Computer Modelling of Energy Structure of Yb3+ and Lu3+ Doped LaF3 Crystals
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. The Influence of Crystal Cell Relaxation on Its Energy Properties
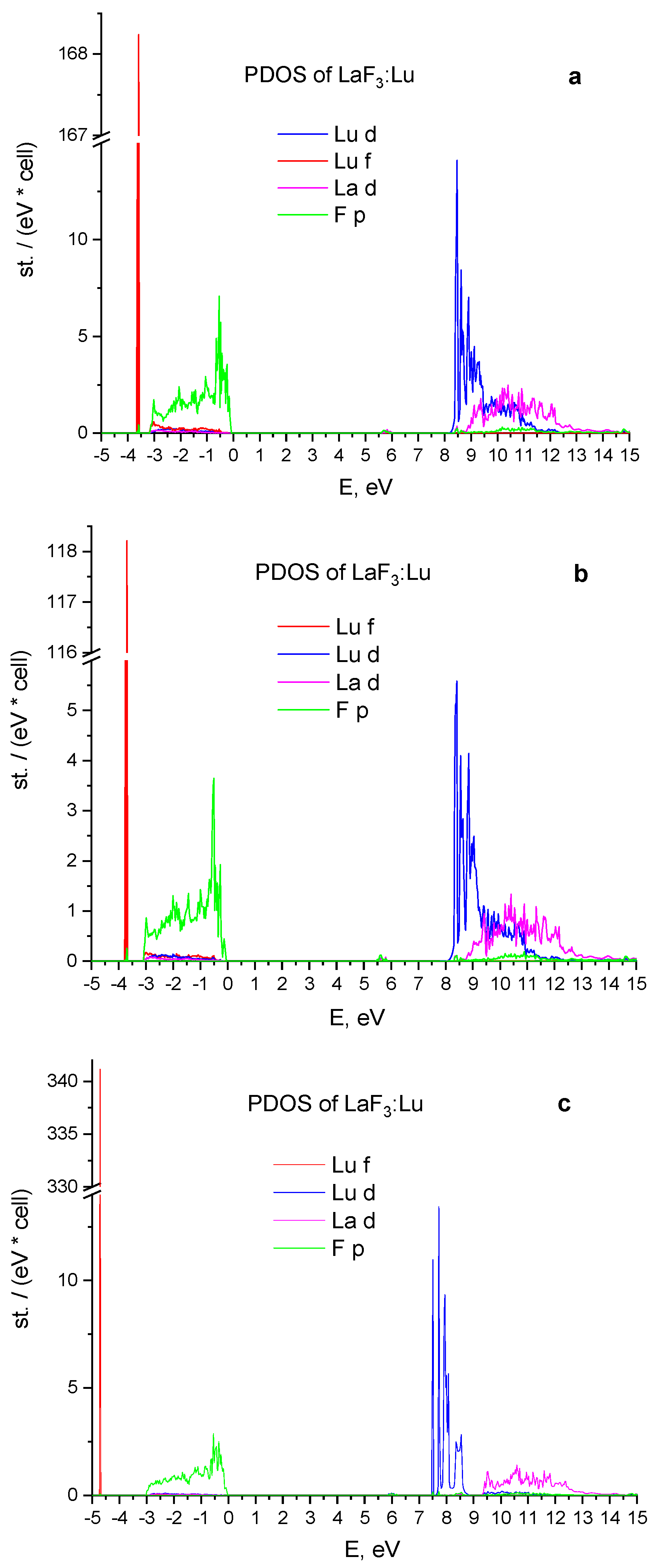
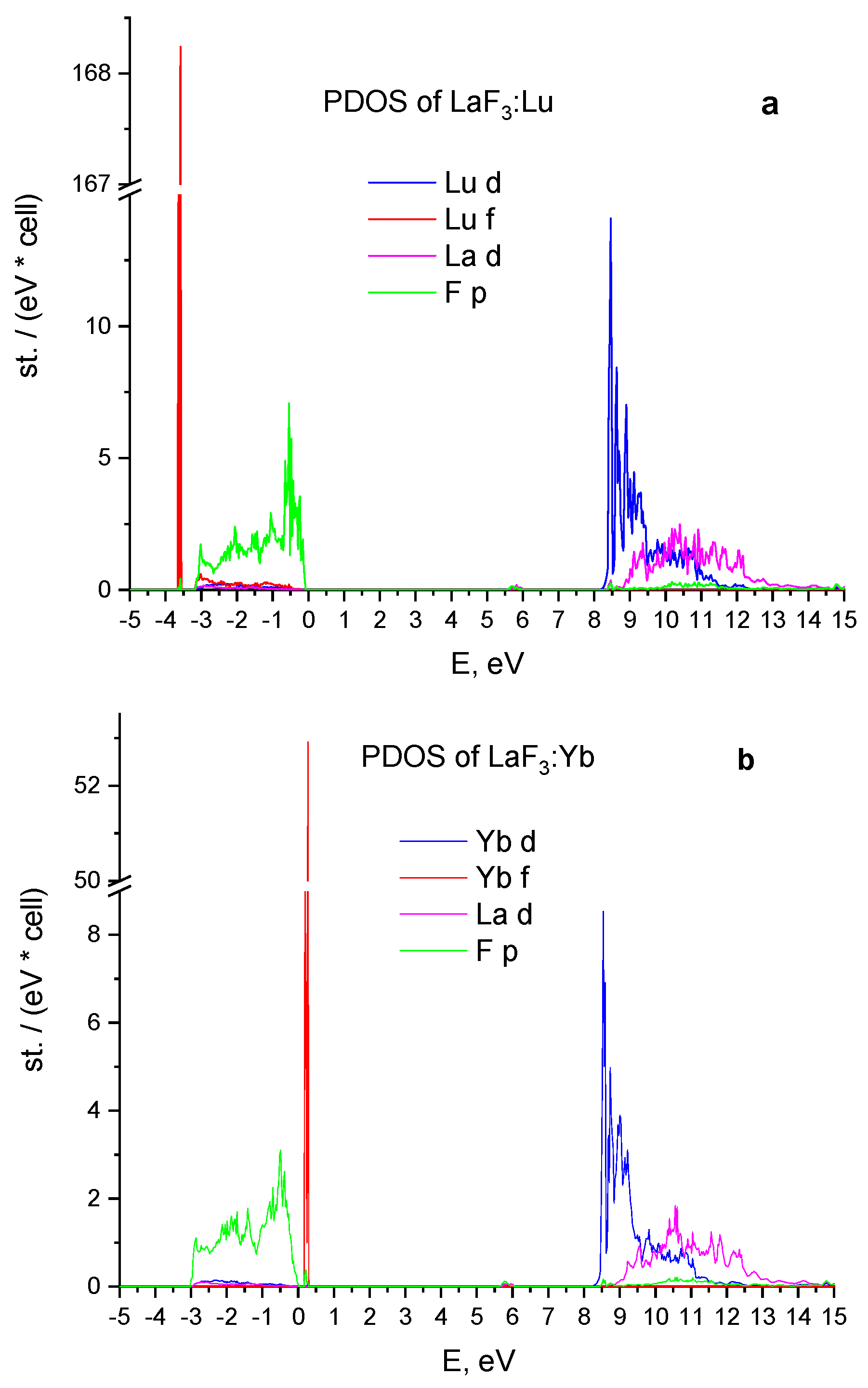
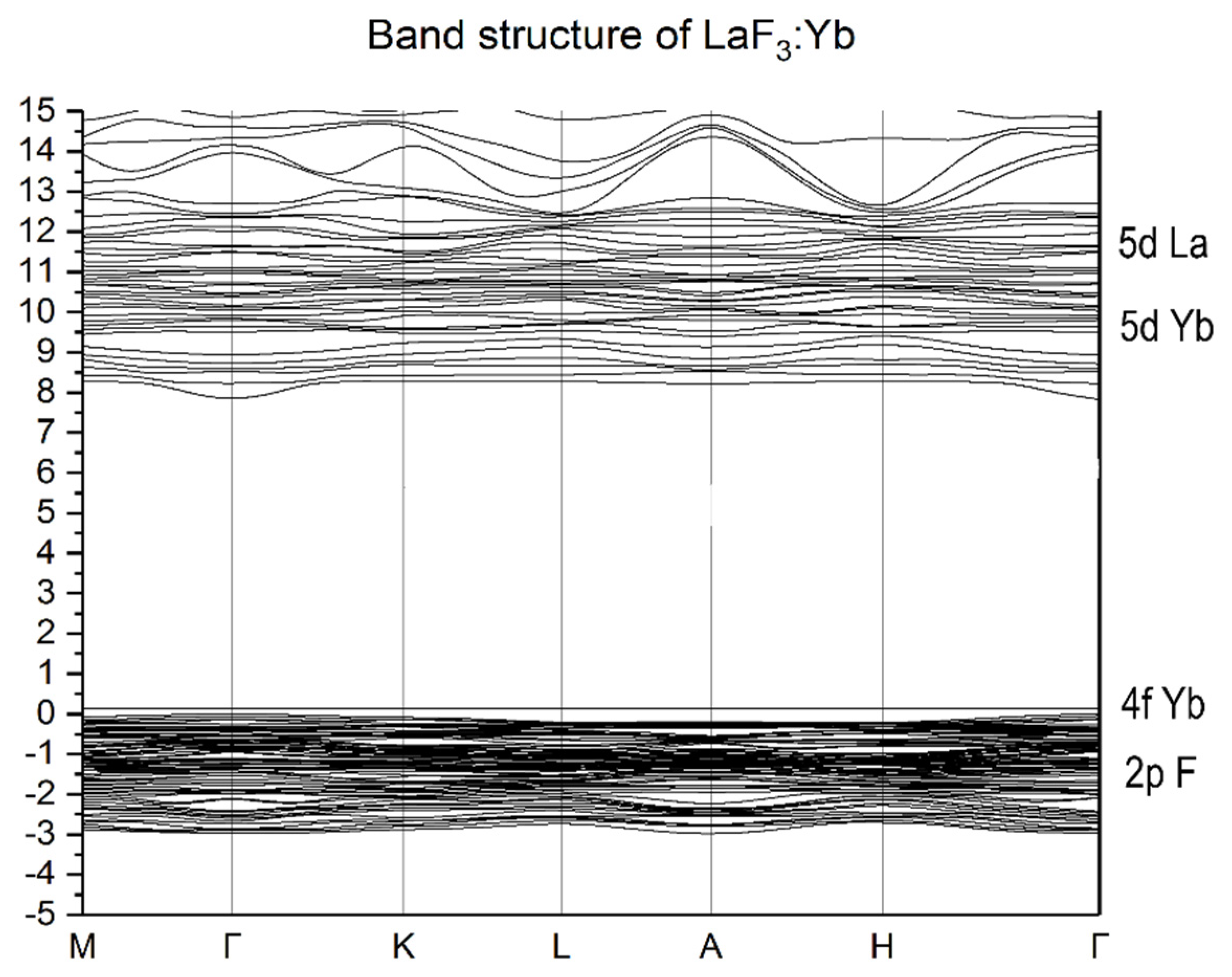
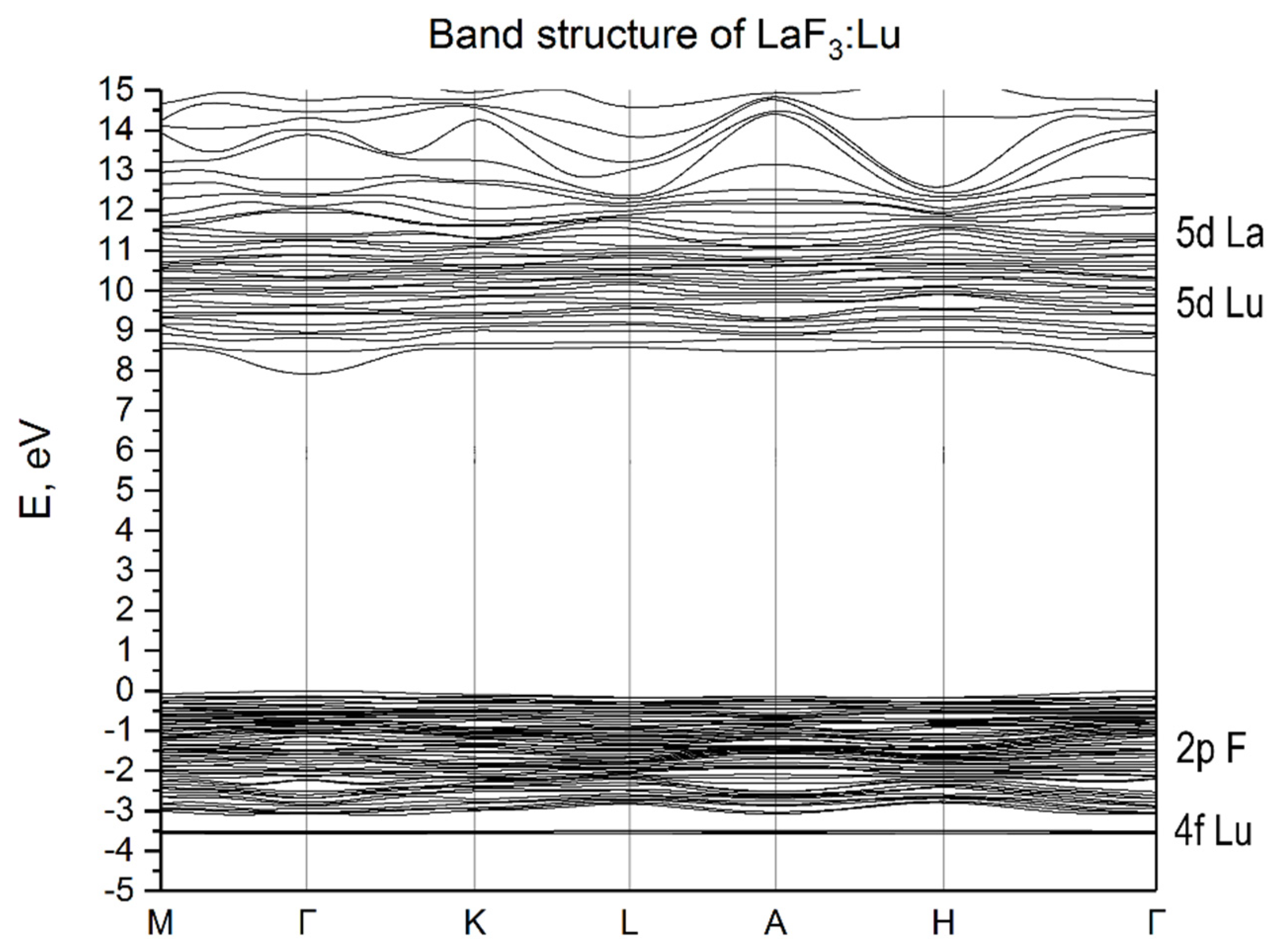
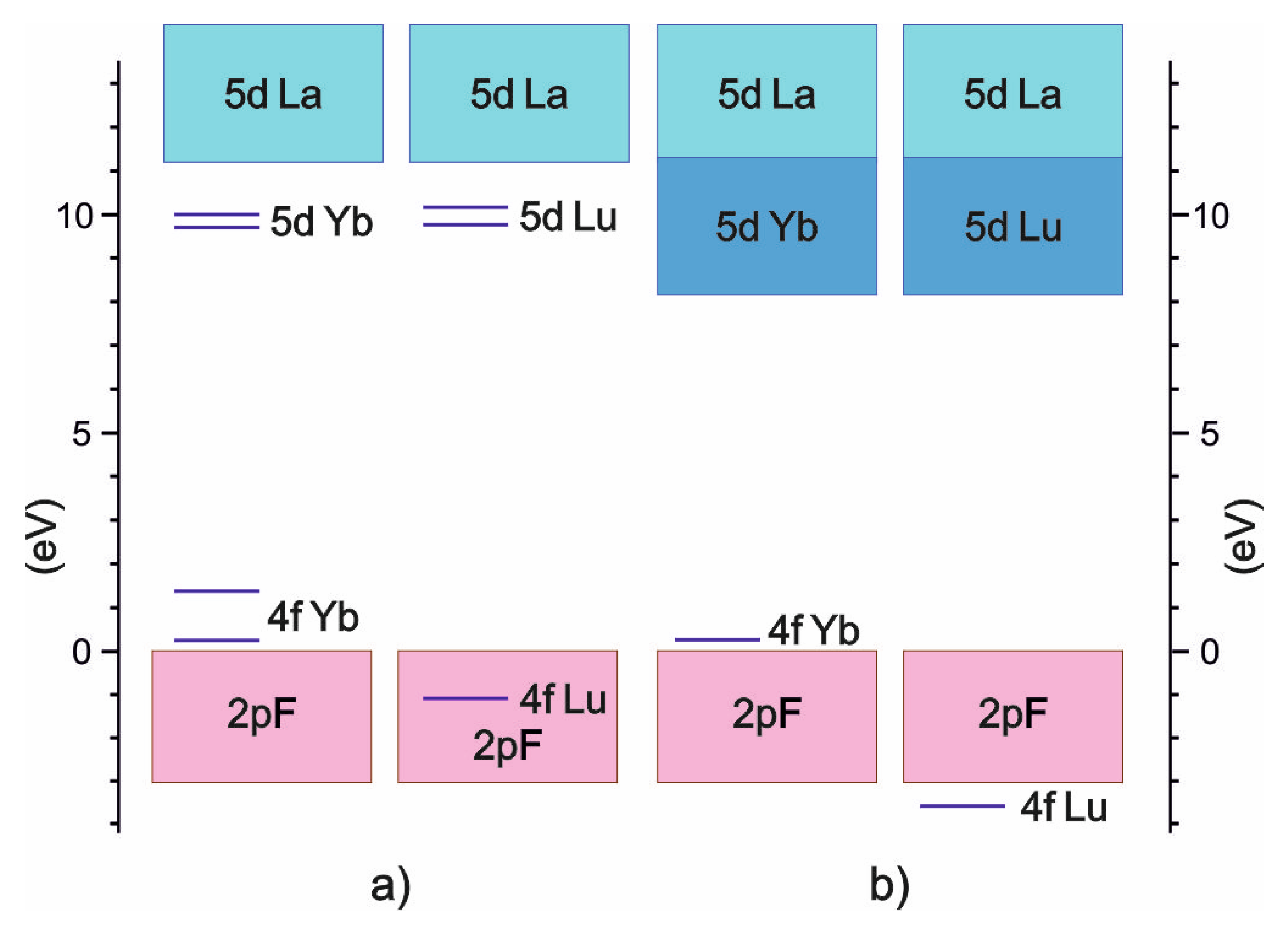
3.2. Energy Properties of LaF3:Lu and LaF3:Yb
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gektin, A.; Korzhik, M. Inorganic Scintillators for Detector Systems; Springer: Berlin, Germany, 2017. [Google Scholar] [CrossRef]
- Dorenbos, P. Ce3+ 5d-centroid shift and vacuum referred 4f-electron binding energies of all lanthanide impurities in 150 different compounds. J. Lumin. 2013, 135, 93–104. [Google Scholar] [CrossRef]
- Blanc, W.; Dujardin, C.; Gâcon, J.C.; Pedrini, C.; Moine, B.; Belsky, A.N.; Kamenskikh, I.; Kirm, M.; Zimmerer, G.; Gǎcon, J.C. On the role of the 4f-Lu level in the scintillation mechanisms of cerium-doped lutetium-based fluoride crystals. Radiat. Eff. Defects Solids 1999, 150, 41–46. [Google Scholar] [CrossRef]
- Guerbous, L.; Krachni, O. The 4f-5d luminescence transitions in cerium-doped LuF3. J. Mod. Opt. 2006, 53, 2043–2053. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Matveev, A.; Rösch, N. The DFT+ U method in the linear combination of Gaussian-type orbitals framework: Role of 4f orbitals in the bonding of LuF3. Chem. Phys. Lett. 2009, 468, 158–161. [Google Scholar] [CrossRef]
- Kirm, M.; Stryganyuk, G.; Vielhauer, S.; Zimmerer, G.; Makhov, V.; Malkin, B.; Solovyev, O.; Abdulsabirov, R.; Korableva, S. Vacuum-ultraviolet 5d−4f luminescence of Gd3+ and Lu3+ ions in fluoride matrices. Phys. Rev. B 2007, 75, 075111. [Google Scholar] [CrossRef]
- Makhov, V.; Kirm, M.; Stryganyuk, G.; Vielhauer, S.; Zimmerer, G.; Malkin, B.; Solovyev, O.; Korableva, S. 5d–4f luminescence of Ce3+, Gd3+ and Lu3+ in LiCaAlF6. J. Lumin. 2012, 132, 418–424. [Google Scholar] [CrossRef]
- Hewes, A.; Sarver, J. Infrared excitation processes for the visible luminescence of Er3+, Ho3+, and Tm3+ in Yb3+-sensitized rare-earth trifluorides. Phys. Rev. 1969, 186, 427–436. [Google Scholar] [CrossRef]
- Stryganyuk, G.; Zazubovich, S.; Voloshinidraskii, S.; Zimmerer, M.; Peters, R.; Petermann, K. Charge transfer luminescence of Yb3 + ions in LiY1-xYbxP4O12 phosphates. J. Phys Condens. Matter. 2007, 19, 036202. [Google Scholar] [CrossRef][Green Version]
- Chornodolskyy, Y. Energy band structure peculiarities and luminescent parameters of CeX3 (X = Cl, Br, I) crystals. J. Lumin. 2021, 237, 118147. [Google Scholar] [CrossRef]
- Kochan, O.; Chornodolskyy, Y.; Selech, J.; Karnaushenko, V.; Przystupa, K.; Kotlov, A.; Demkiv, T.; Vistovskyy, V.; Stryhanyuk, H.; Rodnyi, P.; et al. Energy Structure and Luminescence of CeF3 Crystals. Materials 2021, 14, 4243. [Google Scholar] [CrossRef] [PubMed]
- Karnaushenko, V.; Chornodolskyy, Y.; Syrotyuk, S.; Voloshinovskii, A. Electronic energy structure of LaF3:Ce crystal. Bull. Lviv. Univ. Ser. Phys. 2019, 56, 133–139. [Google Scholar] [CrossRef][Green Version]
- Burke, K. Perspective on density functional theory. Chem. Phys. 2012, 136, 150901. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P. Projector augmented-wave method. Phys. Rev. 1994, 50, 17953. [Google Scholar] [CrossRef] [PubMed]
- Himmetoglu, B.; Floris, A.; de Gironcoli, S.; Cococcioni, M. Hubbard-corrected DFT energy functionals: The LDA+ U description of correlated systems. Int. J. Quantum Chem. 2014, 114, 14–49. [Google Scholar] [CrossRef]
- Saini, S.; Tashi, N.; Auluck, S. Electronic and optical properties of rare earth trifluorides RF3 (R = La, Ce, Pr, Nd, Gd and Dy). Mater. Chem. Phys. 2011, 129, 349–355. [Google Scholar] [CrossRef]
- The Materials Project. MateriaLs Data ON LaF3 by MateriaLs Project. United States: N. 2020. Available online: https://materialsproject.org/materials/mp-905 (accessed on 1 September 2022).
- Head, J.; Zerner, M. A Broyden—Fletcher—Goldfarb—Shanno optimization procedure for molecular geometries. Chem. Phys. Lett. 1985, 122, 264–270. [Google Scholar] [CrossRef]
- Schlegel, H. Optimization of equilibrium geometries and transition structures. J. Comput. Chem. 1982, 3, 214–218. [Google Scholar] [CrossRef]
- Topsakal, M.; Wentzcovitch, R. Accurate projected augmented wave (PAW) datasets for rare-earth elements (RE = La–Lu). Comput. Mater. Sci. 2014, 95, 263–270. [Google Scholar] [CrossRef]
- Karnaushenko, V.; Chornodolskyy, Y.; Vistovskyy, V.; Syrotyuk, S.; Voloshinovskii, A. Energy band structure of LaF3:Sm and LaF3:Pm crystals. J. Phys. Stud. 2020, 24, 4703. [Google Scholar] [CrossRef]
- Chuklina, N.; Piskunov, S.; Popov, N.V.; Mysovsky, A.; Popov, A.I. Comparative quantum chemistry study of the F-center in lanthanum trifluoride. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2020, 474, 57–62. [Google Scholar] [CrossRef]
| a, Å | b, Å | c, Å | α | β | γ | |
|---|---|---|---|---|---|---|
| Initial | 7.25 | 7.25 | 7.39 | 90 | 90 | 120 |
| Optimized | 7.22 | 7.22 | 7.36 | 90 | 90 | 120 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chornodolskyy, Y.; Karnaushenko, V.; Selech, J.; Vistovskyy, V.; Demkiv, T.; Przystupa, K.; Syrotyuk, S.; Voloshinovskii, A. Computer Modelling of Energy Structure of Yb3+ and Lu3+ Doped LaF3 Crystals. Materials 2022, 15, 7937. https://doi.org/10.3390/ma15227937
Chornodolskyy Y, Karnaushenko V, Selech J, Vistovskyy V, Demkiv T, Przystupa K, Syrotyuk S, Voloshinovskii A. Computer Modelling of Energy Structure of Yb3+ and Lu3+ Doped LaF3 Crystals. Materials. 2022; 15(22):7937. https://doi.org/10.3390/ma15227937
Chicago/Turabian StyleChornodolskyy, Yaroslav, Vladyslav Karnaushenko, Jaroslaw Selech, Vitaliy Vistovskyy, Taras Demkiv, Krzysztof Przystupa, Stepan Syrotyuk, and Anatolii Voloshinovskii. 2022. "Computer Modelling of Energy Structure of Yb3+ and Lu3+ Doped LaF3 Crystals" Materials 15, no. 22: 7937. https://doi.org/10.3390/ma15227937
APA StyleChornodolskyy, Y., Karnaushenko, V., Selech, J., Vistovskyy, V., Demkiv, T., Przystupa, K., Syrotyuk, S., & Voloshinovskii, A. (2022). Computer Modelling of Energy Structure of Yb3+ and Lu3+ Doped LaF3 Crystals. Materials, 15(22), 7937. https://doi.org/10.3390/ma15227937







