Abstract
The structure, phase composition and spectral luminescence properties of single crystal and ceramic specimens of (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 solid solutions synthesized using uniaxial compaction and slip casting techniques have been compared. The ceramic specimens have been synthesized from crushed single crystal specimens of similar composition. It has been shown that the crystalline structures of the ceramic and single crystal specimens are identical and cubic. The ceramic specimens synthesized using different methods prove to have close microstructure patterns. The spectral luminescence properties of Eu3+ ions in the (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramic specimens are similar to those of the single crystals with similar composition. The (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramic specimens prove to have uncontrolled Cr3+:Al2O3 impurities due to the synthesis conditions.
1. Introduction
Zirconia-based materials exhibit a wide range of physicochemical properties that provide the possibility of their use in various applications [1,2,3]. For example, the high oxygen-ionic conductivity of these materials makes them suitable as solid-state electrolytes in solid oxide fuel cells, oxygen gages, oxygen pumps, etc. [3,4,5].
Zirconia-based solid state electrolytes are most typically synthesized using various ceramic technologies [6,7,8,9,10,11]. The synthesis method and conditions greatly affect the structural, physical, mechanical and transport properties of the ceramics. The structure, phase composition and density of the ceramic electrolytes depend largely on the precursor particle synthesis method. Ceramic material parameters such as grain size, porosity and component distribution homogeneity in the bulk and at the grain boundaries may dramatically change the electrophysical parameters of solid state electrolyte materials.
Precursor particles for ZrO2-Y2O3 ceramics are produced using solid state synthesis, laser ablation, hydrothermal techniques, sol–gel technologies, combustion, co-deposition of metal hydroxides, co-crystallization of salts, etc. The most widely used methods are solid state synthesis [12] and various modifications of sol–gel synthesis [13,14,15].
It was of interest within the scope of this work to implement zirconia ceramic synthesis from molten powder obtained by crushing of ZrO2-Y2O3 single crystals. The (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 composition of the source single crystals was chosen because our earlier studies of the transport properties of ZrO2-Y2O3 crystals showed that crystals containing 9 mol.% Y2O3 stabilizing oxide have the highest ionic conductivity [16]. Eu3+ ions were introduced in the ZrO2-Y2O3 single crystals as a spectroscopic probe for studying the local neighborhood of the stabilizing oxide ions [17,18,19,20,21,22].
Thus, the aim of this work was to synthesize (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramics from crushed single crystals of similar composition by uniaxial compaction and slip casting, to study its structure, phase composition and the local neighborhood of the Eu3+ and Y3+ ions in this ceramic and to compare these parameters with those of single crystal specimens with similar composition.
2. Materials and Methods
The test (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 solid solution single crystals were grown using directional melt crystallization at a 10 mm/h rate in a 130 mm diameter water-cooled crucible with direct high-frequency heating on a Kristall-407 plant (frequency 5.28 MHz, power 60 kW) (Moscow, Russia) [23,24]. The charge was prepared from zirconium oxide (ZrO2), yttrium oxide (Y2O3) and europium oxide (Eu2O3) with a purity of min. 99.96 wt.%.
Some of the as-synthesized (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 solid solution single crystals were mechanically crushed to powder less than 1 mm in size, which was used for the synthesis of ceramic specimens. The preliminarily crushed (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 powder was ground with oleic acid addition in a drum with lining and grinding bodies made from stabilized zirconium dioxide (Y2O3 stabilizer) («Fritsch», Idar-Oberstein, Germany). The final powder had a grain size of ~40 µm and a specific surface area of ~8000 cm2/g.
The ceramic specimens were synthesized using two methods, i.e., uniaxial compaction and slip casting of thin films on a moving substrate.
Casting powders were prepared for the uniaxial compaction of ceramic specimens. Granulated powder was compacted in the form of discs (radius ~15 mm, thickness ~0.48 mm) at a 15 kN pressure. The specimens were air heat treated at 1680 °C for 2 h in furnaces with lanthanum chromite heaters and VK-97 closed corundum ceramic crucibles («Nabertherm», Lilienthal, Germany). The density of the specimens was ~5.85 g/cm2, i.e., 98% of the density of single crystals with similar composition (~5.98 g/cm2). The ceramic specimens synthesized by uniaxial compaction are shown in Figure 1a.
Figure 1.
(ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramic specimens synthesized from crushed single crystals of similar composition using (a) uniaxial compaction and (b) slip casting.
Slip casting of films on a moving ribbon (substrate) was implemented using a special plant designed by JSC «ECON» (JSC «ECON», Obninsk, Russia). The 7 mm × 7 mm × 0.3 mm pieces were placed on porous aluminum oxide kiln furniture and air heat treated at 1680 °C for 2 h. The density of the specimens was ~5.86 g/cm2, i.e., 98% of the density of single crystals with similar composition (~5.98 g/cm2). The ceramic specimens synthesized by slip casting are shown in Figure 1b.
The density of the (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 single crystal and ceramic specimens was measured by hydrostatic weighing on a Sartogosm CE224-C balance (St. Petersburg, Russia).
The surface morphology and elemental composition of the ceramic specimens were studied using scanning electron microscopy (SEM) and energy dispersion spectroscopy under a Quanta TM 3D 200i scanning electron microscope equipped with a microanalysis system (EDS) (FEI Company, Hillsboro, OR, USA). The SEM images were taken at a 20 kV accelerating voltage under high vacuum conditions (~10−3 Pa).
The phase composition of the ceramic specimens was studied using X-ray diffraction on an Empyrean diffractometer from PANalytical B.V. Co. (CuKα radiation, λ = 1.5414 Å) with a vertical goniometer and a PIXcel 3D detector (PANalytical B.V. Co, Almelo, Netherlands). The diffraction patterns were identified using the JSPDS PDF 2 1911 database.
The phase composition was also studied using Raman spectroscopy on a NTEGRA SPECTRA instrument from NT-MDT Co. with a 632.8 nm He-Ne laser as the excitation source (NT-MDT Co., Zelenograd, Russia). The Raman spectra were recorded in the reflection scheme with a resolution of 0.8 cm−1.
The local neighborhood of the stabilizing oxide ions was studied using optical spectroscopy with Eu3+ ions being used as a spectroscopic probe. The T = 300 K luminescence spectra were recorded using an FHR 1000 spectrophotometer from Horiba Co. (Horiba Co., Kyoto, Japan) and a Hamamatsu R928B photomultiplier («Hamamatsu Photonics», Naka Ward, Japan) was used as a radiation detector. The excitation sources were YVO4:Nd (λexc. = 532 nm) and LiYF4:Nd (λexc. = 527 nm) lasers.
The 473 nm luminescence spectra were recorded for different areas of the ceramic specimen on a NTEGRA SPECTRA instrument from NT-MDT Co. (NT-MDT Co., Zelenograd, Russia). at room temperature. Confocal microscopic images were taken from 50 × 50 µm areas of the specimens.
The excitation spectra were recorded on a RF-5301PC spectrofluorometer from Shimadzu Co. (Shimadzu Co., Kyoto, Japan) with a 150 W xenon lamp used as an excitation source and a R212-14 photomultiplier used as a radiation detector.
3. Results
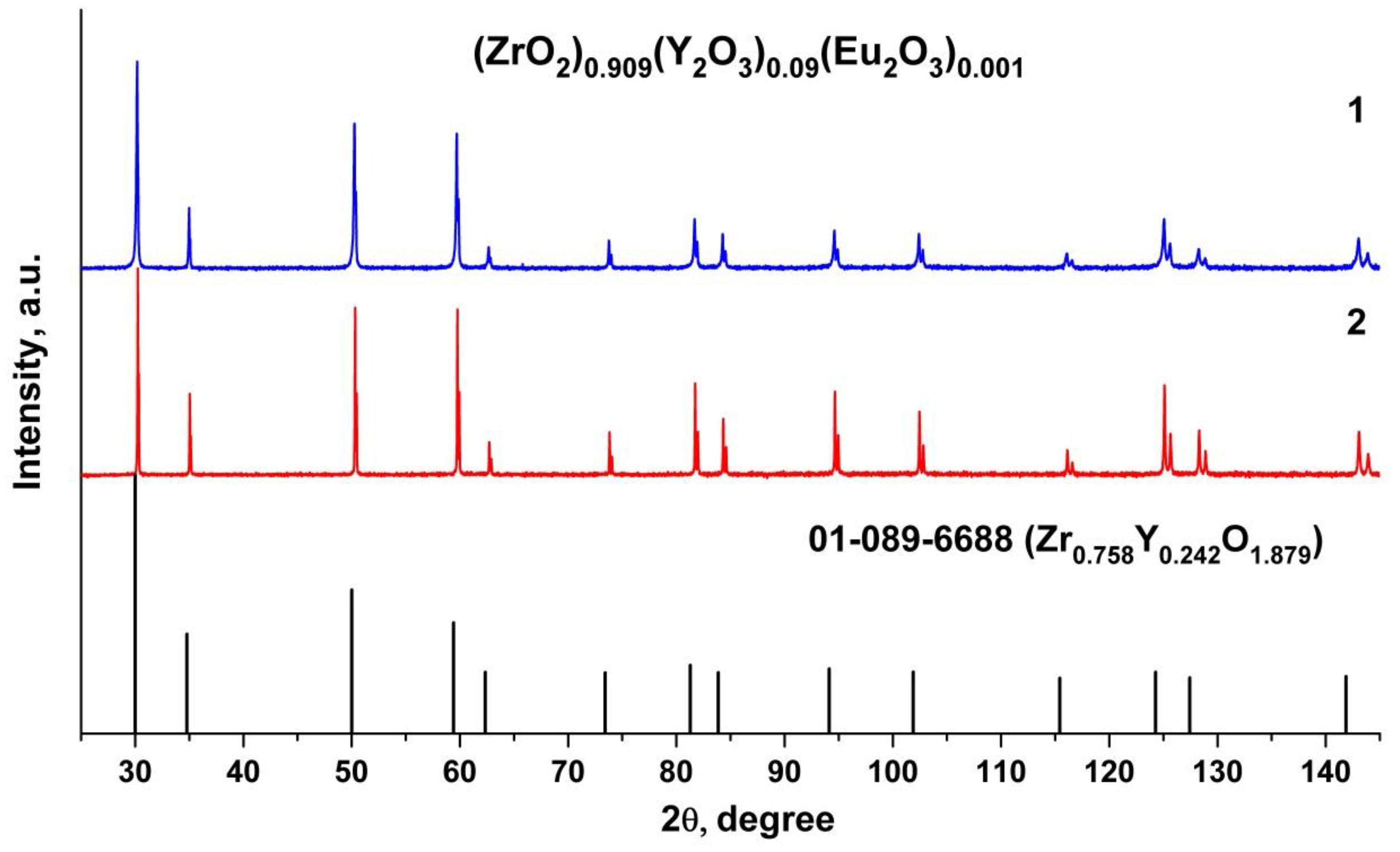
X-ray phase analysis data for the test (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramic specimens (Figure 2) suggest that the material is single-phase and has a fluorite-type cubic structure.
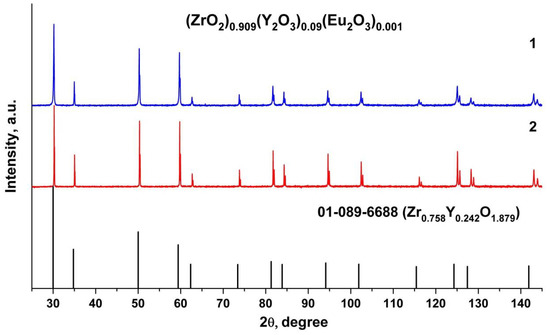
Figure 2.
Fragments of the X-ray diffraction patterns of (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramic solid solutions synthesized using different methods from crushed single crystals: (1) uniaxial compaction; (2) slip casting.
The test ceramic specimens synthesized using different methods (uniaxial compaction and slip casting) from crushed single crystals have close unit cell parameters, as summarized in Table 1. The lattice parameter of the (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramics is somewhat smaller than that of the single crystal material with similar composition.

Table 1.
Phase composition and lattice parameters of (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 single crystals and ceramics synthesized using different methods from crushed single crystals.
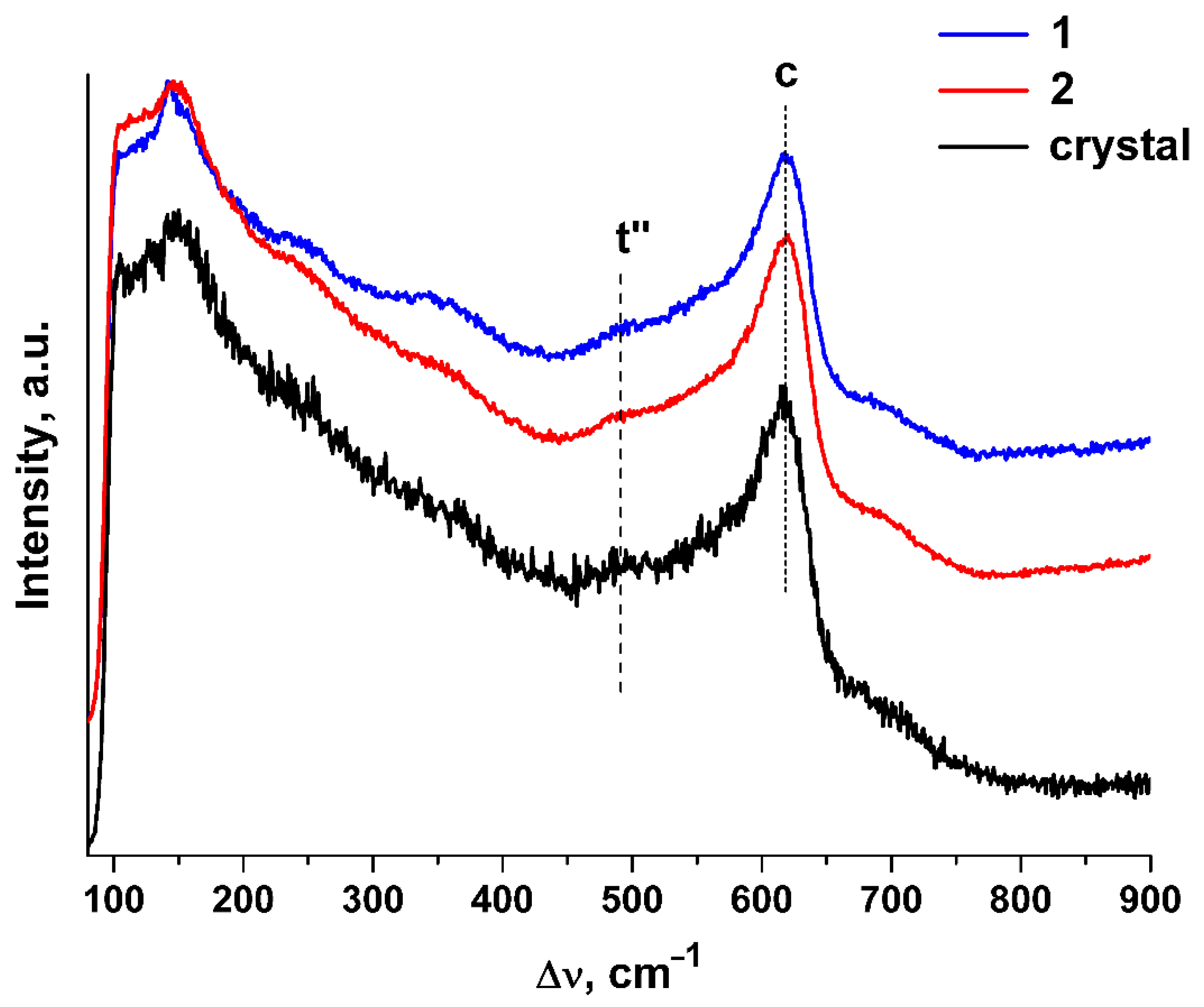
The Raman spectra of the (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramics synthesized using different methods are shown in Figure 3. For comparison, the Raman spectrum of single crystals with similar composition is also shown.
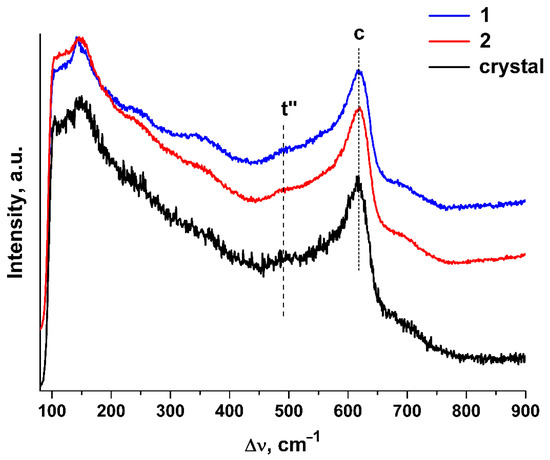
Figure 3.
Raman spectra of (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 single crystal and ceramic solid solutions synthesized using different methods from crushed single crystals: (1) uniaxial compaction; (2) slip casting. λexc. = 632.8 nm, T = 300 K.
The shape and positions of the bands in the Raman spectra of the ceramic specimens are close to those in the Raman spectra of the single crystals with similar composition. The Raman spectra of both the single crystal and ceramic specimens contain, along with the band typical of the cubic phase (c), a band near ~483 cm−1 that is typical of the t″ phase [25,26,27]. The structure of the t″ phase is close to cubic except for a small shift of the oxygen ions along a certain direction relative to their positions in the fluorite structure.
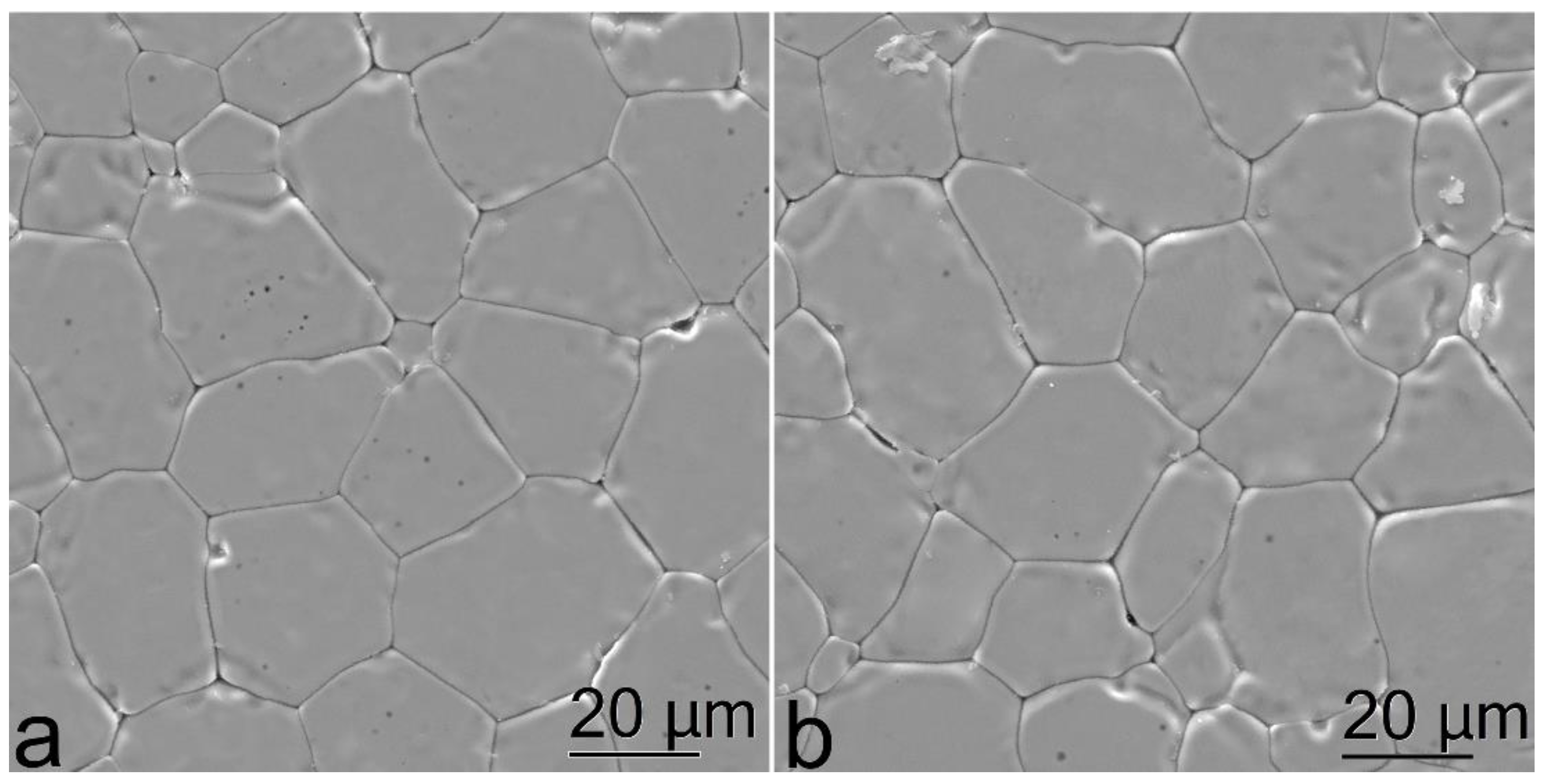
Figure 4 shows the surface SEM images of the test (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramic specimens.
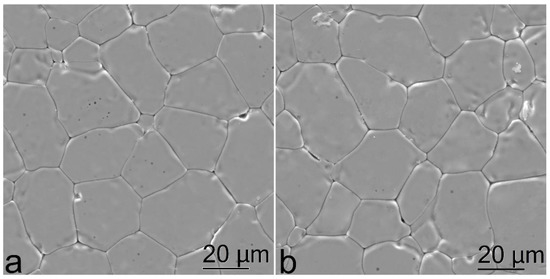
Figure 4.
Microstructure images of the (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramic specimens synthesized using different methods from crushed single crystals: (a) uniaxial compaction; (b) slip casting.
The SEM data suggest that the microstructures of the ceramic specimens synthesized using different methods slightly differ. The ceramic specimens synthesized using uniaxial compaction (Figure 4a) have a rough surface. The grain sizes could only be determined after specimen polishing. On the contrary, the ceramic specimens synthesized using slip casting on a ribbon had a smoother surface and their microstructure could be visualized under optical or electron microscope without preliminary polishing. The grain size was approx. 10 to 40 μm for the specimens synthesized using different methods.
Energy dispersion elemental analysis of the ceramic materials suggests that their chemical composition is identical to that of the single crystal specimens except for the presence of aluminum on the specimen surfaces.
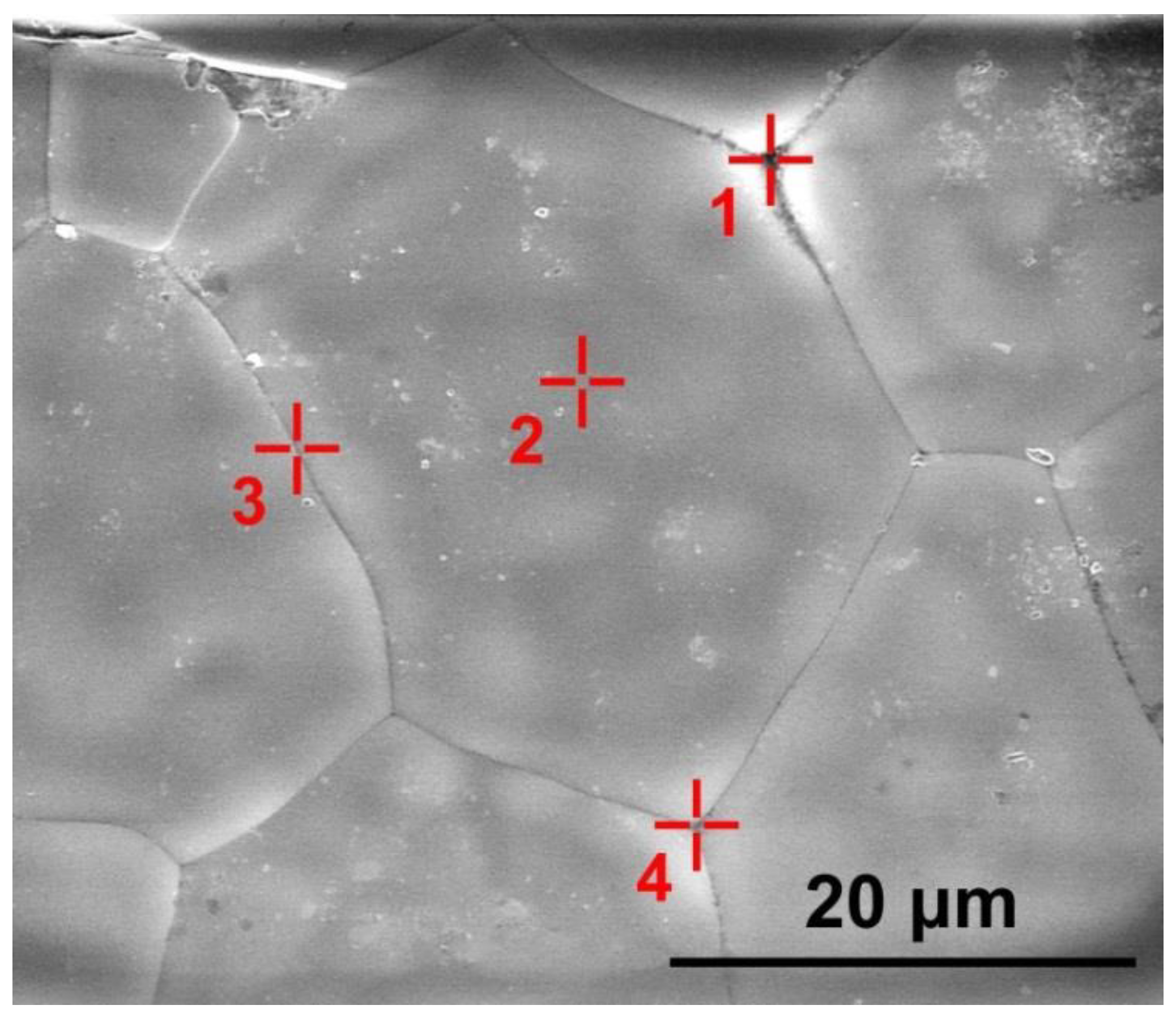
The presence and distribution of the uncontrolled aluminum oxide impurity was studied for different ceramic specimen areas. The content of europium oxide in the material is commensurable with the energy dispersion method error and therefore was not measured. Figure 5 shows the grain structure of the ceramic specimen synthesized using slip casting. Table 2 shows experimentally measured concentrations of ZrO2, Y2O3 and Al2O3 oxides in this ceramic specimen for the areas shown in Figure 5.
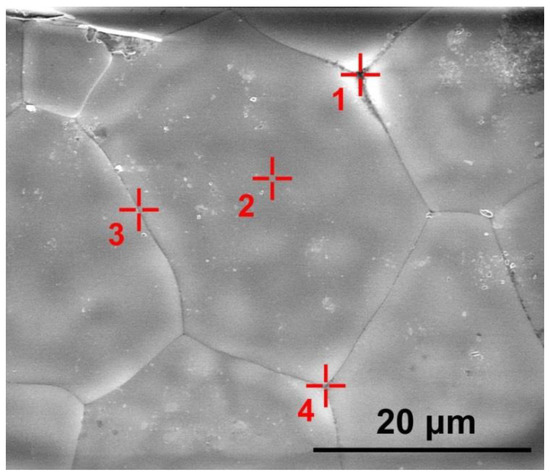
Figure 5.
SEM image of the (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramic specimen synthesized using slip casting. The  symbols show areas (1, 2, 3, 4) in which the ZrO2, Y2O3 and Al2O3 oxide concentrations were measured.
symbols show areas (1, 2, 3, 4) in which the ZrO2, Y2O3 and Al2O3 oxide concentrations were measured.
 symbols show areas (1, 2, 3, 4) in which the ZrO2, Y2O3 and Al2O3 oxide concentrations were measured.
symbols show areas (1, 2, 3, 4) in which the ZrO2, Y2O3 and Al2O3 oxide concentrations were measured.

Table 2.
Elemental analysis data for (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramics synthesized using slip casting.
Table 2 suggests that the Al2O3 concentration at the ceramic grain surfaces was 0.6 mol.%. The oxide distribution along the grain boundaries was local (the Al2O3 concentration being within 1–3 mol.%). In the area corresponding to the intergrain volume (area 1 in Figure 5) between three ceramic grains, the Al2O3 concentration was 3.3 mol.%.
It can be hypothesized that the origin of the aluminum impurity is the corundum crucible that the specimens contacted during annealing. Analysis of literary data reveals evidence of the low solubility of aluminum oxide in ZrO2-Y2O3. It was reported [28,29,30,31] that the solubility limit of Al2O3 in ZrO2-9 mol.% Y2O3 at T = 1700 °C is 0.7 mol.%. At higher Al2O3 concentrations, aluminum oxide in the ZrO2-Y2O3 ceramics is present in the form of particles located inside the grains and at the grain boundaries or forms grain-boundary phases with high Al2O3 content.
Taking into account the results of this work and earlier studies [28,29,30,31], one can conclude that some of the Al2O3 impurity is dissolved in the ceramics whereas most of the impurity is localized at the grain boundaries and, to a smaller extent, on the ceramic surface in the form of inclusions. Al2O3 solubility in (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramic solid solutions can be the cause of the decrease in the lattice parameter of the solid solutions in comparison to the single crystals of similar composition.
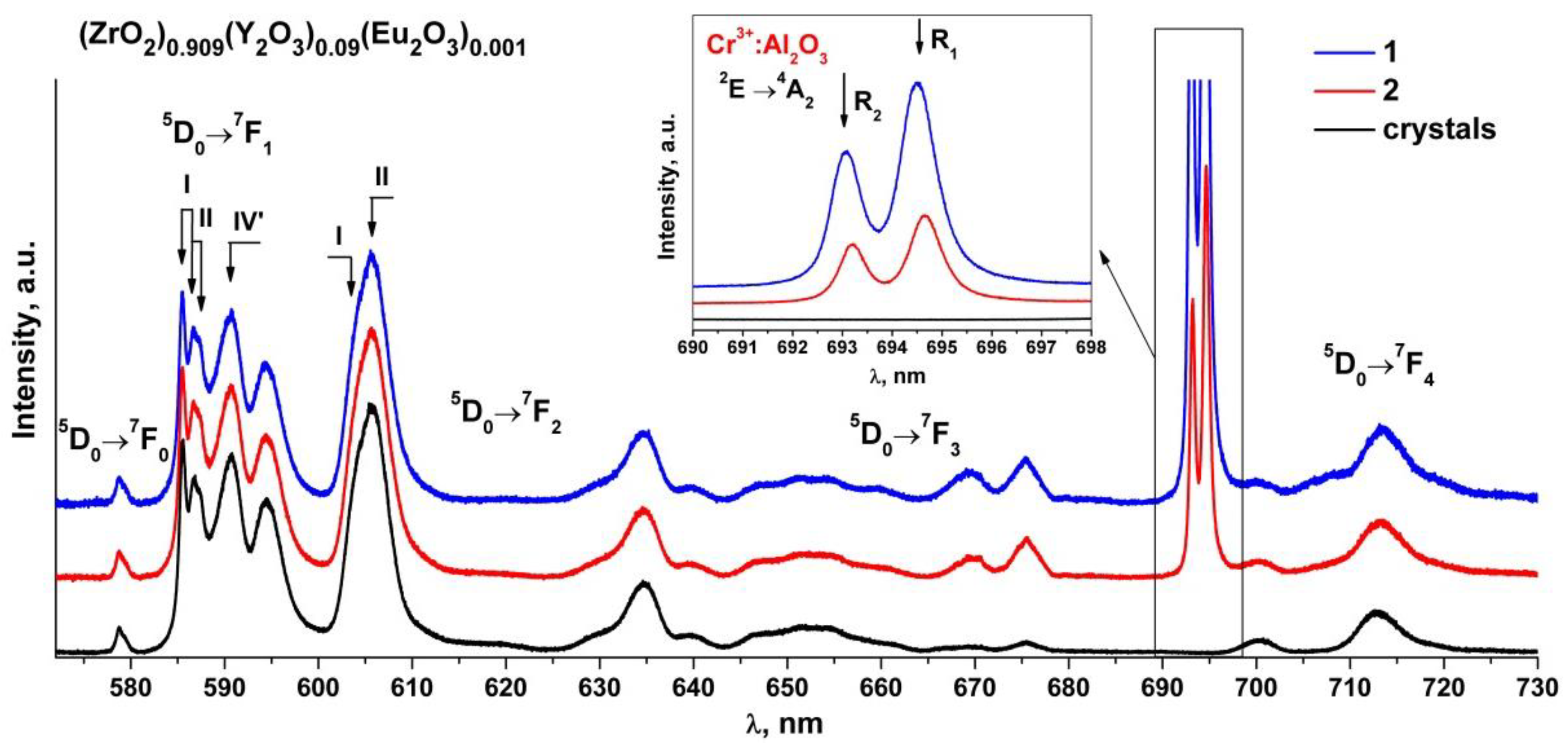
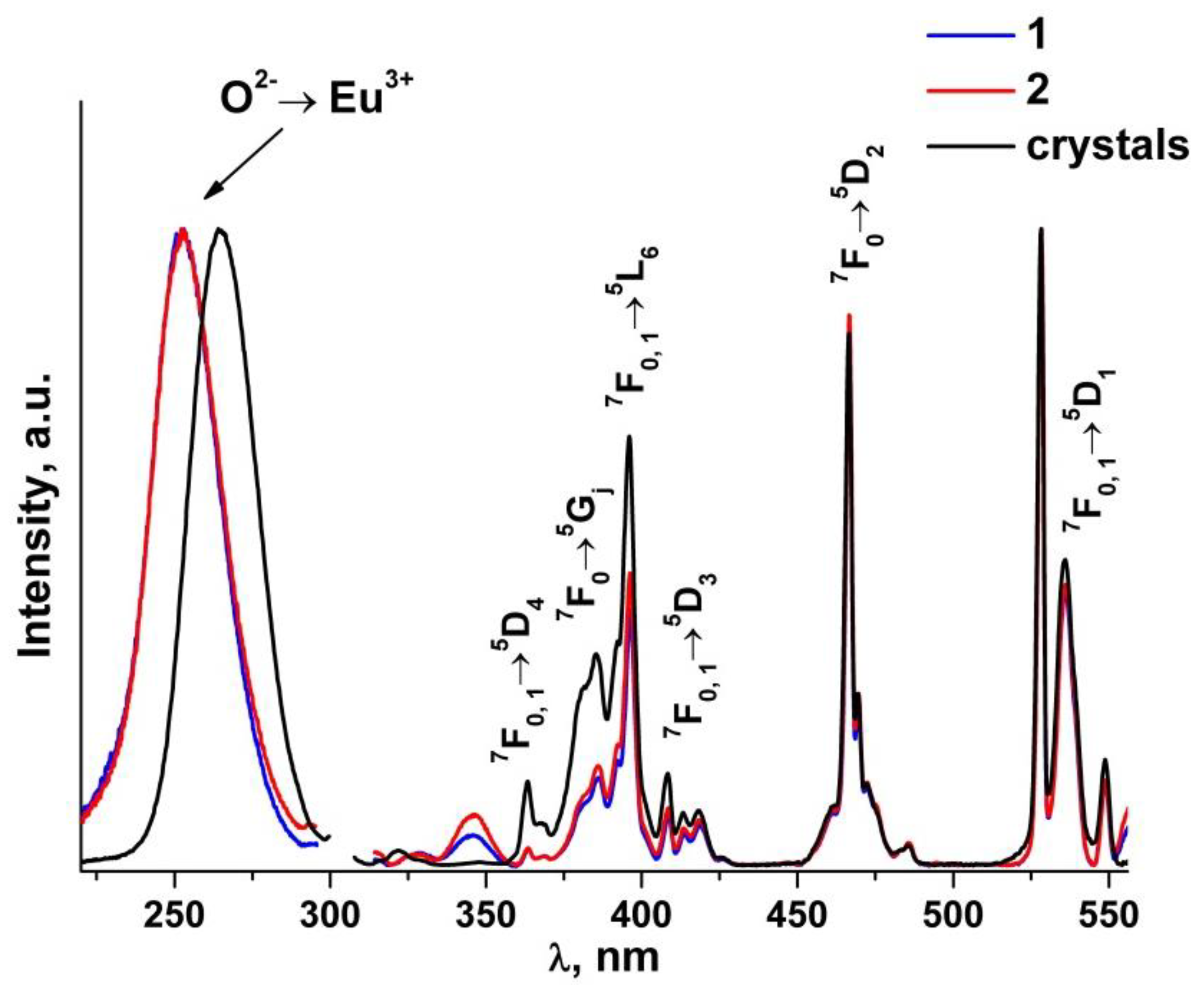
Figure 6 shows the luminescence spectra of the (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 single crystal and ceramic solid solutions recorded with excitation of the 5D1 level of the Eu3+ ions by a 532 nm laser at room temperature.
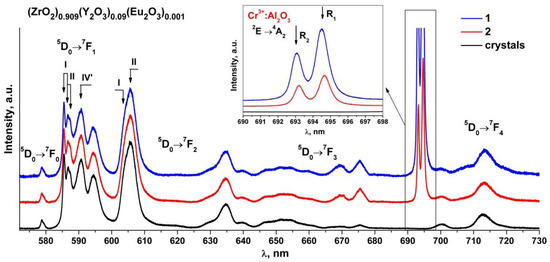
Figure 6.
Luminescence spectra of (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 single crystal and ceramic solid solutions synthesized using different methods from crushed single crystals: (1) uniaxial compaction; (2) slip casting. λ = 532 nm, T = 300 K. Inset: luminescence bands (R1 and R2) of Cr3+ ions in Al2O3 for the 2E→4A2 transition.
Analysis of the luminescence spectra for the transitions between the 5D0 and 7FJ(0-4) multiplets of the Eu3+ ions in the (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramic solid solutions recorded with excitation of the 5D1 level of the Eu3+ ions by a 532 nm laser at T = 300 K shows that they contain the same spectral bands as the respective spectra of the (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 single crystal solid solutions. The luminescence spectra of the (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 single crystals for the 5D0→7F0-2 transitions of the Eu3+ ions, which are superimpositions of the bands of the Eu3+ optical centers with different neighborhoods, were reported earlier [17]. The numbers I, II and IV’ in Figure 6 mark the respective Eu3+ optical centers. Optical center I is where the Eu3+ ions have one oxygen vacancy and are surrounded by seven oxygen atoms. Optical center II is where the Eu3+ ions do not have oxygen vacancies in the first coordination shell but have one oxygen vacancy in the second coordination shell. Optical center IV’ represents the centers in which oxygen vacancies are located in the farthest coordination shells of the Eu3+ ions.
It can be seen from Figure 6 that the intensity ratio of the spectral bands for the 5D0→7F0-2 transitions of the Eu3+ ions pertaining to different optical centers in the synthesized (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramic specimens is the same as the respective intensity ratio of the optical centers for the single crystal material of similar composition. The difference in the luminescence spectra for the ceramics is the presence of two intense and narrow bands near 693 and 694.5 nm and a change in the shape of the spectral bands in the 665–730 nm region. Taking into account the results of earlier studies [17,18,19,20,21,22], one can conclude that these changes in the luminescence spectra of the ceramics are not caused by the optical transitions of the Eu3+ ions. It can also be seen from Figure 6 that the bands near 693 and 694.5 nm for different ceramic specimens have different intensities relative to the luminescence bands of the Eu3+ ions.
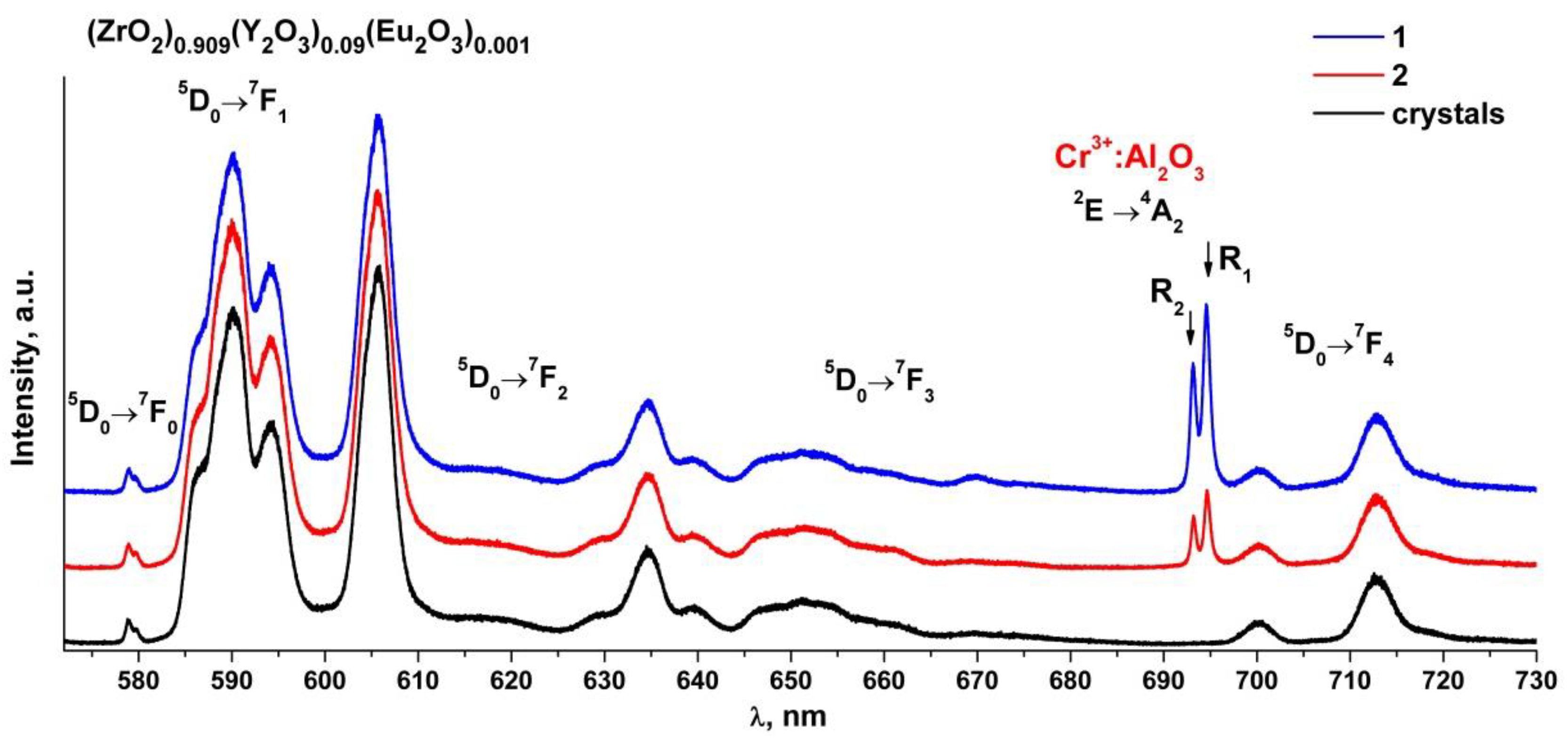
Figure 7 shows the luminescence spectra of the (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 single crystal and ceramic solid solutions recorded with excitation of the 5D1 level of the Eu3+ ions by a 527 nm laser at room temperature.
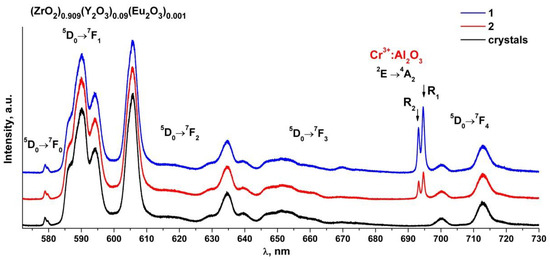
Figure 7.
Luminescence spectra of (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 single crystal and ceramic solid solutions synthesized using different methods from crushed single crystals: (1) uniaxial compaction; (2) slip casting. λ = 527 nm, T = 300 K.
Analysis of the luminescence spectra of the Eu3+ ions in the (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 single crystal and ceramic solid solutions shown in Figure 7 shows that the luminescence spectra of the ceramic specimens are identical to each other and to the luminescence spectrum of the single crystal specimen. The luminescence spectra of the ceramic specimens differ from those of the single crystal specimen through the presence of two narrow bands at 693 and 694.5 nm whose intensities differ for the two ceramic specimens. It should be noted that the intensities of the bands in the luminescence spectrum of the Eu3+ ions under excitation by a 527 nm laser are lower than the intensities of the bands in the luminescence spectrum recorded with 532 nm excitation.
Analysis of literary data shows that the Cr3+: Al2O3 luminescence spectrum excited by a 532 nm laser contains two intense and narrow bands near 692.8 and 694.2 nm (the so-called R-bands) caused by the 2E→4A2 transition of the Cr3+ ions and also contains several additional weak bands near 660, 670, 706 and 714 nm (the N-bands and the sidebands) [32,33,34]. The positions of these bands are similar to those of the bands found in the luminescence spectra of the (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramics (Figure 6 and Figure 7). Thus, all the ceramic specimens contained a chromium impurity along with aluminum oxide, as suggested by the presence of additional bands in the luminescence spectra of the ceramic specimens.
The chromium impurity probably originates from the use of lanthanum chromite (LaCrO3) heaters during specimen heat treatment in insufficiently closed aluminum oxide (Al2O3) crucibles. Since the ceramic specimens were sintered at a high temperature (1680 °C), chromium ions could evaporate from the heater material LaCrO3. The volatility of chromium in LaCrO3 at high temperatures is a well-known problem [35]. It was hypothesized that chromium evaporation occurs by the following reaction:
Chromium oxide may also reduce to metallic chromium at above 1200 °C [36].
According to literary data, Cr2O3 solubility in Al2O3 is unlimited [37], and given that the luminescence spectra (Figure 6 and Figure 7) only contain bands typical of the Cr3+ ions in Al2O3, one can conclude that Cr2O3 interacts mainly with Al2O3 to form the Cr2O3-Al2O3 solid solution. The chromium impurity seems to not enter into the ZrO2-Y2O3-Eu2O3 solid solution since the experimental luminescence spectra did not contain the wide band in the 700–1100 nm region which is typical of Cr3+ ion luminescence in ZrO2-Y2O3 [38,39]. Furthermore, Cr2O3 solubility in ZrO2-Y2O3 was quite low, only 0.7 mol.% for the ZrO2-8 mol.% Y2O3 ceramics at T = 1450 °C [36,40].
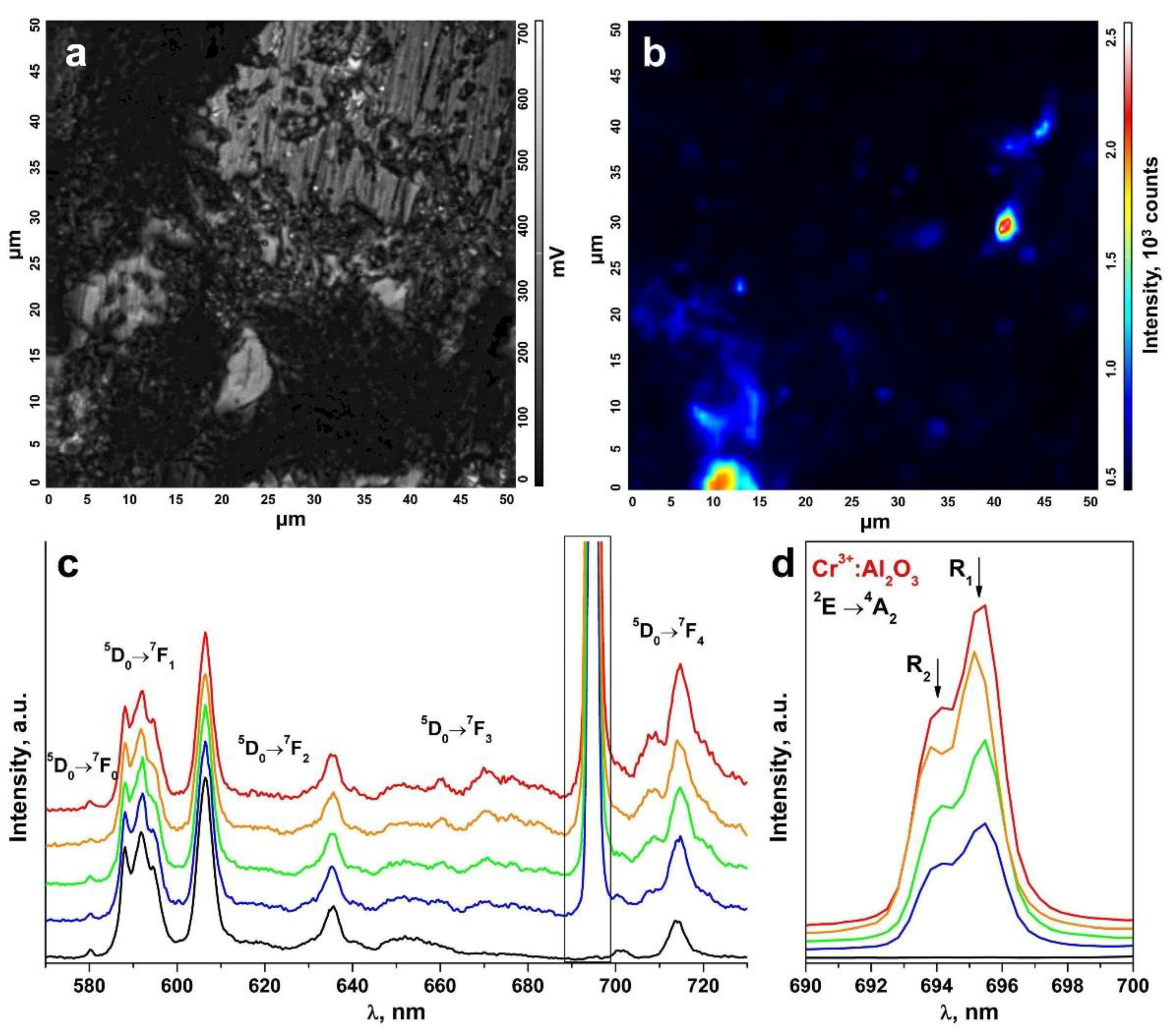
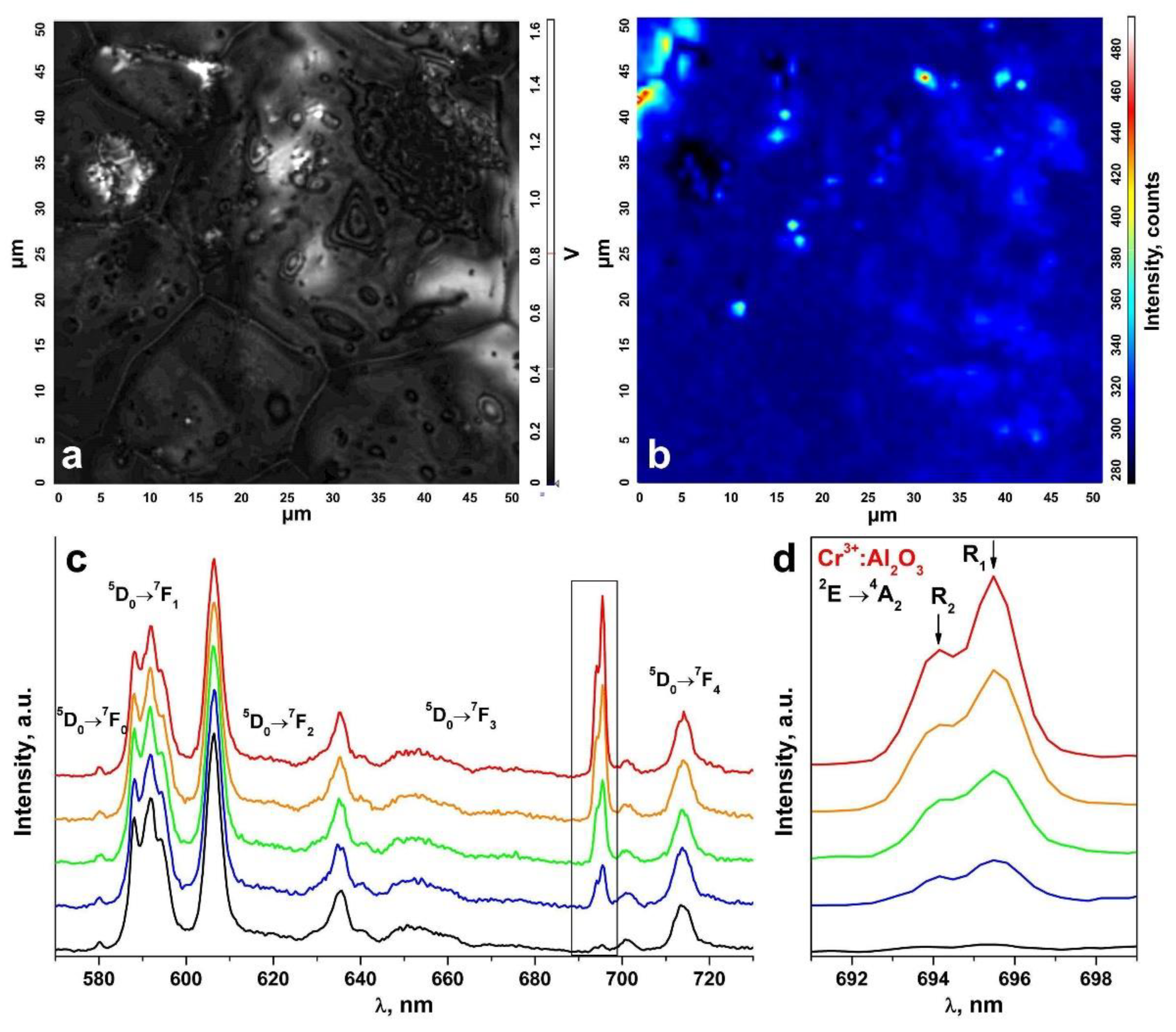
An inhomogeneous distribution of the uncontrolled impurity in the ceramic specimens is suggested by the change in the R1 and R2 luminescence band intensities of the Cr3+ ions in Al2O3 in comparison to the luminescence bands of the Eu3+ ions in different ceramic surface areas (Figure 8 and Figure 9). The R1 and R2 luminescence band intensity distribution map for the Cr3+ ions in Al2O3 in a 50 μm × 50 μm surface area (Figure 8a and Figure 9a) is shown in Figure 8b and Figure 9b. Figure 8c and Figure 9c show the luminescence spectra for different surface areas of the test ceramics excited with a 473 nm laser. Figure 8d and Figure 9d separately show the R1 and R2 luminescence bands of the Cr3+ ions in Al2O3 for the 2E→4A2 transition.
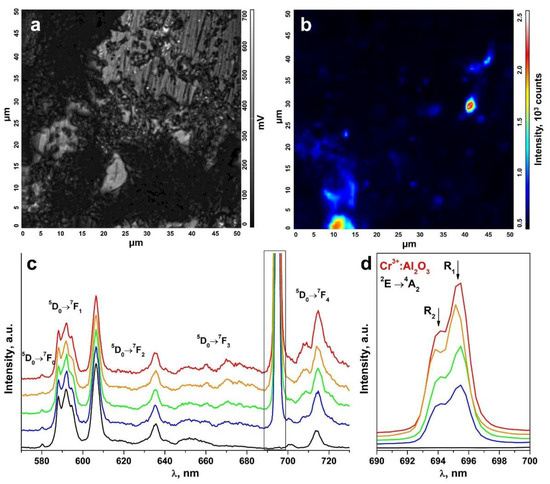
Figure 8.
(a) Surface image of a ceramic specimen synthesized using uniaxial compaction; (b) R1 and R2 luminescence band intensity distribution map for the Cr3+ ions in Al2O3 on the surface area shown in Figure 8a; (c) luminescence spectra of the ceramic specimen excited with a λ = 473 nm laser (T = 300 K) corresponding to the intensity scale in Figure 8b; (d) R1 and R2 luminescence bands of the Cr3+ ions in Al2O3 for the 2E→4A2 transition.
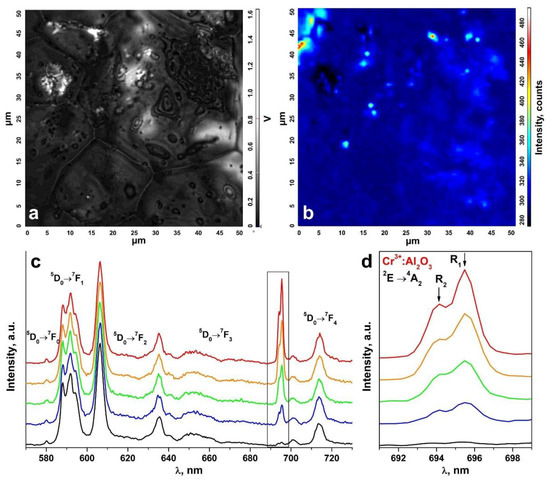
Figure 9.
(a) Surface image of a ceramic specimen synthesized using slip casting; (b) R1 and R2 luminescence band intensity distribution map for the Cr3+ ions in Al2O3 on the surface area shown in Figure 9a; (c) luminescence spectra of the ceramic specimen excited with a λ = 473 nm laser (T = 300 K) corresponding to the intensity scale in Figure 9b; (d) R1 and R2 luminescence bands of the Cr3+ ions in Al2O3 for the 2E→4A2 transition.
These spectra are shown in relative units normalized to unity relative to the luminescence band peaking at 606.4 nm caused by the 5D0→7F2 transition of the Eu3+ ions. The color of the R1 and R2 luminescence bands of the Cr3+ ions in Figure 8c,d and Figure 9c,d corresponds to the color in the scale shown to the right of the intensity distribution maps in Figure 8b and Figure 9b characterizing the intensity of these bands.
Thus, analysis of the R1 and R2 luminescence band intensity distribution maps for the Cr3+ ions in Al2O3 on the ceramic specimen surfaces, as shown in Figure 8b and Figure 9b, showed that the uncontrolled Cr3+: Al2O3 impurities in the test ceramic specimens were in the form of discrete inclusions.
Figure 10 shows the luminescence excitation spectra of (ZrO2)0.879(Y2O3)0.12(Eu2O3)0.001 single crystal and (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramic solid solutions. The recording wavelength of 606 nm corresponds to the position of the most intense 5D0→7F2 transition band of the Eu3+ ions.
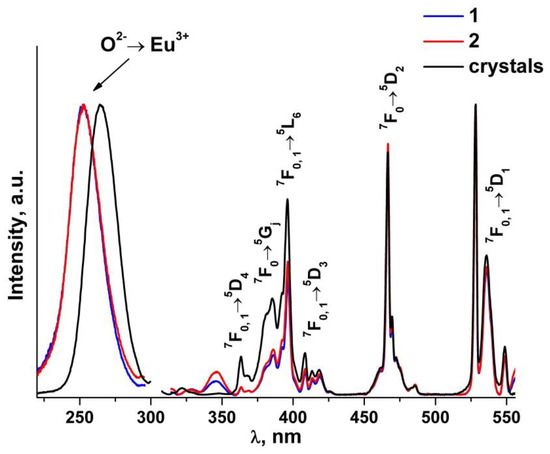
Figure 10.
Luminescence excitation spectra of (ZrO2)0.879(Y2O3)0.12(Eu2O3)0.001 single crystal and (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramic solid solutions synthesized using different methods from crushed single crystals: (1) uniaxial compaction; (2) slip casting. λem = 606 nm, T = 300 K.
The excitation spectra of the (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramics (Figure 10) contain a sufficiently wide symmetrical band in the 220 to 290 nm region peaking at ~253 nm, which according to earlier data [41] originates from the O2−→Eu3+ charge transfer transition. For the (ZrO2)0.879(Y2O3)0.12(Eu2O3)0.001 single crystal, this band is at a greater wavelength (~265 nm). Along with the O2−→Eu3+ charge transfer band, the luminescence excitation spectra of the (ZrO2)0.879(Y2O3)0.12(Eu2O3)0.001 single crystal and (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramic solid solutions contain bands corresponding to intraconfigurational f-f transitions between the multiplets of the 7F main state and the 5D, 5L and 5G excited states of the Eu3+ ions. The most intense band is the one at 528 nm that pertains to the 7F0, 1→5D2 transition. The difference in the spectral band intensities between the single crystals and the ceramics for the group of optical transitions in the 380–430 nm region seems to be caused by stronger scattering from the non-transparent ceramic specimens.
4. Discussion
In this work ceramic specimens were synthesized using uniaxial compaction and slip casting from powders of crushed (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 single crystals grown by directional melt crystallization in a cold skull. The ceramic specimens were sintered in air at 1680 °C for 2 h. The density of the as-synthesized ceramic specimens was 98% of the density of the single crystal specimens.
The (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramic solid solutions synthesized using different methods from crushed single crystals are single-phase and have a fluorite-type cubic structure with close lattice parameters. For uniaxially compacted ceramics, a = 5.1381(3) Å; for slip cast ceramics, a = 5.1368(3) Å. Raman spectroscopy showed that the structure of the test ceramic specimens consists of a t″ phase.
The surface morphology of the ceramic specimens synthesized using different methods differs, but the microstructures of the specimens are quite similar. The (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 slip cast ceramic specimens have more homogeneous surfaces. The grain size of the ceramic specimens was approx. 10 to 40 µm.
Comparison of the luminescence spectra of the Eu3+ ions in the (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramic and single crystal solid solutions did not reveal any tangible difference. The luminescence excitation spectra of the Eu3+ ions in the (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramics are similar in band shape and position to the luminescence excitation spectra of the Eu3+ ions in the single crystals except for the O2−→ Eu3+ charge transfer band’s shift towards smaller wavelengths.
Elemental analysis revealed the presence of aluminum impurities, and optical spectroscopic study of the (ZrO2)0.909(Y2O3)0.09(Eu2O3)0.001 ceramic specimens showed the presence of uncontrolled Cr3+:Al2O3 impurities originating from conditions related to the synthesis process.
Author Contributions
Conceptualization, P.R., E.L. and N.T.; Formal analysis, A.K., V.M., V.K., A.N., M.B. and E.C.; Investigation, N.L., V.K., F.M. and A.N.; Methodology, P.R.; Resources, M.B., A.K., E.L., V.M., N.T. and E.C.; Supervision, E.L.; Validation, P.R. and N.T; Visualization, N.L. and F.M.; Writing—original draft, N.L. and P.R.; Writing—review and editing, N.L., P.R., M.B., V.K., E.L., F.M., V.M. and N.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Moscow Polytechnic University within the framework of the grant named after Pyotr Kapitsa.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are available within the manuscript.
Acknowledgments
The authors are grateful to V.P. Mishkin for help in studying the surface morphology and elemental composition of the ceramic specimens by scanning electron microscopy (SEM) and energy dispersion spectroscopy.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationship that could have appeared to influence the work reported in this paper.
References
- Chen, Y.W.; Moussi, J.; Drury, J.L.; Wataha, J.C. Zirconia in biomedical applications. Expert Rev. Med. Devices 2016, 13, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Peuchert, U.; Okano, Y.; Menke, Y.; Reichel, S.; Ikesue, A. Transparent cubic-ZrO2 ceramics for application as optical lenses. J. Eur. Ceram. Soc. 2009, 29, 283–291. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Mansilla, Y.; Arce, M.; Oliver Gonzalez, C.; Troiani, H.; Serquis, A. Synthesis and characterization of ZrO2 and YSZ thin films. Mater. Today Proc. 2019, 14, 92–95. [Google Scholar] [CrossRef]
- Fergus, J.W. Electrolytes for solid oxide fuel cells. J. Power Sources 2006, 162, 30–40. [Google Scholar] [CrossRef]
- Dou, J.; Li, H.; Xu, L.; Zhang, L.; Wang, G. Preparation of YSZ solid electrolyte by slip casting and its properties. Rare Met. 2009, 28, 372–377. [Google Scholar] [CrossRef]
- Shukla, M.; Ghosh, S.; Dandapat, N.; Mandal, A.K.; Balla, V.K. Comparative Study on Conventional Sintering with Microwave Sintering and Vacuum Sintering of Y2O3-Al2O3-ZrO2 Ceramics. J. Mater. Sci. Chem. Eng. 2016, 4, 71–78. [Google Scholar]
- Vasile, B.S.; Andronescu, E.; Ghitulica, C.; Vasile, O.R.; Curechiu, L.; Scurtu, R.; Vasile, E.; Trusca, R.; Pall, L.; Aldica, V. Microstructure and electrical properties of zirconia and composite nanostructured ceramics sintered by different methods. Ceram. Int. 2013, 39, 2535–2543. [Google Scholar] [CrossRef]
- Oguntuyi, S.D.; Johnson, O.T.; Shongwe, M.B.; Jeje, S.O.; Rominiyi, A.L. The effects of sintering additives on the ceramic matrix composite of ZrO2: Microstructure, densification, and mechanical properties—A review. Adv. Appl. Ceram. 2021, 120, 319–335. [Google Scholar] [CrossRef]
- Anselmi-Tamburini, U.; Garay, J.E.; Munir, Z.A. Fast low-temperature consolidation of bulk nanometric ceramic materials. Scriptamaterialia 2006, 54, 823–828. [Google Scholar] [CrossRef]
- Binner, J.; Vaidhyanathan, B.; Wang, J.; Price, D.; Reading, M. Evidence for non-thermal microwave effects using single and multimode hybrid conventional microwave systems. J. Microw. Power Electromagn. Energy 2007, 42, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.; Najib, W.B.; Chen, W.; Bonanos, N. Electrical Conductivity of 10 mol% Sc2O3–1 mol% M2O3–ZrO2 Ceramics. J. Am. Ceram. Soc. 2012, 95, 1–8. [Google Scholar] [CrossRef]
- Abbas, H.A.; Argirusis, C.; Kilo, M.; Wiemhöfer, H.D.; Hammad, F.F.; Hanaf, Z.M. Preparation and conductivity of ternary scandia-stabilised zirconia. Solid State Ion. 2011, 184, 6–9. [Google Scholar] [CrossRef]
- Galkin, V.S.; Konakov, V.G.; Shorohov, A.V.; Solovieva, E.N. Synthesis of nanopowders in the systems of Ce2O3-ZrO2, Y2O3-ZrO2, and Y2O3-Ce2O3-ZrO2 for fabrication of oxygen sensors. Rev. Adv. Mater. Sci. 2005, 10, 353–356. [Google Scholar]
- Courtin, E.; Boy, P.; Piquero, T.; Vulliet, J.; Poirot, N.; Laberty-Robert, C. A composite sol–gel process to prepare a YSZ electrolyte for Solid Oxide Fuel Cells. J. Power Sources 2012, 206, 77–83. [Google Scholar] [CrossRef]
- Borik, M.A.; Zaharov, D.M.; Kulebyakin, A.V.; Kuritsyna, I.E.; Lomonova, E.E.; Larina, N.A.; Milovich, F.O.; Myzina, V.A.; Ryabochkina, P.A.; Tabachkova, N.Y.; et al. Single crystal solid state electrolytes based on yttria, ytterbia and gadolinia doped zirconia. Mater. Chem. Phys. 2022, 277, 12549. [Google Scholar] [CrossRef]
- Agarkov, D.A.; Borik, M.A.; Volkova, T.V.; Eliseeva, G.A.; Kulebyakin, A.V.; Larina, N.A.; Lomonova, E.E.; Myzina, V.A.; Ryabochkina, P.A.; Tabachkova, N.Y. Phase composition and local structure of scandia and yttria stabilized zirconia solid solution. J. Lumin. 2020, 222, 117170. [Google Scholar] [CrossRef]
- Borik, M.A.; Volkova, T.V.; Lomonova, E.E.; Myzina, V.A.; Ryabochkina, P.A.; Tabachkova, N.Y.; Chabushkin, A.N. Spectroscopy of Optical Centers of Eu3+ Ions in Partially Stabilized and Stabilized Zirconium Crystals. Opt. Spectrosc. 2017, 122, 580–587. [Google Scholar] [CrossRef]
- Borik, M.A.; Volkova, T.V.; Kuritzyna, I.E.; Larina, N.A.; Lomonova, E.E.; Myzina, V.A.; Ryabochkina, P.A.; Tabachkova, N.Y. Specific Features of the Local Structure and Transport Properties of ZrO2–Sc2O3–Y2O3 and ZrO2–Sc2O3–Yb2O3Crystals. Opt. Spectrosc. 2018, 125, 898–902. [Google Scholar] [CrossRef]
- Borik, M.A.; Volkova, T.V.; Lomonova, E.E.; Myzina, V.A.; Ryabochkina, P.A.; Tabachkova, N.Y.; Chabushkin, A.N.; Kyashkin, V.M.; Khrushchalina, S.A. Spectroscopy of optical centers of Eu3+ ions in ZrO2-Gd2O3-Eu2O3 crystals. J. Lumin. 2018, 200, 66–73. [Google Scholar] [CrossRef]
- Dexpert-Ghys, J.; Faucher, M.; Caro, P. Site selective spectroscopy and structural analysis of yttria-doped zirconias. J. Solid State Chem. 1984, 54, 179–192. [Google Scholar] [CrossRef]
- Yugami, H.; Koike, A.; Ishigame, M.; Suemoto, T. Relationship between local structures and ionic conductivity in ZrO2-Y2O3 studied by site-selective spectroscopy. Phys. Rev. B Condens. Matter. 1991, 44, 9214–9222. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrov, V.I.; Osiko, V.V.; Prokhorov, A.M.; Tatarintsev, V.M. Synthesis and crystal growth of refractory materials by RF melting in a cold container. Curr. Top. Mater.Sci. 1978, 1, 421–480. [Google Scholar]
- Osiko, V.V.; Borik, M.A.; Lomonova, E.E. Synthesis of refractory materials by skull melting technique. In Springer Handbook of Crystal Growth; Dhanaraj, G., Byrappa, K., Prasad, V., Dudley, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 433–477. [Google Scholar]
- Hemberger, Y.; Wichtner, N.; Berthold, C.; Nickel, K.G. Quantification of yttria in stabilized zirconia by Raman spectroscopy. Int. J. Appl. Ceram. Technol. 2016, 13, 116–124. [Google Scholar] [CrossRef]
- Yashima, M.; Sasaki, S.; Kakihana, M.; Yamaguchi, Y.; Arashi, H.; Yoshimura, M. Oxygen-induced structural change of the tetragonal phase around the tetragonal-cubic phase boundary in ZrO2–YO1.5 solid solutions. Acta Crystallogr. B Struct.Sci. 1994, B50, 663–672. [Google Scholar] [CrossRef]
- Yashima, M.; Ohtake, K.; Kakihana, M.; Arashi, H.; Yoshimura, M. Determination of tetragonal-cubic phase boundary of Zr1 – xRxO2 – x/2 (R = Nd, Sm, Y, Er and Yb) by Raman scattering. J. Phys. Chem. Solids. 1996, 57, 17–24. [Google Scholar] [CrossRef]
- Guo, X.; Yuan, R. Roles of alumina in zirconia-based solid electrolyte. J. Mater. Sci. 1995, 30, 923–931. [Google Scholar] [CrossRef]
- Navarro, L.M.; Recio, P.; Jurado, J.R.; Duran, P. Preparation and properties evaluation of zirconia-based/Al2O3 composites as electrolytes for solid oxide fuel cell systems. J. Mater. Sci. 1995, 30, 1949–1960. [Google Scholar] [CrossRef]
- Yu, F.; Xiao, J.; Lei, L.; Cai, W.; Zhang, Y.; Liu, J.; Liu, M. Effects of doping alumina on the electrical and sintering performances of yttrium-stabilized-zirconia. Solid State Ion. 2016, 289, 28–34. [Google Scholar] [CrossRef]
- Hassan, A.A.E.; Menzler, N.H.; Blass, G.; Ali, M.E.; Buchkremer, H.P.; Stöver, D. Influence of alumina dopant on the properties of yttria-stabilized zirconia for SOFC applications. J. Mater. Sci. 2002, 37, 3467–3475. [Google Scholar] [CrossRef]
- Kusuma, H.H.; Astuti, B.; Ibrahim, Z. Absorption and emission properties of ruby (Cr:Al2O3) single crystal. IOP Conf. Series J. Phys. Conf. Ser. 2019, 1170, 012054. [Google Scholar] [CrossRef]
- Guguschev, C.; Götze, J.; Göbbels, M. Cathodoluminescence microscopy and spectroscopy of synthetic ruby crystals grown by the optical floating zone technique. Am. Mineral. 2010, 95, 449–455. [Google Scholar] [CrossRef]
- Kostyukov, A.; Baronskiy, M.; Rastorguev, A.; Snytnikov, V.; Snytnikov, V.; Zhuzhgov, A.; Ishchenko, A. Photoluminescence of Cr3+ in nanostructured Al2O3 synthesized by evaporation using a continuous wave CO2 laser. RSC Adv. 2016, 6, 2072–2078. [Google Scholar] [CrossRef]
- Gupta, S.; Mahapatra, M.K.; Singh, P. Lanthanum chromite based perovskites for oxygen transport membrane. Mater. Sci. Eng. R Rep. 2015, 90, 1–36. [Google Scholar] [CrossRef]
- Jayaratna, M.; Yoshimura, M.; Somiya, S. Hot pressing of Y2O3-stabilized ZrO2 with Cr2O3 additions. J. Mater. Sci. 1986, 21, 591–596. [Google Scholar] [CrossRef]
- Ristić, M.; Popović, S.; Musić, S. Structural properties of the system Al2O3-Cr2O3. Mater. Lett. 1993, 16, 309–312. [Google Scholar] [CrossRef]
- Gutzov, S.; Wasgestian, F.; Barthel, T.; Assmus, W. Chromium as a probe in amorphous and single crystal zirconia. Z. Phys. Chem. 1998, 205, 41–55. [Google Scholar] [CrossRef]
- Alonso, P.J.; Alcalá, R.; Casas-Gonzalez, J.; Cases, R.; Orera, V.M. Spectroscopy of chromium (III) in yttrium-stabilized ZrO2. J. Phys. Chem. Solids 1989, 50, 1185–1191. [Google Scholar] [CrossRef]
- Štefanić, G.; Popović, S.; Musić, S. Influence of Cr2O3 on the stability of low temperature t-ZrO2. Mater. Lett. 1998, 36, 240–244. [Google Scholar] [CrossRef]
- Garcia-Hipolito, M.; Martinez, E.; Alvarez-Fregoso, O.; Falcony, C.; Aguilar-Frutis, M.A. Preparation and characterization of Eu doped zirconia luminescent films synthesized by the pyrosol technique. J. Mater. Sci. Lett. 2001, 20, 1799–1801. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).