Sodium Dodecyl Sulfate–Mediated Graphene Sensor for Electrochemical Detection of the Antibiotic Drug: Ciprofloxacin
Abstract
1. Introduction
2. Materials and Methods
2.1. Used Chemicals and Reagents
2.2. Equipment and Instruments Used
2.3. Preparation of Standard Tablet and Urine Samples
2.4. Preparation and Evaluation of Fabricated Electrode
3. Results and Discussions
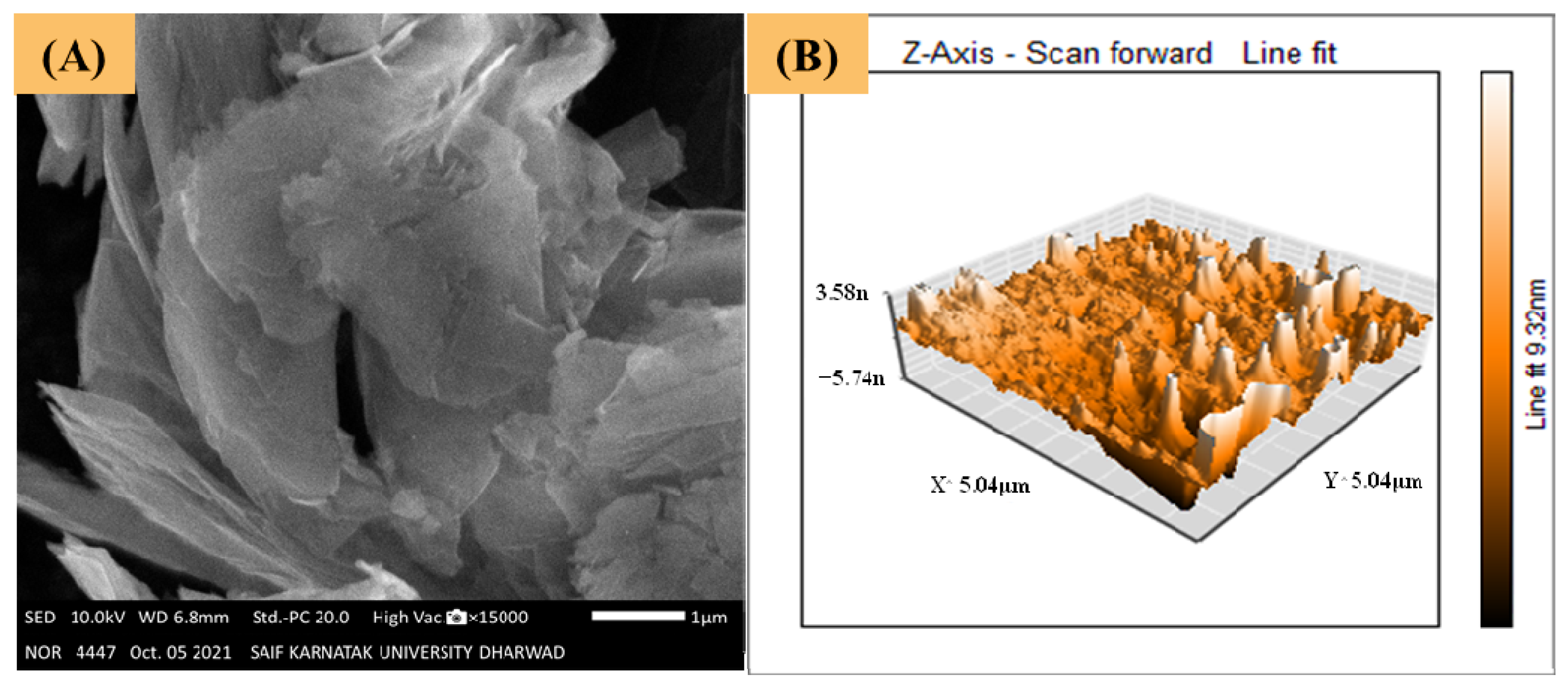
3.1. Topography and Morphology Studies
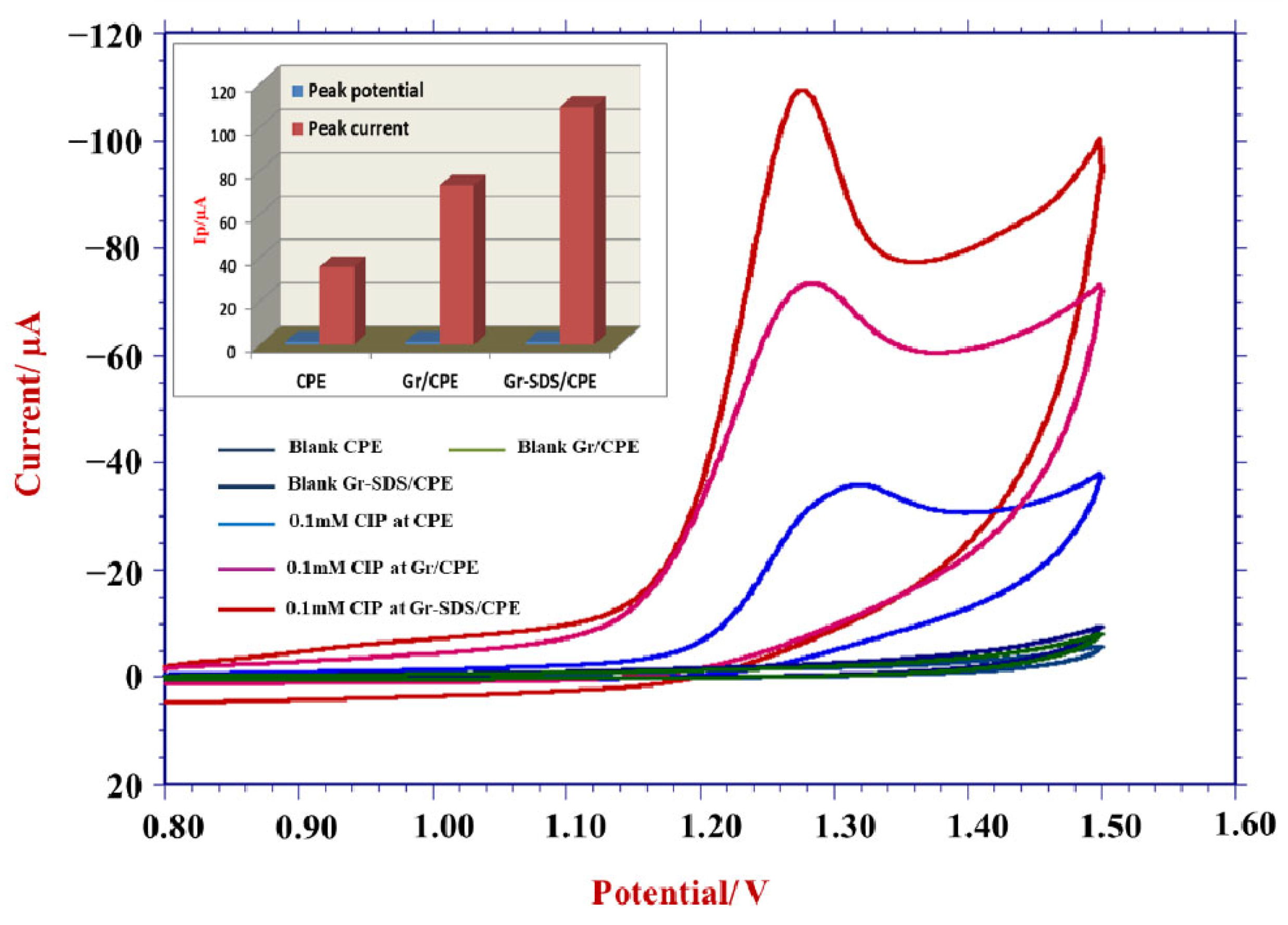
3.2. Impact of Electrode Modification
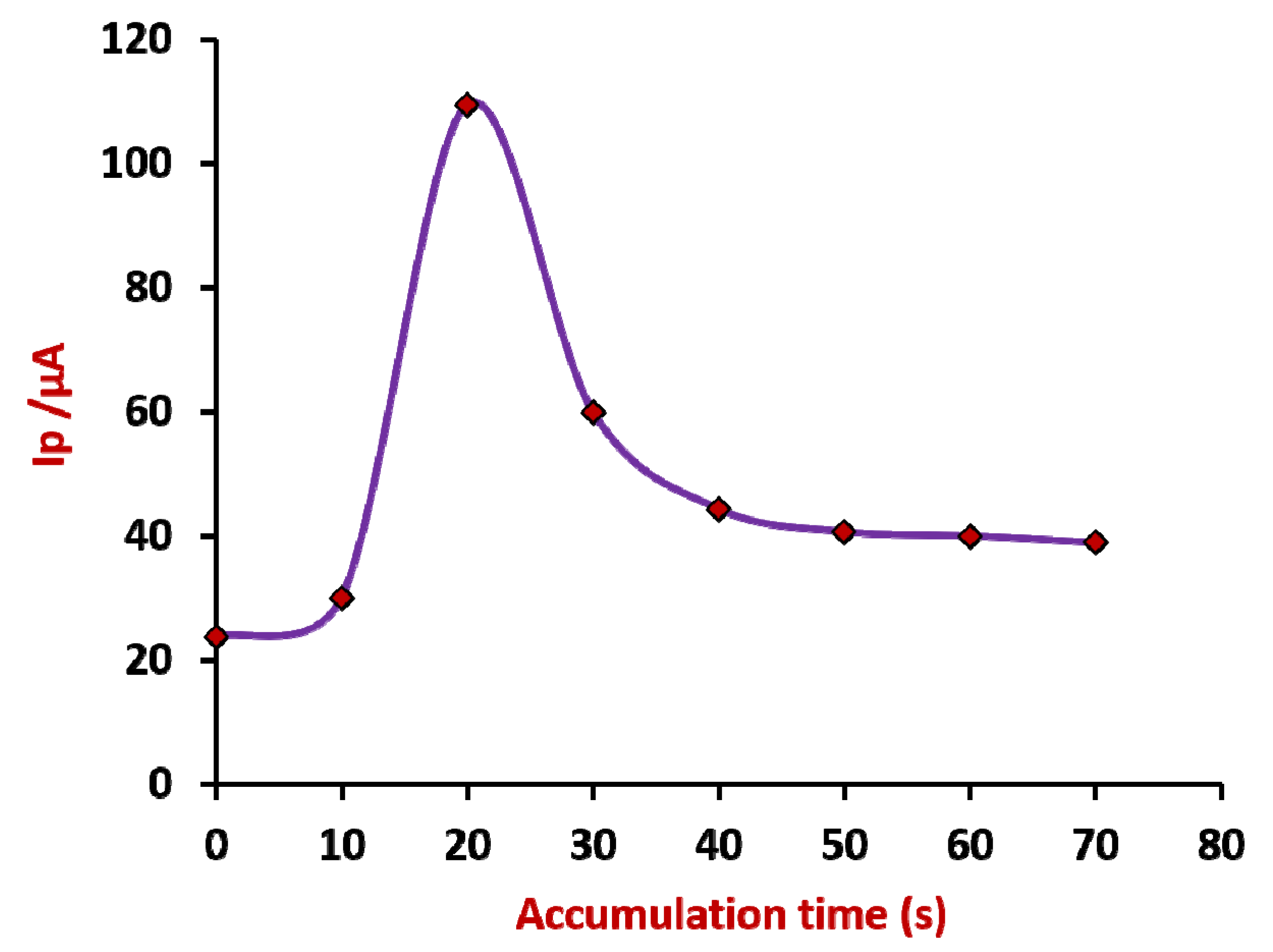
3.3. Accumulation Time
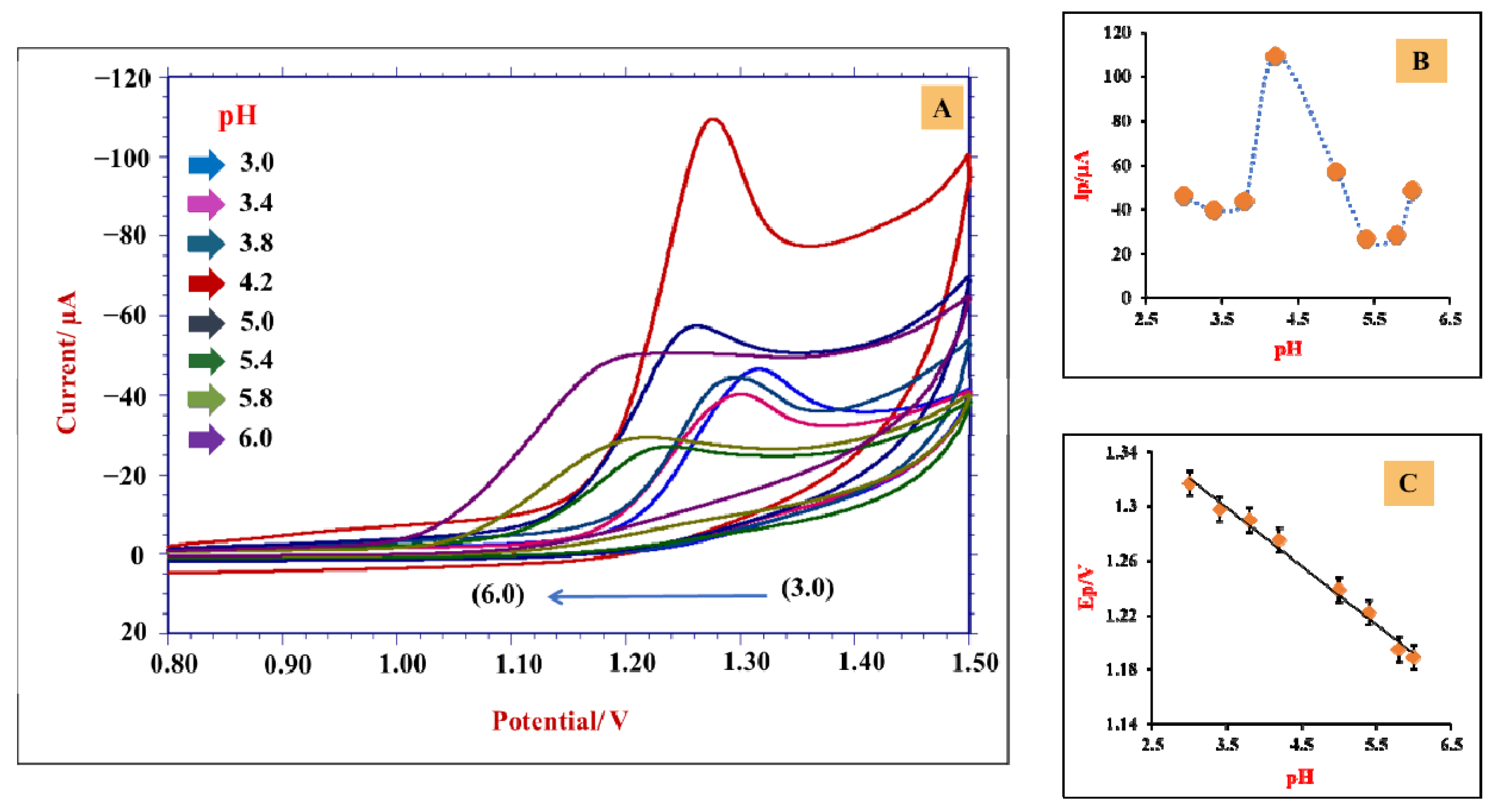
3.4. Impact of an Electrolytic Solution
3.5. Influence of Scan Rate
3.6. Plausible Electro-Oxidation Reaction of CIP
4. Analytical Applications
4.1. Quantification of CIP
4.2. Impact of Interferers
4.3. Recovery Studies
4.4. Stability of SDS·Gr/CPE
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kawas, G.; Marouf, M.; Mansour, O.; Sakur, A.A. Analytical methods of ciprofloxacin and its combinations review. Res. J. Pharm. Tech. 2018, 11, 2139–2148. [Google Scholar] [CrossRef]
- Osonwa, U.E.; Ugochukwu, J.I.; Ajaegbu, E.E.; Chukwu, K.I.; Azevedo, R.B.; Esimone, C.O. Enhancement of antibacterial activity of ciprofloxacin hydrochloride by complexation with sodium cholate. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 233–237. [Google Scholar] [CrossRef]
- Cheng, A.-C.; Turnidge, J.; Collignon, P.; Looke, D.; Barton, M.; Gottlieb, T. Control of Fluoroquinolone Resistance through Successful Regulation. Emerg. Infect. Dis. 2012, 18, 1453–1460. [Google Scholar] [CrossRef]
- Salehniya, H.; Amiri, M.; Mansoori, Y. Positively charged carbon nanoparticulate/sodium dodecyl sulfate bilayer electrode for extraction and voltammetric determination of ciprofloxacin in real samples. RSC Adv. 2016, 6, 30867–30874. [Google Scholar] [CrossRef]
- Scherer, R.; Pereira, J.; Firme, J.; Lemos, M.; Lemos, M. Determination of Ciprofloxacin in Pharmaceutical Formulations Using HPLC Method with UV Detection. Indian J. Pharm. Sci. 2014, 76, 541–544. [Google Scholar] [PubMed]
- Vella, J.; Busuttil, F.; Bartolo, N.S.; Sammut, S.; Ferrito, V.; Serracino-Inglott, A.; Azzopardi, L.M.; LaFerla, G. A simple HPLC–UV method for the determination of ciprofloxacin in human plasma. J. Chromatogr. B 2015, 989, 80–85. [Google Scholar] [CrossRef]
- Neckel, U.; Joukhadar, C.; Frossard, M.; Jager, W.; Muller, M.; Mayer, B.X. Simultaneous determination of levofloxacin and ciprofloxacin in microdialysates and plasma by high-performance liquid chromatography. Anal. Chim. Acta 2003, 463, 199–206. [Google Scholar] [CrossRef]
- Vilchez, J.L.; Araujo, L.; Prieto, A.; Navalon, A. Determination of ciprofloxacin and enoxacin in human serum samples by micellar liquid chromatography. Anal. Chim. Acta 2004, 516, 135–140. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, Y.; Liu, M. Vortex-assisted acid-induced cloud point extraction coupled with spectrofluorometry for the determination of fluoroquinolonesin environmental water samples. Spectrosc. Lett. 2014, 47, 206–213. [Google Scholar] [CrossRef]
- Li, Z.; Cui, Z.; Tang, Y. Fluorometric determination of ciprofloxacin using molecularly imprinted polymer and polystyrene microparticles doped with europium (III)(DBM)3phen. Microchim. Acta 2019, 186, 334. [Google Scholar] [CrossRef]
- Pascual-Reguera, M.I.; Perez Parra, G.; Molina Diaz, A. A single spectroscopic flow through sensing device for determination of ciprofloxacin. J. Pharm. Biomed. Anal. 2004, 35, 689–695. [Google Scholar] [CrossRef]
- Kalunke, R.M.; Grasso, G.; D’Ovidio, R.; Dragone, R.; Frazzoli, C. Detection of ciprofloxacin residues in cow milk: A novel and rapid optical b-galactosidase-based screening assay. Microchem. J. 2018, 136, 128–132. [Google Scholar] [CrossRef]
- Koska, I.; Purgat, K.; Kubalczyk, P. Simple, fast and reliable CE method for simultaneous determination of ciprofloxacin and ofloxacin in human urine. Sci. Rep. 2022, 12, 7729. [Google Scholar] [CrossRef] [PubMed]
- Shetti, N.P.; Shanbhag, M.M.; Malode, S.J.; Srivastava, R.K.; Reddy, K.R. Amberlite XAD-4 modified electrodes for highly sensitive electrochemical determination of nimesulide in human urine. Microchem. J. 2020, 153, 104389. [Google Scholar] [CrossRef]
- Sawkar, R.R.; Shanbhag, M.M.; Tuwar, S.M.; Shetti, N.P. Silica gel-based electrochemical sensor for tinidazole. Sens. Int. 2022, 3, 100192. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Arotiba, O.A. Simultaneous determination of cholesterol, ascorbic acid and uric acid as three essential biological compounds at a carbon paste electrode modified with copper oxide decorated reduced graphene oxide nanocomposite and ionic liquid. J. Colloid Interface Sci. 2020, 560, 208–212. [Google Scholar] [CrossRef]
- Patil, V.B.; Sawkar, R.R.; Ilager, D.; Shetti, N.P.; Tuwar, S.M.; Aminabhavi, T.M. Glucose based sensor for the trace level detection of acetoaminophen in pharmaceutical and biological samples. Electrochem. Sci. Adv. 2021, 2, e202100117. [Google Scholar] [CrossRef]
- Sawkar, R.R.; Patil, V.B.; Tuwar, S.M. Electrochemical oxidation of Atorvastatin using graphene oxide and surfactant-based sensor. Mater. Today Proc. 2022, in press. [Google Scholar] [CrossRef]
- Patil, V.B.; Malode, S.J.; Shetti, N.P.; Baghayeri, M.; Mangasuli, S.N.; Tuwar, S.M.; Mondal, K. An electrochemical electrode to detect theophylline based on copper oxide nanoparticles composited with graphene oxide. Micromachines 2022, 13, 1166. [Google Scholar] [CrossRef]
- Bukkitgar, S.D.; Shetti, N.P. Electrochemical sensor for the determination of anticancer drug 5-fluorouracil at glucose modified electrode. ChemistrySelect 2016, 1, 771–777. [Google Scholar] [CrossRef]
- Rejithamol, R.; Beena, S. Carbon Paste Electrochemical Sensors for the Detection of Neurotransmitters. Front. Sens. 2022, 3, 901628. [Google Scholar] [CrossRef]
- Shiba, S.; Kamata, T.; Kato, D.; Niwa, O. Electroanalysis with carbon film-based electrodes. In Nanocarbons for Electroanalysis; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Zhang, Z.; Ohta, S.; Shiba, S.; Niwa, O. Nanocarbon film electrodes for electroanalysis and electrochemical sensors. Curr. Opin. Electrochem. 2022, 35, 101045. [Google Scholar] [CrossRef]
- Munoz, J.; Baeza, M. Customized bio-functionalization of nanocomposite carbon paste electrodes for electrochemical sensing: A mini review. Electroanalysis 2017, 29, 1660–1669. [Google Scholar] [CrossRef]
- Moscoso, R.; Alvarez-Lueje, A.; Squella, J.A. Nanostructured interfaces containing MWCNT and nitro aromatics: A new tool to determine Nimesulide. Microchem. J. 2020, 159, 105361. [Google Scholar] [CrossRef]
- Radic, J.; Buljac, M.; Genorio, B.; Gricar, E.; Kolar, M. A Novel Reduced Graphene Oxide Modified Carbon Paste Electrode for Potentiometric Determination of Trihexyphenidyl Hydrochloride in Pharmaceutical and Biological Matrices. Sensors 2021, 21, 2955. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sanchez, C.; Montiel-Gonzalez, F.; Rodriguez-Gonzalez, V. Electrochemical sensing of acetaminophen using a practical carbon paste electrode modified with a graphene oxide-Y2O3 nanocomposite. J. Taiwan Inst. Chem. Eng. 2019, 96, 382–389. [Google Scholar] [CrossRef]
- Frag, E.Y.; El Badry Mohamed, M.; Mohamed, G.G.; Ebrahim, M.S. Selective potentiometric sensors for the determination of butenafine hydrochloride in a cream formulation. Microchem. J. 2020, 157, 104870. [Google Scholar] [CrossRef]
- Ashrafi, A.M.; Richtera, L. Preparation and Characterization of Carbon Paste Electrode Bulk-Modified with Multiwalled Carbon Nanotubes and Its Application in a Sensitive Assay of Antihyperlipidemic Simvastatin in Biological Samples. Molecules 2019, 24, 2215. [Google Scholar] [CrossRef]
- Malode, S.J.; Prabhu, K.; Shetti, N.P.; Reddy, K.R. Highly sensitive electrochemical assay for selective detection of Aminotriazole based on TiO2/poly (CTAB) modified sensor. Environ. Technol. Innov. 2021, 21, 101222. [Google Scholar] [CrossRef]
- Prabhu, K.; Malode, S.J.; Veerapur, R.S.; Shetti, N.P. Clay-based carbon sensor for electro-oxidation of nimesulide. Mater. Chem. Phys. 2021, 272, 124992. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Safaei, M.; Zhang, K.; Van Le, Q.; Varma, R.S.; Jang, H.O.; Shokouhimehr, M. Developments and applications of nanomaterial-based carbon paste electrodes. RSC Adv. 2020, 10, 21561–21581. [Google Scholar] [CrossRef]
- Reddy, Y.V.; Shin, J.H.; Palakollu, V.N.; Sravani, B.; Choi, C.H.; Park, K.; Kim, S.K.; Madhavi, G.; Park, J.P.; Shetti, N.P. Strategies, advances, and challenges associated with the use of graphene-based nanocomposites for electrochemical biosensors. Adv. Colloid Interface Sci. 2022, 304, 102664. [Google Scholar] [CrossRef]
- Ilager, D.; Shetti, N.P.; Reddy, K.R.; Tuwar, S.M.; Aminabhavi, T.M. Nanostructured graphitic carbon nitride (g-C3N4)-CTAB modified electrode for the highly sensitive detection of amino-triazole and linuron herbicides. Environ. Res. 2022, 204, 111856. [Google Scholar] [CrossRef]
- Bukkitgar, S.D.; Shetti, N.P. Fabrication of a TiO2 and clay nanoparticle composite electrode as a sensor. Anal. Methods 2017, 9, 4387–4393. [Google Scholar] [CrossRef]
- Kumar, S.; Bukkitgar, S.D.; Singh, S.; Pratibha; Singh, V.; Reddy, K.R.; Shetti, N.P.; Reddy, C.V.; Sadhu, V.; Naveen, S. Electrochemical Sensors and Biosensors Based on Graphene Functionalized with Metal Oxide Nanostructures for Healthcare Applications. ChemistrySelect 2019, 4, 5322–5337. [Google Scholar] [CrossRef]
- Miraki, M.; Karimi-Maleh, H.; Taher, M.A.; Cheraghi, S.; Karimi, F.; Agarwal, S.; Gupta, V.K. Voltammetric amplified platform based on ionic liquid/NiO nanocomposite for determination of benserazide and levodopa. J. Mol. Liq. 2019, 278, 672–676. [Google Scholar] [CrossRef]
- Khodadadi, A.; Faghih-Mirzaei, E.; Karimi-Maleh, H.; Abbaspourrad, A.; Agarwal, S.; Gupta, V.K. A new epirubicin biosensor based on amplifying DNA interactions with polypyrrole andnitrogen-doped reduced graphene: Experimental and docking theoretical investigations. Sens. Actuators B Chem. 2019, 284, 568–574. [Google Scholar] [CrossRef]
- Killedar, L.S.; Shanbhag, M.M.; Shetti, N.P.; Malode, S.J.; Veerapur, R.S.; Reddy, K.R. Novel graphene-nanoclay hybrid electrodes for electrochemical determination of theophylline. Microchem. J. 2021, 165, 106115. [Google Scholar] [CrossRef]
- Ilager, D.; Shetti, N.P.; Foucaud, Y.; Badawi, M.; Aminabhavi, T.M. Graphene/g-carbon nitride (GO/g-C3N4) nanohybrids as a sensor material for the detection of methyl parathion and carbendazim. Chemosphere 2022, 292, 133450. [Google Scholar] [CrossRef]
- Heerema, S.J.; Dekker, C. Graphene nanodevices for DNA sequencing. Nat. Nanotechnol. 2016, 11, 127–136. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, X. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Tao, Y.; Sui, Z.-Y.; Han, B.-H. Advanced porous graphene materials: From in-plane pore generation to energy storage applications. J. Mater. Chem. A 2020, 8, 6125–6143. [Google Scholar] [CrossRef]
- Dasari Shareena, T.P.; McShan, D.; Dasmahapatra, A.K. A Review on Graphene-Based Nano-materials in Biomedical Applications and Risks in Environment and Health. Nano-Micro Lett. 2018, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, P.; Ouyang, Z.; Zhang, M.; Lin, Z.; Li, J.; Su, Z.; Wei, G. Nanoscale graphene doped with highly dispersed silver nanoparticles: Quick synthesis, facile fabrication of 3D membrane-modified electrode, and super performance for electrochemical sensing. Adv. Funct. Mater. 2016, 26, 2122–2134. [Google Scholar] [CrossRef]
- Song, H.E.; Zhang, X.; Liu, Y.; Su, Z. Developing Graphene-Based Nanohybrids for Electrochemical Sensing. Chem. Rec. 2019, 19, 534–549. [Google Scholar] [CrossRef]
- Lin, D.; Su, Z.; Wei, G. Three-dimensional porous reduced graphene oxide decorated with MoS2 quantum dots for electrochemical determination of hydrogen peroxide. Mater. Today Chem. 2018, 7, 76–83. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Shetti, N.P.; Malode, S.J.; Veerapur, R.S.; Reddy, K.R. Cholesterol intercalated 2D graphene oxide sheets fabricated sensor for voltametric analysis of theophylline. FlatChem 2021, 28, 100255. [Google Scholar] [CrossRef]
- Housaindokht, M.R.; Janati-Fard, F.; Ashraf, N. Recent advances in applications of surfactant-based voltammetric sensors. J. Surfact Deterg. 2021, 24, 873–895. [Google Scholar] [CrossRef]
- Amrutha, B.M.; Manjunatha, J.G.; Bhatt, A.S.; Hareesha, N. Electrochemical analysis of evans blue by surfactant modified carbon nanotube paste electrode. J. Mater. Environ. Sci. 2019, 10, 668–676. [Google Scholar]
- Nayak, D.S.; Shetti, N.P. Voltammetric Response and Determination of an anti-inflammatory drug at a cationic surfactant-modified glassy carbon electrode. J. Surf. Deterg. 2016, 19, 1071–1079. [Google Scholar] [CrossRef]
- Zhai, H.; Wang, S.; Zhou, J.; Pan, J.; Tong, Y.; Mei, Q.; Zhou, Q. A Simple and Sensitive Electrochemical Sensor for 3-Nitrotyrosine Based on Electrochemically Anodic Pretreated Glassy Carbon Electrode in Anionic Surfactant Medium. J. Electrochem. Soc. 2019, 166, B1426. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; Leddy, J.; Zoski, C.G. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Sawkar, R.R.; Patil, V.B.; Shanbhag, M.M.; Shetti, N.P.; Tuwar, S.M.; Aminabhavi, T.M. Detection of ketorolac drug using pencil graphite electrode. Biomed. Eng. Adv. 2021, 2, 100009. [Google Scholar] [CrossRef]
- Shetti, N.P.; Nayak, D.S.; Malode, S.J.; Kulkarni, R.M.; Kulkarni, D.M.; Teggi, R.A.; Joshi, V.V. Electrooxidation and determination of flufenamic acid at graphene oxide modifed carbon electrode. Surf. Interfaces 2017, 9, 107–113. [Google Scholar] [CrossRef]
- Dutton, P.L. Redox potentiometry: Determination of midpoint potentials of oxidation-reduction components of biological electron-transfer systems. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1978; pp. 411–435. [Google Scholar]
- Sawkar, R.R.; Shanbhag, M.M.; Tuwar, S.M.; Veerapur, R.S.; Shetti, N.P. Glucose incorporated graphite matrix for electroanalysis of trimethoprim. Biosensors 2022, 12, 909. [Google Scholar] [CrossRef]
- Alam, A.U.; Jamal Deen, M. Bisphenol a electrochemical sensor using graphene oxide and β-Cyclodextrin-Functionalized Multi-Walled Carbon Nanotubes. Anal. Chem. 2020, 92, 5532–5539. [Google Scholar] [CrossRef]
- Xie, A.-J.; Chen, Y.; Luo, S.-P.; Tao, Y.-W.; Jin, Y.-S.; Li, W.-W. Electrochemical detection of ciprofloxacin based on graphene modified glassy carbon electrode. Mater. Technol. 2015, 30, 362–367. [Google Scholar] [CrossRef]
- Sawkar, R.R.; Shanbhag, M.M.; Tuwar, S.M.; Mondal, K.; Shetti, N.P. Zinc Oxide–Graphene nanocomposite-based sensor for the electrochemical determination of cetirizine. Catalysts 2022, 12, 1166. [Google Scholar] [CrossRef]
- Ipte, P.R.; Kumar, S.; Satpati, A.K. Electrochemical synthesis of carbon nano spheres and its application for detection of ciprofloxacin. J. Environ. Sci. Health A 2019, 55, 142–150. [Google Scholar] [CrossRef]
- Shan, J.; Liu, Y.; Li, R.; Wu, C.; Zhu, L.; Zhang, J. Indirect electrochemical determination of ciprofloxacin by anodic stripping voltammetry of Cd (II) on graphene-modified electrode. J. Electroanal. Chem. 2015, 738, 123–129. [Google Scholar] [CrossRef]
- Pushpanjali, P.A.; Manjunatha, J.G.; Shreenivas, M.T. The electrochemical resolution of Ciprofloxacin, Riboflavin and estriol using anionic surfactant and polymer-modified carbon paste electrode. ChemistrySelect 2019, 4, 13427–13433. [Google Scholar] [CrossRef]
- Tajeu, K.Y.; Ebunang, D.V.T.; Tonleu, R.C.T.; Jiokeng, S.L.Z.; Ymele, E.; Tonle, I.K. Electroanalytical application of thiol-grafted laponite to the sensitive quantification of ciprofloxacin antibiotic. J. Appl. Electrochem. 2021, 51, 435–446. [Google Scholar] [CrossRef]

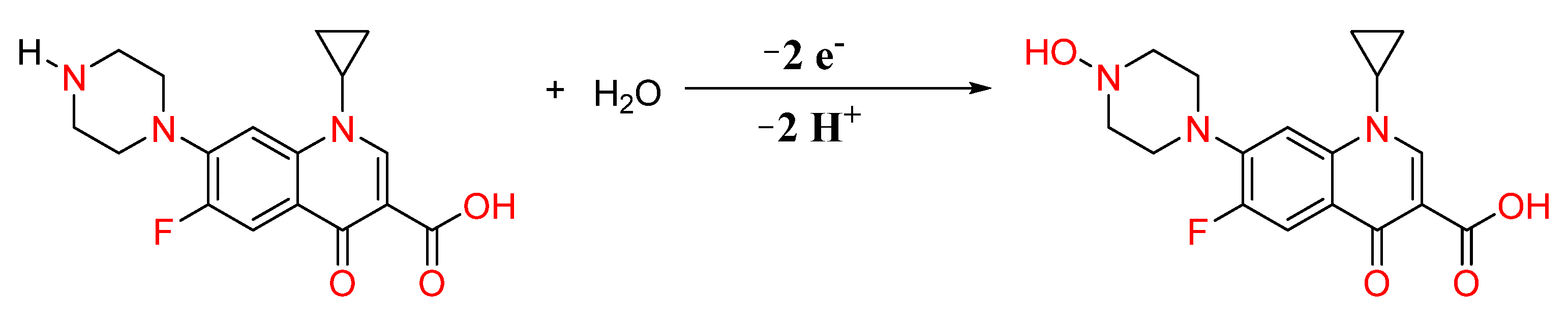
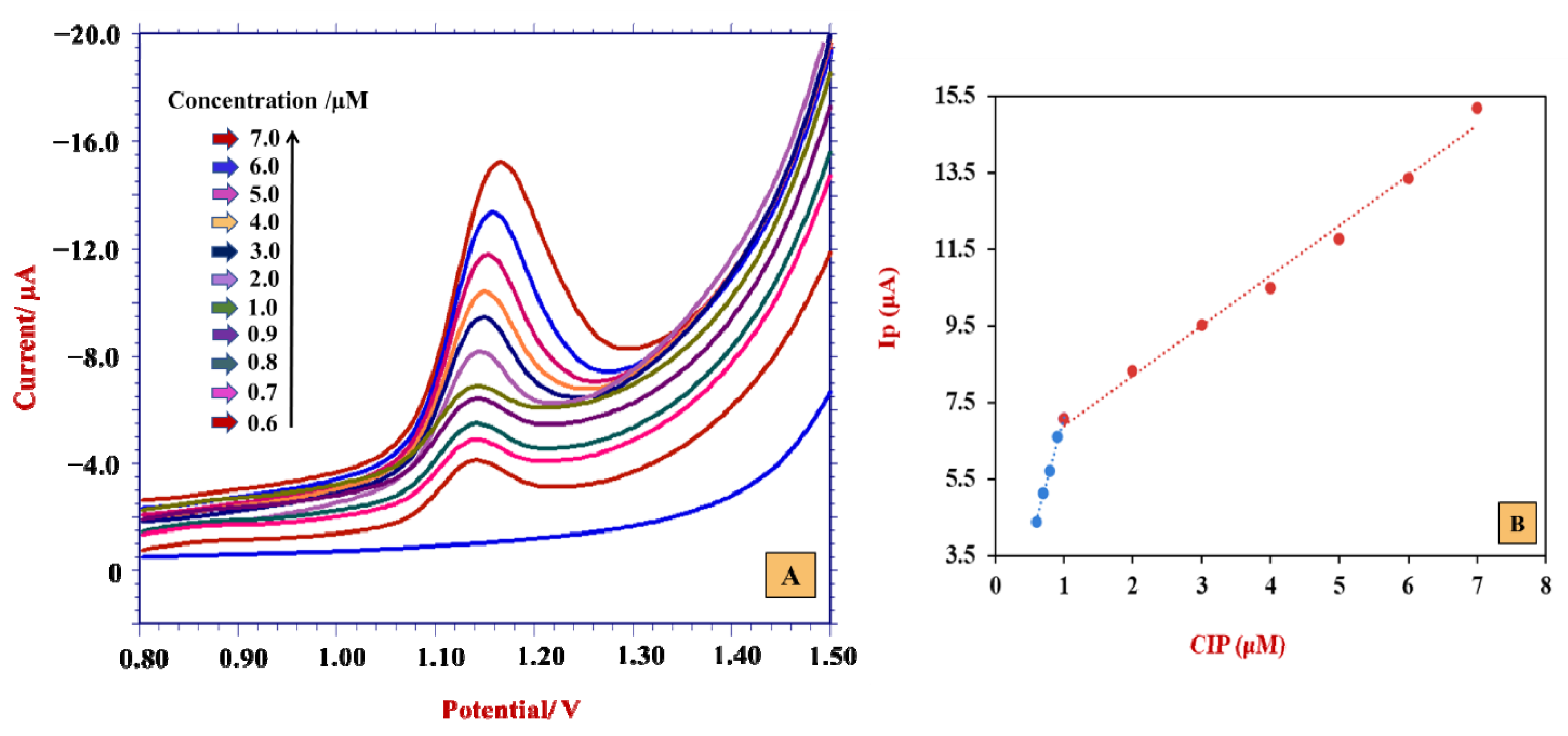

| Calibration Characteristics | CIP |
|---|---|
| Linearity range | 0.3 × 10−6 M to 100.0 × 10−6 M |
| Concentration range | 0.6 × 10−6 M to 1.0 × 10−6 M |
| Value of slope | 6.92 |
| Standard deviation of slope | 0.083 |
| Value of intercept | 0.232 |
| Standard deviation of intercept | 0.067 |
| Regression coefficient | 0.992 |
| Total data points | 05 |
| LD | 0.03 μM |
| LQ | 0.1 μM |
| Electrode | LD (µM) | LQ (µM) | Linear Range (μM) | pH | Reference |
|---|---|---|---|---|---|
| GNs-GCE (DPV) | 0.02 | - | 0.5–200 | 2.0 | [59] |
| Carbon nanospheres (DPV) | 0.15 | - | 0.5–5 | 7.0 | [61] |
| Cd(II)/graphene-based electrode (ASV) | 0.05 | - | 0.1–10 | 3.6 | [62] |
| Surfactant-CPE (DPV) | 0.18 | 0.61 | 2.0–4.0 | 6.5 | [63] |
| Thiol-grafted laponite GCE (DPV) | 0.26 | - | 10–110 | 2.0 | [64] |
| SDS·Gr/CPE (DPV) | 0.029 | 0.096 | 0.3–100 | 4.2 | Present method |
| Mentioned amount (mg) | 500 |
| Amount obtained (mg) a | 471 |
| RSD (%) | 3.04 |
| Added (mg) | 1.0 |
| Obtained (mg) a | 0.93 |
| Recovered (%) | 93.8 |
| Sample No. | Spiked (×10−5 M) | Obtained (×10−5 M) | Recovery (%) |
|---|---|---|---|
| #1 | 0.1 | 0.09 | 98.7 |
| #2 | 0.2 | 0.19 | 95.0 |
| #3 | 0.3 | 0.28 | 93.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawkar, R.R.; Shanbhag, M.M.; Tuwar, S.M.; Mondal, K.; Shetti, N.P. Sodium Dodecyl Sulfate–Mediated Graphene Sensor for Electrochemical Detection of the Antibiotic Drug: Ciprofloxacin. Materials 2022, 15, 7872. https://doi.org/10.3390/ma15227872
Sawkar RR, Shanbhag MM, Tuwar SM, Mondal K, Shetti NP. Sodium Dodecyl Sulfate–Mediated Graphene Sensor for Electrochemical Detection of the Antibiotic Drug: Ciprofloxacin. Materials. 2022; 15(22):7872. https://doi.org/10.3390/ma15227872
Chicago/Turabian StyleSawkar, Rakesh R., Mahesh M. Shanbhag, Suresh M. Tuwar, Kunal Mondal, and Nagaraj P. Shetti. 2022. "Sodium Dodecyl Sulfate–Mediated Graphene Sensor for Electrochemical Detection of the Antibiotic Drug: Ciprofloxacin" Materials 15, no. 22: 7872. https://doi.org/10.3390/ma15227872
APA StyleSawkar, R. R., Shanbhag, M. M., Tuwar, S. M., Mondal, K., & Shetti, N. P. (2022). Sodium Dodecyl Sulfate–Mediated Graphene Sensor for Electrochemical Detection of the Antibiotic Drug: Ciprofloxacin. Materials, 15(22), 7872. https://doi.org/10.3390/ma15227872







