1. Introduction
Fresh produce is a significant part of a healthy diet. However, foodborne illness outbreaks have occurred frequently and are increasingly recognized worldwide. This reflects the growing concern regarding safety and hygiene problems in fresh produce. A large number of foodborne outbreaks derive from fresh fruits and vegetables. In 2019, many outbreaks of foodborne illness due to
Salmonella,
Escherichia coli O157:H7
and Listeria monocytogenes were attributed to the consumption of fruits, seeded vegetables, vegetable row crops, and sprouts in the United States [
1].
An important bacterial pathogen that can contaminate fresh produce is
L. monocytogenes, which can lead to listeriosis. Approximately 1600 people succumb to listeriosis each year, and 260 deaths may occur, based on an estimated report from the U.S. Centers for Disease Control and Prevention [
2]. The hospitalization rate of listeriosis can reach 94%. Pregnant women and newborns are high-risk groups for listeriosis [
3]. Economically, the annual cost of
L. monocytogenes control can reach approximately 2.4 billion USD [
4].
L. monocytogenes can be found on many fresh fruits and vegetables. Additionally,
L. monocytogenes can be found in a variety of environments, including soil, water, other food products, humans, and animals [
5]. Fresh produce such as raw vegetables can be contaminated by
L. monocytogenes from manure used as soil amendments. Additionally, increased
L. monocytogenes contamination is detected in fields with more recently cultivated soil and more recent worker activity than the fields with less recent practices [
6].
The U.S. Food and Drug Administration (FDA) states that
L. monocytogenes has been found in raw, unpasteurized milk and cheeses, ice cream, raw or processed vegetables, raw or processed fruits, raw or undercooked poultry, hotdogs, sausages, deli meats, and raw or smoked fish, and even in raw pet food [
3]. Meats and dairy products can also contain
L. monocytogenes because animals may carry the organism without appearing ill or environmental contamination can occur during meat processing [
7]. This pathogen has been found on the surface of some raw fruits or vegetables and can also become internalized. Bardsley et al. (2019) reported that, on frozen whole and sliced cucumbers,
L. monocytogenes can survive for more than 120 d [
8]. Thus, it is necessary to evaluate cucumber conditions at each step of the supply chain to reduce potential
Listeria contamination. Zhu et al. (2017) summarized several studies that reported on the prevalence rates of
L. monocytogenes in fresh produce, including 21.9% for cucumber [
9]. If
L. monocytogenes contaminates the surface of fresh avocados, then they may enter the avocado pulp from the stem or stem scar. When exposed to the hydrocooled water with
L. monocytogenes, the pathogen inside the edible parts of avocados can range from 5.90 to 7.19 log CFU/g due to the bacteria’s transmission from the bottom end to the inside of fresh whole avocados [
10].
To reduce potential
L. monocytogenes contamination, the FDA advises that all fruits and vegetables should be washed under running water just before eating, cutting, cooking and peeling. For firm produce such as melons and cucumbers, consumers may need to scrub the surfaces using a clean brush. In addition to using water to remove
L. monocytogenes, the FDA recommends keeping refrigerated foods under 40°F to reduce risk of
Listeria infection by slowing down or halting the growth of
L. monocytogenes. Other prevention measures include cleaning refrigerators frequently to remove food spills, which may harbor
L. monocytogenes, and washing hands and kitchen surfaces regularly to avoid the cross-contamination of
L. monocytogenes among different food impact surfaces, such as stainless-steel surfaces used by food-processing industries. In food-processing factories, cleaners can be effectively applied to remove this pathogen on etched stainless-steel surfaces [
11].
During the last three decades, cavitation has become a more common treatment methodology to remove bacteria from object surfaces. The definition of cavitation is the formation of vapor cavities, such as bubbles in a liquid, caused by forces acting on the liquid. Cavitation will occur when a rapid change in pressure acts on a liquid to form cavities [
12]. Many researchers have studied the cavitation phenomenon and examined the effects of cavitation on industrial systems. Franc and Michel (2010) discuss the history of the study of bubble dynamics, including cavitation [
13].
Cavitation and bubble dynamics have a wide range of practical applications in many fields, including hydraulic, mechanical and naval engineering, oil exploration, clinical medicine, sonochemistry, and ultrasonic cleaning for electrical and medical microdevices [
14]. In wastewater, hydrodynamic cavitation can successfully remove pharmaceuticals, cyanobacteria, algae,
Legionella and Rotavirus in an energy-efficient way [
15]. The application of physical cavitation bubbles was found to remove and inactivate
Listeria on the surface of fresh Roma tomatoes and cantaloupes. Lee et al., (2018) reported that
L. monocytogenes on the surface of fresh Roma tomatoes and cantaloupe was detached and inactivated using a physical continuous bubble stream. The efficacy of the cavitation bubble stream (14 L air/min for 1 min) was 2.89 and 2.63 log pathogen reductions for Roma tomatoes and cantaloupe, respectively [
12].
The goal of this research was to evaluate the removal of a biological contaminant from a food or food-contact surface by the shear forces generated from the impact of air bubbles. Therefore, a low-intensity bubble process, with air being injected into water, was developed to determine the efficacy of microbubbles for detaching loosely or firmly attached cells of L. monocytogenes from stainless steel (a food-contact surface) and the surface of fresh cucumber and avocado. Moreover, cross-contamination from inoculated to uninoculated cucumbers and avocados was evaluated to determine the level of cross-contamination that may occur. Finally, a quality evaluation was performed on cucumber and avocado after bubble treatment to compare changes in naturally occurring bacterial populations that may lead to microbial spoilage during storage.
2. Materials and Methods
2.1. Inoculation of Test Surfaces with L. monocytogenes
L. monocytogenes (strain LCDC, serotype 4b) was transferred from frozen (−80 °C) storage to Tryptic Soy Broth (TSB) and incubated at 35 °C for 24 h. Then, the culture was transferred to fresh TSB and incubated at 35 °C for 24 h. This culture was subsequently used to inoculate stainless steel, cucumbers and avocados. Culture identity and purity was confirmed by colony appearance on Rapid L’mono agar (Bio-Rad Laboratories, Richmond, CA, USA) and with the Microgen Listeria-ID biochemical identification kit (Microbiology International, Frederick, MD, USA). Additionally, this culture was confirmed to be able to adhere to the test materials and could be recovered 24 and 48 h after inoculation at higher concentrations than three other test strains of L. monocytogenes.
Stainless-steel sheet (type 304, #2 Finish; Speedy Metals, Milwaukee, WI, USA) was cut into 1 × 2-inch coupons. Slicing cucumbers (Cucumis sativas) and avocados (Persea americana cultivar Hass) were purchased from local supermarkets. The degree of maturity for all cucumbers and all avocados was likely similar, since growers and packers had determined they were suitable for distribution. These markets generally sell these produce items within three days after they are made available to the consumer. The Hass avocado is the most common cultivar of commercial avocados. Before the bacterial inoculation onto cucumbers, wax was removed from the cucumber surfaces by washing them with peptone water and drying them gently with paper wipes. Additionally, cucumbers and avocados were sprayed with 70% ethanol and then wiped and air-dried to reduce background microflora.
Stainless-steel coupons were spot inoculated with 0.1 mL of a L. monocytogenes culture (~2 × 108 CFU/mL). The spots of inoculation were marked before inoculation. Inoculums were allowed to dry for 1, 24, or 48 h at room temperature (22 °C) in a sealed box with two small beakers of a sodium chloride solution to maintain the humidity from 50% to 60%. Cucumbers and avocados were also spot-inoculated with 0.1 mL of a L. monocytogenes culture (~2 × 108 CFU/mL) and stored at 10 °C. Inoculums were allowed to dry for 1, 24, or 48 h.
2.2. Bubble Treatments for Stainless Steel
An air compressor (Campbell Hausfeld, Cincinnati, OH, USA, 120 V, 60 Hz, 2 A, 1 gallon tank, maximum rated 110 psi) was used to deliver air through a bubble diffuser (Model MBD75 (Diffuser Area 6-1/8″ × 1-1/8″, Range up to 0.75 LPM, Flow Rate @ 50 PSI 2.2 LPM, Gas Inlet Connection 1/4″ Hose Barb); Pentair, Golden Hills, MN, USA) submerged in the bottom of a water tank. The manufacturer states that this diffuser produces bubbles with an approximately 100–500 micron diameter in water, hereafter referred to as microbubbles. A Masterflex flow meter (Cole-Parmer, Vernon Hills, IL, USA) was used to regulate air flow to 1.0 L/min.
After stainless-steel coupons were dried (1, 24, or 48 h), but before the application of bubble treatments, the inoculated steel coupons were rinsed with 10 mL peptone water to remove loosely attached cells. These rinsates were collected and further analyzed as described in
Section 2.4. Stainless steel coupons were individually placed into a 13 L plastic tank containing 4.5 L distilled water. Plastic forceps were used to hold the rectangle stainless-steel coupon when applying bubbles to the inoculated surface.
Stainless-steel coupons were treated for 0, 1, 2, 5 or 10 min using the bubble diffuser with a 1.0 L/min air flow rate. Three steel samples were tested for each combination of inoculum drying time and bubble treatment time. After treatment with a stream of heavy bubbles, stainless-steel coupons were collected in a sterile bag with peptone water and sonicated (Aquasonic Ultrasonic Cleaner, Volts: 117/120, 50/60 Hz) for 2 min at room temperature. Solutions were then diluted with peptone water and plated onto Oxford agar (Neogen, Lansing, MI, USA) plates. Plates were incubated at 35 °C for 48 h. Three replications of the above process were conducted for a total of 108 samples.
2.3. Bubble Treatment of Cucumber and Avocado Surfaces
After inoculated cucumbers and avocados were dried (1, 24, or 48 h), but before the application of bubble treatments, they were rinsed with 10 mL peptone water to remove loosely attached cells. These rinses were collected and further analyzed as described in
Section 2.4. Rinsed cucumbers and avocados were individually placed into a 13 L plastic tank containing 4.5 L distilled water. Two elastic rubber wires on the top the opposite edges of the tank were used to hold the cucumbers in place over the bubble stream. A compressed air supply was used to deliver 1.0 L/min of air through a bubble diffuser as described in
Section 2.2. A stream of bubbles (~0.1–0.5 mm diameter) was applied for either 0, 1, 2, 5 or 10 min. Three cucumbers and three avocados were tested for each combination of inoculum drying time (3) and bubble treatment time (5) for each of the three replications.
2.4. Recovery of L. monocytogenes from Inoculated Surfaces
Before the application of bubble treatments, inoculated steel coupons, cucumbers and avocados were rinsed with 10 mL distilled water to remove loosely attached cells. The rinses were collected, diluted with peptone water and plated onto Oxford agar plates. Plates were incubated at 35 °C for 48 h and enumerated. Then, the rinsed test materials were treated with a bubble application for 0, 1, 2, 5, or 10 min. Firmly attached cells were removed from the steel coupons, cucumbers and avocados by placing the sample in a sterile bag or cup with added peptone water. Containers were then sonicated for 2 min at room temperature (22 °C). Solutions were then diluted and plated onto Oxford agar plates and incubated at 35 °C for 48 h. Finally, the cell counts of the solutions after sonication of bubble-treated samples were compared to the cell counts of the solutions after sonication where samples were not treated with bubbles (0 min treatment).
2.5. Cross-Contamination from Inoculated to Un-Inoculated Produce
To simulate the cross-contamination of produce with L. monocytogenes, one inoculated cucumber and one un-inoculated cucumber were added to a water tank. All cucumbers were wiped to remove wax and reduce background microflora, as described previously. Inoculated cucumbers received 0.1 mL of a L. monocytogenes (LCDC) culture and allowed to dry for 1, 24 or 48 h before they were placed into a water tank with one un-inoculated cucumber. A heavy bubble stream from the bubble diffuser was applied for 2 or 10 min with an airflow rate of 1.0 L/min. As a control, pairs of inoculated and uninoculated cucumbers were placed in the water tank for 1 min, but without a bubble application. Then, each sample cucumber was placed into separate sterile bags, and L. monocytogenes were recovered from the sample surface, as described previously. Oxford agar plates were incubated at 35 °C for 48 h. A similar inoculation, treatment and recovery protocol was carried out for avocados. Three sample pairs of cucumbers and avocados were tested for each combination of inoculum drying time and bubble treatment time. These experiments were replicated three times for both cucumbers and avocados. The proportion of cells that were transferred to the uninoculated samples was determined.
2.6. Microbial Quality Evaluation of Treated Cucumbers and Avocados after Refrigerated Storage (10 °C)
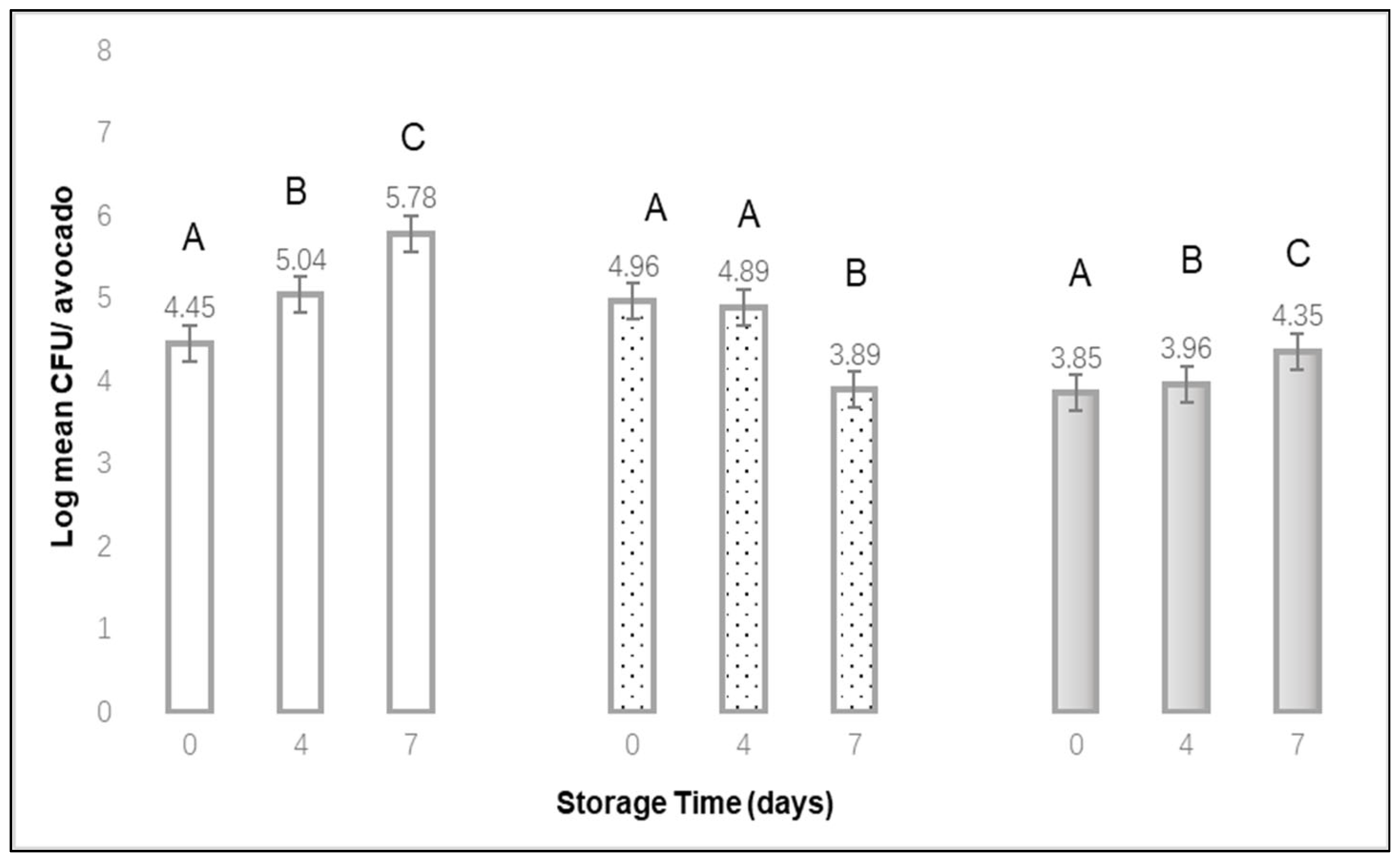
Fresh cucumbers and avocados were stored in a refrigerator at 10 °C for up to 7 d. After storage for 0, 1, 4, or 7 d, three cucumbers and three avocados were individually placed in a sterile bag, diluted with peptone water, and solutions were plated onto Tryptic Soy Agar plates. Additional cucumbers and avocados were treated to a heavy bubble stream, as described previously, for 2 or 10 min. These samples were stored for either 0, 1, 4 or 7 d at 10 °C. After storage, three cucumbers and three avocados (from each storage time) were individually placed in a sterile bag, diluted with peptone water, and solutions were plated onto Tryptic Soy Agar plates. All plates were incubated at 35 °C for 24 h, then enumerated. The above process was repeated for 3 replications.
2.7. Data Analysis
L. monocytogenes cell counts recovered from fresh produce and stainless-steel coupons were compared to the cell counts of original inoculation on produce and stainless steel to determine the pathogen detachment from the surface of fresh produce and stainless steel after microbubble treatment. A one-way analysis of variance (ANOVA) was used to determine significant differences between means for each variable tested at a statistical significance of α = 0.05. All calculations were performed with JMP 16.1 Statistical Software (SAS Institute, Inc., Cary, NC, USA).
4. Discussion
Based on this study, a stream of targeted by microbubbles (<0.5 mm dia.) from a bubble diffuser (1.0 L air/min) can detach cells of L. monocytogenes from steel and produce surfaces. Extended bubble treatment times (up to 10 min) promoted greater removal of L. monocytogenes from stainless steel coupons, since significantly fewer L. monocytogenes were recovered from stainless steel after 10 min bubble treatments compared with no bubble treatment. The bubble application used in this study may reduce L. monocytogenes on stainless by nearly three logs (2.95 log CFU per sample) after an inoculum was dried for 48 h and loosely attached cells were removed. For cucumber and avocado, a 5 min application of the same heavy bubble stream removed at least 1.0 log more of the firmly attached bacteria than submerging the produce in water without bubbles.
Lee et al. (2018) applied cavitating bubbles to inoculated fresh Roma tomatoes and cantaloupes using larger bubble sizes (~1–3 mm diameter) and higher air flows (3.5–14 L/min) than were used in this study [
12]. Those authors reported that
L. monocytogenes significantly decreased on Roma tomatoes (~1.0 log CFU after 30 s and ~1.2 log CFU after 60 s treatment) with a 14 L/min airflow rate compared to no cavitating bubbles.
L. monocytogenes significantly decreased on cantaloupes (0.65 log CFU for 30 s and ~0.74 log CFU for 60 s treatment) when the maximum air-flow rate was used. In the study reported here, the log reduction in
L. monocytogenes on cucumber and avocado surfaces also increased with increasing bubble treatment time, even though the airflow rate was lower, the treatment times longer, and the bubble diameters smaller. An important difference in these studies is that Lee et al. (2018) did not remove loosely attached cells prior to any bubble treatments. Here, in our study with cucumbers, avocados and stainless-steel coupons, the number of loosely attached cells recovered and enumerated after 1, 24 or 48 h drying was always higher than the quantity that could be recovered from the samples that were not treated with bubbles (treatment time = 0 min).
Chlorine is commonly applied to wash and clean fresh produce due to its bactericidal capacity and economical cost. When chlorine makes contact with wash water during washing, it yields an oxidizer, HOCI, which can inactivate pathogens effectively [
16]. The common dosage of commercial chlorine in industry ranges from around 50 to 200 mg/L, but 100 mg/L is the most common dosage used in industry [
17]. The application of this dosage of chlorine requires a short impact time, of about 1 to 2 min, and a preferred pH of from 6.0 to 7.5 is used to maintain the stabilization of HOCI in order to prevent chemicals from corroding the processing equipment. Banach et al. (2015) showed that a five-log CFU of
E. coli on fresh spinach can be completely removed with wash water containing a level of 7 mg/L free chlorine [
16]. Although some chlorine solutions have been shown to inactivate pathogens at a higher level than reported in the present study using streams of microbubbles, some regulators, consumers, and food industries believe that an over-reliance on chlorine rinses by food industries can lead to the excessive formation of chlorate residues that can harm human health.
Other researchers have compared the addition of several antimicrobial chemicals to the treatment water of fresh produce regarding their ability to inactivate or reduce pathogens, including
Listeria monocytogenes. Chlorine, peracetic acid (PAA), chlorine dioxide (CIO
2), ozone, and electrolyzed oxidizing water (EOW) are some of the most common antimicrobial agents [
18]. Rodgers et al. (2004) showed that both
E. coli O157:H7 and
L. monocytogenes can be inactivated over five logs when exposed to aqueous chemical sanitizers, including ozone, ClO
2, chlorine, and PAA, for a 2–5 min exposure time [
19]. Additionally, ozonation can be an excellent alternative for chlorine, as an economical and environmentally friendly disinfectant for this industry [
20]. Additionally, ultraviolet light energy could be an effective measure by which industry could clean fresh produce. Yaun et al. (2004) reported using ultraviolet energy (24 mW/cm
2) to remove
Salmonella spp. or
Escherichia coli on the surface of fresh produce, including red apples, leaf lettuce, and tomatoes. These researchers reported that both
Salmonella (2.65 log reduction) and
E. coli (2.79 log reduction) can be inactivated to a similar level [
21].
The present study was designed to evaluate microbubbles for removal or detachment of L. monocytogenes on stainless steel, avocado and cucumber surfaces. Even though microbubbles may reduce the concentration of L. monocytogenes on steel and some produce surfaces, they may or may not enhance microbial inactivation. Nevertheless, microbubbles can be an environmentally friendly alternative to chlorine for reducing the microbial surface contamination of fresh produce, which will be odorless and possibly less corrosive to equipment.
Based on the results, the cross-contamination of
L. monocytogenes from inoculated to clean cucumbers and avocados increased with extended bubble treatment time (2 vs. 10 min). The transfer rate to cucumbers and avocados was also higher when inoculum was dried for 24 h versus 1 or 48 h. This result could be explained by differences in the total number of firmly or loosely attached cells that occurred with each drying time. The variable numbers of attached cells on inoculated cucumbers and avocados influence the number of cells that detach and adhere to uninoculated produce. The percentage of cells that were transferred between cucumbers was always higher than the percentage transfer between avocados, even though the initial inoculums were similar. Compared with cucumbers, the cross-contamination of avocados by
L. monocytogenes was less affected by bubble treatments. In Lee et al. (2018), the cross-contamination of
L. monocytogenes on Roma tomatoes was not significantly different with bubble treatment time (30 or 60 s) in terms of the transfer between inoculated to uninoculated Roma tomatoes, but significantly different between airflow rates (0, 7 or 14 SLPM) [
12].
The application of a bubble stream similar to that used in this study could be used in a produce-packing house prior to the packaging and shipment of some produce to retail markets. This technique is not likely to be employed by consumers due to the need to purchase equipment that will be only occasionally used. After the consumer’s retail purchase, the shelf-life of fresh, whole cucumbers is approximately 7 d when refrigerated (1–10 °C), and the shelf-life of fresh avocados is ~3–4 d at room temperature (20–25 °C) and ~7–10 d when refrigerated [
22,
23]. In this study, extending refrigerated storage from 4 to 7 d resulted in variable increases or decreases in surface bacteria growth and changes in quality for cucumbers and avocados. While an extended bubble treatment time appears to have higher effectiveness for removing bacteria from cucumbers, the surface aerobic plate count concentrations did not decrease over time. Similarly, for avocados, surface aerobic plate counts increased with longer storage times, and surface concentrations could initially be reduced by a bubble treatment, by less than one log CFU per unit. Extended bubble treatment times (>10 min, for example) for raw fruit or vegetables could damage the surface or skin in such a way that bacteria are able to infiltrate. Additionally, submerging some produce in water without bubbles could lead to a disruption in the integrity of the skin or surface. For this project, we did not microscopically examine the physical surface of the test produce.
The efficacy required by a bubble stream to detach bacteria from an irregular surface will vary according to the speed at which a bubble moves through water, its impact angle with a surface and the forces exerted on a surface. Microbubble applications can be more effective for surface cleaning if the angle of contact between the bubbles and the surface and the shear stress exerted on a surface can be measured and controlled [
24,
25]. Therefore, variations in the distance a bubble travels before surface contact, contact angles, total bubble volume or air-flow rate used, as well as the bubble diameter range, can be adjusted and compared to further characterize a non-thermal aeration process to enhance food safety.
We demonstrated that a stream of injected air bubbles can effectively reduce the population of L. monocytogenes on stainless steel, raw cucumbers and raw avocados. As the bubble treatment times increases, more of these bacteria can be removed from individual fruits, but there is a risk of increased bacterial transfer to other nearby fruits. While extended bubble treatment times appear to have higher effectiveness for removing aerobic bacteria populations from cucumbers and avocados, these populations did not further decrease over 7 d of refrigerated storage.
Additional research is needed to understand and optimize an injected air-bubble treatment for raw produce that can consistently, and significantly, reduce surface microbial concentrations. More specific to the study reported here, the detachment of other Listeria serotypes or other bacterial pathogens associated with raw fruits or vegetables could be studied. Additionally, the application of a bubble stream may enhance the removal of non-pathogenic or spoilage organisms, which could increase shelf-life under some storage conditions. In addition to cucumbers and avocados, the surface microbial concentrations of melons, tomatoes and papaya could be evaluated, for example, since a consumer could either eat the outer surfaces, or the surface bacteria could be transferred to interior edible portions during slicing.
 , 24 h
, 24 h  , or 48 h
, or 48 h  , and different bubble treatment times from 0 to 10 min (Mean of: 3 replications × 3 samples each). A,B,C,D Denote significant differences in recovery (p < 0.01) between treatment times for each of the inoculum drying times.
, and different bubble treatment times from 0 to 10 min (Mean of: 3 replications × 3 samples each). A,B,C,D Denote significant differences in recovery (p < 0.01) between treatment times for each of the inoculum drying times.
 , 24 h
, 24 h  , or 48 h
, or 48 h  , and different bubble treatment times from 0 to 10 min (Mean of: 3 replications × 3 samples each). A,B,C,D Denote significant differences in recovery (p < 0.01) between treatment times for each of the inoculum drying times.
, and different bubble treatment times from 0 to 10 min (Mean of: 3 replications × 3 samples each). A,B,C,D Denote significant differences in recovery (p < 0.01) between treatment times for each of the inoculum drying times.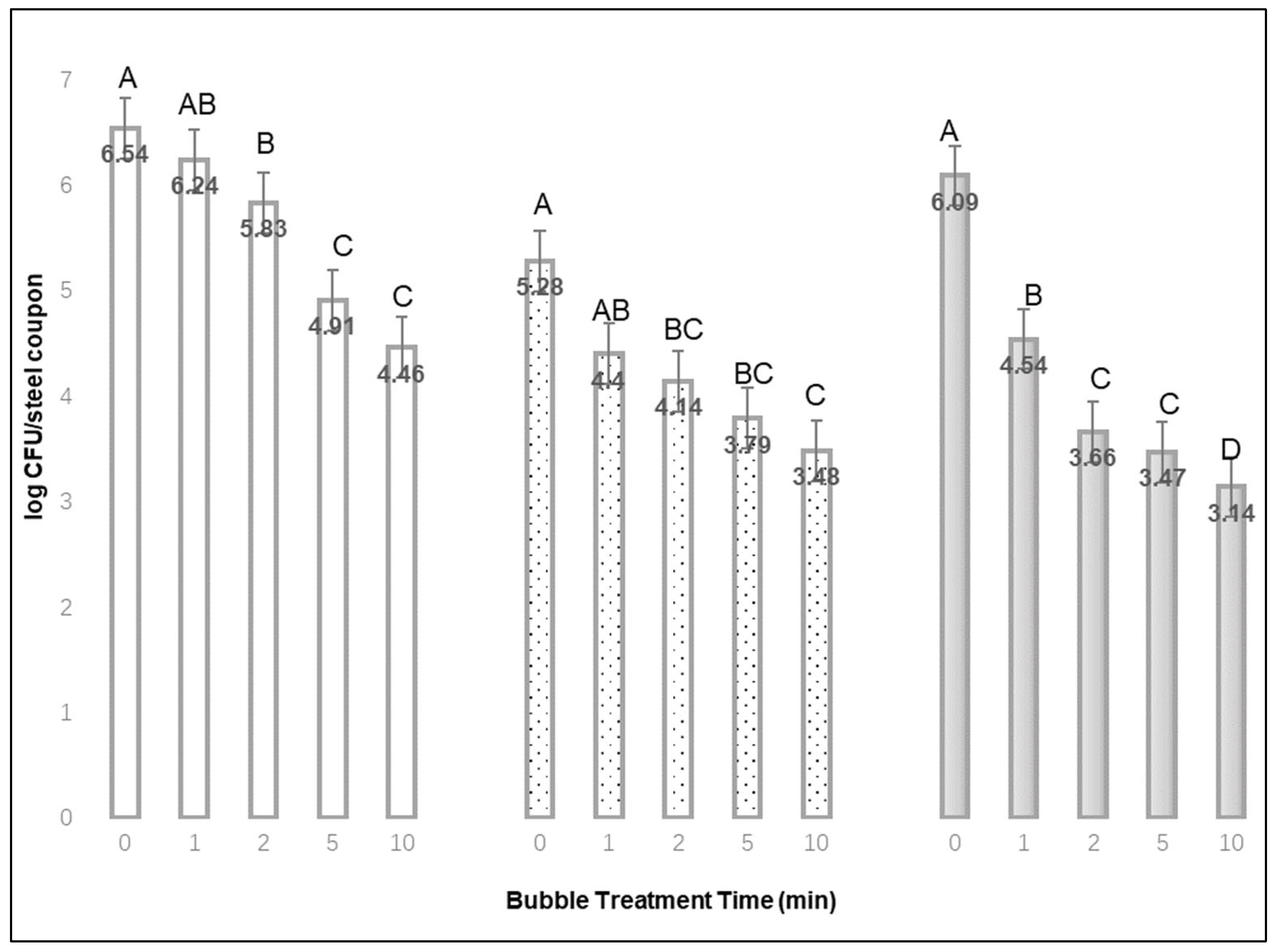
 , 24 h
, 24 h  , or 48 h
, or 48 h  , and different bubble treatment times from 0 to 10 min (Mean of 3 replications × 3 samples each). A,B,C,D,E Denote significant differences in recovery (p < 0.01) between treatment times for each of the inoculum drying times.
, and different bubble treatment times from 0 to 10 min (Mean of 3 replications × 3 samples each). A,B,C,D,E Denote significant differences in recovery (p < 0.01) between treatment times for each of the inoculum drying times.
 , 24 h
, 24 h  , or 48 h
, or 48 h  , and different bubble treatment times from 0 to 10 min (Mean of 3 replications × 3 samples each). A,B,C,D,E Denote significant differences in recovery (p < 0.01) between treatment times for each of the inoculum drying times.
, and different bubble treatment times from 0 to 10 min (Mean of 3 replications × 3 samples each). A,B,C,D,E Denote significant differences in recovery (p < 0.01) between treatment times for each of the inoculum drying times.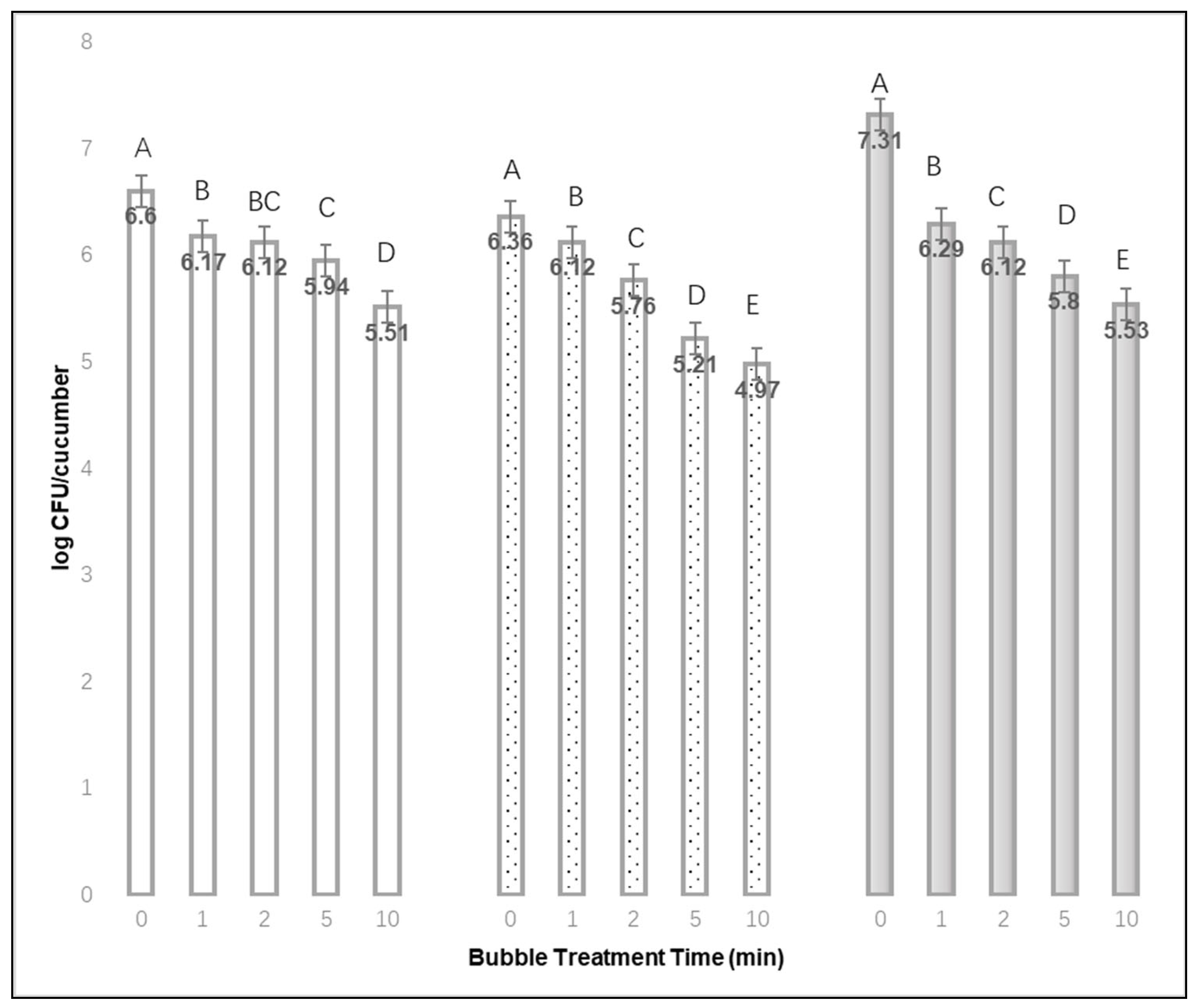
 , 24 h
, 24 h  , or 48 h
, or 48 h  , and different bubble treatment times from 0 to 10 min (mean of 3 replications × 3 samples each). A,B,C,D,E Denote significant differences in recovery (p < 0.01) between treatment times for each of the inoculum drying times.
, and different bubble treatment times from 0 to 10 min (mean of 3 replications × 3 samples each). A,B,C,D,E Denote significant differences in recovery (p < 0.01) between treatment times for each of the inoculum drying times.
 , 24 h
, 24 h  , or 48 h
, or 48 h  , and different bubble treatment times from 0 to 10 min (mean of 3 replications × 3 samples each). A,B,C,D,E Denote significant differences in recovery (p < 0.01) between treatment times for each of the inoculum drying times.
, and different bubble treatment times from 0 to 10 min (mean of 3 replications × 3 samples each). A,B,C,D,E Denote significant differences in recovery (p < 0.01) between treatment times for each of the inoculum drying times.
 , 2 min
, 2 min  , or 10 min
, or 10 min  bubble stream. (mean of 3 replications × 3 samples each). A,B,C Denote significant differences in recovery (p < 0.01) between storage times for each of the bubble treatment times.
bubble stream. (mean of 3 replications × 3 samples each). A,B,C Denote significant differences in recovery (p < 0.01) between storage times for each of the bubble treatment times.
 , 2 min
, 2 min  , or 10 min
, or 10 min  bubble stream. (mean of 3 replications × 3 samples each). A,B,C Denote significant differences in recovery (p < 0.01) between storage times for each of the bubble treatment times.
bubble stream. (mean of 3 replications × 3 samples each). A,B,C Denote significant differences in recovery (p < 0.01) between storage times for each of the bubble treatment times.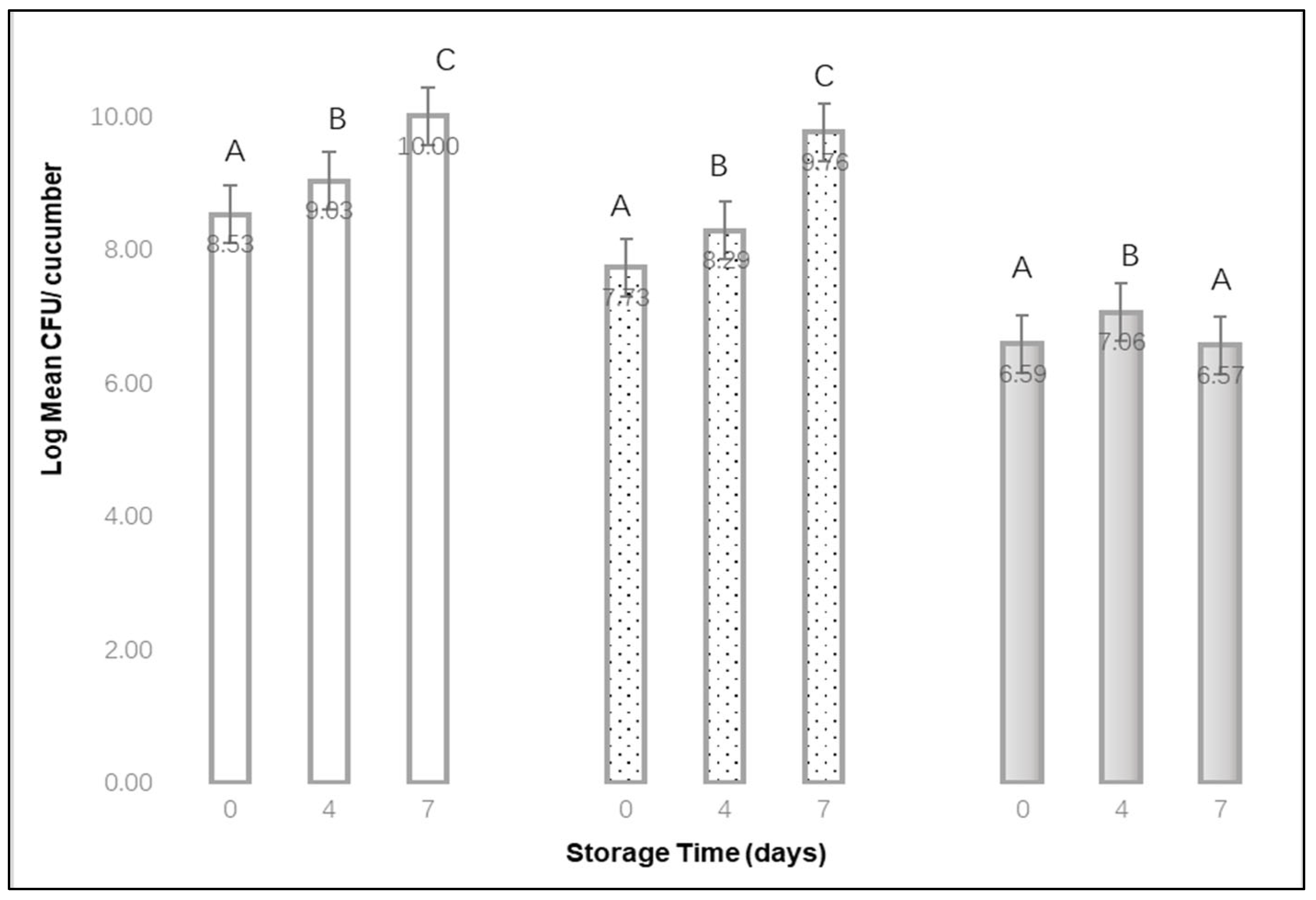
 , 2 min
, 2 min  , or 10 min
, or 10 min  bubble stream (mean of 3 replications × 3 samples). A,B,C Denote significant differences in recovery (p < 0.01) between storage times for each of the bubble treatment times.
bubble stream (mean of 3 replications × 3 samples). A,B,C Denote significant differences in recovery (p < 0.01) between storage times for each of the bubble treatment times.
 , 2 min
, 2 min  , or 10 min
, or 10 min  bubble stream (mean of 3 replications × 3 samples). A,B,C Denote significant differences in recovery (p < 0.01) between storage times for each of the bubble treatment times.
bubble stream (mean of 3 replications × 3 samples). A,B,C Denote significant differences in recovery (p < 0.01) between storage times for each of the bubble treatment times.