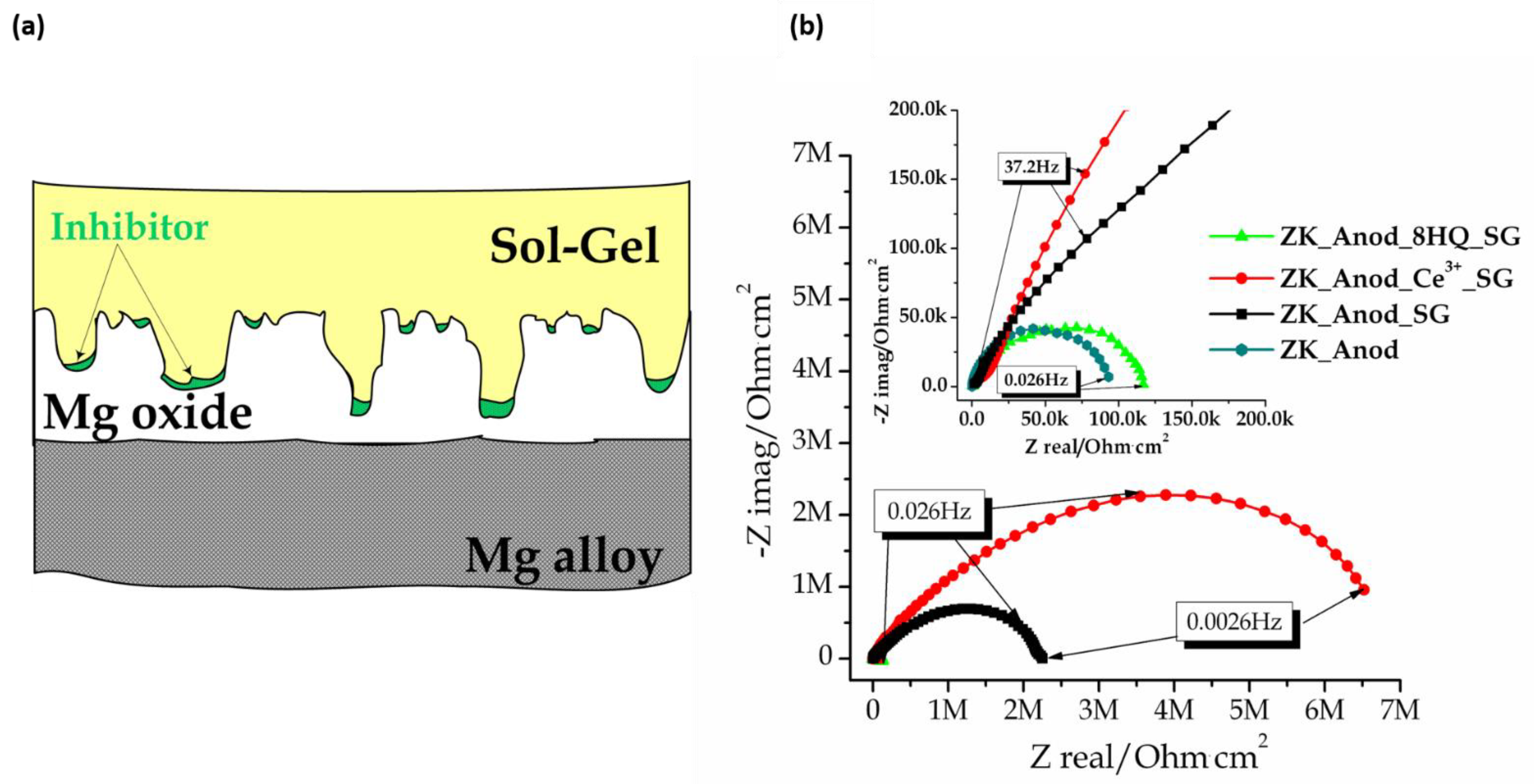
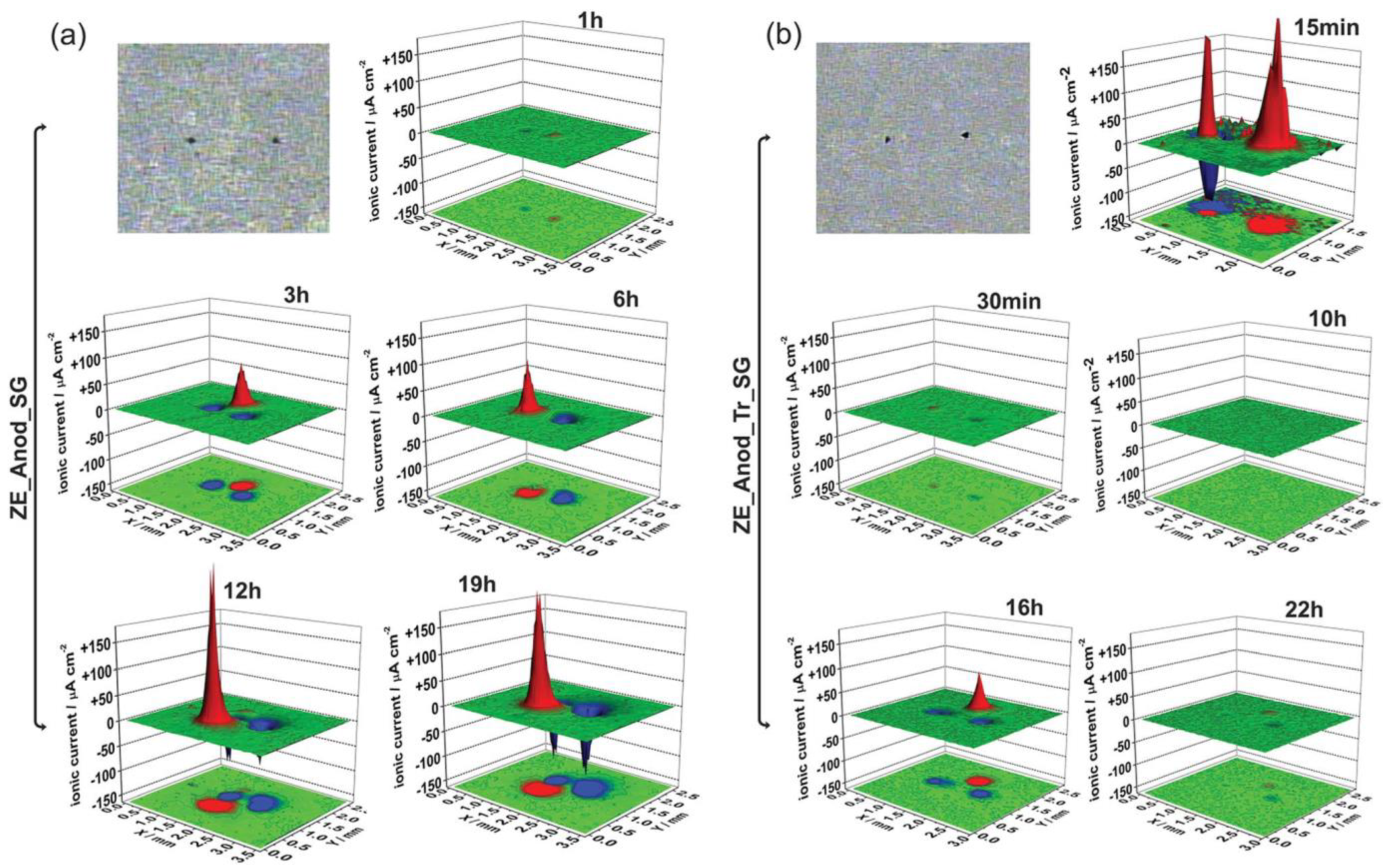
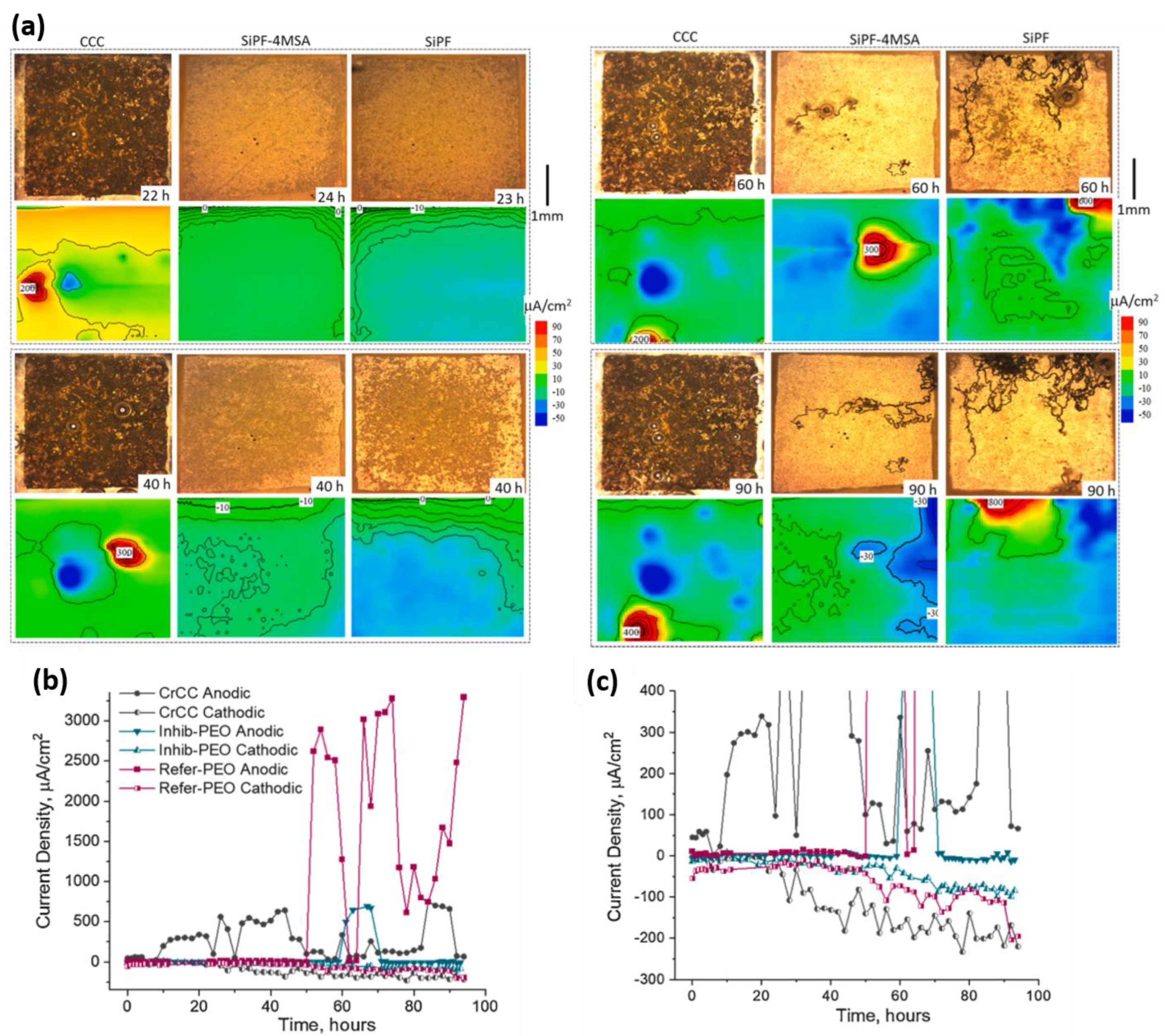
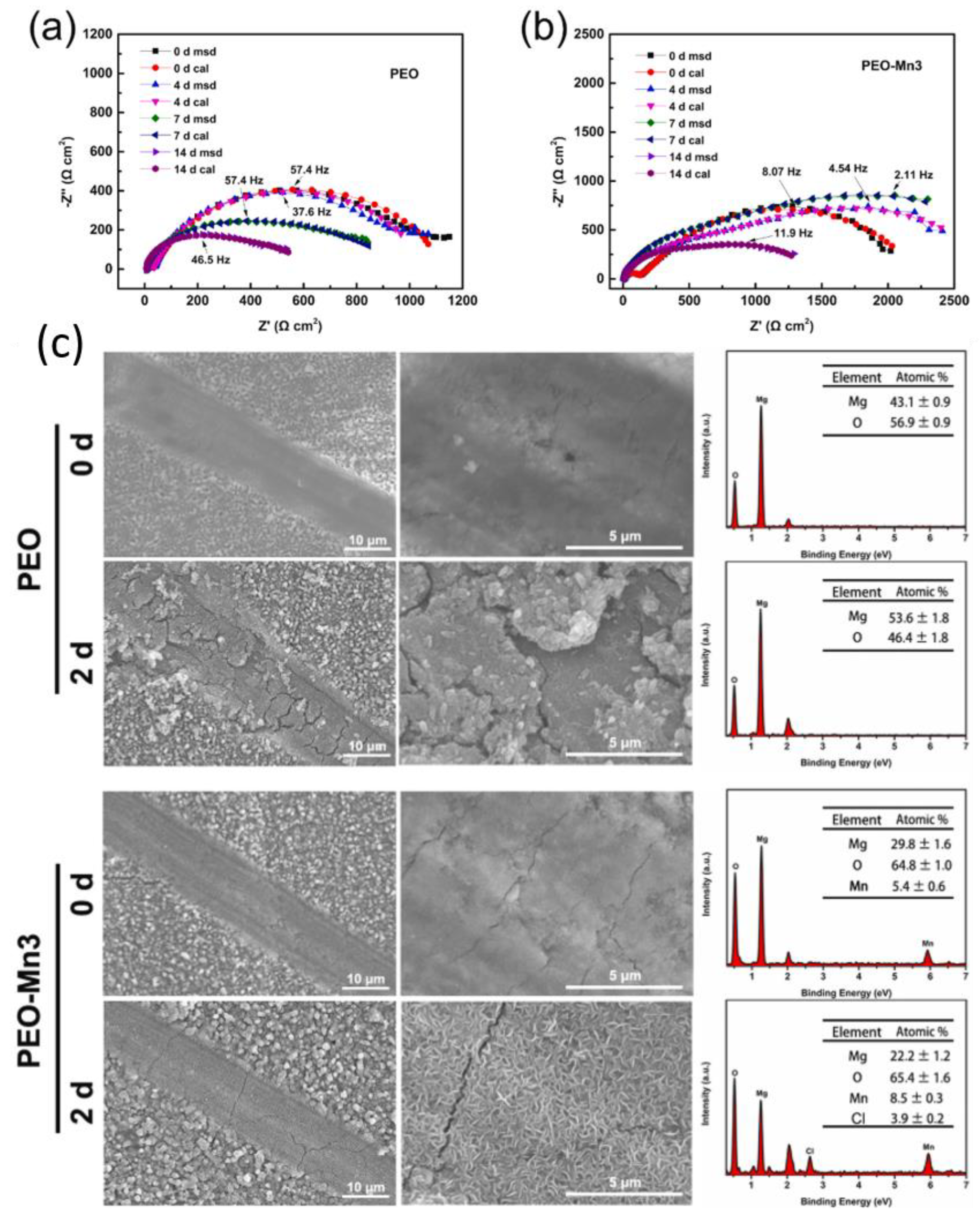
5. Combining Inhibitors with Other Corrosion Protection Strategies
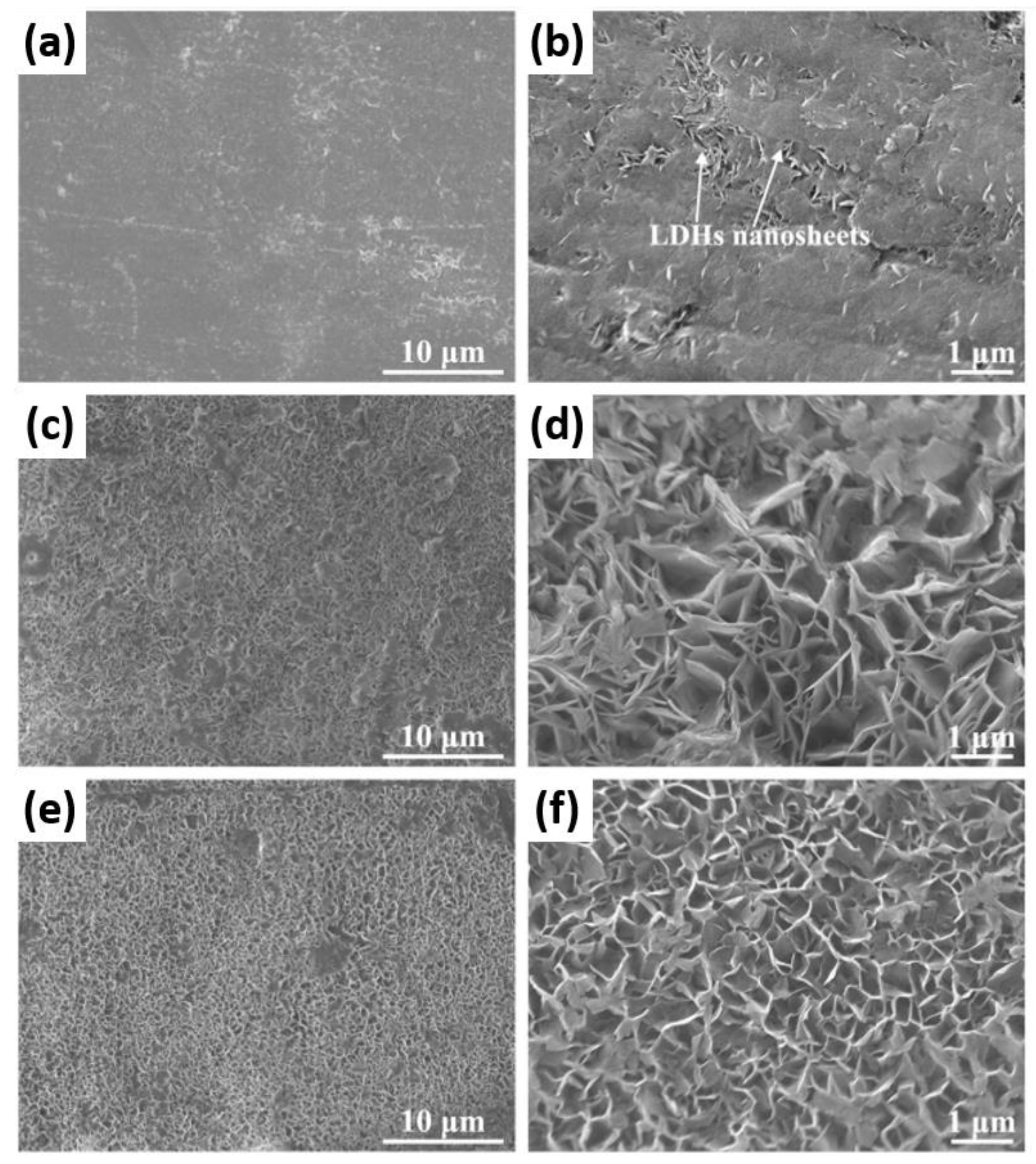
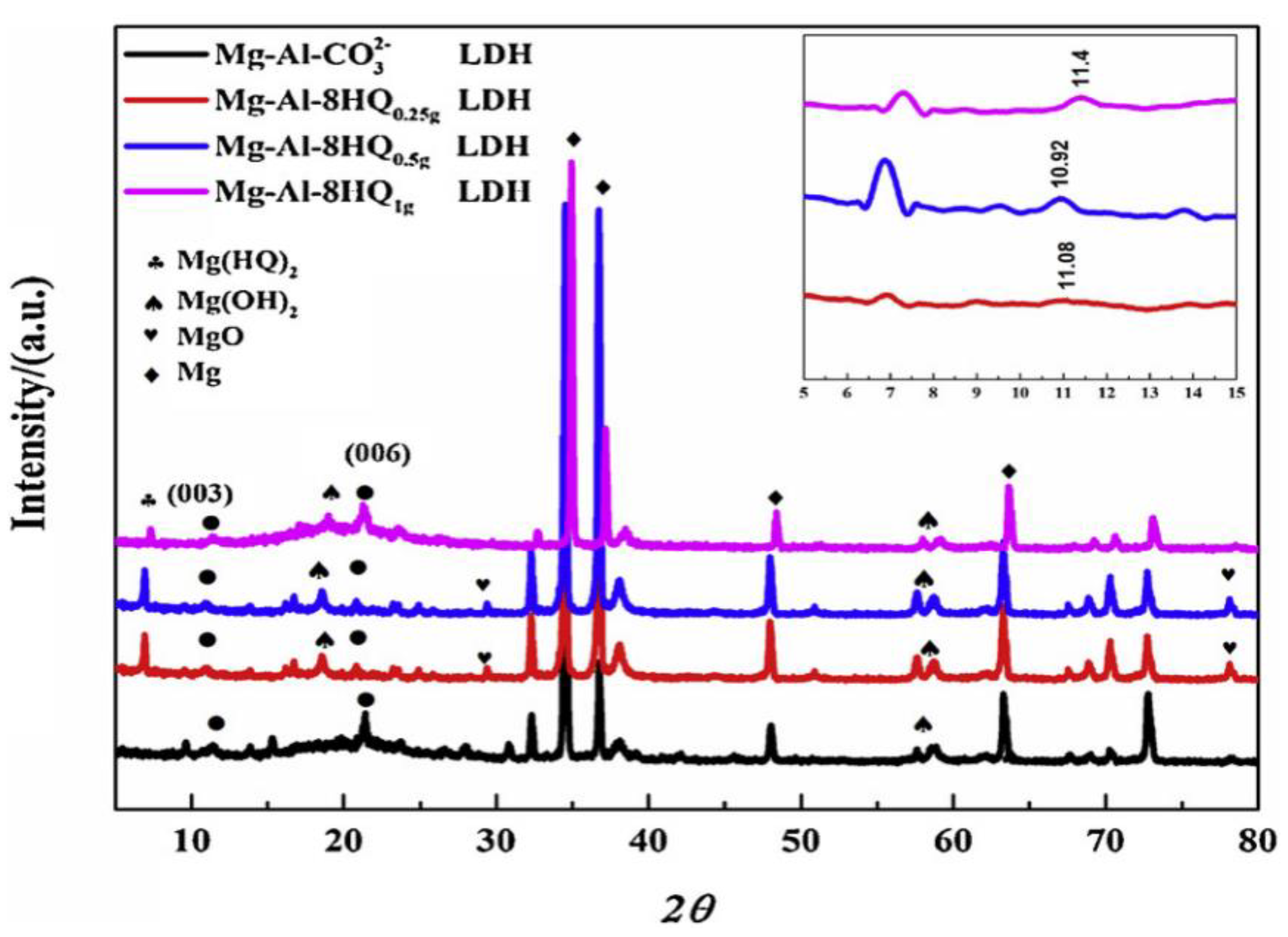
- LDH
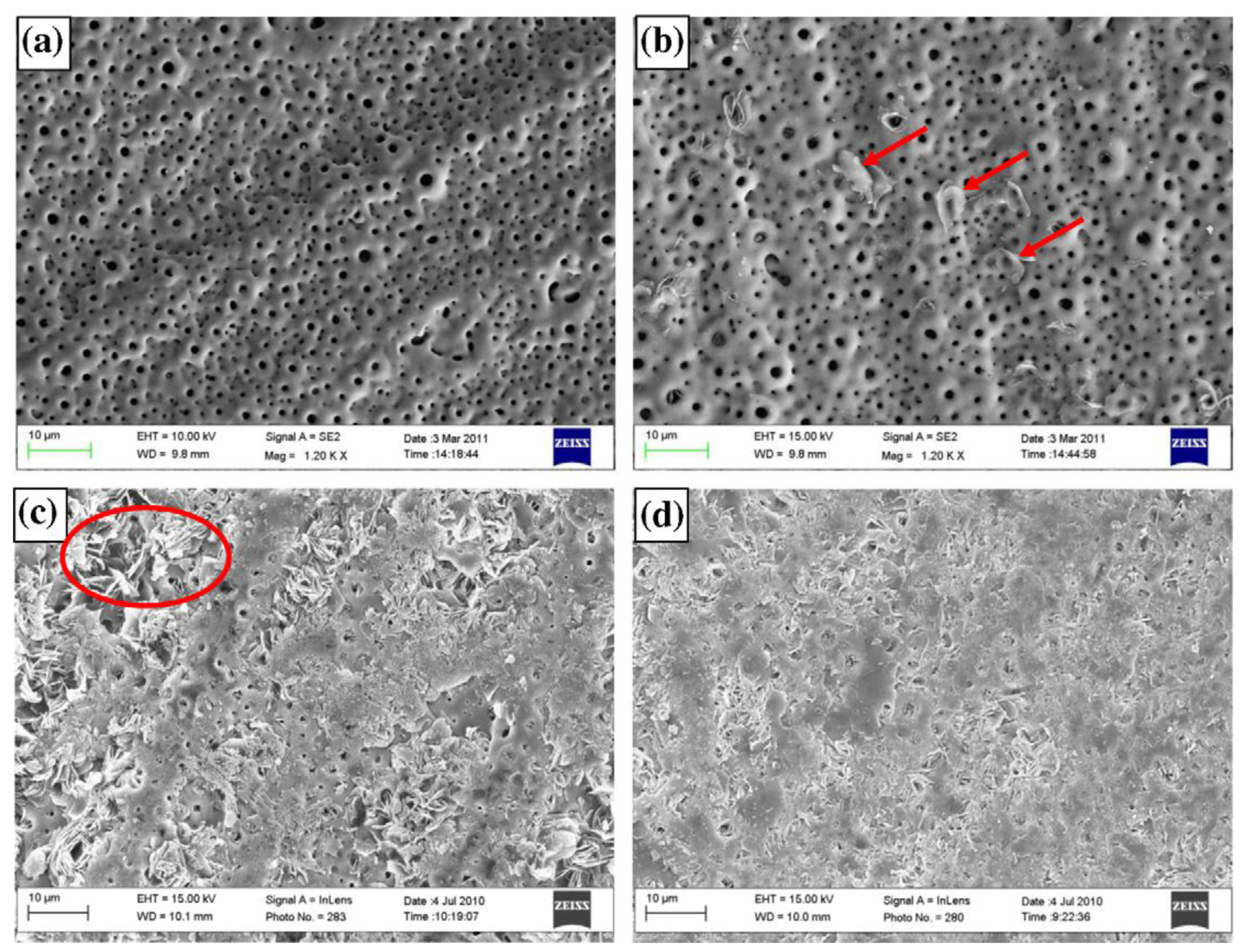
- PEO
6. Summary and Perspectives
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Handbook, A. Corrosion–Fundamentals, Testing and Protection; ASM International, The Materials Information Society: Materials Park, OH, USA, 2003; Volume 13A. [Google Scholar]
- Flick, E.W. Corrosion Inhibitors: An Industrial Guide; Noyes Publications: Park Ridge, NJ, USA, 1987. [Google Scholar]
- Sastri, V.S. Green Corrosion Inhibitors: Theory and Practice; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- ECHA. Authorisation List. 2017. Available online: https://echa.europa.eu/authorisation-list (accessed on 1 September 2017).
- ECHA. Authorisation List with Sunset Dates. 2017. Available online: https://echa.europa.eu/authorisation-list?p_p_id=disslists_WAR_disslistsportlet&p_p_lifecycle=1&p_p_state=normal&p_p_mode=view&p_p_col_id=column-1&p_p_col_pos=1&p_p_col_count=2&_disslists_WAR_disslistsportlet_javax.portlet.action=searchDissLists (accessed on 1 September 2017).
- ASD-Eurospace. Space Chromate Task Force Concludes REACH Authorisation Dossier Development for Chromium Trioxide. 2016. Available online: https://chemycal.com/news/7049d07a-149c-4759-a159-685a5aee3224/Space_Chromate_Task_Force_concludes_REACH_authorisation_dossier_development_for_chromium_trioxide (accessed on 1 May 2016).
- ECHA Recommends Authorising Critical Continued Uses of Chromium Trioxide under Strict Conditions. 2016. Available online: https://echa.europa.eu/view-article/-/journal_content/title/echa-recommends-authorising-critical-continued-uses-of-chromium-trioxide-under-strict-conditions (accessed on 1 May 2016).
- Vaghefinazari, B.; Wierzbicka, E.; Visser, P.; Posner, R.; Jordens, G.; Arrabal, R.; Matykina, E.; Mohedano, M.; Blawert, C.; Zheludkevich, M.L.; et al. Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: PART I. Pre-treatment and Conversion Coatings. Materials, 2022; accepted. [Google Scholar]
- Wierzbicka, E.; Vaghefinazari, B.; Mohedano, M.; Visser, P.; Posner, R.; Jordens, G.; Blawert, C.; Zheludkevich, M.L.; Lamaka, S.V.; Matykina, E.; et al. Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: Part II. PEO and anodizing. Materials, 2022; accepted. [Google Scholar]
- Lamaka, S.V.; Vaghefinazari, B.; Mei, D.; Petrauskas, R.P.; Hoche, D.; Zheludkevich, M.L. Comprehensive screening of Mg corrosion inhibitors. Corros. Sci. 2017, 128, 224–240. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Twite, R.L.; Bierwagen, G.P. Review of alternatives to chromate for corrosion protection of aluminum aerospace alloys. Prog. Org. Coat. 1998, 33, 91–100. [Google Scholar] [CrossRef]
- Kendig, M.; Davenport, A.J.; Isaacs, H. The mechanism of corrosion inhibition by chromate conversion coatings from X-ray absorption near edge spectroscopy (XANES). Corros. Sci. 1993, 34, 41–49. [Google Scholar] [CrossRef]
- Schmutz, P.; Guillaumin, V.; Lillard, R.; Lillard, J.; Frankel, G. Influence of dichromate ions on corrosion processes on pure magnesium. J. Electrochem. Soc. 2003, 150, B99. [Google Scholar] [CrossRef]
- Kendig, M.; Jeanjaquet, S.; Addison, R.; Waldrop, J. Role of hexavalent chromium in the inhibition of corrosion of aluminum alloys. Surf. Coat. Technol. 2001, 140, 58–66. [Google Scholar] [CrossRef]
- Pommiers, S.; Frayret, J.; Castetbon, A.; Potin-Gautier, M. Alternative conversion coatings to chromate for the protection of magnesium alloys. Corros. Sci. 2014, 84, 135–146. [Google Scholar] [CrossRef]
- Zhang, X.; van den Bos, C.; Sloof, W.G.; Hovestad, A.; Terryn, H.; de Wit, J.H.W. Comparison of the morphology and corrosion performance of Cr(VI)- and Cr(III)-based conversion coatings on zinc. Surf. Coat. Technol. 2005, 199, 92–104. [Google Scholar] [CrossRef]
- Zhao, J.; Xia, L.; Sehgal, A.; Lu, D.; McCreery, R.L.; Frankel, G.S. Effects of chromate and chromate conversion coatings on corrosion of aluminum alloy 2024-T3. Surf. Coat. Technol. 2001, 140, 51–57. [Google Scholar] [CrossRef]
- Williams, G.; Grace, R.; Woods, R.M. Inhibition of the localized corrosion of mg alloy AZ31 in chloride containing electrolyte. Corrosion 2015, 71, 184–198. [Google Scholar] [CrossRef]
- Chen, X.; Birbilis, N.; Abbott, T. Review of corrosion-resistant conversion coatings for magnesium and its alloys. Corrosion 2011, 67, 035005-1. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, H.N.; Grace, R. Inhibition of magnesium localised corrosion in chloride containing electrolyte. Electrochim. Acta 2010, 55, 7824–7833. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Chen, R.; Zhang, F.; Han, E.H. Formation mechanism of phosphate conversion film on Mg-8.8Li alloy. Corros. Sci. 2009, 51, 62–69. [Google Scholar] [CrossRef]
- Zhou, W.; Shan, D.; Han, E.-H.; Ke, W. Structure and formation mechanism of phosphate conversion coating on die-cast AZ91D magnesium alloy. Corros. Sci. 2008, 50, 329–337. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Gonzalez, J.; Mei, D.; Feyerabend, F.; Willumeit-Römer, R.; Zheludkevich, M.L. Local pH and Its Evolution Near Mg Alloy Surfaces Exposed to Simulated Body Fluids. Adv. Mater. Interfaces 2018, 5, 1800169. [Google Scholar] [CrossRef]
- Kartsonakis, I.A.; Stanciu, S.G.; Matei, A.A.; Karaxi, E.K.; Hristu, R.; Karantonis, A.; Charitidis, C.A. Evaluation of the protective ability of typical corrosion inhibitors for magnesium alloys towards the Mg ZK30 variant. Corros. Sci. 2015, 100, 194–208. [Google Scholar] [CrossRef]
- Liu, D.; Song, Y.W.; Shan, D.Y.; Han, E.H. Comparison of the Inhibition Effect of Four Inhibitors on the Corrosion Behaviour of AM60 Magnesium Alloy. Int. J. Electrochem. Sci. 2018, 13, 2219–2235. [Google Scholar] [CrossRef]
- Montemor, M.F.; Simoes, A.M.; Carmezim, M.J. Characterization of rare-earth conversion films formed on the AZ31 magnesium alloy and its relation with corrosion protection. Appl. Surf. Sci. 2007, 253, 6922–6931. [Google Scholar] [CrossRef]
- Richey, F.W.; McCloskey, B.D.; Luntz, A.C. Mg anode corrosion in aqueous electrolytes and implications for Mg-air batteries. J. Electrochem. Soc. 2016, 163, A958–A963. [Google Scholar] [CrossRef]
- Wang, S.; Li, Q.; Zhong, X.; Li, L.; Chen, F.; Luo, F.; Dai, Y.; Gao, H.; Liu, F.; Zhang, H. Effects of NO3- in NaCl solution on corrosion protection of AZ91D magnesium alloy coated with silane films. Trans. IMF 2012, 90, 78–85. [Google Scholar] [CrossRef]
- Snihirova, D.; Wang, L.; Lamaka, S.V.; Wang, C.; Deng, M.; Vaghefinazari, B.; Hoche, D.; Zheludkevich, M.L. Synergistic Mixture of Electrolyte Additives: A Route to a High-Efficiency Mg-Air Battery. J. Phys. Chem. Lett. 2020, 11, 8790–8798. [Google Scholar] [CrossRef]
- Eaves, D.; Williams, G.; McMurray, H.N. Inhibition of self-corrosion in magnesium by poisoning hydrogen recombination on iron impurities. Electrochim. Acta 2012, 79, 1–7. [Google Scholar] [CrossRef]
- Birbilis, N.; Williams, G.; Gusieva, K.; Samaniego, A.; Gibson, M.A.; McMurray, H.N. Poisoning the corrosion of magnesium. Electrochem. Commun. 2013, 34, 295–298. [Google Scholar] [CrossRef]
- Liu, R.L.; Hurley, M.F.; Kvryan, A.; Williams, G.; Scully, J.R.; Birbilis, N. Controlling the corrosion and cathodic activation of magnesium via microalloying additions of Ge. Sci. Rep. 2016, 6, 28747. [Google Scholar] [CrossRef]
- Williams, G.; Dafydd, H.A.-L.; McMurray, H.N.; Birbilis, N. The influence of arsenic alloying on the localised corrosion behaviour of magnesium. Electrochim. Acta 2016, 219, 401–411. [Google Scholar] [CrossRef]
- Glover, C.; Liu, R.; McNally, E.; Mahboubi, S.; McDermid, J.; Kish, J.; Birbilis, N.; McMurray, H.; Williams, G. Toward a Physical Description of the Role of Germanium in Moderating Cathodic Activation of Magnesium. Corrosion 2021, 77, 134–147. [Google Scholar] [CrossRef]
- Yuwono, J.A.; Birbilis, N.; Liu, R.; Ou, Q.; Bao, Q.; Medhekar, N.V. Aqueous Electrochemical Activity of the Mg Surface: The Role of Group 14 and 15 Microalloying Elements. J. Electrochem. Soc. 2017, 164, C918–C929. [Google Scholar] [CrossRef]
- Gore, P.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Raja, V.S. Anodic activation of Mg in the presence of In3+ ions in dilute sodium chloride solution. Electrochim. Acta 2019, 293, 199–210. [Google Scholar] [CrossRef]
- Bao, T.; Hou, L.; Sun, J.; Liu, X.; Du, H.; Wei, H.; Wei, Y. Effect of Indium on the Negative Difference Effect for Magnesium Alloy. J. Electrochem. Soc. 2021, 168, 031515. [Google Scholar] [CrossRef]
- Wang, L.Q.; Snihirova, D.; Deng, M.; Wang, C.; Hoche, D.; Lamaka, S.V.; Zheludkevich, M.L. Indium chloride as an electrolyte additive for primary aqueous Mg batteries. Electrochim. Acta 2021, 373, 137916. [Google Scholar] [CrossRef]
- Mercier, D.; Światowska, J.; Protopopoff, E.; Zanna, S.; Seyeux, A.; Marcus, P. Inhibition of Mg Corrosion by Sulfur Blocking of the Hydrogen Evolution Reaction on Iron Impurities. J. Electrochem. Soc. 2020, 167, 121504. [Google Scholar] [CrossRef]
- Xu, J.; Yang, Q.; Javed, M.S.; Gong, Y.; Aslam, M.K.; Chen, C. The effects of NaF concentration on electrochemical and corrosion behavior of AZ31B magnesium alloy in a composite electrolyte. RSC Adv. 2017, 7, 5880–5887. [Google Scholar] [CrossRef]
- El-Taib Heakal, F.; Tantawy, N.S.; Shehata, O.S. Impact of chloride and fluoride additions on surface reactivity and passivity of AM60 magnesium alloy in buffer solution. Corros. Sci. 2012, 64, 153–163. [Google Scholar] [CrossRef]
- Heakal, F.E.-T.; Fekry, A.; Fatayerji, M. Influence of halides on the dissolution and passivation behavior of AZ91D magnesium alloy in aqueous solutions. Electrochim. Acta 2009, 54, 1545–1557. [Google Scholar] [CrossRef]
- Barros, C.; Muzeau, B.; L’hostis, V.; François, R. Impact of fluoride concentration on general corrosion of Mg-Zr alloy in a Na-geopolymer and alkaline solutions. Corros. Sci. 2020, 176, 109009. [Google Scholar] [CrossRef]
- Qiu, Y.; Tu, X.; Lu, X.; Yang, J. A novel insight into synergistic corrosion inhibition of fluoride and DL-malate as a green hybrid inhibitor for magnesium alloy. Corros. Sci. 2022, 199, 110177. [Google Scholar] [CrossRef]
- Cheng, M.-Q.; Wahafu, T.; Jiang, G.-F.; Liu, W.; Qiao, Y.-Q.; Peng, X.-C.; Cheng, T.; Zhang, X.-L.; He, G.; Liu, X.-Y. A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Okulov, I.V.; Lamaka, S.V.; Wada, T.; Yubuta, K.; Zheludkevich, M.L.; Weissmüller, J.; Markmann, J.; Kato, H. Nanoporous magnesium. Nano Res. 2018, 11, 6428–6435. [Google Scholar] [CrossRef]
- Prince, L.; Rousseau, M.A.; Noirfalise, X.; Dangreau, L.; Coelho, L.B.; Olivier, M.G. Inhibitive effect of sodium carbonate on corrosion of AZ31 magnesium alloy in NaCl solution. Corros. Sci. 2021, 179, 109131. [Google Scholar] [CrossRef]
- Liu, H.; Cao, F.; Song, G.-L.; Zheng, D.; Shi, Z.; Dargusch, M.S.; Atrens, A. Review of the atmospheric corrosion of magnesium alloys. J. Mater. Sci. Technol. 2019, 35, 2003–2016. [Google Scholar] [CrossRef]
- Mei, D.; Lamaka, S.V.; Gonzalez, J.; Feyerabend, F.; Willumeit-Römer, R.; Zheludkevich, M.L. The role of individual components of simulated body fluid on the corrosion behavior of commercially pure Mg. Corros. Sci. 2019, 147, 81–93. [Google Scholar] [CrossRef]
- Zaghloul, B.; Glover, C.; Scully, J.; Kish, J. Inhibiting Corrosion of Mg Alloy AZ31B-H24 Sheet Metal with Lithium Carbonate. Corrosion 2021, 77, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, B.; Kish, J. Corrosion Inhibition of Mg Alloy ZEK100 Sheet Metal by Dissolved Lithium Carbonate. J. Electrochem. Soc. 2021, 168, 081507. [Google Scholar] [CrossRef]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. Effect of [Ca 2+] and [PO43-] levels on the formation of calcium phosphate conversion coatings on die-cast magnesium alloy AZ91D. Corros. Sci. 2012, 55, 226–232. [Google Scholar] [CrossRef]
- Su, Y.; Guo, Y.; Huang, Z.; Zhang, Z.; Li, G.; Lian, J.; Ren, L. Preparation and corrosion behaviors of calcium phosphate conversion coating on magnesium alloy. Surf. Coat. Technol. 2016, 307, 99–108. [Google Scholar] [CrossRef]
- Jiang, P.; Blawert, C.; Scharnagl, N.; Zheludkevich, M.L. Influence of water purity on the corrosion behavior of Mg0.5ZnX (X= Ca, Ge) alloys. Corros. Sci. 2019, 153, 62–73. [Google Scholar] [CrossRef]
- Gonzalez, J.; Lamaka, S.V.; Mei, D.; Scharnagl, N.; Feyerabend, F.; Zheludkevich, M.L.; Willumeit-Römer, R. Mg Biodegradation Mechanism Deduced from the Local Surface Environment under Simulated Physiological Conditions. Adv. Healthc. Mater. 2021, 10, 2100053. [Google Scholar] [CrossRef]
- Iannuzzi, M.; Frankel, G.S. Mechanisms of corrosion inhibition of AA2024-T3 by vanadates. Corros. Sci. 2007, 49, 2371–2391. [Google Scholar] [CrossRef]
- Hurley, B.L.; Ralston, K.D.; Buchheit, R.G. Corrosion inhibition of zinc by aqueous vanadate species. J. Electrochem. Soc. 2014, 161, C471. [Google Scholar] [CrossRef]
- Feng, Z.; Hurley, B.; Li, J.; Buchheit, R. Corrosion inhibition study of aqueous vanadate on Mg alloy AZ31. J. Electrochem. Soc. 2018, 165, C94. [Google Scholar] [CrossRef]
- Mingo, B.; Guo, Y.; Leiva-Garcia, R.; Connolly, B.J.; Matthews, A.; Yerokhin, A. Smart Functionalization of Ceramic-Coated AZ31 Magnesium Alloy. ACS Appl. Mater. Interfaces 2020, 12, 30833–30846. [Google Scholar] [CrossRef] [PubMed]
- Kharitonov, D.S.; Zimowska, M.; Ryl, J.; Zieliński, A.; Osipenko, M.A.; Adamiec, J.; Wrzesińska, A.; Claesson, P.M.; Kurilo, I.I. Aqueous molybdate provides effective corrosion inhibition of WE43 magnesium alloy in sodium chloride solutions. Corros. Sci. 2021, 190, 109664. [Google Scholar] [CrossRef]
- Zeng, R.-C.; Liu, Z.-G.; Zhang, F.; Li, S.-Q.; Cui, H.-Z.; Han, E.-H. Corrosion of molybdate intercalated hydrotalcite coating on AZ31 Mg alloy. J. Mater. Chem. A 2014, 2, 13049–13057. [Google Scholar] [CrossRef]
- Osipenko, M.A.; Kharytonau, D.S.; Kasach, A.A.; Ryl, J.; Adamiec, J.; Kurilo, I.I. Inhibitive Effect of Sodium Molybdate on Corrosion of AZ31 Magnesium Alloy in Chloride Solutions. Electrochim. Acta 2022, 414, 140175. [Google Scholar] [CrossRef]
- Feng, Z.; Hurley, B.; Zhu, M.; Yang, Z.; Hwang, J.; Buchheit, R. Corrosion Inhibition of AZ31 Mg Alloy by Aqueous Selenite (SeO32−). J. Electrochem. Soc. 2019, 166, C520. [Google Scholar] [CrossRef]
- Feng, Z.; Xu, C.C.; Zhang, D.; Buchheit, R. Corrosion Protective Film Formation on Mg Alloy AZ31 by Exposure to Dilute Selenite Solutions. Materials 2021, 14, 286. [Google Scholar] [CrossRef]
- Whanger, P.; Vendeland, S.; Park, Y.-C.; Xia, Y. Metabolism of subtoxic levels of selenium in animals and humans. Ann. Clin. Lab. Sci. 1996, 26, 99–113. [Google Scholar]
- Spallholz, J.E. On the nature of selenium toxicity and carcinostatic activity. Free Radic. Biol. Med. 1994, 17, 45–64. [Google Scholar] [CrossRef]
- Navarro-Alarcon, M.; López-Martınez, M. Essentiality of selenium in the human body: Relationship with different diseases. Sci. Total Environ. 2000, 249, 347–371. [Google Scholar] [CrossRef]
- Cui, Z.; Ge, F.; Lin, Y.; Wang, L.; Lei, L.; Tian, H.; Yu, M.; Wang, X. Corrosion behavior of AZ31 magnesium alloy in the chloride solution containing ammonium nitrate. Electrochim. Acta 2018, 278, 421–437. [Google Scholar] [CrossRef]
- Wang, J.L.; Ke, C.; Pohl, K.; Birbilis, N.; Chen, X.B. The Unexpected Role of Benzotriazole in Mitigating Magnesium Alloy Corrosion: A Nucleating Agent for Crystalline Nanostructured Magnesium Hydroxide Film. J. Electrochem. Soc. 2015, 162, C403–C411. [Google Scholar] [CrossRef]
- Fukumura, K.; Shiraishi, T. Surface-Treating Agent for Magnesium-Based Part and Method of Surface Treatment. U.S.6569264B1, 27 May 2003. [Google Scholar]
- Fukumura, K.; Yu, Y.; Kajimoto, M.; Hama, H.; Hamauzu, T.; Yagi, H. Rust Preventive for Magnesium and/or Magnesium Alloy. U.S.20070080319A1, 12 April 2007. [Google Scholar]
- Supplit, R.; Koch, T.; Schubert, U. Evaluation of the anti-corrosive effect of acid pickling and sol–gel coating on magnesium AZ31 alloy. Corros. Sci. 2007, 49, 3015–3023. [Google Scholar] [CrossRef]
- Karavai, O.V.; Bastos, A.C.; Zheludkevich, M.L.; Taryba, M.G.; Lamaka, S.V.; Ferreira, M.G.S. Localized electrochemical study of corrosion inhibition in microdefects on coated AZ31 magnesium alloy. Electrochim. Acta 2010, 55, 5401–5406. [Google Scholar] [CrossRef]
- Semiletov, A.M. Metals Passivation by Aqueous Solutions of Organic Acids and Trialkoxysilanes. Ph.D. Thesis, Moscow State University, Moscow, Russia, 2016. [Google Scholar]
- Ivanou, D.K.; Yasakau, K.A.; Kallip, S.; Lisenkov, A.D.; Starykevich, M.; Lamaka, S.V.; Ferreira, M.G.S.; Zheludkevich, M.L. Active corrosion protection coating for a ZE41 magnesium alloy created by combining PEO and sol-gel techniques. Rsc Adv. 2016, 6, 12553–12560. [Google Scholar] [CrossRef]
- Soltan, A.; Dargusch, M.S.; Shi, Z.; Gerrard, D.; Atrens, A. Influence of commercial corrosion-inhibiting compounds on the atmospheric corrosion of the magnesium alloys EV31A, WE43B, ZE41A and pure magnesium. Mater. Corros. 2021, 72, 672–693. [Google Scholar] [CrossRef]
- Guo, X.; An, M.; Yang, P.; Li, H.; Su, C. Effects of benzotriazole on anodized film formed on AZ31B magnesium alloy in environmental-friendly electrolyte. J. Alloys Compd. 2009, 482, 487–497. [Google Scholar] [CrossRef]
- Ostanina, T.N.; Rudoi, V.M.; Ovsyannikova, A.N.; Malkov, V.B. Magnesium alloys spontaneous dissolution features under external anodic polarization in presence of inhibitors. Russ. J. Electrochem. 2010, 46, 707–713. [Google Scholar] [CrossRef]
- Huang, D.; Hu, J.; Song, G.-L.; Guo, X. Inhibition effect of inorganic and organic inhibitors on the corrosion of Mg–10Gd–3Y–0.5Zr alloy in an ethylene glycol solution at ambient and elevated temperatures. Electrochim. Acta 2011, 56, 10166–10178. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Shabani, B.; Aligholipour, B.; Seifzadeh, D. The effect of some Schiff bases on the corrosion of aluminum in hydrochloric acid solution. Appl. Surf. Sci. 2006, 252, 4039–4047. [Google Scholar] [CrossRef]
- Gomma, G.K.; Wahdan, M.H. Schiff bases as corrosion inhibitors for aluminium in hydrochloric acid solution. Mater. Chem. Phys. 1995, 39, 209–213. [Google Scholar] [CrossRef]
- Yurt, A.; Ulutas, S.; Dal, H. Electrochemical and theoretical investigation on the corrosion of aluminium in acidic solution containing some Schiff bases. Appl. Surf. Sci. 2006, 253, 919–925. [Google Scholar] [CrossRef]
- Yurt, A.; Balaban, A.; Kandemir, S.U.; Bereket, G.; Erk, B. Investigation on some Schiff bases as HCl corrosion inhibitors for carbon steel. Mater. Chem. Phys. 2004, 85, 420–426. [Google Scholar] [CrossRef]
- Hosseini, M.; Mertens, S.F.L.; Ghorbani, M.; Arshadi, M.R. Asymmetrical Schiff bases as inhibitors of mild steel corrosion in sulphuric acid media. Mater. Chem. Phys. 2003, 78, 800–808. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Shaabani, B.; Seifzadeh, D. Corrosion inhibition of mild steel by some schiff base compounds in hydrochloric acid. Appl. Surf. Sci. 2005, 239, 154–164. [Google Scholar] [CrossRef]
- Behpour, M.; Ghoreishi, S.M.; Salavati-Niasari, M.; Ebrahimi, B. Evaluating two new synthesized S-N Schiff bases on the corrosion of copper in 15% hydrochloric acid. Mater. Chem. Phys. 2008, 107, 153–157. [Google Scholar] [CrossRef]
- Ma, H.; Chen, S.; Niu, L.; Zhao, S.; Li, S.; Li, D. Inhibition of copper corrosion by several Schiff bases in aerated halide solutions. J. Appl. Electrochem. 2002, 32, 65–72. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M. Recent progresses in Schiff bases as aqueous phase corrosion inhibitors: Design and applications. Coord. Chem. Rev. 2021, 446, 214105. [Google Scholar] [CrossRef]
- Seifzadeh, D.; Bezaatpour, A.; Joghani, R.A. Corrosion inhibition effect of N, N′-bis (2-pyridylmethylidene)-1,2-diiminoethane on AZ91D magnesium alloy in acidic media. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2014, 24, 3441–3451. [Google Scholar] [CrossRef]
- Thirugnanaselvi, S.; Kuttirani, S.; Emelda, A.R. Effect of Schiff base as corrosion inhibitor on AZ31 magnesium alloy in hydrochloric acid solution. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2014, 24, 1969–1977. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, S.; Feng, W.; Li, Y.; Cheng, Y. Eelectrochemical study of inhibition effect of a schiff base towards magnesium alloy corrosion. Int. J. Electrochem. Sci. 2016, 11, 6043–6051. [Google Scholar] [CrossRef]
- Ma, L.; Li, W.; Zhu, S.; Wang, L.; Guan, S. Corrosion inhibition of Schiff bases for Mg-Zn-Y-Nd alloy in normal saline: Experimental and theoretical investigations. Corros. Sci. 2021, 184, 109268. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Zheludkevich, M.L.; Yasakau, K.A.; Montemor, M.F.; Ferreira, M.G.S. High effective organic corrosion inhibitors for 2024 aluminium alloy. Electrochim. Acta 2007, 52, 7231–7247. [Google Scholar] [CrossRef]
- Gao, H.; Li, Q.; Dai, Y.; Luo, F.; Zhang, H.X. High efficiency corrosion inhibitor 8-hydroxyquinoline and its synergistic effect with sodium dodecylbenzenesulphonate on AZ91D magnesium alloy. Corros. Sci. 2010, 52, 1603–1609. [Google Scholar] [CrossRef]
- Zong, Q.; Wang, L.; Sun, W.; Liu, G. Active deposition of bis (8-hydroxyquinoline) magnesium coating for enhanced corrosion resistance of AZ91D alloy. Corros. Sci. 2014, 89, 127–136. [Google Scholar] [CrossRef]
- Argade, G.R.; Sanders, S.; Mohandass, G.; Alsaleh, A.; D’Souza, F.; Golden, T.D.; Mishra, R.S. Corrosion Inhibition Study of Mg-Nd-Y High Strength Magnesium Alloy Using Organic Inhibitor. J. Mater. Eng. Perform. 2019, 28, 852–862. [Google Scholar] [CrossRef]
- Seifzadeh, D.; Hamzedoust-Hasankiadeh, S.; Shamkhali, A. Electrochemical and DFT studies of 8-hydroxyquinoline as corrosion inhibitor for AZ61 magnesium alloy in acidic media. Prot. Met. Phys. Chem. Surf. 2013, 49, 229–239. [Google Scholar] [CrossRef]
- Slavcheva, E.; Schmitt, G. Screening of new corrosion inhibitors via electrochemical noise analysis. Mater. Corros. 2002, 53, 647–655. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Knörnschild, G.; Snihirova, D.V.; Taryba, M.G.; Zheludkevich, M.L.; Ferreira, M.G.S. Complex anticorrosion coating for ZK30 magnesium alloy. Electrochim. Acta 2009, 55, 131–141. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Kim, M.J.; Kim, Y.G.; Ko, Y.G. Fabrication of graphene oxide/8-hydroxyquinolin/inorganic coating on the magnesium surface for extraordinary corrosion protection. Prog. Org. Coat. 2019, 137, 105314. [Google Scholar] [CrossRef]
- Vaghefinazari, B.; Lamaka, S.V.; Blawert, C.; Serdechnova, M.; Scharnagl, N.; Karlova, P.; Wieland, D.C.F.; Zheludkevich, M.L. Exploring the corrosion inhibition mechanism of 8-hydroxyquinoline for a PEO-coated magnesium alloy. Corros. Sci. 2022, 203, 110344. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A.; Ebenso, E.E. Quinoline and its derivatives as corrosion inhibitors: A review. Surf. Interfaces 2020, 21, 100634. [Google Scholar] [CrossRef]
- Verma, C.; Rhee, K.Y.; Quraishi, M.A.; Ebenso, E.E. Pyridine based N-heterocyclic compounds as aqueous phase corrosion inhibitors: A review. J. Taiwan Inst. Chem. Eng. 2020, 117, 265–277. [Google Scholar] [CrossRef]
- Lavanya, K.; Saranya, J.; Chitra, S. Recent reviews on quinoline derivatives as corrosion inhibitors. Corros. Rev. 2018, 36, 365–371. [Google Scholar] [CrossRef]
- Menger, F.M.; Keiper, J.S. Gemini surfactants. Angew. Chem. Int. Ed. 2000, 39, 1906–1920. [Google Scholar] [CrossRef]
- Rocca, E.; Steinmetz, J. Inhibition of lead corrosion with saturated linear aliphatic chain monocarboxylates of sodium. Corros. Sci. 2001, 43, 891–902. [Google Scholar] [CrossRef]
- McCafferty, E. Introduction to Corrosion Science; Springer: New York, NY, USA, 2010. [Google Scholar]
- Frignani, A.; Grassi, V.; Zucchi, F.; Zanotto, F. Mono-carboxylate conversion coatings for AZ31 Mg alloy protection. Mater. Corros. 2011, 62, 995–1002. [Google Scholar] [CrossRef]
- Daloz, D.; Rapin, C.; Steinmetz, P.; Michot, G. Corrosion Inhibition of Rapidly Solidified Mg-3% Zn-15% Al Magnesium Alloy with Sodium Carboxylates. Corrosion 1998, 54, 444–450. [Google Scholar] [CrossRef]
- Zucchi, F.; Grassi, V.; Zanotto, F. Sodium monocarboxylates as inhibitors of AZ31 alloy corrosion in a synthetic cooling water. Mater. Corros. 2009, 60, 199–205. [Google Scholar] [CrossRef]
- Dinodi, N.; Shetty, A.N. Alkyl carboxylates as efficient and green inhibitors of magnesium alloy ZE41 corrosion in aqueous salt solution. Corros. Sci. 2014, 85, 411–427. [Google Scholar] [CrossRef]
- Szillies, S.; Thissen, P.; Tabatabai, D.; Feil, F.; Fürbeth, W.; Fink, N.; Grundmeier, G. Formation and stability of organic acid monolayers on magnesium alloy AZ31: The role of alkyl chain length and head group chemistry. Appl. Surf. Sci. 2013, 283, 339–347. [Google Scholar] [CrossRef]
- Mesbah, A.; Juers, C.; Lacouture, F.; Mathieu, S.; Rocca, E.; François, M.; Steinmetz, J. Inhibitors for magnesium corrosion: Metal organic frameworks. Solid State Sci. 2007, 9, 322–328. [Google Scholar] [CrossRef]
- Kuznetsov, Y.I. Organic corrosion inhibitors: Where are we now? A review. Part II. Passivation and the role of chemical structure of carboxylates. Int. J. Corros. Scale Inhib. 2016, 5, 282–375. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Ju, P.; Chen, Y.; Yang, J.; Qian, K.; Zhang, T.; Wang, F. Unveiling the inhibition mechanism of an effective inhibitor for AZ91 Mg alloy. Corros. Sci. 2019, 148, 264–271. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Arrabal, R.; Viejo, F.; Matykina, E. Corrosion behaviour of magnesium/aluminium alloys in 3.5wt.% NaCl. Corros. Sci. 2008, 50, 823–834. [Google Scholar] [CrossRef]
- Bland, L.G.; Scully, L.C.; Scully, J.R. Assessing the corrosion of multi-phase Mg-Al alloys with high al content by electrochemical impedance, mass loss, hydrogen collection, and inductively coupled plasma optical emission spectrometry solution analysis. Corrosion 2017, 73, 526–543. [Google Scholar] [CrossRef] [PubMed]
- Frignani, A.; Grassi, V.; Zanotto, F.; Zucchi, F. Inhibition of AZ31 Mg alloy corrosion by anionic surfactants. Corros. Sci. 2012, 63, 29–39. [Google Scholar] [CrossRef]
- Yang, X.; Pan, F.; Zhang, D. A study on corrosion inhibitor for magnesium alloy. In Materials Science Forum; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2009; Volume 610–613, pp. 920–926. [Google Scholar]
- Li, Y.; Lu, X.; Wu, K.; Yang, L.; Zhang, T.; Wang, F. Exploration the inhibition mechanism of sodium dodecyl sulfate on Mg alloy. Corros. Sci. 2020, 168, 108559. [Google Scholar] [CrossRef]
- Li, Y.; Lu, X.; Mei, D.; Zhang, T.; Wang, F. Passivation of corrosion product layer on AM50 Mg by corrosion inhibitor. J. Magnes. Alloy. 2022, 10, 2563–2573. [Google Scholar] [CrossRef]
- Song, H.; Xu, Z.; Benabou, L.; Yin, Z.; Guan, H.; Yan, H.; Chao, L.; Hu, Z.; Wang, X. Sodium dodecyl sulfate (SDS) as an effective corrosion inhibitor for Mg-8Li-3Al alloy in aqueous NaCl: A combined experimental and theoretical investigation. J. Magnes. Alloy. 2021. [Google Scholar] [CrossRef]
- Abd El Rehim, S.S.; Amin, M.A.; Moussa, S.O.; Ellithy, A.S. The corrosion inhibition of aluminum and its copper alloys in 1.0 M H2SO4 solution using linear-sodium dodecyl benzene sulfonate as inhibitor. Mater. Chem. Phys. 2008, 112, 898–906. [Google Scholar] [CrossRef]
- Kellou-Kerkouche, F.; Benchettara, A.; Amara, S. Effect of sodium dodecyl benzene sulfonate on the corrosion inhibition of Fe-1Ti-20C alloy in 0.5M H2SO4. Mater. Chem. Phys. 2008, 110, 26–33. [Google Scholar] [CrossRef]
- Li, L.; Pan, F.; Lei, J. Environmental Friendly Corrosion Inhibitors for Mg Alloys. In Magnesium Alloys-Corrosion and Surface Treatments; Czerwinski, F., Ed.; InTech: London, UK, 2011. [Google Scholar]
- Lin, J.; Battocchi, D.; Bierwagen, G.P. Inhibitors for prolonging corrosion protection of Mg-rich primer on Al alloy 2024-T3. J. Coat. Technol. Res. 2017, 14, 497–504. [Google Scholar] [CrossRef]
- Chen, J.; He, J.; Li, L. Spectroscopic insight into the role of SDBS on the interface evolution of Mg in NaCl corrosive medium. Corros. Sci. 2021, 182, 109215. [Google Scholar] [CrossRef]
- Tezak, D.; Strajnar, F.; Sarcevic, D.; Milat, O.; Stubicar, M. Solid/liquid equilibria in aqueous system of dodecyl benzene sulphonate and alkaline earch ions. Croat. Chem. Acta 1984, 57, 93–107. [Google Scholar]
- Soltan, A.; Dargusch, M.S.; Shi, Z.; Jones, F.; Wood, B.; Gerrard, D.; Atrens, A. Effect of corrosion inhibiting compounds on the corrosion behaviour of pure magnesium and the magnesium alloys EV31A, WE43B and ZE41A. J. Magnes. Alloy. 2021, 9, 432–455. [Google Scholar] [CrossRef]
- Sun, J.; Howlett, P.C.; MacFarlane, D.R.; Lin, J.; Forsyth, M. Synthesis and physical property characterisation of phosphonium ionic liquids based on P(O)2(OR)2− and P(O)2(R)2− anions with potential application for corrosion mitigation of magnesium alloys. Electrochim. Acta 2008, 54, 254–260. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Latham, J.-A.; MacFarlane, D.R.; Howlett, P.C.; Forsyth, M. A review of ionic liquid surface film formation on Mg and its alloys for improved corrosion performance. Electrochim. Acta 2013, 110, 501–510. [Google Scholar] [CrossRef]
- Galiński, M.; Lewandowski, A.; Stepniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Gao, X.; Ma, D.; Huang, Q.; Ren, T.; Li, G.; Guo, L. Pyrazole ionic liquid corrosion inhibitor for magnesium alloy: Synthesis, performances and theoretical explore. J. Mol. Liq. 2022, 353, 118769. [Google Scholar] [CrossRef]
- Gao, X.; Huang, Q.; Ma, D.; Jiang, Y.; Ren, T.; Guo, X.; Zhang, J.; Guo, L. Improving environmental adaptability and long-term corrosion resistance of Mg alloys by pyrazole ionic liquids: Experimental and theoretical studies. J. Mol. Liq. 2021, 333, 115964. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Y.; Gao, S.; Guo, X.; Zhang, J. Experimental and theoretical studies on corrosion inhibition behavior of three imidazolium-based ionic liquids for magnesium alloys in sodium chloride solution. J. Mol. Liq. 2021, 345, 116998. [Google Scholar] [CrossRef]
- Gao, X.; Wu, Y.; Huang, Q.; Jiang, Y.; Ma, D.; Ren, T. The inhibition behavior of novel ionic liquids for magnesium alloy in NaCl solution: Experimental and theoretical investigation. J. Mol. Liq. 2021, 324, 114732. [Google Scholar] [CrossRef]
- Su, H.; Wu, Y.; Zhang, Y.; Jiang, Y.; Ding, Y.; Wang, L.; Zhang, J. Enhancing the long-term anti-corrosion property of Mg alloy by quaternary phosphonium salt: Integrated experimental and theoretical approaches. Corros. Sci. 2021, 178, 109010. [Google Scholar] [CrossRef]
- Zhang, Y.; Yaxu, W.; Jiang, Y.; Wang, L.; Zhang, J. Adsorbed film and synergistic effect of Benzyltriphenylphosphonium chloride and l-Histidine for magnesium alloys corrosion in NaCl. J. Alloys Compd. 2020, 849, 156230. [Google Scholar] [CrossRef]
- Su, H.; Wang, L.; Wu, Y.; Zhang, Y.; Zhang, J. Insight into inhibition behavior of novel ionic liquids for magnesium alloy in NaCl solution: Experimental and theoretical investigation. Corros. Sci. 2020, 165, 108410. [Google Scholar] [CrossRef]
- Su, H.; Liu, Y.; Gao, X.; Qian, Y.; Li, W.; Ren, T.; Wang, L.; Zhang, J. Corrosion inhibition of magnesium alloy in NaCl solution by ionic liquid: Synthesis, electrochemical and theoretical studies. J. Alloys Compd. 2019, 791, 681–689. [Google Scholar] [CrossRef]
- Kurchavov, D.; Haddad, M.; Lair, V.; Volovitch, P. Mg-alloys in water–hydrophilic ionic liquid mixtures: Is there a negative difference effect? Corros. Sci. 2022, 200, 110178. [Google Scholar] [CrossRef]
- McNulty, R.E.; Hanawalt, J.D. Some Corrosion Characteristics of High Purity Magnesium Alloys. Trans. Electrochem. Soc. 1942, 81, 423–433. [Google Scholar] [CrossRef]
- Cain, T.; Madden, S.B.; Birbilis, N.; Scully, J.R. Evidence of the enrichment of transition metal elements on corroding magnesium surfaces using rutherford backscattering spectrometry. J. Electrochem. Soc. 2015, 162, C228–C237. [Google Scholar] [CrossRef]
- Lysne, D.; Thomas, S.; Hurley, M.F.; Birbilis, N. On the Fe enrichment During anodic polarization of Mg and its impact on hydrogen evolution. J. Electrochem. Soc. 2015, 162, C396–C402. [Google Scholar] [CrossRef]
- Höche, D.; Blawert, C.; Lamaka, S.V.; Scharnagl, N.; Mendis, C.; Zheludkevich, M.L. The effect of iron re-deposition on the corrosion of impurity-containing magnesium. Phys. Chem. Chem. Phys. 2015, 18, 1279–1291. [Google Scholar] [CrossRef]
- Michailidou, E.; McMurray, H.N.; Williams, G. Quantifying the Role of Transition Metal Electrodeposition in the Cathodic Activation of Corroding Magnesium. J. Electrochem. Soc. 2018, 165, C195–C205. [Google Scholar] [CrossRef]
- Mercier, D.; Światowska, J.; Zanna, S.; Seyeux, A.; Marcus, P. Role of Segregated Iron at Grain Boundaries on Mg Corrosion. J. Electrochem. Soc. 2018, 165, C42–C49. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Höche, D.; Petrauskas, R.P.; Blawert, C.; Zheludkevich, M.L. A new concept for corrosion inhibition of magnesium: Suppression of iron re-deposition. Electrochem. Commun. 2016, 62, 5–8. [Google Scholar] [CrossRef]
- Vaghefinazari, B.; Snihirova, D.; Wang, C.; Wang, L.; Deng, M.; Höche, D.; Lamaka, S.V.; Zheludkevich, M.L. Exploring the effect of sodium salt of Ethylenediaminetetraacetic acid as an electrolyte additive on electrochemical behavior of a commercially pure Mg in primary Mg-air batteries. J. Power Sources 2022, 527, 231176. [Google Scholar] [CrossRef]
- Martell, A.E.; Smith, R.M. Critical Stability Constants: Second Supplement, V. 6; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1989; Volume 6. [Google Scholar]
- Martell, A.E.; Smith, R.M. Critical Stability Constants, Other Organic Ligands, V. 3; Springer: New York, NY, USA, 1977; Volume 3. [Google Scholar]
- Martell, A.E.; Smith, R.M. Critical Stability Constants: First Supplement, V. 5; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1982; Volume 5. [Google Scholar]
- Lamaka, S.; Höche, D.; Blawert, C.; Zheludkevich, M. Corrosion Inhibitor Composition for Magnesium or Magnesium Alloys. WO/2017/064185, 20 April 2017. [Google Scholar]
- Lamaka, S.V.; Höche, D.; Blawert, C.; Zheludkevich, M.L. Corrosion Inhibitor Composition for Magnesium or Magnesium Alloys. EP15189674.3, 14 October 2015. [Google Scholar]
- Dean, J.A. Lange’s Chemistry Handbook, 15th ed.; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- Maltseva, A.; Lamaka, S.V.; Yasakau, K.A.; Mei, D.; Kurchavov, D.; Zheludkevich, M.L.; Lefèvre, G.; Volovitch, P. In situ surface film evolution during Mg aqueous corrosion in presence of selected carboxylates. Corros. Sci. 2020, 171, 108484. [Google Scholar] [CrossRef]
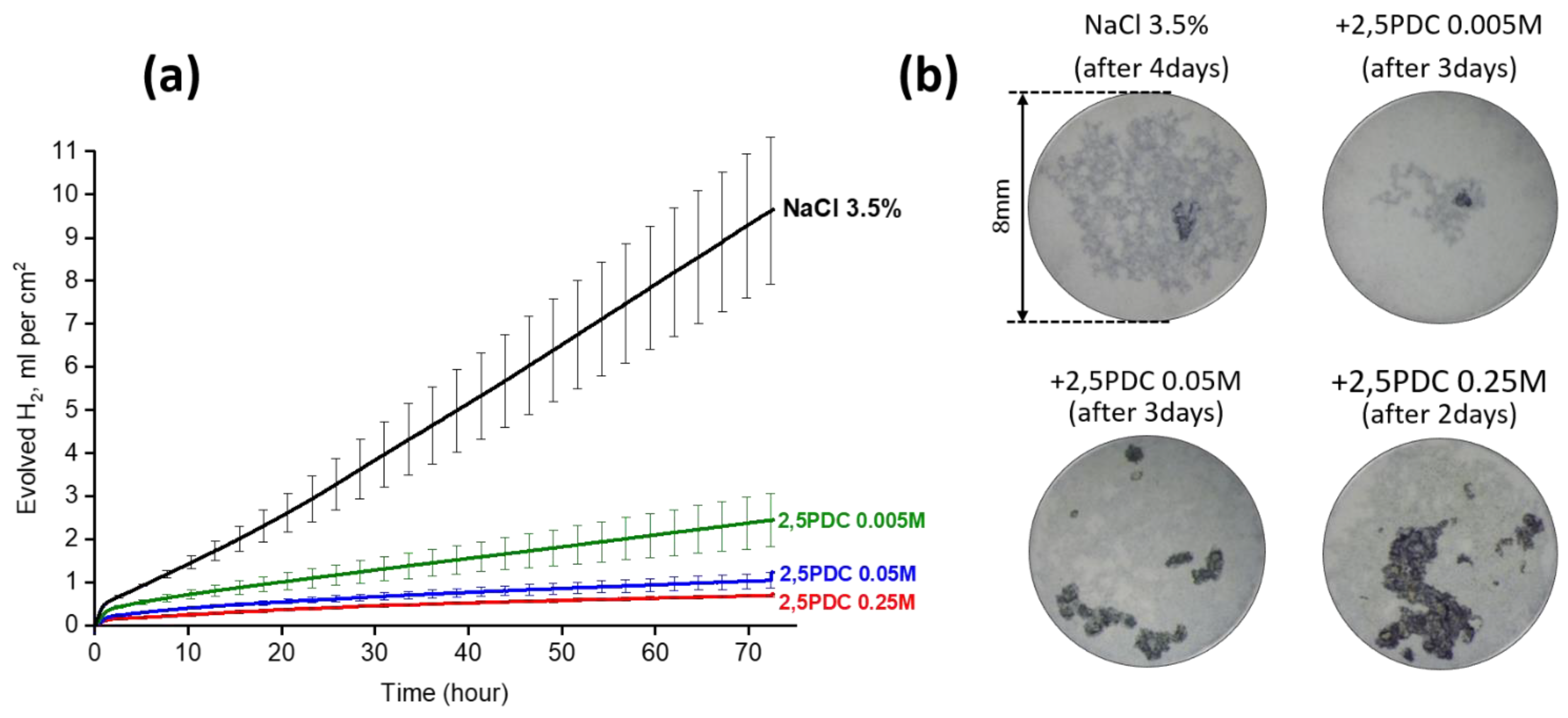
- Vaghefinazari, B.; Wang, C.; Mercier, D.; Mei, D.; Seyeux, A.; Marcus, P.; Blawert, C.; Lamaka, S.V.; Zheludkevich, M.L. Adverse effect of 2,5PDC corrosion inhibitor on PEO coated magnesium. Corros. Sci. 2021, 192, 109830. [Google Scholar] [CrossRef]
- Fockaert, L.I.; Würger, T.; Unbehau, R.; Boelen, B.; Meißner, R.H.; Lamaka, S.V.; Zheludkevich, M.L.; Terryn, H.; Mol, J.M.C. ATR-FTIR in Kretschmann configuration integrated with electrochemical cell as in situ interfacial sensitive tool to study corrosion inhibitors for magnesium substrates. Electrochim. Acta 2020, 345, 136166. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, P.; Qiu, Y.; Jao, C.-Y.; Blawert, C.; Lamaka, S.; Bouali, A.; Lu, X.; Zheludkevich, M.L.; Li, W. Experimental and quantum chemical studies of carboxylates as corrosion inhibitors for AM50 alloy in pH neutral NaCl solution. J. Magnes. Alloy. 2021, 10, 555–568. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Maltseva, A.; Lamaka, S.V.; Mei, D.; Orvi, H.; Volovitch, P.; Ferreira, M.G.S.; Zheludkevich, M.L. The effect of carboxylate compounds on Volta potential and corrosion inhibition of Mg containing different levels of iron. Corros. Sci. 2022, 194, 109937. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, T.; Wang, F. New understanding on the mechanism of organic inhibitors for magnesium alloy. Corros. Sci. 2022, 192, 110118. [Google Scholar] [CrossRef]
- Winkler, D.A.; Breedon, M.; White, P.; Hughes, A.; Sapper, E.; Cole, I. Using high throughput experimental data and in silico models to discover alternatives to toxic chromate corrosion inhibitors. Corros. Sci. 2016, 106, 229–235. [Google Scholar] [CrossRef]
- Winkler, D.A.; Breedon, M.; Hughes, A.E.; Burden, F.R.; Barnard, A.S.; Harvey, T.G.; Cole, I. Towards chromate-free corrosion inhibitors: Structure–property models for organic alternatives. Green Chem. 2014, 16, 3349–3357. [Google Scholar] [CrossRef]
- Galvão, T.L.; Novell-Leruth, G.; Kuznetsova, A.; Tedim, J.o.; Gomes, J.R. Elucidating structure–property relationships in aluminum alloy corrosion inhibitors by machine learning. J. Phys. Chem. C 2020, 124, 5624–5635. [Google Scholar] [CrossRef]
- Fernandez, M.; Breedon, M.; Cole, I.S.; Barnard, A.S. Modeling corrosion inhibition efficacy of small organic molecules as non-toxic chromate alternatives using comparative molecular surface analysis (CoMSA). Chemosphere 2016, 160, 80–88. [Google Scholar] [CrossRef]
- Würger, T.; Mei, D.; Vaghefinazari, B.; Winkler, D.A.; Lamaka, S.V.; Zheludkevich, M.L.; Meißner, R.H.; Feiler, C. Exploring structure-property relationships in magnesium dissolution modulators. npj Mater. Degrad. 2021, 5, 2. [Google Scholar] [CrossRef]
- Schiessler, E.J.; Würger, T.; Lamaka, S.V.; Meißner, R.H.; Cyron, C.J.; Zheludkevich, M.L.; Feiler, C.; Aydin, R.C. Predicting the inhibition efficiencies of magnesium dissolution modulators using sparse machine learning models. npj Comput. Mater. 2021, 7, 1–9. [Google Scholar] [CrossRef]
- Feiler, C.; Mei, D.; Vaghefinazari, B.; Würger, T.; Meißner, R.H.; Luthringer-Feyerabend, B.J.C.; Winkler, D.A.; Zheludkevich, M.L.; Lamaka, S.V. In silico screening of modulators of magnesium dissolution. Corros. Sci. 2020, 163, 108245. [Google Scholar] [CrossRef]
- Würger, T.; Feiler, C.; Musil, F.; Feldbauer, G.B.V.; Höche, D.; Lamaka, S.V.; Zheludkevich, M.L.; Meißner, R.H. Data Science Based Mg Corrosion Engineering. Front. Mater. 2019, 6, 53. [Google Scholar] [CrossRef]
- Zeller-Plumhoff, B.; Gile, M.; Priebe, M.; Slominska, H.; Boll, B.; Wiese, B.; Würger, T.; Willumeit-Römer, R.; Meißner, R.H. Exploring key ionic interactions for magnesium degradation in simulated body fluid—A data-driven approach. Corros. Sci. 2021, 182, 109272. [Google Scholar] [CrossRef]
- Feiler, C.; Mei, D.; Luthringer-Feyerabend, B.; Lamaka, S.; Zheludkevich, M. Rational design of effective Mg degradation modulators. Corrosion 2021, 77, 204–208. [Google Scholar] [CrossRef]
- Galvão, T.L.P.; Ferreira, I.; Kuznetsova, A.; Novell-Leruth, G.; Song, C.; Feiler, C.; Lamaka, S.V.; Rocha, C.; Maia, F.; Zheludkevich, M.L.; et al. CORDATA: An open data management web application to select corrosion inhibitors. NPJ Mater. Degrad. 2022, 6, 48. [Google Scholar] [CrossRef]
- Muster, T.; Sullivan, H.; Lau, D.; Alexander, D.; Sherman, N.; Garcia, S.; Harvey, T.; Markley, T.; Hughes, A.; Corrigan, P. A combinatorial matrix of rare earth chloride mixtures as corrosion inhibitors of AA2024-T3: Optimisation using potentiodynamic polarisation and EIS. Electrochim. Acta 2012, 67, 95–103. [Google Scholar] [CrossRef]
- Hu, J.; Huang, D.; Song, G.L.; Guo, X. The synergistic inhibition effect of organic silicate and inorganic Zn salt on corrosion of Mg-10Gd-3Y magnesium alloy. Corros. Sci. 2011, 53, 4093–4101. [Google Scholar] [CrossRef]
- Tawfik, S.M. Alginate surfactant derivatives as an ecofriendly corrosion inhibitor for carbon steel in acidic environments. RSC Adv. 2015, 5, 104535–104550. [Google Scholar] [CrossRef]
- Sangeetha, Y.; Meenakshi, S.; Sundaram, C.S. Investigation of corrosion inhibitory effect of hydroxyl propyl alginate on mild steel in acidic media. J. Appl. Polym. Sci. 2016, 133, 43004. [Google Scholar] [CrossRef]
- Obot, I.B.; Onyeachu, I.B.; Kumar, A.M. Sodium alginate: A promising biopolymer for corrosion protection of API X60 high strength carbon steel in saline medium. Carbohydr. Polym. 2017, 178, 200–208. [Google Scholar] [CrossRef]
- Hassan, R.; Zaafarany, I.; Gobouri, A.; Takagi, H. A revisit to the corrosion inhibition of aluminum in aqueous alkaline solutions by water-soluble alginates and pectates as anionic polyelectrolyte inhibitors. Int. J. Corros. 2013, 2013, 508596. [Google Scholar] [CrossRef]
- Zaafarany, I. Corrosion inhibition of aluminum in aqueous alkaline solutions by alginate and pectate water-soluble natural polymer anionic polyelectrolytes. Port. Electrochim. Acta 2012, 30, 419–426. [Google Scholar] [CrossRef]
- Umoren, S.A.; Eduok, U.M. Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: A review. Carbohydr. Polym. 2016, 140, 314–341. [Google Scholar] [CrossRef] [PubMed]
- Dang, N.; Wei, Y.H.; Hou, L.F.; Li, Y.G.; Guo, C.L. Investigation of the inhibition effect of the environmentally friendly inhibitor sodium alginate on magnesium alloy in sodium chloride solution. Mater. Corros. 2015, 66, 1354–1362. [Google Scholar] [CrossRef]
- Hou, L.; Dang, N.; Yang, H.; Liu, B.; Li, Y.; Wei, Y.; Chen, X.B. A combined inhibiting effect of sodium alginate and sodium phosphate on the corrosion of magnesium alloy AZ31 in NaCl solution. J. Electrochem. Soc. 2016, 163, C486–C494. [Google Scholar] [CrossRef]
- Li, Y.; Ba, Z.X.; Li, Y.L.; Ge, Y.; Zhu, X.C. Influence of sodium alginate inhibitor addition on the corrosion protection performance of AZ91D magnesium alloy in NaCl solution. Anti-Corros. Methods Mater. 2017, 64, 486–491. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, Z.; Feng, Y.; Li, Q. Synergistic corrosion inhibition of sodium phosphate and sodium dodecyl sulphate on magnesium alloy AZ91 in 3.5 wt% NaCl solution. Mater. Today Commun. 2022, 31, 103568. [Google Scholar] [CrossRef]
- Liu, D.; Han, E.H.; Song, Y.; Shan, D. Enhancing the self-healing property by adding the synergetic corrosion inhibitors of Na3PO4 and 2-mercaptobenzothiazole into the coating of Mg alloy. Electrochim. Acta 2019, 323, 134796. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Lourenço, M.M.; Ivanov, D.K.; Zheludkevich, M.L.; Ferreira, M.G.S.; Hack, T. Fault-tolerant composite protective coating for WE43 magnesium alloy. In Proceedings of the IMA 2014 World Annual Magnesium Conference, München, Germany, 1–3 June 2014; pp. 116–122. [Google Scholar]
- Shchukin, D.G. Container-based multi-functional self-healing polymer coatings. Polym. Chem. 2013, 4, 4871–4877. [Google Scholar]
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol-gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Zhong, X.; Li, Q.; Hu, J.; Zhang, S.; Chen, B.; Xu, S.; Luo, F. A novel approach to heal the sol-gel coating system on magnesium alloy for corrosion protection. Electrochim. Acta 2010, 55, 2424–2429. [Google Scholar] [CrossRef]
- Galio, A.F.; Lamaka, S.V.; Zheludkevich, M.L.; Dick, L.F.P.; Müller, I.L.; Ferreira, M.G.S. Inhibitor-doped sol–gel coatings for corrosion protection of magnesium alloy AZ31. Surf. Coat. Technol. 2010, 204, 1479–1486. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Mahdavian, M.; Naderi, R. Superior corrosion protection and adhesion strength of epoxy coating applied on AZ31 magnesium alloy pre-treated by PEO/Silane with inorganic and organic corrosion inhibitors. Corros. Sci. 2021, 178, 109065. [Google Scholar] [CrossRef]
- Montemor, M.F.; Ferreira, M.G.S. Electrochemical study of modified bis-[triethoxysilylpropyl] tetrasulfide silane films applied on the AZ31 Mg alloy. Electrochim. Acta 2007, 52, 7486–7495. [Google Scholar] [CrossRef]
- Zanotto, F.; Grassi, V.; Frignani, A.; Zucchi, F. Protection of the AZ31 magnesium alloy with cerium modified silane coatings. Mater. Chem. Phys. 2011, 129, 1–8. [Google Scholar] [CrossRef]
- Hernández-Barrios, C.A.; Saavedra, J.A.; Higuera, S.L.; Coy, A.E.; Viejo, F. Effect of cerium on the physicochemical and anticorrosive features of TEOS-GPTMS sol-gel coatings deposited on the AZ31 magnesium alloy. Surf. Interfaces 2020, 21, 100671. [Google Scholar] [CrossRef]
- Van Soestbergen, M.; Baukh, V.; Erich, S.; Huinink, H.; Adan, O. Release of cerium dibutylphosphate corrosion inhibitors from highly filled epoxy coating systems. Prog. Org. Coat. 2014, 77, 1562–1568. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R.G.; Ho, D.L.; Forsyth, M. Inhibition of AA2024-T3 on a phase-by-phase basis using an environmentally benign inhibitor, cerium dibutyl phosphate. Electrochem. Solid State Lett. 2005, 8, C180–C183. [Google Scholar] [CrossRef]
- Calado, L.M.; Taryba, M.G.; Morozov, Y.; Carmezim, M.J.; Montemor, M.F. Novel smart and self-healing cerium phosphate-based corrosion inhibitor for AZ31 magnesium alloy. Corros. Sci. 2020, 170, 108648. [Google Scholar] [CrossRef]
- Calado, L.M.; Taryba, M.G.; Morozov, Y.; Carmezim, M.J.; Montemor, M. Cerium phosphate-based inhibitor for smart corrosion protection of WE43 magnesium alloy. Electrochim. Acta 2021, 365, 137368. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Moradi-Alavian, S.; Esrafili, M.D.; Kazempour, A. Hybrid sol-gel coatings based on silanes-amino acids for corrosion protection of AZ91 magnesium alloy: Electrochemical and DFT insights. Prog. Org. Coat. 2019, 131, 191–202. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Zheludkevich, M.; Yasakau, K.; Lamaka, S.; Ferreira, M.G.S.; Möhwald, H. Layer-by-layer assembled nanocontainers for self-healing corrosion protection. Adv. Mater. 2006, 18, 1672–1678. [Google Scholar] [CrossRef]
- Garcia-Heras, M.; Jimenez-Morales, A.; Casal, B.; Galvan, J.C.; Radzki, S.; Villegas, M.A. Preparation and electrochemical study of cerium–silica sol–gel thin films. J. Alloys Compd. 2004, 380, 219–224. [Google Scholar] [CrossRef]
- Zhong, X.; Li, Q.; Hu, J.; Yang, X.; Luo, F.; Dai, Y. Effect of cerium concentration on microstructure, morphology and corrosion resistance of cerium-silica hybrid coatings on magnesium alloy AZ91D. Prog. Org. Coat. 2010, 69, 52–56. [Google Scholar] [CrossRef]
- Adsul, S.H.; Siva, T.; Sathiyanarayanan, S.; Sonawane, S.H.; Subasri, R. Self-healing ability of nanoclay-based hybrid sol-gel coatings on magnesium alloy AZ91D. Surf. Coat. Technol. 2017, 309, 609–620. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Moradi-Alavian, S.; Kazempour, A. Salt-nanoparticle systems incorporated into sol-gel coatings for corrosion protection of AZ91 magnesium alloy. Prog. Org. Coat. 2019, 135, 475–482. [Google Scholar] [CrossRef]
- Adsul, S.H.; Bagale, U.D.; Sonawane, S.H.; Subasri, R. Release rate kinetics of corrosion inhibitor loaded halloysite nanotube-based anticorrosion coatings on magnesium alloy AZ91D. J. Magnes. Alloy. 2021, 9, 202–215. [Google Scholar] [CrossRef]
- Ding, C.; Xu, J.; Tong, L.; Gong, G.; Jiang, W.; Fu, J. Design and Fabrication of a Novel Stimulus-Feedback Anticorrosion Coating Featured by Rapid Self-Healing Functionality for the Protection of Magnesium Alloy. ACS Appl. Mater. Interfaces 2017, 9, 21034–21047. [Google Scholar] [CrossRef] [PubMed]
- Samadianfard, R.; Seifzadeh, D.; Habibi-Yangjeh, A. Sol-gel coating filled with SDS-stabilized fullerene nanoparticles for active corrosion protection of the magnesium alloy. Surf. Coat. Technol. 2021, 419, 127292. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Kardar, P.; Arabi, A.M.; Amini, R.; Pasbakhsh, P. The active corrosion performance of silane coating treated by praseodymium encapsulated with halloysite nanotubes. Prog. Org. Coat. 2020, 138, 105404. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Lamaka, S.V.; Yasakau, K.A.; Zheludkevich, M.L.; Ferreira, M.G.S.; Möhwald, H. Active Anticorrosion Coatings with Halloysite Nanocontainers. J. Phys. Chem. C 2008, 112, 958–964. [Google Scholar] [CrossRef]
- Mei, D.; Lamaka, S.V.; Feiler, C.; Zheludkevich, M.L. The effect of small-molecule bio-relevant organic components at low concentration on the corrosion of commercially pure Mg and Mg-0.8Ca alloy: An overall perspective. Corros. Sci. 2019, 153, 258–271. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Liu, Z.; Peng, W.-X.; Yoysefi, N. Amino acid and TiO2 nanoparticles mixture inserted into sol-gel coatings: An efficient corrosion protection system for AZ91 magnesium alloy. Prog. Org. Coat. 2019, 136, 105296. [Google Scholar] [CrossRef]
- Upadhyay, V.; Bergseth, Z.; Kelly, B.; Battocchi, D. Silica-Based Sol-Gel Coating on Magnesium Alloy with Green Inhibitors. Coatings 2017, 7, 86. [Google Scholar] [CrossRef]
- Abdolah Zadeh, M.; van der Zwaag, S.; Garcia, S.J. Self-healing corrosion-protective sol-gel coatings based on extrinsic and intrinsic healing approaches. In Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2016; Volume 273, pp. 185–218. [Google Scholar]
- Bouali, A.C.; Serdechnova, M.; Blawert, C.; Tedim, J.; Ferreira, M.G.S.; Zheludkevich, M.L. Layered double hydroxides (LDHs) as functional materials for the corrosion protection of aluminum alloys: A review. Appl. Mater. Today 2020, 21, 100857. [Google Scholar] [CrossRef]
- Yun, S.K.; Pinnavaia, T.J. Water content and particle texture of synthetic hydrotalcite-like layered double hydroxides. Chem. Mater. 1995, 7, 348–354. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, F.; Fang, L.; Guan, T.; Hu, J.; Zhang, S. A comparative study and optimization of corrosion resistance of ZnAl layered double hydroxides films intercalated with different anions on AZ31 Mg alloys. Surf. Coat. Technol. 2019, 358, 594–603. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, F.; Lu, J.-C.; Zeng, R.-C.; Li, S.-Q.; Song, L.; Zeng, J.-M. A comparison of corrosion inhibition of magnesium aluminum and zinc aluminum vanadate intercalated layered double hydroxides on magnesium alloys. Front. Mater. Sci. 2018, 12, 198–206. [Google Scholar] [CrossRef]
- Wu, L.; Ding, X.; Zheng, Z.; Tang, A.; Zhang, G.; Atrens, A.; Pan, F. Doublely-doped Mg-Al-Ce-V2O74-LDH composite film on magnesium alloy AZ31 for anticorrosion. J. Mater. Sci. Technol. 2021, 64, 66–72. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, L.; Zheludkevich, M.L.; Chen, Y.; Serdechnova, M.; Yao, W.; Blawert, C.; Atrens, A.; Pan, F. MgAl-V2O7 4-LDHs/(PEI/MXene) 10 composite film for magnesium alloy corrosion protection. J. Mater. Sci. Technol. 2021, 91, 28–39. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Xie, Z.-H.; Yu, G. Duplex coating combining layered double hydroxide and 8-quinolinol layers on Mg alloy for corrosion protection. Electrochim. Acta 2018, 283, 1845–1857. [Google Scholar] [CrossRef]
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Asl, V.Z.; Zhao, J.; Anjum, M.J.; Wei, S.; Wang, W.; Zhao, Z. The effect of cerium cation on the microstructure and anti-corrosion performance of LDH conversion coatings on AZ31 magnesium alloy. J. Alloys Compd. 2020, 821, 153248. [Google Scholar]
- Song, Y.; Tang, Y.; Fang, L.; Wu, F.; Zeng, X.; Hu, J.; Zhang, S.F.; Jiang, B.; Luo, H. Enhancement of corrosion resistance of AZ31 Mg alloys by one-step in situ synthesis of ZnAl-LDH films intercalated with organic anions (ASP, La). J. Magnes. Alloy. 2021, 9, 658–667. [Google Scholar] [CrossRef]
- Chen, J.; Fang, L.; Wu, F.; Xie, J.; Hu, J.; Jiang, B.; Luo, H. Corrosion resistance of a self-healing rose-like MgAl-LDH coating intercalated with aspartic acid on AZ31 Mg alloy. Prog. Org. Coat. 2019, 136, 105234. [Google Scholar] [CrossRef]
- Anjum, M.J.; Zhao, J.; Asl, V.Z.; Yasin, G.; Wang, W.; Wei, S.; Zhao, Z.; Khan, W.Q. In-situ intercalation of 8-hydroxyquinoline in Mg-Al LDH coating to improve the corrosion resistance of AZ31. Corros. Sci. 2019, 157, 1–10. [Google Scholar] [CrossRef]
- Anjum, M.J.; Zhao, J.-M.; Asl, V.Z.; Malik, M.U.; Yasin, G.; Khan, W.Q. Green corrosion inhibitors intercalated Mg:Al layered double hydroxide coatings to protect Mg alloy. Rare Met. 2021, 40, 2254–2265. [Google Scholar] [CrossRef]
- Li, L.-X.; Xie, Z.-H.; Fernandez, C.; Wu, L.; Cheng, D.; Jiang, X.-H.; Zhong, C.-J. Development of a thiophene derivative modified LDH coating for Mg alloy corrosion protection. Electrochim. Acta 2020, 330, 135186. [Google Scholar] [CrossRef]
- Song, Y.; Wang, H.; Liu, Q.; Li, G.; Wang, S.; Zhu, X. Sodium dodecyl sulfate (SDS) intercalated MgAl layered double hydroxides film to enhance the corrosion resistance of AZ31 magnesium alloy. Surf. Coat. Technol. 2021, 422, 127524. [Google Scholar] [CrossRef]
- Yang, Q.; Tabish, M.; Wang, J.; Zhao, J. Enhanced Corrosion Resistance of Layered Double Hydroxide Films on Mg Alloy: The Key Role of Cationic Surfactant. Materials 2022, 15, 2028. [Google Scholar] [CrossRef]
- Huang, M.; Lu, G.; Pu, J.; Qiang, Y. Superhydrophobic and smart MgAl-LDH anti-corrosion coating on AZ31 Mg surface. J. Ind. Eng. Chem. 2021, 103, 154–164. [Google Scholar] [CrossRef]
- Zhang, R.F.; Zhang, S.F.; Yang, N.; Yao, L.J.; He, F.X.; Zhou, Y.P.; Xu, X.; Chang, L.; Bai, S.J. Influence of 8-hydroxyquinoline on properties of anodic coatings obtained by micro arc oxidation on AZ91 magnesium alloys. J. Alloys Compd. 2012, 539, 249–255. [Google Scholar] [CrossRef]
- Cao, F.h.; Cao, J.l.; Zhang, Z.; Zhang, J.q.; Cao, C.n. Plasma electrolytic oxidation of AZ91D magnesium alloy with different additives and its corrosion behavior. Mater. Corros. 2007, 58, 696–703. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Z.; Yang, F.; Zhang, Z. Environmental friendly anodizing of AZ91D magnesium alloy in alkaline borate-benzoate electrolyte. J. Alloys Compd. 2011, 509, 6440–6446. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloy. 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Golabadi, M.; Rouhaghdam, A.S. Effect of lanthanum nitrate on the microstructure and electrochemical behavior of PEO coatings on AZ31 Mg alloy. J. Alloys Compd. 2017, 719, 242–255. [Google Scholar] [CrossRef]
- Blawert, C.; Heitmann, V.; Dietzel, W.; Nykyforchyn, H.M.; Klapkiv, M.D. Influence of electrolyte on corrosion properties of plasma electrolytic conversion coated magnesium alloys. Surf. Coat. Technol. 2007, 201, 8709–8714. [Google Scholar] [CrossRef]
- Mingo, B.; Arrabal, R.; Mohedano, M.; Llamazares, Y.; Matykina, E.; Yerokhin, A.; Pardo, A. Influence of sealing post-treatments on the corrosion resistance of PEO coated AZ91 magnesium alloy. Appl. Surf. Sci. 2018, 433, 653–667. [Google Scholar] [CrossRef]
- Mohedano, M.; Blawert, C.; Zheludkevich, M.L. Cerium-based sealing of PEO coated AM50 magnesium alloy. Surf. Coat. Technol. 2015, 269, 145–154. [Google Scholar] [CrossRef]
- Van Phuong, N.; Fazal, B.R.; Moon, S. Cerium-and phosphate-based sealing treatments of PEO coated AZ31 Mg alloy. Surf. Coat. Technol. 2017, 309, 86–95. [Google Scholar] [CrossRef]
- Laleh, M.; Kargar, F.; Rouhaghdam, A.S. Investigation of rare earth sealing of porous micro-arc oxidation coating formed on AZ91D magnesium alloy. J. Rare Earths 2012, 30, 1293–1297. [Google Scholar] [CrossRef]
- Mohedano, M.; Pérez, P.; Matykina, E.; Pillado, B.; Garcés, G.; Arrabal, R. PEO coating with Ce-sealing for corrosion protection of LPSO Mg–Y–Zn alloy. Surf. Coat. Technol. 2020, 383, 125253. [Google Scholar] [CrossRef]
- Pezzato, L.; Babbolin, R.; Cerchier, P.; Marigo, M.; Dolcet, P.; Dabalà, M.; Brunelli, K. Sealing of PEO coated AZ91magnesium alloy using solutions containing neodymium. Corros. Sci. 2020, 173, 108741. [Google Scholar] [CrossRef]
- Pezzato, L.; Brunelli, K.; Babbolin, R.; Dolcet, P.; Dabalà, M. Sealing of PEO coated AZ91 magnesium alloy using La-based solutions. Int. J. Corros. 2017, 2017, 5305218. [Google Scholar] [CrossRef]
- Sun, M.; Matthews, A.; Yerokhin, A. Plasma electrolytic oxidation coatings on cp-Mg with cerium nitrate and benzotriazole immersion post-treatments. Surf. Coat. Technol. 2018, 344, 330–341. [Google Scholar] [CrossRef]
- Mohedano, M.; Blawert, C.; Zheludkevich, M. Silicate-based plasma electrolytic oxidation (PEO) coatings with incorporated CeO2 particles on AM50 magnesium alloy. Mater. Des. 2015, 86, 735–744. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Sabour Rouhaghdam, A. Microstructural, protective, inhibitory and semiconducting properties of PEO coatings containing CeO2 nanoparticles formed on AZ31 Mg alloy. Surf. Coat. Technol. 2018, 352, 561–580. [Google Scholar] [CrossRef]
- Malayoglu, U.; Tekin, K.C.; Shrestha, S. Influence of post-treatment on the corrosion resistance of PEO coated AM50B and AM60B Mg alloys. Surf. Coat. Technol. 2010, 205, 1793–1798. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Protective properties of inhibitor-containing composite coatings on a Mg alloy. Corros. Sci. 2016, 102, 348–354. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Localized corrosion of the Mg alloys with inhibitor-containing coatings: SVET and SIET studies. Corros. Sci. 2016, 102, 269–278. [Google Scholar] [CrossRef]
- Kaseem, M.; Ko, Y.G. Formation of flower-like structures for optimizing the corrosion resistance of Mg alloy. Mater. Lett. 2018, 221, 196–200. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Ma, Y.; Song, G.-L.; Zheng, D.; Jiang, B.; Atrens, A.; Pan, F. Active corrosion protection by a smart coating based on a MgAl-layered double hydroxide on a cerium-modified plasma electrolytic oxidation coating on Mg alloy AZ31. Corros. Sci. 2018, 139, 370–382. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Ko, Y.G. Self-assembly of hierarchical N-heterocycles-inorganic materials into three-dimensional structure for superior corrosion protection. Chem. Eng. J. 2019, 356, 850–856. [Google Scholar] [CrossRef]
- Liu, D.; Song, Y.; Shan, D.; Han, E.H. Self-Healing Coatings Prepared by Loading Interphase Inhibitors into MAO Coating of AM60 Mg Alloy. J. Electrochem. Soc. 2018, 165, C412–C421. [Google Scholar] [CrossRef]
- Lu, X.P.; Blawert, C.; Tolnai, D.; Subroto, T.; Kainer, K.U.; Zhang, T.; Wang, F.H.; Zheludkevich, M.L. 3D reconstruction of plasma electrolytic oxidation coatings on Mg alloy via synchrotron radiation tomography. Corros. Sci. 2018, 139, 395–402. [Google Scholar] [CrossRef]
- Curran, J.A.; Clyne, T.W. Porosity in plasma electrolytic oxide coatings. Acta Mater. 2006, 54, 1985–1993. [Google Scholar] [CrossRef]
- Dong, K.; Song, Y.; Shan, D.; Han, E.-H. Corrosion behavior of a self-sealing pore micro-arc oxidation film on AM60 magnesium alloy. Corros. Sci. 2015, 100, 275–283. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, E.; Wu, L.; Ma, W.; Yang, H.; Tang, A.; Pan, F. Corrosion protection properties of different inhibitors containing PEO/LDHs composite coating on magnesium alloy AZ31. Sci. Rep. 2021, 11, 2774. [Google Scholar] [CrossRef]
- Peng, F.; Wang, D.; Tian, Y.; Cao, H.; Qiao, Y.; Liu, X. Sealing the pores of PEO coating with Mg-Al layered double hydroxide: Enhanced corrosion resistance, cytocompatibility and drug delivery ability. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Blawert, C.; Lamaka, S.V.; Snihirova, D.; Lu, X.; Di, S.; Zheludkevich, M.L. Corrosion protection properties of inhibitor containing hybrid PEO-epoxy coating on magnesium. Corros. Sci. 2018, 140, 99–110. [Google Scholar] [CrossRef]
- Zoubi, W.A.; Min, J.H.; Ko, Y.G. Hybrid organic-inorganic coatings via electron transfer behaviour. Sci. Rep. 2017, 7, 7063. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, E.; Vaghefinazari, B.; Lamaka, S.V.; Zheludkevich, M.L.; Mohedano, M.; Moreno, L.; Visser, P.; Rodriguez, A.; Velasco, J.; Arrabal, R.; et al. Flash-PEO as an alternative to chromate conversion coatings for corrosion protection of Mg alloy. Corros. Sci. 2021, 180, 109189. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, X.; Lamaka, S.V.; Ju, P.; Blawert, C.; Zhang, T.; Wang, F.; Zheludkevich, M.L. Active protection of Mg alloy by composite PEO coating loaded with corrosion inhibitors. Appl. Surf. Sci. 2020, 504, 144462. [Google Scholar] [CrossRef]
- Wu, W.X.; Wang, W.P.; Lin, H.C. A study on corrosion behavior of micro-arc oxidation coatings doped with 2-aminobenzimidazole loaded halloysite nanotubes on AZ31 magnesium alloys. Surf. Coat. Technol. 2021, 416, 127116. [Google Scholar] [CrossRef]
- Sun, M.; Yerokhin, A.; Bychkova, M.Y.; Shtansky, D.V.; Levashov, E.A.; Matthews, A. Self-healing plasma electrolytic oxidation coatings doped with benzotriazole loaded halloysite nanotubes on AM50 magnesium alloy. Corros. Sci. 2016, 111, 753–769. [Google Scholar] [CrossRef]
- Mingo, B.; Guo, Y.; Němcová, A.; Gholinia, A.; Mohedano, M.; Sun, M.; Matthews, A.; Yerokhin, A. Incorporation of halloysite nanotubes into forsterite surface layer during plasma electrolytic oxidation of AM50 Mg alloy. Electrochim. Acta 2019, 299, 772–788. [Google Scholar] [CrossRef]
- Li, Y.; Lu, X.; Serdechnova, M.; Blawert, C.; Zheludkevich, M.L.; Qian, K.; Zhang, T.; Wang, F. Incorporation of LDH nanocontainers into plasma electrolytic oxidation coatings on Mg alloy. J. Magnes. Alloy. 2021, in press. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Serdechnova, M.; Tang, A.; Wang, C.; Blawert, C.; Pan, F.; Zheludkevich, M.L. In-situ LDHs growth on PEO coatings on AZ31 magnesium alloy for active protection: Roles of PEO composition and conversion solution. J. Magnes. Alloy. 2021, in press. [Google Scholar] [CrossRef]
- Chen, J.; Lin, W.; Liang, S.; Zou, L.; Wang, C.; Wang, B.; Yan, M.; Cui, X. Effect of alloy cations on corrosion resistance of LDH/MAO coating on magnesium alloy. Appl. Surf. Sci. 2019, 463, 535–544. [Google Scholar] [CrossRef]
- Song, Z.; Xie, Z.; Ding, L.; Zhang, Y.; Hu, X. Preparation of corrosion-resistant MgAl-LDH/Ni composite coating on Mg alloy AZ31B. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 632, 127699. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, F.; Qiu, J.; Tan, J.; Zhang, X.; Chen, S.; Qian, S.; Liu, X. Regulating corrosion reactions to enhance the anti-corrosion and self-healing abilities of PEO coating on magnesium. Corros. Sci. 2021, 192, 109840. [Google Scholar] [CrossRef]
- Visser, P.; Gonzalez-Garcia, Y.; Mol, J.M.C.; Terryn, H. Mechanism of Passive Layer Formation on AA2024-T3 from Alkaline Lithium Carbonate Solutions in the Presence of Sodium Chloride. J. Electrochem. Soc. 2018, 165, C60–C70. [Google Scholar] [CrossRef]
- Visser, P.; Liu, Y.; Zhou, X.; Hashimoto, T.; Thompson, G.E.; Lyon, S.B.; Van Der Ven, L.G.J.; Mol, A.J.M.C.; Terryn, H.A. The corrosion protection of AA2024-T3 aluminium alloy by leaching of lithium-containing salts from organic coatings. Faraday Discuss. 2015, 180, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Visser, P.; Marcoen, K.; Trindade, G.F.; Abel, M.L.; Watts, J.F.; Hauffman, T.; Mol, J.M.C.; Terryn, H. The chemical throwing power of lithium-based inhibitors from organic coatings on AA2024-T3. Corros. Sci. 2019, 150, 194–206. [Google Scholar] [CrossRef]
- Marcoen, K.; Visser, P.; Trindade, G.F.; Abel, M.L.; Watts, J.F.; Mol, J.M.C.; Terryn, H.; Hauffman, T. Compositional study of a corrosion protective layer formed by leachable lithium salts in a coating defect on AA2024-T3 aluminium alloys. Prog. Org. Coat. 2018, 119, 65–75. [Google Scholar] [CrossRef]
- Visser, P.; Terryn, H.; Mol, J.M.C. Active corrosion protection of various aluminium alloys by lithium-leaching coatings. Surf. Interface Anal. 2019, 51, 1276–1287. [Google Scholar] [CrossRef]
- Visser, P.; Lutz, A.; Mol, J.M.C.; Terryn, H. Study of the formation of a protective layer in a defect from lithium-leaching organic coatings. Prog. Org. Coat. 2016, 99, 80–90. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaghefinazari, B.; Wierzbicka, E.; Visser, P.; Posner, R.; Arrabal, R.; Matykina, E.; Mohedano, M.; Blawert, C.; Zheludkevich, M.L.; Lamaka, S.V. Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: Part III—Corrosion Inhibitors and Combining Them with Other Protection Strategies. Materials 2022, 15, 8489. https://doi.org/10.3390/ma15238489
Vaghefinazari B, Wierzbicka E, Visser P, Posner R, Arrabal R, Matykina E, Mohedano M, Blawert C, Zheludkevich ML, Lamaka SV. Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: Part III—Corrosion Inhibitors and Combining Them with Other Protection Strategies. Materials. 2022; 15(23):8489. https://doi.org/10.3390/ma15238489
Chicago/Turabian StyleVaghefinazari, Bahram, Ewa Wierzbicka, Peter Visser, Ralf Posner, Raúl Arrabal, Endzhe Matykina, Marta Mohedano, Carsten Blawert, Mikhail L. Zheludkevich, and Sviatlana V. Lamaka. 2022. "Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: Part III—Corrosion Inhibitors and Combining Them with Other Protection Strategies" Materials 15, no. 23: 8489. https://doi.org/10.3390/ma15238489
APA StyleVaghefinazari, B., Wierzbicka, E., Visser, P., Posner, R., Arrabal, R., Matykina, E., Mohedano, M., Blawert, C., Zheludkevich, M. L., & Lamaka, S. V. (2022). Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: Part III—Corrosion Inhibitors and Combining Them with Other Protection Strategies. Materials, 15(23), 8489. https://doi.org/10.3390/ma15238489