Near Infrared Reflection and Hydrophobic Properties of Composite Coatings Prepared from Hollow Glass Microspheres Coated with Needle-Shaped Rutile Shell
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HGM Pretreatment
2.3. Preparation of Rutile-Coated HGM
2.4. Preparation of Reflective Composite Coating
2.5. Characterization
3. Results and Discussion
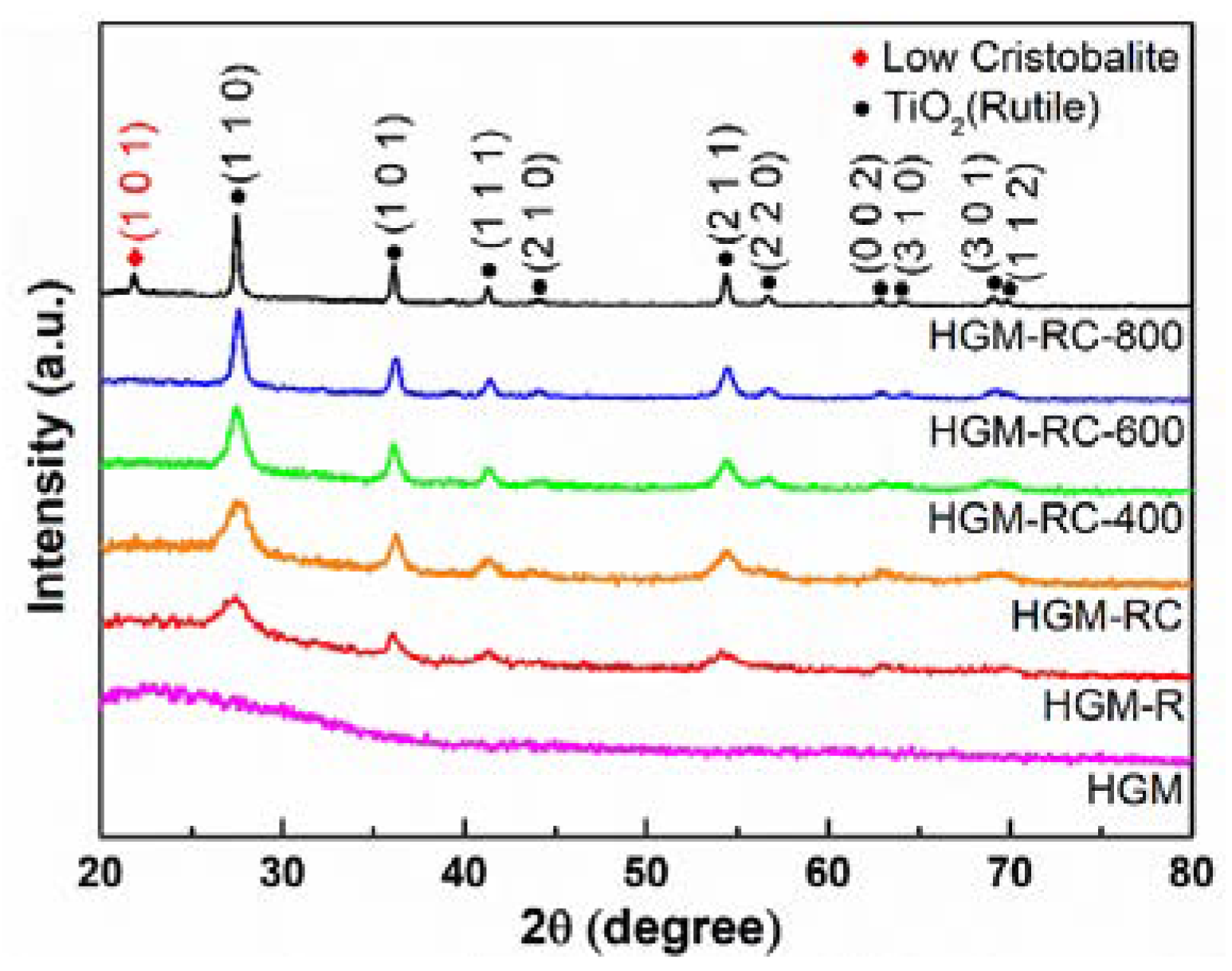
3.1. Phase Composition of the As-Prepared Samples
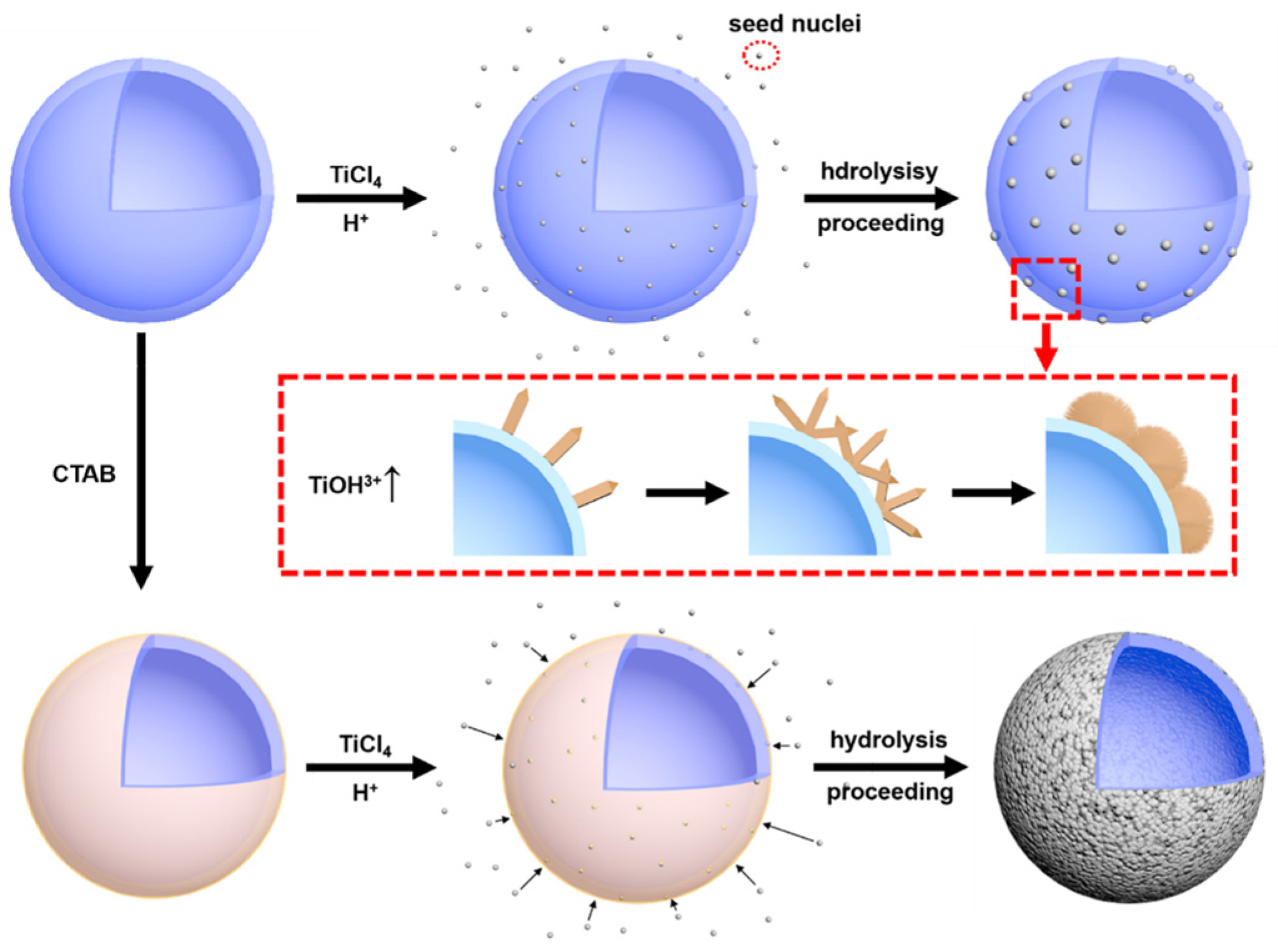
3.2. Micro-Morphology Analysis and Growth Mechanism of Rutile-Coated HGM
3.3. Formation Mechanism of Rutile-TiO2-Coated HGM
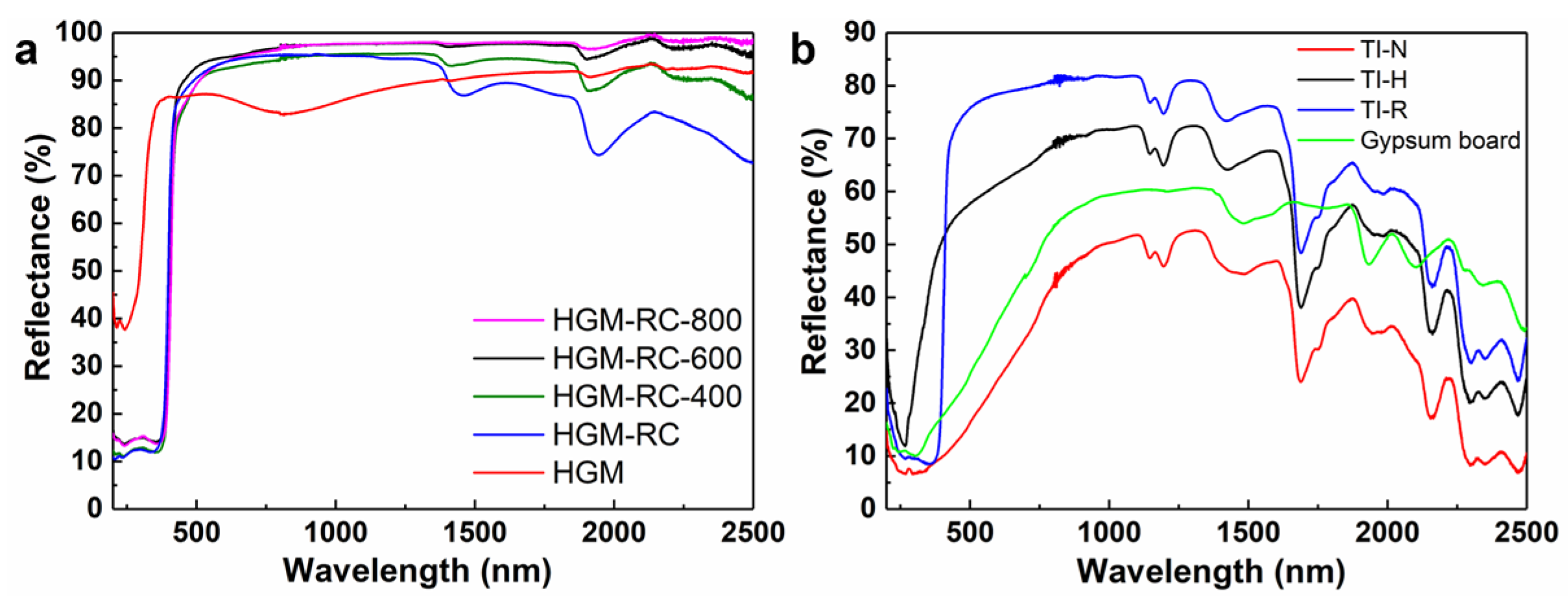
3.4. Near Infrared Reflective Properties of Rutile-TiO2-Coated HGM
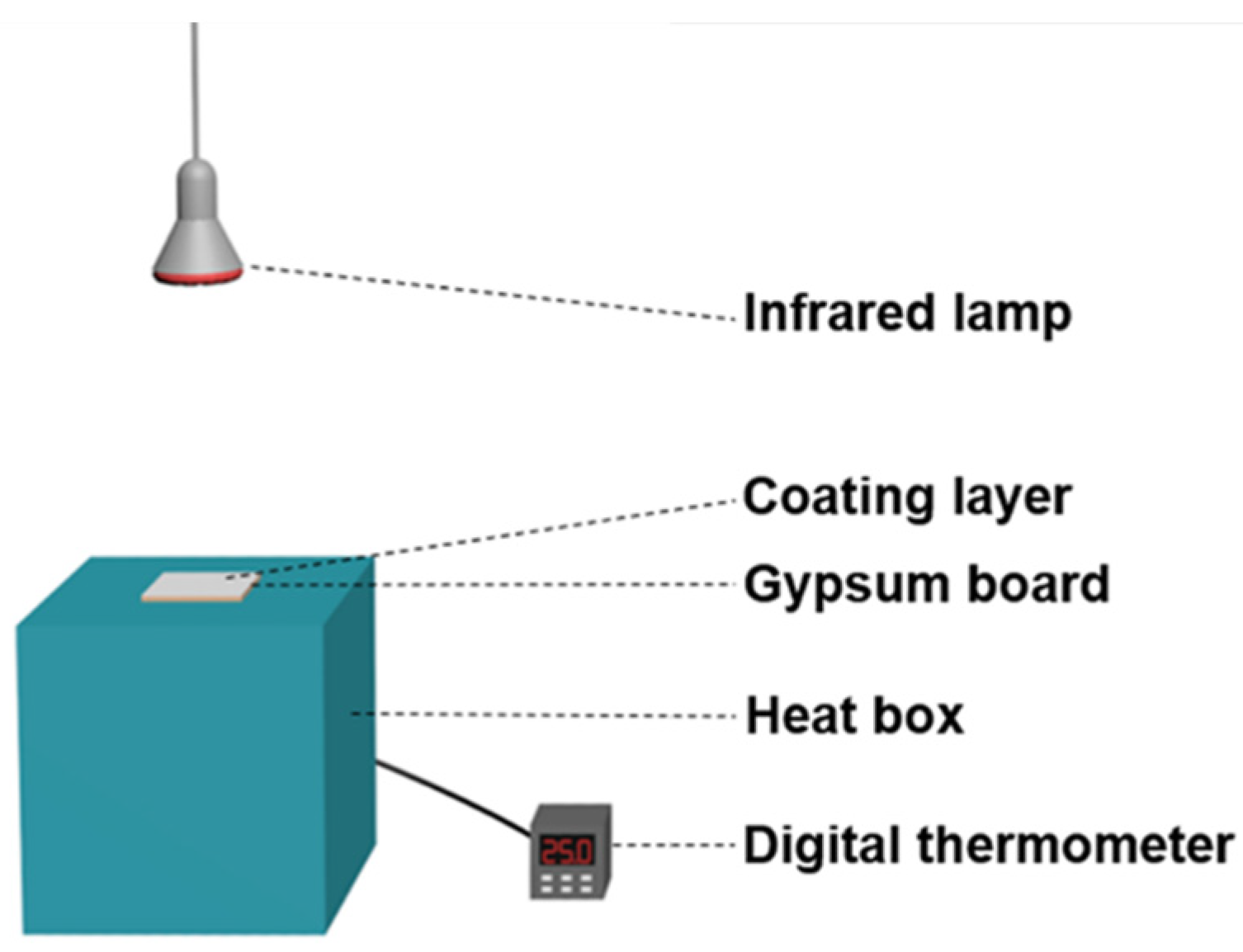
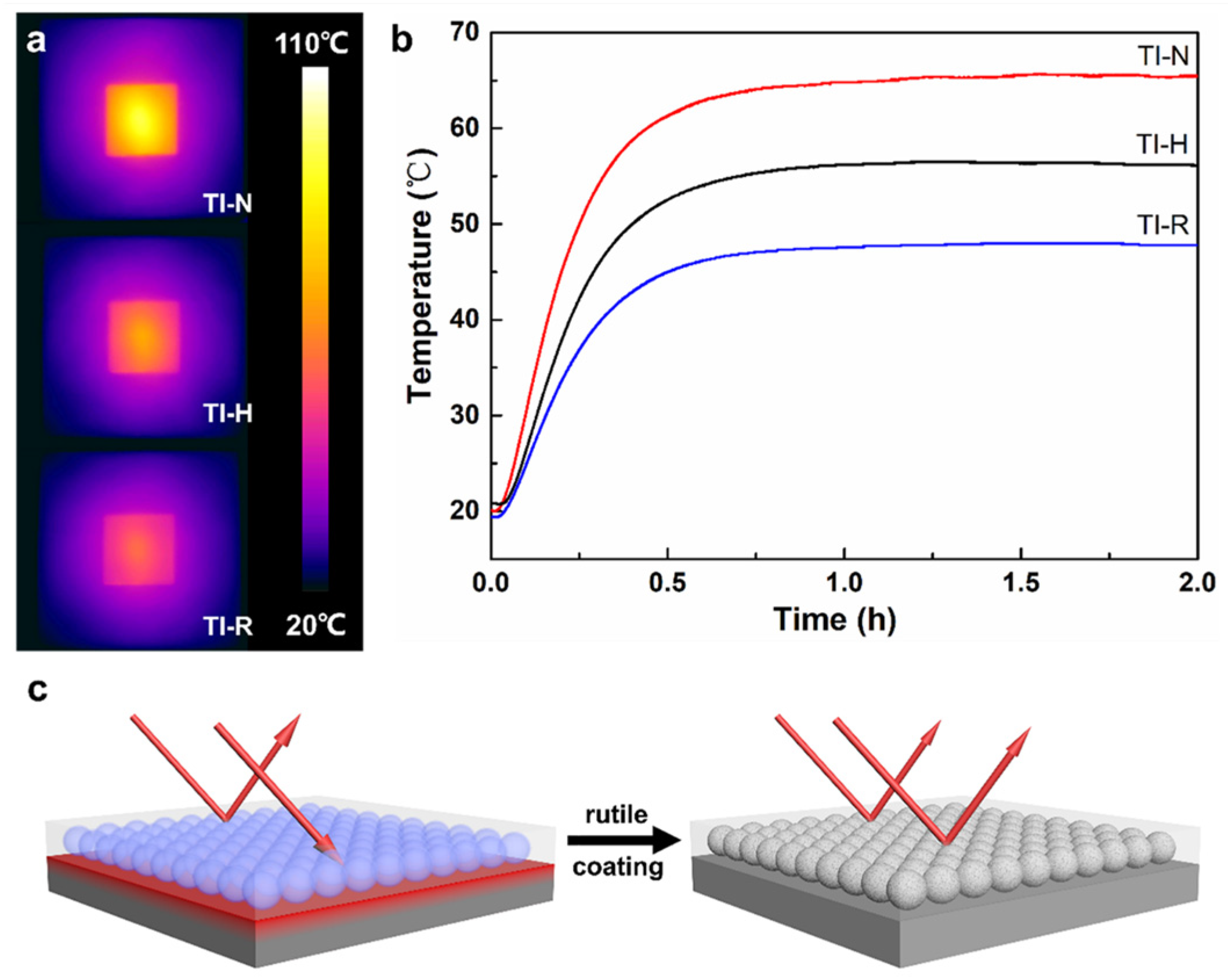
3.5. Thermal Insulation Properties of the Reflective Coatings
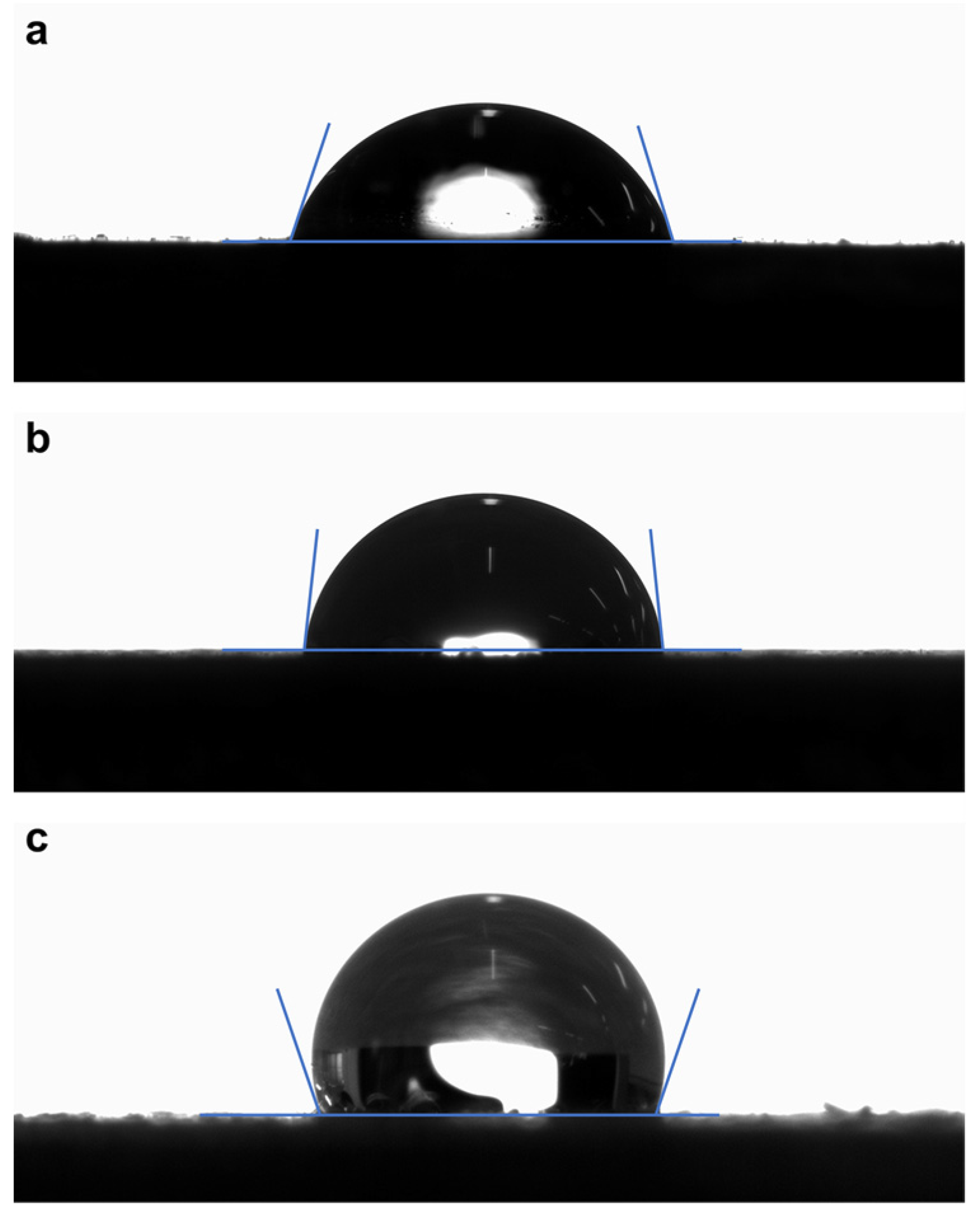
3.6. Surface Hydrophobic Properties of Coatings of the Reflective Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rizwan, A.M.; Dennis, L.Y.C.; Liu, C. A review on the generation, determination and mitigation of Urban Heat Island. J. Environ. Sci. 2008, 20, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Santamouris, M.; Ding, L.; Fiorito, F.; Oldfield, P.; Osmond, P.; Paolini, R.; Prasad, D.; Synnefa, A. Passive and active cooling for the outdoor built environment—Analysis and assessment of the cooling potential of mitigation technologies using performance data from 220 large scale projects. Sol. Energy 2017, 154, 14–33. [Google Scholar] [CrossRef]
- Tian, Q.; Yao, W.; Wu, W.; Jiang, C. NIR light-activated upconversion semiconductor photocatalysts. Nanoscale Horiz. 2019, 4, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Bing, L.; Jing, Y.; An, Z.; Zhang, J. Effect of microstructure and physical parameters of hollow glass microsphere on insulation performance. Mater. Lett. 2011, 65, 1992–1994. [Google Scholar]
- Yung, K.C.; Zhu, B.L.; Yue, T.M.; Xie, C.S. Preparation and properties of hollow glass microsphere-filled epoxy-matrix composites. Compos. Sci. Technol. 2009, 69, 260–264. [Google Scholar] [CrossRef]
- Nan, X.; Dai, J.; Zhu, Z.; Xiang, H.; Wu, P. Synthesis and characterization of hollow glass–ceramics microspheres. Ceram. Int. 2011, 37, 2663–2667. [Google Scholar]
- Hu, Y.; Mei, R.; An, Z.; Zhang, J. Silicon rubber/hollow glass microsphere composites: Influence of broken hollow glass microsphere on mechanical and thermal insulation property. Compos. Sci. Technol. 2013, 79, 64–69. [Google Scholar] [CrossRef]
- Hu, Y.; Zhong, H.; Wang, Y.; Yang, H. Development of an Antimony Doped Tin Oxide/TiO2 Double-Layers Coated HGM: A High Reflectivity and Low Transmittance Building Thermal Conservation Material. Energy Procedia 2017, 105, 4128–4132. [Google Scholar]
- Yuan, J.; An, Z.; Li, B.; Zhang, J. Facile aqueous synthesis and thermal insulating properties of low-density glass/TiO2 core/shell composite hollow spheres. Particuology 2012, 10, 475–479. [Google Scholar] [CrossRef]
- Wong, A.; Daoud, W.A.; Liang, H.-h.; Szeto, Y.S. Application of rutile and anatase onto cotton fabric and their effect on the NIR reflection/surface temperature of the fabric. Sol. Energy Mater. Sol. Cells 2015, 134, 425–437. [Google Scholar] [CrossRef]
- Long, J.; Jiang, C.; Zhu, J.; Song, Q.; Hu, J. Controlled TiO2 coating on hollow glass microspheres and their reflective thermal insulation properties. Particuology 2020, 49, 33–39. [Google Scholar] [CrossRef]
- Wu, C.; Wang, W.; Ji, H. Preparation and Properties of TiO2-Coated Hollow Glass Microspheres as Thermal Insulation Materials for Energy-Saving Buildings. Trans. Tianjin Univ. 2020, 26, 283–291. [Google Scholar] [CrossRef]
- Srisittipokakun, N.; Kaewkhao, J.; Chewpraditkul, W.; Limsuwan, P. Comparative study of optical and spectroscopic properties of lead and bismuth on borosilicate glasses. Procedia Eng. 2012, 32, 699–705. [Google Scholar] [CrossRef]
- Yan, H.; Wang, Y.; An, Z.; Zhang, J.; Yang, H. The super-hydrophobic IR-reflectivity TiO2 coated hollow glass microspheres synthesized by soft-chemistry method. J. Phys. Chem. Solids. 2016, 98, 43–49. [Google Scholar]
- Gao, Q.; Wu, X.; Xia, Z.; Fan, Y. Coating mechanism and near-infrared reflectance property of hollow fly ash bead/TiO2 composite pigment. Powder Technol. 2017, 305, 433–439. [Google Scholar] [CrossRef]
- Song, G.; Liang, J.; Liu, F.; Peng, T.; Rao, G. Preparation and phase transformation of anatase–rutile crystals in metal doped TiO2/muscovite nanocomposites. Thin Solid Film. 2005, 491, 110–116. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, P.; Jiang, P.; Guo, Z.; Liu, W.; Lu, Q.; Cao, W. The formation mechanism of TiO2 polymorphs under hydrothermal conditions based on the structural evolution of [Ti(OH)h(H2O)6−h]4−h monomers. J. Mater. Chem. C. 2019, 7, 5764–5771. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, X.; Ma, Y.; Li, D.; Fan, Y.; Du, C. Effect of Sn4+ doping on the photoactivity inhibition and near infrared reflectance property of mica-titania pigments for a solar reflective coating. Ceram. Int. 2016, 42, 17148–17153. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, F.; Hao, J.; Liu, W. Stable biomimetic super-hydrophobic engineering materials. J. Am. Chem. Soc. 2005, 127, 15670–15671. [Google Scholar] [CrossRef]
- Lee, J.H.; Yang, Y.S. Effect of HCl concentration and reaction time on the change in the crystalline state of TiO2 prepared from aqueous TiCl4 solution by precipitation. J. Eur. Ceram. Soc. 2005, 25, 3573–3578. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Jia, Z. Morphological control and photodegradation behavior of rutile TiO2 prepared by a low-temperature process. Mater. Lett. 2006, 60, 1753–1757. [Google Scholar] [CrossRef]
- Wang, M.Q.; Yan, J.; Cui, H.P.; Du, S.G. Low temperature preparation and characterization of TiO2 nanoparticles coated glass beads by heterogeneous nucleation method. Mater. Charact. 2013, 76, 39–47. [Google Scholar] [CrossRef]
- Byun, H.Y.; Vittal, R.; Kim, D.Y.; Kim, K.J. Beneficial role of cetyltrimethylammonium bromide in the enhancement of photovoltaic properties of dye-sensitized rutile TiO2 solar cells. Langmuir 2004, 20, 6853–6857. [Google Scholar] [CrossRef] [PubMed]
- ASTM G173-03; Standard Tables for Reference Solar Irradiance: Direct Normal and Hemispherical on 37° Tilted Surface. ASTM: West Conshohocken, PA, USA, 2020.
- Pfaff, G.; Reynders, P. Angle-dependent optical effects deriving from submicron structures of films and pigments. Chem. Rev. 1999, 99, 1963–1982. [Google Scholar] [CrossRef]
- Kim, D.J.; Hahn, S.H.; Oh, S.H.; Kim, E.J. Influence of calcination temperature on structural and optical properties of TiO2 thin films prepared by sol–gel dip coating. Mater. Lett. 2002, 57, 355–360. [Google Scholar] [CrossRef]
- Qi, Y.L.; Chen, S.J.; Sun, H.X.; Zhang, J. A multifunctional cool material with contamination-resistant and anti-icing characters based on the application of fluorinated TiO2. Sol. Energ. Mat. Sol. C. 2019, 201, 110091. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Q.; Xiong, S.; Wu, X.; Kuang, J.; Liu, W.; Cao, W. Near Infrared Reflection and Hydrophobic Properties of Composite Coatings Prepared from Hollow Glass Microspheres Coated with Needle-Shaped Rutile Shell. Materials 2022, 15, 8310. https://doi.org/10.3390/ma15238310
Zheng Q, Xiong S, Wu X, Kuang J, Liu W, Cao W. Near Infrared Reflection and Hydrophobic Properties of Composite Coatings Prepared from Hollow Glass Microspheres Coated with Needle-Shaped Rutile Shell. Materials. 2022; 15(23):8310. https://doi.org/10.3390/ma15238310
Chicago/Turabian StyleZheng, Qianfang, Shanxia Xiong, Xiaowei Wu, Jianlei Kuang, Wenxiu Liu, and Wenbin Cao. 2022. "Near Infrared Reflection and Hydrophobic Properties of Composite Coatings Prepared from Hollow Glass Microspheres Coated with Needle-Shaped Rutile Shell" Materials 15, no. 23: 8310. https://doi.org/10.3390/ma15238310
APA StyleZheng, Q., Xiong, S., Wu, X., Kuang, J., Liu, W., & Cao, W. (2022). Near Infrared Reflection and Hydrophobic Properties of Composite Coatings Prepared from Hollow Glass Microspheres Coated with Needle-Shaped Rutile Shell. Materials, 15(23), 8310. https://doi.org/10.3390/ma15238310




