Easy, Fast Self-Heating Polyurethane Nanocomposite with the Introduction of Thermally Annealed Carbon Nanotubes Using Near-Infrared Lased Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Highly Thermally Annealed SWNTs (H-SWNTs)
2.2. Synthesis of Shape Memory PU Block Copolymer and Preparation of Nanocomposite
2.3. Characterizations
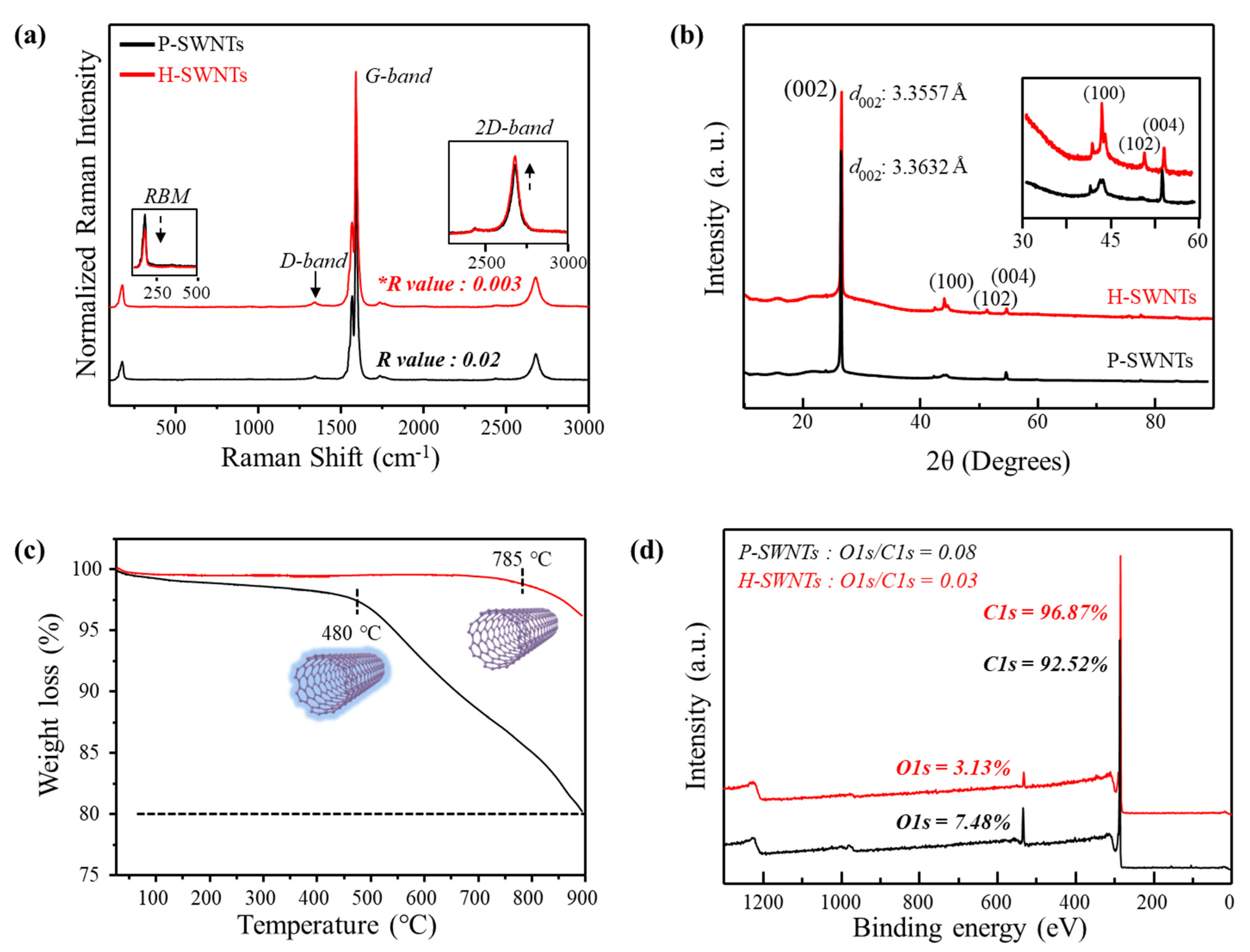
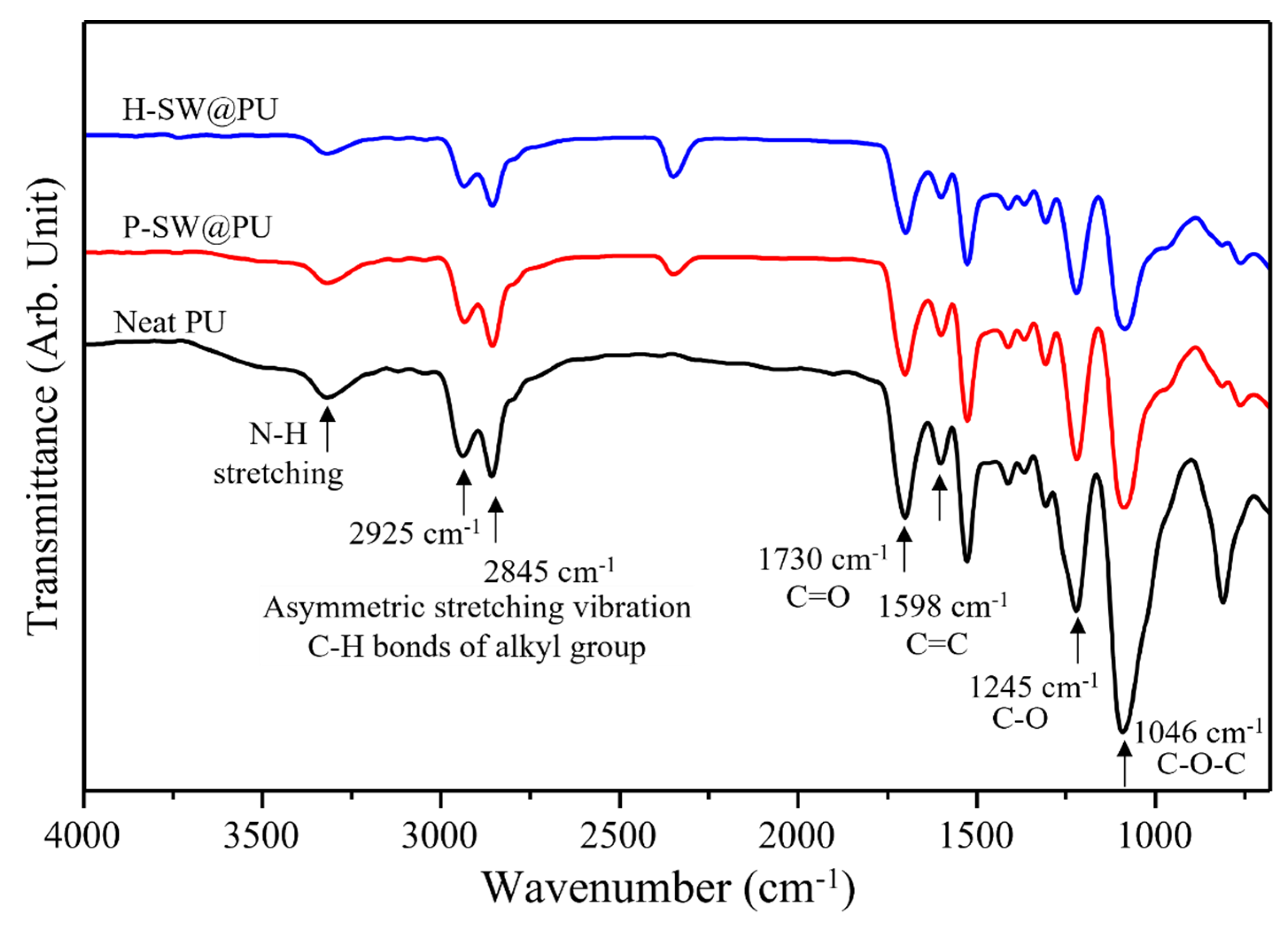
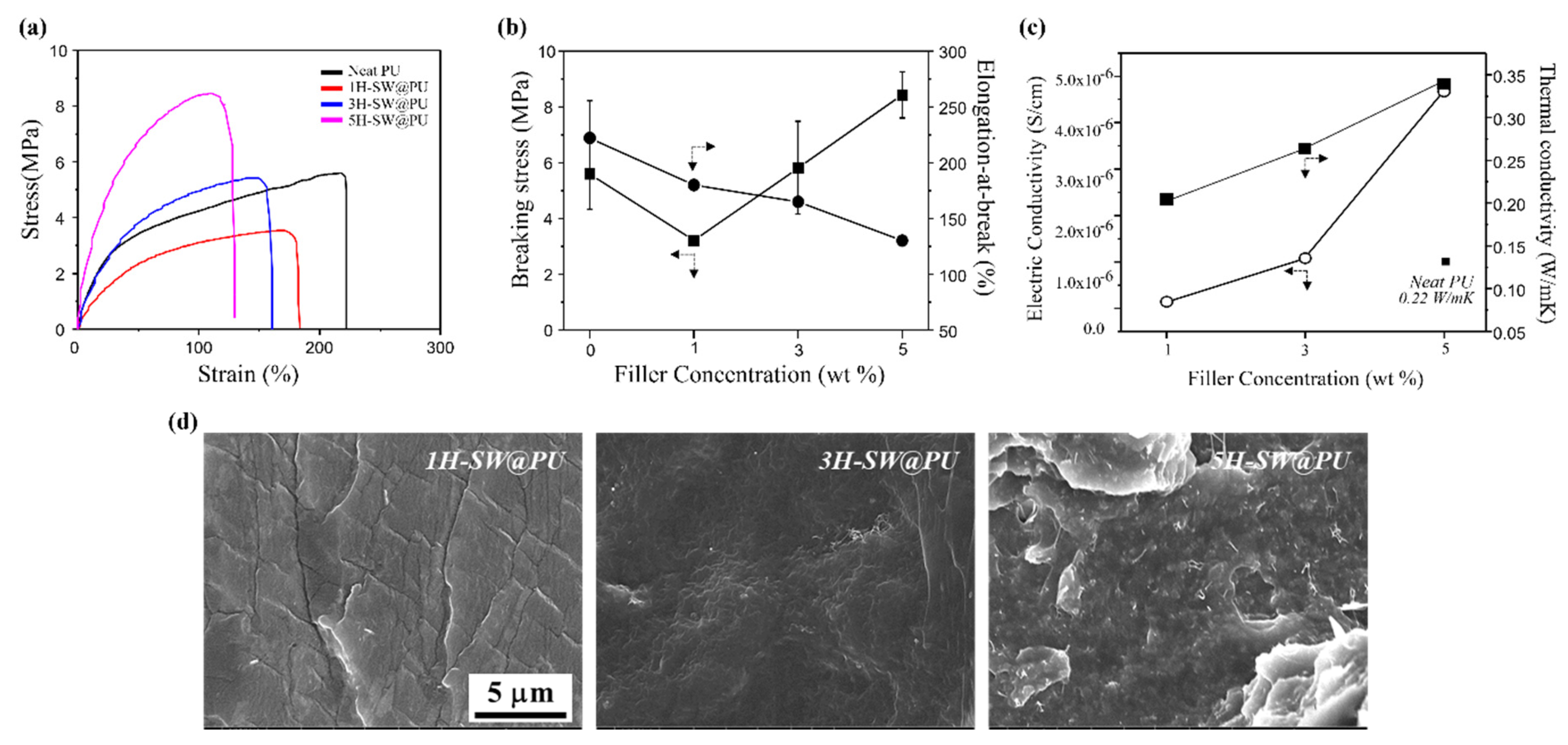
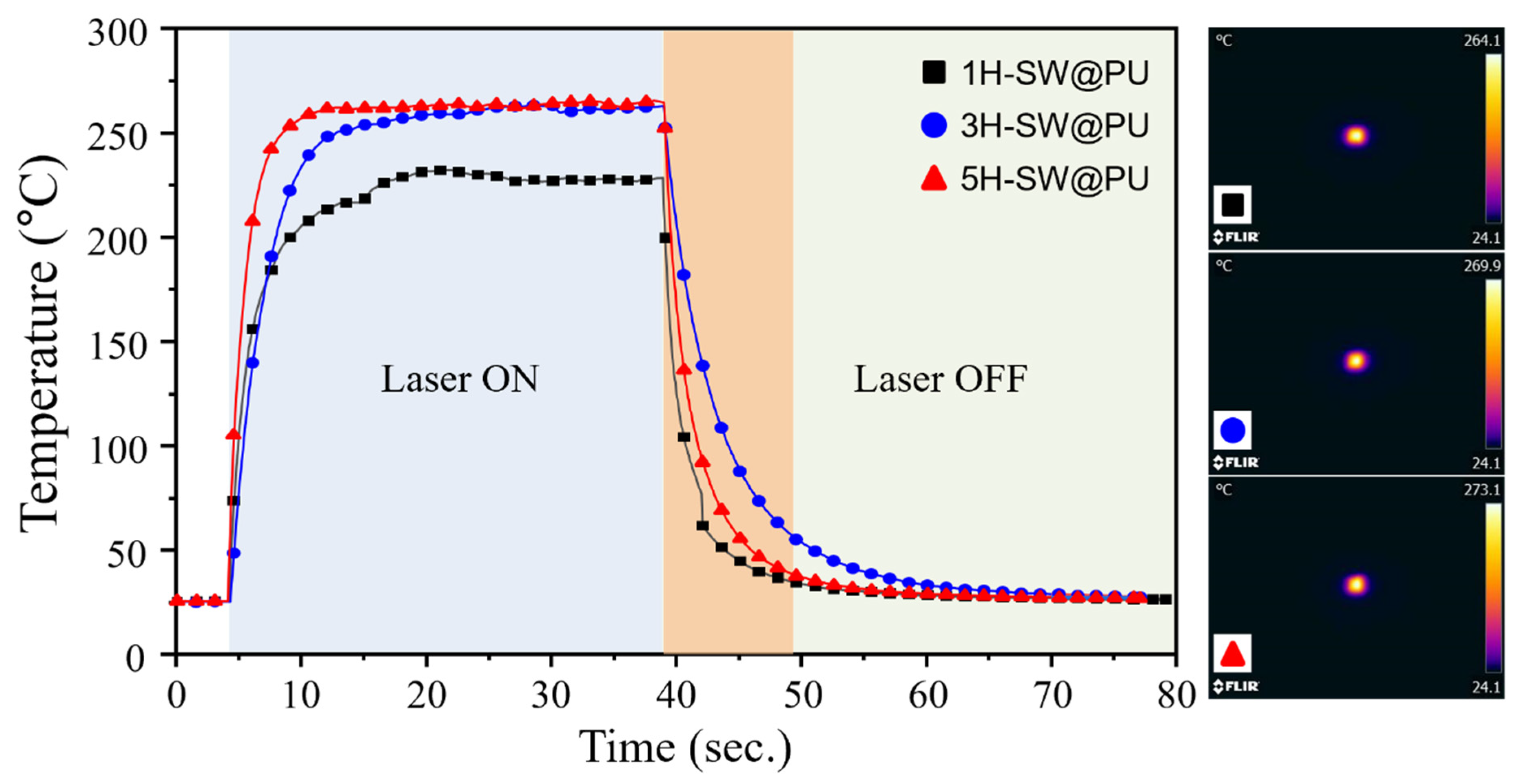
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Du, H.; Zhao, Y.; Li, Q.; Wang, J.; Kang, M.; Wang, X.; Xiang, H. Synthesis and characterization of waterborne polyurethane adhesive from MDI and HDI. J. Appl. Polym. Sci. 2008, 110, 1396–1402. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Raju, K. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar]
- Aznar, A.C.; Pardini, O.; Amalvy, J. Glossy topcoat exterior paint formulations using water-based polyurethane/acrylic hybrid binders. Prog. Org. Coat. 2006, 55, 43–49. [Google Scholar] [CrossRef]
- Varghese, S.; Gatos, K.; Apostolov, A.; Karger-Kocsis, J. Morphology and mechanical properties of layered silicate reinforced natural and polyurethane rubber blends produced by latex compounding. J. Appl. Polym. Sci. 2004, 92, 543–551. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Y.; Li, S.; Xu, M.; He, Y.; Shi, Z.; Li, B. A novel strategy for simultaneously improving the fire safety, water resistance and compatibility of thermoplastic polyurethane composites through the construction of biomimetic hydrophobic structure of intumescent flame retardant synergistic system. Compos. B Eng. 2019, 176, 107218. [Google Scholar] [CrossRef]
- Ha, Y.-M.; Kim, Y.-O.; Kim, Y.-N.; Kim, J.; Lee, J.-S.; Cho, J.W.; Endo, M.; Muramatsu, H.; Kim, Y.A.; Jung, Y.C. Rapidly self-heating shape memory polyurethane nanocomposite with boron-doped single-walled carbon nanotubes using near-infrared laser. Compos. B Eng. 2019, 175, 107065. [Google Scholar] [CrossRef]
- Ha, Y.-M.; Kim, Y.N.; Kim, Y.-O.; So, C.; Lee, J.-S.; Kim, J.; Jung, Y.C. Enhanced mechanical properties and thermal conductivity of polyimide nanocomposites incorporating individualized boron-doped graphene. Carbon Lett. 2020, 30, 457–464. [Google Scholar] [CrossRef]
- Ha, Y.-M.; Lim, D.; Lee, S.Y.; Kim, J.; Kim, J.H.; Kim, Y.A.; Jung, Y.C. Enhanced thermal conductivity and mechanical properties of polyurethane composites with the introduction of thermally annealed carbon nanotubes. Macromol. Res. 2017, 25, 1015–1021. [Google Scholar] [CrossRef]
- Tayfun, U.; Kanbur, Y.; Abacı, U.; Güney, H.Y.; Bayramlı, E. Mechanical, electrical, and melt flow properties of polyurethane elastomer/surface-modified carbon nanotube composites. J. Compos. Mater. 2017, 51, 1987–1996. [Google Scholar] [CrossRef]
- Špírková, M.; Duszová, A.; Poręba, R.; Kredatusová, J.; Bureš, R.; Fáberová, M.; Šlouf, M. Thermoplastic polybutadiene-based polyurethane/carbon nanofiber composites. Compos. B Eng. 2014, 67, 434–440. [Google Scholar] [CrossRef]
- Pantano, A.; Modica, G.; Cappello, F. Multiwalled carbon nanotube reinforced polymer composites. Mater. Sci. Eng. A 2008, 486, 222–227. [Google Scholar] [CrossRef]
- Kausar, A.; Rafique, I.; Muhammad, B. Review of applications of polymer/carbon nanotubes and epoxy/CNT composites. Polym.-Plast. Technol. Eng. 2016, 55, 1167–1191. [Google Scholar] [CrossRef]
- Odom, T.W.; Huang, J.-L.; Kim, P.; Lieber, C.M. Atomic structure and electronic properties of single-walled carbon nanotubes. Nature 1998, 391, 62–64. [Google Scholar] [CrossRef]
- Ouyang, M.; Huang, J.-L.; Lieber, C.M. Fundamental electronic properties and applications of single-walled carbon nanotubes. Acc. Chem. Res. 2002, 35, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Bekyarova, E.; Itkis, M.E.; Cabrera, N.; Zhao, B.; Yu, A.; Gao, J.; Haddon, R.C. Electronic properties of single-walled carbon nanotube networks. J. Am. Chem. Soc. 2005, 127, 5990–5995. [Google Scholar] [CrossRef]
- Javey, A.; Shim, M.; Dai, H. Electrical properties and devices of large-diameter single-walled carbon nanotubes. Appl. Phys. Lett. 2002, 80, 1064–1066. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Terentjev, E.M. Dispersion of carbon nanotubes: Mixing, sonication, stabilization, and composite properties. Polymers 2012, 4, 275–295. [Google Scholar] [CrossRef]
- Harito, C.; Bavykin, D.V.; Yuliarto, B.; Dipojono, H.K.; Walsh, F.C. Polymer nanocomposites having a high filler content: Synthesis, structures, properties, and applications. Nanoscale 2019, 11, 4653–4682. [Google Scholar] [CrossRef]
- Kim, Y.; Muramatsu, H.; Hayashi, T.; Endo, M.; Terrones, M.; Dresselhaus, M. Thermal stability and structural changes of double-walled carbon nanotubes by heat treatment. Chem. Phys. Lett. 2004, 398, 87–92. [Google Scholar] [CrossRef]
- Yudasaka, M.; Ichihashi, T.; Kasuya, D.; Kataura, H.; Iijima, S. Structure changes of single-wall carbon nanotubes and single-wall carbon nanohorns caused by heat treatment. Carbon 2003, 41, 1273–1280. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Han, L.; Song, Q. Enhancing both strength and toughness of carbon/carbon composites by heat-treated interface modification. J. Mater. Sci. Technol. 2019, 35, 383–393. [Google Scholar] [CrossRef]
- Fujisawa, K.; Hayashi, T.; Endo, M.; Terrones, M.; Kim, J.H.; Kim, Y.A. Effect of boron doping on the electrical conductivity of metallicity-separated single walled carbon nanotubes. Nanoscale 2018, 10, 12723–12733. [Google Scholar] [CrossRef] [PubMed]
- Redlich, P.; Loeffler, J.; Ajayan, P.; Bill, J.; Aldinger, F.; Rühle, M. B C N nanotubes and boron doping of carbon nanotubes. Chem. Phys. Lett. 1996, 260, 465–470. [Google Scholar] [CrossRef]
- Siregar, S.; Oktamuliani, S.; Saijo, Y. A theoretical model of laser heating carbon nanotubes. Nanomaterials 2018, 8, 580. [Google Scholar] [CrossRef]
- Singh, R.; Torti, S.V. Carbon nanotubes in hyperthermia therapy. Adv. Drug Deliv. Rev. 2013, 65, 2045–2060. [Google Scholar] [CrossRef]
- Heister, E.; Brunner, E.W.; Dieckmann, G.R.; Jurewicz, I.; Dalton, A.B. Are carbon nanotubes a natural solution? Applications in biology and medicine. ACS Appl. Mater. Interfaces 2013, 5, 1870–1891. [Google Scholar] [CrossRef]
- Han, B.; Zhang, Y.L.; Chen, Q.D.; Sun, H.B. Carbon-based photothermal actuators. Adv. Funct. Mater. 2018, 28, 1802235. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.A.; Ambulo, C.P.; Lee, H.B.; Kim, S.H.; Naik, V.V.; Haines, C.S.; Aliev, A.E.; Ovalle-Robles, R.; Baughman, R.H. Intelligently actuating liquid crystal elastomer-carbon nanotube composites. Adv. Funct. Mater. 2019, 29, 1905063. [Google Scholar] [CrossRef]
- Shen, X.; Viney, C.; Wang, C.; Lu, J.Q. Greatly enhanced thermal contraction at room temperature by carbon nanotubes. Adv. Funct. Mater. 2014, 24, 77–85. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, B.; Zhang, X.; Hu, N.; Li, K.; Chiavaioli, F.; Gui, X.; Guo, T. Plasmonic fiber-optic photothermal anemometers with carbon nanotube coatings. J. Lightwave Technol. 2019, 37, 3373–3380. [Google Scholar] [CrossRef]
- Suzuki, D.; Takida, Y.; Kawano, Y.; Minamide, H.; Terasaki, N. Carbon nanotube-based, serially connected terahertz sensor with enhanced thermal and optical efficiencies. Sci. Technol. Adv. Mater. 2022, 23, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Takeya, T.; Kimura, T.; Dresselhaus, G.; Dresselhaus, M. Raman intensity of single-wall carbon nanotubes. Phys. Rev. B 1998, 57, 4145. [Google Scholar] [CrossRef]
- Raravikar, N.R.; Keblinski, P.; Rao, A.M.; Dresselhaus, M.S.; Schadler, L.S.; Ajayan, P.M. Temperature dependence of radial breathing mode Raman frequency of single-walled carbon nanotubes. Phys. Rev. B 2002, 66, 235424. [Google Scholar] [CrossRef]
- Yudasaka, M.; Kataura, H.; Ichihashi, T.; Qin, L.-C.; Kar, S.; Iijima, S. Diameter enlargement of HiPco single-wall carbon nanotubes by heat treatment. Nano Lett. 2001, 1, 487–489. [Google Scholar] [CrossRef][Green Version]
- McGuire, K.; Gothard, N.; Gai, P.; Dresselhaus, M.; Sumanasekera, G.; Rao, A. Synthesis and Raman characterization of boron-doped single-walled carbon nanotubes. Carbon 2005, 43, 219–227. [Google Scholar] [CrossRef]
- Maultzsch, J.; Reich, S.; Thomsen, C.; Webster, S.; Czerw, R.; Carroll, D.; Vieira, S.; Birkett, P.; Rego, C.A. Raman characterization of boron-doped multiwalled carbon nanotubes. Appl. Phys. Lett. 2002, 81, 2647–2649. [Google Scholar] [CrossRef]
- Maniwa, Y.; Fujiwara, R.; Kira, H.; Tou, H.; Kataura, H.; Suzuki, S.; Achiba, Y.; Nishibori, E.; Takata, M.; Sakata, M. Thermal expansion of single-walled carbon nanotube (SWNT) bundles: X-ray diffraction studies. Phys. Rev. B 2001, 64, 241402. [Google Scholar] [CrossRef]
- Moon, J.-M.; An, K.H.; Lee, Y.H.; Park, Y.S.; Bae, D.J.; Park, G.-S. High-yield purification process of singlewalled carbon nanotubes. J. Phys. Chem. B 2001, 105, 5677–5681. [Google Scholar] [CrossRef]
- Zhang, M.; Yudasaka, M.; Bandow, S.; Iijima, S. Thermogravimetric analysis for the array of C60 molecules formed in single-wall carbon nanotube. Chem. Phys. Lett. 2003, 369, 680–683. [Google Scholar] [CrossRef]
- Bertoncini, M.; Coelho, L.A.F.; Maciel, I.O.; Pezzin, S.H. Purification of Single-Wall carbon nanotubes by heat treatment and supercritical extraction. Mater. Res. 2011, 14, 380–383. [Google Scholar] [CrossRef][Green Version]
- Tjong, S.C. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R Rep. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Jung, Y.C.; Kim, H.H.; Kim, Y.A.; Kim, J.H.; Cho, J.W.; Endo, M.; Dresselhaus, M.S. Optically active multi-walled carbon nanotubes for transparent, conductive memory-shape polyurethane film. Macromolecules 2010, 43, 6106–6112. [Google Scholar] [CrossRef]
- Rwei, S.-P.; Wang, L. Synthesis and electrical, rheological and thermal characterization of conductive polyurethane. Colloid Polym. Sci. 2007, 285, 1313–1319. [Google Scholar] [CrossRef]

| Samples | Mol% | Filler Contents (wt%) | ||
|---|---|---|---|---|
| MDI | PTMG | BD | ||
| Pure PU | 4.0 | 1.0 | 3.0 | - |
| Pristine SWCNTs/PU (P-SW/PU) | 1 | |||
| 3 | ||||
| 5 | ||||
| *HTT SWCNTs/PU (H-SW/PU) | 1 | |||
| 3 | ||||
| 5 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.; Ryu, S.; Kim, Y.N.; Ha, Y.-M.; Tewari, C.; Kim, S.Y.; Kim, J.K.; Jung, Y.C. Easy, Fast Self-Heating Polyurethane Nanocomposite with the Introduction of Thermally Annealed Carbon Nanotubes Using Near-Infrared Lased Irradiation. Materials 2022, 15, 8463. https://doi.org/10.3390/ma15238463
Jeong H, Ryu S, Kim YN, Ha Y-M, Tewari C, Kim SY, Kim JK, Jung YC. Easy, Fast Self-Heating Polyurethane Nanocomposite with the Introduction of Thermally Annealed Carbon Nanotubes Using Near-Infrared Lased Irradiation. Materials. 2022; 15(23):8463. https://doi.org/10.3390/ma15238463
Chicago/Turabian StyleJeong, Hyunsung, Sooyeon Ryu, Young Nam Kim, Yu-Mi Ha, Chetna Tewari, Seong Yun Kim, Jung Kyu Kim, and Yong Chae Jung. 2022. "Easy, Fast Self-Heating Polyurethane Nanocomposite with the Introduction of Thermally Annealed Carbon Nanotubes Using Near-Infrared Lased Irradiation" Materials 15, no. 23: 8463. https://doi.org/10.3390/ma15238463
APA StyleJeong, H., Ryu, S., Kim, Y. N., Ha, Y.-M., Tewari, C., Kim, S. Y., Kim, J. K., & Jung, Y. C. (2022). Easy, Fast Self-Heating Polyurethane Nanocomposite with the Introduction of Thermally Annealed Carbon Nanotubes Using Near-Infrared Lased Irradiation. Materials, 15(23), 8463. https://doi.org/10.3390/ma15238463









