One-Step Synthesis of LiCo1-1.5xYxPO4@C Cathode Material for High-Energy Lithium-ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of LiCo1-1.5xYxPO4@C Cathode Material
2.2. Material Characterization
2.3. Electrochemical Performance
3. Results and Discussion
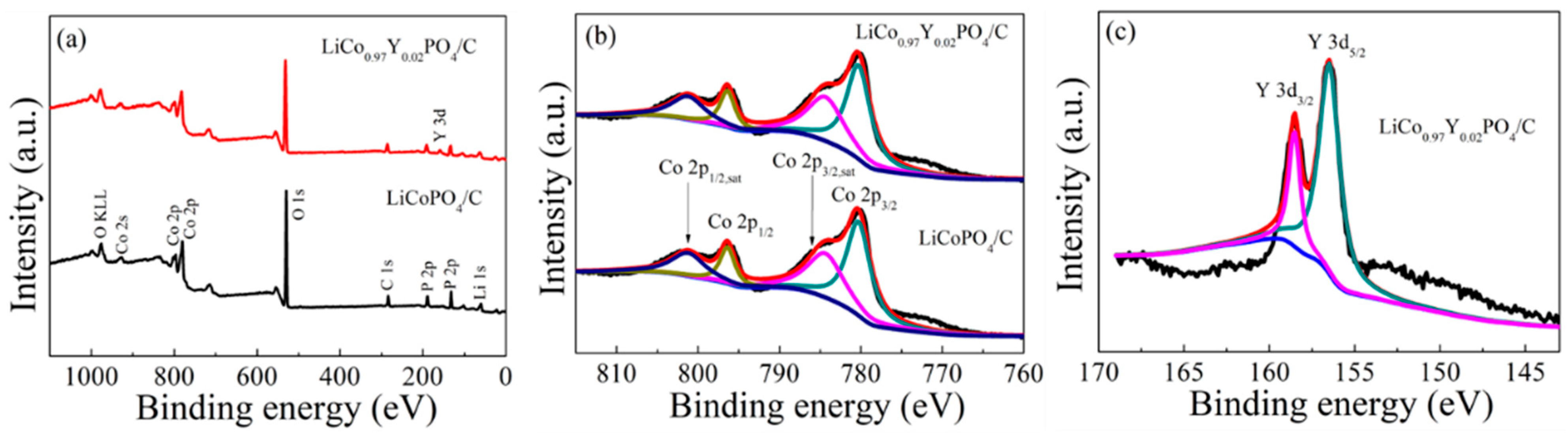
3.1. Composition and Morphology of LiCo1-1.5xYxPO4@C Cathode Material
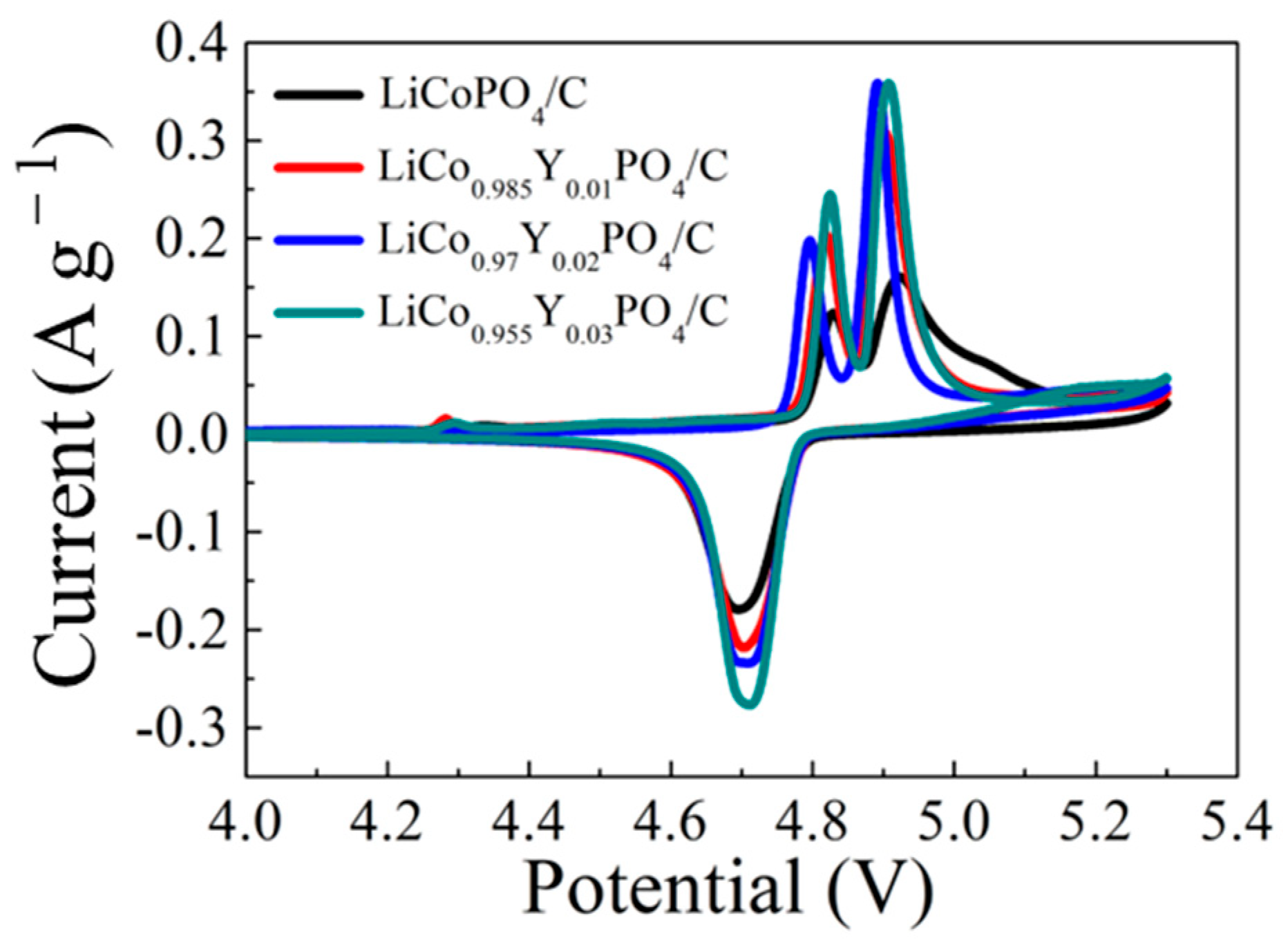
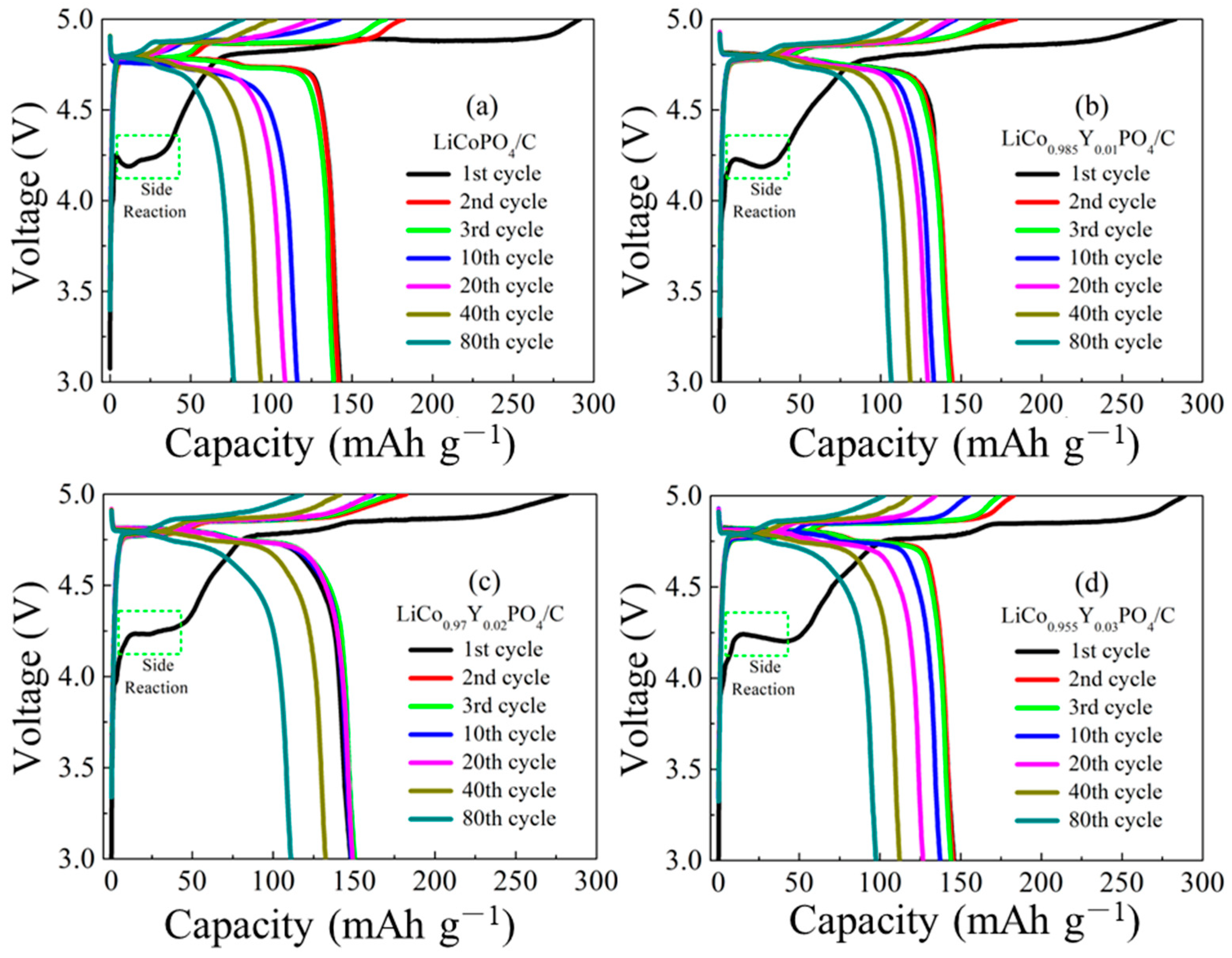
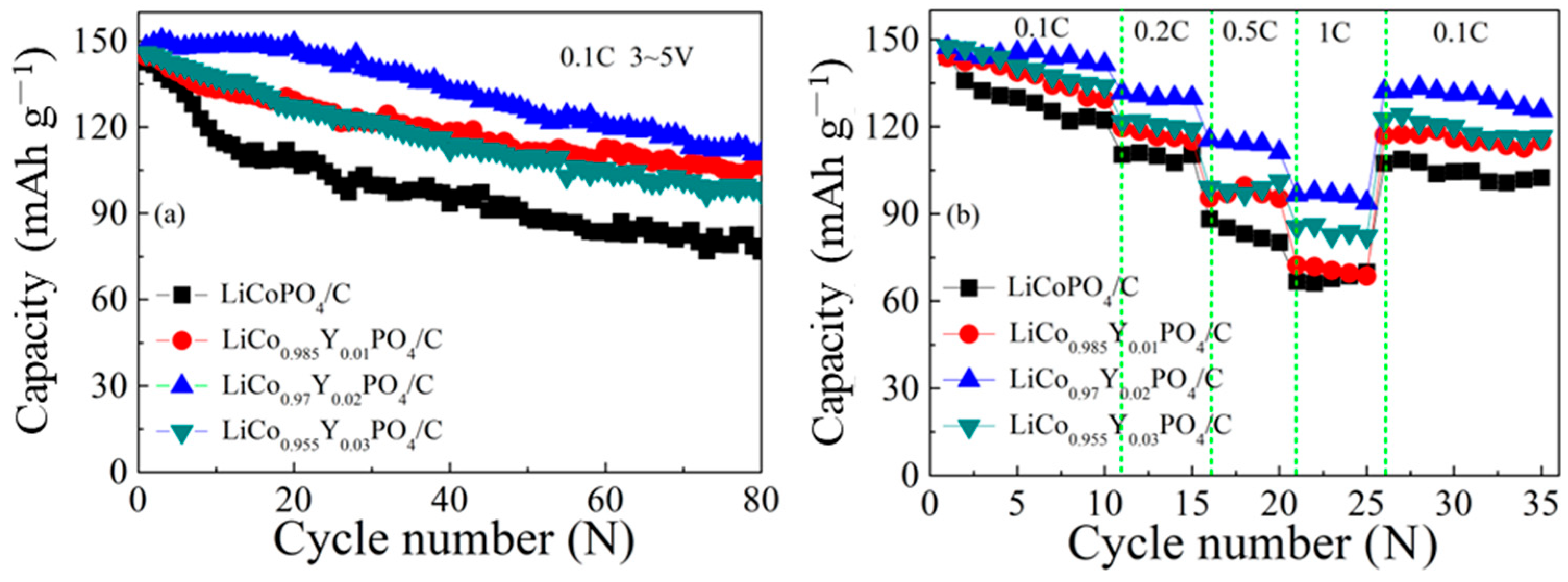
3.2. Electrochemical Performances of LiCo1-1.5xYxPO4@C Electrodes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pramanik, A.; Manche, A.G.; Clulow, R.; Lightfoot, P.; Armstrong, A.R. Exploiting anion and cation redox chemistry in lithium-rich perovskite oxalate: A novel next-generation Li/Na-ion battery electrode. Dalton Trans. 2022, 51, 12467–12475. [Google Scholar] [CrossRef] [PubMed]
- Pandit, B.; Goda, E.S.; Ubaidullah, M.; Shaikh, S.F.; Nakate, U.T.; Khedulkar, A.P.; Rana, A.u.H.S.; Kumar, D.; Doong, R.-A. Hexagonal δ-MnO2 nanoplates as efficient cathode material for potassium-ion batteries. Ceram. Int. 2022, 48, 28856–28863. [Google Scholar] [CrossRef]
- Linnell, S.F.; Kim, E.J.; Choi, Y.-S.; Hirsbrunner, M.; Imada, S.; Pramanik, A.; Cuesta, A.F.; Miller, D.N.; Fusco, E.; Bode, B.E.; et al. Enhanced oxygen redox reversibility and capacity retention of titanium-substituted Na4/7[□1/7Ti1/7Mn5/7]O2 in sodium-ion batteries. J. Mater. Chem. A 2022, 10, 9941–9953. [Google Scholar] [CrossRef]
- Pramanik, A.; Bradford, A.J.; Lee, S.L.; Lightfoot, P.; Armstrong, A.R. Na2Fe (C2O4)(HPO4): A promising new oxalate-phosphate based mixed polyanionic cathode for Li/Na ion batteries. J. Phys. Mater. 2021, 4, 024004–024013. [Google Scholar] [CrossRef]
- Pandit, B.; Rondiya, S.R.; Dzade, N.Y.; Shaikh, S.F.; Kumar, N.; Goda, E.S.; Al-Kahtani, A.A.; Mane, R.S.; Mathur, S.; Salunkhe, R.R. High Stability and Long Cycle Life of Rechargeable Sodium-Ion Battery Using Manganese Oxide Cathode: A Combined Density Functional Theory (DFT) and Experimental Study. ACS Appl. Mater. Interfaces 2021, 13, 11433–11441. [Google Scholar] [CrossRef]
- Nitti, A.; Carfora, R.; Assanelli, G.; Notari, M.; Pasini, D. Single-Chain Polymer Nanoparticles for Addressing Morphologies and Functions at the Nanoscale: A Review. ACS Appl. Nano Mater. 2022. [Google Scholar] [CrossRef]
- Callegari, D.; Colombi, S.; Nitti, A.; Simari, C.; Nicotera, I.; Ferrara, C.; Mustarelli, P.; Pasini, D.; Quartarone, E. Autonomous Self-Healing Strategy for Stable Sodium-Ion Battery: A Case Study of Black Phosphorus Anodes. ACS Appl. Mater. Interfaces 2021, 13, 13170–13182. [Google Scholar] [CrossRef]
- Lapping, J.; Borkiewicz, O.; Wiaderek, K.; Allen, J.L.; Jow, T.R.; Cabana, J. Structural Changes and Reversibility Upon Deintercalation of Li from LiCoPO4 Derivatives. ACS Appl. Mater. Interfaces 2020, 12, 20570–20578. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Lee, U.; Poese, B.; Allen, J.L. Effect of oxygen partial pressure on the discharge capacity of LiCoPO4. J. Power Source 2005, 144, 226–230. [Google Scholar] [CrossRef]
- Zhang, M.; Garcia-Araez, N.; Hector, A.L. Understanding and development of olivine LiCoPO4 cathode materials for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 14483–14517. [Google Scholar] [CrossRef]
- Bramnik, N.N.; Nikolowski, K.; Baehtz, C.; Bramnik, K.G.; Ehrenberg, H. Phase transitions occurring upon lithium insertion-extraction of LiCoPO4. Chem. Mater. 2007, 19, 908–915. [Google Scholar] [CrossRef]
- Kosova, N.V.; Podgornova, O.A.; Devyatkina, E.T.; Podugolnikov, V.R.; Petrov, S.A. Effect of Fe2+ substitution on the structure and electrochemistry of LiCoPO4 prepared by mechanochemically assisted carbothermal reduction. J. Mater. Chem. A 2014, 2, 20697–20705. [Google Scholar] [CrossRef]
- Markevich, E.; Salitra, G.; Fridman, K.; Sharabi, R.; Gershinsky, G.; Garsuch, A.; Semrau, G.; Schmidt, M.A.; Aurbach, D. Fluoroethylene Carbonate as an Important Component in Electrolyte Solutions for High-Voltage Lithium Batteries: Role of Surface Chemistry on the Cathode. Langmuir 2014, 30, 7414–7424. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ming, H.; Qiu, J.; Yu, Z.; Li, M.; Zhang, S.; Yang, Y. Improving cycling performance of LiCoPO4 cathode material by adding tris (trimethylsilyl) borate as electrolyte additive. J. Electroanal. Chem. 2017, 802, 8–14. [Google Scholar] [CrossRef]
- Wu, X.; Meledina, M.; Barthel, J.; Liu, Z.; Tempel, H.; Kungl, H.; Mayer, J.; Eichel, R.-A. Investigation of the Li–Co antisite exchange in Fe-substituted LiCoPO4 cathode for high-voltage lithium ion batteries. Energy Storage Mater. 2019, 22, 138–146. [Google Scholar] [CrossRef]
- Kreder, K.J., III; Assat, G.; Manthiram, A. Aliovalent Substitution of V3+ for Co2+ in LiCoPO4 by a Low-Temperature Microwave-Assisted Solvothermal Process. Chem. Mater. 2016, 28, 1847–1853. [Google Scholar] [CrossRef]
- Lapping, J.G.; Delp, S.A.; Allen, J.L.; Allen, J.L.; Freeland, J.W.; Johannes, M.D.; Hu, L.; Tran, D.T.; Jow, T.R.; Cabana, J. Changes in electronic structure upon Li deintercalation from LiCoPO4 derivatives. Chem. Mater. 2018, 30, 1898–1906. [Google Scholar] [CrossRef]
- Wolfenstine, J. Electrical conductivity of doped LiCoPO4. J. Power Source 2006, 158, 1431–1435. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Read, J.; Allen, J.L. Effect of carbon on the electronic conductivity and discharge capacity LiCoPO4. J. Power Source 2007, 163, 1070–1073. [Google Scholar] [CrossRef]
- Wu, X.; Lin, Y.; Ji, Y.; Zhou, D.; Liu, Z.; Sun, X. Insights into the Enhanced Catalytic Activity of Fe-Doped LiCoPO4 for the Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2020, 3, 2959–2965. [Google Scholar] [CrossRef]
- Wu, X.; Meledina, M.; Tempel, H.; Kungl, H.; Mayer, J.; Eichel, R.-A. Morphology-controllable synthesis of LiCoPO4 and its influence on electrochemical performance for high-voltage lithium ion batteries. J. Power Source 2020, 450, 227726–227733. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, X.; Fang, Z.; Zhu, Y.; Xie, Y.; Cha, J.J.; Yu, G. General facet-controlled synthesis of single-crystalline {010}-oriented LiMPO4 (M = Mn, Fe, Co) nanosheets. Chem. Mater. 2017, 29, 10526–10533. [Google Scholar] [CrossRef]
- Ludwig, J.; Marino, C.; Haering, D.; Stinner, C.; Gasteiger, H.A.; Nilges, T. Morphology-controlled microwave-assisted solvothermal synthesis of high-performance LiCoPO4 as a high-voltage cathode material for Li-ion batteries. J. Power Source 2017, 342, 214–223. [Google Scholar] [CrossRef]
- Wu, B.R.; Xu, H.L.; Mu, D.B.; Shi, L.L.; Jiang, B.; Gai, L.; Wang, L.; Liu, Q.; Ben, L.B.; Wu, F. Controlled solvothermal synthesis and electrochemical performance of LiCoPO4 submicron single crystals as a cathode material for lithium ion batteries. J. Power Source 2016, 304, 181–188. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, J.; Yu, Z.; Ming, H.; Li, M.; Zhang, S.; Yang, Y. AlF3-modified LiCoPO4 for an advanced cathode towards high energy lithium-ion battery. Ceram. Int. 2018, 44, 1312–1320. [Google Scholar] [CrossRef]
- Okita, N.; Kisu, K.; Iwama, E.; Sakai, Y.; Lim, Y.; Takami, Y.; Sougrati, M.T.; Brousse, T.; Rozier, P.; Simon, P. Stabilizing the Structure of LiCoPO4 Nanocrystals via Addition of Fe3+: Formation of Fe3+ Surface Layer, Creation of Diffusion-Enhancing Vacancies, and Enabling High-Voltage Battery Operation. Chem. Mater. 2018, 30, 6675–6683. [Google Scholar] [CrossRef]
- Örnek, A. An impressive approach to solving the ongoing stability problems of LiCoPO4 cathode: Nickel oxide surface modification with excellent core–shell principle. J. Power Source 2017, 356, 1–11. [Google Scholar] [CrossRef]
- Ikuhara, Y.H.; Gao, X.; Fisher, C.A.; Kuwabara, A.; Moriwake, H.; Kohama, K.; Iba, H.; Ikuhara, Y. Atomic level changes during capacity fade in highly oriented thin films of cathode material LiCoPO4. J. Mater. Chem. A 2017, 5, 9329–9338. [Google Scholar] [CrossRef]
- Wu, J.; Tsai, C.-J. Qualitative modeling of the electrolyte oxidation in long-term cycling of LiCoPO4 for high-voltage lithium-ion batteries. Electrochim. Acta 2021, 368, 137585–137594. [Google Scholar] [CrossRef]
- Aboraia, A.M.; Shapovalov, V.V.; Guda, A.A.; Butova, V.V.; Soldatov, A. One-pot coating of LiCoPO4/C by a UiO-66 metal–organic framework. RSC Adv. 2020, 10, 35206–35213. [Google Scholar] [CrossRef]
- Wu, X.; Rohman, F.; Meledina, M.; Tempel, H.; Schierholz, R.; Kungl, H.; Mayer, J.; Eichel, R.-A. Analysis of the effects of different carbon coating strategies on structure and electrochemical behavior of LiCoPO4 material as a high-voltage cathode electrode for lithium ion batteries. Electrochim. Acta 2018, 279, 108–117. [Google Scholar] [CrossRef]
- Maeyoshi, Y.; Miyamoto, S.; Munakata, H.; Kanamura, K. Effect of conductive carbon additives on electrochemical performance of LiCoPO4. J. Power Source 2018, 376, 18–25. [Google Scholar] [CrossRef]
- Maeyoshi, Y.; Miyamoto, S.; Noda, Y.; Munakata, H.; Kanamura, K. Effect of organic additives on characteristics of carbon-coated LiCoPO4 synthesized by hydrothermal method. J. Power Source 2017, 337, 92–99. [Google Scholar] [CrossRef]
- Muñoz-García, A.B.; Tirri, B.; Capone, I.; Matic, A.; Pavone, M.; Brutti, S. Structural evolution of disordered LiCo1/3 Fe1/3 Mn1/3 PO4 in lithium batteries uncovered. J. Mater. Chem. A 2020, 8, 19641–19653. [Google Scholar] [CrossRef]
- Liu, D.; Kim, C.; Perea, A.; Joël, D.; Zhu, W.; Collin-Martin, S.; Forand, A.; Dontigny, M.; Gagnon, C.; Demers, H. High-Voltage Lithium-Ion Battery Using Substituted LiCoPO4: Electrochemical and Safety Performance of 1.2 Ah Pouch Cell. Materials 2020, 13, 4450. [Google Scholar] [CrossRef]
- Kim, E.J.; Miller, D.N.; Irvine, J.T.; Armstrong, A.R. Enhanced Cycling Performance of Magnesium-Doped Lithium Cobalt Phosphate. ChemElectroChem 2019, 6, 4885–4892. [Google Scholar] [CrossRef]
- Kreder, K.J.; Manthiram, A. Vanadium-Substituted LiCoPO4 Core with a Monolithic LiFePO4 Shell for High-Voltage Lithium-Ion Batteries. ACS Energy Lett. 2017, 2, 64–69. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, W.; Kim, C.; Cho, M.; Guerfi, A.; Delp, S.; Allen, J.; Jow, T.; Zaghib, K. High-energy lithium-ion battery using substituted LiCoPO4: From coin type to 1 Ah cell. J. Power Source 2018, 388, 52–56. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Qiu, J.; Yu, Z.; Ming, H.; Li, M.; Zhang, S.; Yang, Y. Cr-Substituted LiCoPO4 Core with a Conductive Carbon Layer towards High-Voltage Lithium-Ion Batteries. J. Solid State Chem. 2017, 258, 32–41. [Google Scholar] [CrossRef]
- Brutti, S.; Manzi, J.; Meggiolaro, D.; Vitucci, F.M.; Trequattrini, F.; Paolone, A.; Palumbo, O. Interplay between local structure and transport properties in iron-doped LiCoPO4 olivines. J. Mater. Chem. A 2017, 5, 14020–14030. [Google Scholar] [CrossRef]
- Fukutsuka, T.; Nakagawa, T.; Miyazaki, K.; Abe, T. Electrochemical properties of LiCoPO4-thin film electrodes in LiF-based electrolyte solution with anion receptors. J. Power Source 2016, 306, 753–757. [Google Scholar] [CrossRef]
- Freiberg, A.; Metzger, M.; Haering, D.; Bretzke, S.; Puravankara, S.; Nilges, T.; Stinner, C.; Marino, C.; Gasteiger, H.A. Anodic Decomposition of Trimethylboroxine as Additive for High Voltage Li-Ion Batteries. J. Electrochem. Soc. 2014, 161, A2255–A2261. [Google Scholar] [CrossRef]
- Manzi, J.; Brutti, S. Surface chemistry on LiCoPO4 electrodes in lithium cells: SEI formation and self-discharge. Electrochim. Acta 2016, 222, 1839–1846. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Yang, X.; Liu, L.; Chen, L.; Wei, J. Improved electrochemical performance of 5 V LiCoPO4 cathode materials via yttrium doping. Solid State Ion. 2014, 255, 84–88. [Google Scholar] [CrossRef]
- Allen, J.L.; Thompson, T.; Sakamoto, J.; Becker, C.R.; Jow, T.R.; Wolfenstine, J. Transport properties of LiCoPO4 and Fe-substituted LiCoPO4. J. Power Source 2014, 254, 204–208. [Google Scholar] [CrossRef]
- Serment, B.; Corucho, L.; Demourgues, A.; Hadziioannou, G.; Brochon, C.; Cloutet, E.; Gaudon, M. Tailoring the Chemical Composition of LiMPO4 (M = Mg, Co, Ni) Orthophosphates to Design New Inorganic Pigments from Magenta to Yellow Hue. Inorg. Chem. 2019, 58, 7499–7510. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.G.; Frith, J.T.; Hector, A.L.; Lodge, A.W.; Owen, J.R.; Nicklin, C.; Rawle, J. In situ phase behaviour of a high capacity LiCoPO4 electrode during constant or pulsed charge of a lithium cell. Chem. Commun. 2016, 52, 14169–14172. [Google Scholar] [CrossRef]
- Allen, J.L.; Allen, J.L.; Thompson, T.; Delp, S.A.; Wolfenstine, J.; Jow, T.R. Cr and Si Substituted-LiCo0.9Fe0.1PO4: Structure, full and half Li-ion cell performance. J. Power Source 2016, 327, 229–234. [Google Scholar]
- Zhang, M.; Garcia-Araez, N.; Hector, A.L.; Owen, J.R.; Palgrave, R.G.; Palmer, M.G.; Soulé, S. Solvothermal water-diethylene glycol synthesis of LiCoPO4 and effects of surface treatments on lithium battery performance. RSC Adv. 2019, 9, 740–752. [Google Scholar] [CrossRef] [PubMed]
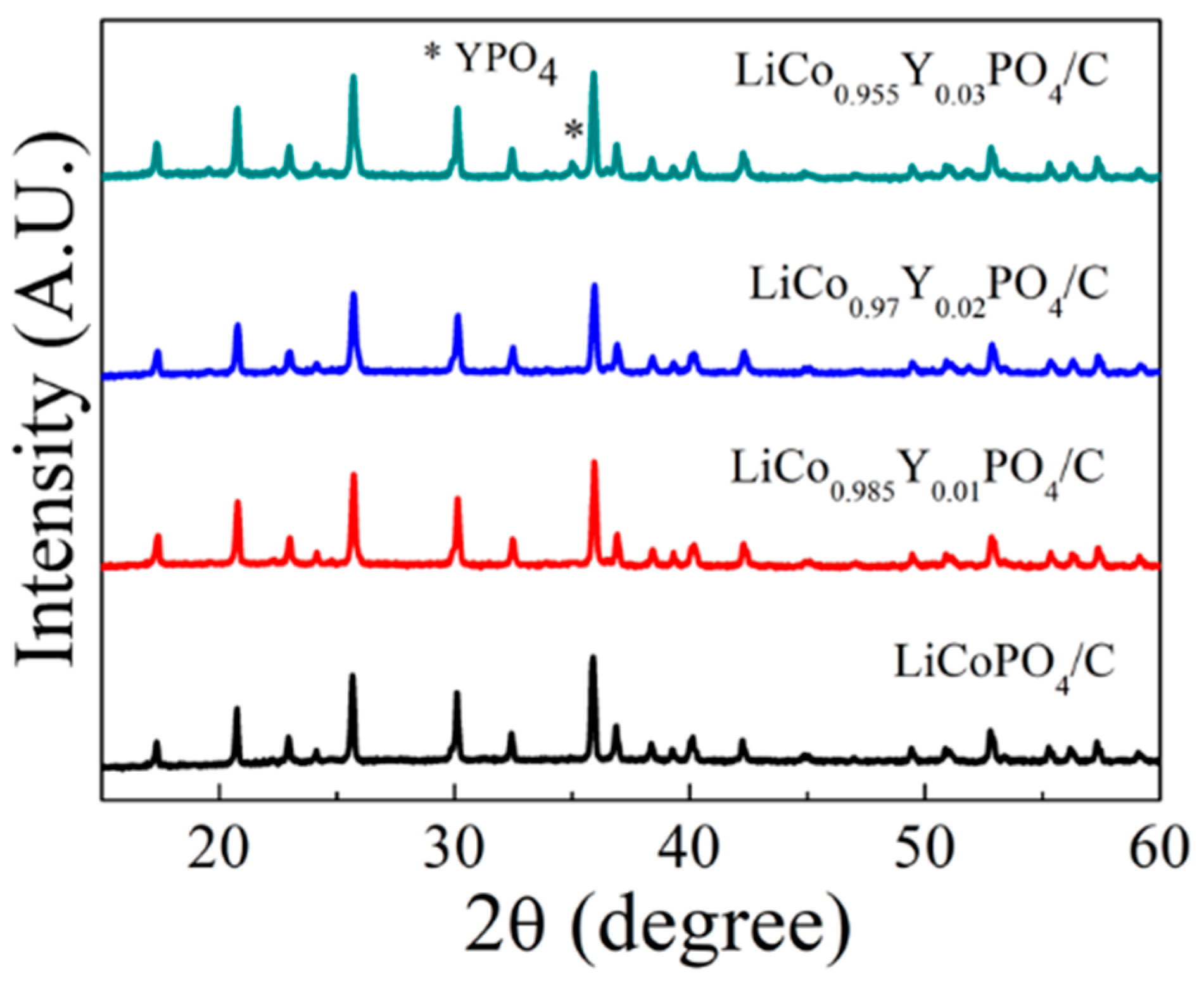

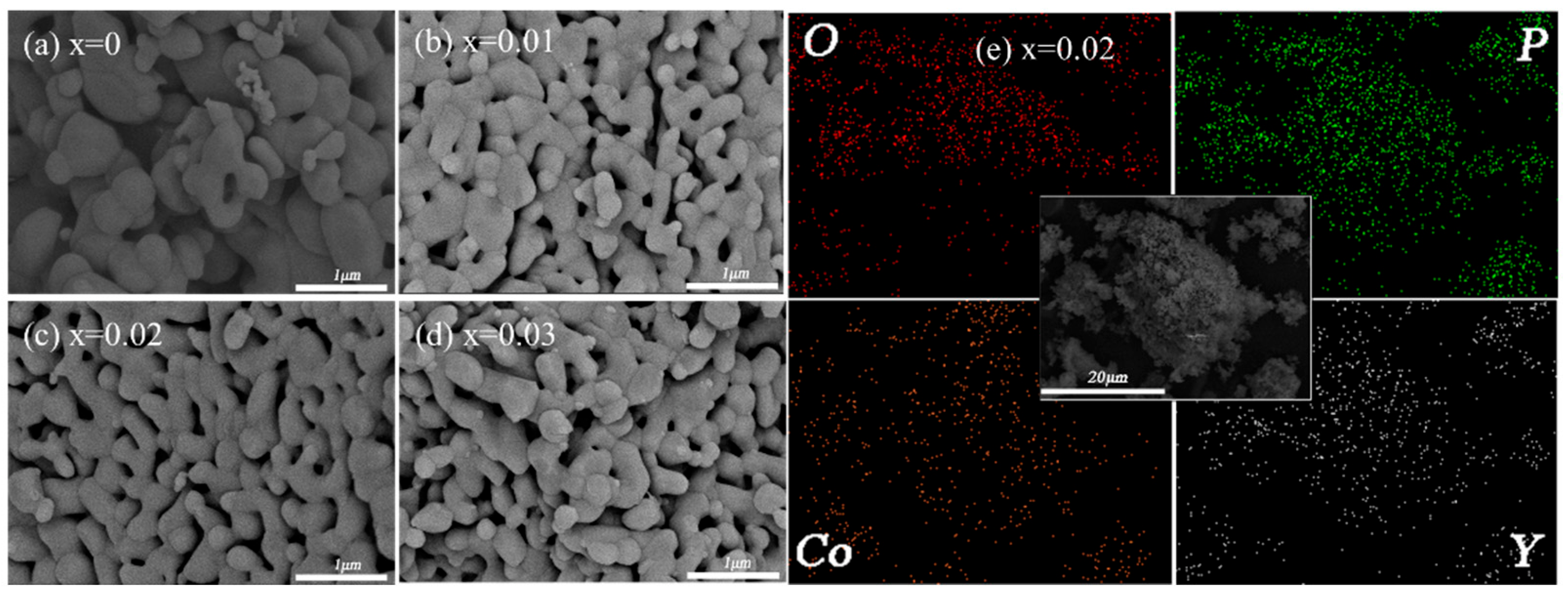
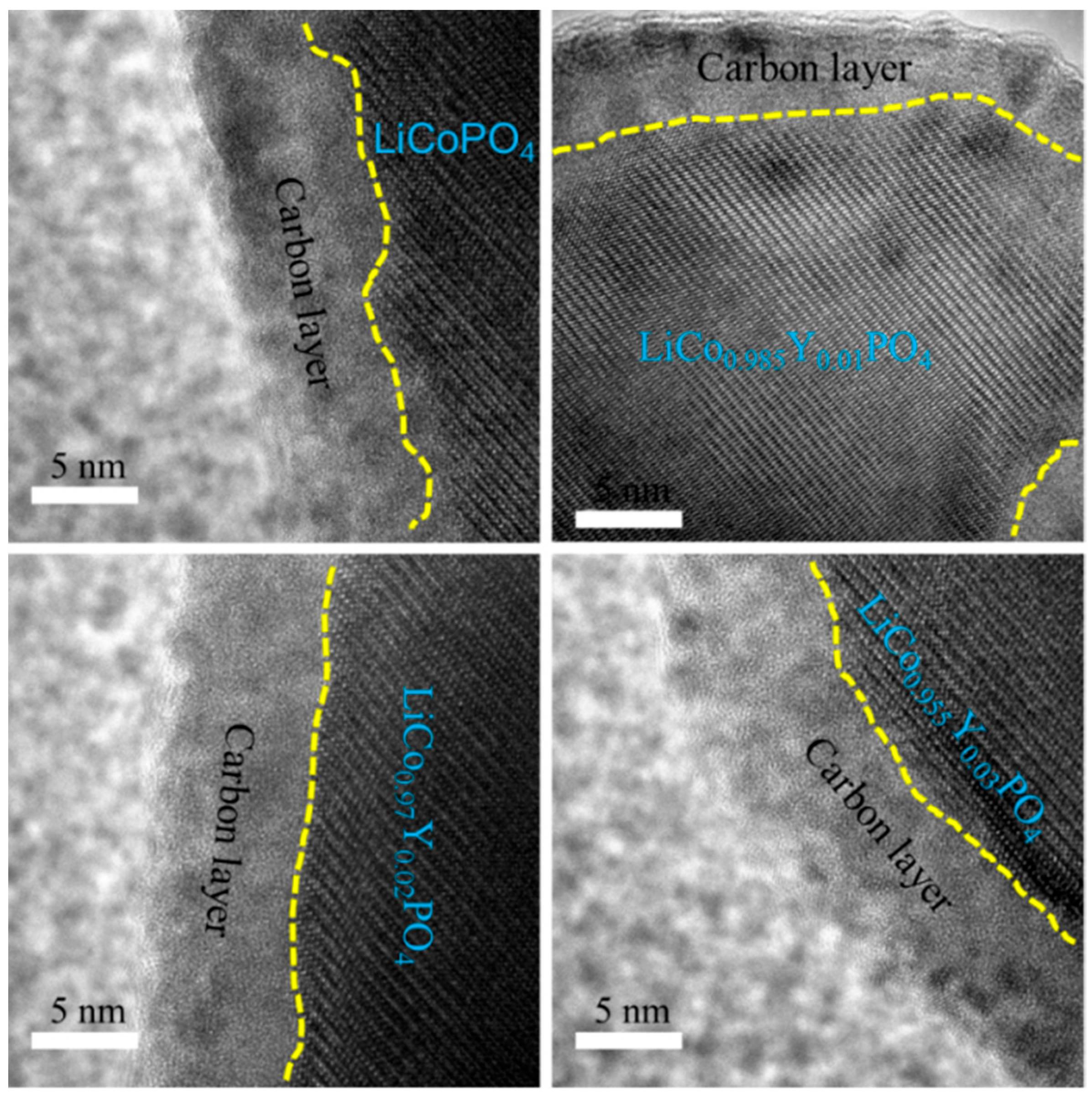
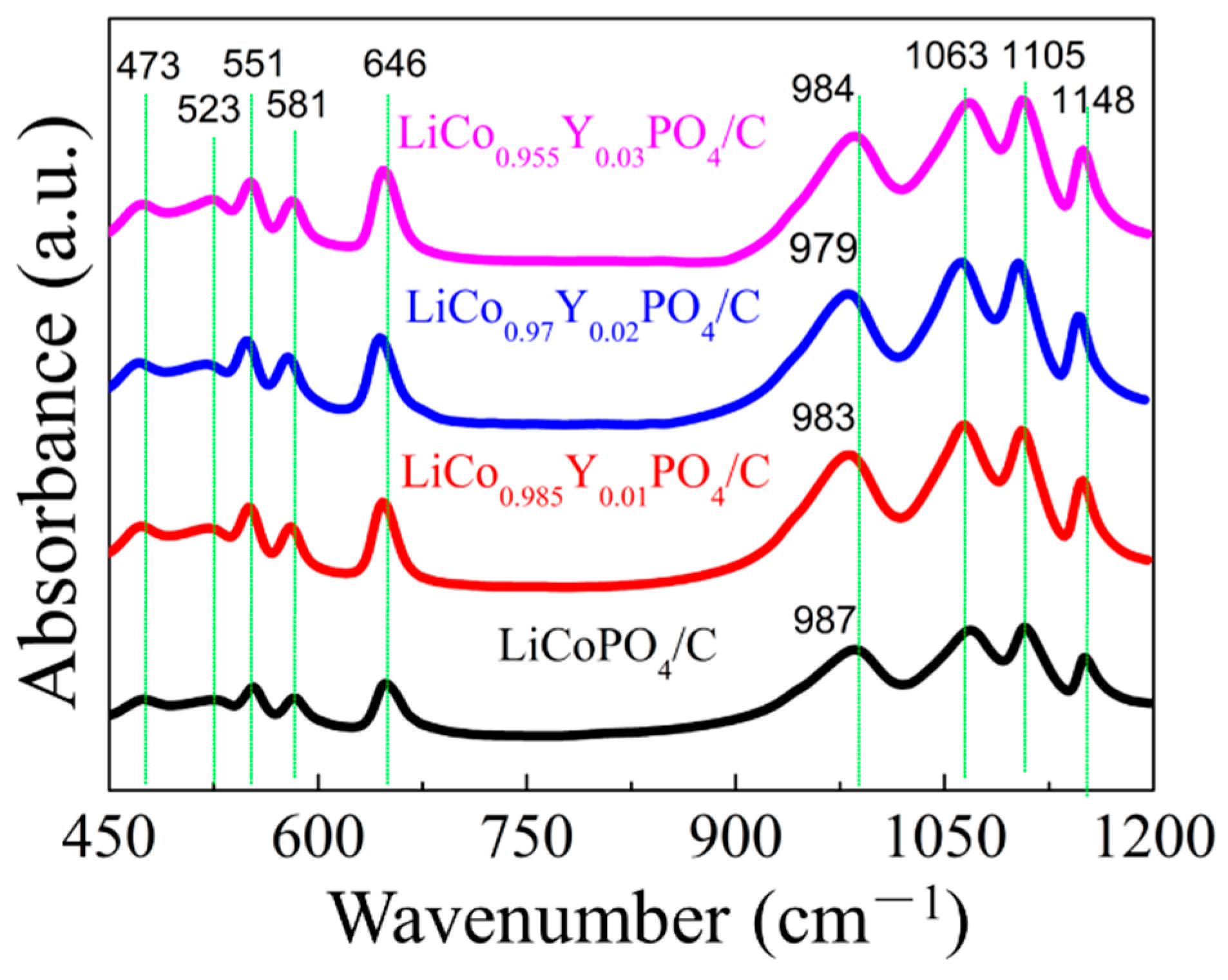
| Samples | a (Å) | b (Å) | c (Å) | V (Å3) | Rwp | Rp | GOF |
|---|---|---|---|---|---|---|---|
| LiCoPO4 (89-6192) | 10.2021 | 5.9227 | 4.7003 | 284.01 | |||
| LiCoPO4@C | 10.2007 | 5.9220 | 4.6987 | 283.84 | 3.85 | 2.71 | 1.73 |
| LiCo0.985Y0.01PO4@C | 10.2027 | 5.9245 | 4.6999 | 284.09 | 3.96 | 2.74 | 1.78 |
| LiCo0.97Y0.02PO4@C | 10.2058 | 5.9275 | 4.6995 | 284.30 | 4.02 | 2.82 | 1.84 |
| LiCo0.955Y0.03PO4@C | 10.2077 | 5.9288 | 4.6996 | 284.42 | 4.07 | 2.92 | 1.89 |
| Samples | Oxidation Potential (V) | Reduction Potential (V) | Polarization Potential (V) | |
|---|---|---|---|---|
| LiCoPO4@C | 4.829 | 4.924 | 4.695 | 0.229 |
| LiCo0.985Y0.01PO4/C | 4.82 | 4.903 | 4.704 | 0.199 |
| LiCo0.97Y0.02PO4/C | 4.796 | 4.892 | 4.71 | 0.182 |
| LiCo0.955Y0.03PO4/C | 4.827 | 4.908 | 4.712 | 0.196 |
| Samples | Rate | Initial Discharge Capacity (mAh g−1) | Capacity Retention (%) | Cycles | Method |
|---|---|---|---|---|---|
| Our work | 0.1C | 148 | 75 | 80 | Y-Substituted and carbon coating |
| Ref. [33] | 0.1C | 135 | 52 | 30 | Carbon coating |
| Ref. [49] | 0.1C | 147 | 69 | 40 | Carbon coating |
| Ref. [31] | 0.1C | 120 | 75 | 20 | Carbon coating |
| Ref. [32] | 0.1C | 124 | 56 | 100 | Carbon coating |
| Ref. [16] | 0.1C | 97 | 85 | 20 | V-Substituted |
| Ref. [37] | 0.1C | 145 | 52 | 20 | V-Substituted |
| Ref. [44] | 0.1C | 153 | 21 | 30 | Y-Substituted |
| Ref. [15] | 0.1C | 124 | 80 | 20 | Fe-Substituted |
| Ref. [36] | 0.1C | 88 | 22 | 20 | Mg-Substituted |
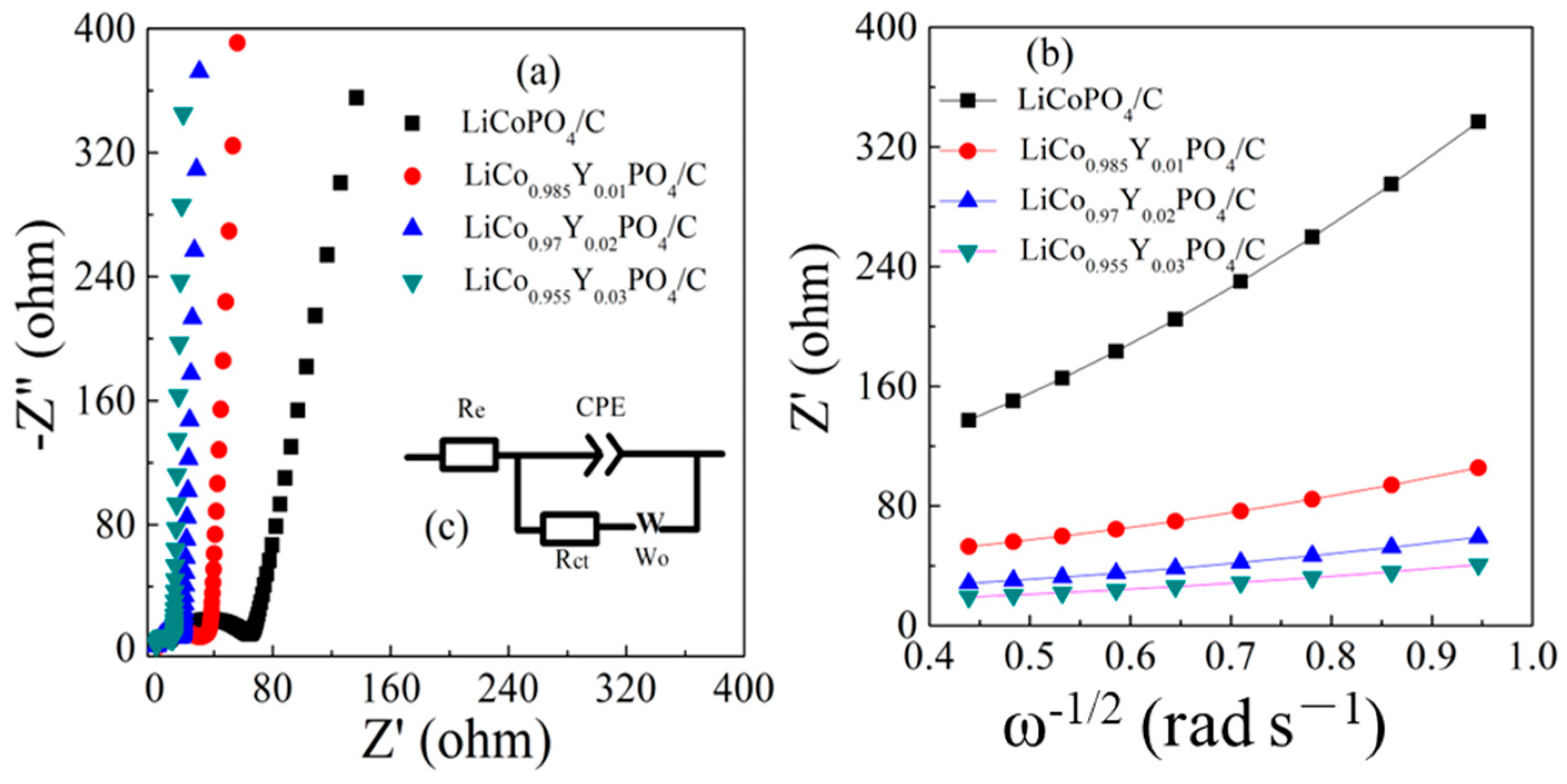
| Samples | Re (Ω) | Rct (Ω) | σ | D Li+ (cm2 s−1) |
|---|---|---|---|---|
| LiCoPO4@C | 1.6 | 59.15 | 391 | 7.11 × 10−16 |
| LiCo0.985Y0.01PO4/C | 1.33 | 25.41 | 103 | 1.02 × 10−14 |
| LiCo0.97Y0.02PO4/C | 1.41 | 23.66 | 60 | 3.02 × 10−14 |
| LiCo0.955Y0.03PO4/C | 1.53 | 21.03 | 42 | 6.16 × 10−14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Qiu, J.; Li, M.; Zhu, X.; Wen, Y.; Li, B. One-Step Synthesis of LiCo1-1.5xYxPO4@C Cathode Material for High-Energy Lithium-ion Batteries. Materials 2022, 15, 7325. https://doi.org/10.3390/ma15207325
Wang Y, Qiu J, Li M, Zhu X, Wen Y, Li B. One-Step Synthesis of LiCo1-1.5xYxPO4@C Cathode Material for High-Energy Lithium-ion Batteries. Materials. 2022; 15(20):7325. https://doi.org/10.3390/ma15207325
Chicago/Turabian StyleWang, Yue, Jingyi Qiu, Meng Li, Xiayu Zhu, Yuehua Wen, and Bin Li. 2022. "One-Step Synthesis of LiCo1-1.5xYxPO4@C Cathode Material for High-Energy Lithium-ion Batteries" Materials 15, no. 20: 7325. https://doi.org/10.3390/ma15207325
APA StyleWang, Y., Qiu, J., Li, M., Zhu, X., Wen, Y., & Li, B. (2022). One-Step Synthesis of LiCo1-1.5xYxPO4@C Cathode Material for High-Energy Lithium-ion Batteries. Materials, 15(20), 7325. https://doi.org/10.3390/ma15207325







