Nitration of 2,6,8,12-Tetraacetyl-2,4,6,8,10,12-Hexaazaisowurtzitane Derivatives
Abstract
:1. Introduction
- -
- 4,10-di(2-ethoxyacetyl)-2,6,8,12-tetraacetyl-2,4,6,8,10,12-hexaazaisowurtzitane (2);
- -
- 4-(3,4-dibromothiophenecarbonyl)-2,6,8,12-tetraacetyl-2,4,6,8,10,12-hexaazaisowurtzitane (thiowurtzine, 3);
- -
- 4-(3,4-dibromothiophenecarbonyl)-10-(2-ethoxyacetyl)-2,6,8,12-tetraacetyl-2,4,6,8,10,12-hexaazaisowurtzitane (4);
- -
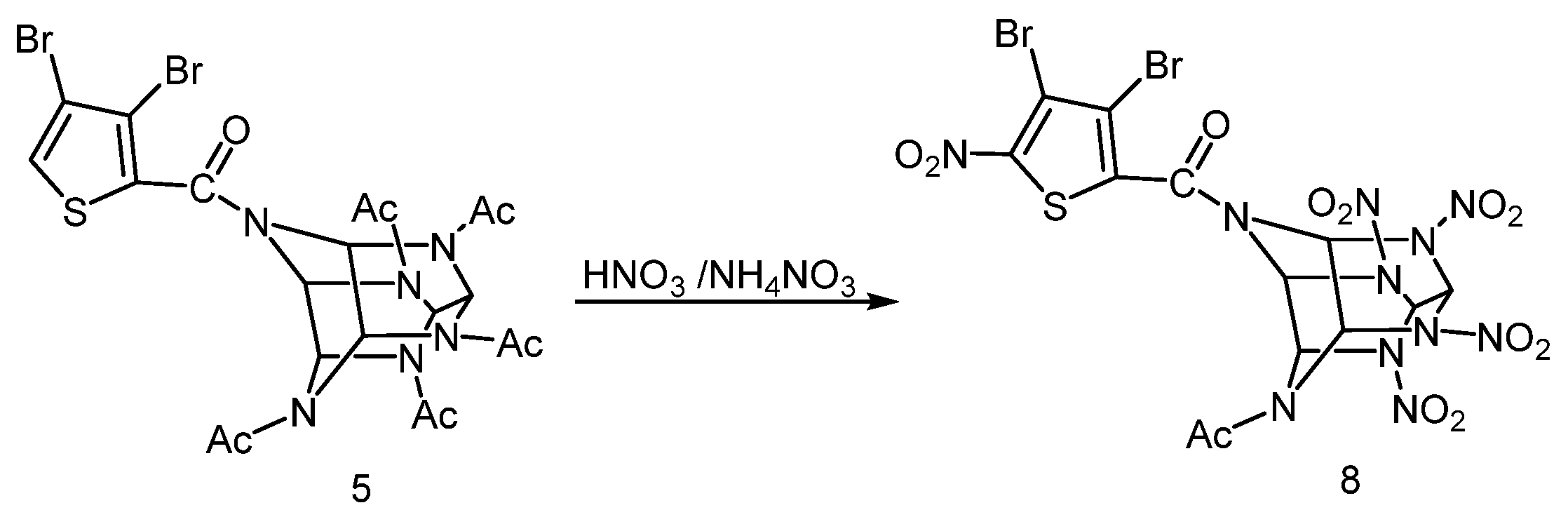
- 4-(3,4-dibromothiophenecarbonyl)-2,6,8,10,12-pentaacetyl-2,4,6,8,10,12-hexaazaisowurtzitane (5);
- -
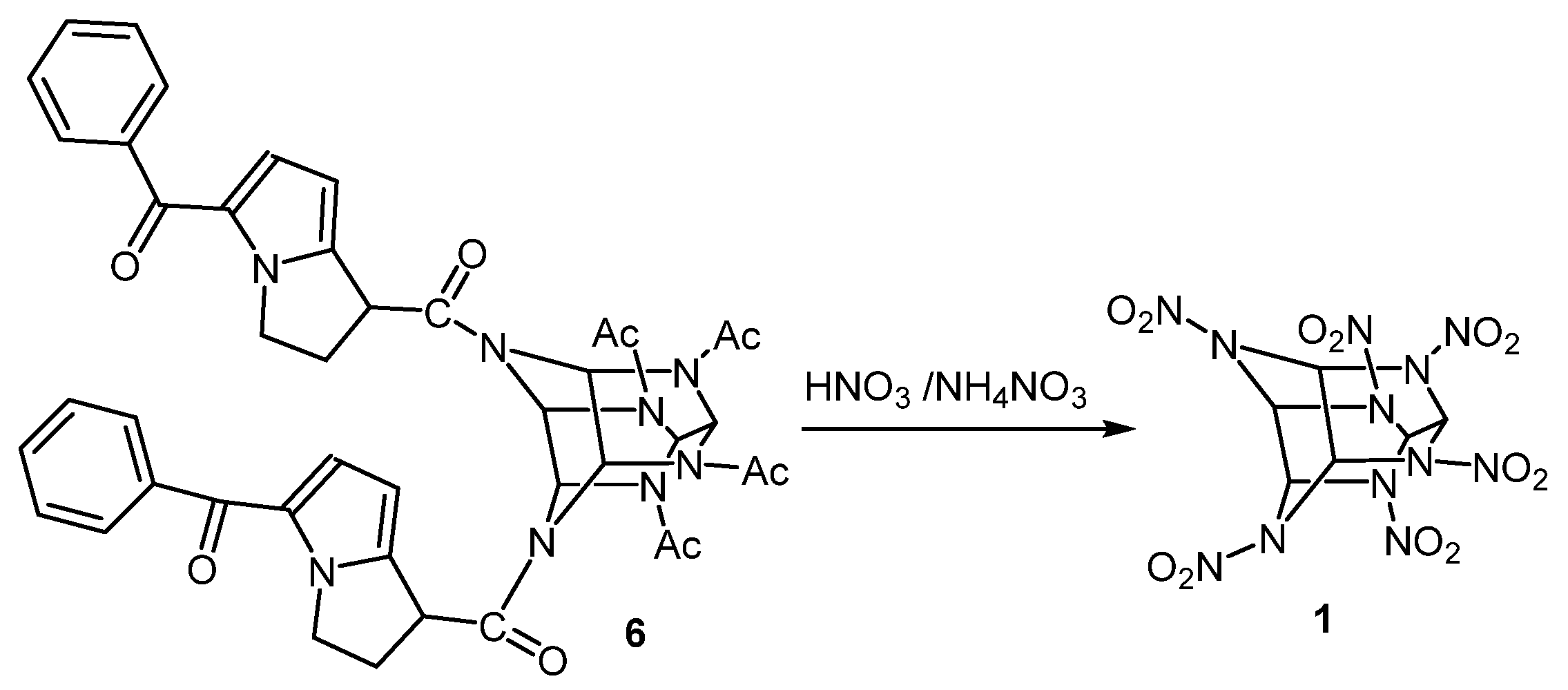
- 4,10-bis((±)-5-benzoyl-2,3-dihydro-1Н-pyrrolo [1,2-а]pyrrol-1-carbonyl)-2,6,8,12-tetraacetyl-2,4,6,8,10,12-hexaazaisowurtzitane (6).
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nielsen, A.T.; Chafin, A.P.; Christian, S.L.; Moore, D.W.; Nadler, M.; Nissan, R.; Vanderah, D.; Gilardi, R.; George, C.; Flippen-Anderson, J. Synthesis of polyazapolycyclic caged polynitramins. Tetrahedron 1998, 54, 11793–11812. [Google Scholar] [CrossRef]
- Il’yasov, S.G.; Chikina, M.V. A novel approach to synthesis of hexaazaisowurtzitane derivatives. Tetrahedron Lett. 2013, 54, 1931–1932. [Google Scholar] [CrossRef]
- Latypov, N.; Wellmar, U.; Goede, P.; Bellamy, A. Synthesis and scale-Up of 2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane from 2,6,8,12-tetraacetyl-4,10-dibenzyl-2,4,6,8,10,12-hexaazaisowurtzitane (HNIW, CL-20). Org. Process Res. Dev. 2000, 4, 156–158. [Google Scholar] [CrossRef]
- Kalashnikov, A.A.; Sysolyatin, S.V.; Surmacheva, I.A.; Surmacheva, V.N.; Lapina, Y.T. Debenzylation of 2,6,8,12-tetraacetyl-4,10-dibenzyl-2,4,6,8,10,12-hexaazatetracyclo [5.5.0.03,11.05,9]dodecane. Rus. Chem. Bull. 2009, 58, 2164–2168. [Google Scholar] [CrossRef]
- Ramazani, A.; Azizkhani, V.; Joo, S.W. Heteropolyacids: As efficient catalysts for the nitration of 2,4,6,8,10,12-hexaacetyl-2,4,6,8,10,12-hexaazaisowurtzitane. Rev. Roum. Chim. 2019, 64, 569–575. [Google Scholar] [CrossRef]
- Yanhua, L.; Qing, L.; Xiaoting, R.; Danyang, Y.; Yingyuan, G.; Ning, D.; Jinxuan, H. A Kind of Method that Three-Step Reaction Prepares CL 20. CN Patent 107353293A, 17 November 2017. [Google Scholar]
- Qian, H.; Lu, C.; Ye, Z. Synthesis of CL-20 by clean nitrating agent dinitrogen pentoxide. J. Indian Chem. Soc. 2008, April, 434–439. [Google Scholar]
- Kalashnikov, А.И.; Sysolyatin, S.V.; Sakovich, G.V.; Dubkov, A.S.; Kulagina, D.А. Nitrolysis of 4,10-dibenzyl-2,6,8,12-tetraacetyl-2,4,6,8,10,12-hexaazatetracyclo[5,5,0,3,11,5,9]dodecane. Rus. Chem. Bull. 2017, 3, 531–536. [Google Scholar] [CrossRef]
- Kalashnikov, A.I.; Sysolyatin, S.V.; Surmachev, V.N. Nitration of acyl derivatives of 2,4,6,8,10,12-hexaazaisowurtzitane. Prop. Explos. Pyrotech. 2019, 44, 1472. [Google Scholar] [CrossRef]
- Surmachev, V.N.; Kubasova, V.A.; Zimin, D.E. A study on nitration of 4,10-dibenzyl-2,6,8,12-tetraacetyl-2,4,6,8,10,12-hexaazaisowurtzitane. Prop. Explos. Pyrotech. 2020, 45, 1841. [Google Scholar] [CrossRef]
- Krylova, S.G.; Povet’eva, T.N.; Zueva, E.P.; Suslov, N.I.; Amosova, E.N.; Razina, T.G.; Lopatina, K.A.; Rybalkina, O.Y.; Nesterova, Y.V.; Afanas’Eva, O.G.; et al. Analgesic activity of hexaazaisowurtzitane derivatives. Bull. Exp. Biol. Med. 2019, 166, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, K.A.; Krylova, S.G.; Safonova, E.A.; Zueva, E.P.; Kulagina, D.A.; Churin, А.A.; Fomina, T.I.; Sysolyatinm, S.V. A new analgesic agent based on hexaazaisowurzitan: Feasibility of using in managing patients with cancer. Sib. J. Oncol. 2020, 19, 76–81. [Google Scholar] [CrossRef]
- Krylova, S.G.; Lopatina, K.А.; Zueva, Е.P.; Safonova, Е.А.; Rybalkina, О.Y.; Povet’yeva, Т.N.; Suslov, N.I.; Kulagina, D.А.; Sysolyatin, S.V.; Zhdanov, V.V. Hexaazaizowurtzitane derivate as a new analgesic for chronic pain syndrome relief in experimental rheumatoid artritis. Rus. J. Pain 2020, 18, 5. [Google Scholar] [CrossRef]
- Bryushinina, O.S.; Yanovskaya, E.A.; Abdrashitova, N.Y.; Zyuz’kova, Y.G.; Frelikh, G.A.; Lopatina, K.A.; Zueva, E.P.; Krylova, S.G.; Kulagina, D.A.; Sysolyatin, S.V.; et al. Pharmacokinetics of a new analgesic on the basis of hexaazaisowurtzitane derivative. Bull. Exp. Biol. Med. 2021, 170, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Kulagina, D.A.; Sysolyatin, S.V.; Kalashnikov, A.I.; Malykhin, V.V.; Sonina, E.G.; Shevchenko, A.V.; Krylova, S.G.; Lopatina, K.A.; Zueva, E.P.; Razina, T.G.; et al. Synthesis and analgesic activity of 4,10-bis((±)-5-benzoyl-2,3-dihydro-1hpyrrolo[1,2-a]pyrrole-1-carbonyl)-2,6,8,12-tetraacetyl-2,4,6,8,10,12-hexaazatetracyclo[5,5,3,11,5,9]dodecane. Pharm. Chem. J. 2021, 54, 1140–1144. [Google Scholar] [CrossRef]
- Krylova, S.G.; Lopatina, K.A.; Nesterova, Y.V.; Povet’eva, T.N.; Kul’Pin, P.V.; Afanas’Eva, O.G.; Kulagina, D.A.; Safonova, E.A.; Zueva, E.P.; Suslov, N.I.; et al. Some aspects of investigation of the central mechanism of antinociceptive effect of a new analgetic from the hexaazaisowurtzitane group. Bull. Exp. Biol. Med. 2021, 170, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Krylova, S.G.; Lopatina, K.A.; Zueva, Е.P.; Safonova, Е.А.; Povet’eva, T.N.; Nesterova, Y.V.; Afanas’yeva, О.G.; Kul’pin, P.V.; Suslov, N.I.; Kulagina, D.А.; et al. Analgesic action of hexaazaisowurtzitane derivative in somatic pain models caused by TRPA1 and TRPV1 Ion channels activation. Bull. Sib. Med. 2020, 19, 110–118. [Google Scholar] [CrossRef]
- Tomilova, E.; Kurgachev, D.; Kulagina, D.; Sysolyatin, S.; Krylova, S.; Novikov, D. Development of HPLC-method for simultaneous determination of API and related components in thiowurtzine: A new non-narcotic analgesic. Chromatographia 2021, 84, 147–154. [Google Scholar] [CrossRef]
- Aguero, S.; Megy, S.; Eremina, V.; Kalashnikov, A.; Krylova, S.; Kulagina, D.; Lopatina, K.; Fournier, M.; Povetyeva, T.; Vorozhtsov, A.; et al. Discovery of a novel non-narcotic analgesic derived from the CL-20 explosive: Synthesis, pharmacology, and target identification of thiowurtzine, a potent inhibitor of the opioid receptors and the ion channels. ACS Omega 2021, 23, 15400. [Google Scholar] [CrossRef] [PubMed]
- Krylova, S.G.; Pove’teva, T.N.; Lopatina, K.A.; Nesterova, Yu.V.; Zueva, E.P.; Afanas’eva, O.G.; Kulpin, P.V.; Kiseleva, E.A.; Suslov, N.I.; Kulagina, D.A.; et al. Risk of developing drug abuse in administration of a new hexaazaisowurtzitane derivative-based analgesic (experimental study). Bull. Sib. Med. 2022, 21, 54–62. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikina, M.V.; Kulagina, D.A.; Sysolyatin, S.V. Nitration of 2,6,8,12-Tetraacetyl-2,4,6,8,10,12-Hexaazaisowurtzitane Derivatives. Materials 2022, 15, 7880. https://doi.org/10.3390/ma15227880
Chikina MV, Kulagina DA, Sysolyatin SV. Nitration of 2,6,8,12-Tetraacetyl-2,4,6,8,10,12-Hexaazaisowurtzitane Derivatives. Materials. 2022; 15(22):7880. https://doi.org/10.3390/ma15227880
Chicago/Turabian StyleChikina, Maya V., Daria A. Kulagina, and Sergey V. Sysolyatin. 2022. "Nitration of 2,6,8,12-Tetraacetyl-2,4,6,8,10,12-Hexaazaisowurtzitane Derivatives" Materials 15, no. 22: 7880. https://doi.org/10.3390/ma15227880
APA StyleChikina, M. V., Kulagina, D. A., & Sysolyatin, S. V. (2022). Nitration of 2,6,8,12-Tetraacetyl-2,4,6,8,10,12-Hexaazaisowurtzitane Derivatives. Materials, 15(22), 7880. https://doi.org/10.3390/ma15227880





