Abstract
As an edible oil, palm oil is also safe and reliable in dyeing, and the residual palm oil after dyeing can be recycled and used continuously, which is green and environmentally friendly and has great research prospects. In this research, raw ramie yarn, used for traditional grass cloth, was dyed in a palm oil medium using Reactive Blue 194. Studying the adsorption and diffusion behaviour in the dyeing process is necessary. Additionally, the kinetics and isotherm model of dyeing raw ramie yarn with Reactive Blue 194 in palm oil is studied, and the adsorption behaviour between them is discussed. For a better understanding, the raw ramie yarn dyeing adsorption behaviour was also carried out in a water medium. It was found that the dyeing rates in palm oil are distinctly faster than in water. Kinetics data suggested that the pseudo-second-order model fitted for both dyeing mediums (palm oil and water) of the adsorption of the Reactive Blue 194 dye onto raw ramie yarn. Afterward, the adsorption isotherms’ results denote that the Langmuir model was suitable for palm oil dyeing medium while the Freundlich model was suited for water medium. Overall, this study has demonstrated that raw ramie yarn dyeing in a palm oil medium could be a sustainable colouration route for textile fibres with a greater dye exhaustion percentage.
1. Introduction
Traditional grass cloth, also named Xiabu, refers to the woven fabric produced from raw ramie yarns by handwork (Figure 1), including the manual preparation of raw ramie yarns. Traditional grass cloth differs from modern grass cloth, whose manufacturing process is finished by modern textile machines. Traditional grass cloth has special significance in Chinese textile history as it has existed for more than 6000 years [1]. It is rare to use traditional grass cloth as garment fabric now, but it is commonly used to produce all kinds of decorative items [2,3]. In 2008, the manufacturing process of traditional grass cloth was recorded in the National Intangible Cultural Heritage List, established by the government of China. To maintain and develop the manufacturing technics of traditional grass cloth, the utilization of colourful raw ramie yarns to produce a special pattern of traditional grass cloth is a potential option.

Figure 1.
Preparation of traditional grass cloth.
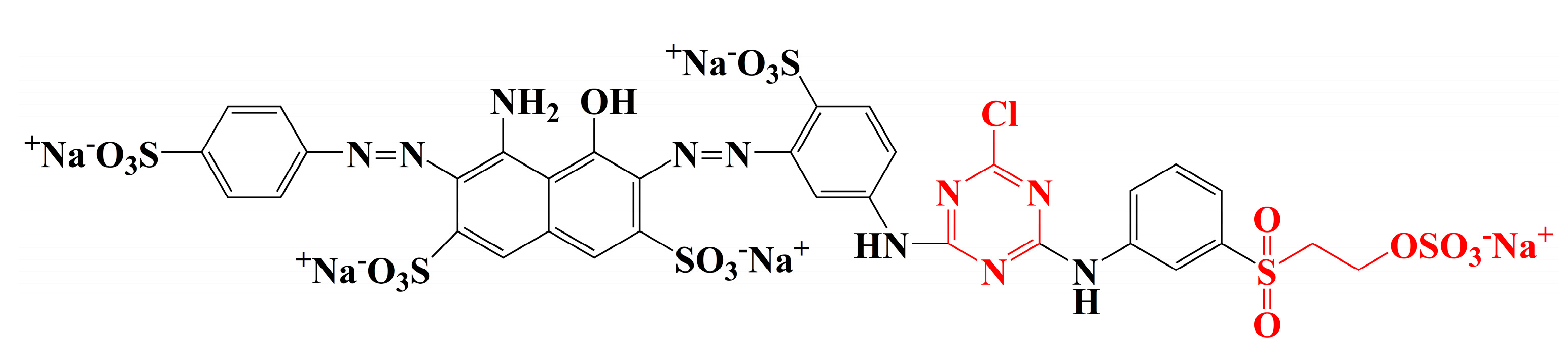
Raw ramie yarns are prepared by tearing the ramie phloem components into small yarns and drying them, and their main constituents include cellulosic fibres and gummy materials [4]. The gummy materials bind each cellulosic fibre [5], resulting in a hard and rough surface with a ditch-like cavity, which contributes a special and unique style to traditional grass cloth. The molecular structures of the gummy components [6] (hemicelluloses, pectin, and lignin) are similar to cellulose, which have free hydroxyl groups and hydrophilic property [7]. Therefore, reactive dye is beneficial for the colouration of raw ramie yarn because it not only offers varieties of brilliant colour, but also gives excellent washfastness by covalently bonding [8,9,10]. Generally, reactive dyes [8,11] of the dichlorotriazinyl group, vinyl sulphone group, or monochlorotriazinyl group are mainly applied for cellulosic fibre dyeing. Dichlorotriazinyl reactive dye can be fixed with cellulosic fibre at low temperatures (30–50 °C) and is mostly used in pad-bath dyeing. However, the residual monochlorotriazinyl group is potentially hydrolysed, and it then generates acidic conditions to accelerate the hydrolyses of a covalent bond between the reactive dye and fibre. Thus, the dichlorotriazinyl reactive dye is gradually abandoned. Vinyl sulphone reactive dye is fixed at medium temperature (50–70 °C), but the stability of the covalent bond between reactive dye and fibre is the poorest among these three reactive dyes. Thereby, reactive dye with a sole vinyl sulphone group is rarely used. For monochlorotriazinly reactive dye, although the covalent bond is the most stable, the required fixation temperature is high (80–100 °C). Thus, the monochlorotriazinyl reactive dye is widely used for printing. Therefore, in the reactive dye category, bi-functional reactive dye includes one monochlorotriazinyl reactive group and one vinyl sulphone reactive group, widely applied in the dyeing of cellulosic fibre because of the medium fixation temperature and the stable covalent bond; for example, Reactive Blue 194 (Figure 2). However, the problems of high water consumption and water pollution, as well as the fact that the dye utilization rate concerning reactive dyeing is unsatisfactory, make it urgent to find a method to solve these problems [12,13]. To reduce water consumption, salt, and alkali usage in reactive dyeing, the dyeing process using vegetable oil as a medium is gradually being tested and approved by researchers [14,15,16,17], which is a cleaner dyeing production and is effective in sorting environmental pollution problems out. However, after reactive dyeing in cottonseed oil, the transparent cottonseed oil became muddy, especially for the dyeing at high pH and high temperature, which was caused by the transformation of the saturated fatty acids from unsaturated ones. This transformation challenges the recycling and reusing of cottonseed oil.
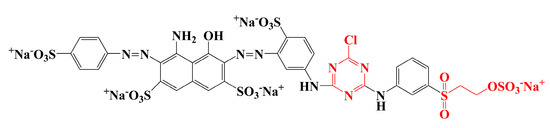
Figure 2.
Molecular structure of Reactive Blue 194.
Palm oil is one of the vegetable oils extracted from palm trees. The high yield and low production cost of palm oil make it a competitive vegetable oil [18]. Palm oil has high heat and oxidative stabilities, and it is widely used for food frying [19]. Thus, it is not harmful to humans. Since palm oil has fewer unsaturated fatty acids than other liquid vegetable oils [20], it has resistance to high temperature and high pH conditions. Therefore, using palm oil as a medium in the reactive dyeing of cellulosic fibre can avoid the muddy problem of cottonseed oil after reactive dyeing.
In our previous paper [21], traditional grass cloth dyeing with reactive dye in palm oil medium exhibits a good dyeing performance, including a high dye exhaustion percentage (95.4%) and acceptable dye fixation rate (50.6%). Reactive dyeing in palm oil has some advantages as compared to in water [22]. First, palm oil dyeing is a waterless dyeing process, so it decreases water consumption. Second, the elimination of electrolyte addition in dyeing reduces chemical consumption. Third, the solid reactive dyes in palm oil are exhausted fast and large in the wet substance. Forth, the dispersion of solid reactive dyes in palm oil medium avoids the problem of reactive dye hydrolysis in the dye bath during dyeing; thus, it is effective in the promotion of total dye fixation and eases the reuse of residual dye baths. Finally, it is easy to recycle palm oil, which can be collected by static separation of residual palm oil dye bath with water addition. It is worth noting that after the dyeing, high efficient soaping auxiliary is essential to wash off the palm oil adsorbed in the dyed substance, as well as the unfixed reactive dye. The washed water containing a little palm oil is discharged to the wastewater treatment station. However, palm oil is environmentally friendly and biodegradable [23], so aquatic creatures are unharmed when it is in the water.
The cleaner production of raw ramie yarn dyeing in palm oil medium is framed. The present work is the first to discover the dye adsorption behaviours, including kinetics and isotherm models, in the dyeing of raw ramie yarn in a palm oil medium compared to that in aqueous dyeing. However, the dye fixation treatment was not considered because it prompts further dye exhaustion, i.e., second exhaustion, and consequently interferes with the analysis of the dyeing kinetics and isotherm models. These investigations are beneficial to better understanding the palm oil dyeing process.
2. Materials and Methods
2.1. Materials
Raw ramie yarn was purchased from the local market. Palm oil was obtained from Feidong Jufeng Grain and Oil Food Factory. Reactive Blue 194 was purchased from Shanghai Jiaying Chemical Company (Shanghai, China).
2.2. Dyeing Process
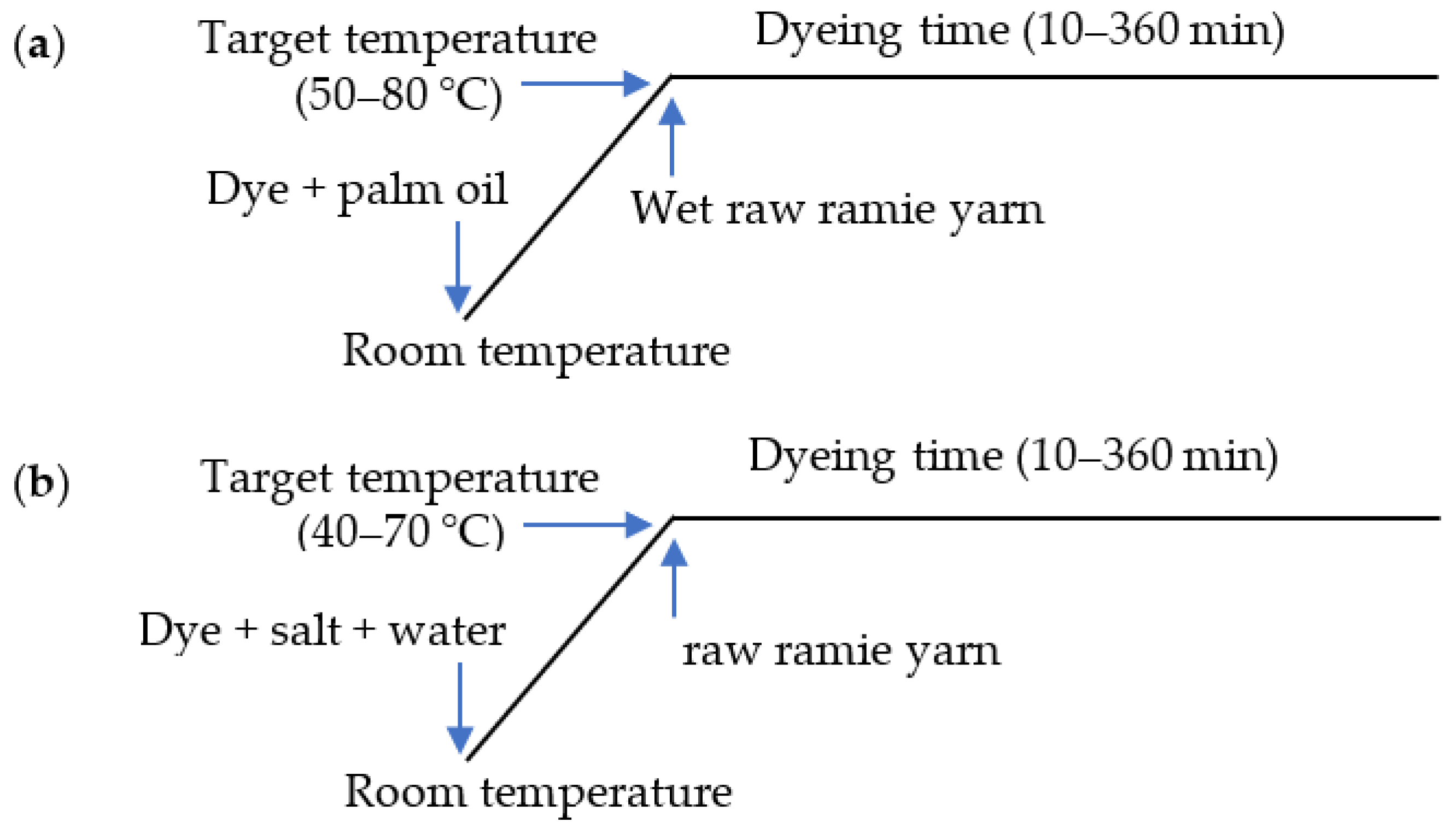
In palm oil medium dyeing, before dyeing, the raw ramie yarn (2 g) was wetted by dipping in water and then padding twice with a 140% pick-up rate. For the kinetic dyeing analysis, the wet raw ramie yarn was dyed with Reactive Blue 194 (0.06 g) in palm oil (40 mL) at a variety of dyeing temperatures from 50–80 °C for 10–360 min with a swing dyeing machine (Foshan Ronggui Huibao Dyeing and Finishing Machinery Factory, Foshan, China). For the thermodynamic dyeing analysis, the wet ramie yarn was dyed with a variety of Reactive Blue 194 dye mass from 0.004 to 0.08 g in palm oil (40 mL) at various dyeing temperatures from 50–80 °C for 360 min with the swing dyeing machine. The dyeing profile is shown in Figure 3a.
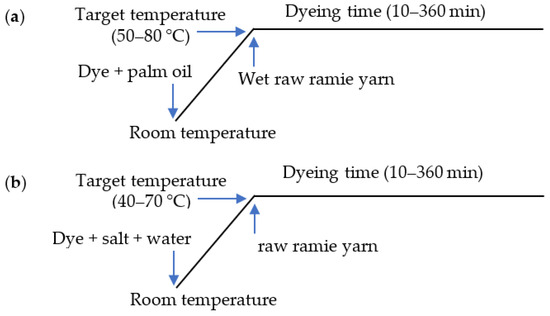
Figure 3.
Dyeing profile of raw ramie yarn with Reactive Blue 194 in (a) palm oil and (b) water media.
In water medium dyeing, the raw ramie yarn (2 g) was dyed with Reactive Blue 194 (0.06 g) in water (40 mL) with 40 g L−1 of NaCl at a variety of dyeing temperatures from 40–70 °C for 10–360 min using a swing dyeing machine (Foshan Ronggui Huibao Dyeing and Finishing Machinery Factory, Foshan, China). For the thermodynamic dyeing analysis, the raw ramie yarn was dyed with a variety of Reactive Blue 194 dye mass from 0.004 to 0.08 g in water (40 mL) with 40 g L−1 of NaCl at a variety of dyeing temperatures from 40–70 °C for 360 min using the swing dyeing machine. The dyeing profile is shown in Figure 3b.
2.3. Dye Exhaustion Percentage
After palm oil dyeing, the excessive dye solution in the dyed sample was removed by hand squeezing and collected with the residual palm oil dye solution. Distilled water (100 mL) was added to the residual palm oil dye solution, followed by a stirring to completely dissolve the reactive dye in the water. Then the aqueous dye solution was separated from the palm oil medium by static separation at 30 °C until the top layer (palm oil) displayed clean (about 3 days). The bottom layer, i.e., aqueous dye solution, was collected to detect its light absorbance with a UV-Vis spectrophotometer (Cary 300, Agilent Technologies, Melbourne, Australia) at its max wavelength (600 nm).
The dye exhaustion percentage (E%) of palm oil dyeing or aqueous dyeing was calculated according to Equation (1). A0 and A1 refer to the light absorbance of the aqueous dye solution before and after dyeing, respectively [24,25].
2.4. X-ray Diffraction Analysis
The X-ray diffraction (XRD) analysis of the raw ramie yarns scissored into powders was carried out with an X-ray diffractometer (Rigaku Ultima III, Tokyo, Japan). The XRD pattern was analysed and deconvoluted using the FitYK 1.3.1 software to obtain the characteristic peaks. The crystalline index (CI) of the sample was calculated from the areas of crystalline (Ic) and amorphous (Ia) regions by Equation (2) [26].
3. Results and Discussion
3.1. Dye Adsorption Behaviour
In reactive dyeing of cellulosic fibre, alkali is essential to fix the adsorbed dye with fibre, which fixation treatment prompts further dye exhaustion, i.e., second exhaustion. To evade interference in the analysis of the dyeing kinetics and isotherm models from the second exhaustion, alkali addition was not considered in the present work.
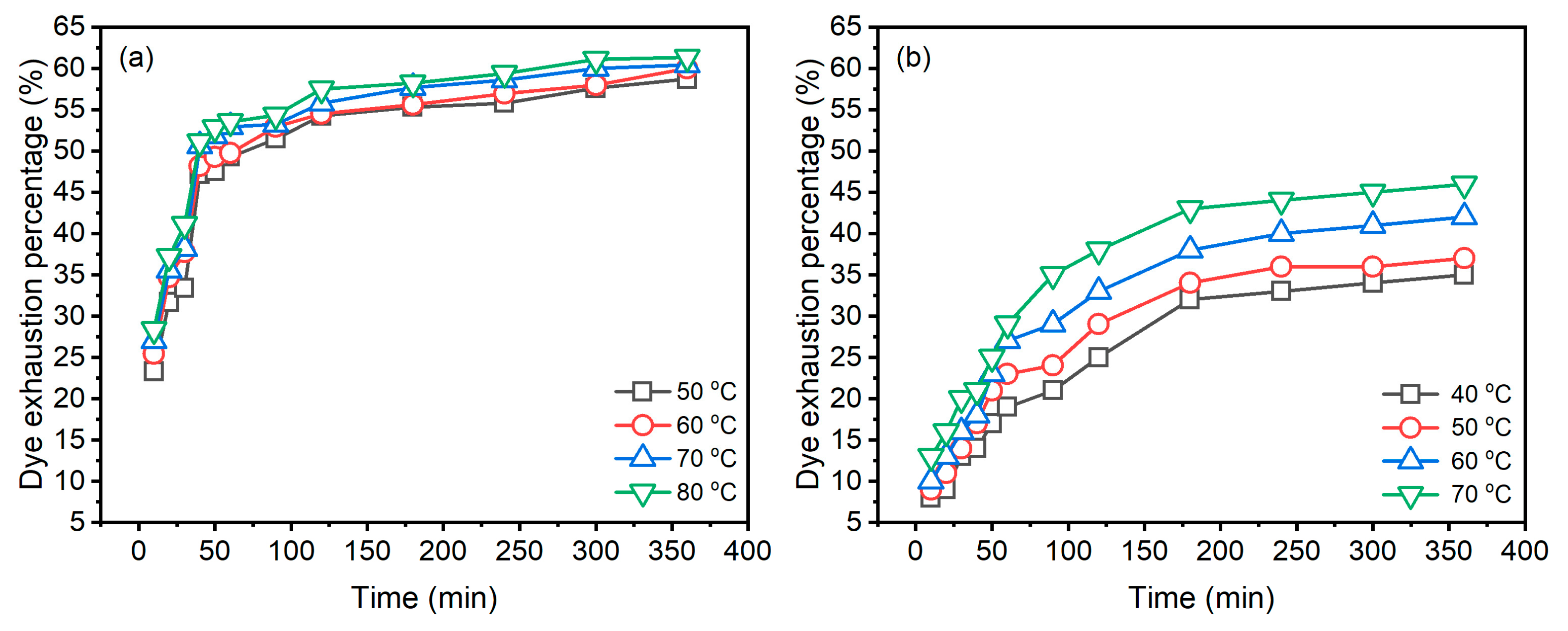
The dye adsorption behaviours of Reactive Blue 194 in the dyeing of raw ramie yarn in palm oil and water media at different temperatures are shown in Figure 4. In the palm oil medium dyeing (Figure 4a), it is obvious that the dye was exhausted quickly in raw ramie yarn in 50 min; subsequently, the dye adsorption of dyeings increased gradually and tended to be in equilibrium. While in the water medium dyeing (Figure 4b), the dye was adsorbed fast in the first 180 min of the dyeing period and then slightly slow in the later dyeing period. In comparing the dye adsorption behaviour between the dyeings in palm oil and water media, the dye uptake rates in palm oil are distinctly faster than in water. The E% values of dyeings in palm oil at 50, 60, 70, and 80 °C for 50 min were 47.5, 49.3, 51.9, and 52.8%, respectively; whereas the E% values of aqueous dyeing at 40, 50, 60, and 70 °C for 180 min were 32.3, 34.5, 38.2, and 43.1%, respectively.
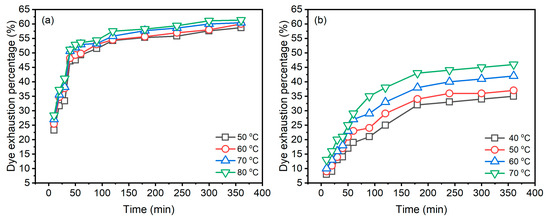
Figure 4.
Dye exhaustion of Reactive Blue 194 in the dyeing of raw ramie yarn in (a) palm oil medium and (b) water medium.
During the palm oil dyeing, the aqueous soluble Reactive Blue 194 dye in the palm oil was adsorbed in the wet raw ramie yarn at once, and the dye preferred staying in the wet substance rather than transferring into the palm oil. Also, the water adsorbed in the wet raw ramie yarn was repulsed to transfer to the palm oil dye bath because the palm oil medium is hydrophobic. Thus, this dye adsorption was irreversible, i.e., the dye only transferred from the palm oil dye bath to the wet raw ramie yarn [17]. Therefore, in the first 50 min dyeing, the dye was dramatically adsorbed on the wet raw ramie yarn. During the aqueous dyeing, the main components of raw ramie yarn, as well as the Reactive Blue 194, became negative substances caused by ionization. Thereby, a repulsing force [27] between the raw ramie yarn and the dye declined the dye adsorption amount and the dye adsorption rate. Adding salt (NaCl, 40 g L−1) in the aqueous dyeing reduced the repulsing force, so the dye exhaustion was promoted. Whereas, in palm oil dyeing, the solid reactive dye was adsorbed first in water content in the wet raw ramie yarn owing to the hydrophilic property of reactive dye. Thus, salt is not essential in palm oil dyeing. In addition, in aqueous dyeing, the dye adsorbed in raw ramie yarn was desorbed and transferred to an aqueous dye bath, i.e., the dye adsorption process was reversible. Therefore, compared to palm oil dyeing, the adsorbed dye amount and the dyeing rate in aqueous dyeing was lower and slower.
When the palm oil dyeing extended to 360 min, the E% of dyeings at 50, 60, 70, and 80 °C only increased to 58.7, 60.0, 60.4, and 61.3%, respectively. While extending the aqueous dyeing time to 360 min from 180 min, the E% of dyeings at 40, 50, 60, and 70 °C increased to 35.4, 37.2, 42.3, and 46.4%, respectively. The increment of E% of aqueous dyeing was higher than that of palm oil dyeing. The amount of dye adsorption in raw ramie yarn in 180 min aqueous dyeing was low, which means that there were many dyes in the dyebath. Meanwhile, adding NaCl (40 g L−1) assisted dye adsorption. Furthermore, the aqueous dyeing process is reversible dye adsorption. Thus, the dye adsorption was gradual and increased in the later dyeing period, in contrast to the dye adsorption in palm oil dyeing. However, for 360 min dyeing, the E% of palm oil dyeing was higher than that of aqueous dyeing.
The dyeing temperature supplies energy for distributing the dye in the dye bath [27], increasing dye transfer from the dye bath to raw ramie fibre. Thus, the higher the dyeing temperature, the higher the dye adsorption, identified by the palm oil and especially the aqueous dyeing. The E% curves (Figure 4b) of different dyeing temperatures are more distinct in aqueous dyeing, while the E% curves in palm oil dyeing are closer, suggesting that, in palm oil dyeing, the influence of dyeing temperature on dye absorption was not sensitive due to rapid dye exhaustion.
3.2. Kinetic Analysis of Dye Adsorption
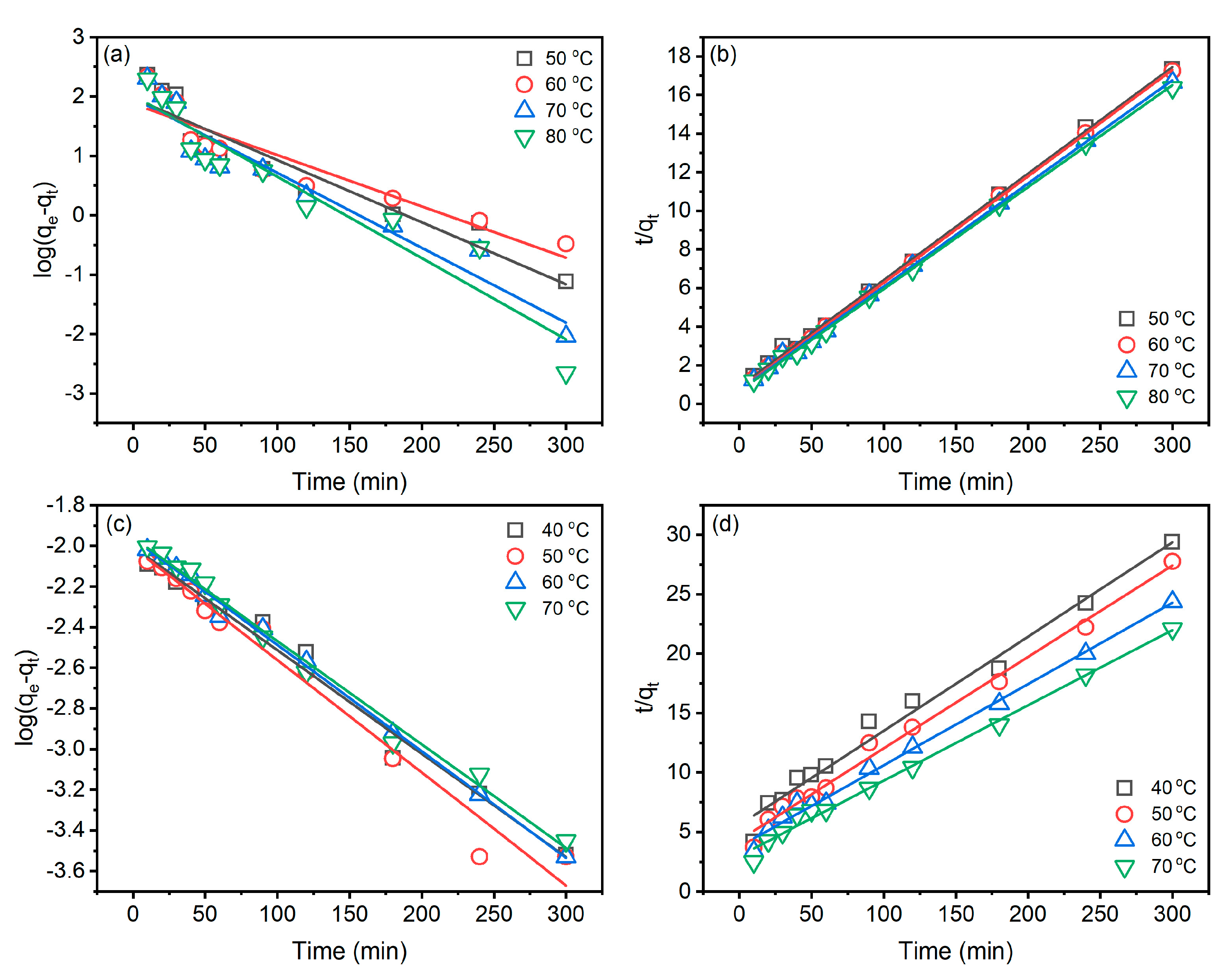
Analysis of the kinetic characteristics of dye adsorption in dyeing is helpful for the control and optimization of the dyeing process. The pseudo-first-order and pseudo-second-order models were selected to characterize, and the results are shown in Figure 5 and Table 1. The pseudo-first-order [28] and pseudo-second-order kinetic models [29] are worked out in Equations (3) and (4), respectively.
where qe (mg g−1) is the amount of dye adsorbed at equilibrium, qt (mg g−1) denotes the amount of dye adsorbed at time t (min) and K1 (min−1) is the rate constant of the pseudo-first-order model, K2 (g mg−1 min−1) is the rate constant of the pseudo-second-order model.
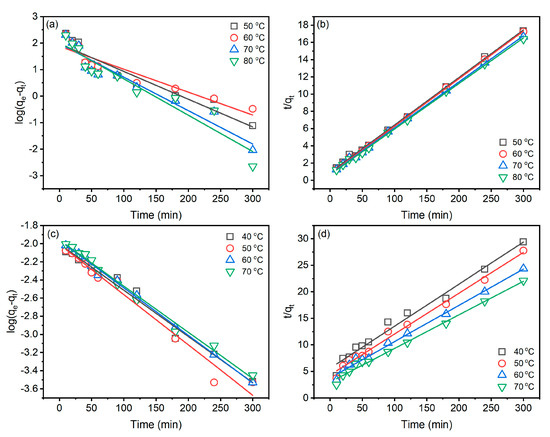
Figure 5.
Kinetic characteristics of Reactive Blue 194 dye adsorption in the dyeing of raw ramie yarn in palm oil medium: (a) pseudo-first-order and (b) pseudo-second-order and in water medium: (c) pseudo-first-order and (d) pseudo-second-order.

Table 1.
Kinetic model of Reactive Blue 194 dyeing raw ramie yarn.
In the palm oil dyeing (Table 1), linear fit correlation coefficients () of the pseudo-second-order kinetic are all greater than 0.99, while that () of the pseudo-first-order kinetic range from 0.8763 to 0.9280, which are lower than those of the pseudo-first-order kinetic model. In addition, the qe(cal1) of the pseudo-first-order model is much lower than qe(exp.), while qe(cal2) of the pseudo-second-order model is close to qe(exp.). Therefore, the adsorption behaviour of Reactive Blue 194 in palm oil dyeing of raw ramie yarn fits the pseudo-second-order kinetic model. In the aqueous dyeing, the linear fit correlation coefficients of pseudo-first-order () and pseudo-second-order () are excellent and close, but the is little better than since values are all higher than 0.9900. Therefore, the adsorption behaviour of Reactive Blue 194 in aqueous dyeing of raw ramie yarn fits the pseudo-second-order kinetic model as well [30].
The pseudo-second-order model describes the adsorption identified by two adsorption processes; the first adsorption is fast and quick, an equilibrium situation, whereas the second adsorption takes a long time and is obtuse [31,32]. In Figure 4a, the dye adsorption behaviours fit the pseudo-second-order model description. In the aqueous dyeing, after 180 min dyeing, the dye adsorption still substantially increased, although the dye adsorption rate was lower than in 180 min dyeing. Thus, the increment of dye adsorption leads to the fuzzy kinetic model.
3.3. Half-Dyeing Time and Dye Diffusion Coefficient
The half-dyeing time (t1/2) is the time that the dye mass adsorbed in the substance is equal to half of the dye mass adsorbed at equilibrium, i.e., qt = 1/2 qe. It can be calculated from the rate constant of the Pseudo-second-order model, K2, according to Equation (5) [33].
In Table 2, the t1/2 values of palm oil dyeing and aqueous dyeing were reduced with increased dyeing temperature. The shorter t1/2 indicates that the dye adsorption is faster, which is confirmed by the E% curves in Figure 4. Comparing the t1/2 values between the dyeings in palm oil and in water media, the t1/2 value of palm oil dyeing shows that dye adsorption is faster in palm oil dyeing.

Table 2.
Half-dyeing time and dye diffusion coefficient of Reactive Blue 194 dyeing raw ramie yarn.
During the dyeing, the dye on the surface of raw ramie yarn diffused into the fibre interior. Thus, the diffusion coefficient was an important value for characterising the dye adsorption behaviours. The dye diffusion coefficient (D) of the raw ramie yarn dyeing in palm oil and water media at t1/2 can be calculated by the simplified Hill equation (Equation (6)) [34], and the results are listed in Table 2.
where D (m2 min−1) is the dye diffusion coefficient at t1/2; r (m) is the radius of raw ramie yarn (68.9 ± 13.9 μm) [7].
The D increased from 1.8576 × 10−11 to 2.3838 × 10−11 m2 min−1 with an increase in dyeing temperatures from 50 to 80 °C in palm oil dyeing, and increased from 0.4218 × 10−11 to 0.6046 × 10−11 m2 min−1 with an increase of dyeing temperatures from 40 to 70 °C in aqueous dyeing. The dye diffusion in palm oil dyeing is 3 to 4 times faster than in aqueous dyeing. The tendency can be explained by the fact that at a higher dyeing temperature, the raw ramie yarn swelled well, and the dye movement in the dye solution and the fibre was accelerated [35].
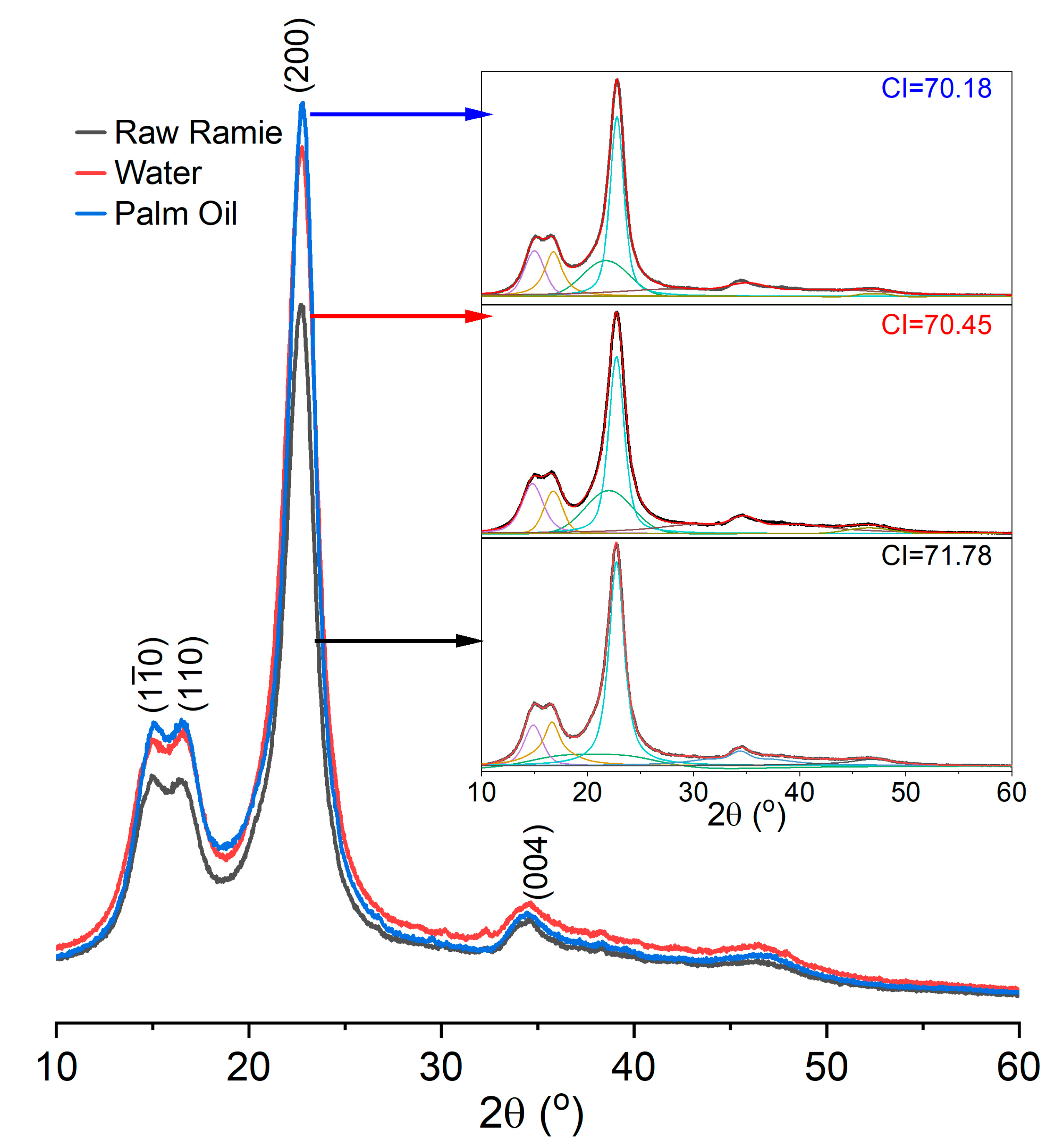
In addition, the crystallinity of the raw ramie yarn influences the dye diffusion in the fibre’s interior. The XRD curves of the raw ramie yarn dyed at 80 °C for 360 min in a palm oil medium, the one dyed at 70 °C for 360 min in water, and the original one have displayed in Figure 6, and peaks were deconvoluted using the Gaussian peak fitting function. Afterward, the area under the peaks (different colours) was determined to calculate the crystalline index (CI) using Equation (2). The XRD curves of these samples are similar and fit the cellulose I pattern [36], in which intense peaks at 15.0, 16.6, 22.7, and 34.4° correspond to the (10), (110), (200), and (004) lattice planes, respectively. Besides, the crystallinities of these three samples ranged from 70–72, indicating that the morphological structure of raw ramie yarn was not damaged by the dyeing conditions. Therefore, the fast and high dye adsorption behaviour in the palm oil dyeing of raw ramie yarn was mainly due to irreversible adsorption.
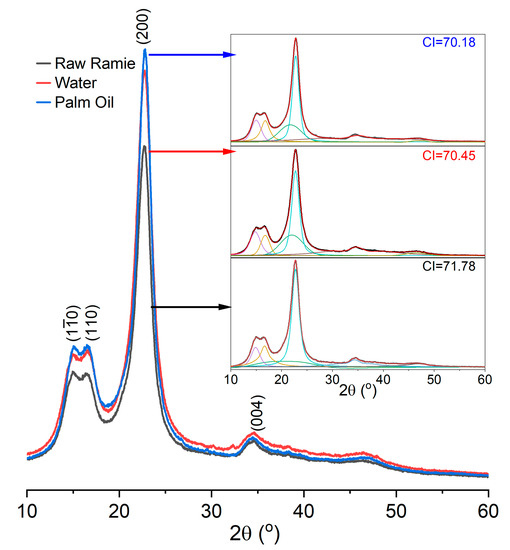
Figure 6.
XRD patterns of original raw ramie yarn and raw ramie yarn dyed in the palm oil medium and the water medium, and original raw ramie yarn.
3.4. Adsorption Isotherms
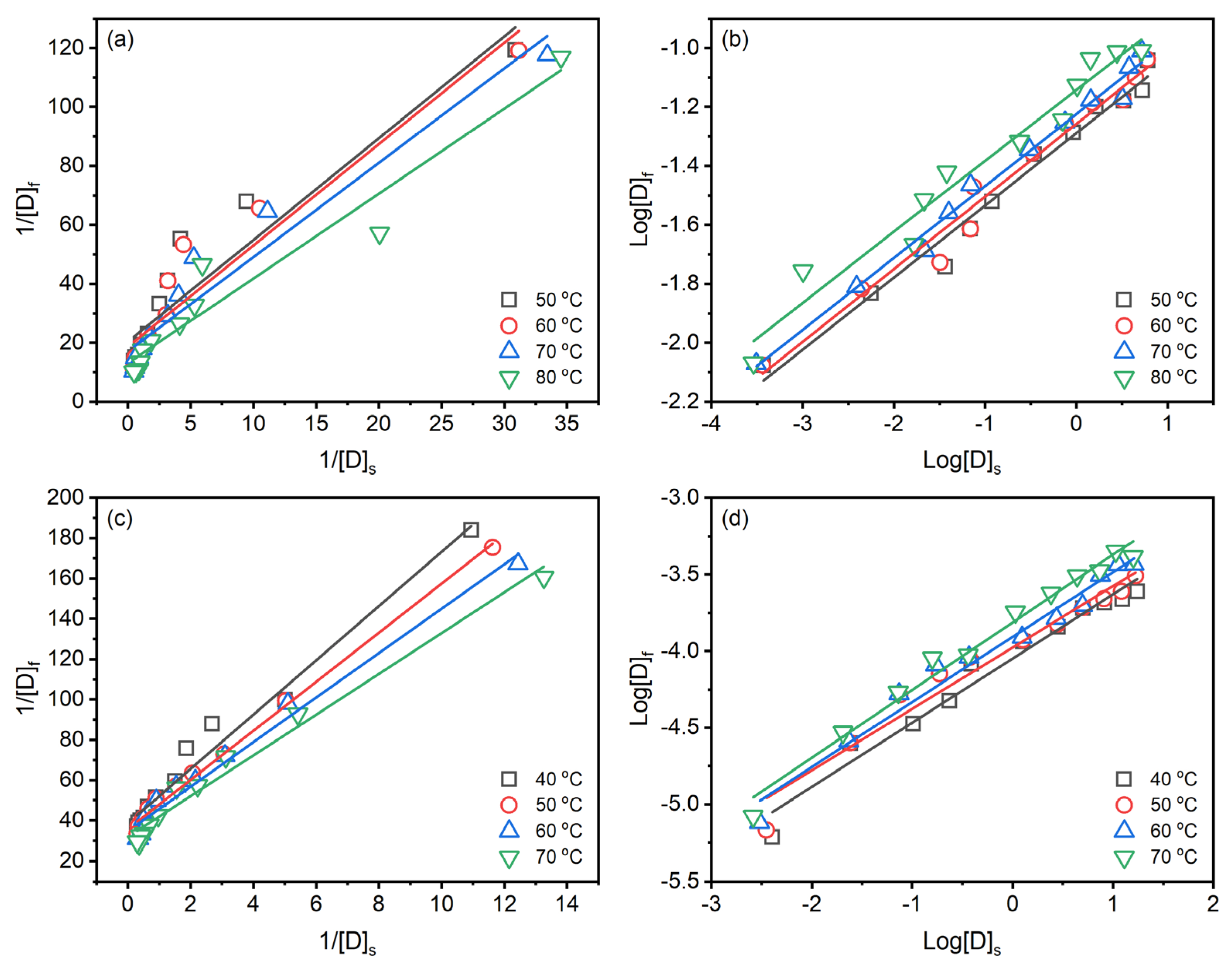
Langmuir [37] (Equation (7)) and Freundlich [38] (Equation (8)) isotherms are widely used in fitting the dye adsorption in textile dyeing.
where [D]f (g g−1) and [D]s (g L−1) are the dye concentration in the fibre and dye solution at equilibrium time; [S]f (g g−1) is the Langmuir constant related to adsorption capacity; KL is the Langmuir equilibrium constant. KF is the Freundlich equilibrium constant, and n is the constant correlated to the adsorption intensity of the adsorbent.
Langmuir isotherm assumes the adsorption occurs on a homogeneous surface by monolayer adsorption without interaction between adsorbed molecules [39]. In contrast, Freundlich isotherm assumes that adsorption occurs on heterogeneous surfaces by monolayer and multilayer adsorption [40]. Both isotherms can be chemisorption and physisorption. In the case of reactive dyeing, it is hard to clear distinct the sole chemical or physical adsorption since the reactive group of the reactive dye can partly chemically react with the substance to form covalent bonds [41]. The Langmuir and Freundlich isotherms for dyeing raw ramie yarn with Reactive Blue 194 in palm oil medium are shown in Figure 7a,b, and Table 3, and those for dyeing in water medium are shown in Figure 7c,d, and Table 4. The linear relationship of the Langmuir isotherm (Figure 7a,c) and Freundlich isotherm (Figure 7b,d) is high fitness, which average correlation coefficients higher than 0.9200, suggesting that the dyeing of raw ramie yarn in the palm oil medium and water medium both chemical and physical adsorptions existed. In the dyeing of raw ramie yarn in the palm oil medium, the average correlation coefficient of the Freundlich isotherm () is higher than that of Langmuir isotherm (). It hints that the dye adsorption tended to be physical adsorption, more fittable to the Freundlich isotherm [42]. While the dyeing of raw ramie yarn in a water medium exhibited a competition of chemical and physical adsorption because the average correlation coefficients of the Langmuir isotherm () and Freundlich isotherm () are 0.9833 and 0.9714, respectively. In the aqueous dyeings, the addition of 40 g L−1 of NaCl promoted dye adsorption, which enhanced the potential to form covalent bonds between the dye and raw ramie yarn, i.e., chemical adsorption [43,44].
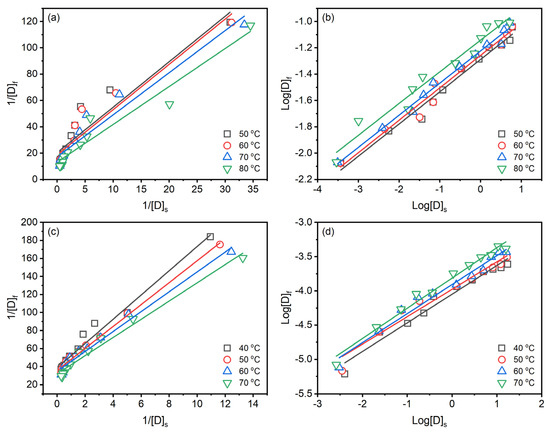
Figure 7.
Isotherms of dyeing of raw ramie yarn with Reactive Blue 194 in palm oil medium: (a) Langmuir and (b) Freundlich, and in water medium: (c) Langmuir and (d) Freundlich.

Table 3.
Parameters of Langmuir and Freundlich isotherms of dyeing of raw ramie yarn with Reactive Blue 194 in palm oil medium.

Table 4.
Parameters of Langmuir and Freundlich isotherms of dyeing of raw ramie yarn with Reactive Blue 194 in water medium.
4. Conclusions
This study investigated using palm oil and water mediums for the environmentally friendly sustainable dyeing of grass cloth fibre utilizing commercial Reactive Blue 194 dye. The adsorption behaviour of reactive dye on raw ramie yarn was investigated to better understand the dyeing process of reactive dye in palm oil and water media, which ultimately optimizes the dyeing techniques. The dyeing of grass cloth yarns by reactive blue 194 dyes in palm oil and water medium is in accordance with the quasi-secondary kinetic model. The half-dyeing time becomes smaller by increasing the temperature. In the palm oil dyeing system, the dyeing temperature greatly influences its activation energy. The adsorption isotherm of reactive blue 194 dye on grass cloth yarn in palm oil is more consistent with the Freundlich isotherm adsorption model, while the Langmuir isotherm adsorption model was suitable for water dyeing. The affinity of reactive blue 194 dye for dyeing grass cloth yarn in palm oil increased with the increase of temperature, the trend of dye transfer from the dye solution to the fibre was greater, and the dyeing process was a heat absorption reaction. Besides, it was found that the adsorption rates in palm oil are distinctly faster than in water, indicating that a palm oil dyeing medium could be an alternative to a water dyeing medium with a greater dye exhaustion performance, beneficial for the textile dyeing industry in the future regarding environmental sustainability.
Author Contributions
Conceptualization: L.L. (Lina Lin), L.L. (Le Li) and Y.C.; Methodology: L.X., C.Z. and X.L.; Formal analysis and investigation: L.L (Le Li)., Y.Z. and Y.C.; Writing—original draft preparation: L.L. (Lina Lin), M.N., M.N.P. and Y.C.; Writing—review and editing: Y.C., M.N.P., V.N. and M.I.H.M.; Supervision: Y.C. and V.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Opening Project of the Hubei Provincial Engineering Laboratory for Clean Production and High Value Utilization of Bio-based Textile Materials, Project Number: SWJ202205.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request (Yingjie Cai, Y.C.).
Acknowledgments
We would like to express our sincere gratitude for the support from the Sanitary Environmental Engineering Division (SEED) and grants (FARB projects) from the University of Salerno, Italy, coordinated by V. Naddeo. Grant Number: 300393FRB22NADDE.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liao, J.; Yang, X. Study on the evolution of grass cloth. Asian Soc. Sci. 2016, 12, 109. [Google Scholar]
- Luo, G.; Lin, L. Research on the grass cloth embroidery regeneration development strategies in the information age. In Proceedings of the 2021 World Automation Congress (WAC), Taipei, Taiwan, 1–5 August 2021; IEEE: New York, NY, USA, 2021; pp. 254–259. [Google Scholar]
- Luo, G.; Lin, L.; Li, A. Cultural and Creative Tourism Product Design Strategies to Enhance the Brand Value of Intangible Cultural Heritage under Artificial Intelligence Technology: Focused on Grass Cloth Embroidery Example. In Proceedings of the 2021 International Conference on Forthcoming Networks and Sustainability in AIoT Era (FoNeS-AIoT), Nicosia, Turkey, 27–28 December 2021; IEEE: New York, NY, USA, 2021; pp. 160–167. [Google Scholar]
- Huang, H.; Tang, Q.; Lin, G.; Yu, C.; Wang, H.; Li, Z. High-efficiency and recyclable ramie cellulose fiber degumming enabled by deep eutectic solvent. Ind. Crops Prod. 2021, 171, 113879. [Google Scholar] [CrossRef]
- Lin, G.; Tang, Q.; Huang, H.; Yu, J.; Li, Z.; Ding, B. Process optimization and comprehensive utilization of recyclable deep eutectic solvent for the production of ramie cellulose fibers. Cellulose 2022, 29, 3689–3701. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Z.; Yu, C. Property of ramie fiber degummed with Fenton reagent. Fibers Polym. 2017, 18, 1891–1897. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, C.; Jiang, T.; Hossain, M.Y.; Zhu, W.; Pervez, M.N.; Hoque, M.I.U.; Khan, I.; Long, X.; Cai, Y. Dyeing of raw ramie yarn with Reactive Orange 5 dye. Ind. Crops Prod. 2022, 176, 114315. [Google Scholar] [CrossRef]
- Lewis, D.M. The chemistry of reactive dyes and their application processes. In Handbook of Textile and Industrial Dyeing; Clark, M., Ed.; Woodhead Publishing: Sawston, UK, 2011; Volume 1, pp. 303–364. [Google Scholar]
- Inamdar, U.Y.; Pervez, M.N.; Navik, R.G.; Peng, X.; Cai, Y. Low-temperature bleaching of cotton fabric by activated peroxide system. Emerg. Mater. Res. 2017, 6, 387–395. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, W.; Zhang, C.; Hossain, M.Y.; Oli, Z.B.S.; Pervez, M.N.; Sarker, S.; Hoque, M.I.U.; Cai, Y.; Naddeo, V. Combination of wet fixation and drying treatments to improve dye fixation onto spray-dyed cotton fabric. Sci. Rep. 2021, 11, 15403. [Google Scholar] [CrossRef]
- Lewis, D.M. Developments in the chemistry of reactive dyes and their application processes. Coloration Technol. 2014, 130, 382–412. [Google Scholar] [CrossRef]
- Anis, P.; Toprak, T.; Kutlu, E. Sericin assisted eco-friendly reactive dyeing for cotton fabric. Cellulose 2019, 26, 6317–6331. [Google Scholar] [CrossRef]
- Tang, P.; Lockett, L.-M.E.; Zhang, M.; Sun, G. Modification of cotton fabrics with 2-diethylaminoethyl chloride for salt-free dyeing with anionic dyes. Cellulose 2021, 28, 6699–6712. [Google Scholar] [CrossRef]
- Liu, L.; Mu, B.; Li, W.; Yang, Y. Cost-effective reactive dyeing using spent cooking oil for minimal discharge of dyes and salts. J. Clean. Prod. 2019, 227, 1023–1034. [Google Scholar] [CrossRef]
- Liu, L.; Mu, B.; Li, W.; Yang, Y. Semistable Emulsion System Based on Spent Cooking Oil for Pilot-Scale Reactive Dyeing with Minimal Discharges. ACS Sustain. Chem. Eng. 2019, 7, 13698–13707. [Google Scholar] [CrossRef]
- Liu, L.; Mu, B.; Li, W.; Xu, H.; Yang, J.; Yang, Y. Clean cotton dyeing in circulated dyebath of waste cooking oil: A feasible industrialization strategy for pollution minimization. J. Clean. Prod. 2021, 278, 123799. [Google Scholar] [CrossRef]
- Mu, B.; Li, W.; Xu, H.; Emanuel, L.; Yang, Y. Salt-free and environment-friendly reactive dyeing of cotton in cottonseed oil/water system. Cellulose 2019, 26, 6379–6391. [Google Scholar] [CrossRef]
- Tan, K.; Lee, K.; Mohamed, A.; Bhatia, S. Palm oil: Addressing issues and towards sustainable development. Renew. Sustain. Energy Rev. 2009, 13, 420–427. [Google Scholar] [CrossRef]
- Matthäus, B. Use of palm oil for frying in comparison with other high-stability oils. Eur. J. Lipid Sci. Technol. 2007, 109, 400–409. [Google Scholar] [CrossRef]
- Hinrichsen, N. Commercially available alternatives to palm oil. Lipid Technol. 2016, 28, 65–67. [Google Scholar] [CrossRef]
- Lin, L.; Xiao, L.; Li, L.; Zhang, C.; Pervez, M.N.; Naddeo, V.; Zhang, Y.; Islame, M.S.; Cai, Y.; Hassan, M.M. Sustainable and eco-friendly dyeing of traditional grass cloth with a reactive dye in palm oil medium. RSC Adv. 2022, 46, 29767–29776. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, T.; Li, L.; Pervez, M.N.; Zhang, C.; Yan, C.; Cai, Y.; Naddeo, V. Sustainable traditional grass cloth fiber dyeing using the Taguchi L16 (4^4) orthogonal design. Sci. Rep. 2022, 12, 13833. [Google Scholar] [CrossRef]
- Masra, S.M.W.; Arief, Y.Z.; Sahari, S.K.; Muhammad, M.S.; Rigit, A.R.H.; Rahman, M.R. A Systematic Review on Promising Development of Palm Oil and Its Nanofluid as a Biodegradable Oil Insulation Alternative. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 302–318. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, T.; Liang, Y.; Zhu, W.; Inamdar, U.Y.; Pervez, M.N.; Navik, R.; Yang, X.; Cai, Y.; Naddeo, V. Combination of Pre- and Post-Mercerization Processes for Cotton Fabric. Materials 2022, 15, 2092. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Jiang, T.; Liang, Y.; Pervez, M.N.; Navik, R.; Gao, B.; Cai, Y.; Hassan, M.M.; Kumari, N.; Naddeo, V. Influence of Sequential Liquid Ammonia and Caustic Mercerization Pre-Treatment on Dyeing Performance of Knit Cotton Fabric. Materials 2022, 15, 1758. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhu, W.; Zhang, C.; Navik, R.; Ding, X.; Mia, M.S.; Pervez, M.N.; Mondal, M.I.H.; Lin, L.; Cai, Y. Post-treatment of reactive dyed cotton fabrics by caustic mercerization and liquid ammonia treatment. Cellulose 2021, 28, 7435–7453. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, W.; Hossain, M.Y.; Sarker, S.; Pervez, M.N.; Mondal, M.I.H.; Yan, C.; Cai, Y.; Naddeo, V. Toward improved performance of reactive dyeing on cotton fabric using process sensitivity analysis. Int. J. Cloth. Sci. Technol. 2022, 34, 469–484. [Google Scholar] [CrossRef]
- Pervez, M.N.; Chen, C.; Li, Z.; Naddeo, V.; Zhao, Y. Tuning the structure of cerium-based metal-organic frameworks for efficient removal of arsenic species: The role of organic ligands. Chemosphere 2022, 303, 134934. [Google Scholar] [CrossRef]
- Talukder, M.E.; Pervez, M.N.; Jianming, W.; Gao, Z.; Stylios, G.K.; Hassan, M.M.; Song, H.; Naddeo, V. Chitosan-functionalized sodium alginate-based electrospun nanofiber membrane for As(III) removal from aqueous solution. J. Environ. Chem. Eng. 2021, 9, 106693. [Google Scholar] [CrossRef]
- Hossain, M.Y.; Zhu, W.; Pervez, M.N.; Yang, X.; Sarker, S.; Hassan, M.M.; Hoque, M.I.U.; Naddeo, V.; Cai, Y. Adsorption, kinetics, and thermodynamic studies of cacao husk extracts in waterless sustainable dyeing of cotton fabric. Cellulose 2021, 28, 2521–2536. [Google Scholar] [CrossRef]
- Cai, Y.; Huang, Y.; Liu, F.; He, L.; Lin, L.; Zeng, Q. Liquid ammonia dyeing of cationic ramie yarn with triazinyl reactive dyes. Cellulose 2014, 21, 3841–3849. [Google Scholar] [CrossRef]
- Pervez, M.N.; Fu, D.; Wang, X.; Bao, Q.; Yu, T.; Naddeo, V.; Tian, H.; Cao, C.; Zhao, Y. A bifunctional α-FeOOH@GCA nanocomposite for enhanced adsorption of arsenic and photo Fenton-like catalytic conversion of As(III). Environ. Technol. Innov. 2021, 22, 101437. [Google Scholar] [CrossRef]
- Ho, Y.-S. McKay, Gordon, Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Wang, X.; Song, Z.; Hao, J.; Mingmei, X. Dyeing thermodynamics of acrylic acid photografted linen fabric with cationic dyes. J. Text. Res. 2012, 33, 49–52. [Google Scholar]
- Inyinbor, A.A.; Adekola, F.A.; Olatunji, G.A. Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp. Water Resour. Ind. 2016, 15, 14–27. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Cheng, X.; Ren, X.; Huang, T.S. Self-assembled antibacterial coating by N-halamine polyelectrolytes on a cellulose substrate. J. Mater. Chem. B 2015, 3, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Fan, J.; Zhou, W.; Gao, B.; Yue, Q.; Kang, Q. Adsorption kinetics and isotherm of anionic dyes onto organo-bentonite from single and multisolute systems. J. Hazard. Mater. 2009, 172, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.; Mathew, S.; Kurian, S.; Saravanakumar, M.; Ealias, A.M.; George, G. Facile synthesis, growth process, characterisation of a nanourchin-structured α-MnO2 and their application on ultrasonic-assisted adsorptive removal of cationic dyes: A half-life and half-capacity concentration approach. Ultrason. Sonochem. 2018, 49, 175–189. [Google Scholar] [CrossRef]
- Sari, A.; Mendil, D.; Tuzen, M.; Soylak, M. Biosorption of palladium(II) from aqueous solution by moss (Racomitrium lanuginosum) biomass: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2009, 162, 874–879. [Google Scholar] [CrossRef]
- Ramesh, A.; Hasegawa, H.; Sugimoto, W.; Maki, T.; Ueda, K. Adsorption of gold(III), platinum(IV) and palladium(II) onto glycine modified crosslinked chitosan resin. Bioresour. Technol. 2008, 99, 3801–3809. [Google Scholar] [CrossRef]
- Cai, Y.; Liang, Y.; Navik, R.; Zhu, W.; Zhang, C.; Pervez, M.N.; Wang, Q. Improved reactive dye fixation on ramie fiber in liquid ammonia and optimization of fixation parameters using the Taguchi approach. Dye. Pigment. 2020, 183, 108734. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Remadevi, R.; Rojas, O.J.; Wang, X.; Naebe, M. Kinetics and equilibrium adsorption of methylene blue onto cotton gin trash bioadsorbents. Cellulose 2020, 27, 6485–6504. [Google Scholar] [CrossRef]
- Gao, B.; Huang, X.; Jiang, T.; Pervez, M.N.; Zhu, W.; Hassan, M.M.; Cai, Y.; Naddeo, V. Sustainable dyeing of ramie fiber with ternary reactive dye mixtures in liquid ammonia. RSC Adv. 2022, 12, 19253–19264. [Google Scholar] [CrossRef]
- Yuan, C.; Lou, K.; Yu, L.; Pervez, M.N.; Su, S.; Huang, J.; Peng, X.; Cai, Y. Electrolyte influence on sorption behaviours of Direct Blue 71 dye on ramie fibre. MATEC Web Conf. 2017, 108, 03003. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).