Characteristics of Mortar Containing Oyster Shell as Fine Aggregate
Abstract
1. Introduction
2. Experimental Plan
2.1. Experimental Scope and Method
2.2. Materials
2.2.1. Oyster Shells
2.2.2. Ordinary Portland Cement
2.3. Mix Design
3. Experiment Results and Analysis
3.1. OSA
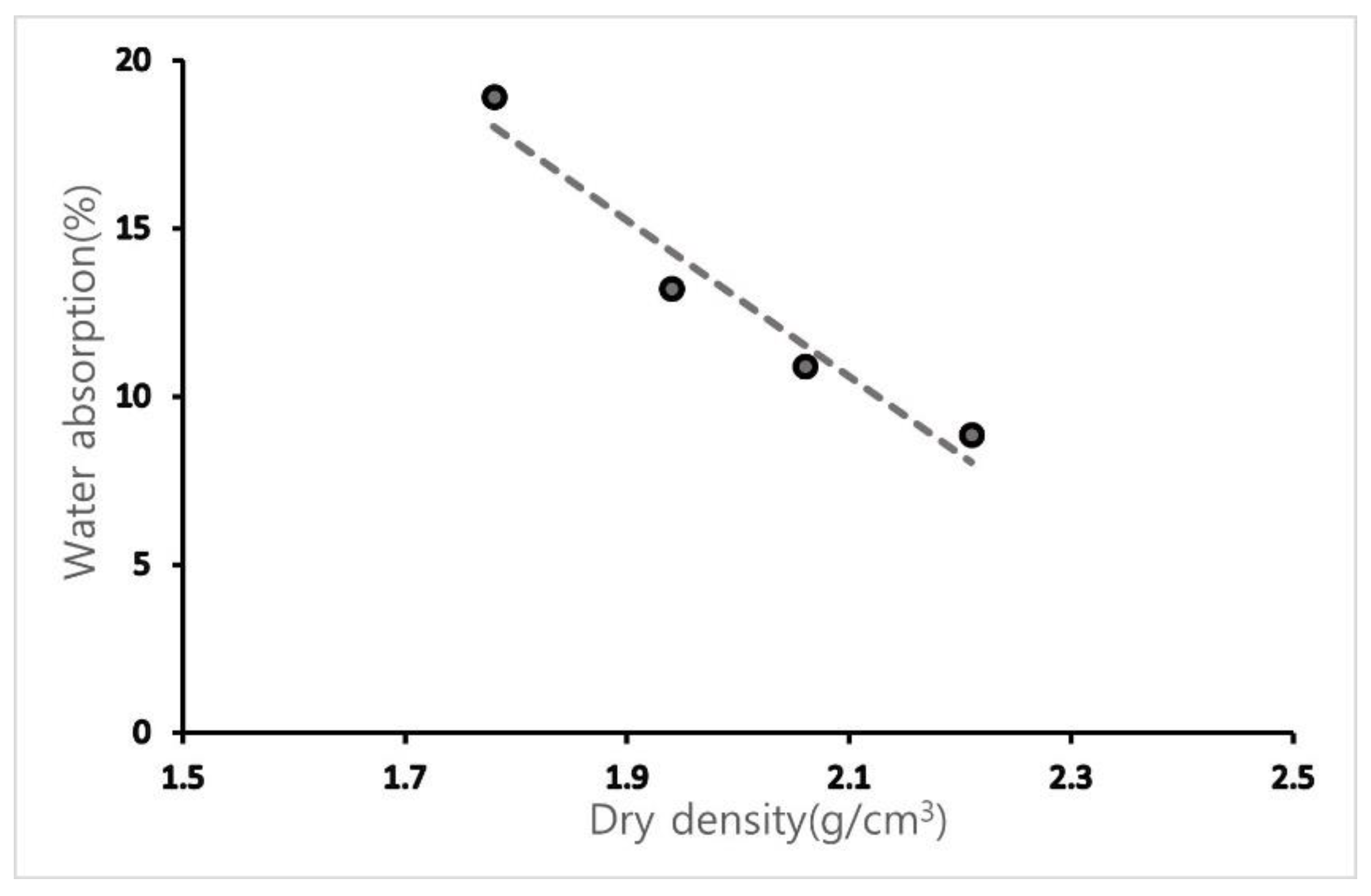
3.1.1. Density and Water Absorption
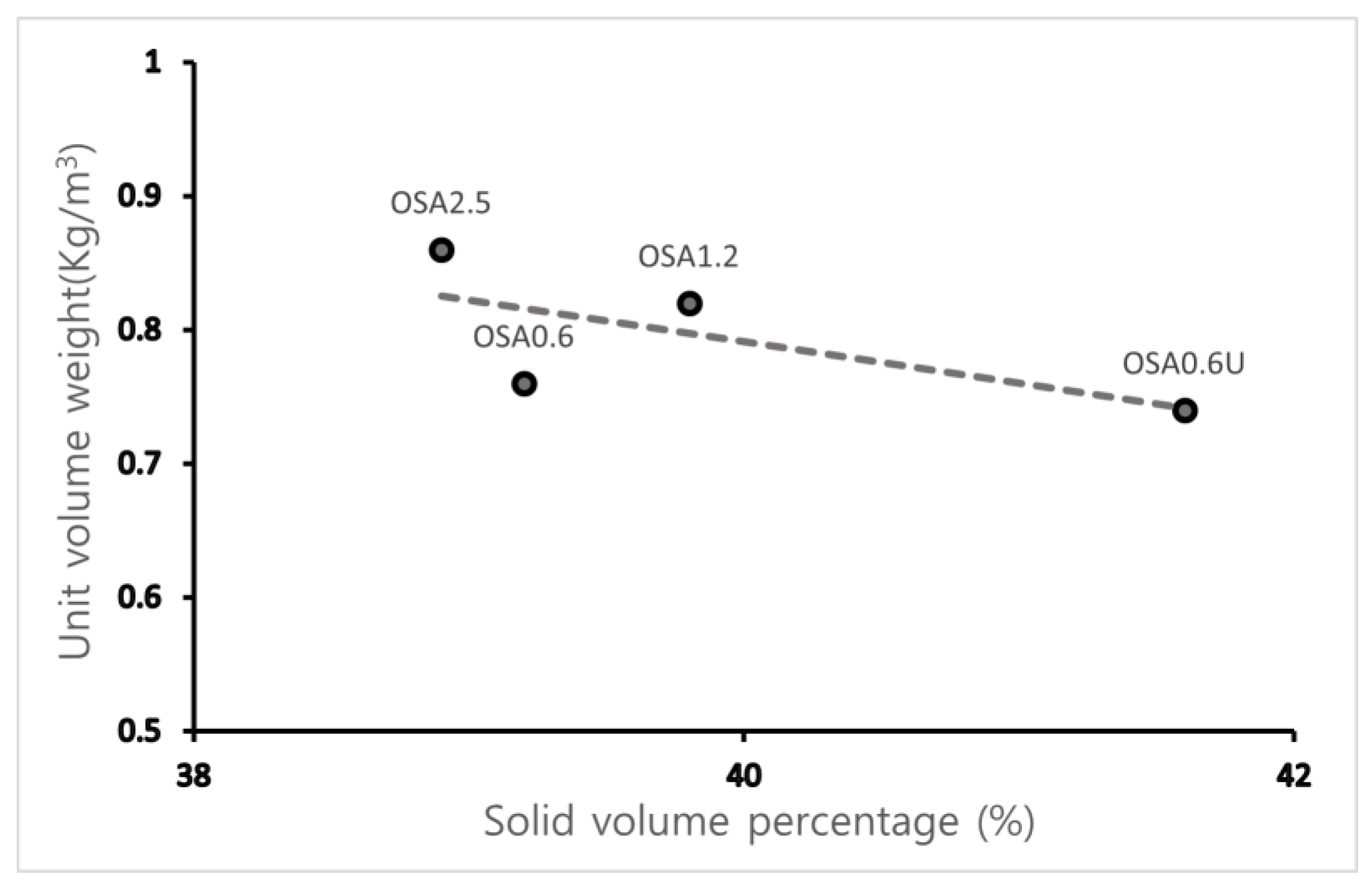
3.1.2. Unit Volume Weight and Solid Volume Percentage
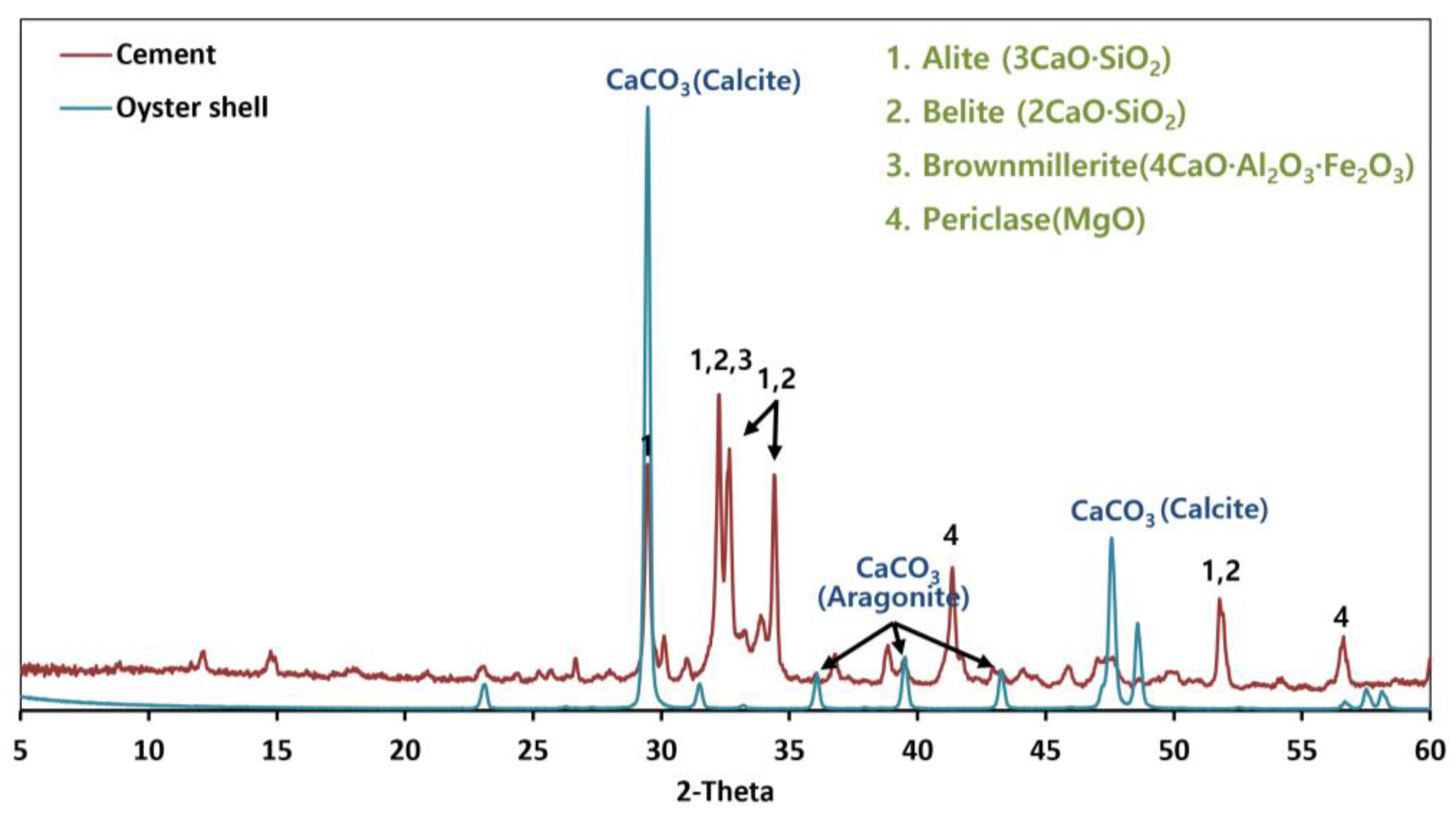
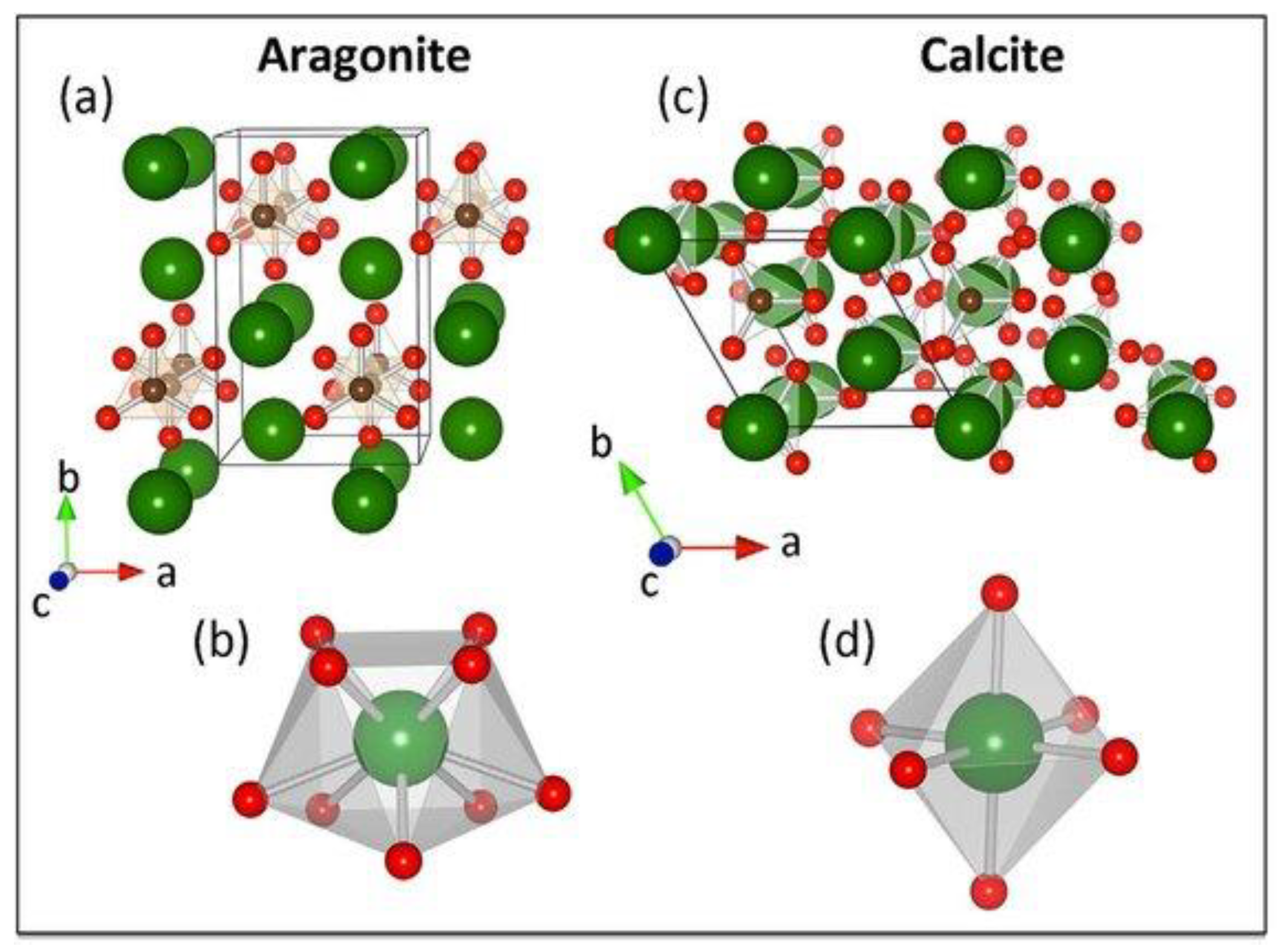
3.1.3. Crystal Structure Analysis
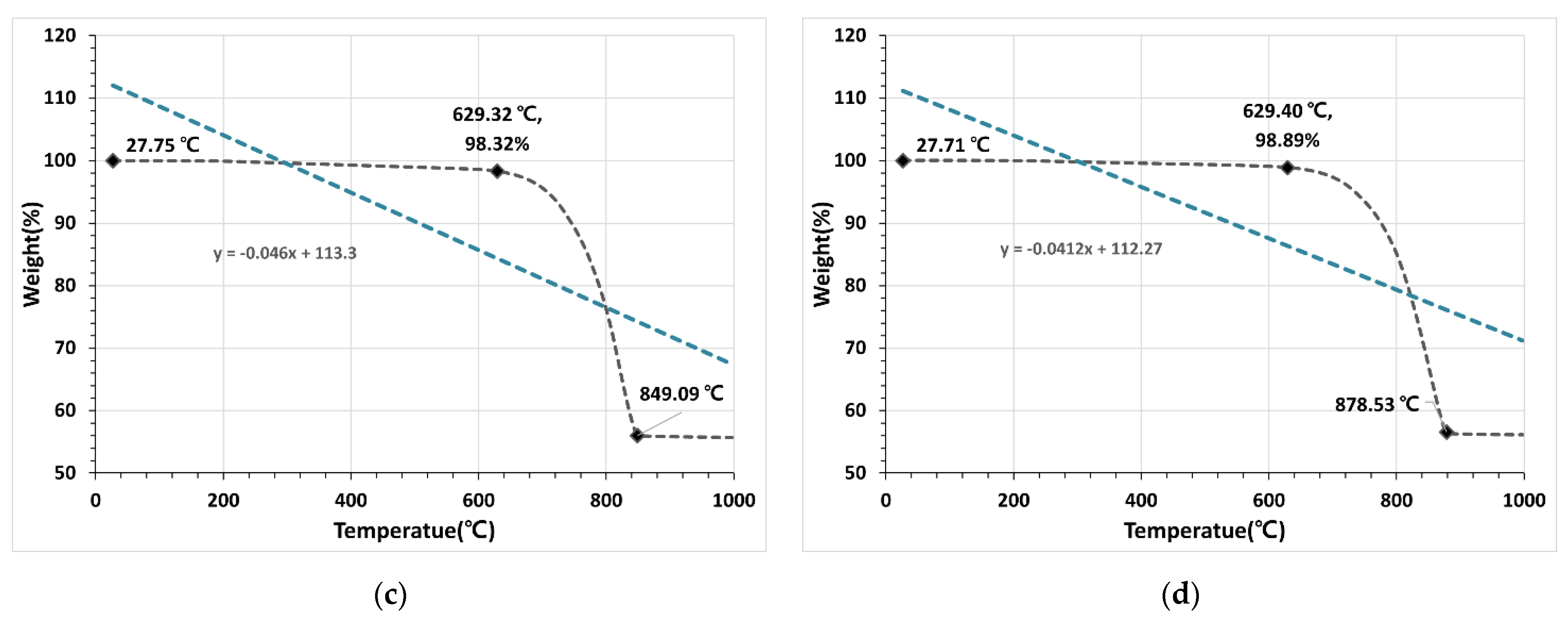
3.1.4. TGA
3.2. Mortar
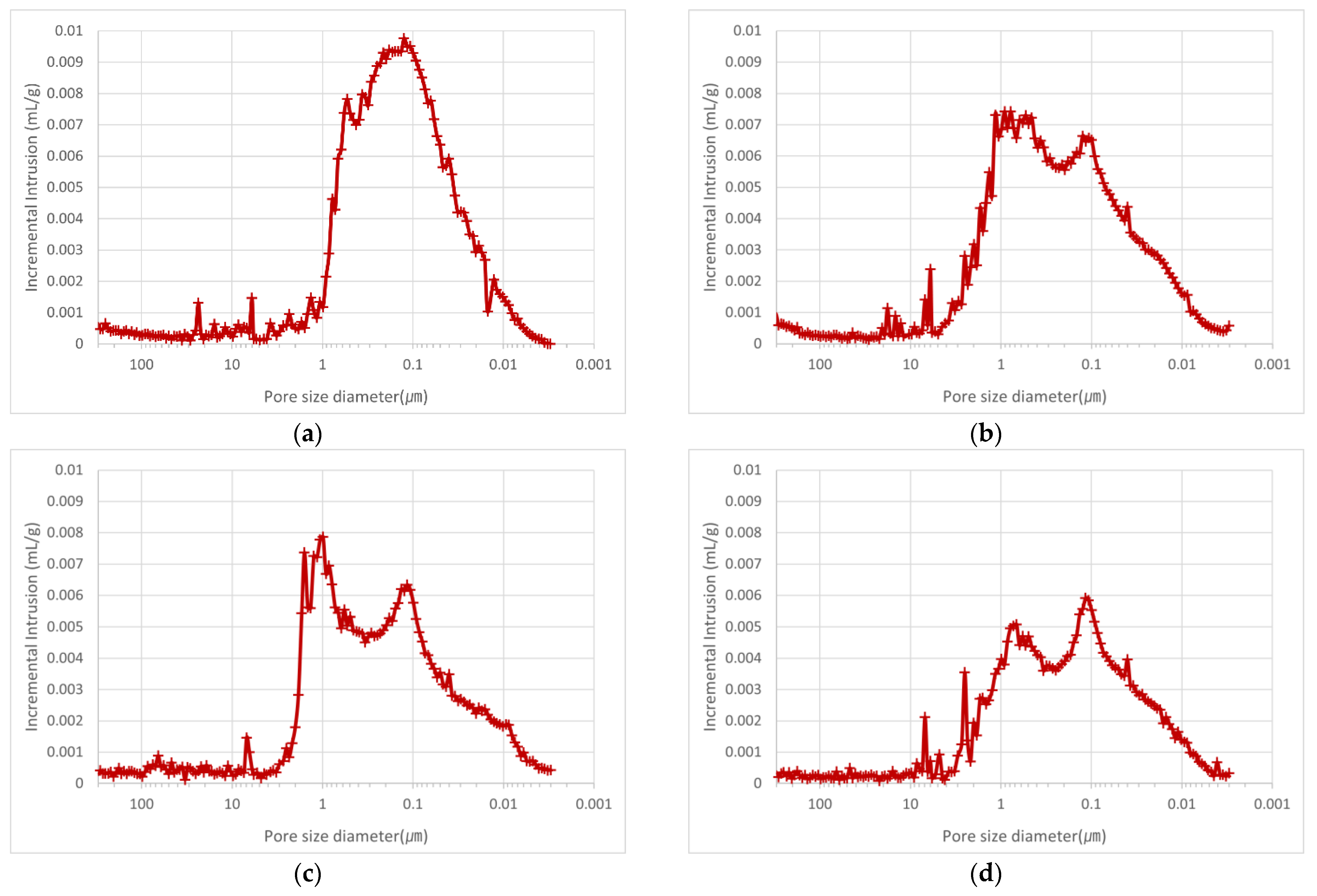
3.2.1. Porosity
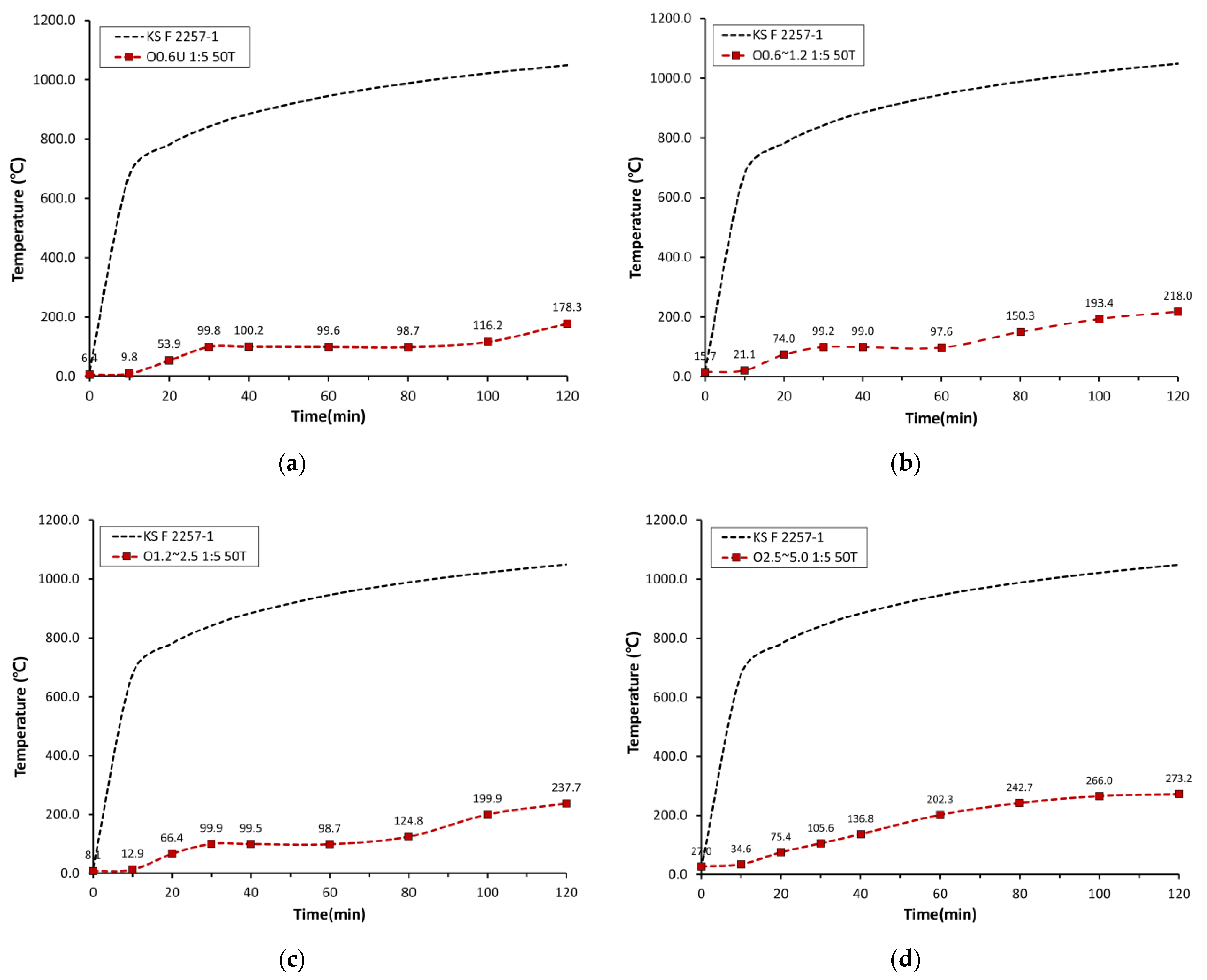
3.2.2. Back-Side Temperatures Obtained in the Heating Test
4. Conclusions
- (1)
- The TGA results showed that the decarboxylation reaction occurred at 630 °C for OSA0.6U, which had the smallest particle size, and at 688 °C for OSA2.5, which had the largest particle size, indicating that the temperature at which decarboxylation reaction started depended on the OSA particle size. This effect was attributed to the increase in endothermic reaction rate with decreasing particle size because of the increase in specific surface area, i.e., the heat-receiving area.
- (2)
- The TGA results combined with the heating test results suggested that CO2 would be generated at different temperatures in boards containing OSAs with different particle sizes because of the differences in the endothermic reaction temperature. Therefore, the differences in the back-side temperatures of the boards were attributed to the differences in the insulation effect caused by the thermal decomposition of calcium carbonate and the subsequent generation of micropores and the fire-extinguishing effect caused by the generation of CO2.
- (3)
- The porosity area and porosity of the OSA-containing mortar increased with decreasing particle size. This was attributed to the effect of the micropores in the OSA and the effect of greater water usage to satisfy the flow standard because of the increase in water absorption with decreasing particle size. The experimental results showed that the porosity and porosity area were proportional and that the back-side temperature decreased with increasing porosity and porosity area.
- (4)
- As with the conclusion of the existing literature, high-performance concrete for strength is not always a good decision. Durability and strength of concrete require a low porosity. The low porosity supports all characteristics of concrete with the exception of fire resistance. The lesser the porosity, the higher the risk of spalling, having a final impact on the safety of the infrastructure users [28]. Therefore, the relationship between concrete strength and fire resistance performance is reviewed, and an optimal mixture application study is required as necessary.
- (5)
- In a follow-up study, the relationship between the insulation effect and particle size should be further examined by comparing the pore distributions before and after heating. Based on the experimental results, it was concluded that the use of OSA with small particle size is optimal for increasing the fire resistance of mortar boards.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- An, J.H.; Yeo, I.H.; Park, J.S. A Experimental Study on Fire Resistance Performance of Board Encased Steel Frame Used in Modular House. J. Korean Soc. Hazard Mitig. 2015, 15, 224–226. [Google Scholar] [CrossRef]
- Won, J.P.; Choi, S.W.; Park, C.G.; Park, H.K. Evaluation of Wet-Mixed High Strength Sprayed Polymer Mortar for Fire Resistance. J. Korea Concr. Inst. 2006, 18, 559–560. [Google Scholar] [CrossRef]
- Youm, K.S.; Jeon, H.G. Fire Resistance Performance of High Strength Concrete Columns with Fireproof Gypsum Board. J. Korea Concr. Inst. 2010, 22, 229–230. [Google Scholar] [CrossRef]
- Choi, S.W.; Kang, T.H.; Lee, C.H.; Kim, H.S.; Ahn, B.C.; Chang, S.H. Fire resistance assessment of segment lining with PP fiber amount. J. Korean Tunn. Undergr. Space Assoc. 2021, 23, 304–305. [Google Scholar] [CrossRef]
- Jung, U.I.; Kim, B.J. Remaining Strength of Fireproof Mortar using the Oyster Shell as a Fine Aggregate. J. Korea Inst. Build. Constr. 2017, 17, 411–413. [Google Scholar] [CrossRef][Green Version]
- Yoon, G.L.; Kim, B.T.; Kim, B.O.; Han, S.H. Chemical-mechanical characteristics of crushed oyster-shell. Waste Manag. 2003, 23, 825–834. [Google Scholar] [CrossRef]
- Falade, F. An Investigation of Periwinkle Shells as Coarse Aggregate in Concrete. Build. Environ. 1995, 30, 573–577. [Google Scholar] [CrossRef]
- Okafor, F.O. An Investigation on the Use of Superplasticizer in Palm Kernel Shells Aggregate Concrete. Cem. Concr. Res. 1991, 21, 551–557. [Google Scholar] [CrossRef]
- Okpala, D.C. Palm Kernel Shells as a Lightweight Aggregate in Concrete. Build. Environ. 1990, 25, 291–296. [Google Scholar] [CrossRef]
- Yang, E.I.; Kim, M.Y.; Park, H.G.; Yi, S.T. Effect of partial replacement of sand with dry oyster shell on the long-term performance of concrete. Constr. Build. Mater. 2010, 24, 758–765. [Google Scholar] [CrossRef]
- Eo, S.H.; Yi, S.T. Effect of oyster shell as an aggregate replacement on the characteristics of concrete. Mag. Concr. Res. 2015, 67, 833–842. [Google Scholar] [CrossRef]
- Jung, U.I. An Experimental Study on the Strength Development of Concrete Using of the Oyster Shells. Ph.D. Thesis, Kongju National University, Gongju, Korea, 23 February 2018. [Google Scholar]
- KS F 2504; Testing Method for Density and Absorption of Fine Aggregate. Korean Industrial Standards: Eumseong, Korea, 2020.
- KS F 2505; Standard Test Method for Bulk Density and Solid Contents in Aggregates. Korean Industrial Standards: Eumseong, Korea, 2017.
- KS L ISO679; Methods of Testing Cements—Determination of Strength. Korean Industrial Standards: Eumseong, Korea, 2021.
- KS F 2257-1; Methods of Fire Resistance Test for Elements of Building Construction-general Requirements. Korean Industrial Standards: Eumseong, Korea, 2019.
- Sun, J.; Huang, Y. Modeling the Simultaneous Effects of Particle Size and Porosity in Simulating Geo-Materials. Materials 2022, 15, 1576. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.U.; Lee, N.J.; Ahn, J.W. Sustainable Management of Oyster Shell By-Products and Recent Research Techniques. J. Energy Eng. 2018, 27, 3–10. [Google Scholar] [CrossRef]
- KS L 5201; Portland Cement. Korean Industrial Standards: Eumseong, Korea, 2021.
- Kim, J.W.; Lee, B.H.; Kim, J.C.; Kim, H.N. Particle Size Analysis of Nano-sized Talc Prepared by Mechanical Milling Using High-energy Ball Mill. J. Mineral. Soc. Korea 2018, 31, 48–54. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, M.H. Formation and Control of Calcium Carbonate Films having Aragonite Crystal Structure by Electro-Chemical Process. J. Korean Inst. Surf. Eng. 2018, 51, 325–331. [Google Scholar] [CrossRef]
- Analia, S.; Dorrit, E.J.; Pieter, G.; Janine, C.S.; Jochen, G. Element substitution by living organisms: The case of manganese in mollusc shell aragonite. Sci. Rep. 2016, 6, 22514. [Google Scholar] [CrossRef]
- Chen, J.; Xiang, L. Controllable synthesis of calcium carbonate polymorphs at different temperatures. Powder Tech. 2009, 189, 64–69. [Google Scholar] [CrossRef]
- Tali, M.; Yitzhak, M. Controlled crystallization of calcium carbonate superstructures in macroemulsions. J. Cryst. Growth 2008, 310, 3552–3556. [Google Scholar] [CrossRef]
- Lee, H.S.; Kwon, S.J. Modeling on Compressive Strength in High Performance Concrete Using Porosity. J. Korea Inst. Struct. Maint. Insp. 2012, 16, 124–133. [Google Scholar] [CrossRef][Green Version]
- Kwon, S.J.; Song, H.W.; Park, S.S. A Study on Change in Cement Mortar Characteristics under Carbonation Based on Tests for Hydration and Porosity. J. Korea Concr. Inst. 2007, 19, 613–621. [Google Scholar] [CrossRef][Green Version]
- Kwon, S.J.; Lee, H.S.; Park, S.G. Effect of Additional Water on Durability and Pore Size Distribution in Cement Mortar. J. Korea Inst. Struct. Maint. Insp. 2012, 16, 75–83. [Google Scholar] [CrossRef]
- Korten, A.; Wetzig, V. Spalling of concrete—Influence of porosity and specimen size and its critical factors regarding safety. Matec Web Conf. 2013, 6, 01010. [Google Scholar] [CrossRef]


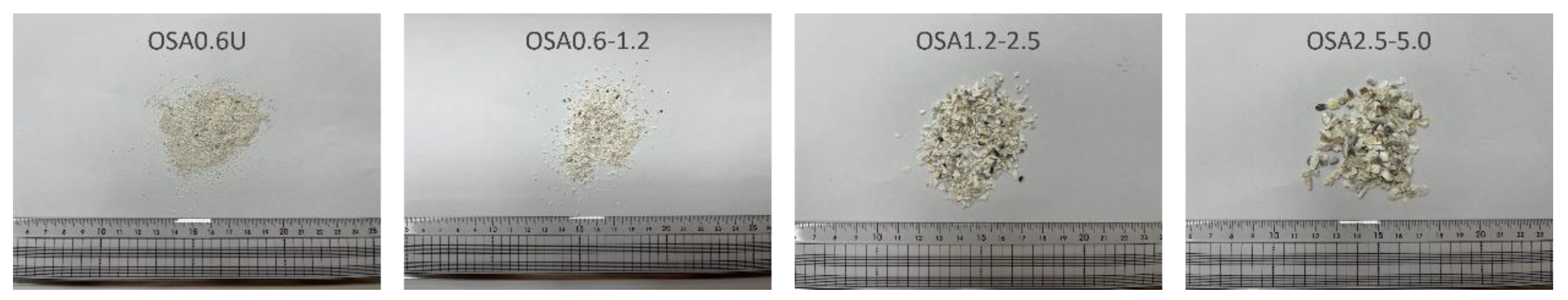


| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | TiO2 | MnO | P2O5 | Ig-loss * | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oyster shell | 0.45 | 0.12 | 0.06 | 53.66 | 0.26 | 0.06 | 0.55 | <0.01 | 0.01 | 0.16 | 44.56 |
| ID | Cement (g) | W/C | Aggregate (g) | |||
|---|---|---|---|---|---|---|
| OSA0.6U | OSA0.6 | OSA1.2 | OSA2.5 | |||
| O0.6U-M | 324.7 | 0.5 | 1090.48 | |||
| O0.6~1.2-M | 1188.50 | |||||
| O1.2~2.5-M | 1262.01 | |||||
| O2.5~5.0-M | 1353.91 | |||||
| ID | Dry Density (g/cm3) | Water Absorption (%) |
|---|---|---|
| OSA0.6U | 1.78 | 18.93 |
| OSA0.6 | 1.94 | 13.22 |
| OSA1.2 | 2.06 | 10.89 |
| OSA2.5 | 2.21 | 8.87 |
| ID | Unit Volume Weight (kg/m3) | Solid Volume Percentage (%) |
|---|---|---|
| OSA0.6U | 0.74 | 41.6 |
| OSA0.6 | 0.76 | 39.2 |
| OSA1.2 | 0.82 | 39.8 |
| OSA2.5 | 0.86 | 38.9 |
| Measurement Target | Porosity (%) | Porosity Area (m2/g) |
|---|---|---|
| O0.6U-M | 51.4 | 26.3 |
| O0.6~1.2-M | 50.5 | 24.4 |
| O1.2~2.5-M | 47.1 | 23.7 |
| O2.5~5.0-M | 43.4 | 20.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, U.-I.; Kim, B.-J. Characteristics of Mortar Containing Oyster Shell as Fine Aggregate. Materials 2022, 15, 7301. https://doi.org/10.3390/ma15207301
Jung U-I, Kim B-J. Characteristics of Mortar Containing Oyster Shell as Fine Aggregate. Materials. 2022; 15(20):7301. https://doi.org/10.3390/ma15207301
Chicago/Turabian StyleJung, Ui-In, and Bong-Joo Kim. 2022. "Characteristics of Mortar Containing Oyster Shell as Fine Aggregate" Materials 15, no. 20: 7301. https://doi.org/10.3390/ma15207301
APA StyleJung, U.-I., & Kim, B.-J. (2022). Characteristics of Mortar Containing Oyster Shell as Fine Aggregate. Materials, 15(20), 7301. https://doi.org/10.3390/ma15207301







