Effects of Na2CO3/Na2SiO3 Ratio and Curing Temperature on the Structure Formation of Alkali-Activated High-Carbon Biomass Fly Ash Pastes
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Paste Design and Sample Preparation
2.3. Test Methods
3. Results and Discussion
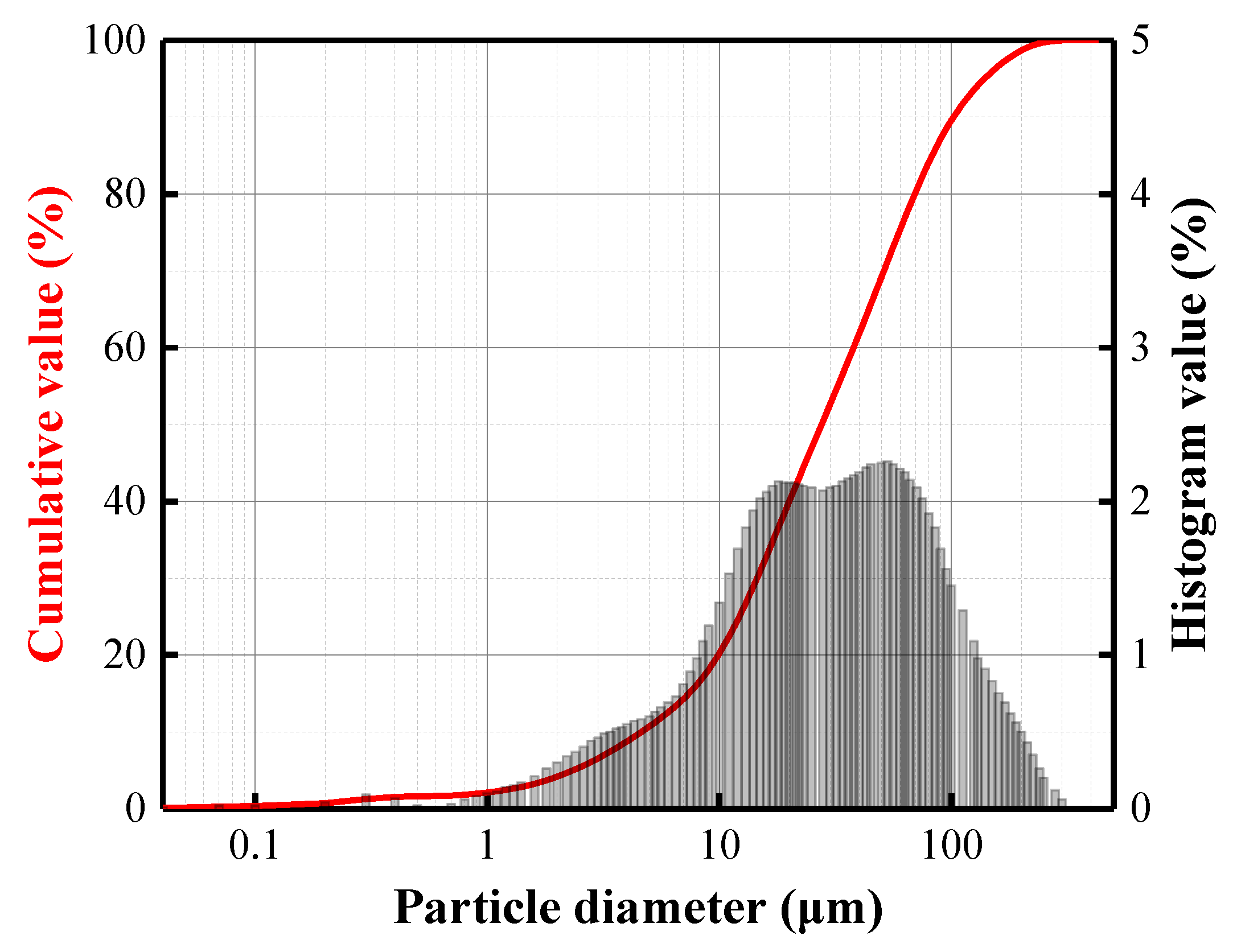
3.1. Parameters of BFA
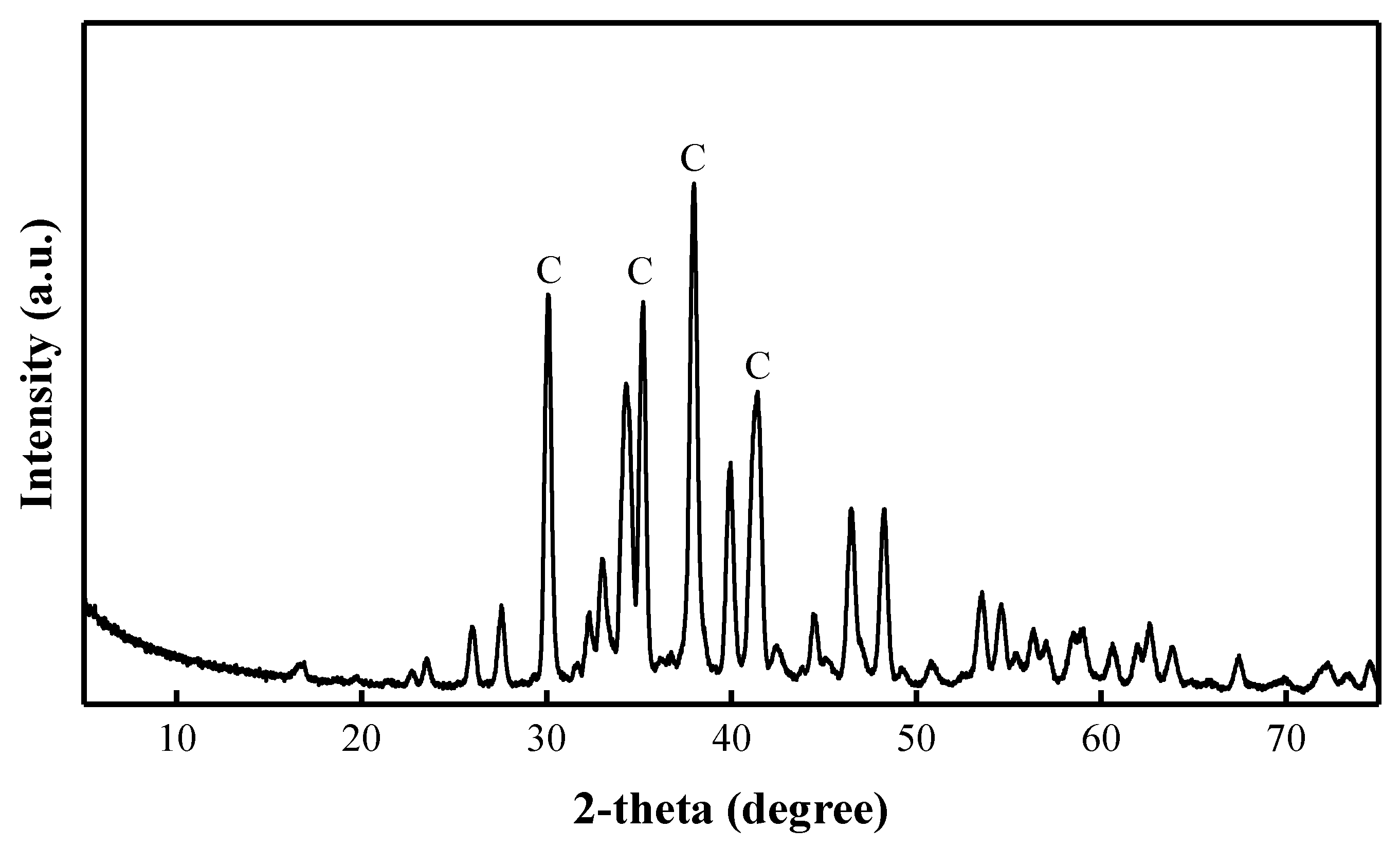
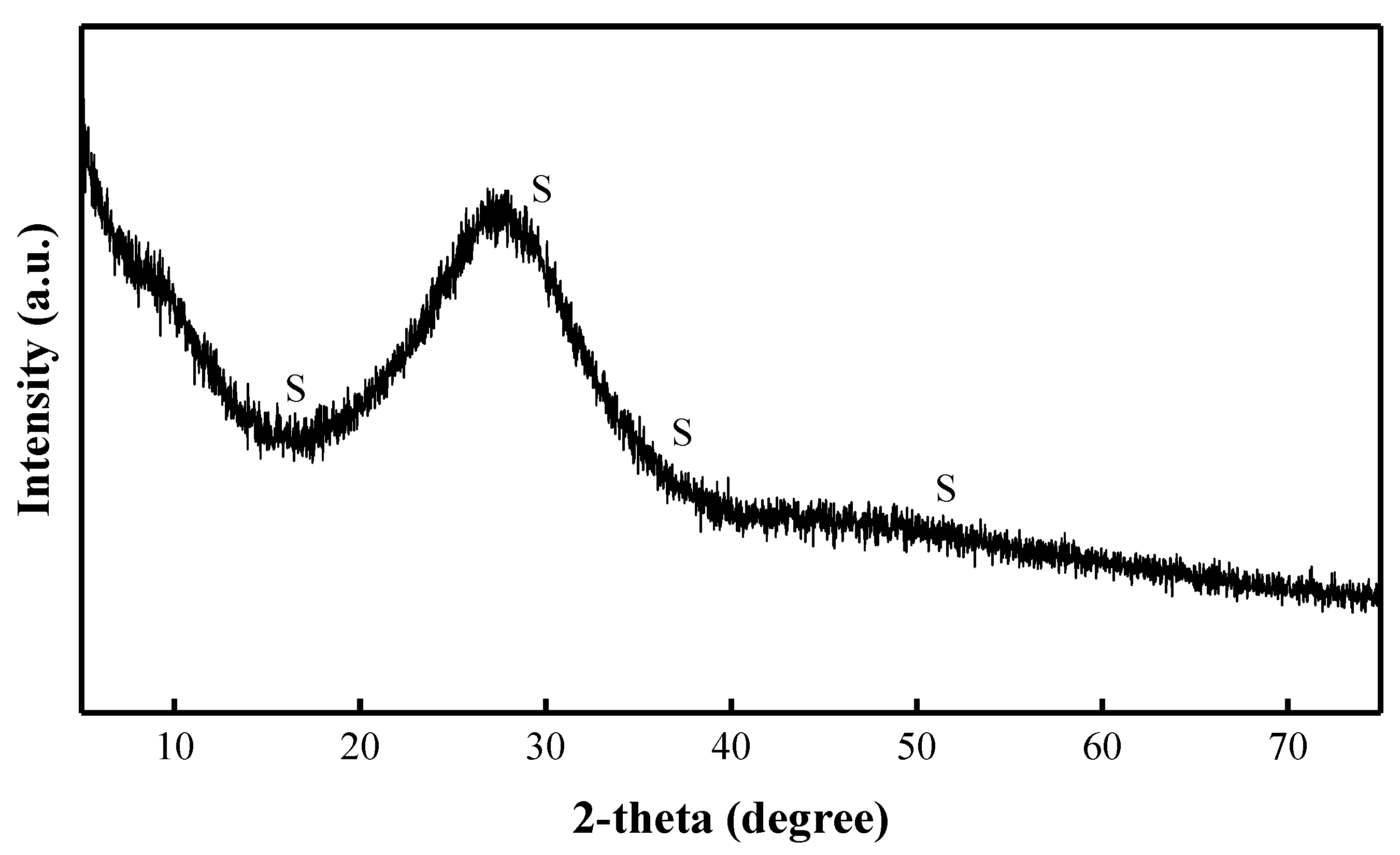
3.2. Activation Solution Characterization
3.3. Initial and Final Setting Times of BFA-Based AAM Pastes
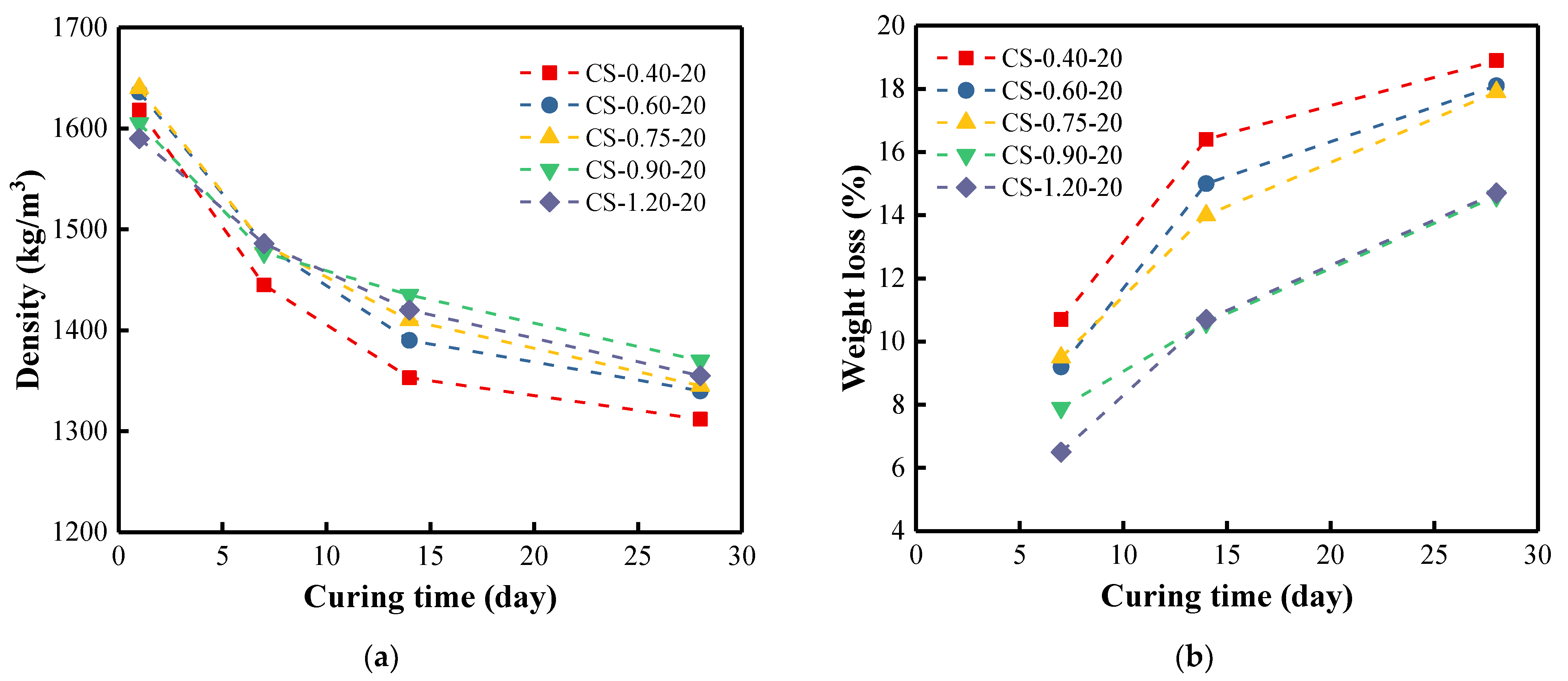
3.4. Density of BFA-Based AAM Pastes
3.5. UPV of BFA-Based AAM Pastes
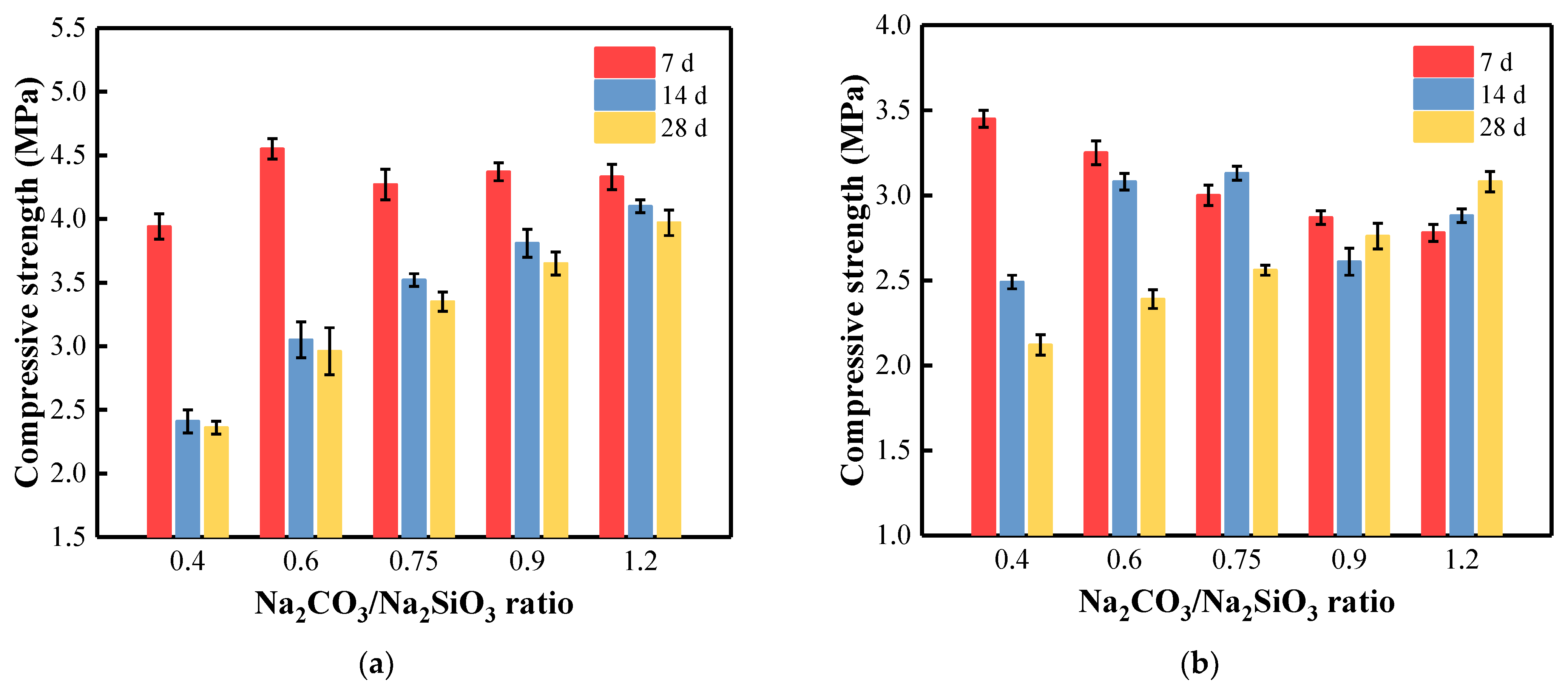
3.6. Compressive Strength of BFA-Based AAM Pastes
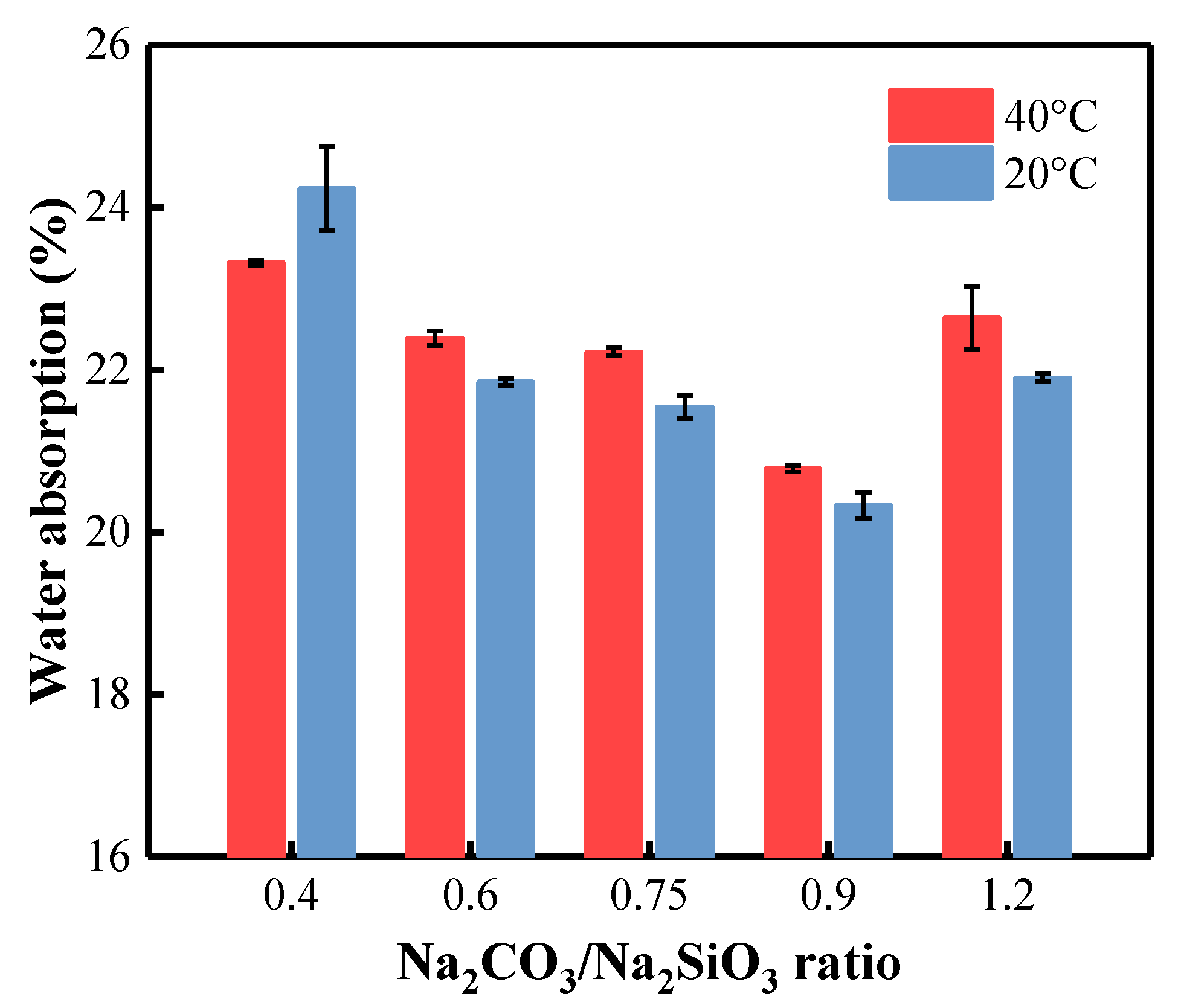
3.7. Water Absorption of BFA-Based AAM Pastes
3.8. XRD Patterns of BFA-Based AAM Pastes
3.9. TG/DTA Test of BFA-Based AAM Pastes
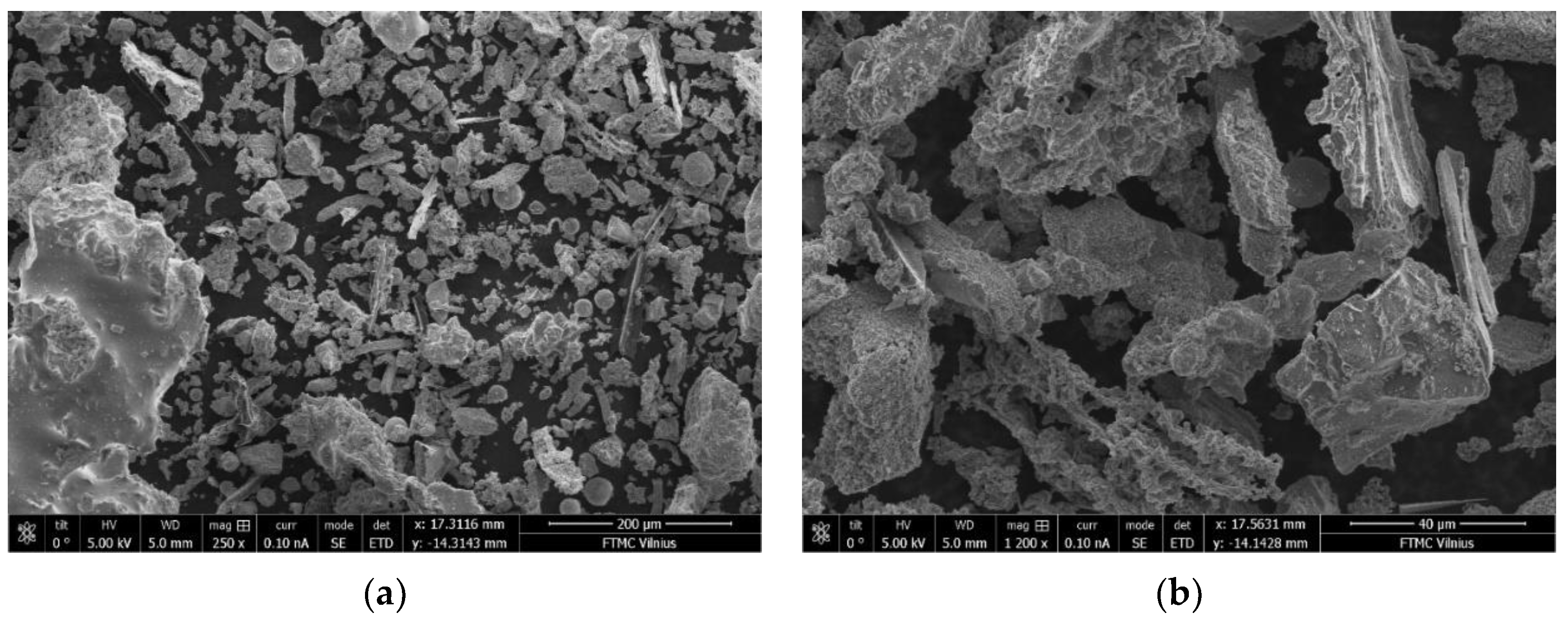
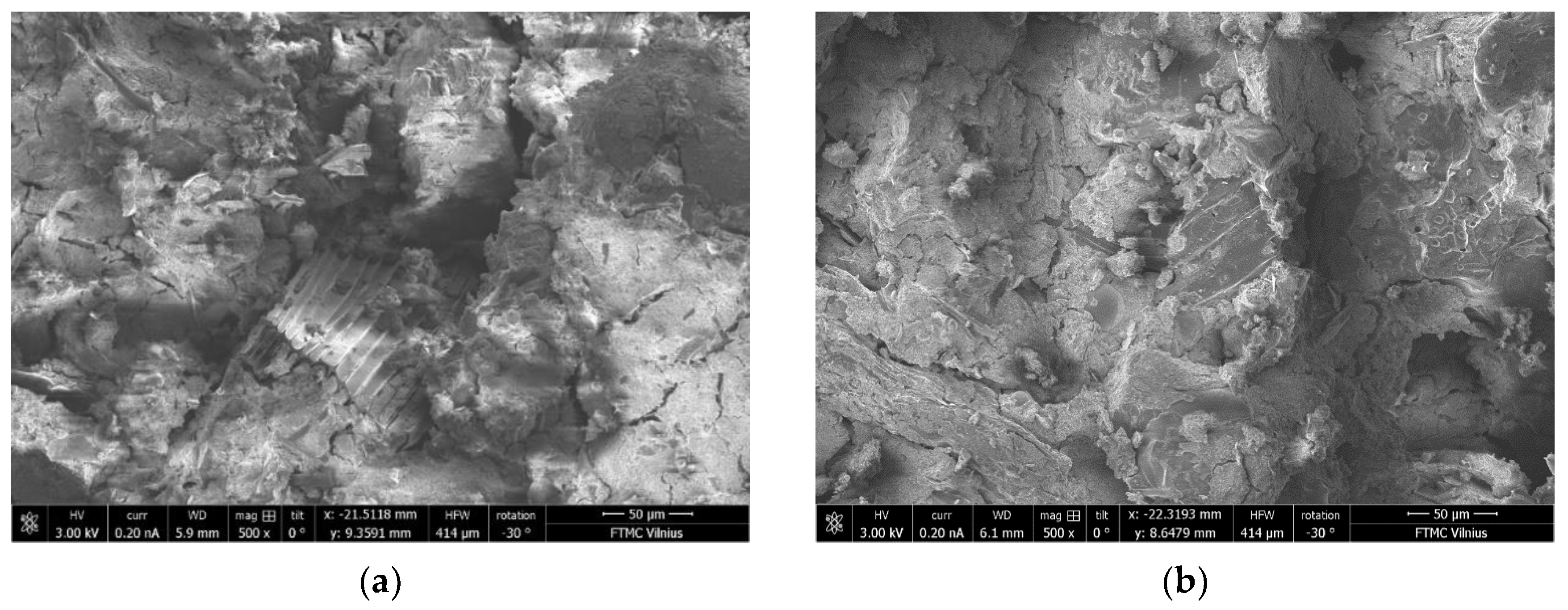
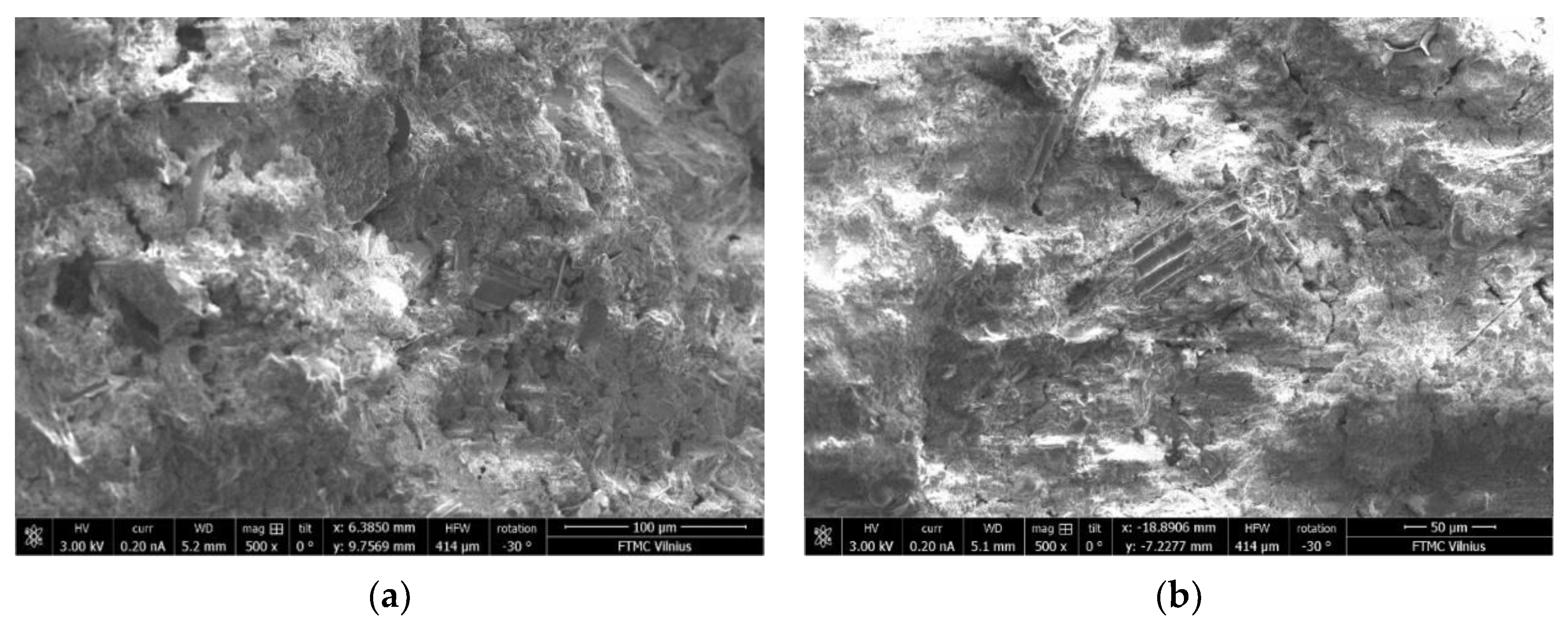
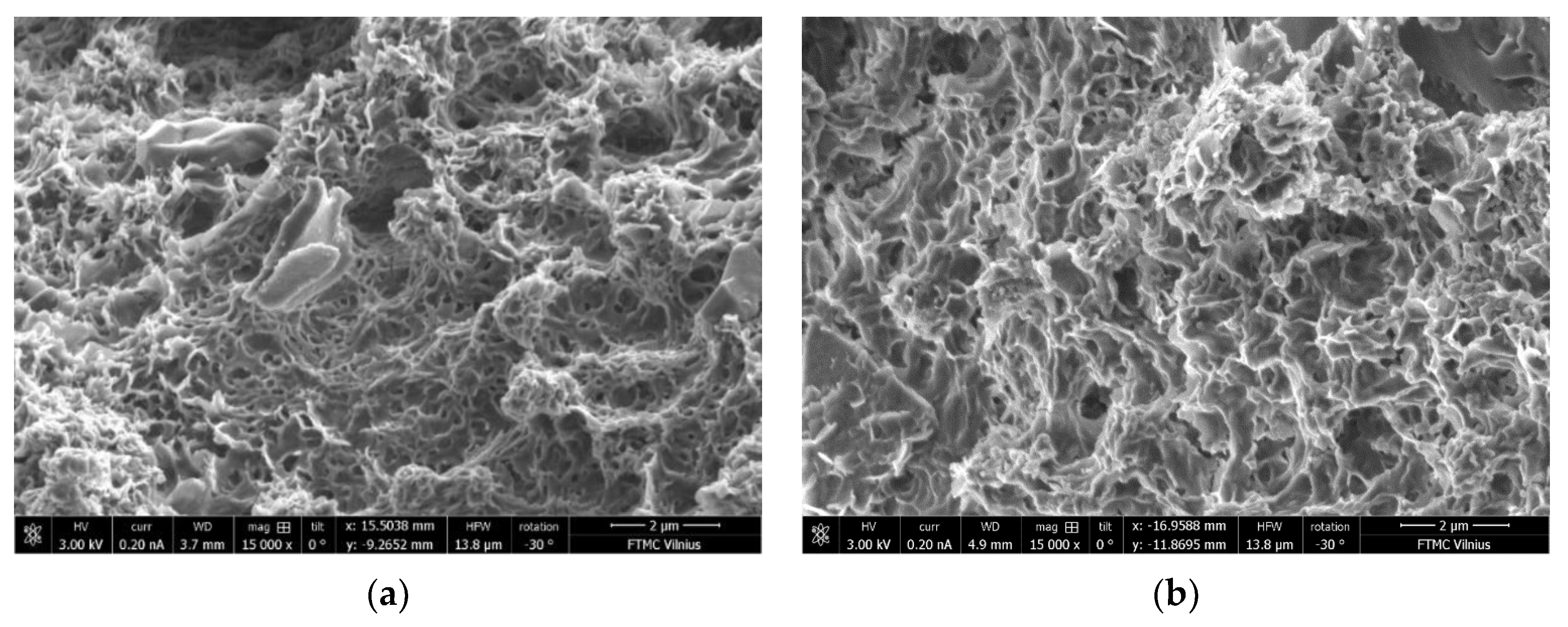
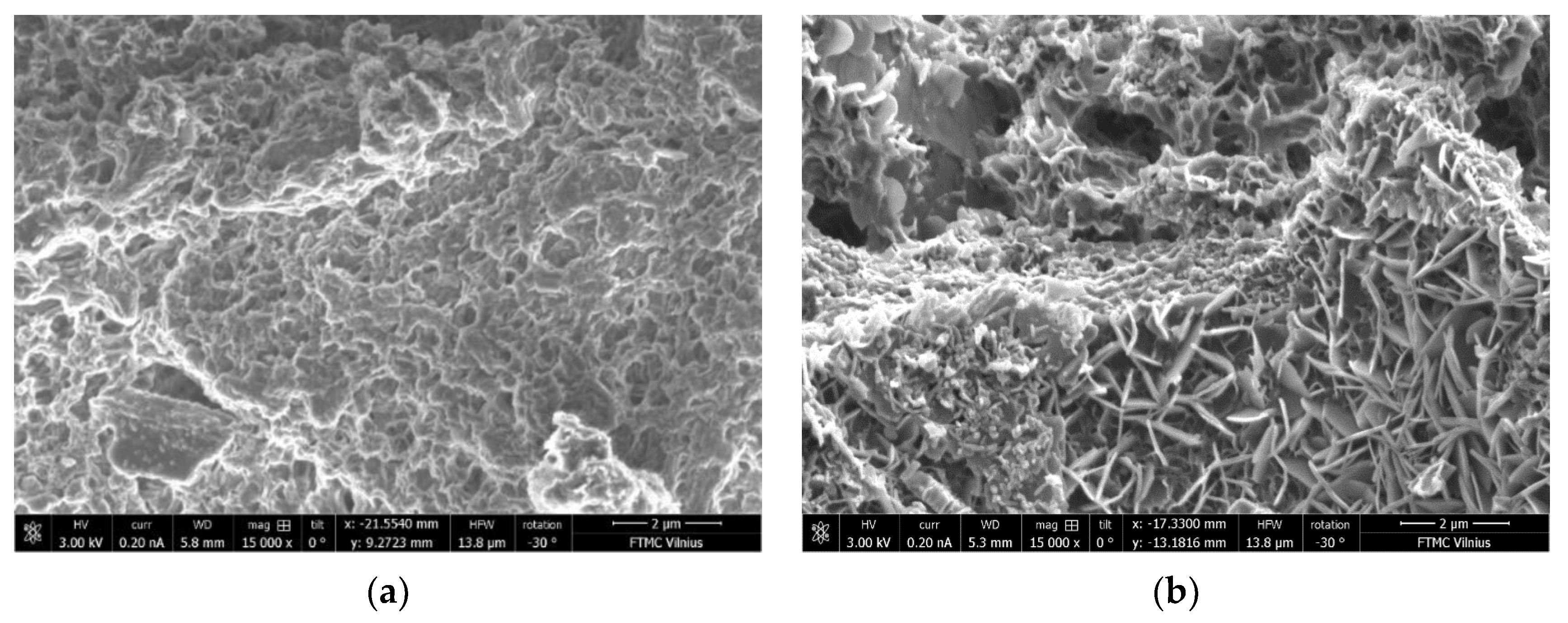
3.10. Microstructure of BFA-Based AAM Pastes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cuenca, J.; Rodríguez, J.; Martín-Morales, M.; Sánchez-Roldán, Z.; Zamorano, M. Effects of Olive Residue Biomass Fly Ash as Filler in Self-Compacting Concrete. Constr. Build. Mater. 2013, 40, 702–709. [Google Scholar] [CrossRef]
- Demirbas, A. Potential Applications of Renewable Energy Sources, Biomass Combustion Problems in Boiler Power Systems and Combustion Related Environmental Issues. Prog. Energy Combust. Sci. 2005, 31, 171–192. [Google Scholar] [CrossRef]
- Chaunsali, P.; Uvegi, H.; Osmundsen, R.; Laracy, M.; Poinot, T.; Ochsendorf, J.; Olivetti, E. Mineralogical and Microstructural Characterization of Biomass Ash Binder. Cem. Concr. Compos. 2018, 89, 41–51. [Google Scholar] [CrossRef]
- IEA. Key World Energy Statistics 2016; IEA: Paris, France, 2016. [Google Scholar] [CrossRef]
- Scurlock, J.M.O.; Hall, D.O. The Contribution of Biomass to Global Energy Use (1987). Biomass 1990, 21, 75–81. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook 2010; OECD Publishing: Paris, France, 2010. [Google Scholar] [CrossRef]
- U.S. EIA. Assumptions to the Annual Energy Outlook 2015; U.S. EIA: Washington, DC, USA, 2015.
- Hughes, E. Biomass Cofiring: Economics, Policy and Opportunities. Biomass Bioenergy 2000, 19, 457–465. [Google Scholar] [CrossRef]
- IRENA. Renewable Power Generation Costs in 2014; IRENA: Bonn, Germany, 2015. [Google Scholar]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Chemical Composition of Biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An Overview of the Behaviour of Biomass during Combustion: Part II. Ash Fusion and Ash Formation Mechanisms of Biomass Types. Fuel 2014, 117, 152–183. [Google Scholar] [CrossRef]
- Chaunsali, P.; Uvegi, H.; Traynor, B.; Olivetti, E. Leaching Characteristics of Biomass Ash-Based Binder in Neutral and Acidic Media. Cem. Concr. Compos. 2019, 100, 92–98. [Google Scholar] [CrossRef]
- World Energy Council. World Energy Resources 2016; World Energy Council: London, UK, 2016.
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Composition and Application of Biomass Ash. Part 1. Phase-Mineral and Chemical Composition and Classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Bernal, S.A.; Rodríguez, E.D.; Mejia De Gutiérrez, R.; Provis, J.L.; Delvasto, S. Activation of Metakaolin/Slag Blends Using Alkaline Solutions Based on Chemically Modified Silica Fume and Rice Husk Ash. Waste Biomass Valoriz. 2012, 3, 99–108. [Google Scholar] [CrossRef]
- Billong, N.; Melo, U.C.; Kamseu, E.; Kinuthia, J.M.; Njopwouo, D. Improving Hydraulic Properties of Lime-Rice Husk Ash (RHA) Binders with Metakaolin (MK). Constr. Build. Mater. 2011, 25, 2157–2161. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Martirena Hernández, J.F.; Middendorf, B.; Gehrke, M.; Budelmannt, H. Use of Wastes of the Sugar Industry as Pozzolana in Lime-Pozzolana Binders: Study of the Reaction. Cem. Concr. Res. 1998, 28, 1525–1536. [Google Scholar] [CrossRef]
- Nair, D.G.; Fraaij, A.; Klaassen, A.A.K.; Kentgens, A.P.M. A Structural Investigation Relating to the Pozzolanic Activity of Rice Husk Ashes. Cem. Concr. Res. 2008, 38, 861–869. [Google Scholar] [CrossRef]
- Yeboah, N.N.N.; Shearer, C.R.; Burns, S.E.; Kurtis, K.E. Characterization of Biomass and High Carbon Content Coal Ash for Productive Reuse Applications. Fuel 2014, 116, 438–447. [Google Scholar] [CrossRef]
- Cordeiro, G.C.; Toledo Filho, R.D.; De Moraes Rego Fairbairn, E. Use of Ultrafine Rice Husk Ash with High-Carbon Content as Pozzolan in High Performance Concrete. Mater. Struct. Constr. 2009, 42, 983–992. [Google Scholar] [CrossRef]
- Medina, J.M.; Sáez del Bosque, I.F.; Frías, M.; Sánchez de Rojas, M.I.; Medina, C. Characterisation and Valorisation of Biomass Waste as a Possible Addition in Eco-Cement Design. Mater. Struct. Constr. 2017, 50, 207. [Google Scholar] [CrossRef]
- Mehta, P.K.; Pitt, N. Energy and Industrial Materials from Crop Residues. Resour. Recover. Conserv. 1976, 2, 23–38. [Google Scholar] [CrossRef]
- Detphan, S.; Chindaprasirt, P. Preparation of Fly Ash and Rice Husk Ash Geopolymer. Int. J. Miner. Metall. Mater. 2009, 16, 720–726. [Google Scholar] [CrossRef]
- Castaldelli, V.N.; Akasaki, J.L.; Melges, J.L.P.; Tashima, M.M.; Soriano, L.; Borrachero, M.V.; Monzó, J.; Payá, J. Use of Slag/Sugar Cane Bagasse Ash (SCBA) Blends in the Production of Alkali-Activated Materials. Materials 2013, 6, 3108–3127. [Google Scholar] [CrossRef]
- Ali, S.H.; Emran, M.Y.; Gomaa, H. Rice Husk-Derived Nanomaterials for Potential Applications. In Waste Recycling Technologies for Nanomaterials Manufacturing; Topics in Mining, Metallurgy and Materials Engineering; Springer: Cham, Switzerland, 2021; pp. 541–588. [Google Scholar] [CrossRef]
- Freeman, E.; Gao, Y.M.; Hurt, R.; Suuberg, E. Interactions of Carbon-Containing Fly Ash with Commercial Air-Entraining Admixtures for Concrete. Fuel 1997, 76, 761–765. [Google Scholar] [CrossRef]
- Wang, S.; Miller, A.; Llamazos, E.; Fonseca, F.; Baxter, L. Biomass Fly Ash in Concrete: Mixture Proportioning and Mechanical Properties. Fuel 2008, 87, 365–371. [Google Scholar] [CrossRef]
- Basu, M.; Pande, M.; Bhadoria, P.B.S.; Mahapatra, S.C. Potential Fly-Ash Utilization in Agriculture: A Global Review. Prog. Nat. Sci. 2009, 19, 1173–1186. [Google Scholar] [CrossRef]
- Rajamma, R.; Senff, L.; Ribeiro, M.J.; Labrincha, J.A.; Ball, R.J.; Allen, G.C.; Ferreira, V.M. Biomass Fly Ash Effect on Fresh and Hardened State Properties of Cement Based Materials. Compos. Part B Eng. 2015, 77, 1–9. [Google Scholar] [CrossRef]
- Maschio, S.; Tonello, G.; Piani, L.; Furlani, E. Fly and Bottom Ashes from Biomass Combustion as Cement Replacing Components in Mortars Production: Rheological Behaviour of the Pastes and Materials Compression Strength. Chemosphere 2011, 85, 666–671. [Google Scholar] [CrossRef]
- Sherwood, P.T. Alternative Materials in Road Construction: A Guide to the Use of Recycled and Secondary Aggregates, 2nd ed.; T. Telford: London, UK, 2001. [Google Scholar]
- Forteza, R.; Far, M.; Seguí, C.; Cerdá, V. Characterization of Bottom Ash in Municipal Solid Waste Incinerators for Its Use in Road Base. Waste Manag. 2004, 24, 899–909. [Google Scholar] [CrossRef]
- Kumar, S.; Patil, C.B. Estimation of Resource Savings Due to Fly Ash Utilization in Road Construction. Resour. Conserv. Recycl. 2006, 48, 125–140. [Google Scholar] [CrossRef]
- Elinwa, A.U.; Mahmood, Y.A. Ash from Timber Waste as Cement Replacement Material. Cem. Concr. Compos. 2002, 24, 219–222. [Google Scholar] [CrossRef]
- Siddique, R. Utilization of Wood Ash in Concrete Manufacturing. Resour. Conserv. Recycl. 2012, 67, 27–33. [Google Scholar] [CrossRef]
- Martirena, F.; Middendorf, B.; Day, R.L.; Gehrke, M.; Roque, P.; Martínez, L.; Betancourt, S. Rudimentary, Low Tech Incinerators as a Means to Produce Reactive Pozzolan out of Sugar Cane Straw. Cem. Concr. Res. 2006, 36, 1056–1061. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W.; Luo, W. Fresh Properties, Mechanical Strength and Microstructure of Fly Ash Geopolymer Paste Reinforced with Sawdust. Constr. Build. Mater. 2016, 111, 600–610. [Google Scholar] [CrossRef]
- Shi, C.; Jiménez, A.F.; Palomo, A. New Cements for the 21st Century: The Pursuit of an Alternative to Portland Cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-Activated Binders: A Review. Part 2. About Materials and Binders Manufacture. Constr. Build. Mater. 2008, 22, 1315–1322. [Google Scholar] [CrossRef]
- Fernández-JIMÉNEZ, A.; Palomo, A.; Criado, M. Activación Alcalina de Cenizas Volantes. Estudio Comparativo Entre Activadores Sódicos y Potásicos. Mater. Constr. 2006, 56, 51–65. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Fly Ash-Based Geopolymers: The Relationship between Composition, Pore Structure and Efflorescence. Cem. Concr. Res. 2014, 64, 30–41. [Google Scholar] [CrossRef]
- Xue, X.; Liu, Y.L.; Dai, J.G.; Poon, C.S.; Zhang, W.D.; Zhang, P. Inhibiting Efflorescence Formation on Fly Ash–Based Geopolymer via Silane Surface Modification. Cem. Concr. Compos. 2018, 94, 43–52. [Google Scholar] [CrossRef]
- Dung, N.T.; Hooper, T.J.N.; Unluer, C. Accelerating the Reaction Kinetics and Improving the Performance of Na2CO3 Activated GGBS Mixes. Cem. Concr. Res. 2019, 126, 105927. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Brough, A.R.; Atkinson, A. Sodium Silicate-Based, Alkali-Activated Slag Mortars—Part I. Strength, Hydration and Microstructure. Cem. Concr. Res. 2002, 32, 865–879. [Google Scholar] [CrossRef]
- Brough, A.R.; Holloway, M.; Sykes, J.; Atkinson, A. Sodium Silicate-Based Alkali-Activated Slag Mortars. Part II. The Retarding Effect of Additions of Sodium Chloride or Malic Acid. Cem. Concr. Res. 2000, 30, 1375–1379. [Google Scholar] [CrossRef]
- Rashad, A.M. A Comprehensive Overview about the Influence of Different Additives on the Properties of Alkali-Activated Slag—A Guide for Civil Engineer. Constr. Build. Mater. 2013, 47, 29–55. [Google Scholar] [CrossRef]
- Živica, V. Effects of Type and Dosage of Alkaline Activator and Temperature on the Properties of Alkali-Activated Slag Mixtures. Constr. Build. Mater. 2007, 21, 1463–1469. [Google Scholar] [CrossRef]
- Bian, Z.; Jin, G.; Ji, T. Effect of Combined Activator of Ca(OH)2 and Na2CO3 on Workability and Compressive Strength of Alkali-Activated Ferronickel Slag System. Cem. Concr. Compos. 2021, 123, 104179. [Google Scholar] [CrossRef]
- Mobasher, N.; Bernal, S.A.; Provis, J.L. Structural Evolution of an Alkali Sulfate Activated Slag Cement. J. Nucl. Mater. 2016, 468, 97–104. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Myers, R.J.; San Nicolas, R.; van Deventer, J.S.J. Role of Carbonates in the Chemical Evolution of Sodium Carbonate-Activated Slag Binders. Mater. Struct. Constr. 2014, 48, 517–529. [Google Scholar] [CrossRef]
- Yuan, B.; Straub, C.; Segers, S.; Yu, Q.L.; Brouwers, H.J.H. Sodium Carbonate Activated Slag as Cement Replacement in Autoclaved Aerated Concrete. Ceram. Int. 2017, 43, 6039–6047. [Google Scholar] [CrossRef]
- Kim, M.S.; Jun, Y.; Lee, C.; Oh, J.E. Use of CaO as an Activator for Producing a Price-Competitive Non-Cement Structural Binder Using Ground Granulated Blast Furnace Slag. Cem. Concr. Res. 2013, 54, 208–214. [Google Scholar] [CrossRef]
- Jin, F.; Al-Tabbaa, A. Strength and Drying Shrinkage of Slag Paste Activated by Sodium Carbonate and Reactive MgO. Constr. Build. Mater. 2015, 81, 58–65. [Google Scholar] [CrossRef]
- Yang, K.H.; Cho, A.R.; Song, J.K.; Nam, S.H. Hydration Products and Strength Development of Calcium Hydroxide-Based Alkali-Activated Slag Mortars. Constr. Build. Mater. 2012, 29, 410–419. [Google Scholar] [CrossRef]
- Ishwarya, G.; Singh, B.; Deshwal, S.; Bhattacharyya, S.K. Effect of Sodium Carbonate/Sodium Silicate Activator on the Rheology, Geopolymerization and Strength of Fly Ash/Slag Geopolymer Pastes. Cem. Concr. Compos. 2019, 97, 226–238. [Google Scholar] [CrossRef]
- Noushini, A.; Babaee, M.; Castel, A. Suitability of Heat-Cured Low-Calcium Fly Ash-Based Geopolymer Concrete for Precast Applications. Mag. Concr. Res. 2015, 68, 163–177. [Google Scholar] [CrossRef]
- Singh, B.; Rahman, M.R.; Paswan, R.; Bhattacharyya, S.K. Effect of Activator Concentration on the Strength, ITZ and Drying Shrinkage of Fly Ash/Slag Geopolymer Concrete. Constr. Build. Mater. 2016, 118, 171–179. [Google Scholar] [CrossRef]
- Xu, H.; Gong, W.; Syltebo, L.; Izzo, K.; Lutze, W.; Pegg, I.L. Effect of Blast Furnace Slag Grades on Fly Ash Based Geopolymer Waste Forms. Fuel 2014, 133, 332–340. [Google Scholar] [CrossRef]
- Chithiraputhiran, S.; Neithalath, N. Isothermal Reaction Kinetics and Temperature Dependence of Alkali Activation of Slag, Fly Ash and Their Blends. Constr. Build. Mater. 2013, 45, 233–242. [Google Scholar] [CrossRef]
- Yuan, B.; Yu, Q.L.; Brouwers, H.J.H. Evaluation of Slag Characteristics on the Reaction Kinetics and Mechanical Properties of Na2CO3 Activated Slag. Constr. Build. Mater. 2017, 131, 334–346. [Google Scholar] [CrossRef]
- Duran Atiş, C.; Bilim, C.; Çelik, Ö.; Karahan, O. Influence of Activator on the Strength and Drying Shrinkage of Alkali-Activated Slag Mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Effect of Admixtures on Properties of Alkali-Activated Slag Concrete. Cem. Concr. Res. 2000, 30, 1367–1374. [Google Scholar] [CrossRef]
- Puertas, F.; Torres-Carrasco, M. Use of Glass Waste as an Activator in the Preparation of Alkali-Activated Slag. Mechanical Strength and Paste Characterisation. Cem. Concr. Res. 2014, 57, 95–104. [Google Scholar] [CrossRef]
- Yuan, B.; Yu, Q.L.; Brouwers, H.J.H. Reaction Kinetics, Reaction Products and Compressive Strength of Ternary Activators Activated Slag Designed by Taguchi Method. Mater. Des. 2015, 86, 878–886. [Google Scholar] [CrossRef]
- Kovtun, M.; Kearsley, E.P.; Shekhovtsova, J. Chemical Acceleration of a Neutral Granulated Blast-Furnace Slag Activated by Sodium Carbonate. Cem. Concr. Res. 2015, 72, 1–9. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Early Age Strength and Workability of Slag Pastes Activated by NaOH and Na2CO3. Cem. Concr. Res. 1998, 28, 655–664. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Puertas, F. Setting of Alkali-Activated Slag Cement. Influence of Activator Nature. Adv. Cem. Res. 2001, 13, 115–121. [Google Scholar] [CrossRef]
- Xu, H.; Provis, J.L.; Van Deventer, J.S.J.; Krivenko, P.V. Characterization of Aged Slag Concretes. ACI Mater. J. 2008, 105, 131–139. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, J.G.; Puertas, F. Alkali-Activated Slag Mortars: Mechanical Strength Behaviour. Cem. Concr. Res. 1999, 29, 1313–1321. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and Drying Shrinkage of Reactive MgO Modified Alkali-Activated Slag Paste. Constr. Build. Mater. 2014, 51, 395–404. [Google Scholar] [CrossRef]
- Silva, G.J.B.; Santana, V.P.; Wójcik, M. Investigation on Mechanical and Microstructural Properties of Alkali-Activated Materials Made of Wood Biomass Ash and Glass Powder. Powder Technol. 2021, 377, 900–912. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. A Calorimetric Study of Early Hydration of Alkali-Slag Cements. Cem. Concr. Res. 1995, 25, 1333–1346. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Puertas, F. Effect of Activator Mix on the Hydration and Strength Behaviour of Alkali-Activated Slag Cements. Adv. Cem. Res. 2003, 15, 129–136. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. Some Factors Affecting Early Hydration of Alkali-Slag Cements. Cem. Concr. Res. 1996, 26, 439–447. [Google Scholar] [CrossRef]
- Ke, X.; Bernal, S.A.; Provis, J.L. Controlling the Reaction Kinetics of Sodium Carbonate-Activated Slag Cements Using Calcined Layered Double Hydroxides. Cem. Concr. Res. 2016, 81, 24–37. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Reaction Kinetics, Gel Character and Strength of Ambient Temperature Cured Alkali Activated Slag-Fly Ash Blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef]
- Hubler, M.H.; Thomas, J.J.; Jennings, H.M. Influence of Nucleation Seeding on the Hydration Kinetics and Compressive Strength of Alkali Activated Slag Paste. Cem. Concr. Res. 2011, 41, 842–846. [Google Scholar] [CrossRef]
- Burciaga-Díaz, O.; Betancourt-Castillo, I. Characterization of Novel Blast-Furnace Slag Cement Pastes and Mortars Activated with a Reactive Mixture of MgO-NaOH. Cem. Concr. Res. 2018, 105, 54–63. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and Hydration Properties of Reactive MgO-Activated Ground Granulated Blastfurnace Slag Paste. Cem. Concr. Compos. 2015, 57, 8–16. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Abdollahzadeh, A.; Al-Tabbaa, A. Effects of Different Reactive MgOs on the Hydration of MgO-Activated GGBS Paste. J. Mater. Civ. Eng. 2015, 27, B4014001. [Google Scholar] [CrossRef]
- Dung, N.T.; Unluer, C. Influence of Nucleation Seeding on the Performance of Carbonated MgO Formulations. Cem. Concr. Compos. 2017, 83, 1–9. [Google Scholar] [CrossRef]
- Dung, N.T.; Unluer, C. Development of MgO Concrete with Enhanced Hydration and Carbonation Mechanisms. Cem. Concr. Res. 2018, 103, 160–169. [Google Scholar] [CrossRef]
- Dung, N.T.; Unluer, C. Performance of Reactive MgO Concrete under Increased CO2 Dissolution. Cem. Concr. Res. 2019, 118, 92–101. [Google Scholar] [CrossRef]
- Carrillo-Beltran, R.; Corpas-Iglesias, F.A.; Terrones-Saeta, J.M.; Bertoya-Sol, M. New Geopolymers from Industrial By-Products: Olive Biomass Fly Ash and Chamotte as Raw Materials. Constr. Build. Mater. 2021, 272, 121924. [Google Scholar] [CrossRef]
- Quarcioni, V.A.; Chotoli, F.F.; Coelho, A.C.V.; Cincotto, M.A. Indirect and Direct Chapelle’s Methods for the Determination of Lime Consumption in Pozzolanic Materials. Rev. IBRACON Estrut. Mater. 2015, 8, 1–7. [Google Scholar] [CrossRef]
- Berra, M.; Mangialardi, T.; Paolini, A.E. Reuse of Woody Biomass Fly Ash in Cement-Based Materials. Constr. Build. Mater. 2015, 76, 286–296. [Google Scholar] [CrossRef]
- Shi, C.; Roy, D.; Krivenko, P. Alkali-Activated Cements and Concretes, 1st ed.; Taylor & Francis: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Straka, P.; Havelcová, M. Polycyclic Aromatic Hydrocarbons and Other Organic Compounds in Ashes from Biomass Combustion. Acta Geodyn. Geomater. 2012, 9, 481–490. [Google Scholar]
- Roberts, A.F. A Review of Kinetics Data for the Pyrolysis of Wood and Related Substances. Combust. Flame 1970, 14, 261–272. [Google Scholar] [CrossRef]
- Sharypov, V.I.; Grishechko, L.I.; Tarasova, L.S.; Baryshnikov, S.V.; Celzard, A.; Kuznetsov, B.N. Investigation of Thermal Decomposition of Lignin Samples Isolated from Aspen Wood by Various Methods. J. Sib. Fed. Univ. Chem. 2011, 3, 221–232. [Google Scholar]
- Cabrera, M.; Galvin, A.P.; Agrela, F.; Carvajal, M.D.; Ayuso, J. Characterisation and Technical Feasibility of Using Biomass Bottom Ash for Civil Infrastructures. Constr. Build. Mater. 2014, 58, 234–244. [Google Scholar] [CrossRef]
- Maschowski, C.; Kruspan, P.; Garra, P.; Talib Arif, A.; Trouvé, G.; Gieré, R. Physicochemical and Mineralogical Characterization of Biomass Ash from Different Power Plants in the Upper Rhine Region. Fuel 2019, 258, 116020. [Google Scholar] [CrossRef]
- Abdalqader, A.F.; Jin, F.; Al-Tabbaa, A. Characterisation of Reactive Magnesia and Sodium Carbonate-Activated Fly Ash/Slag Paste Blends. Constr. Build. Mater. 2015, 93, 506–513. [Google Scholar] [CrossRef]
- Islam, A.; Alengaram, U.J.; Jumaat, M.Z.; Bashar, I.I. The Development of Compressive Strength of Ground Granulated Blast Furnace Slag-Palm Oil Fuel Ash-Fly Ash Based Geopolymer Mortar. Mater. Des. 2014, 56, 833–841. [Google Scholar] [CrossRef]
- Wallah, S.E.; Rangan, B.V. Low-Calcium Fly Ash-Based Geopolymer Concrete: Long-Term Properties. 2006. Available online: http://hdl.handle.net/20.500.11937/34322 (accessed on 6 May 2022).
- Askarian, M.; Tao, Z.; Samali, B.; Adam, G.; Shuaibu, R. Mix Composition and Characterisation of One-Part Geopolymers with Different Activators. Constr. Build. Mater. 2019, 225, 526–537. [Google Scholar] [CrossRef]
- Puertas, F.; Martínez-Ramírez, S.; Alonso, S.; Vázquez, T. Alkali-Activated Fly Ash/Slag Cements. Strength Behaviour and Hydration Products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Emdadi, Z.; Asim, N.; Amin, M.H.; Yarmo, M.A.; Maleki, A.; Azizi, M.; Sopian, K. Development of Green Geopolymer Using Agricultural and Industrialwaste Materials with High Water Absorbency. Appl. Sci. 2017, 7, 514. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J.; Shaikh, F.U.A. Synthesis of Heat and Ambient Cured One-Part Geopolymer Mixes with Different Grades of Sodium Silicate. Ceram. Int. 2015, 41, 5696–5704. [Google Scholar] [CrossRef]
- Perná, I.; Šupová, M.; Hanzlíček, T.; Špaldoňová, A. The Synthesis and Characterization of Geopolymers Based on Metakaolin and High LOI Straw Ash. Constr. Build. Mater. 2019, 228, 116765. [Google Scholar] [CrossRef]
- Košnář, Z.; Mercl, F.; Perná, I.; Tlustoš, P. Investigation of Polycyclic Aromatic Hydrocarbon Content in Fly Ash and Bottom Ash of Biomass Incineration Plants in Relation to the Operating Temperature and Unburned Carbon Content. Sci. Total Environ. 2016, 563–564, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.E.; Monteiro, P.J.M.; Jun, S.S.; Choi, S.; Clark, S.M. The Evolution of Strength and Crystalline Phases for Alkali-Activated Ground Blast Furnace Slag and Fly Ash-Based Geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]
- Troëdec, M.L.; Dalmay, P.; Patapy, C.; Peyratout, C.; Smith, A.; Chotard, T. Mechanical Properties of Hemp-lime Reinforced Mortars: Influence of the Chemical Treatment of Fibers. J. Compos. Mater. 2011, 45, 2347–2357. [Google Scholar] [CrossRef]
- Johnson, D.B.; Robb, W.A. Gaylussite: Thermal Properties by Simultaneous Thermal Analysis. Am. Mineral. 1973, 58, 778–784. [Google Scholar]
- Siriwardane, R.V.; Poston, J.A., Jr.; Robinson, C.; Simonyi, T. Effect of Additives on Decomposition of Sodium Carbonate: Precombustion CO2 Capture Sorbent Regeneration. Energy Fuels 2011, 25, 1284–1293. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, H.G. Thermal and Carbothermic Decomposition of Na2CO3 and Li2CO3. Metall. Mater. Trans. B 2001, 32, 17–24. [Google Scholar] [CrossRef]



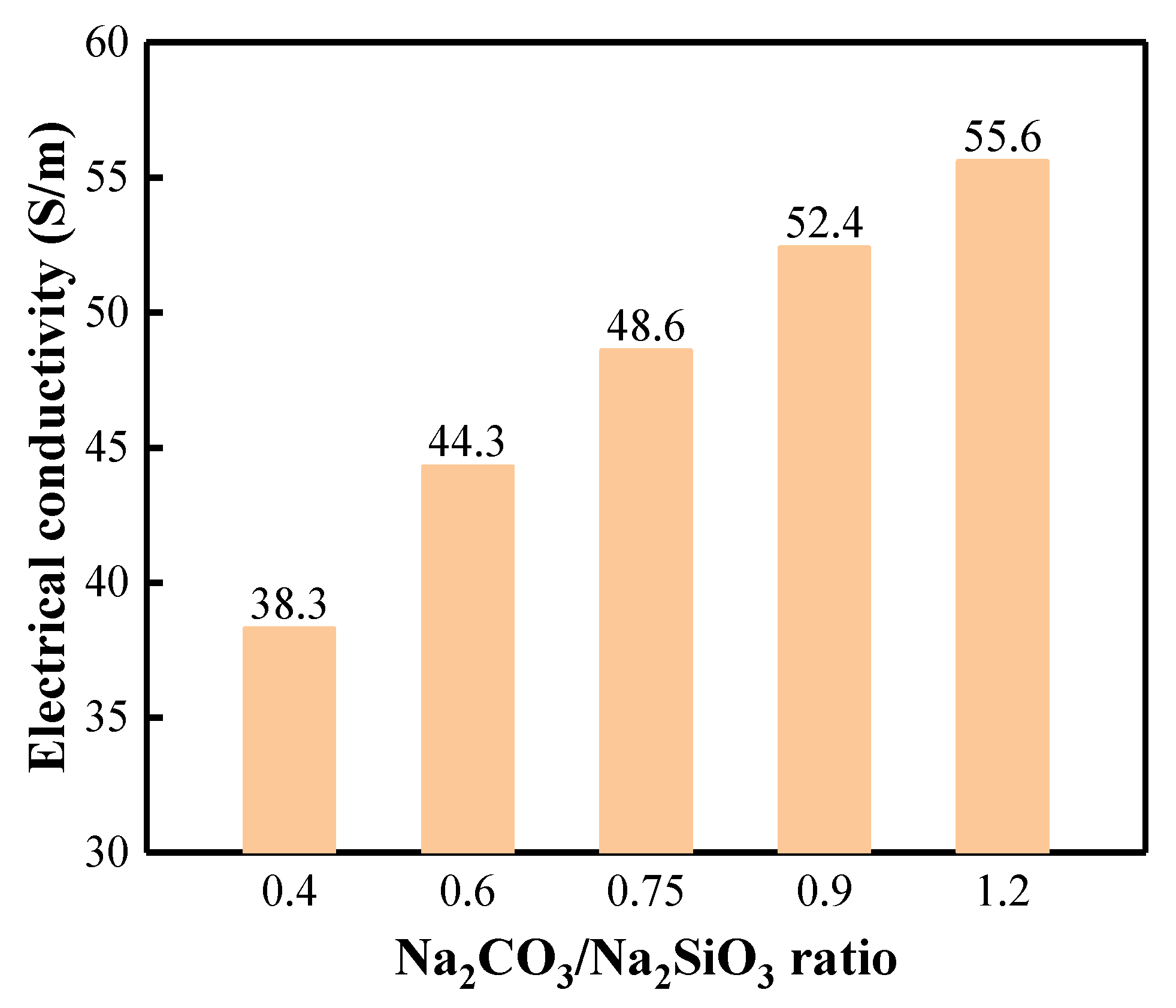
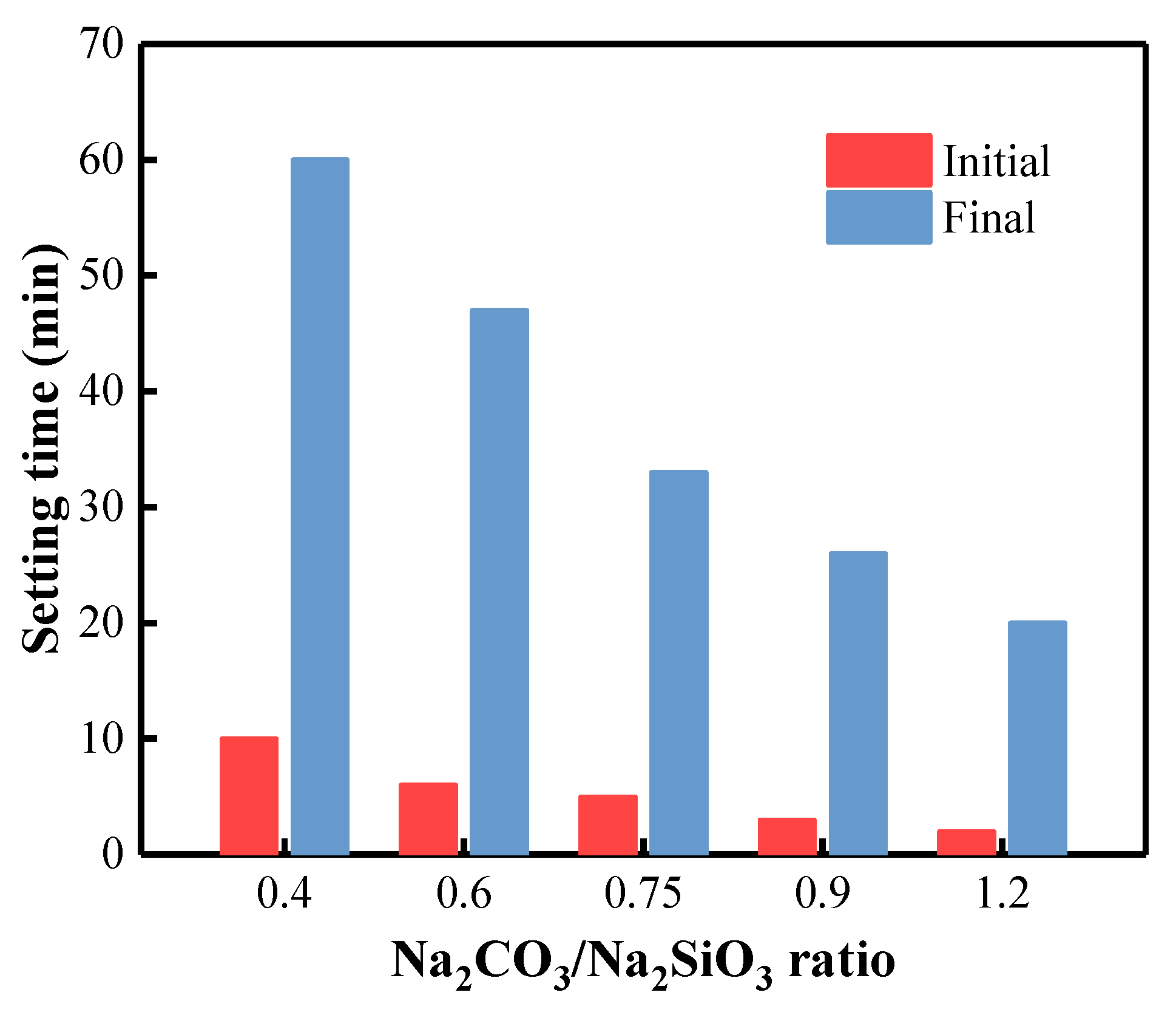




| Element | Concentration (%) | Compound Formula | Concentration (%) |
|---|---|---|---|
| C | 20.40 | C | 20.40 |
| O | 33.96 | O | 5.992 |
| Na | 0.192 | Na2O | 0.259 |
| Mg | 2.155 | MgO | 3.574 |
| Al | 1.390 | Al2O3 | 2.627 |
| Si | 10.71 | SiO2 | 22.91 |
| P | 1.182 | P2O5 | 2.707 |
| S | 0.354 | SO3 | 0.883 |
| Cl | 0.098 | Cl | 0.098 |
| K | 3.324 | K2O | 4.003 |
| Ca | 22.51 | CaO | 31.50 |
| Ti | 0.127 | TiO2 | 0.212 |
| Cr | 0.011 | Cr2O3 | 0.016 |
| Mn | 1.602 | MnO | 2.068 |
| Fe | 1.624 | Fe2O3 | 2.322 |
| Ni | 0.010 | NiO | 0.013 |
| Cu | 0.013 | CuO | 0.017 |
| Zn | 0.080 | ZnO | 0.100 |
| Rb | 0.016 | Rb2O | 0.017 |
| Sr | 0.077 | SrO | 0.091 |
| Y | 0.015 | Y2O3 | 0.019 |
| Ba | 0.154 | BaO | 0.172 |
| Group | Sample | Na2CO3 (wt% of BFA) | Na2SiO3 (wt% of BFA) | Na2CO3/Na2SiO3 Solute Ratio | Na2CO3/Na2SiO3 Solution Ratio | Curing Temperature (°C) |
|---|---|---|---|---|---|---|
| 1 | CS-0.40-40 | 3.00 | 7.50 | 0.40 | 0.67 | 40 |
| CS-0.60-40 | 3.75 | 6.25 | 0.60 | 1.00 | 40 | |
| CS-0.75-40 | 4.17 | 5.56 | 0.75 | 1.25 | 40 | |
| CS-0.90-40 | 4.50 | 5.00 | 0.90 | 1.50 | 40 | |
| CS-1.20-40 | 5.00 | 4.15 | 1.20 | 2.00 | 40 | |
| 2 | CS-0.40-20 | 3.00 | 7.50 | 0.40 | 0.67 | 20 |
| CS-0.60-20 | 3.75 | 6.25 | 0.60 | 1.00 | 20 | |
| CS-0.75-20 | 4.17 | 5.56 | 0.75 | 1.25 | 20 | |
| CS-0.90-20 | 4.50 | 5.00 | 0.90 | 1.50 | 20 | |
| CS-1.20-20 | 5.00 | 4.15 | 1.20 | 2.00 | 20 |
| Activator Solution | pH | EC, S/m |
|---|---|---|
| Na2SiO3 | 11.90 | 31.8 |
| 10% Na2CO3 | 11.36 | 71.4 |
| 20% Na2CO3 | 11.38 | 85.9 |
| 30% Na2CO3 | 11.38 | 79.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Pundienė, I.; Pranckevičienė, J.; Kligys, M. Effects of Na2CO3/Na2SiO3 Ratio and Curing Temperature on the Structure Formation of Alkali-Activated High-Carbon Biomass Fly Ash Pastes. Materials 2022, 15, 8354. https://doi.org/10.3390/ma15238354
Zhu C, Pundienė I, Pranckevičienė J, Kligys M. Effects of Na2CO3/Na2SiO3 Ratio and Curing Temperature on the Structure Formation of Alkali-Activated High-Carbon Biomass Fly Ash Pastes. Materials. 2022; 15(23):8354. https://doi.org/10.3390/ma15238354
Chicago/Turabian StyleZhu, Chengjie, Ina Pundienė, Jolanta Pranckevičienė, and Modestas Kligys. 2022. "Effects of Na2CO3/Na2SiO3 Ratio and Curing Temperature on the Structure Formation of Alkali-Activated High-Carbon Biomass Fly Ash Pastes" Materials 15, no. 23: 8354. https://doi.org/10.3390/ma15238354
APA StyleZhu, C., Pundienė, I., Pranckevičienė, J., & Kligys, M. (2022). Effects of Na2CO3/Na2SiO3 Ratio and Curing Temperature on the Structure Formation of Alkali-Activated High-Carbon Biomass Fly Ash Pastes. Materials, 15(23), 8354. https://doi.org/10.3390/ma15238354






