Salicylic Acid Co-Precipitation with Alginate via Supercritical Atomization for Cosmetic Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sodium Alginate-Salicylic Acid Solutions Preparation and SAA Apparatus Description
2.3. Characterization Techniques of Sodium Alginate-Salicylic Acid Microparticles
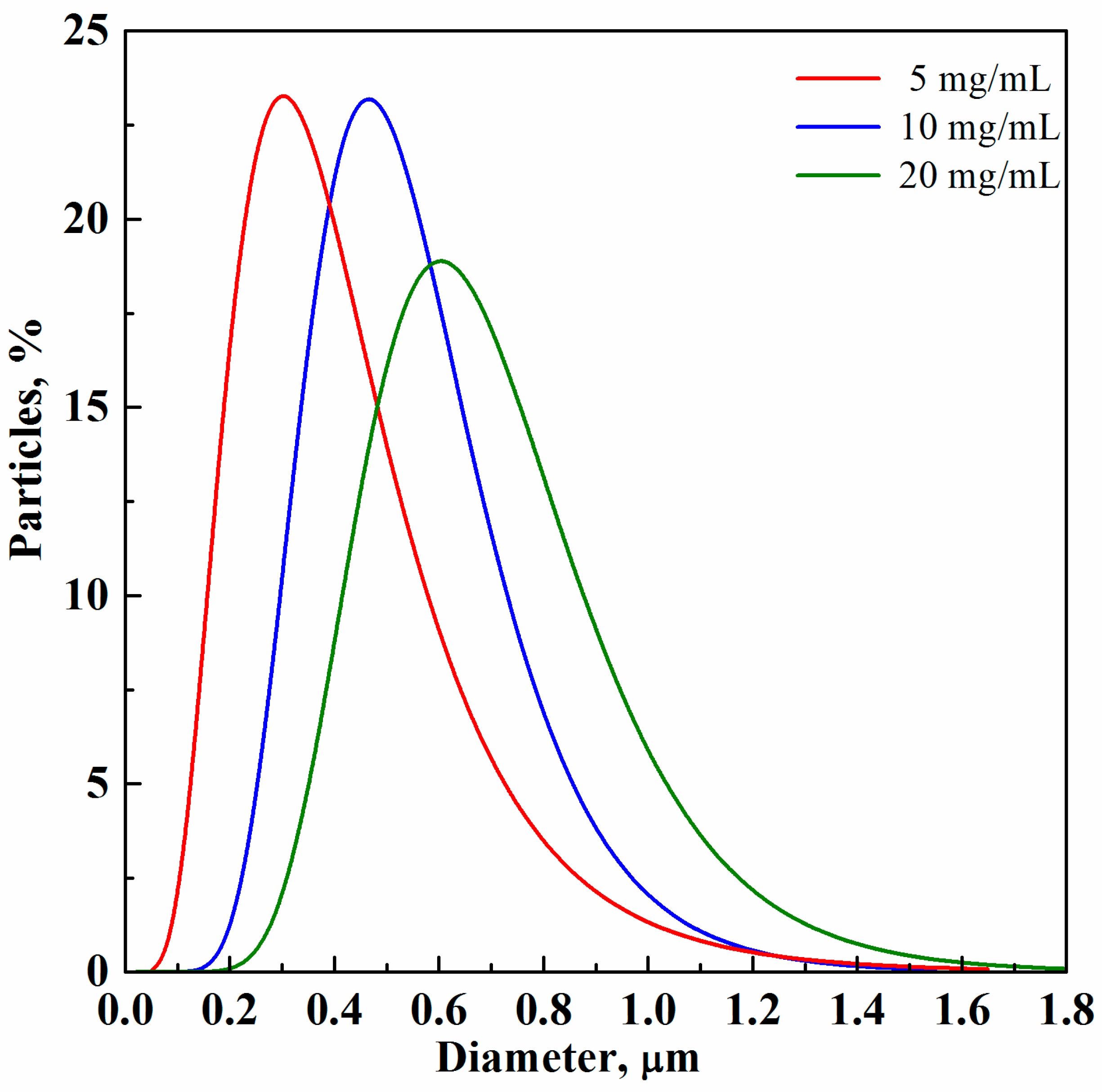
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kouassi, M.-C.; Grisel, M.; Gore, E. Multifunctional active ingredient-based delivery systems for skincare formulations: A review. Colloids Surf. B 2022, 217, 112676. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.K.; Fok, L. Evidence of microbeads from personal care product contaminating the sea. Mar. Pollut. Bull. 2016, 109, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Bom, S.; Jorge, J.; Ribeiro, H.M.; Marto, J. A step forward on sustainability in the cosmetics industry: A review. J. Clean. Prod. 2019, 225, 270–290. [Google Scholar] [CrossRef]
- Amberg, N.; Fogarassy, C. Green consumer behavior in the cosmetics market. Resources 2019, 8, 137. [Google Scholar] [CrossRef]
- Ghazali, E.; Soon, P.C.; Mutum, D.S.; Nguyen, B. Health and cosmetics: Investigating consumers’ values for buying organic personal care products. J. Retail. Consum. Serv. 2017, 39, 154–163. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Kozlowska, J.; Prus, W.; Stachowiak, N. Microparticles based on natural and synthetic polymers for cosmetic applications. Int. J. Biol. Macromol. 2019, 129, 952–956. [Google Scholar] [CrossRef]
- Bialik-Wąs, K.; Miastkowska, M.; Sapuła, P.; Pluta, K.; Malina, D.; Chwastowski, J.; Barczewski, M. Bio-hybrid hydrogels incorporated into a system of salicylic acid-pH/thermosensitive nanocarriers intended for cutaneous wound-healing processes. Pharmaceutics 2022, 14, 773. [Google Scholar] [CrossRef]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Bae, S.B.; Nam, H.C.; Park, W.H. Electrospraying of environmentally sustainable alginate microbeads for cosmetic additives. Int. J. Biol. Macromol. 2019, 133, 278–283. [Google Scholar] [CrossRef]
- Oh, G.-W.; Kim, S.C.; Kim, T.-H.; Jung, W.Q. Characterization of an oxidized alginate-gelatin hydrogel incorporating a COS-salicylic acid conjugate for wound healing. Carbohydr. Polym. 2021, 252, 117145. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Yadav, K.; Singh, D.; Singh, M.R. Topical delivery of fluocinolone acetonide integrated NLCs and salicylic acid enriched gel: A potential and synergistic approach in the management of psoriasis. J. Drug Deliv. Sci. Technol. 2021, 61, 102282. [Google Scholar] [CrossRef]
- Saoji, V.; Madke, B. Efficacy of salicylic acid peel in dermatophytosis. Indian J. Dermatol. Venereol. Leprol. 2022, 87, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Santos, L. Delivery systems for cosmetics—from manufacturing to the skin of natural antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Gama, M.; Peixoto, D.; Sousa-Oliveira, I.; Ferreira-Faria, I.; Zeinali, M.; Abbaspour-Ravasjani, S.; Mascarenhas-Melo, F.; Hamishehkar, H.; Veiga, F. Nanocarrier-based dermopharmaceutical formulations for the topical management of atopic dermatitis. Int. J. Pharm. 2022, 618, 121656. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Lee, S.H.; Chow, P.S.; Macbeath, C. Microemulsion composed of combination of skin beneficial oils as vehicle: Development of resveratrol-loaded microemulsion based formulations for skin care applications. Colloids Surf. B Biointerfaces 2020, 194, 111161. [Google Scholar] [CrossRef] [PubMed]
- Adami, R.; Liparoti, S.; Reverchon, E. A new supercritical assisted atomization configuration, for the micronization of thermolabile compounds. Chem. Eng. J. 2011, 173, 55–61. [Google Scholar] [CrossRef]
- Reverchon, E.; Scognamiglio, M.; Baldino, L. The Nanostructure of polymer-active principle microparticles produced by supercritical CO2 assisted processing. Nanomaterials 2022, 12, 1401. [Google Scholar] [CrossRef]
- Reverchon, E.; Scognamiglio, M.; Baldino, L. Lycopene extract from tomato concentrate and its co-precipitation with PVP using hybrid supercritical processes. J. CO2 Util. 2022, 64, 102157. [Google Scholar] [CrossRef]
- Akien, G.R.; Poliakoff, M. A critical look at reactions in class I and II gas-expanded liquids using CO2 and other gases. Green Chem. 2009, 11, 1083–1100. [Google Scholar] [CrossRef]
- Guastaferro, M.; Baldino, L.; Cardea, S.; Reverchon, E. Supercritical assisted electrospray/spinning to produce PVP+quercetin microparticles and microfibers. J. Taiwan Inst. Chem. Eng. 2020, 117, 278–286. [Google Scholar] [CrossRef]
- De Marco, I. Production of carrier/antioxidant particles by Supercritical Assisted Atomization as an adjuvant treatment of the COVID-19 pathology. J. Supercrit. Fluids 2022, 186, 105604. [Google Scholar] [CrossRef] [PubMed]
- De Marco, I.; Franco, P. Effect of the carrier on the coprecipitation of curcumin through supercritical-assisted atomization. ChemEngineering 2021, 5, 59. [Google Scholar] [CrossRef]
- Adami, R.; Russo, P.; Amante, C.; De Soricellis, C.; Della Porta, G.; Reverchon, E.; Del Gaudio, P. Supercritical antisolvent technique for the production of breathable naringin powder. Pharmaceutics 2022, 14, 1623. [Google Scholar] [CrossRef] [PubMed]
- Di Capua, A.; Adami, R.; Cosenza, E.; Jalaber, V.; Crampon, C.; Badens, E.; Reverchon, E. β-Carotene/PVP microspheres produced by Supercritical Assisted Atomization. Powder Technol. 2019, 346, 228–236. [Google Scholar] [CrossRef]
- OșSullivan, A.; Ryan, K.M.; Padrela, L. Production of biopharmaceutical dried-powders using supercritical CO2 technology. J. Supercrit. Fluids 2022, 187, 105645. [Google Scholar] [CrossRef]
- Wu, H.-T.; Chuang, Y.-H.; Lin, H.-C.; Hu, T.-C.; Tu, Y.-J.; Chien, L.-J. Immediate release formulation of inhaled beclomethasone dipropionate-hydroxypropyl-beta-cyclodextrin composite particles produced using supercritical assisted atomization. Polymers 2022, 14, 2114. [Google Scholar] [CrossRef]
- Gangapurwala, G.; Vollrath, A.; De San Luis, A.; Schubert, U.S. PLA/PLGA-based drug delivery systems produced with supercritical CO2—A green future for particle formulation? Pharmaceutics 2020, 12, 1118. [Google Scholar] [CrossRef]
- Svärd, M.; Ahuja, D.; Rasmuson, Å.C. Calorimetric determination of cocrystal thermodynamic stability: Sulfamethazine–salicylic acid case study. Cryst. Growth Des. 2020, 20, 4243–4251. [Google Scholar] [CrossRef]
- Di Donato, P.; Taurisano, V.; Poli, A.; Gomez d’Ayala, G.; Nicolaus, B.; Malinconinco, M.; Santagata, G. Vegetable wastes derived polysaccharides as natural eco-friendly plasticizers of sodium alginate. Carbohydr. Polym. 2020, 229, 115427. [Google Scholar] [CrossRef]
- Ferrándiz, M.; Capablanca, L.; Franco, E.; Mira, E. Microencapsulation of L-ascorbic acid by spray drying using sodium alginate as wall material. J. Encapsulation Adsorpt. Sci. 2016, 6, 64133. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldino, L.; Reverchon, E. Salicylic Acid Co-Precipitation with Alginate via Supercritical Atomization for Cosmetic Applications. Materials 2022, 15, 7634. https://doi.org/10.3390/ma15217634
Baldino L, Reverchon E. Salicylic Acid Co-Precipitation with Alginate via Supercritical Atomization for Cosmetic Applications. Materials. 2022; 15(21):7634. https://doi.org/10.3390/ma15217634
Chicago/Turabian StyleBaldino, Lucia, and Ernesto Reverchon. 2022. "Salicylic Acid Co-Precipitation with Alginate via Supercritical Atomization for Cosmetic Applications" Materials 15, no. 21: 7634. https://doi.org/10.3390/ma15217634
APA StyleBaldino, L., & Reverchon, E. (2022). Salicylic Acid Co-Precipitation with Alginate via Supercritical Atomization for Cosmetic Applications. Materials, 15(21), 7634. https://doi.org/10.3390/ma15217634






