Effect of Reducing Agent on Characteristics and Antibacterial Activity of Copper-Containing Particles in Textile Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Treatment Methods
2.3. Investigative Methods
3. Results and Discussion
3.1. Selection of Solutions for Saturation of Textile Materials with Copper-Containing Particles
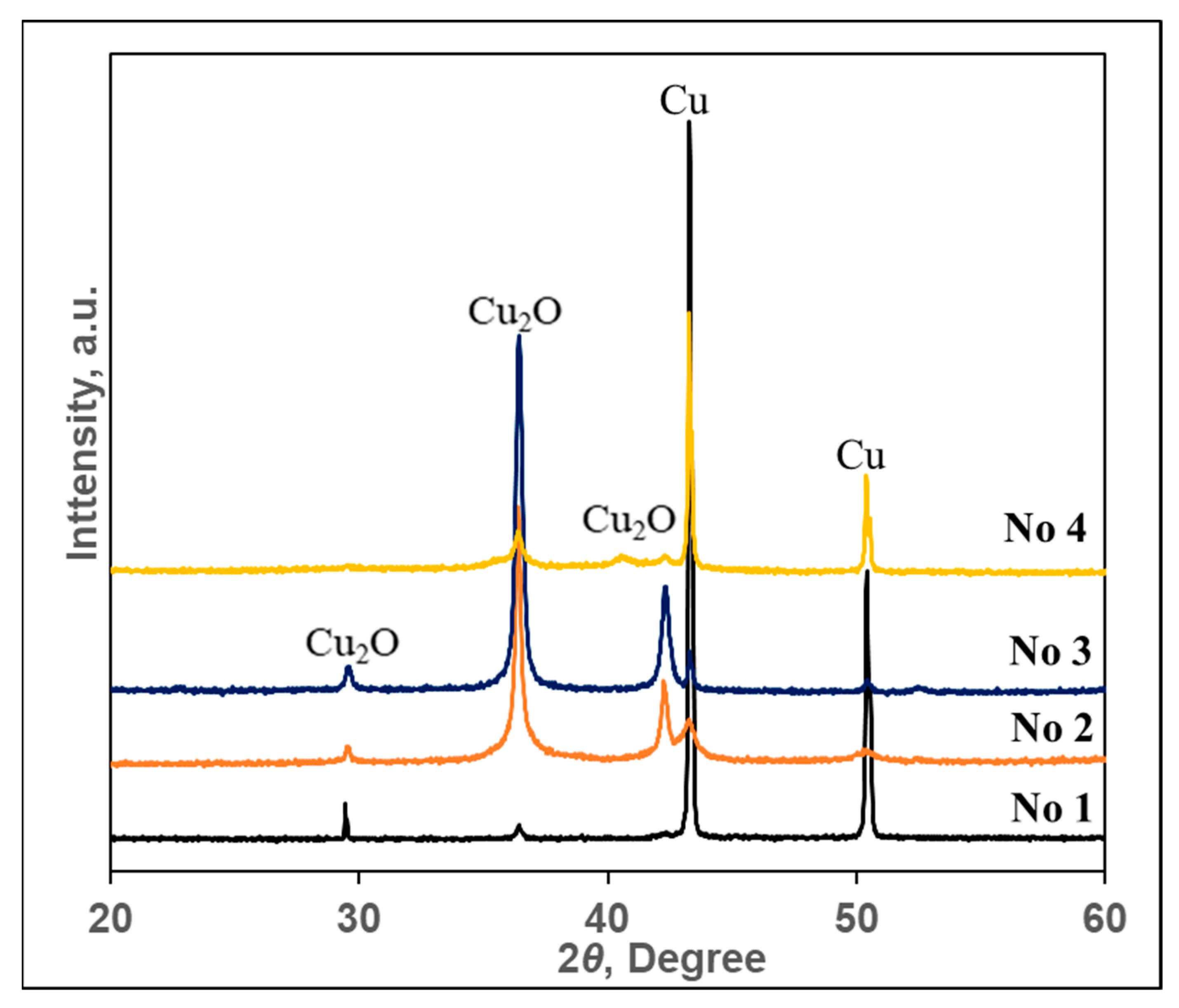
3.2. XRD Analysis of Copper-Containing Particles
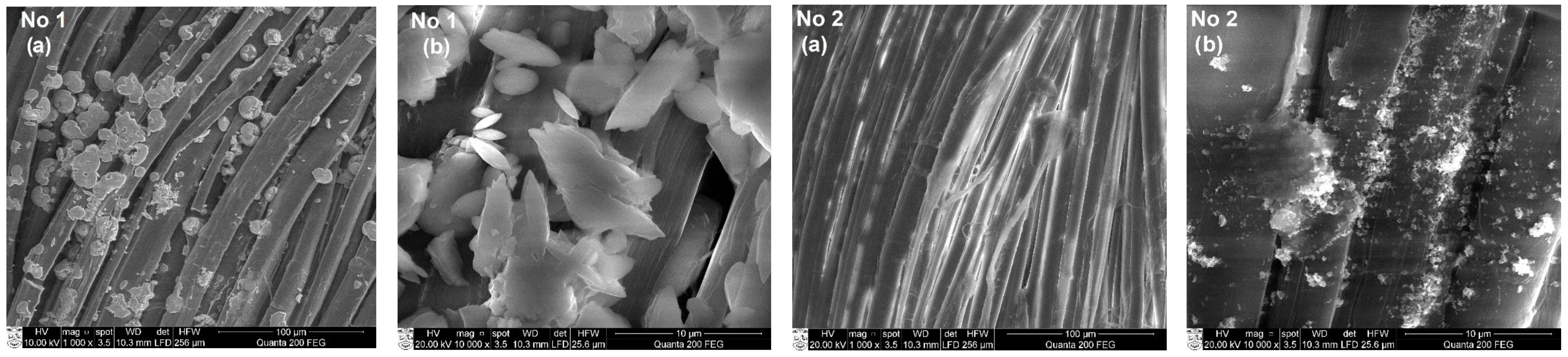
3.3. SEM and EDS Analysis of Textile Samples with Copper-Containing Particles
3.4. Antibacterial Activity of Textil Materials with Copper-Containing Particles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbasov, H. The Effective Thermal Conductivity of Polymer Composites Filled with High Conductive Particles and the Shell Structure. Polym. Compos. 2022, 43, 2593–2601. [Google Scholar] [CrossRef]
- Mehvari, S.; Sanchez-Vicente, Y.; González, S.; Lafdi, K. Conductivity Behaviour under Pressure of Copper Micro-Additive/Polyurethane Composites (Experiment and Modelling). Polymers 2022, 14, 1287. [Google Scholar] [CrossRef] [PubMed]
- Sang, J.; Yuan, Y.; Yang, W.; Zhu, J.; Fu, L.; Li, D.; Zhou, L. Exploring the Underlying Causes of Optimizing Thermal Conductivity of Copper/Diamond Composites by Interface Thickness. J. Alloys Compd. 2022, 891, 161777. [Google Scholar] [CrossRef]
- Ye, R.P.; Lin, L.; Li, Q.; Zhou, Z.; Wang, T.; Russell, C.K.; Adidharma, H.; Xu, Z.; Yao, Y.G.; Fan, M. Recent Progress in Improving the Stability of Copper-Based Catalysts for Hydrogenation of Carbon-Oxygen Bonds. Catal. Sci. Technol. 2018, 8, 3428–3449. [Google Scholar] [CrossRef]
- Atiqah, A.; Jalar, A.; Bakar, M.A.; Ismail, N. Advancement of Printed Circuit Board (PCB) Surface Finishes in Controlling the Intermetallic Compound (IMC) Growth in Solder Joints. In Topics in Mining, Metallurgy and Materials Engineering; Springer: Cham, Switzerland, 2022; pp. 217–238. [Google Scholar]
- O’Hern, C.I.Z.; Djoko, K.Y. Copper Cytotoxicity: Cellular Casualties of Noncognate Coordination Chemistry. MBio 2022, 13, 1–4. [Google Scholar] [CrossRef]
- Chakraborty, N.; Banerjee, J.; Chakraborty, P.; Banerjee, A.; Chanda, S.; Ray, K.; Acharya, K.; Sarkar, J. Green Synthesis of Copper/Copper Oxide Nanoparticles and Their Applications: A Review. Green Chem. Lett. Rev. 2022, 15, 185–213. [Google Scholar] [CrossRef]
- Kim, K.; Huh, J.Y.; Hong, Y.C. Direct Coating of Copper Nanoparticles on Flexible Substrates from Copper Precursors Using Underwater Plasma and Their EMI Performance. Mater. Sci. Eng. B 2021, 265, 114995. [Google Scholar] [CrossRef]
- Taghavi Pourian Azar, G.; Fox, D.; Fedutik, Y.; Krishnan, L.; Cobley, A.J. Functionalised Copper Nanoparticle Catalysts for Electroless Copper Plating on Textiles. Surf. Coatings Technol. 2020, 396, 125971. [Google Scholar] [CrossRef]
- Noman, M.; Shahid, M.; Ahmed, T.; Niazi, M.B.K.; Hussain, S.; Song, F.; Manzoor, I. Use of Biogenic Copper Nanoparticles Synthesized from a Native Escherichia Sp. as Photocatalysts for Azo Dye Degradation and Treatment of Textile Effluents. Environ. Pollut. 2020, 257, 113514. [Google Scholar] [CrossRef]
- Hoon Han, C.; Gil Min, B. Superhydrophobic and Antibacterial Properties of Cotton Fabrics Coated with Copper Nanoparticles through Sonochemical Process. Fibers Polym. 2020, 21, 785–791. [Google Scholar] [CrossRef]
- Gulati, R.; Sharma, S.; Sharma, R.K. Antimicrobial Textile: Recent Developments and Functional Perspective. Polym. Bull. 2022, 79, 5747–5771. [Google Scholar] [CrossRef] [PubMed]
- Moozarm Nia, P.; Pei Meng, W.; Alias, Y. Polyphenol Stabilized Copper Nanoparticle Formulations for Rapid Disinfection of Bacteria and Virus on Diverse Surfaces You May Also like One-Step Electrodeposition of Polypyrrole-Copper Nano Particles for H2O2 Detection. Nanotechnology 2021, 33, 1–9. [Google Scholar] [CrossRef]
- Bisht, N.; Dwivedi, N.; Kumar, P.; Venkatesh, M.; Yadav, A.K.; Mishra, D.; Solanki, P.; Verma, N.K.; Lakshminarayanan, R.; Ramakrishna, S.; et al. Recent Advances in Copper and Copper-Derived Materials for Antimicrobial Resistance and Infection Control. Curr. Opin. Biomed. Eng. 2022, 24, 100408. [Google Scholar] [CrossRef] [PubMed]
- Scully, J.R. The COVID-19 Pandemic, Part 1: Can Antimicrobial Copper-Based Alloys Help Suppress Infectious Transmission of Viruses Originating from Human Contact with High-Touch Surfaces? Corrosion 2020, 76, 523–527. [Google Scholar] [CrossRef]
- Meister, T.L.; Fortmann, J.; Breisch, M.; Sengstock, C.; Steinmann, E.; Köller, M.; Pfaender, S.; Ludwig, A. Nanoscale Copper and Silver Thin Film Systems Display Differences in Antiviral and Antibacterial Properties. Sci. Reports 2022, 12, 7193. [Google Scholar] [CrossRef] [PubMed]
- Abate, C.; Carnamucio, F.; Giuffrè, O.; Foti, C. Metal-Based Compounds in Antiviral Therapy. Biomolecules 2022, 12, 933. [Google Scholar] [CrossRef]
- Govind, V.; Bharadwaj, S.; Sai Ganesh, M.R.; Vishnu, J.; Shankar, K.V.; Shankar, B.; Rajesh, R. Antiviral Properties of Copper and Its Alloys to Inactivate COVID-19 Virus: A Review. BioMetals 2021, 34, 1217–1235. [Google Scholar] [CrossRef]
- Rabiee, N.; Ahmadi, S.; Akhavan, O.; Luque, R. Silver and Gold Nanoparticles for Antimicrobial Purposes against Multi-Drug Resistance Bacteria. Materials 2022, 15, 1–26. [Google Scholar] [CrossRef]
- Mathews, S.; Hans, M.; Mücklich, F.; Solioz, M. Contact Killing of Bacteria on Copper Is Suppressed If Bacterial-Metal Contact Is Prevented and Is Induced on Iron by Copper Ions. Appl. Environ. Microbiol. 2013, 79, 2605–2611. [Google Scholar] [CrossRef]
- Elguindi, J.; Wagner, J.; Rensing, C. Genes Involved in Copper Resistance Influence Survival of Pseudomonas Aeruginosa on Copper Surfaces. J. Appl. Microbiol. 2009, 106, 1448–1455. [Google Scholar] [CrossRef]
- Michels, H.T.; Keevil, C.W.; Salgado, C.D.; Schmidt, M.G. From Laboratory Research to a Clinical Trial: Copper Alloy Surfaces Kill Bacteria and Reduce Hospital-Acquired Infections. Health Environ. Res. Des. J. 2015, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Manzanares-Meza, L.D.; Medina-Contreras, O. SARS-CoV-2 and Influenza: A Comparative Overview and Treatment Implications. Bol. Med. Hosp. Infant. Mex. 2020, 77, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, B.; Prateek; Ranjan, S.; Saraf, M.; Kar, P.; Singh, S.P.; Thakur, V.K.; Singh, A.; Gupta, R.K. Antibacterial and Antiviral Functional Materials: Chemistry and Biological Activity toward Tackling COVID-19-like Pandemics. ACS Pharmacol. Transl. Sci. 2020, 4, 8–54. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.T.; Singh, V.; Vu Ngoc, S.M.; Nguyen, T.L.; Barceló, D. Transmission of SARS-CoV-2 Infections and Exposure in Surfaces, Points and Wastewaters: A Global One Health Perspective. Case Stud. Chem. Environ. Eng. 2022, 5, 100184. [Google Scholar] [CrossRef]
- Coronavirus Disease (COVID-19): Weekly Epidemiological Update (6 July 2022)—World|ReliefWeb. Available online: https://reliefweb.int/report/world/coronavirus-disease-covid-19-weekly-epidemiological-update-6-july-2022 (accessed on 27 July 2022).
- Fernández-Arias, M.; Boutinguiza, M.; Del Val, J.; Covarrubias, C.; Bastias, F.; Gómez, L.; Maureira, M.; Arias-González, F.; Riveiro, A.; Pou, J. Copper Nanoparticles Obtained by Laser Ablation in Liquids as Bactericidal Agent for Dental Applications. Appl. Surf. Sci. 2020, 507, 145032. [Google Scholar] [CrossRef]
- Soganci, T.; Ayranci, R.; Unlu, G.; Acet, M.; Ak, M. Designing Sandwich-Type Single-Layer Graphene Decorated by Copper Nanoparticles for Enhanced Sensing Properties. J. Phys. D Appl. Phys. 2020, 53, 255105. [Google Scholar] [CrossRef]
- Fairushin, I.I.; Saifutdinov, A.I.; Sofronitskiy, A.O. Numerical and Experimental Studies of the Synthesis of Copper Nanoparticles in a High-Pressure Discharge. Short Commun. Plasma Chem. 2020, 54, 164–168. [Google Scholar] [CrossRef]
- Glad, X.; Profili, J.; Cha, M.S.; Hamdan, A. Synthesis of Copper and Copper Oxide Nanomaterials by Electrical Discharges in Water with Various Electrical Conductivities. J. Appl. Phys. 2020, 127, 023302. [Google Scholar] [CrossRef]
- Jahan, I.; Erci, F.; Isildak, I. Facile Microwave-Mediated Green Synthesis of Non-Toxic Copper Nanoparticles Using Citrus Sinensis Aqueous Fruit Extract and Their Antibacterial Potentials. J. Drug Deliv. Sci. Technol. 2021, 61, 102172. [Google Scholar] [CrossRef]
- Netskina, O.V.; Mukha, S.A.; Dmitruk, K.A.; Ishchenko, A.V.; Bulavchenko, O.A.; Pochtar, A.A.; Suknev, A.P.; Komova, O.V. Solvent-Free Method for Nanoparticles Synthesis by Solid-State Combustion Using Tetra(Imidazole)Copper(II) Nitrate. Inorganics 2022, 10, 15. [Google Scholar] [CrossRef]
- Kang, J.; Gao, P.; Zhang, G.; Shi, L.; Zhou, Y.; Wu, J.; Shuang, S.; Zhang, Y. Rapid Sonochemical Synthesis of Copper Nanoclusters with Red Fluorescence for Highly Sensitive Detection of Silver Ions. Microchem. J. 2022, 178, 107370. [Google Scholar] [CrossRef]
- Ren, S.Y.; Wang, W.B.; Hao, Y.G.; Zhang, H.R.; Wang, Z.C.; Chen, Y.L.; Gao, R.D. Stability and Infectivity of Coronaviruses in Inanimate Environments. World J. Clin. Cases 2020, 8, 1391. [Google Scholar] [CrossRef] [PubMed]
- Ashok, B.; Hariram, N.; Siengchin, S.; Rajulu, A.V. Modification of Tamarind Fruit Shell Powder with in Situ Generated Copper Nanoparticles by Single Step Hydrothermal Method. J. Bioresour. Bioprod. 2020, 5, 180–185. [Google Scholar] [CrossRef]
- Yousef, S.; Tatariants, M.; Makarevičius, V.; Lukošiūtė, S.I.; Bendikiene, R.; Denafas, G. A Strategy for Synthesis of Copper Nanoparticles from Recovered Metal of Waste Printed Circuit Boards. J. Clean. Prod. 2018, 185, 653–664. [Google Scholar] [CrossRef]
- Wang, A.; Liu, Z.; Li, S.; Liu, Y.; Zhao, H.; Liu, Y.; Ye, T.; Niu, Y.; Li, W. In-Situ Preparation and Properties of Copper Nanoparticles/Poly(Ionic Liquid) Composites by Click Chemistry within Surfactant-Free Ionic Liquid Microemulsions. J. Mol. Liq. 2021, 342, 117572. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, S.; Shi, T.; Zhong, Y.; Huang, Y.; Li, J.; Liao, G.; Tang, Z. Size Distribution Control of Copper Nanoparticles and Oxides: Effect of Wet-Chemical Redox Cycling. Inorg. Chem. 2019, 58, 2533–2542. [Google Scholar] [CrossRef]
- Pérez-Alvarez, M.; Cadenas-Pliego, G.; Pérez-Camacho, O.; Comparán-Padilla, V.E.; Cabello-Alvarado, C.J.; Saucedo-Salazar, E. Green Synthesis of Copper Nanoparticles Using Cotton. Polymers 2021, 13, 1906. [Google Scholar] [CrossRef]
- Sharma, V.; Basak, S.; Ali, S.W. Synthesis of Copper Nanoparticles on Cellulosic Fabrics and Evaluation of Their Multifunctional Performances. Cellulose 2022, 29, 7973–7988. [Google Scholar] [CrossRef]
- Shahidi, S.; Moazzenchi, B. The Influence of Dyeing on the Adsorption of Silver and Copper Particles as Antibacterial Agents on to Cotton Fabrics. J. Nat. Fibers 2018, 16, 677–687. [Google Scholar] [CrossRef]
- Moozarm Nia, P.; Pei Meng, W.; Alias, Y. Copper Nanoparticle Decorated Non-Woven Polypropylene Fabrics with Durable Superhydrophobicity and Conductivity You May Also like One-Step Electrodeposition of Polypyrrole-Copper Nano Particles for H2O2 Detection. Nanotechnology 2020, 32, 035701. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Y.; Xue, Q.; Wu, X. Synthesis of Highly Stable Dispersions of Nanosized Copper Particles Using L-Ascorbic Acid. Green Chem. 2011, 13, 900–904. [Google Scholar] [CrossRef]
- Ghobadi, N.; Chobin, S.; Rezaee, S.; Shakoury, R. Tuning the Optical and Photocatalytic Features of Copper Selenide Prepared by Chemical Solution Deposition Method. Surf. Interfaces 2020, 21, 100706. [Google Scholar] [CrossRef]
- Ismail, M.I.M. Green Synthesis and Characterizations of Copper Nanoparticles. Mater. Chem. Phys. 2020, 240, 122283. [Google Scholar] [CrossRef]
- Lai, D.; Liu, T.; Jiang, G.; Chen, W. Synthesis of Highly Stable Dispersions of Copper Nanoparticles Using Sodium Hypophosphite. J. Appl. Polym. Sci. 2013, 128, 1443–1449. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.Y.; Li, D.; Yang, Q.B.; Hong, Y. Preparation and Stability of the Nanochains Consistind of Copper Nanoparticles and PVA Nanofiber; World Scientific Publishing Company: Singapore, 2003; pp. 97–102. [Google Scholar]
- Shenoy, U.S.; Shetty, A.N. Simple Glucose Reduction Route for One-Step Synthesis of Copper Nanofluids. Appl. Nanosci. 2014, 4, 47–54. [Google Scholar] [CrossRef]
- Hood, J.R.; Wilkinson, J.M.; Cavanagh, H.M.A. Evaluation of Common Antibacterial Screening Methods Utilized in Essential Oil Research. J. Essent. Oil Res. 2011, 15, 428–433. [Google Scholar] [CrossRef]
- Sweygers, N.; Depuydt, D.E.C.; Eyley, S.; Thielemans, W.; Mosleh, Y.; Ivens, J.; Dewil, R.; Appels, L.; Van Vuure, A.W. Prediction of the Equilibrium Moisture Content Based on the Chemical Composition and Crystallinity of Natural Fibres. Ind. Crop. Prod. 2022, 186, 115187. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Zhang, S.; Gao, Q.; Lu, Q.; Peng, R.; Xu, P.; Shang, H.; Yuan, Y.; Zou, H. Micro-FTIR Combined with Curve Fitting Method to Study Cellulose Crystallinity of Developing Cotton Fibers. Anal. Bioanal. Chem. 2021, 413, 1313–1320. [Google Scholar] [CrossRef]
- Belukhina, O.; Milasiene, D.; Ivanauskas, R. Investigation of the Possibilities of Wool Fiber Surface Modification with Copper Selenide. Materials 2021, 14, 1648. [Google Scholar] [CrossRef]
- Gubała, D.; Harniman, R.; Eloi, J.C.; Wąsik, P.; Wermeille, D.; Sun, L.; Robles, E.; Chen, M.; Briscoe, W.H. Multiscale Characterisation of Single Synthetic Fibres: Surface Morphology and Nanomechanical Properties. J. Colloid Interface Sci. 2020, 571, 398–411. [Google Scholar] [CrossRef]
- Petrény, R.; Almásy, L.; Mészáros, L. Investigation of the Interphase Structure in Polyamide 6–Matrix, Multi-Scale Composites. Compos. Sci. Technol. 2022, 225, 109489. [Google Scholar] [CrossRef]
- Restori, R.; Schwarzenbach, D. Charge Density in Cuprite, Cu2O. Acta Crystallogr. Sect. B Struct. Sci. 1986, 42, 201–208. [Google Scholar] [CrossRef]
- Martis, P.; Fonseca, A.; Mekhalif, Z.; Delhalle, J. Optimization of Cuprous Oxide Nanocrystals Deposition on Multiwalled Carbon Nanotubes. J. Nanoparticle Res. 2010, 12, 439–448. [Google Scholar] [CrossRef]
- Waseda, Y.; Matsubara, E.; Shinoda, K. X-ray Diffraction Crystallography; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Li, Q.; Brady, P.R.; Wang, X. The Effect of PH on Wool Fiber Diameter and Fabric Dimensions. Text. Res. J. 2009, 79, 953–957. [Google Scholar] [CrossRef]
- Monier, M.; Ayad, D.M.; Sarhan, A.A. Adsorption of Cu(II), Hg(II), and Ni(II) Ions by Modified Natural Wool Chelating Fibers. J. Hazard. Mater. 2010, 176, 348–355. [Google Scholar] [CrossRef]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral Surfaces and Coatings and Their Mechanisms of Action. Commun. Mater. 2021, 2, 1–19. [Google Scholar] [CrossRef]
- Jung, S.; Byeon, E.Y.; Kim, D.G.; Lee, D.G.; Ryoo, S.; Lee, S.; Shin, C.W.; Jang, H.W.; Yang, J.Y.; Kim, H.J.; et al. Copper-Coated Polypropylene Filter Face Mask with SARS-CoV-2 Antiviral Ability. Polymers 2021, 13, 1367. [Google Scholar] [CrossRef]
- Sousa, B.C.; Cote, D.L. Antimicrobial Copper Cold Spray Coatings and SARS-CoV-2 Surface Inactivation. MRS Adv. 2020, 5, 2873–2880. [Google Scholar] [CrossRef]
- Purniawan, A.; Lusida, M.I.; Pujiyanto, R.W.; Nastri, A.M.; Permanasari, A.A.; Harsono, A.A.H.; Oktavia, N.H.; Wicaksono, S.T.; Dewantari, J.R.; Prasetya, R.R.; et al. Synthesis and Assessment of Copper-Based Nanoparticles as a Surface Coating Agent for Antiviral Properties against SARS-CoV-2. Sci. Rep. 2022, 12, 4835. [Google Scholar] [CrossRef]
- Foffa, I.; Losi, P.; Quaranta, P.; Cara, A.; Al Kayal, T.; D’Acunto, M.; Presciuttini, G.; Pistello, M.; Soldani, G. A Copper Nanoparticles-Based Polymeric Spray Coating: Nanoshield against Sars-Cov-2. J. Appl. Biomater. Funct. Mater. 2022, 20, 1–6. [Google Scholar] [CrossRef]
- Bashiri Rezaie, A.; Montazer, M.; Mahmoudi Rad, M. Scalable, Eco-Friendly and Simple Strategy for Nano-Functionalization of Textiles Using Immobilized Copper-Based Nanoparticles. Clean Technol. Environ. Policy 2018, 20, 2119–2133. [Google Scholar] [CrossRef]
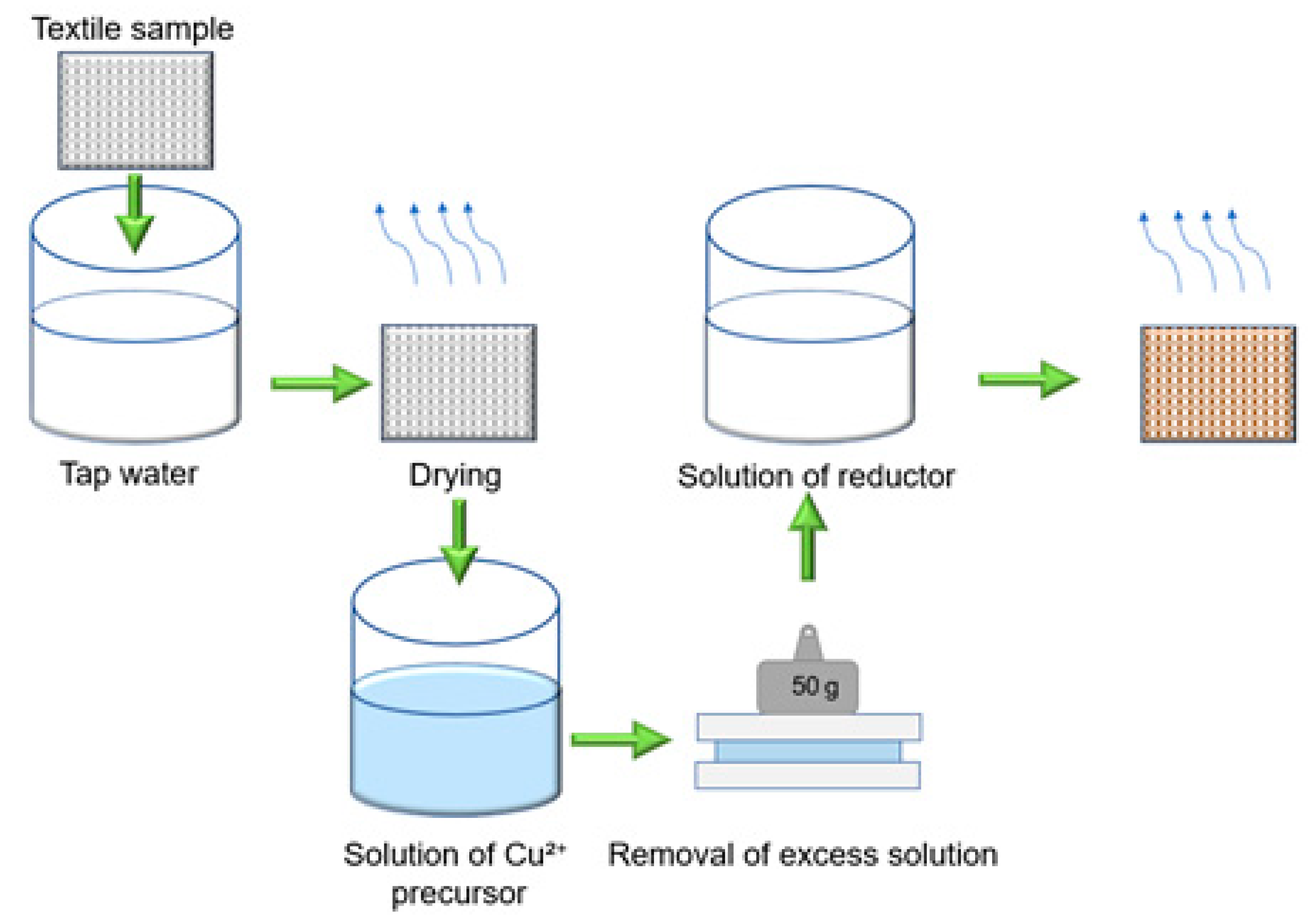
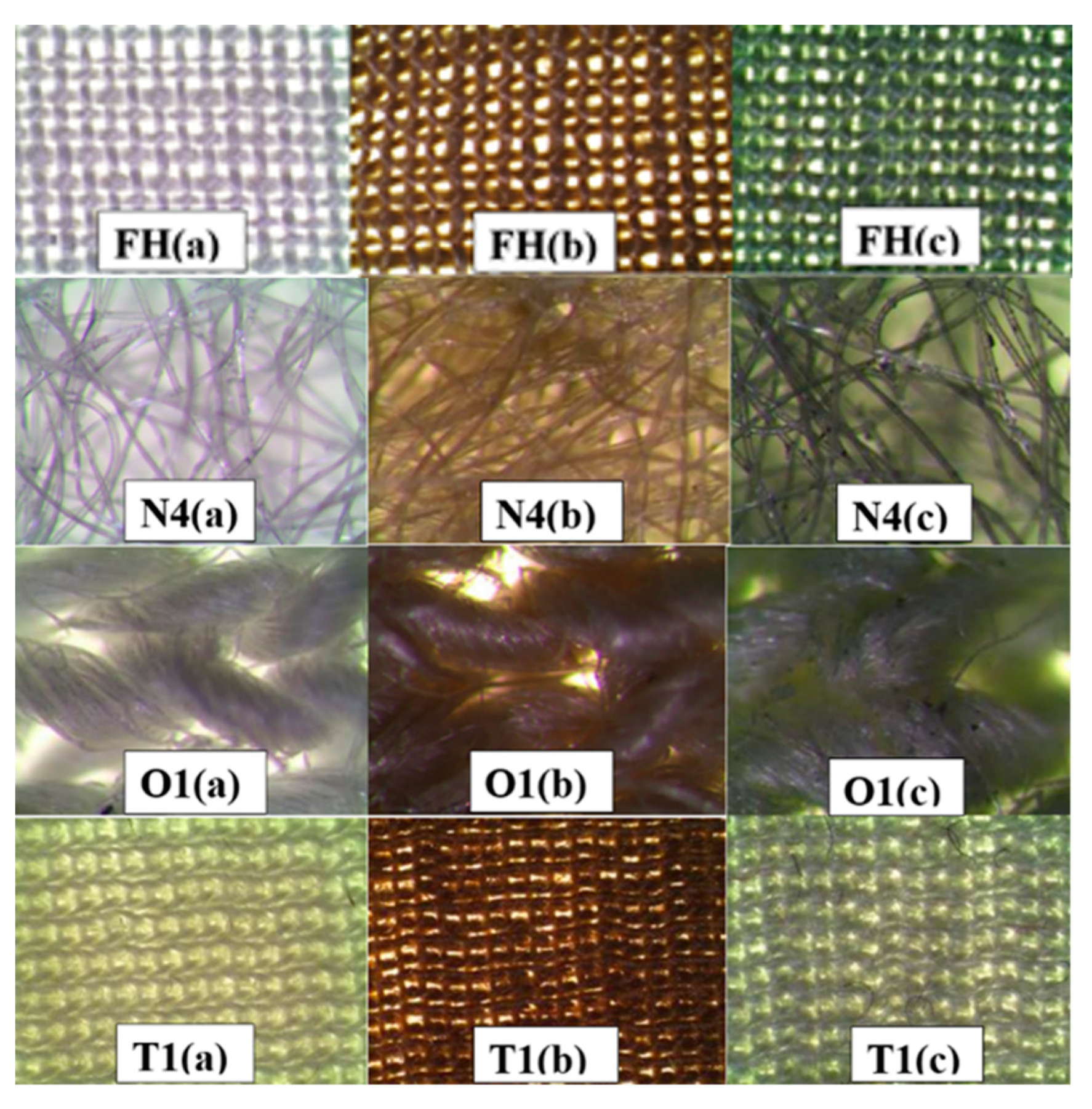
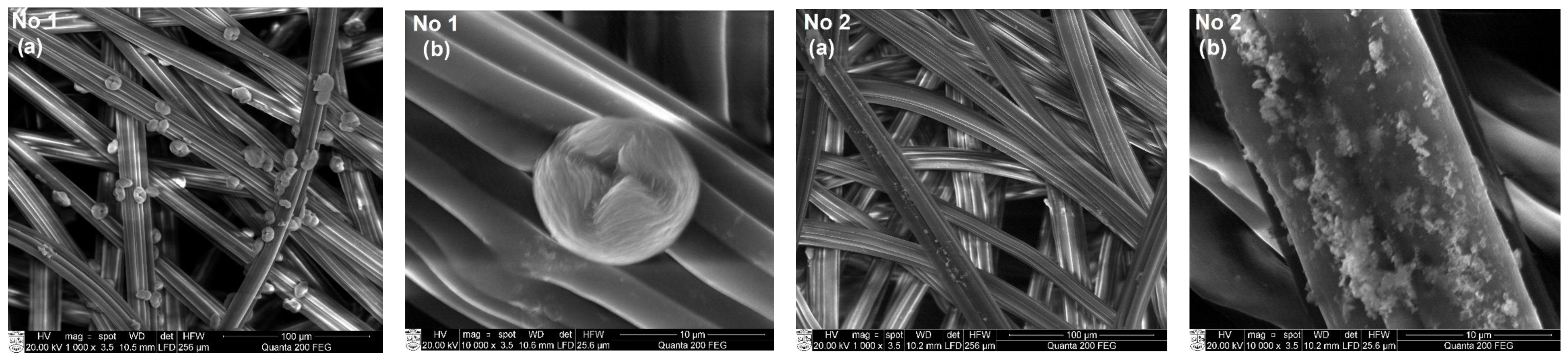

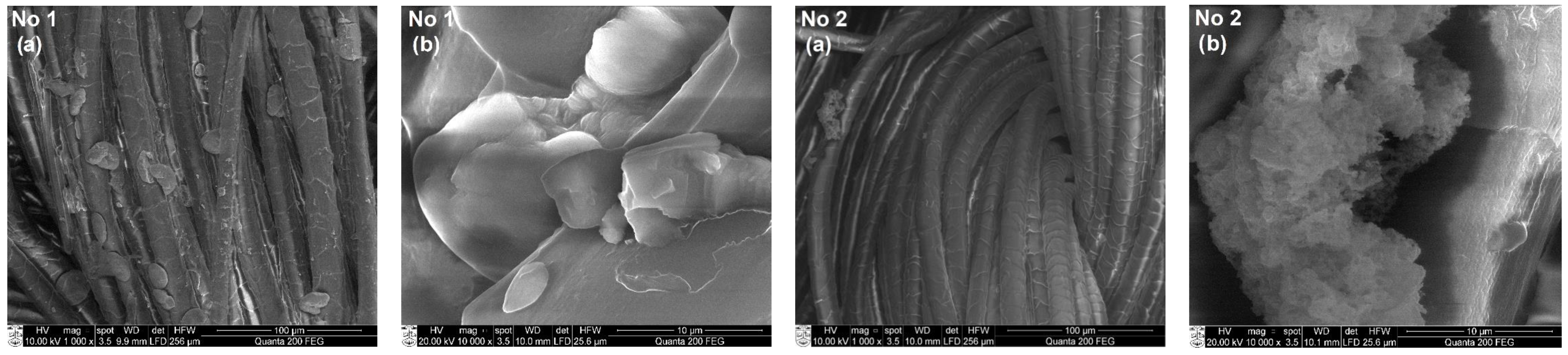
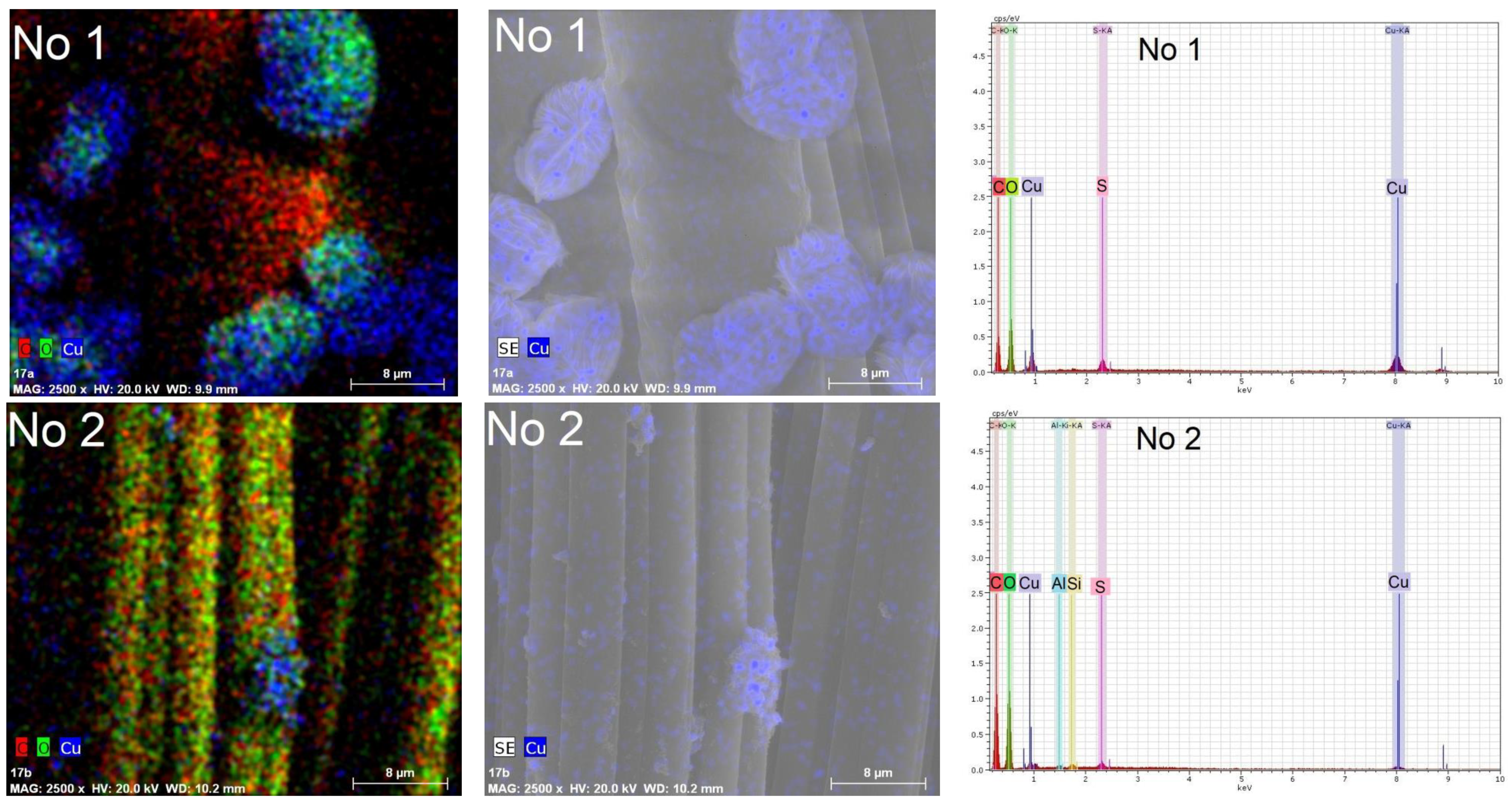

| No. | Marking | Samples | Composition of Textile Materials, % | Weight, g/m2 |
|---|---|---|---|---|
| 1 | FH | Fabric | Flax/hemp, 50/50 | 160 ± 8.0 |
| 2 | F | Fabric | Flax, 100 | 160 ± 8.0 |
| 3 | N1 | Non-woven material (7K7–150) | Viscose, 100 | 150 ± 7.5 |
| 4 | N2 | Non-woven material (1K1A–130) | Polyester (PES), 100 | 130 ± 7.5 |
| 5 | N3 | Non-woven material (9A70W-050-03) | PES/viscose, 60/40 | 50 ± 2.5 |
| 6 | N4 | Non-woven material (9A70W-100-05) | PES/viscose, 60/40 | 100 ± 5.0 |
| 7 | N5 | Non-woven material (9A32W-090-03) | Polyester, 100 | 90 ± 4.5 |
| 8 | N6 | Non-woven material (1K1-220) | Polyester, 100 | 220 ± 11.0 |
| 9 | N7 | Non-woven material (9A70W-040-02) | PES/viscose, 60/40 | 40 ± 2.0 |
| 10 | O1 | Knitted fabric (7061S-1) | Wool/bamboo viscose, 60/40 | 150 ± 7.5 |
| 11 | O2 | Knitted fabric (7021 ENZ) | Cotton/hemp, 70/30 | 195 ± 9.75 |
| 12 | O3 | Knitted fabric (7222PM) | Modal viscose/milk protein fiber/lycra, 57/38/5 | 160 ± 8.0 |
| 13 | O4 | Knitted fabric | Cotton/bamboo viscose, 60/40 | 175 ± 8.75 |
| 14 | O5 | Knitted fabric | PES/hemp, 70/30 | 165 ± 8.25 |
| 15 | O6 | Knitted fabric | Tencel viscose/wool/lycra, 62/34/4 | 165 ± 8.25 |
| 16 | O7 | Knitted fabric | Recycled PES/hemp/PES/lycra, 57/25/16/2 | 270 ± 13.5 |
| 17 | O8 | Knitted fabric | Cotton/PES, 50/50 | 240 ± 12.0 |
| 18 | O9 | Knitted fabric | Bamboo viscose/cotton, 70/30 | 160 ± 8.0 |
| 19 | T1 | Knitted fabric (rib 1 + 1) | Wool, 100 | 180 ± 9.0 |
| 20 | T2 | Knitted fabric (Interlock), unpainted (finishing processes before painting) | Cotton, 100 | 200 ± 10.0 |
| 21 | T3 | Knitted fabric (single jersey) | Polyamide (PA), 100 | 160 ± 8.0 |
| 22 | T4 | Knitted fabric (double knit) | Cotton/PA, 88/12 | 370 ± 18.5 |
| 23 | T5 | Knitted fabric (rib 1 + 1), unpainted (finishing processes before painting) | Wool/acrylic Dralon fibers, NM 50/1, 50/50 | 200 ± 10.0 |
| 24 | T6 | Knitted fabric (single jersey), unpainted (finishing processes before painting) | Bamboo, 100 | 160 ± 8.0 |
| 25 | T7 | Knitted fabric (Interlock) | Wool/PA, 80/20 | 170 ± 8.5 |
| 26 | T8 | Knitted fabric | Cotton/PES, 84/16 | 330 ± 16.5 |
| 27 | T9 | Knitted fabric (Interlock), unpainted (finishing processes before painting | Cotton, 100 | 200 ±10.0 |
| 28 | T10 | Knitted fabric (single jersey) | Tencel viscose, 100 | 190 ± 9.5 |
| 29 | T11 | Knitted fabric (variegated rib) | Cotton/PES, 85/15 | 340 ± 17.0 |
| No | Concentration of CuSO4·5H2O, mol/L | Reductant and Its Concentration, mol/L | Conditions for the Second Stage | |
|---|---|---|---|---|
| Temperature, °C | Duration, minutes | |||
| 1 | 0.5 | C6H8O6, 0.6 | 60 | 60 |
| 2 | 0.05 | NaBH4, 0.15 | 25 | 3 |
| 3 | 0.1 | N2H4·H2O, 0.15 | 25 | 30 |
| 4 | 0.01 | NaH2PO2·H2O, 0.02 | 80 | 120 |
| 5 | 0.125 | NaHSO3, 0.125 | 60 | 10 |
| 6 | 0.1 | C6H12O6, 0.2 | 100 | 20 |
| 2θ (Degree) | Phase | Inter-Plane Distances (d), Å | |
|---|---|---|---|
| Experimental Data | JCPDS Data | ||
| 29.54 | Cu2O | 3.029 | 3.033 |
| 36.39 | Cu2O | 2.466 | 2.465 |
| 42.28 | Cu2O | 2.136 | 2.135 |
| 43.30 | Cu | 2.088 | 2.088 |
| 50.44 | Cu | 1.808 | 1.808 |
| Sample | Inhibition Zone, mm | |
|---|---|---|
| Reducer Solution No. 1 | Reducer Solution No. 2 | |
| FH | 13–16–15 | 16–14–14 |
| N4 | 12–12–13 | 15–16–14 |
| O1 | 10–10–10 | 14–14–14 |
| T1 | 20–22–21 | 18–18–19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanauskas, R.; Ancutienė, I.; Milašienė, D.; Ivanauskas, A.; Bronušienė, A. Effect of Reducing Agent on Characteristics and Antibacterial Activity of Copper-Containing Particles in Textile Materials. Materials 2022, 15, 7623. https://doi.org/10.3390/ma15217623
Ivanauskas R, Ancutienė I, Milašienė D, Ivanauskas A, Bronušienė A. Effect of Reducing Agent on Characteristics and Antibacterial Activity of Copper-Containing Particles in Textile Materials. Materials. 2022; 15(21):7623. https://doi.org/10.3390/ma15217623
Chicago/Turabian StyleIvanauskas, Remigijus, Ingrida Ancutienė, Daiva Milašienė, Algimantas Ivanauskas, and Asta Bronušienė. 2022. "Effect of Reducing Agent on Characteristics and Antibacterial Activity of Copper-Containing Particles in Textile Materials" Materials 15, no. 21: 7623. https://doi.org/10.3390/ma15217623
APA StyleIvanauskas, R., Ancutienė, I., Milašienė, D., Ivanauskas, A., & Bronušienė, A. (2022). Effect of Reducing Agent on Characteristics and Antibacterial Activity of Copper-Containing Particles in Textile Materials. Materials, 15(21), 7623. https://doi.org/10.3390/ma15217623








