Influence of Anodizing Parameters on Tribological Properties and Wettability of Al2O3 Layers Produced on the EN AW-5251 Aluminum Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Material
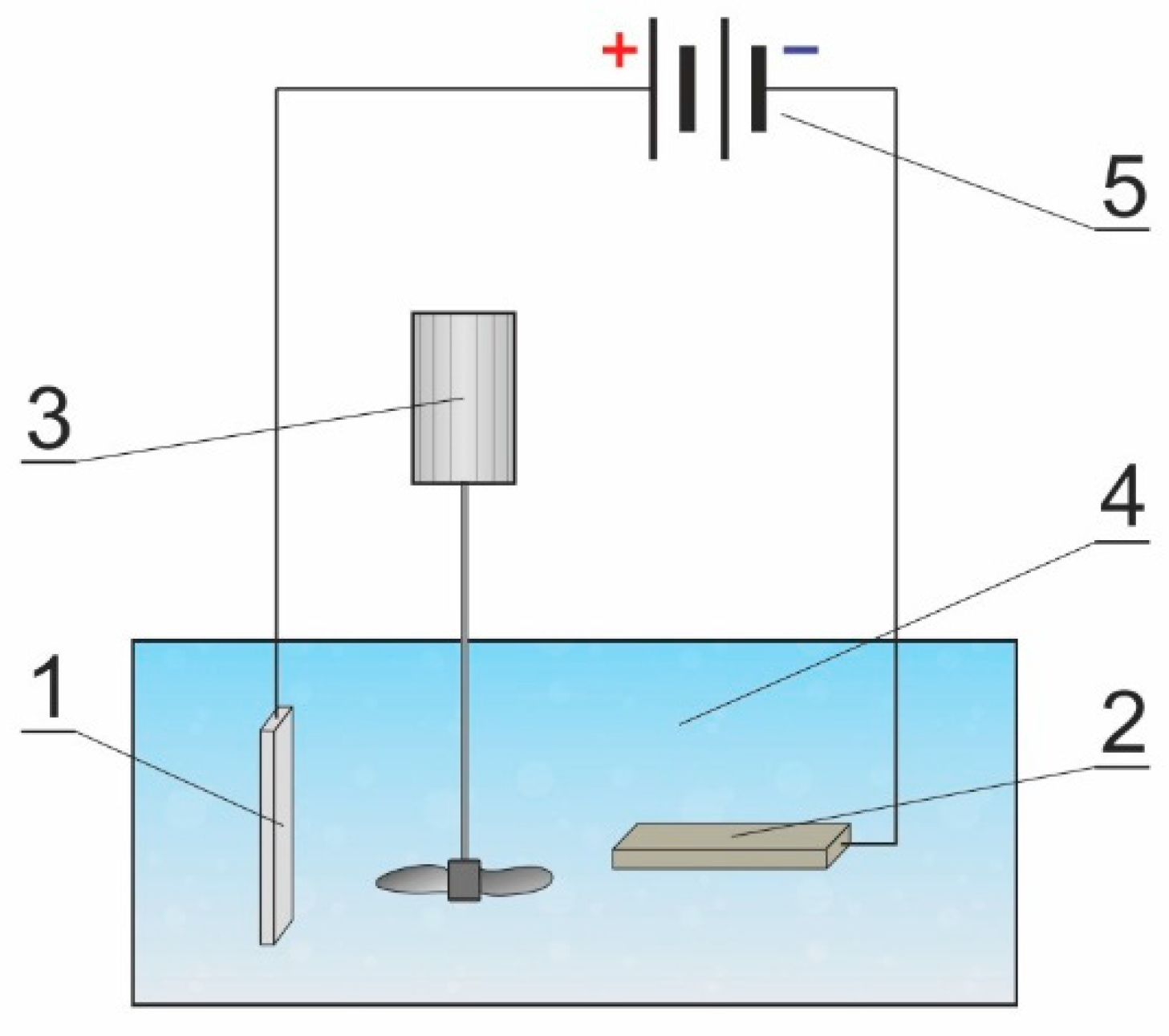
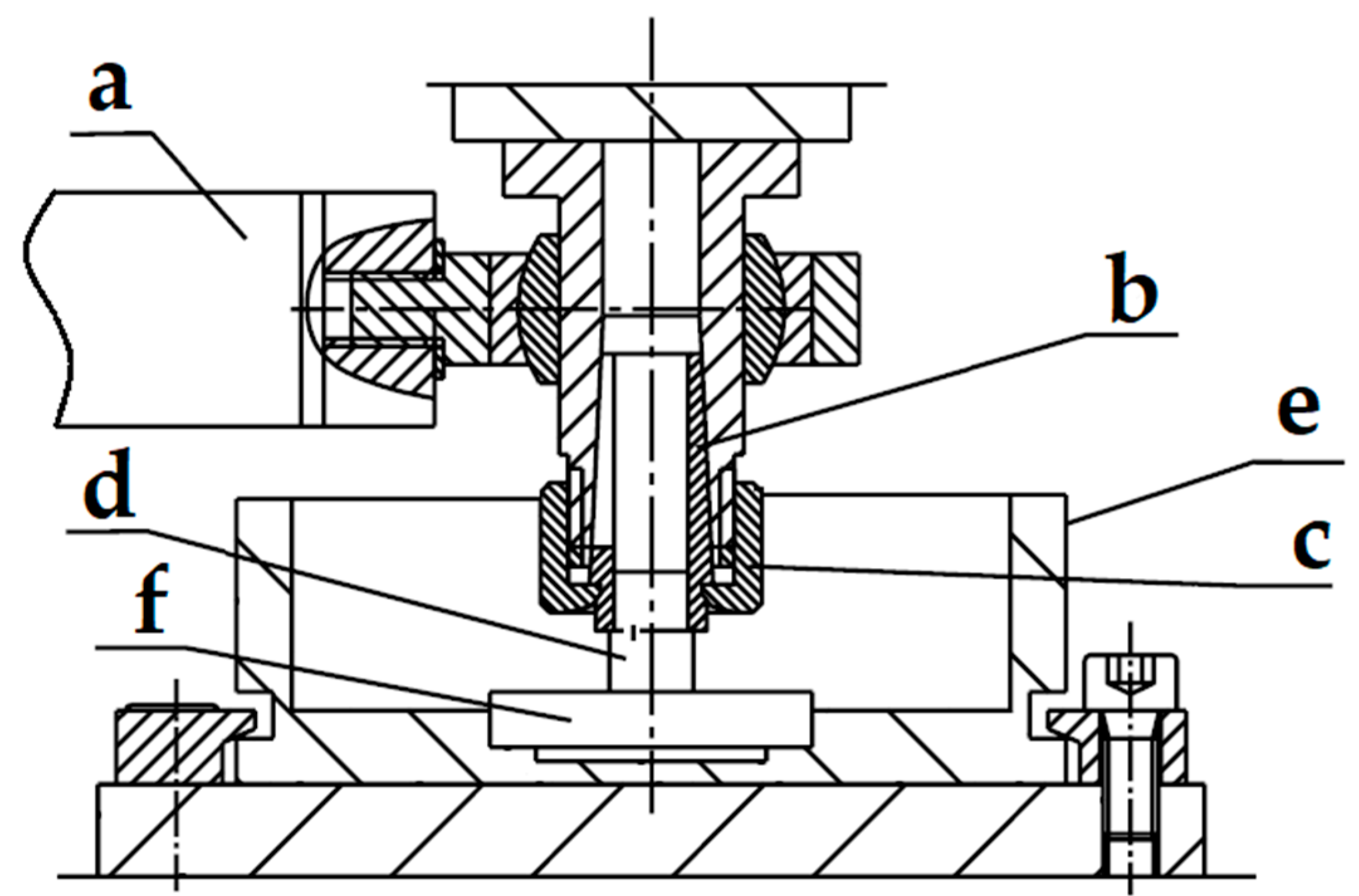
2.2. Research Methodology
3. Results and Discussion
3.1. Structure of the Oxide Layer
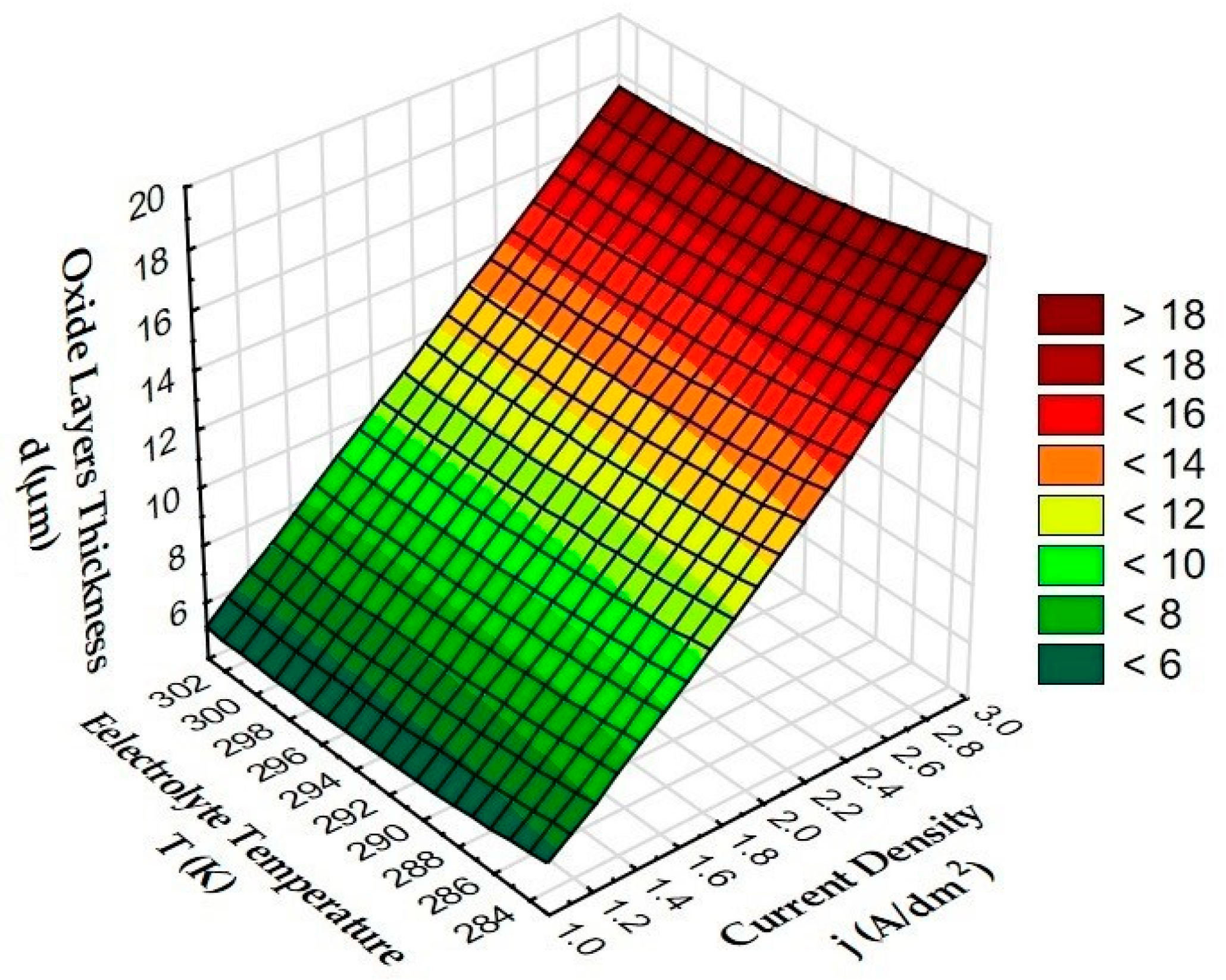
3.2. The Thickness of the Oxide Layer
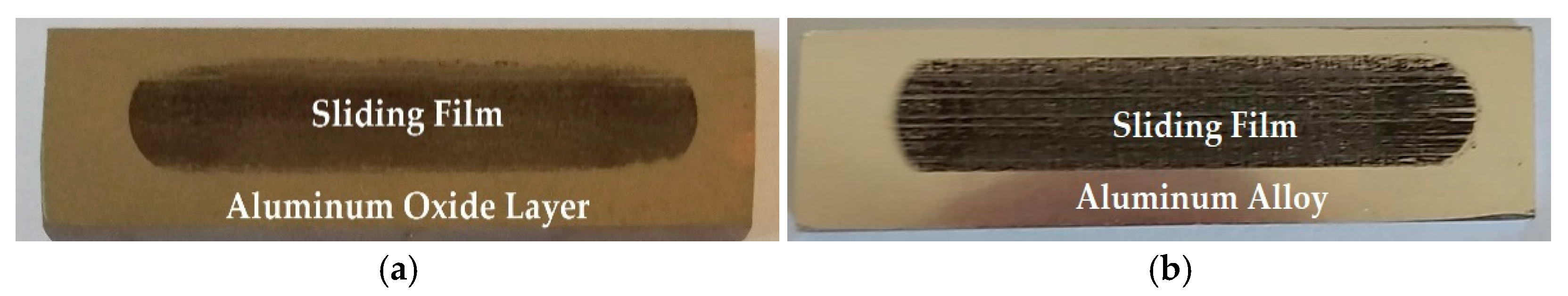
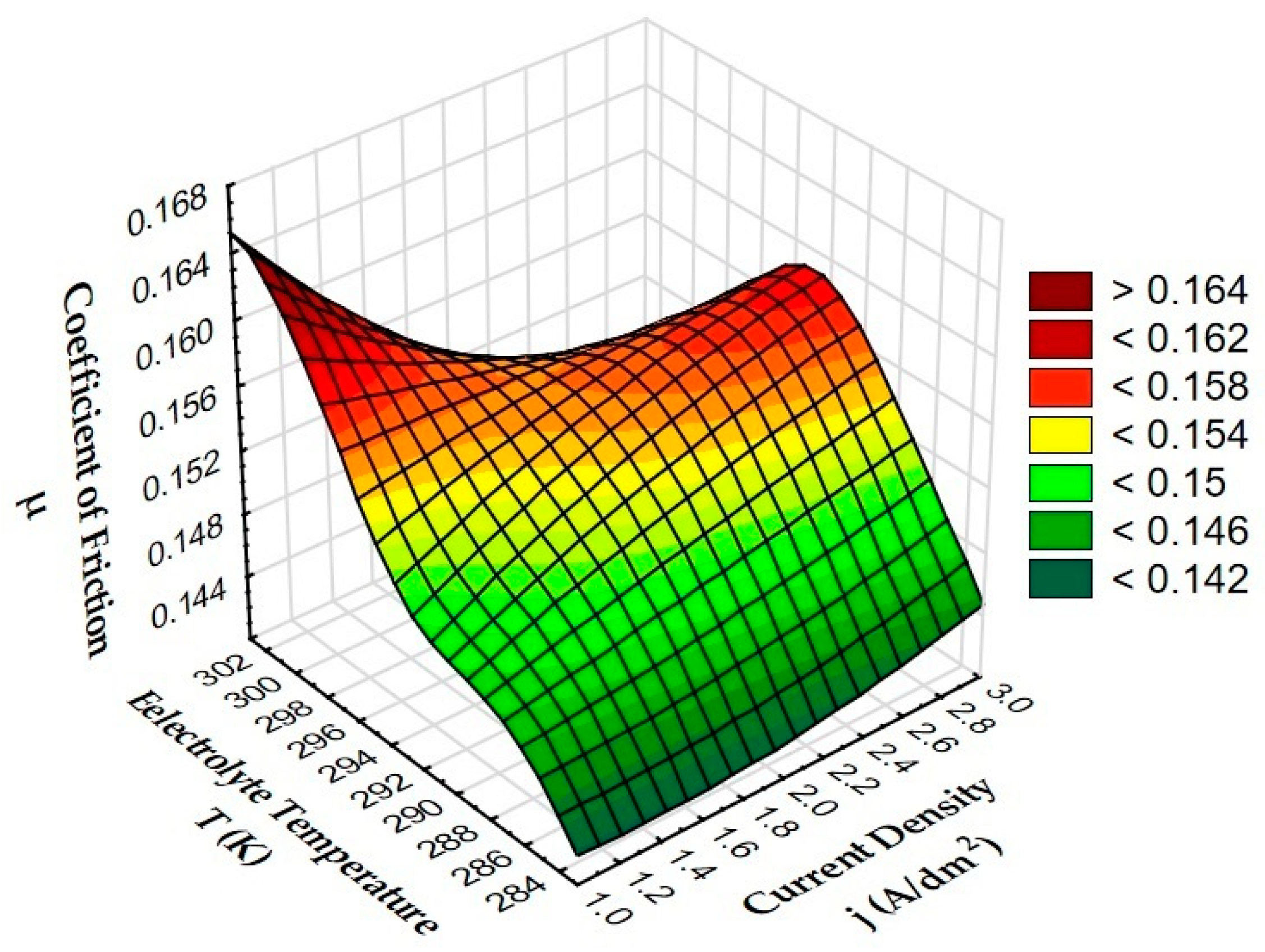
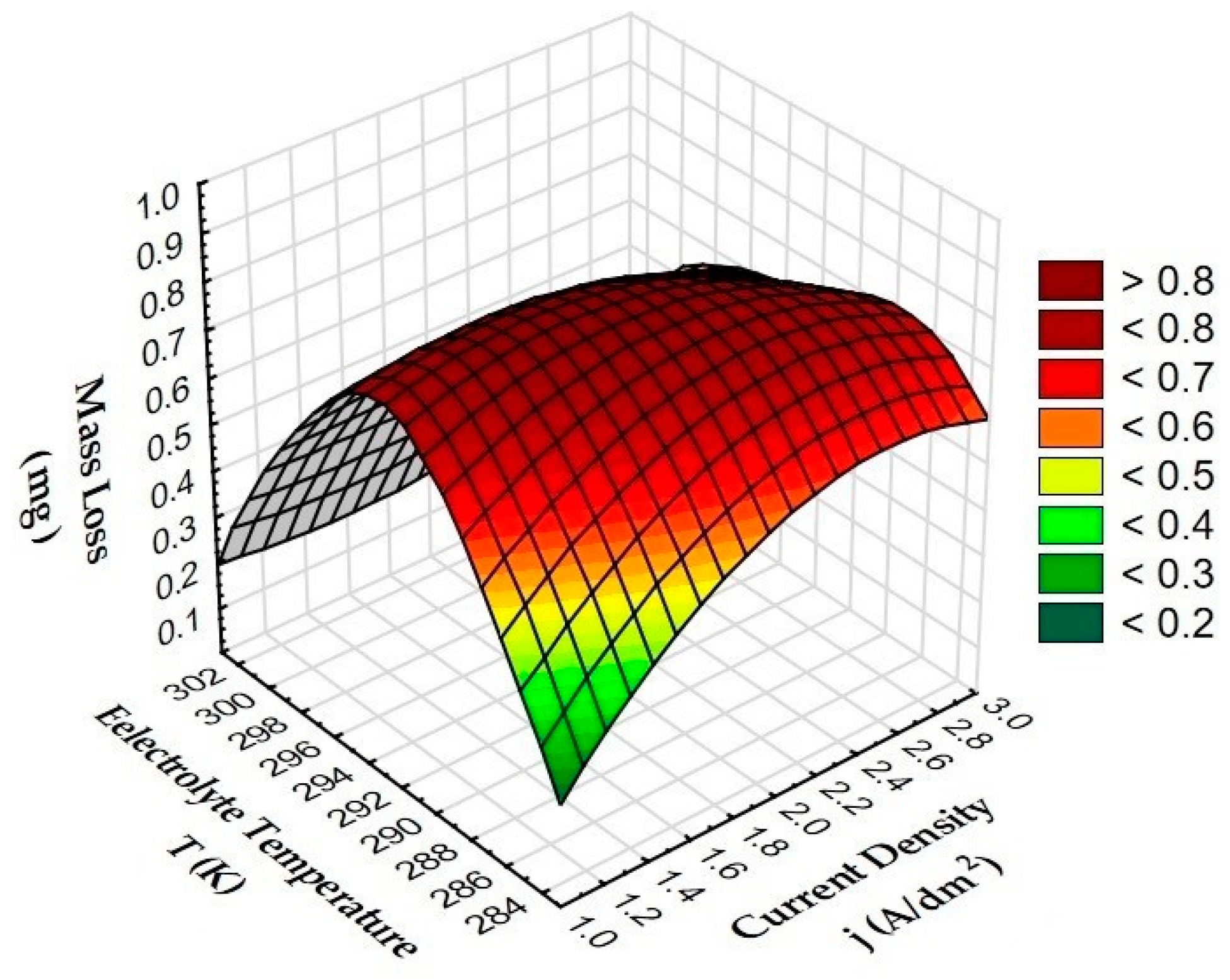
3.3. Tribological Tests
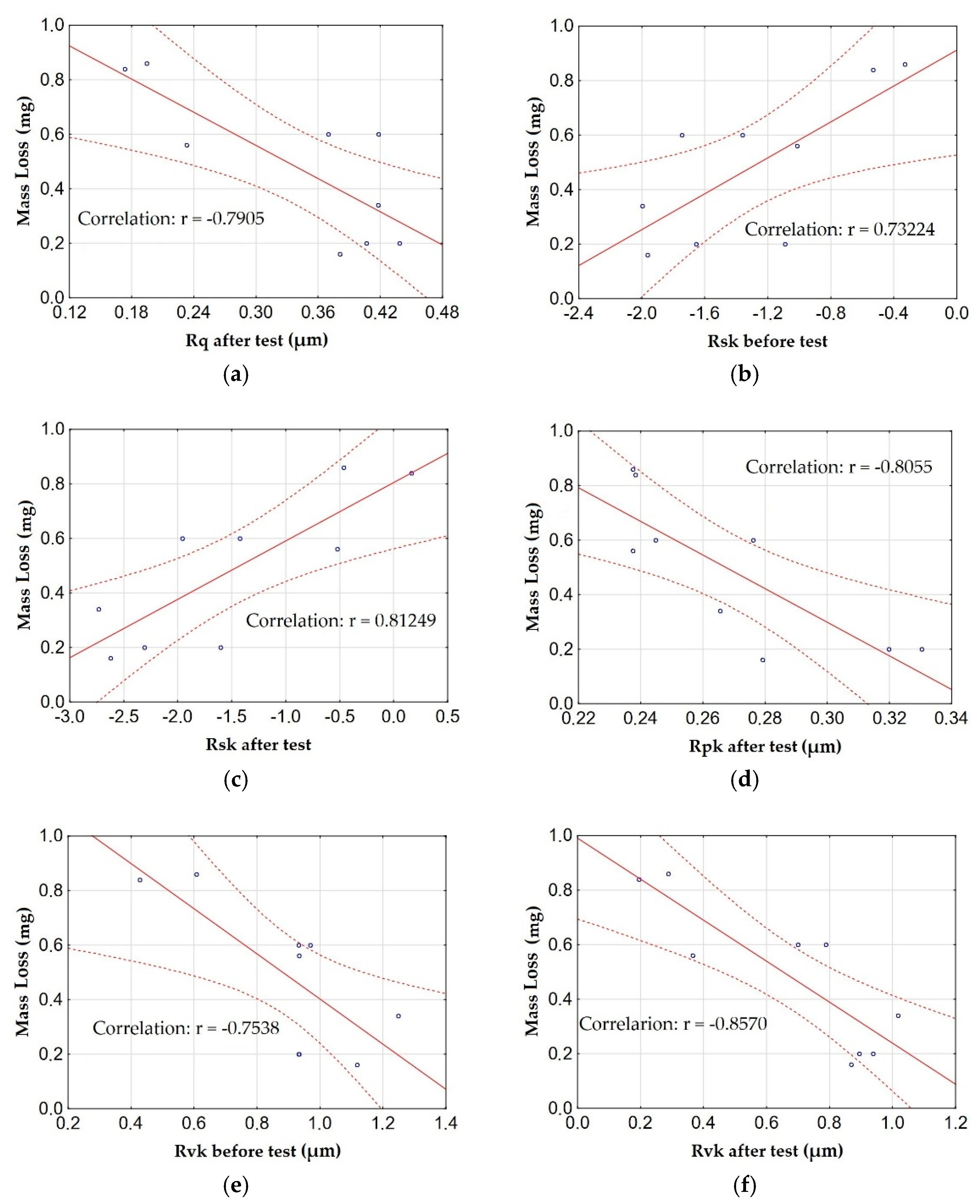
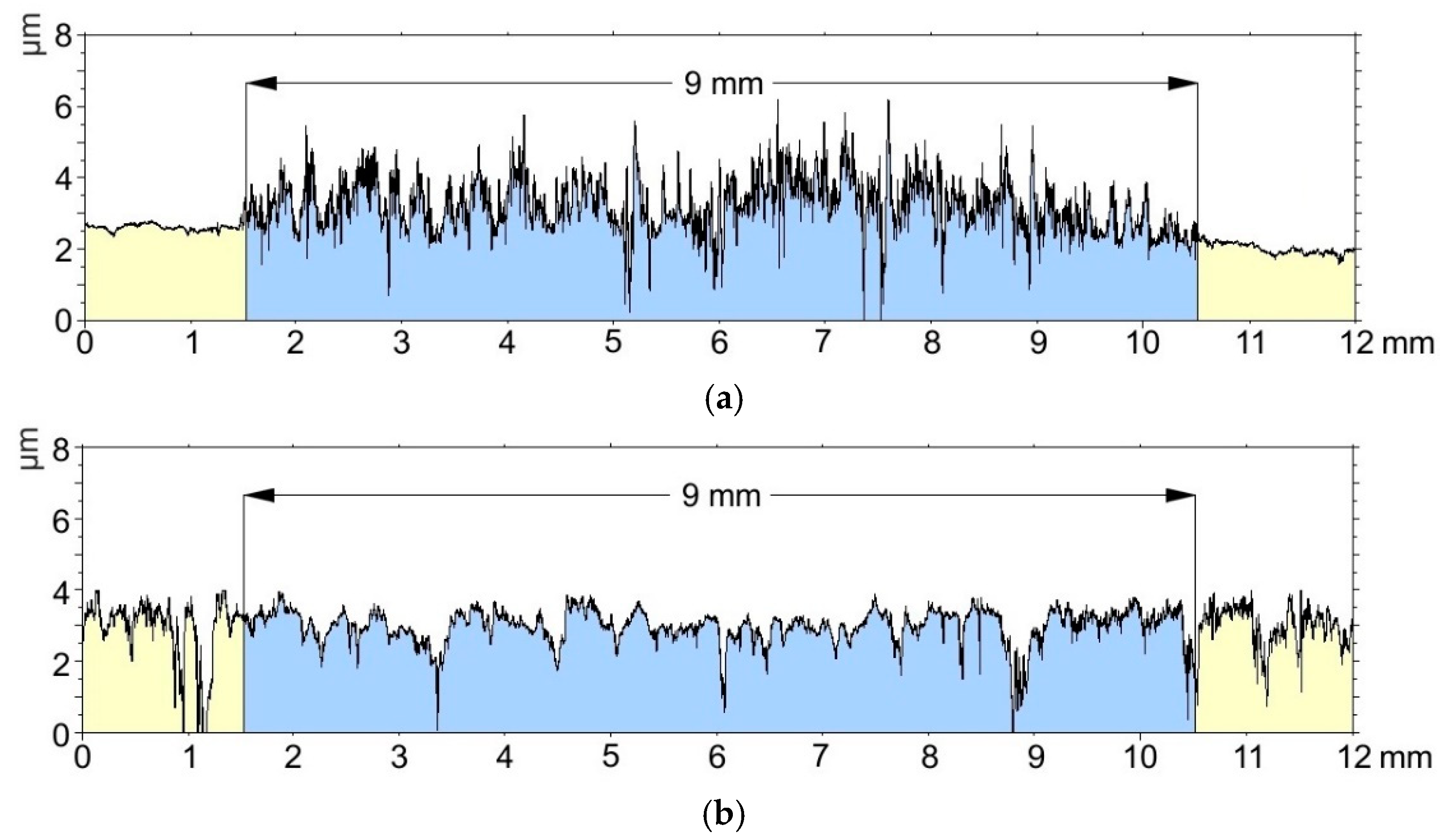
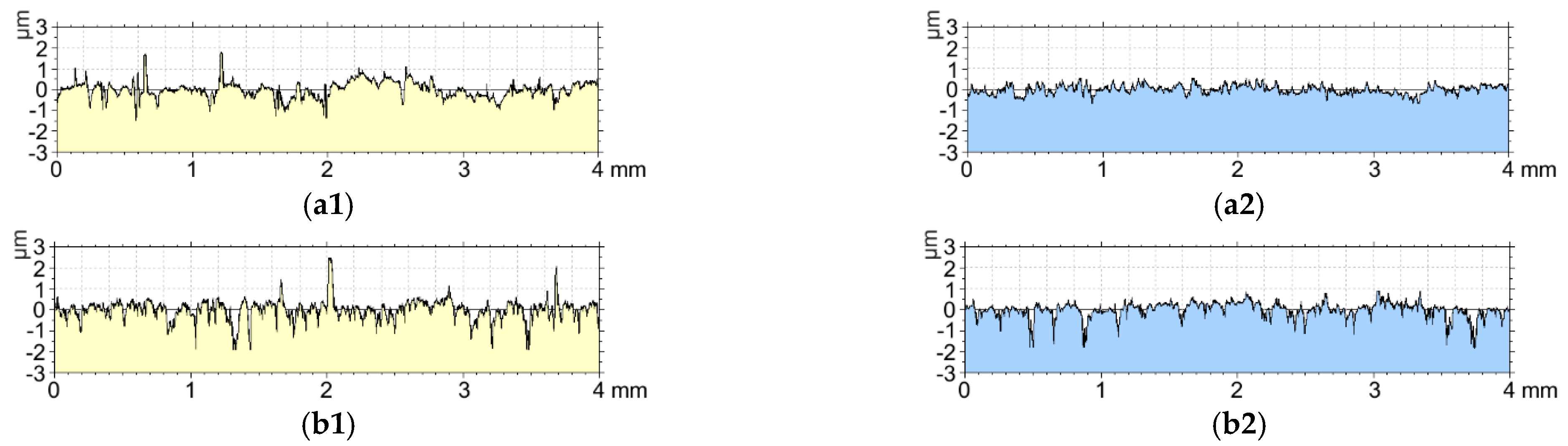
3.4. Surface Roughness Tests
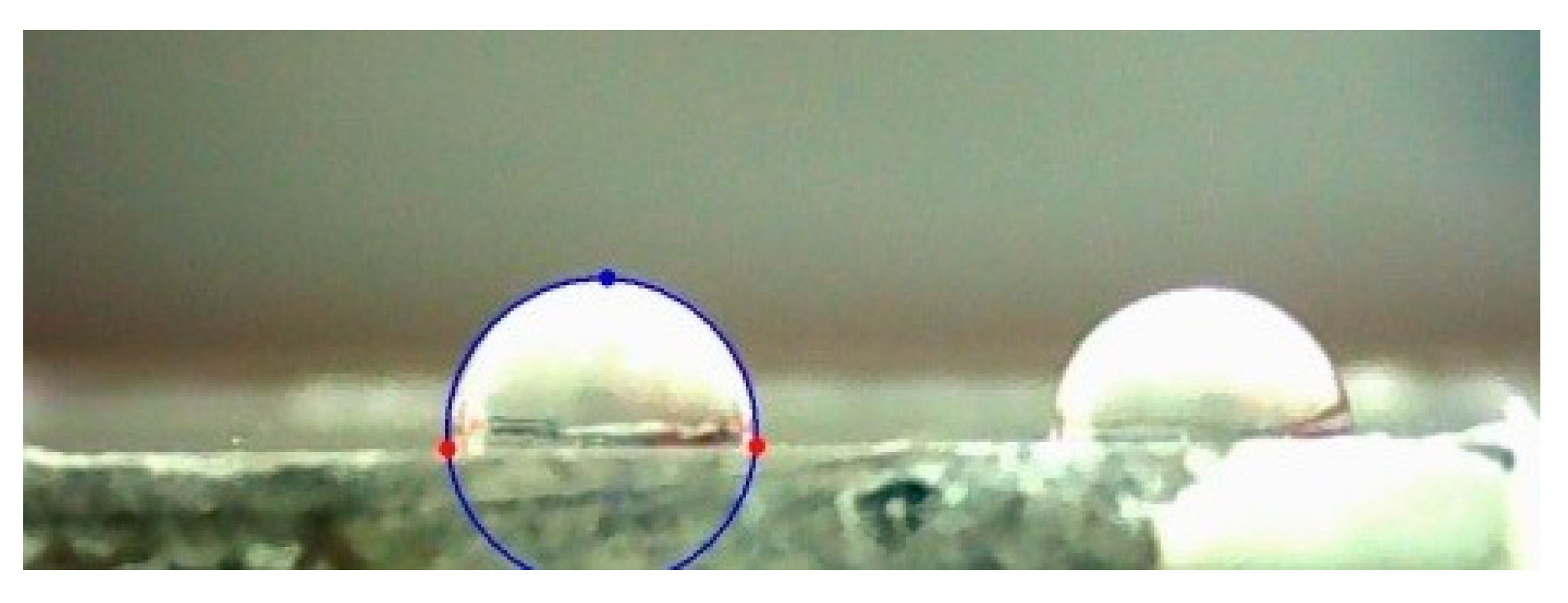
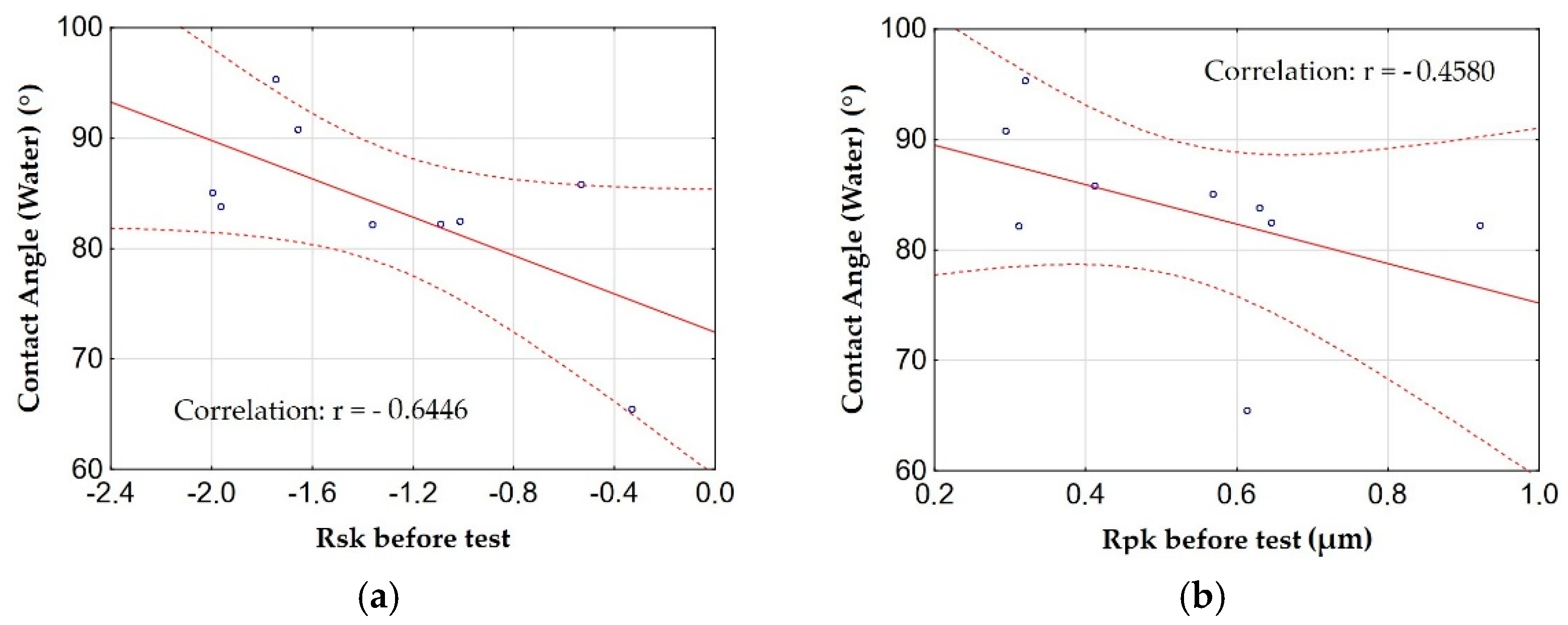
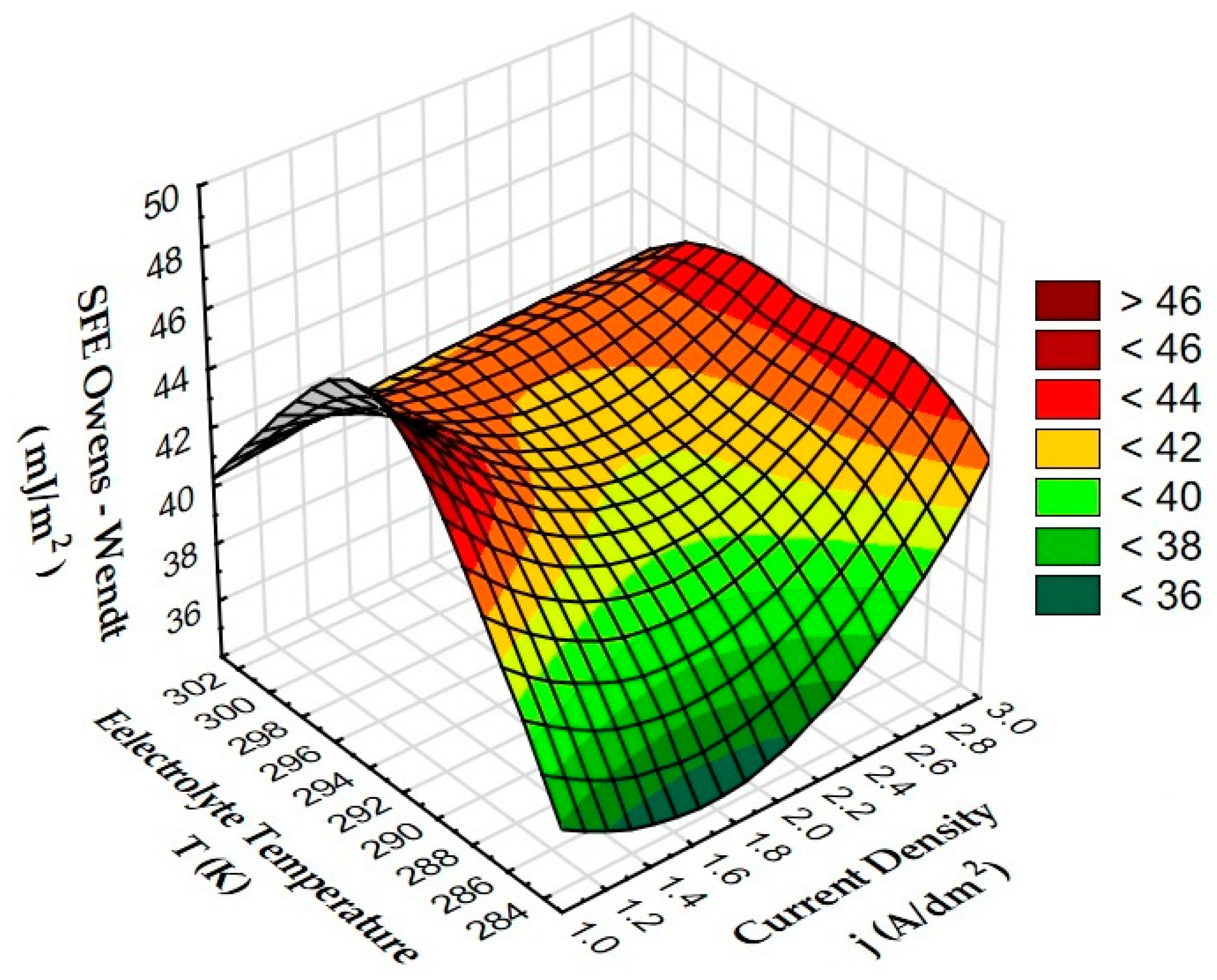
3.5. Measurements of Wettability and Calculation of Surface Free Energy
4. Conclusions
- The research results and their analysis presented in the article confirm the legitimacy of anodizing the EN AW-5251 aluminum alloy in order to improve its sliding properties.
- The anodizing parameters used during the experiment significantly affect the thickness of the Al2O3 layers. Increasing the current density causes a significant increase in the thick-ness of the layer, as a result of increasing the electric charge, while the increase in temperature causes a decrease in the thickness of the layer, as a result of the increased secondary solubility of the Al2O3 layer by the electrolyte with a higher temperature.
- The correlation analysis also showed a significant relationship between the anodizing parameters and the surface roughness of the samples, which in turn affects the wear of the material and the resistance to motion. An increase in the Rsk parameter and a decrease in Rvk causes an increase in the consumption of T7W material, while a decrease in Rsk and an increase in Rvk contribute to a reduction in material consumption. An increase in the Rsk value also increases the friction coefficient. The differences in the roughness parameters result in a different character of the overlapping of the sliding film on the surface of the layers, which in turn translates into differences in material wear and movement resistance.
- The anodizing parameters and surface roughness also affect the wettability of the surface of the layers, which also affects the ability to form and maintain a sliding film. Low current density values (1 and 2 A/dm2) in combination with low electrolyte temperature (283 K) allow for the production of a layer with high contact angles (low wettability). The correlation between the contact angle and the Rsk and Rpk parameters is negative; in other words, an increase in the roughness parameters causes a decrease in the contact angle (increase in wettability).
- The analysis of friction and wear tests showed that the layer with hydrophobic properties, produced at a current density of 1 A/dm2 in an electrolyte at a temperature of 283 K, is the most favorable for sliding associations with T7W material.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vargel, C. Corrosion of Aluminium, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Meyveci, A.; Karacan, I.; Çalıgülü, U.; Durmuş, H. Pin-on-disc characterization of 2xxx and 6xxx aluminium alloys aged by precipitation age hardening. J. Alloys Compd. 2010, 491, 278–283. [Google Scholar] [CrossRef]
- Derakshan, J.; Parsa, M.H.; Jafarian, H.R. Microstructure and mechanical properties variations of pure aluminum subjected to one pass of ECAP-Conform proces. Mater. Sci. Eng. A 2019, 747, 18. [Google Scholar]
- Korzekwa, J.; Bociąga, E.; Bochenek, D. Investigation of selected polymer composite-aluminum oxide coating tribological systems. Materials 2020, 13, 5491. [Google Scholar] [CrossRef] [PubMed]
- Siskou, N.; Charalampidou, C.; Alexopoulos, N.D.; Kourkoulis, S.K. Effect of corrosion exposure on aluminum alloy 2024 for different artificial ageing conditions. Procedia Struct. Integr. 2018, 10, 79–84. [Google Scholar] [CrossRef]
- Sankar, L.; Aruna, G.; Sathish, T.; Parthiban, A.; Vijayan, V.; Kumar, S.; Rajkumar, S.; Mekonnen, A.; Tufa, M. Strength enhancement study on composites of AA6066 Aluminium Alloy with Magnesium Oxide and Coal Ash. Adv. Mater. Sci. Eng. 2021, 2021, 2810106. [Google Scholar]
- Han, B.; Sun, D.; Wan, W.; Dong, C.; Li, D.; Zhao, L.; Wang, H. Correlation among Composition, microstructure and hardness of 7xxx aluminum alloy using original statistical spatial-mapping method. Materials 2022, 15, 5767. [Google Scholar] [CrossRef] [PubMed]
- Bara, M.; Niedźwiedź, M.; Skoneczny, W.; Barylski, A. Nanostructure and morphology of the surface as well as micromechanical and sclerometric properties of Al2O3 layers subjected to thermo-chemical treatment. Materials 2022, 15, 3. [Google Scholar] [CrossRef]
- Lee, W. The anodization of aluminum for nanotechnology applications. Jom J. Min. Met. Mat. S 2010, 62, 57–63. [Google Scholar] [CrossRef]
- Poodt, P.; Lankhorst, A.; Roozeboom, F.; Spee, K.; Maas, D.; Vermeer, M. High-speed spatial atomic-layer deposition of aluminum oxide layers for solar cell passivation. Adv. Mater. 2010, 22, 32. [Google Scholar] [CrossRef]
- Jani, A.M.M.; Losic, D.; Voelcker, N.H. Nanoporous anodic aluminium oxide: Advances in surface engineering and emerging applications. Prog. Mater. Sci. 2013, 58, 5. [Google Scholar]
- Korzekwa, J.; Bara, M.; Pietraszek, J.; Pawlus, P. Tribological behaviour of Al2O3/inorganic fullerene-like WS2 composite layer sliding againstplastic. Int. J. Surf. Sci. Eng. 2016, 10, 570–584. [Google Scholar] [CrossRef]
- Korzekwa, J.; Bara, M.; Kaptacz, S. Al2O3/WS2 surface layers produced on the basis of aluminum alloys for applications in oil-free kinematic systems. Materials 2021, 14, 7738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, F.; Niu, J.; Jiang, Y.G.; Wang, Z.Q. Superhydrophobic surfaces: From structural control to functional application. J. Mater. Chem. 2008, 18, 621–633. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, B.W.; Qu, M.N.; Zhang, J.Y.; He, D.Y. Fabrication of superhydrophobic surfaces on engineering material surfaces with stearic acid. Appl. Surf. Sci. 2008, 254, 2009–2012. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, Y.; Zhang, L.; Sun, J.; Huang, J.; Huang, J.; Chen, J.; Cao, Y. Hydrophilic poly(triphenylamines) with phosphonate groups on the side chains: Synthesis and photovoltaic applications. J. Mater. Chem. 2012, 22, 4329–4336. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Q.; Song, K.; Ren, S.; Lei, T.; Zhang, Q. Using cellulose nanocrystals as a sustainable additive to enhance hydrophilicity, mechanical and thermal properties of poly(vinylidene fluoride)/poly(methyl methacrylate) blend. ACS Sustain. Chem. Eng. 2015, 3, 574–582. [Google Scholar] [CrossRef]
- Li, X.; Liu, K.L.; Wang, M.; Wong, S.Y.; Tiju, W.C.; He, C.B.; Goh, S.H.; Li, J. Improving hydrophilicity, mechanical properties and biocompatibility of poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyvalerate] through blending with poly[(R)-3-hydroxybutyrate]-alt-poly(ethylene oxide). Acta Biomater. 2009, 5, 2002–2012. [Google Scholar] [CrossRef]
- Ghanbari, A.; Attar, M.M. Surface free energy characterization and adhesion performance of mild steel treated based on zirconium conversion coating: A comparative study. Surf. Coat. Technol. 2014, 246, 26–33. [Google Scholar] [CrossRef]
- Sobolewski, S.; Lodes, M.A.; Rosiwal, S.M.; Singer, R.F. Surface energy of growth and seeding side of free standing nanocrystalline diamond foils. Surf. Coat. Technol. 2013, 232, 640–644. [Google Scholar] [CrossRef]
- Schustera, J.M.; Schvezova, C.E.; Rosenberger, M.R. Effect of the contact angle on the morphology, residence time distribution and mass transfer into liquid rivulets: A CFD study. Chem. Eng. Sci. 2018, 176, 356–366. [Google Scholar]
- Thieme, M.; Worch, H. Ultrahydrophobicaluminium surfaces: Properties and EIS measurements of different oxidic and thin-film coated states. J. Solid State Electrochem. 2006, 10, 737–745. [Google Scholar] [CrossRef]
- Wojciechowski, Ł.; Nosal, S. The application of free surface energy measurement to valuation of adhesive scuffing. Eksploat. Niezawodn. 2010, 1, 83–89. [Google Scholar]
- Korzekwa, J.; Skoneczny, W.; Dercz, G.; Bara, M. Wear mechanism of Al2O3/WS2 With PEEK/BG Plastic. J. Tribol. T Asme 2013, 1, 136. [Google Scholar] [CrossRef]
- Zhao, N.-Q.; Jiang, X.-X.; Shi, C.-S.; Li, J.-J.; Zhao, Z.-G.; Du, X.-W. Effects of anodizing conditions on anodic alumina structure. J. Mater. Sci. 2007, 42, 3878–3882. [Google Scholar] [CrossRef]
- Sulka, G.D. Highly ordered anodic porous alumina formation by self-organized anodizing. In Nanostructured Materials in Electrochemistry; Eftekhari, A., Ed.; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2008; pp. 8–20. [Google Scholar]
- Liu, P.; Singh, V.P.; Rajaputra, S. Barrier layer nonuniformity effects in anodized aluminum oxide nanopores on ITO substrates. Nanotechnology 2010, 21, 115303. [Google Scholar] [CrossRef]
- Sattler, K. Handbook of Nanophysics/Functional Nanomaterials; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Bara, M.; Niedźwiedź, M.; Skoneczny, W. Influence of anodizing parameters on surface morphology and surface-free energy of Al2O3 layers produced on EN AW-5251 Alloy. Materials 2019, 12, 5. [Google Scholar] [CrossRef]

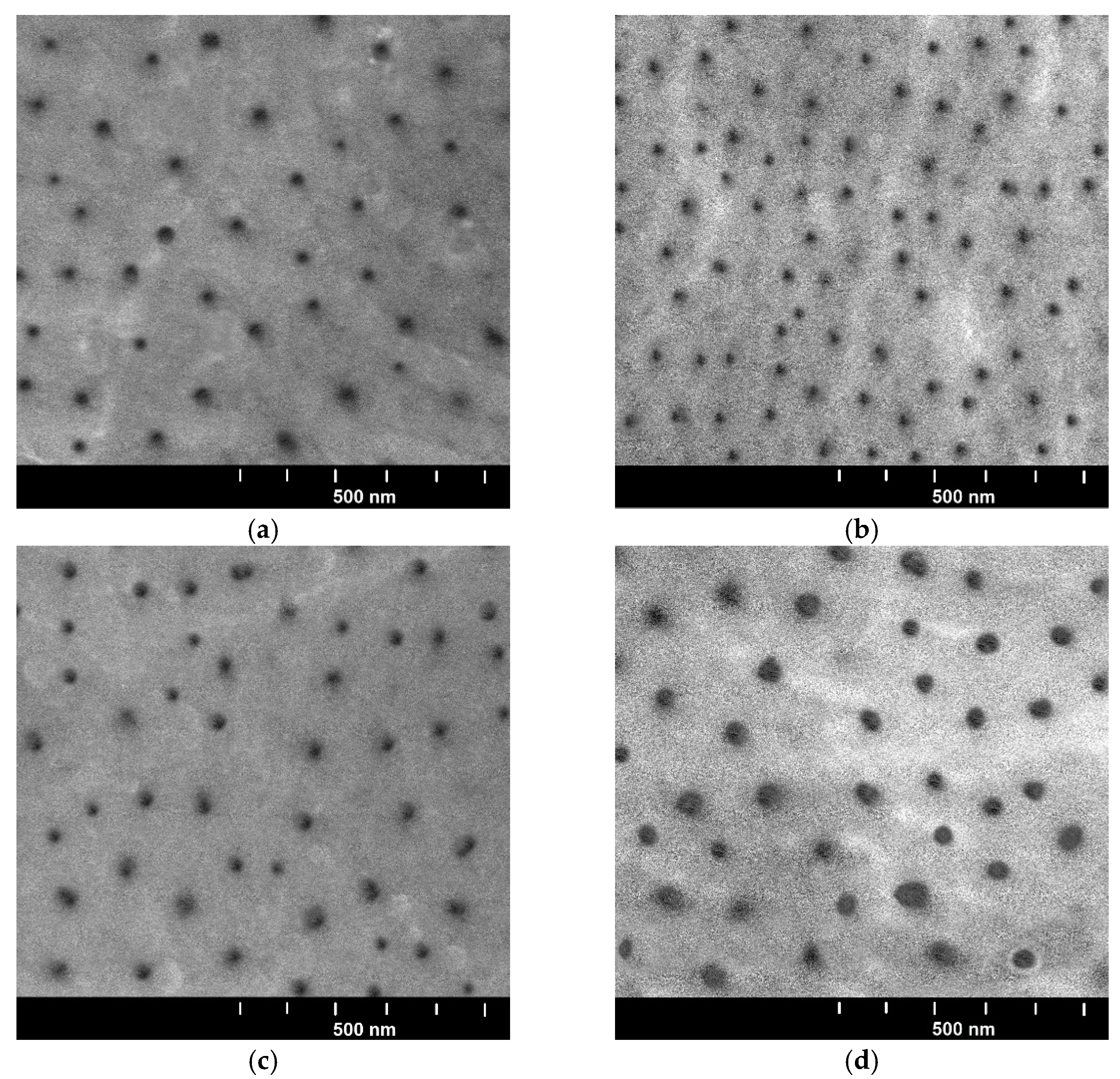





| Sample | Controlled Factors | |||
|---|---|---|---|---|
| On a Natural Scale | On a Standard Scale | |||
| Current Density j (A/dm2) | Electrolyte Temperature T (K) | ×1 | ×2 | |
| A | 1 | 283 | −1 | −1 |
| B | 3 | 283 | 1 | −1 |
| C | 1 | 303 | −1 | 1 |
| D | 3 | 303 | 1 | 1 |
| E | 1 | 293 | −1 | 0 |
| F | 3 | 293 | 1 | 0 |
| G | 2 | 283 | 0 | −1 |
| H | 2 | 303 | 0 | 1 |
| I | 2 | 293 | 0 | 0 |
| Sample | Oxide Layers Thickness d (μm) | Deviation (μm) |
|---|---|---|
| A | 5.8 | 0.5 |
| B | 19.0 | 0.7 |
| C | 5.1 | 0.2 |
| D | 17.6 | 0.4 |
| E | 5.2 | 0.3 |
| F | 17.7 | 0.5 |
| G | 11.9 | 0.5 |
| H | 11.3 | 0.1 |
| I | 11.4 | 0.3 |
| Sample | Coefficient of Friction µ |
|---|---|
| A | 0.1425 |
| B | 0.1443 |
| C | 0.1661 |
| D | 0.1438 |
| E | 0.1463 |
| F | 0.1615 |
| G | 0.1419 |
| H | 0.1513 |
| I | 0.1564 |
| Al | 0.1932 |
| Sample | Mass Loss (mg) |
|---|---|
| A | 0.20 |
| B | 0.60 |
| C | 0.20 |
| D | 0.34 |
| E | 0.86 |
| F | 0.56 |
| G | 0.60 |
| H | 0.16 |
| I | 0.84 |
| Al | 0.60 |
| Sample | Amplitude Parameters | Parameters of the Load Curve (Abott Fireston) | ||||
|---|---|---|---|---|---|---|
| Rq (μm) | Rsk | Rku | Rk (μm) | Rpk (μm) | Rvk (μm) | |
| Before test | ||||||
| A | 0.4625 | −1.6561 | 8.1086 | 0.8090 | 0.2937 | 0.9324 |
| B | 0.5513 | −1.3622 | 6.2085 | 1.0764 | 0.3112 | 0.9693 |
| C | 0.4815 | −1.0896 | 12.1512 | 0.7486 | 0.9222 | 0.9337 |
| D | 0.5452 | −1.9966 | 14.2198 | 0.8502 | 0.5683 | 1.2482 |
| E | 0.3381 | −0.3301 | 11.8179 | 0.4928 | 0.6135 | 0.6071 |
| F | 0.5467 | −1.0133 | 8.6509 | 0.9570 | 0.6454 | 0.9341 |
| G | 0.5392 | −1.7443 | 5.4716 | 1.0826 | 0.3195 | 0.9328 |
| H | 0.5573 | −1.9644 | 16.1986 | 0.7969 | 0.6299 | 1.1172 |
| I | 0.2970 | −0.5330 | 7.3632 | 0.5634 | 0.4120 | 0.4280 |
| Al | 0.0765 | −0.2227 | 9.8823 | 0.1415 | 0.0911 | 0.1162 |
| After test | ||||||
| A | 0.4068 | −1.6021 | 8.7518 | 0.6347 | 0.3303 | 0.9389 |
| B | 0.3700 | −1.4232 | 6.9293 | 0.6591 | 0.2449 | 0.6995 |
| C | 0.4385 | −2.3079 | 12.9483 | 0.5856 | 0.3199 | 0.8947 |
| D | 0.4178 | −2.7333 | 14.1218 | 0.6194 | 0.2655 | 1.0170 |
| E | 0.1946 | −0.4623 | 5.2914 | 0.3416 | 0.2375 | 0.2871 |
| F | 0.2332 | −0.5208 | 4.7619 | 0.4419 | 0.2375 | 0.3654 |
| G | 0.4182 | −1.9585 | 10.4411 | 0.7215 | 0.2762 | 0.7879 |
| H | 0.3809 | −2.6221 | 14.4178 | 0.5178 | 0.2793 | 0.8692 |
| I | 0.1735 | 0.1649 | 4.2484 | 0.3442 | 0.2384 | 0.1948 |
| Al | 0.8276 | −0.7317 | 6.2506 | 1.7494 | 0.9724 | 1.6857 |
| Sample | Contact Angle (Distilled Water) (°) | Deviation (°) | Contact Angle (α-Bromonaphthalene) (°) | Deviation (°) |
|---|---|---|---|---|
| A | 90.80 | 2.70 | 39.88 | 4.44 |
| B | 82.16 | 5.70 | 29.34 | 2.54 |
| C | 82.25 | 5.14 | 37.68 | 3.23 |
| D | 85.06 | 3.67 | 30.12 | 2.43 |
| E | 65.49 | 5.98 | 33.05 | 2.80 |
| F | 82.46 | 6.29 | 27.18 | 2.24 |
| G | 95.33 | 3.86 | 31.54 | 3.75 |
| H | 83.81 | 2.25 | 29.83 | 3.75 |
| I | 85.83 | 4.11 | 32.54 | 2.45 |
| Al | 71.59 | 4.14 | 36.12 | 2.36 |
| Sample | Contact Angle (Glycerin) (°) | Deviation (°) | Contact Angle (Diiodomethane) (°) | Deviation (°) |
|---|---|---|---|---|
| A | 84.25 | 7.69 | 59.05 | 2.26 |
| B | 69.59 | 3.01 | 41.38 | 3.19 |
| C | 80.22 | 5.24 | 44.74 | 6.38 |
| D | 78.80 | 1.36 | 45.76 | 7.09 |
| E | 73.80 | 2.55 | 47.74 | 7.75 |
| F | 69.27 | 2.89 | 46.51 | 3.65 |
| G | 87.03 | 4.10 | 50.52 | 4.98 |
| H | 72.60 | 4.04 | 46.09 | 5.93 |
| I | 88.54 | 2.76 | 47.77 | 3.02 |
| Al | 80.67 | 3.67 | 52.19 | 2.14 |
| Sample | SFE Owens–Wendt (mJ/m2) |
|---|---|
| A | 36.68 |
| B | 42.49 |
| C | 39.75 |
| D | 41.46 |
| E | 48.31 |
| F | 43.04 |
| G | 35.61 |
| H | 41.87 |
| I | 40.48 |
| Al | 49.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedźwiedź, M.; Bara, M.; Skoneczny, W.; Kaptacz, S.; Dercz, G. Influence of Anodizing Parameters on Tribological Properties and Wettability of Al2O3 Layers Produced on the EN AW-5251 Aluminum Alloy. Materials 2022, 15, 7732. https://doi.org/10.3390/ma15217732
Niedźwiedź M, Bara M, Skoneczny W, Kaptacz S, Dercz G. Influence of Anodizing Parameters on Tribological Properties and Wettability of Al2O3 Layers Produced on the EN AW-5251 Aluminum Alloy. Materials. 2022; 15(21):7732. https://doi.org/10.3390/ma15217732
Chicago/Turabian StyleNiedźwiedź, Mateusz, Marek Bara, Władysław Skoneczny, Sławomir Kaptacz, and Grzegorz Dercz. 2022. "Influence of Anodizing Parameters on Tribological Properties and Wettability of Al2O3 Layers Produced on the EN AW-5251 Aluminum Alloy" Materials 15, no. 21: 7732. https://doi.org/10.3390/ma15217732
APA StyleNiedźwiedź, M., Bara, M., Skoneczny, W., Kaptacz, S., & Dercz, G. (2022). Influence of Anodizing Parameters on Tribological Properties and Wettability of Al2O3 Layers Produced on the EN AW-5251 Aluminum Alloy. Materials, 15(21), 7732. https://doi.org/10.3390/ma15217732








