Characteristics of Radio Frequency Dielectric Barrier Discharge Using Argon Doped with Nitrogen at Atmospheric Pressure
Abstract
1. Introduction
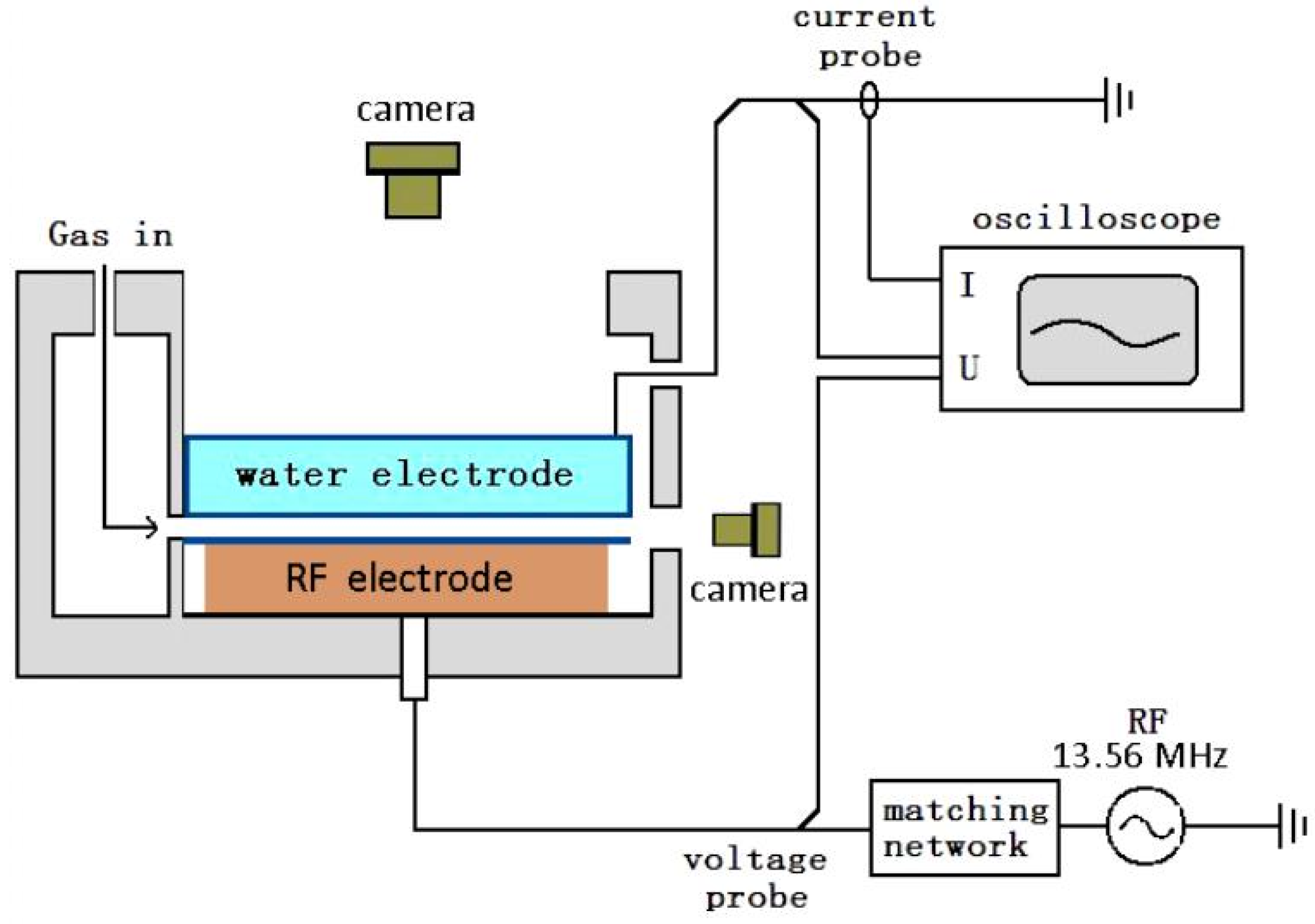
2. Experimental Facility
3. Results and Discussion
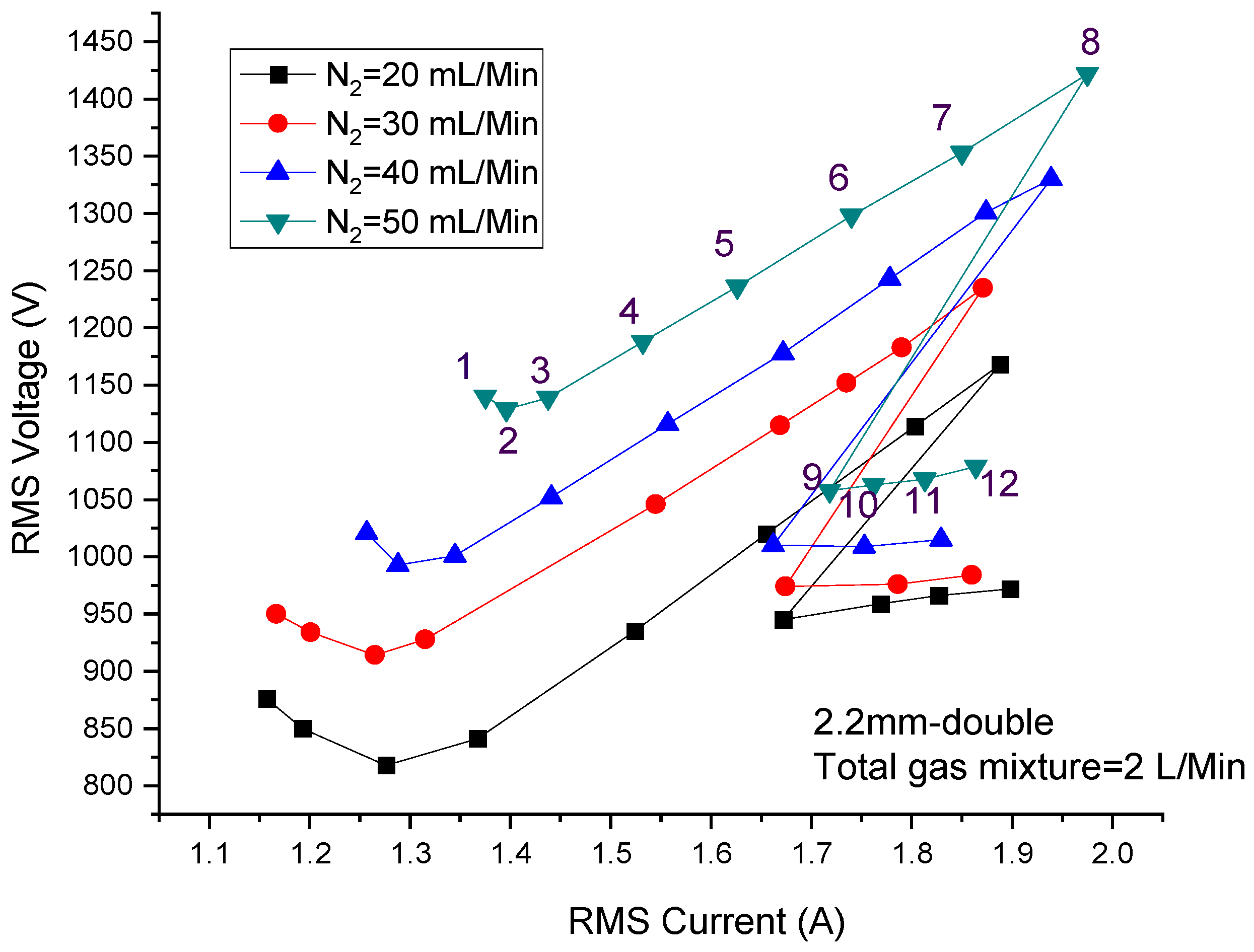
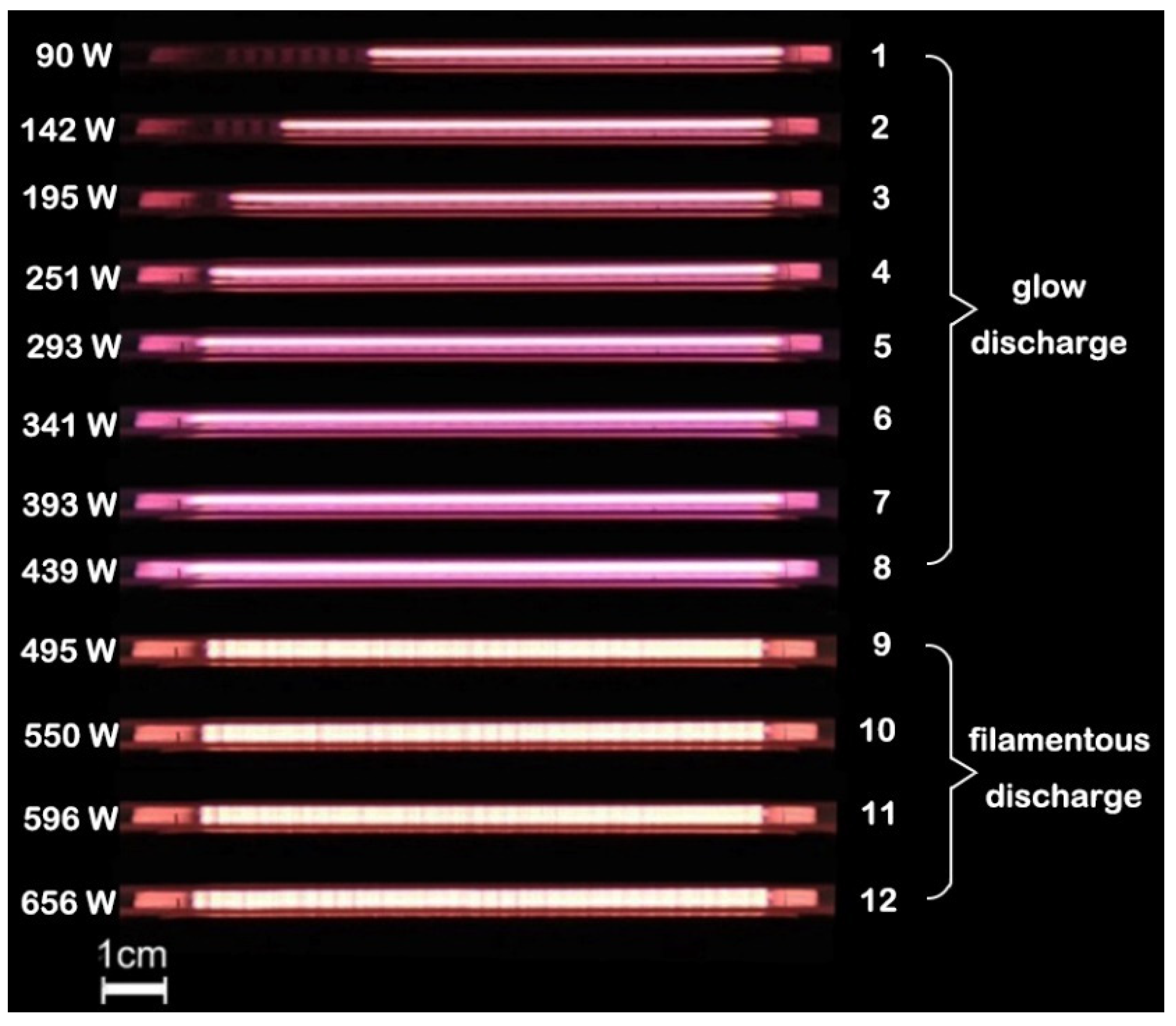
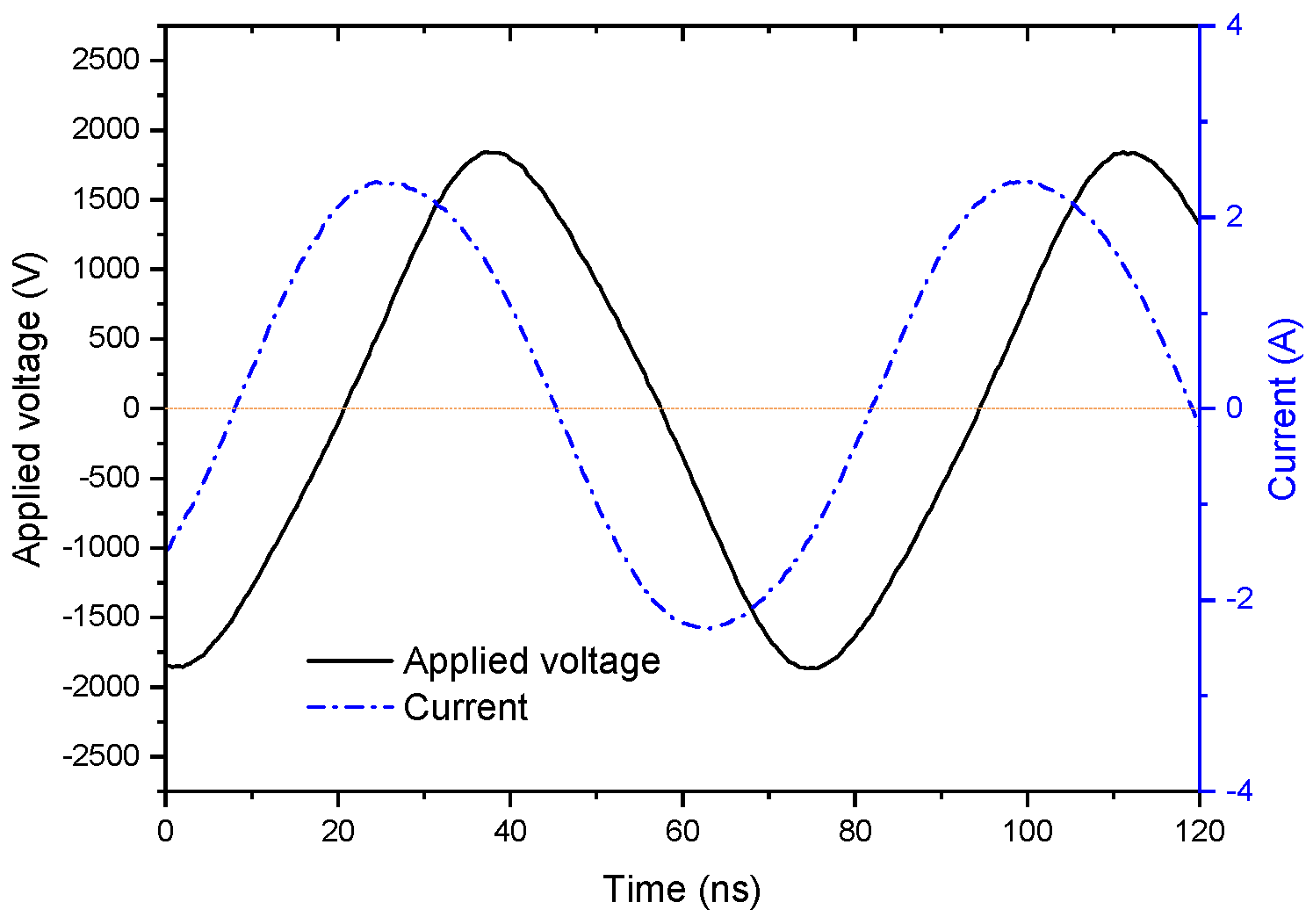
3.1. Effect of Discharge Power on Discharge Characteristics
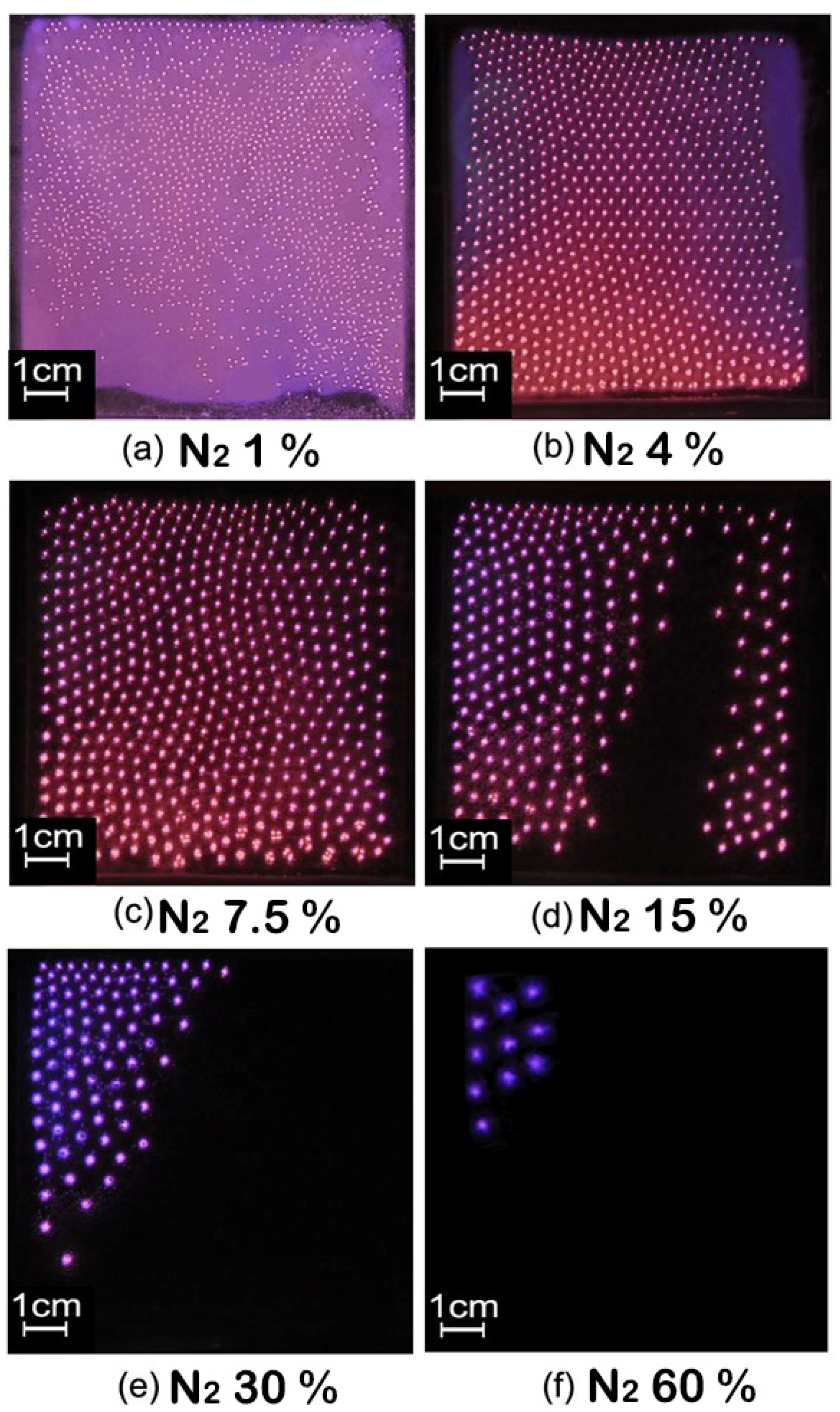
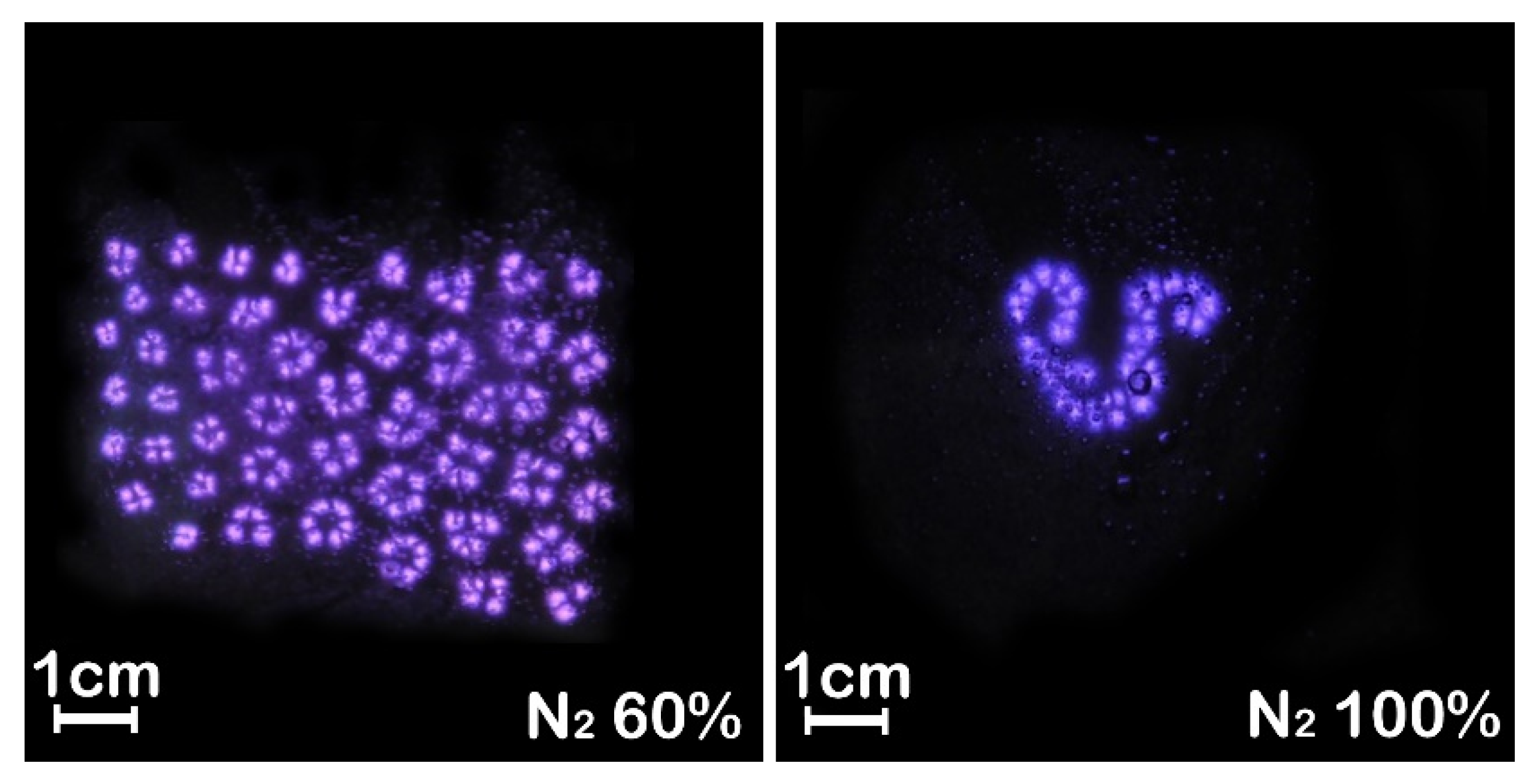
3.2. Effect of Gas Ratio on Self-Organizing Structure of Filamentous Discharge
3.3. Diagnosis of Emission Spectra
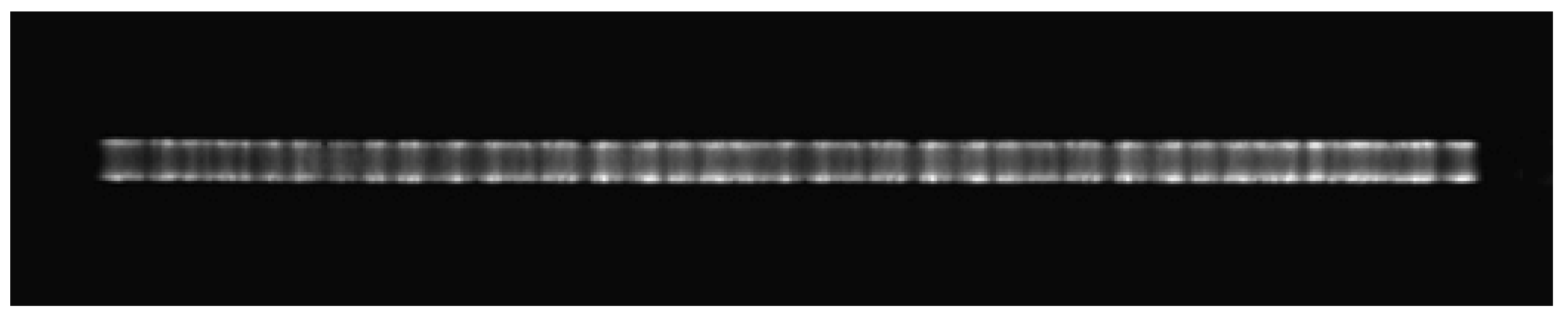
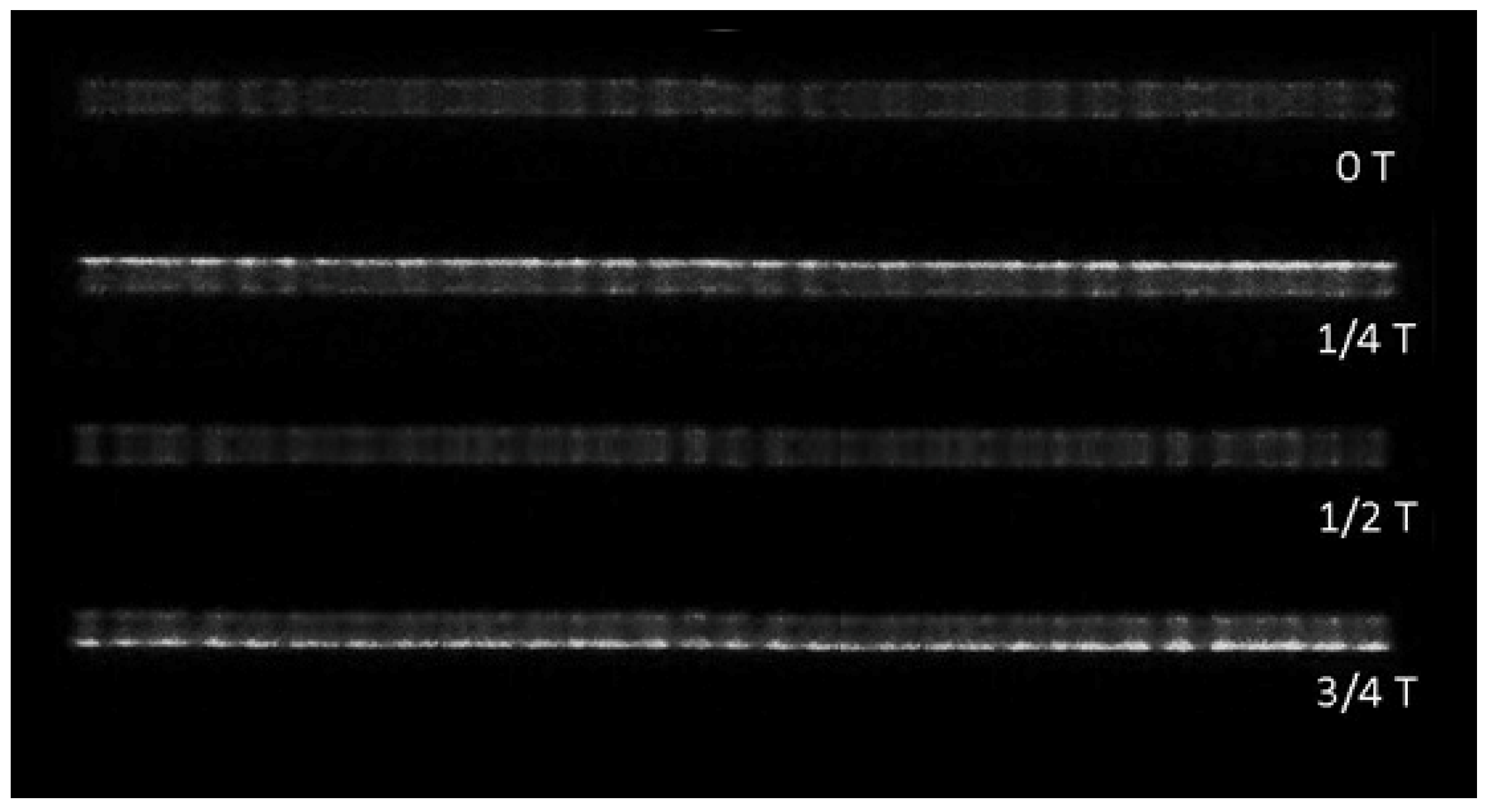
3.4. Diagnosis of Filamentous Discharge by ICCD Camera
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shirai, H.; Kobayashi, T.; Hasegawa, Y. Synthesis of silicon nanocones using RF microplasma at atmospheric pressure. Appl. Phys. Lett. 2005, 87, 143112. [Google Scholar] [CrossRef]
- Tatarova, E.; Bundaleska, N.; Sarrette, J.P.; Ferreira, C.M. Plasmas for environmental issues: From hydrogen production to 2D materials assembly. Plasma Sources Sci. Technol. 2014, 23, 063002. [Google Scholar] [CrossRef]
- Huzum, R.; Nastuta, A.V. Helium Atmospheric Pressure Plasma Jet Source Treatment of White Grapes Juice for Winemaking. Appl. Sci. 2021, 11, 8498. [Google Scholar] [CrossRef]
- Balcon, N.; Benard, N.; Lagmich, Y.; Boeuf, J.-P.; Touchard, G.; Moreau, E. Positive and negative sawtooth signals applied to a DBD plasma actuator–influence on the electric wind. J. Electrost. 2009, 67, 140–145. [Google Scholar] [CrossRef]
- Kunhardt, E.E. Generation of large-volume, atmospheric-pressure, nonequilibrium plasmas. IEEE Trans. Plasma Sci. 2000, 28, 189–200. [Google Scholar] [CrossRef]
- Gandhiraman, R.P.; Singh, E.; Diaz-Cartagena, D.C.; Nordlund, D.; Koehne, J.; Meyyappan, M. Plasma jet printing for flexible substrates. Appl. Phys. Lett. 2016, 108, 123103. [Google Scholar] [CrossRef]
- Li, G.; Li, H.P.; Sun, W.T. Discharge features of radio-frequency, atmospheric-pressure cold plasmas under an intensified local electric field. J. Phys. D Appl. Phys. 2008, 41, 202001. [Google Scholar] [CrossRef]
- Walsh, J.L.; Cao, Z.; Kong, M.G. Atmospheric dielectric-barrier discharges scalable from 1 mm to 1 m. IEEE Trans. Plasma Sci. 2008, 36, 1314. [Google Scholar] [CrossRef]
- Walsh, J.L.; Iza, F.; Kong, M.G. Atmospheric glow discharges from the high-frequency to very high-frequency bands. Appl. Phys. Lett. 2008, 93, 251502. [Google Scholar] [CrossRef]
- Arkhipenko, V.; Callegari, T.; Safronau, Y.; Simonchik, L. Atmospheric-Pressure Air Glow Discharge in a Three-Electrode Configuration. IEEE Trans. Plasma Sci. 2009, 37, 1297–1304. [Google Scholar] [CrossRef]
- Muñoz, J.; Calzada, M.D. Experimental research on surface wave Ar–He discharges at atmospheric pressure. J. Phys. D Appl. Phys. 2008, 41, 135203. [Google Scholar] [CrossRef]
- Shi, J.J.; Kong, M.G. Radio-frequency dielectric-barrier glow discharges in atmospheric argon. Appl. Phys. Lett. 2007, 90, 111502. [Google Scholar] [CrossRef]
- Kanazawa, S.; Kogoma, M.; Moriwaki, T.; Okazaki, S. Stable glow at atmospheric pressure. J. Phys. D Appl. Phys. 1988, 21, 838–840. [Google Scholar] [CrossRef]
- Shi, J.J.; Liu, D.W.; Kong, M.G. Plasma stability control using dielectric barriers in radio-frequency atmospheric pressure glow discharges. Appl. Phys. Lett. 2006, 89, 081502. [Google Scholar] [CrossRef]
- Shi, J.J.; Liu, D.W.; Kong, M.G. Effects of Dielectric Barriers in Radio-Frequency Atmospheric Glow Discharges. IEEE Trans. Plasma Sci. 2007, 35, 137–142. [Google Scholar] [CrossRef]
- Shi, J.J.; Liu, D.W.; Kong, M.G. Mitigating plasma constriction using dielectric barriers in radio-frequency atmospheric pressure glow discharges. Appl. Phys. Lett. 2007, 90, 31505. [Google Scholar] [CrossRef]
- Shi, J.J.; Kong, M.G. Mechanisms of the α and γ modes in radio-frequency atmospheric glow discharges. J. Appl. Phys. 2005, 97, 023306. [Google Scholar] [CrossRef]
- Shi, J.J.; Kong, M.G. Mode transition in radio-frequency atmospheric argon discharges with and without dielectric barriers. Appl. Phys. Lett. 2007, 90, 101502. [Google Scholar]
- Li, B.; Chen, Q.; Liu, Z.W. A large gap of radio frequency dielectric barrier atmospheric pressure glow discharge. Appl. Phys. Lett. 2010, 96, 041502. [Google Scholar] [CrossRef]
- Li, S.; Sun, J.; Sun, R.; Pan, J.; Wang, L.; Chen, C.; Chen, Q.; Liu, Z. Spatiotemporal Characteristics of Radio Frequency Dielectric Barrier Glow Discharge at Atmospheric Pressure. Appl. Sci. 2021, 11, 8430. [Google Scholar] [CrossRef]
- Li, Z.K.; Chen, L.; Wang, M.Q.; Song, P.; Yang, K.; Zeng, W. Diagnosis of Atmospheric Pressure Argon/Air Needle-Ring Dielectric Barrier Discharge Emission Spectrum. Spectrosc. Spectr. Anal. 2017, 41, 3307–3310. [Google Scholar]
- Li, X.; LIU, R.; Gong, D.; Li, X.; Ren, C.; Jia, P. Influence of external parameters on nonlinear behaviors in a helium dielectric-barrier discharge excited by a modulated voltage. Phys. Plasmas 2019, 26, 23514. [Google Scholar] [CrossRef]
- Luo, L.; Wang, Q.; Dai, D.; Zhang, Y.; Li, L. A practical method for controlling the asymmetric mode of atmospheric dielectric barrier discharges. Appl. Sci. 2020, 10, 1341. [Google Scholar] [CrossRef]
- Rueda, V.; Diez, R.; Bente, N.; Piquet, H. Combined Image Processing and Equivalent Circuit Approach for the Diagnostic of Atmospheric Pressure DBD. Appl. Sci. 2022, 12, 8009. [Google Scholar] [CrossRef]
- Massines, F.; Rabehi, A.; Decomps, P. Experimental and theoretical study of a glow discharge at atmospheric pressure controlled by dielectric barrier. J. Appl. Phys. 1998, 83, 2950–2957. [Google Scholar] [CrossRef]
- Shi, J.J.; Kong, M.G. Expansion of the plasma stability range in radio-frequency atmospheric-pressure glow discharges. Appl. Phys. Lett. 2005, 87, 201501. [Google Scholar] [CrossRef]
- Muller, L.; Punset, C.; Ammelt, E.; Purwins, H.-G.; Boeuf, J.P. Self-organized filaments in dielectric barrier glow discharges. IEEE Trans. Plasma Sci. 1999, 27, 20–21. [Google Scholar] [CrossRef]
- Zanin, A.L.; Gurevich, E.L.; Moskalenko, A.S.; Bödeker, H.U.; Purwins, H.-G. Rotating hexagonal pattern in a dielectric barrier discharge system. Phys. Rev. E 2004, 70, 036202. [Google Scholar] [CrossRef]
- Shirafuji, T.; Kitagawa, T.; Wakai, T.; Tachibana, K. Observation of self-organized filaments in a dielectric barrier discharge of Ar gas. Appl. Phys. Lett. 2003, 83, 2309–2311. [Google Scholar] [CrossRef]
- Breazeal, W.; Flynn, K.M.; Gwinn, E.G. Static and dynamic two-dimensional patterns in self-extinguishing discharge avalanches. Phys. Rev. E 1995, 52, 1504–1515. [Google Scholar] [CrossRef]
- Dong, L.F.; Li, B.; Shen, Z.K.; Liu, L. Formation of spiral patterns in dielectric-barrier discharge investigated by an intensified-charge coupled device. Phys. Rev. E 2012, 86, 056217. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.F.; Liu, W.L.; Wang, H.F.; He, Y.F.; Fan, W.L. Honeycomb hexagon pattern in dielectric barrier discharge. Phys. Rev. E 2007, 76, 046210. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Yin, Z.; Wang, L.; Fu, G.; He, Y.; Chai, Z.; Li, X. Square pattern formation in a gas discharge system. Thin Solid Films 2003, 435, 120–123. [Google Scholar] [CrossRef]
- Hussain, S.; Qazi, H.I.A.; Malik, A.A.; Badar, M.A. Glow modes in radio frequency atmospheric discharge operating with and without anodized electrodes. IEEE Trans. Plasma Sci. 2014, 42, 2410. [Google Scholar] [CrossRef]
- Nishime, T.M.C.; Wannicke, N.; Horn, S.; Weltmann, K.-D.; Brust, H. A Coaxial dielectric barrier discharge reactor for treatment of sinter sheat seeds. Appl. Sci. 2020, 10, 7133. [Google Scholar] [CrossRef]

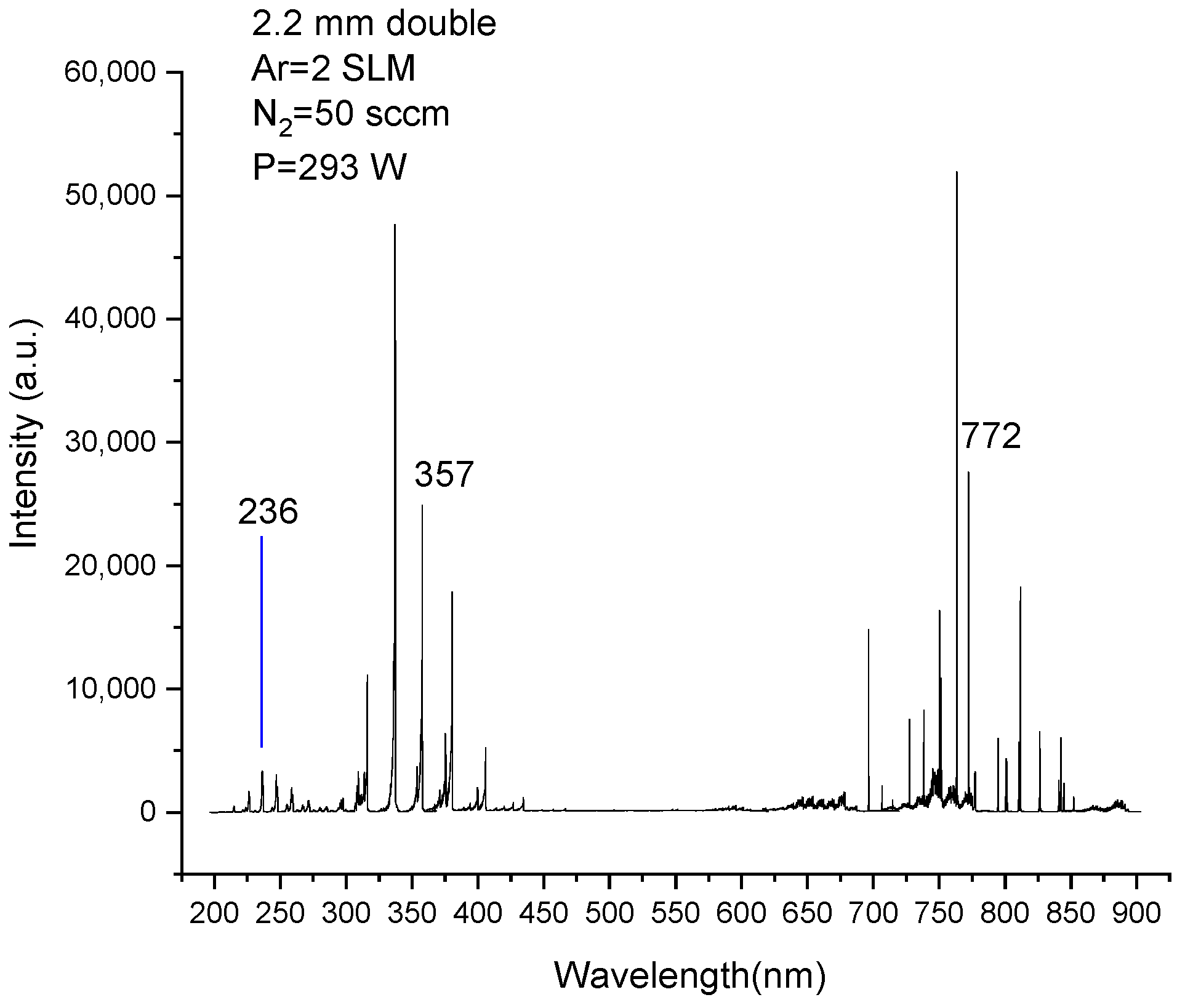

| Transition | λ/nm | Configurations | Aki/107s−1 | Ei–Ek (cm−1) |
|---|---|---|---|---|
| N2(A3∑u+, 0–X1∑g+, 3) | 236 | 2s2p(3P°)3d-2s2p(3P°)4p | 9.12 | 336,206.9–378,432.8 |
| N2 (C3Πu, 0–B3Πg, 1) | 357 | 2s22p(2P°)3p-2s22p(2P°)4s | 1.21 | 168,892.21–196,711.54 |
| Ar (2p7–1s5) | 772 | 3s23p5(2P°3/2)4s-3s23p5(2P°3/2)4p | 0.518 | 93,143.7600–106,087.2598 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Sun, J.; Sun, R.; Pan, J.; Wang, L.; Chen, C.; Chen, Q.; Liu, Z. Characteristics of Radio Frequency Dielectric Barrier Discharge Using Argon Doped with Nitrogen at Atmospheric Pressure. Materials 2022, 15, 7647. https://doi.org/10.3390/ma15217647
Li S, Sun J, Sun R, Pan J, Wang L, Chen C, Chen Q, Liu Z. Characteristics of Radio Frequency Dielectric Barrier Discharge Using Argon Doped with Nitrogen at Atmospheric Pressure. Materials. 2022; 15(21):7647. https://doi.org/10.3390/ma15217647
Chicago/Turabian StyleLi, Sen, Jiazhen Sun, Rui Sun, Jie Pan, Lin Wang, Chen Chen, Qiang Chen, and Zhongwei Liu. 2022. "Characteristics of Radio Frequency Dielectric Barrier Discharge Using Argon Doped with Nitrogen at Atmospheric Pressure" Materials 15, no. 21: 7647. https://doi.org/10.3390/ma15217647
APA StyleLi, S., Sun, J., Sun, R., Pan, J., Wang, L., Chen, C., Chen, Q., & Liu, Z. (2022). Characteristics of Radio Frequency Dielectric Barrier Discharge Using Argon Doped with Nitrogen at Atmospheric Pressure. Materials, 15(21), 7647. https://doi.org/10.3390/ma15217647







