Jujube Shell Based-Porous Carbon Composites Double-Doped by MnO2 and Ti3C2Tx: The Effect of Double Pseudocapacitive Doping on Electrochemical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.3. Structural Characterizations
2.4. Electrochemical Measurements
2.4.1. Electrode Sheet Preparation
2.4.2. Three Electrode System
P = 1000 E/t
2.4.3. Two Electrode System
3. Results and Discussion
3.1. Characteristics of Composites
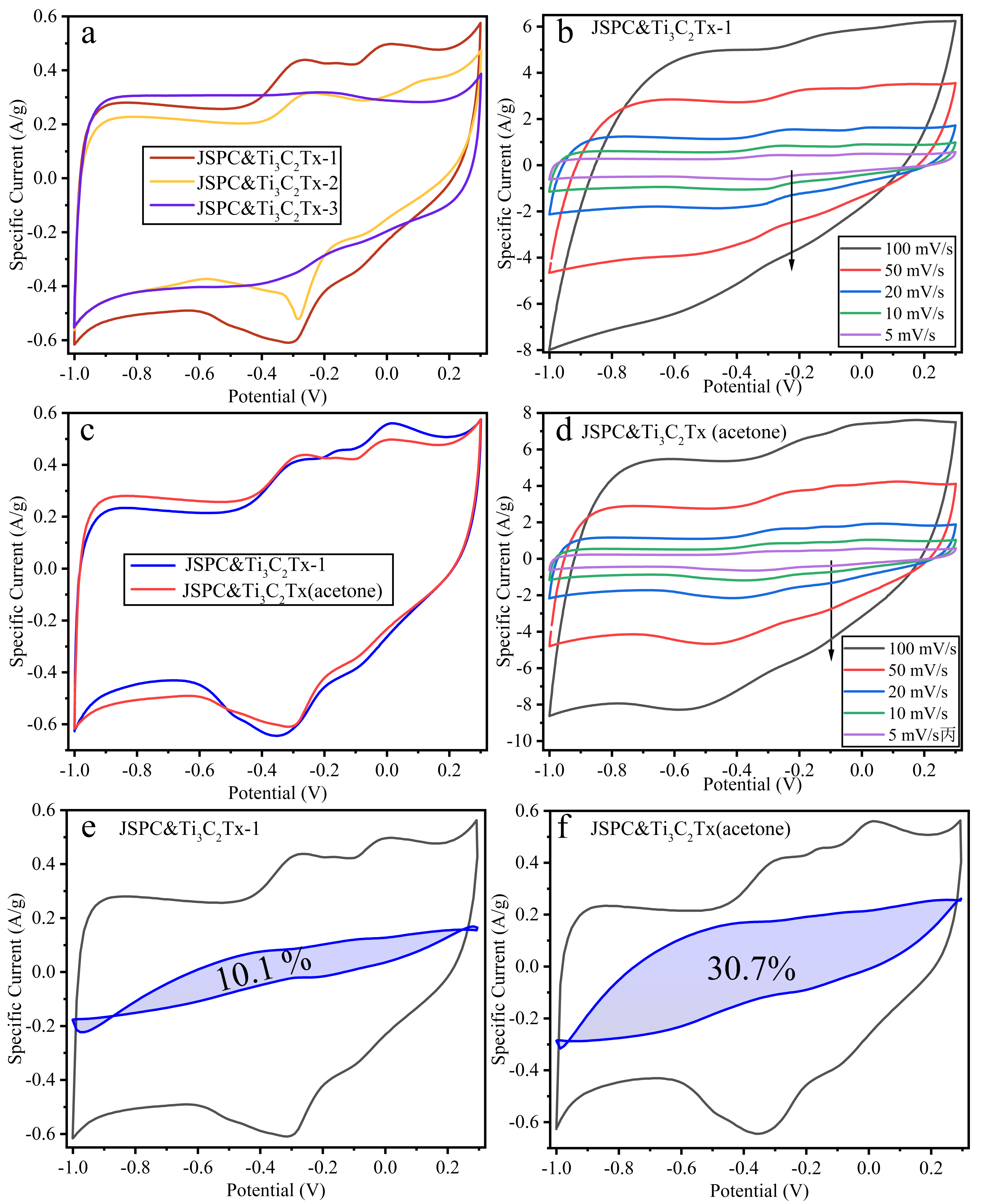
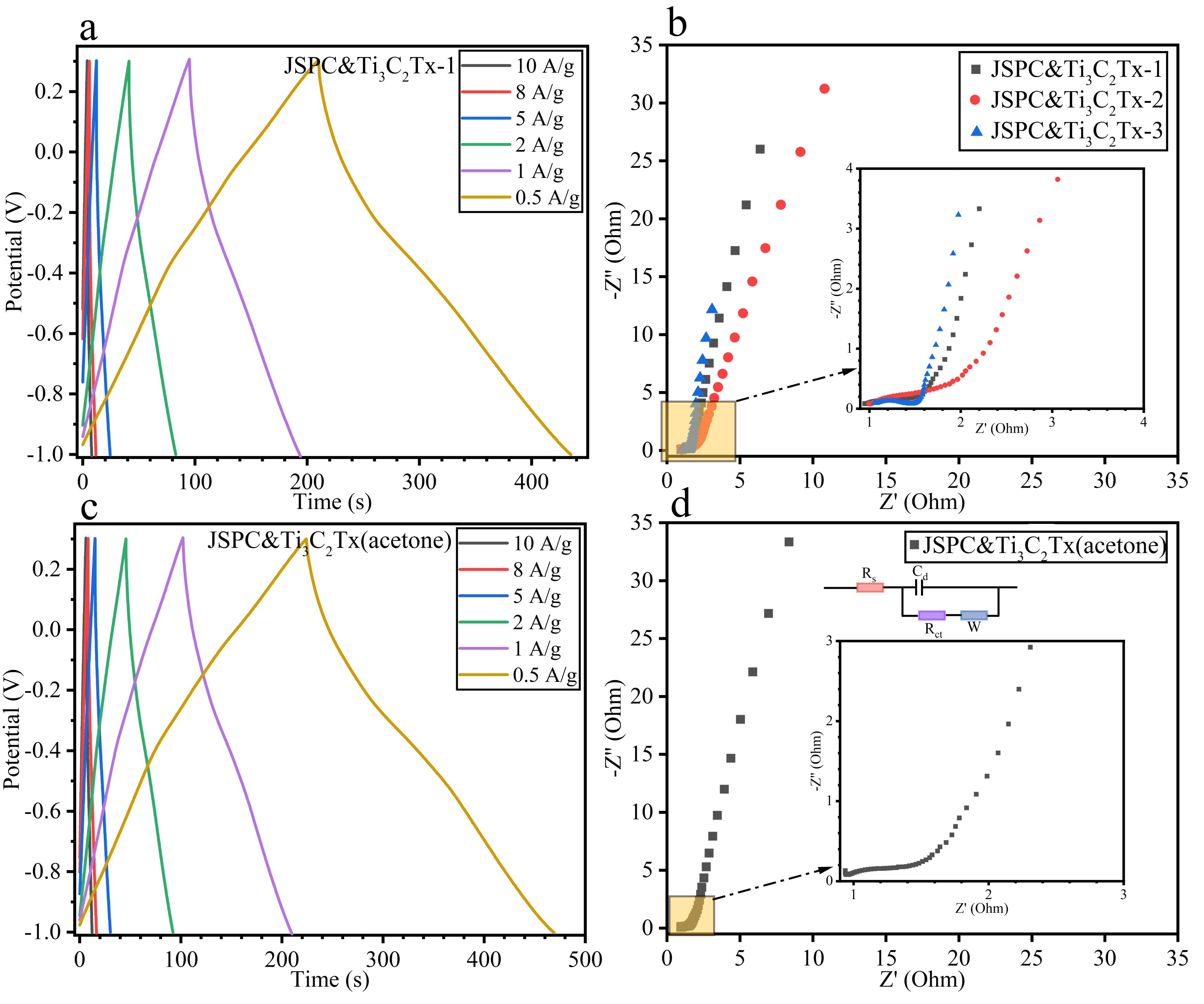
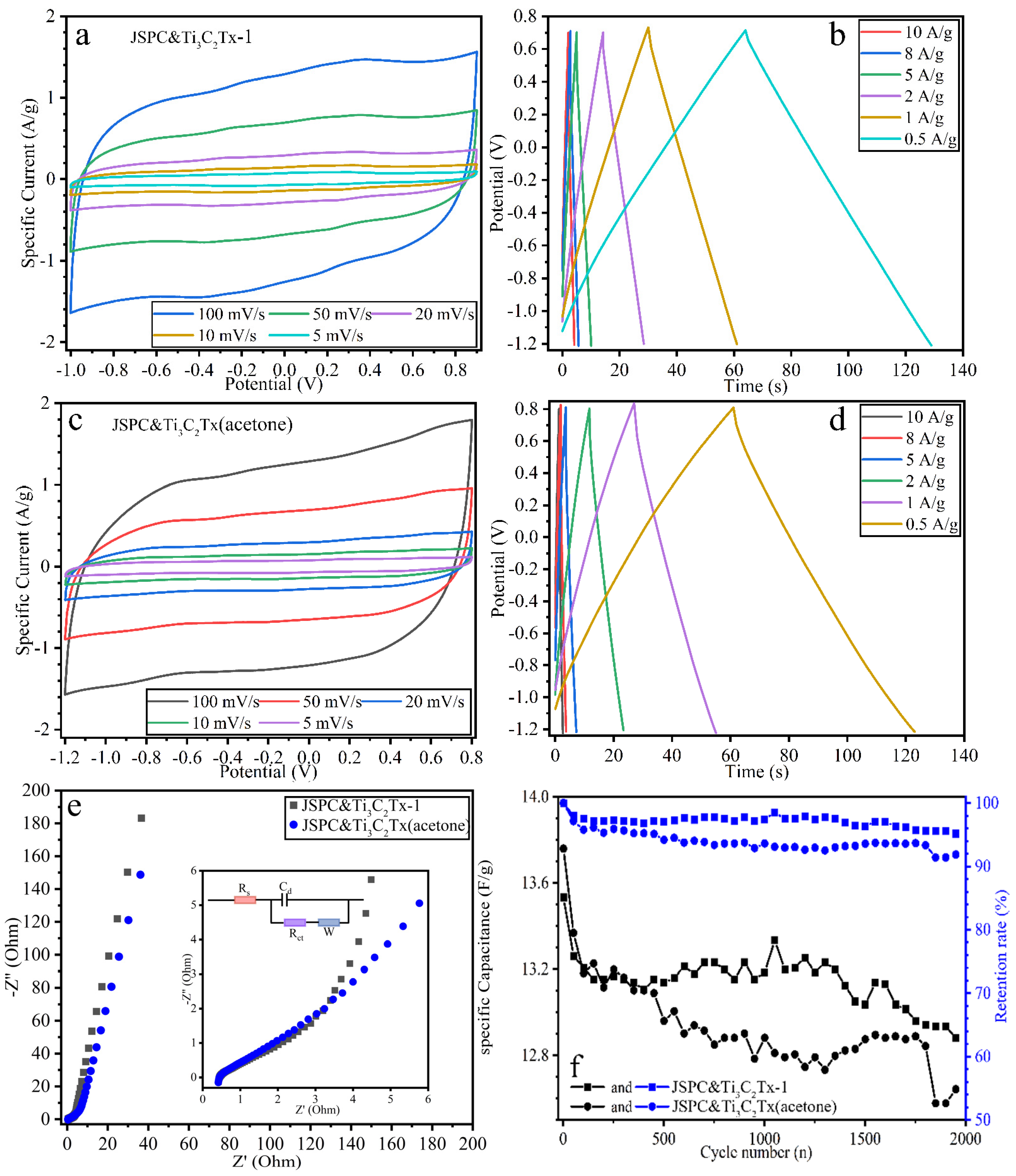
3.2. Electrochemical Properties of Composites
3.3. Electrochemical Properties of the Cell
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Liu, C.-F.; Xu, S.; Cheng, T.; Wang, S.; Lai, W.-Y.; Huang, W. Porous organic polymers for high-performance supercapacitors. Chem. Soc. Rev. 2022, 51, 3181–3225. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Z.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.J.; Fan, X.C.; He, Y. Comprehensive evaluation index system for wind power utilization levels in wind farms in China. Renew. Sustain. Energy Rev. 2017, 69, 461–471. [Google Scholar] [CrossRef]
- Zhan, L.; Bo, Y.; Lin, T.; Fan, Z. Development and outlook of advanced nuclear energy technology. Energy Strategy Rev. 2021, 34, 100630. [Google Scholar] [CrossRef]
- Fan, Q.W.; Fan, X.J.; Fu, P.; Li, Y.; Zhao, Y.X.; Hua, D.L. Anaerobic digestion of wood vinegar wastewater using domesticated sludge: Focusing on the relationship between organic degradation and microbial communities (archaea, bacteria, and fungi). Bioresour. Technol. 2022, 347, 126384. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Pazhamalai, P.; Kim, S.-J. Two-dimensional siloxene nanosheets: Novel high-performance supercapacitor electrode materials. Energy Environ. Sci. 2018, 11, 1595–1602. [Google Scholar] [CrossRef]
- Tang, X.; Liu, D.; Wang, Y.-J.; Cui, L.; Ignaszak, A.; Yu, Y.; Zhang, J. Research advances in biomass-derived nanostructured carbons and their composite materials for electrochemical energy technologies. Prog. Mater. Sci. 2021, 118, 100770. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Z.; Pan, F.; Cui, Y.; Amine, K. High-Performance Anode Materials for Rechargeable Lithium-Ion Batteries. Electrochem. Energy Rev. 2018, 1, 35–53. [Google Scholar] [CrossRef]
- Lokhande, P.E.; Chavan, U.S.; Pandey, A. Materials and Fabrication Methods for Electrochemical Supercapacitors: Overview. Electrochem. Energy Rev. 2020, 3, 155–186. [Google Scholar] [CrossRef]
- Zhao, J.; Burke, A.F. Electrochemical capacitors: Materials, technologies and performance. Energy Storage Mater. 2021, 36, 31–55. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Liu, X.; Chi, H.; Hu, J.; Zhao, H.; Xiao, G. Facile synthesis and electrochemical properties of alicyclic polyimides based carbon microflowers for electrode materials of supercapacitors. J. Energy Storage 2022, 47, 103656. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Chen, Y.; Zhang, H.; Yousaf, M.; Wu, H.; Zou, M.; Cao, A.; Han, R.P.S. Hyperporous Sponge Interconnected by Hierarchical Carbon Nanotubes as a High-Performance Potassium-Ion Battery Anode. Adv. Mater. 2018, 30, 1802074. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shao, Y.; Mei, S.; Lu, Y.; Zhang, M.; Sun, J.-K.; Matyjaszewski, K.; Antonietti, M.; Yuan, J. Polymer-Derived Heteroatom-Doped Porous Carbon Materials. Chem. Rev. 2020, 120, 9363–9419. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-P.; Zhai, Z.-B.; Huang, K.-J.; Zhang, Y.-Y. Energy storage applications of biomass-derived carbon materials: Batteries and supercapacitors. New J. Chem. 2017, 41, 11456–11470. [Google Scholar] [CrossRef]
- Ren, X.; Yuan, Z.; Ma, Y.; Zhang, C.; Qin, C.; Jiang, X. Nitrogen-/Boron-Doped Carbon from Poplar Powder and Carbon Nanotube Composite as Electrode Material for Supercapacitors. Energy Fuels 2022, 36, 2841–2850. [Google Scholar] [CrossRef]
- Purkait, T.; Singh, G.; Singh, M.; Kumar, D.; Dey, R.S. Large area few-layer graphene with scalable preparation from waste biomass for high-performance supercapacitor. Sci. Rep. 2017, 7, 15239. [Google Scholar] [CrossRef]
- El-Kady Maher, F.; Ihns, M.; Li, M.; Jee, Y.H.; Mir, F.M.; Chaney, L.; Andrew, T.L.; Richard, B.K. Engineering three-dimensional hybrid supercapacitors and microsupercapacitors for high-performance integrated energy storage. Proc. Natl. Acad. Sci. USA 2015, 112, 4233–4238. [Google Scholar] [CrossRef]
- Karmur, R.S.; Gogoi, D.; Das, M.R.; Ghosh, N.N. High-Performance Flexible Supercapacitor Device Composed of a Hierarchical 2-D MXene-Ni(OH)2 Nanocomposite and Biomass-Derived Porous Carbon Electrodes. Energy Fuels 2022, 36, 8488–8499. [Google Scholar] [CrossRef]
- Wu, J.-Z.; Li, X.-Y.; Zhu, Y.-R.; Yi, T.-F.; Zhang, J.-H.; Xie, Y. Facile synthesis of MoO2/CNTs composites for high-performance supercapacitor electrodes. Ceram. Int. 2016, 42, 9250–9256. [Google Scholar] [CrossRef]
- Zhang, N.; Fu, C.; Liu, D.; Li, Y.; Zhou, H.; Kuang, Y. Three-Dimensional Pompon-like MnO2/Graphene Hydrogel Composite for Supercapacitor. Electrochim. Acta 2016, 210, 804–811. [Google Scholar] [CrossRef]
- Tran, T.S.; Tripathi, K.M.; Kim, B.N.; You, I.-K.; Park, B.J.; Han, Y.H.; Kim, T. Three-dimensionally assembled Graphene/α-MnO2 nanowire hybrid hydrogels for high performance supercapacitors. Mater. Res. Bull. 2017, 96, 395–404. [Google Scholar] [CrossRef]
- Wang, C.-H.; Zhang, D.-W.; Liu, S.; Yamauchi, Y.; Zhang, F.-B.; Kaneti, Y.V. Ultrathin nanosheet-assembled nickel-based metal–organic framework microflowers for supercapacitor applications. Chem. Commun. 2022, 58, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-Q.; Ren, C.E.; Ling, Z.; Lukatskaya, M.R.; Zhang, C.; Van Aken, K.L.; Barsoum, M.W.; Gogotsi, Y. Flexible MXene/Carbon Nanotube Composite Paper with High Volumetric Capacitance. Adv. Mater. 2015, 27, 339–345. [Google Scholar] [CrossRef]
- Pu, X.J.; Zhao, D.; Fu, C.L.; Chen, Z.X.; Cao, S.N.; Wang, C.S.; Cao, Y.L. Understanding and Calibration of Charge Storage Mechanism in Cyclic Voltammetry Curves. Angew. Chem.-Int. Ed. 2021, 60, 21310–21318. [Google Scholar] [CrossRef]
- Huang, H.; Chu, X.; Xie, Y.; Zhang, B.; Wang, Z.; Duan, Z.; Chen, N.; Xu, Z.; Zhang, H.; Yang, W. Ti3C2Tx MXene-Based Micro-Supercapacitors with Ultrahigh Volumetric Energy Density for All-in-One Si-Electronics. ACS Nano 2022, 16, 3776–3784. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, J.; Song, H. Tailoring Highly N-Doped Carbon Materials from Hexamine-Based MOFs: Superior Performance and New Insight into the Roles of N Configurations in Na-Ion Storage. Small 2018, 14, 1703548. [Google Scholar] [CrossRef]
- Lu, S.; Sui, L.; Liu, Y.; Yong, X.; Xiao, G.; Yuan, K.; Liu, Z.; Liu, B.; Zou, B.; Yang, B. White Photoluminescent Ti3C2 MXene Quantum Dots with Two-Photon Fluorescence. Adv. Sci. 2019, 6, 1801470. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Wang, Y.-Y.; Bai, X.; Yang, H.; Han, J.-P.; Lun, N.; Qi, Y.-X.; Bai, Y.-J. Manganese silicate drapes as a novel electrode material for supercapacitors. RSC Adv. 2016, 6, 105771–105779. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Luo, C.; Fu, Q.; Pan, C. Highly porous graphitic biomass carbon as advanced electrode materials for supercapacitors. Green Chem. 2017, 19, 4132–4140. [Google Scholar] [CrossRef]
- Rebelo, S.L.H.; Guedes, A.; Szefczyk, M.E.; Pereira, A.M.; Araújo, J.P.; Freire, C. Progress in the Raman spectra analysis of covalently functionalized multiwalled carbon nanotubes: Unraveling disorder in graphitic materials. Phys. Chem. Chem. Phys. 2016, 18, 12784–12796. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Jin, Y.; Zhu, J.; Li, H. Highly active TiO2−x−yNxFy visible photocatalyst prepared under supercritical conditions in NH4F/EtOH fluid. Appl. Catal. B Environ. 2009, 89, 543–550. [Google Scholar] [CrossRef]
- Luo, J.; Tao, X.; Zhang, J.; Xia, Y.; Huang, H.; Zhang, L.; Gan, Y.; Liang, C.; Zhang, W. Sn4+ Ion Decorated Highly Conductive Ti3C2 MXene: Promising Lithium-Ion Anodes with Enhanced Volumetric Capacity and Cyclic Performance. ACS Nano 2016, 10, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, J.; Zhu, Q.; Xu, B. Three-Dimensional MXenes for Supercapacitors: A Review. Small Methods 2022, 6, 2101537. [Google Scholar] [CrossRef]
- Quan, C.; Su, R.; Gao, N. Preparation of activated biomass carbon from pine sawdust for supercapacitor and CO2 capture. Int. J. Energy Res. 2020, 44, 4335–4351. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, W.; Lin, H.; Li, Y.; Lu, H.; Wang, Y. Hierarchical porous carbon based on the self-templating structure of rice husk for high-performance supercapacitors. RSC Adv. 2015, 5, 19294–19300. [Google Scholar] [CrossRef]
- Du, J.; Liu, L.; Hu, Z.; Yu, Y.; Zhang, Y.; Hou, S.; Chen, A. Raw-Cotton-Derived N-Doped Carbon Fiber Aerogel as an Efficient Electrode for Electrochemical Capacitors. ACS Sustain. Chem. Eng. 2018, 6, 4008–4015. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, B.; Zhang, D.; Shi, H.; Lei, M.; Li, H.; Wang, K. Strong polar nonaqueous solvent-assisted microwave fabrication of N and P co-doped microporous carbon for high-performance supercapacitor. Appl. Surf. Sci. 2020, 512, 145711. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Ye, J.; Wei, H.; Hao, J.; Mu, J.; Zhao, S.; Hussain, S. Facile synthesis of three-dimensional NiCo2O4 with different morphology for supercapacitors. RSC Adv. 2016, 6, 70077–70084. [Google Scholar] [CrossRef]
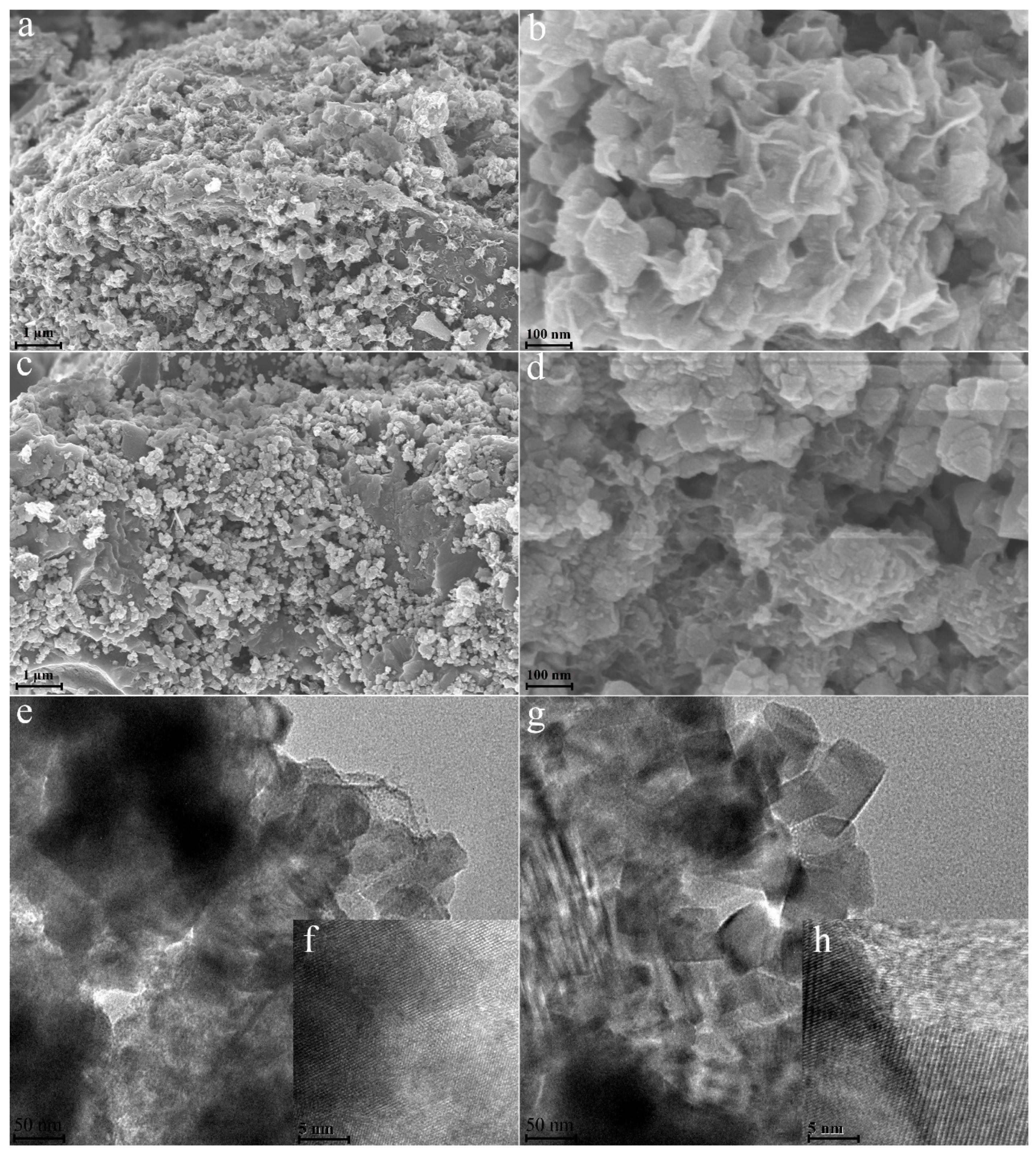
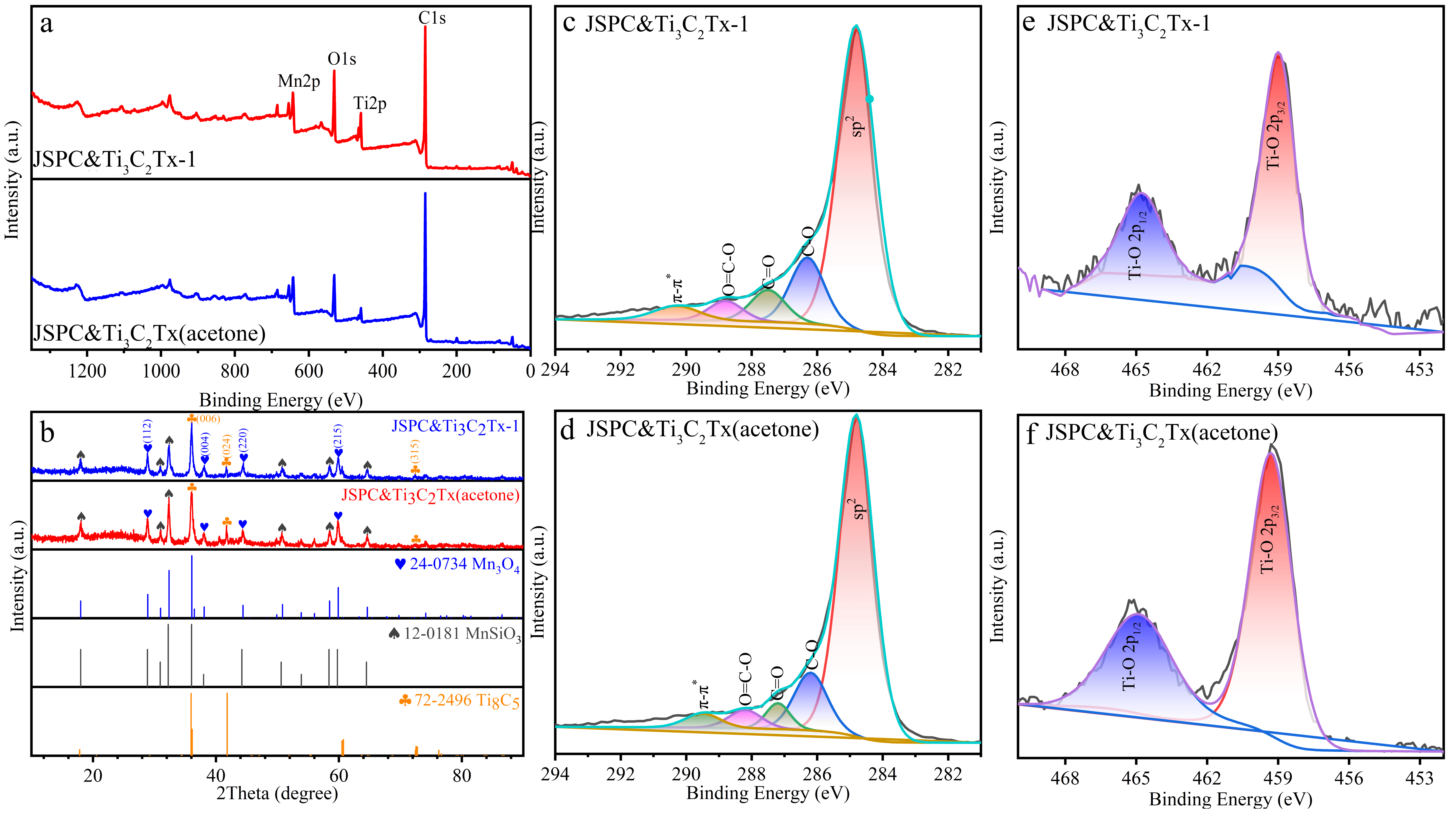
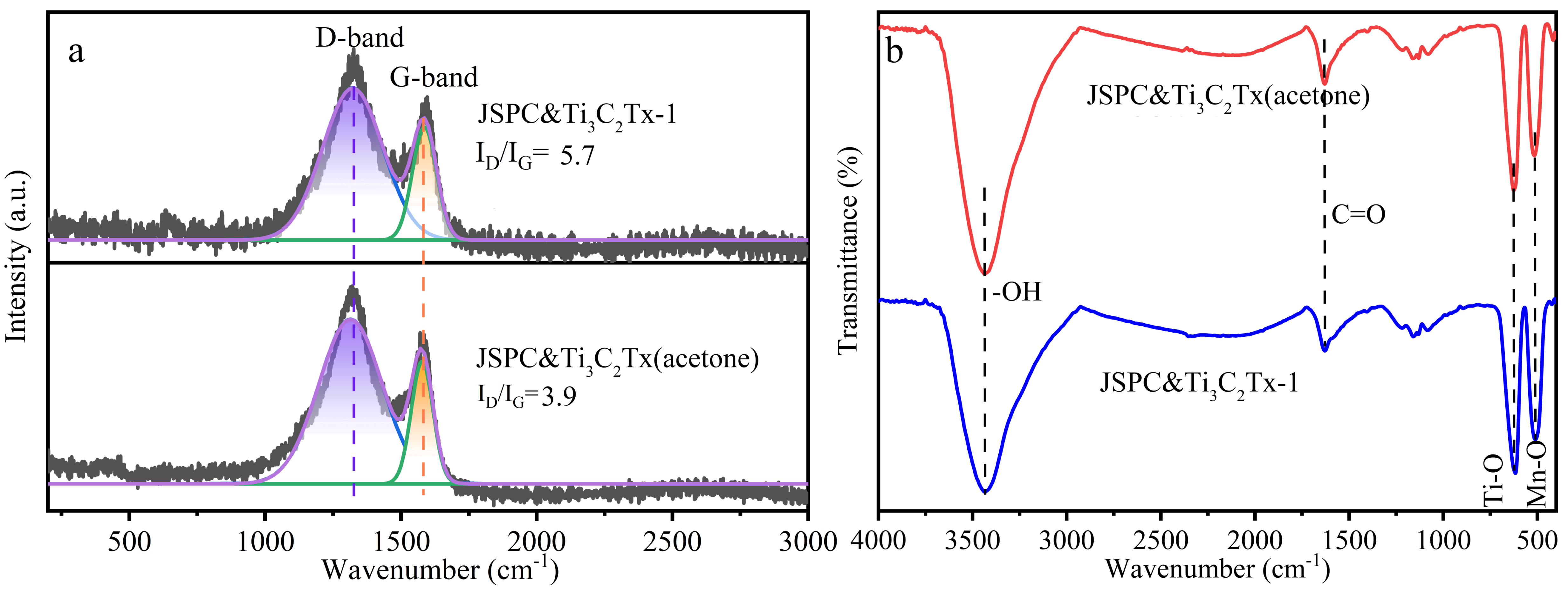
| Atomic (%) | C1s | O1s | Ti2p | Mn2p |
|---|---|---|---|---|
| JSPC&Ti3C2Tx-1 | 79.98 | 13.75 | 2.04 | 4.23 |
| JSPC&Ti3C2Tx (acetone) | 72.84 | 17.94 | 5.03 | 4.2 |
| Electrode | Current Density (A/g) | Specific Capacity (F/g) | Energy Density (Wh/kg) | Power Density (W/kg) | Electrolyte |
|---|---|---|---|---|---|
| JSPC&Ti3C2Tx-1 | 10 | 48.17 | 4.5 | 4100 | 1 M KOH |
| 5 | 57.55 | 8.98 | 2650 | ||
| 1 | 81.67 | 16.33 | 600 | ||
| 0.5 | 89.6 | 19.44 | 312.5 | ||
| JSPC&Ti3C2Tx (acetone) | 10 | 58.17 | 8.74 | 5200 | 1 M KOH |
| 5 | 64.74 | 12.31 | 2925 | ||
| 1 | 86.99 | 18.28 | 615 | ||
| 0.5 | 96.83 | 21.35 | 315 | ||
| Pine sawdust [35] | 0.5 | 175.6 | 24.39 | 254.06 | 1 M KOH |
| Bamboo stalk [30] | 0.5 | 222 | 6.68 | 100.2 | 6 M KOH |
| Rice husk [36] | 0.5 | 278 | 7.4 | 6195 | 6 M KOH |
| Cotton [37] | 0.5 | 365 | 16.1–5.5 | 200–3700 | 6 M KOH |
| Chitosan [38] | 0.5 | 317 | 48.6 | 468.8 | 6 M KOH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Fan, Q.; Yin, X. Jujube Shell Based-Porous Carbon Composites Double-Doped by MnO2 and Ti3C2Tx: The Effect of Double Pseudocapacitive Doping on Electrochemical Properties. Materials 2022, 15, 7532. https://doi.org/10.3390/ma15217532
Sun X, Fan Q, Yin X. Jujube Shell Based-Porous Carbon Composites Double-Doped by MnO2 and Ti3C2Tx: The Effect of Double Pseudocapacitive Doping on Electrochemical Properties. Materials. 2022; 15(21):7532. https://doi.org/10.3390/ma15217532
Chicago/Turabian StyleSun, Xue, Qingwen Fan, and Xiang Yin. 2022. "Jujube Shell Based-Porous Carbon Composites Double-Doped by MnO2 and Ti3C2Tx: The Effect of Double Pseudocapacitive Doping on Electrochemical Properties" Materials 15, no. 21: 7532. https://doi.org/10.3390/ma15217532
APA StyleSun, X., Fan, Q., & Yin, X. (2022). Jujube Shell Based-Porous Carbon Composites Double-Doped by MnO2 and Ti3C2Tx: The Effect of Double Pseudocapacitive Doping on Electrochemical Properties. Materials, 15(21), 7532. https://doi.org/10.3390/ma15217532






