Conductive Membranes Based on Cotton Fabric Coated with Polymers for Electrode Applications
Abstract
1. Introduction
2. Materials and Methods
- -
- the composition of samples 1–14 is based on the polymeric matrix (PVDF (samples 2–5), PEG (samples 6–10 and 14), PVP (samples 5–12 and 13–14), and PVA (sample 6)) and microparticles (GO (samples 2 and 9), Ni (samples 3 and 11), Cu1 (samples 4, 8, 10, 12 and 14) and Cu2 (samples 5–7 and 13));
- -
- the results of the physico-mechanical (Mass per unit area (SR EN 12127:2003), thickness (SR EN ISO 5084:2001), air permeability (SR EN ISO 9237:1999), water vapor permeability (STAS 9005:1979)) and electrical resistance (Rs) evaluations.
- -
- PVDF and Cu1 microparticles (T3), the surface resistance decreases by 10%;
- -
- PVDF and Cu2 microparticles (T4), the surface resistance has the same value of 109 Ω;
- -
- PVDF and Ni microparticles (T2), the surface resistance increases by 200%;
- -
- PVDF and GO microparticles (T1), the surface resistance decreases by 25%.
- -
- distilled water;
- -
- l-histidine monohydrochloride monohydrate;
- -
- sodium chloride;
- -
- disodium hydrogen orthophosphate dodecahydrate;
- -
- disodium hydrogen orthophosphate dihydrate.
- -
- distilled water;
- -
- l-histidine monohydrochloride monohydrate-
- -
- sodium chloride;
- -
- sodium dihydrogen orthophosphate dihydrate (ISO 105-E04:2013).
3. Results
3.1. Characterization of the Samples Treated Using Conductive Membranes
3.1.1. Surface Morphology Using SEM
3.1.2. Surface Topography Using Optical Microscopy
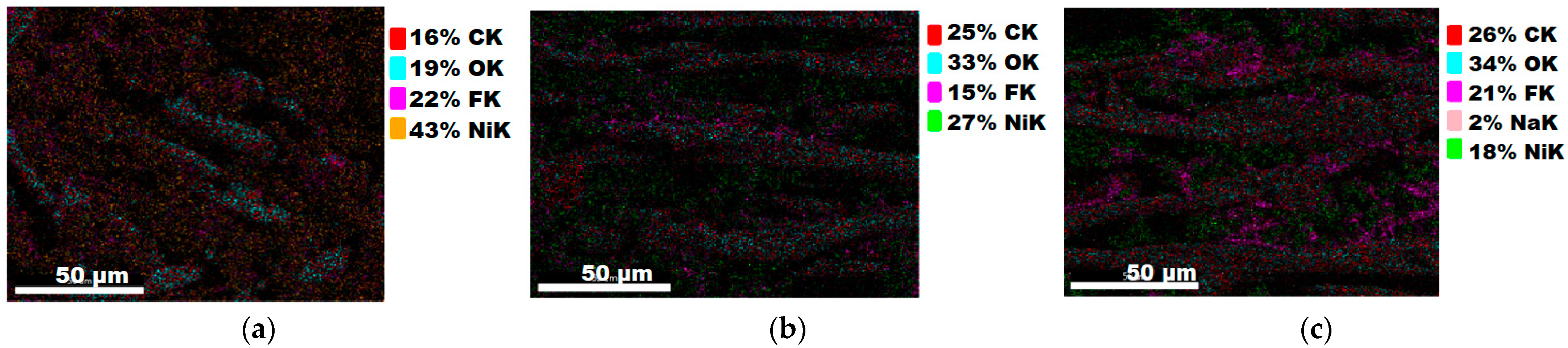
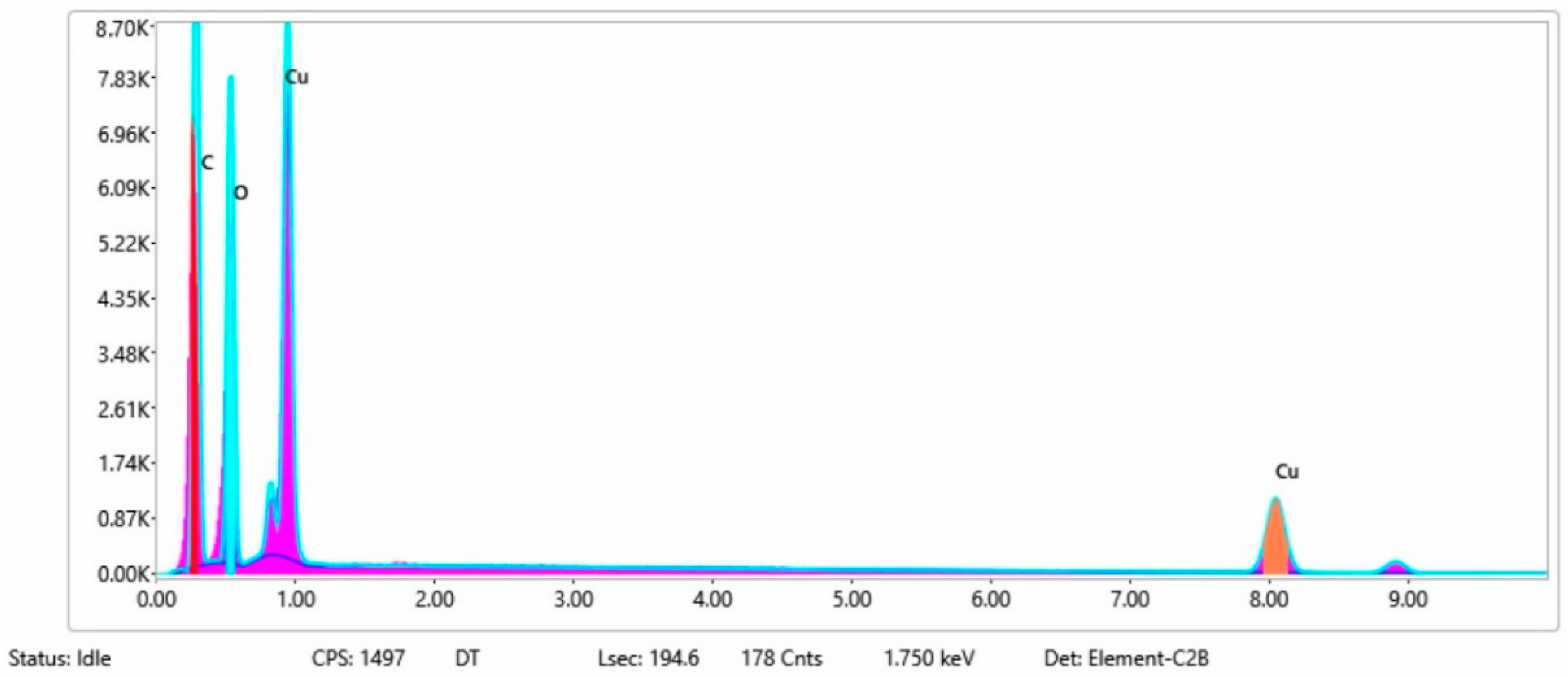
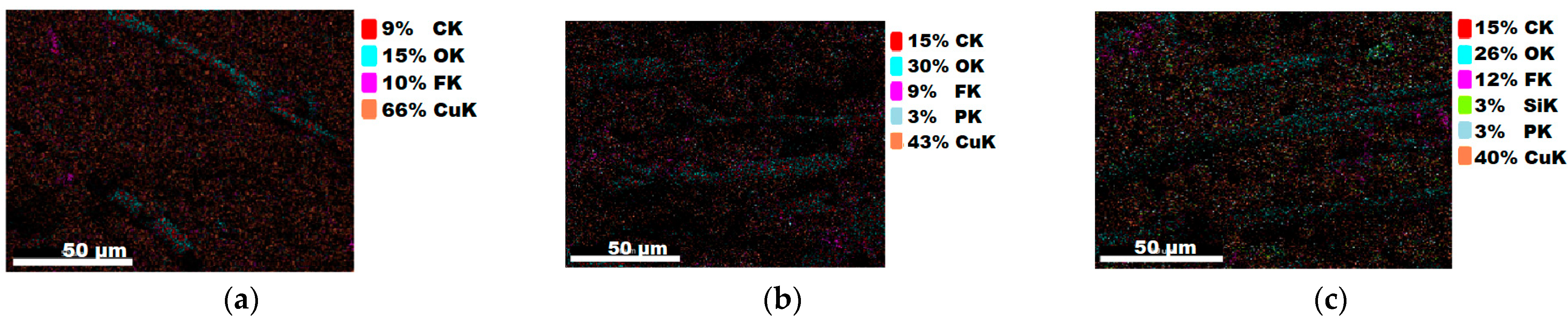
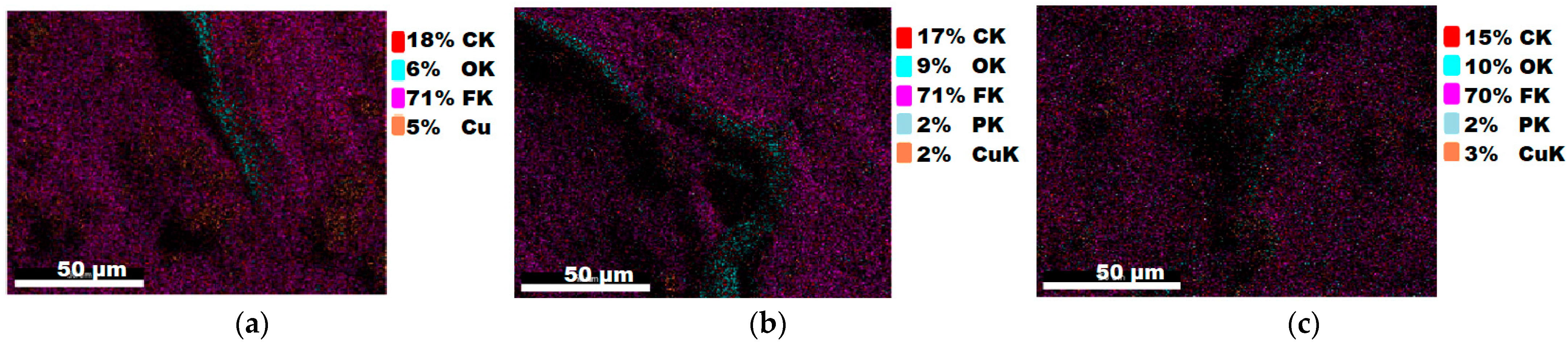
3.1.3. Chemical Composition
- -
- -
- -
4. Discussion
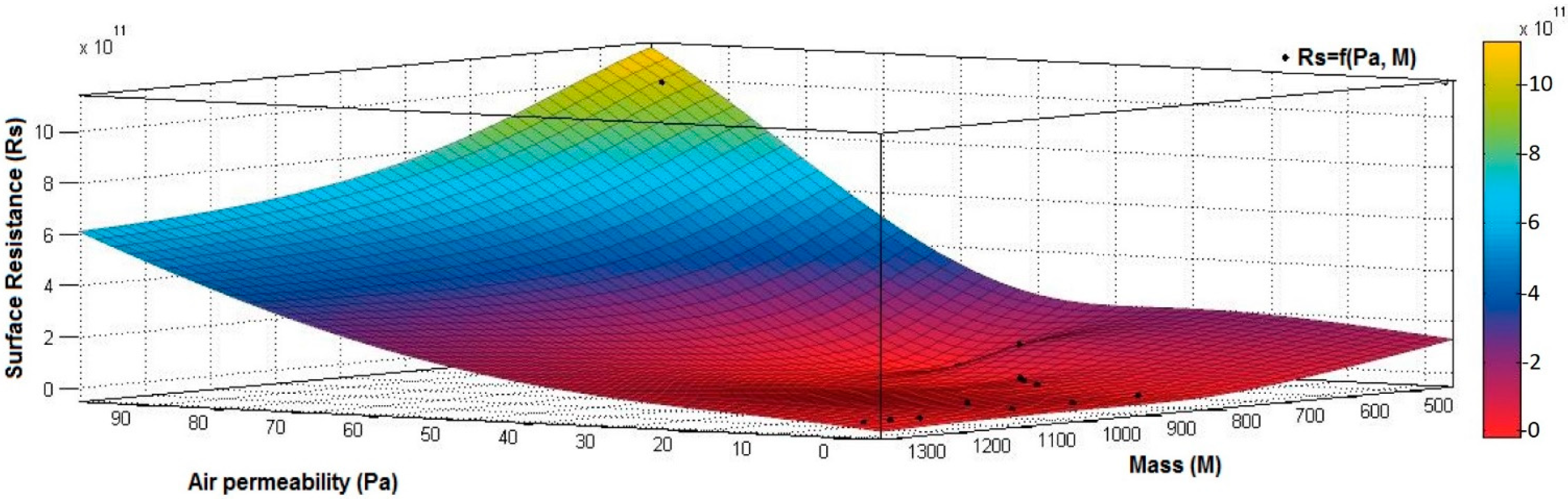
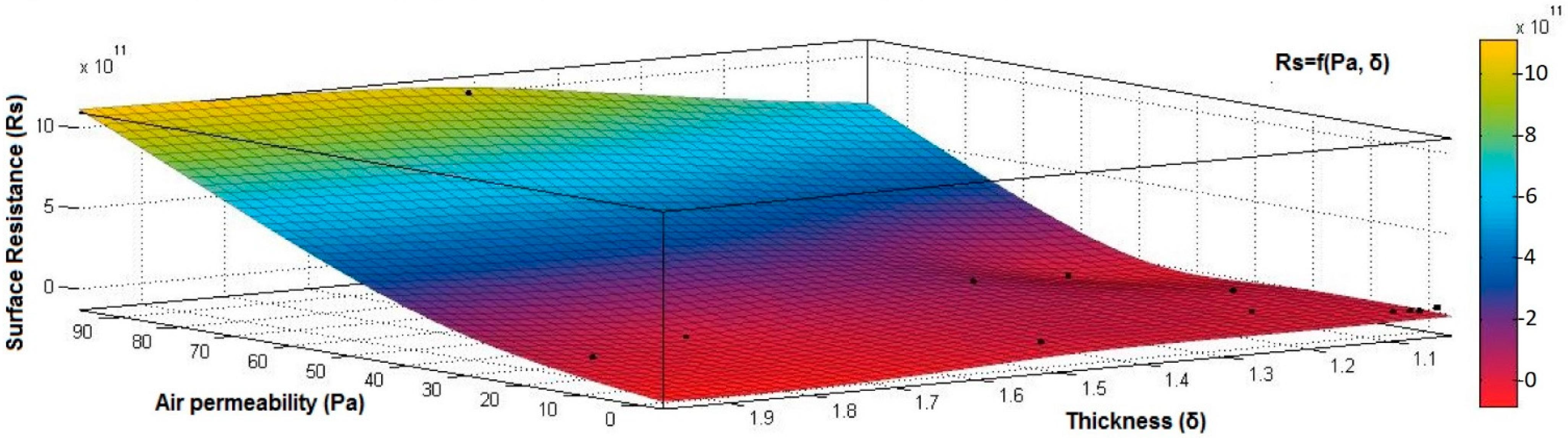
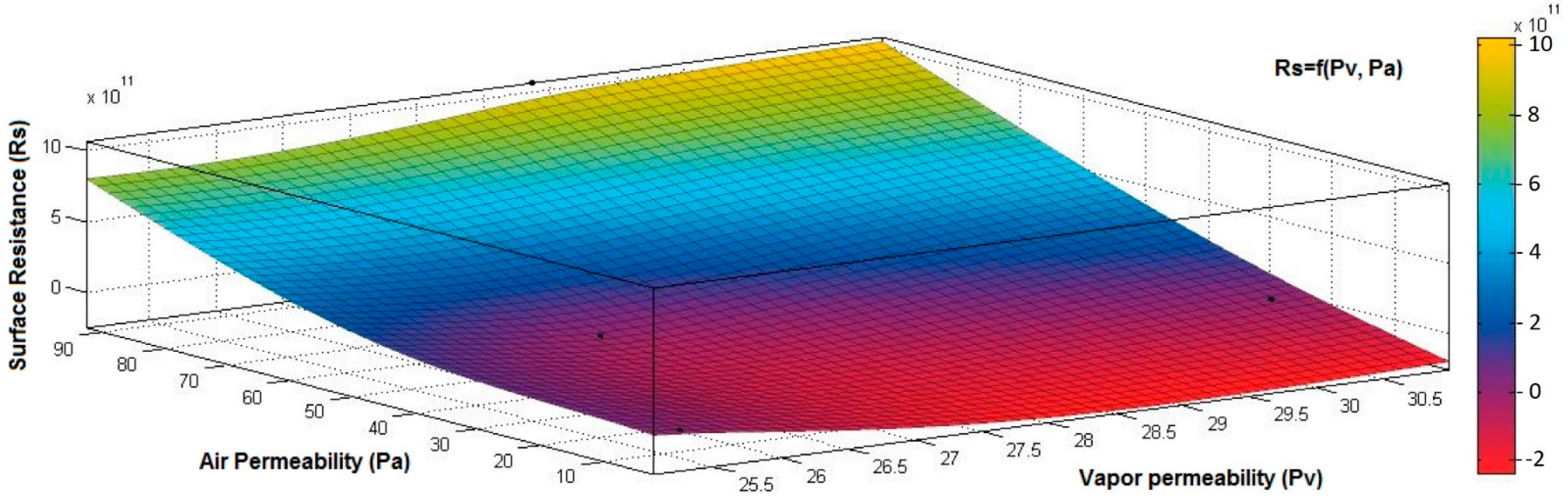
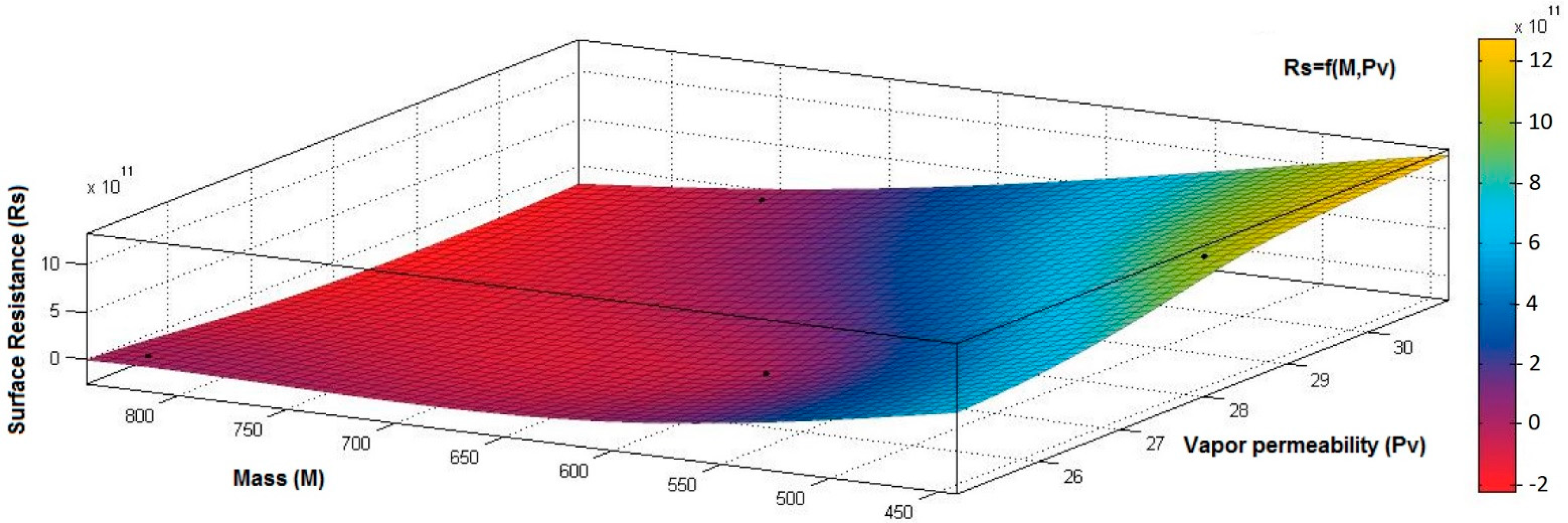
4.1. Correlation between Physicomechanical and Electrical Parameters
4.2. Electrodes Materials Comparison
5. Conclusions
- -
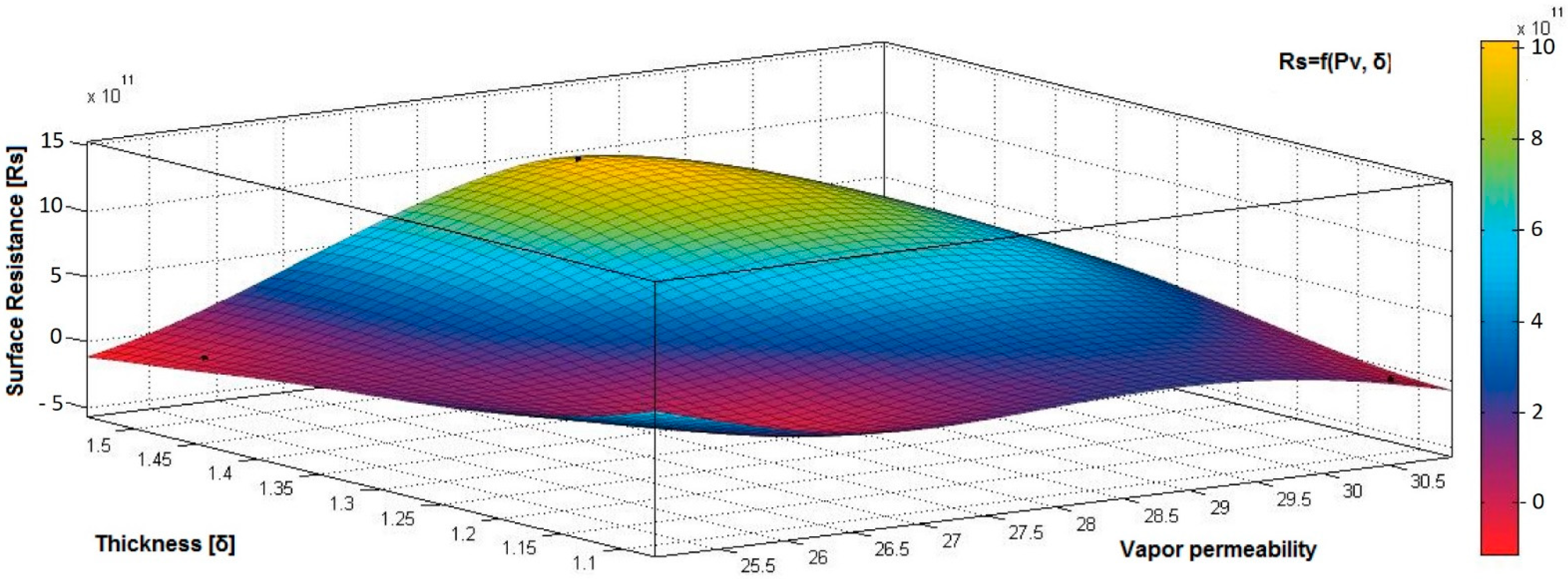
- the correlation coefficient between electrical surface resistance (Rs) and vapor permeability (Pv) is 0.2082 (below 0.3), and this demonstrates that, between Rs and Pv, it is a weak correlation;
- -
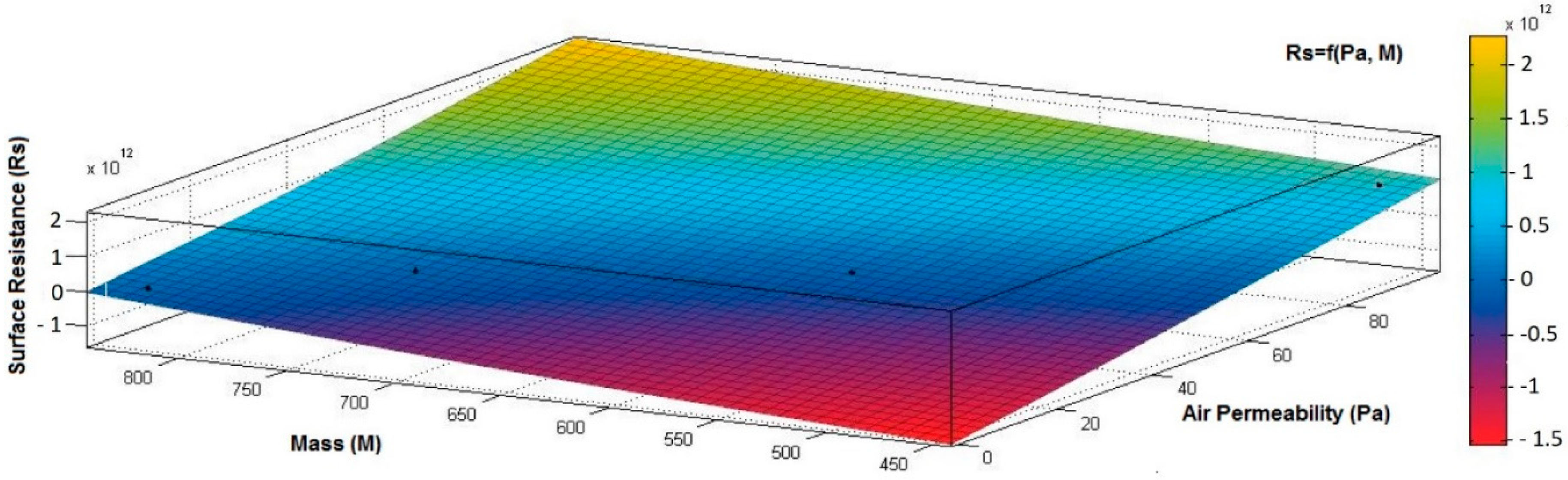
- the correlation coefficient between electrical surface resistance (Rs) and mass (M) is negative (−0.8046) and indicates a strong negative association between Rs and M, suggesting that increasing the M (quantity of conductive paste) will generate a decrease in the Rs;
- -
- the correlation coefficient between Rs and Pa is positive (0.8969), indicating a strong positive association between variables and a direct relationship between Rs and Pa, suggesting that increasing the Pa (air being an excellent electrical insulator) will increase the Rs.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Proto, A.; Fida, B.; Bernabucci, I.; Bibbo, D.; Conforto, S.; Schmid, M.; Penhaker, M. Wearable PVDF transducer for biomechanical energy harvesting and gait cycle detection. In Proceedings of the 2016 IEEE EMBS Conference on Biomedical Engineering and Sciences (IECBES), Kuala Lumpur, Malaysia, 4–8 December 2016; pp. 62–66. [Google Scholar]
- Gupta, S.; Chang, C.; Anbalagan, A.K.; Lee, C.H.; Tai, N.H. Reduced graphene oxide/zinc oxide coated wearable electrically conductive cotton textile for high microwave absorption. Compos. Sci. Technol. 2020, 188, 107994. [Google Scholar] [CrossRef]
- Shateri-Khalilabad, M.; Yazdanshenas, M.E. Fabricating electroconductive cotton textiles using graphene. Carbohydr. Polym. 2013, 96, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.; Liu, S.; Li, W.; Wang, S.; Li, J.; Li, J.; Gong, X. Flexible Polyvinylidene fluoride (PVDF)/MXene (Ti3C2Tx)/Polyimide (P.I.) Wearable Electronic for Body Monitoring, Thermotherapy and Electromagnetic Interference Shielding. Compos. Part A Appl. Sci. Manuf. 2021, 153, 106727. [Google Scholar] [CrossRef]
- Hernández-Rivera, D.; Rodríguez-Roldán, G.; Mora-Martínez, R.; Suaste-Gómez, E. A capacitive humidity sensor based on an electrospun PVDF/graphene membrane. Sensors 2017, 17, 1009. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.; Chowdhury, F.I.; Raza, W.; Qi, X.; Rahman, M.R.; Das, J.; Zabed, H.M. Toward polymer composites based and architectural engineering induced flexible electrodes for lithium-ion batteries. Renew. Sustain. Energy Rev. 2021, 148, 111302. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Cui, Y.; Li, W. Research progress of flexible wearable pressure sensors. Sens. Actuators A Phys. 2021, 330, 112838. [Google Scholar] [CrossRef]
- Zhang, C.; Fan, W.; Wang, S.; Wang, Q.; Zhang, Y.; Dong, K. Recent Progress of Wearable Piezoelectric Nanogenerators. ACS Appl. Electron. Mater. 2021, 3, 2449–2467. [Google Scholar] [CrossRef]
- Xue, L.; Fan, W.; Yu, Y.; Dong, K.; Liu, C.; Sun, Y.; Wang, Q. A Novel Strategy to Fabricate Core-Sheath Structure Piezoelectric Yarns for Wearable Energy Harvesters. Adv. Fiber Mater. 2021, 3, 239–250. [Google Scholar] [CrossRef]
- Xin, Y.; Guo, C.; Qi, X.; Tian, H.; Li, X.; Dai, Q.; Wang, C. Wearable and unconstrained systems based on PVDF sensors in physiological signals monitoring: A brief review. Ferroelectrics 2016, 500, 291–300. [Google Scholar] [CrossRef]
- Fashandi, H.; Abolhasani, M.M.; Sandoghdar, P.; Zohdi, N.; Li, Q.; Naebe, M. Morphological changes towards enhancing piezoelectric properties of PVDF electrical generators using cellulose nanocrystals. Cellulose 2016, 23, 3625–3637. [Google Scholar] [CrossRef]
- Fan, F.R.; Tang, W.; Wang, Z.L. Flexible nanogenerators for energy harvesting and self-powered electronics. Adv. Mater. 2016, 28, 4283–4305. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Bahadur, J.; Panwar, V.; Singh, P.; Rathi, K.; Pal, K. Effective energy harvesting from a single electrode based triboelectric nanogenerator. Sci. Rep. 2016, 6, 38835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S. Flexible Triboelectric Nanogenerators: Enhancement and Applications. In Micro/Nano Integrated Fabrication Technology and Its Applications in Microenergy Harvesting; Springer: Berlin/Heidelberg, Germany, 2016; pp. 93–117. [Google Scholar]
- Li, W.; Torres, D.; Wang, T.; Wang, C.; Sepúlveda, N. Flexible and biocompatible polypropylene ferroelectret nanogenerator (FENG): On the path toward wearable devices powered by human motion. Nano Energy 2016, 30, 649–657. [Google Scholar] [CrossRef]
- Sim, H.J.; Choi, C.; Kim, S.H.; Kim, K.M.; Lee, C.J.; Kim, Y.T.; Kim, S.J. Stretchable triboelectric fiber for self-powered kinematic sensing textile. Sci. Rep. 2016, 6, 35153. [Google Scholar] [CrossRef] [PubMed]
- Fuh, Y.K.; Ho, H.C. Highly flexible self-powered sensors based on printed circuit board technology for human motion detection and gesture recognition. Nanotechnology 2016, 27, 095401. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, H.; Wang, T.; Lee, C. Self-powered liquid triboelectric microfluidic sensor for pressure sensing and finger motion monitoring applications. Nano Energy 2016, 30, 450–459. [Google Scholar] [CrossRef]
- Trung, T.Q.; Lee, N.E. Flexible and stretchable physical sensor integrated platforms for wearable human-activity monitoringand personal healthcare. Adv. Mater. 2016, 28, 4338–4372. [Google Scholar] [CrossRef]
- Bendi, R.; Bhavanasi, V.; Parida, K.; Nguyen, V.C.; Sumboja, A.; Tsukagoshi, K.; Lee, P.S. Self-powered graphene thermistor. Nano Energy 2016, 26, 586–594. [Google Scholar] [CrossRef]
- Xin, Y.; Sun, H.; Tian, H.; Guo, C.; Li, X.; Wang, S.; Wang, C. The use of polyvinylidene fluoride (PVDF) films as sensors for vibration measurement: A brief review. Ferroelectrics 2016, 502, 28–42. [Google Scholar] [CrossRef]
- Khan, R.A.; Ashraf, M.; Javid, A.; Iqbal, K.; Rasheed, A.; Nasir, N. Development of self-polarized PVDF films on carbon fabrics for sensing applications. J. Text. Inst. 2021, 113, 2208–2214. [Google Scholar] [CrossRef]
- Chakhchaoui, N.; Farhan, R.; Chu, Y.M.; Khan, U.; Eddiai, A.; Omari, L.E.H.; Van Langenhove, L. Flexible smart textile coated by PVDF/Graphene oxide with excellent energy harvesting toward a novel class of self-powered sensors: Fabrication, Characterization and Measurements. Preprints 2021, 2021030786. [Google Scholar] [CrossRef]
- Rohit, A.; Kaya, S. A Systematic Study of Wearable Multi-Modal Capacitive Textile Patches. IEEE Sens. J. 2021, 21, 21339475. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, Y.; Zheng, H.; Ramakrishna, S. Piezoelectric Materials for Flexible and Wearable Electronics: A review. Mater. Des. 2021, 211, 110164. [Google Scholar] [CrossRef]
- Katsouras, I.; Asadi, K.; Li, M.; Van Driel, T.B.; Kjaer, K.S.; Zhao, D.; De Leeuw, D.M. The negative piezoelectric effect of the ferroelectric polymer poly (vinylidene fluoride). Nat. Mater. 2016, 15, 78–84. [Google Scholar] [CrossRef]
- Lim, J.; Kim, H.S. Effects of SWCNT/PVDF composite web behavior on acoustic piezoelectric property. Sens. Actuators A Phys. 2021, 330, 112840. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.; Sung, M.; Park, J.; Kim, S.; Choi, Y. Development of EMG-FMG Based Prosthesis With PVDF-Film Vibrational Feedback Control. IEEE Sens. J. 2021, 21, 23597–23607. [Google Scholar] [CrossRef]
- Shateri-Khalilabad, M.; Yazdanshenas, M.E. Preparation of superhydrophobic electroconductive graphene-coated cotton cellulose. Cellulose 2013, 20, 963–972. [Google Scholar] [CrossRef]
- Jang, H.S.; Moon, M.S.; Kim, B.H. Electronic Textiles Fabricated with Graphene Oxide-Coated Commercial Textiles. Coatings 2021, 11, 489. [Google Scholar] [CrossRef]
- Al Faruque, M.A.; Kiziltas, A.; Mielewski, D.; Naebe, M. A facile approach of fabricating electrically conductive knitted fabrics using graphene oxide and textile-based waste material. Polymers 2021, 13, 3003. [Google Scholar] [CrossRef]
- Yun, Y.J.; Hong, W.G.; Kim, W.J.; Jun, Y.; Kim, B.H. A novel method for applying reduced graphene oxide directly to electronic textiles from yarns to fabrics. Adv. Mater. 2013, 25, 5701–5705. [Google Scholar] [CrossRef]
- Yu, R.; Zhu, C.; Wan, J.; Li, Y.; Hong, X. Review of graphene-based textile strain sensors, with emphasis on structure activity relationship. Polymers 2021, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Ma, L.; Patil, A.; Meng, Z.; Liu, S.; Hou, C.; Liu, X.Y. Graphene decorated carbonized cellulose fabric for physiological signal monitoring and energy harvesting. J. Mater. Chem. A 2020, 8, 12665–12673. [Google Scholar] [CrossRef]
- Tissera, N.D.; Wijesena, R.N.; Rathnayake, S.; de Silva, R.M.; de Silva, K.N. Heterogeneous in situ polymerization of polyaniline (PANI) nanofibers on cotton textiles: Improved electrical conductivity, electrical switching, and tuning properties. Carbohydr. Polym. 2018, 186, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Au, W.M.; Li, Y.; Wan, K.M.; Wan, S.H.; Wong, K.S. Design of intelligent garment with transcutaneous electrical nerve stimulation function based on the intarsia knitting technique. Text. Res. J. 2010, 80, 279–286. [Google Scholar] [CrossRef]
- Frydrysiak, M.; Zieba, J.; Tesiorowski, L. Mechatronic line for activation and testing of printed electrodes. Tech. Gaz. 2016, 23, 1003–1010. [Google Scholar]
- Zieba, J.; Frydrysiak, M.; Tesiorowski, L.; Tokarska, M. Textronic matrix of electrode system to electrostimulation. In Proceedings of the IEEE International Symposium on Medical Measurements and Applications Proceedings (MeMeA), Budapest, Hungary, 18–19 May 2012; pp. 1–5. [Google Scholar]
- Ali, A.; Baheti, V.; Militky, J.; Khan, Z. Utility of silver-coated fabrics as electrodes in electrotherapy applications. J. Appl. Polym. Sci. 2018, 135, 46357. [Google Scholar] [CrossRef]
- Euler, L.; Guo, L.; Persson, N.-K. Textile electrodes: Influence of knitting construction and pressure on the contact impedance. Sensors 2021, 21, 1578. [Google Scholar] [CrossRef]
- Ge, J.; Sun, L.; Zhang, F.R.; Zhang, Y.; Shi, L.A.; Zhao, H.Y.; Zhu, H.W.; Jiang, H.L.; Yu, S.H. A stretchable electronic fabric artificial skin with pressure-, lateral strain-, and flexion-sensitive properties. Adv. Mater. 2016, 28, 722–728. [Google Scholar] [CrossRef]
- Huang, F.; Hu, J.; Yan, X. Review of Fiber-or Yarn-Based Wearable Resistive Strain Sensors: Structural Design, Fabrication Technologies and Applications. Textiles 2022, 2, 81–111. [Google Scholar] [CrossRef]
- Dong, K.; Peng, X.; Wang, Z.L. Fiber/fabric-based piezoelectric and triboelectric nanogenerators for flexible/stretchable and wearable electronics and artificial intelligence. Adv. Mater. 2020, 32, 1902549. [Google Scholar] [CrossRef]
- Zhong, J.; Zhang, Y.; Zhong, Q.; Hu, Q.; Hu, B.; Wang, Z.L.; Zhou, J. Fiber-based generator for wearable electronics and mobile medication. ACS Nano 2014, 8, 6273–6280. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Yang, J.; Li, X.; Wu, Y.; Wei, W.; Liu, J.; Chen, J.; Yang, J. Large-scale and washable smart textiles based on triboelectric nanogenerator arrays for self-powered sleeping monitoring. Adv. Funct. Mater. 2018, 28, 1704112. [Google Scholar] [CrossRef]
- Huang, T.; Wang, C.; Yu, H.; Wang, H.; Zhang, Q.; Zhu, M. Human walking-driven wearable all-fiber triboelectric nanogenerator containing electrospun polyvinylidene fluoride piezoelectric nanofibers. Nano Energy 2015, 14, 226–235. [Google Scholar] [CrossRef]
- Abbasipour, M.; Khajavi, R.; Yousefi, A.A.; Yazdanshenas, M.E.; Razaghian, F.; Akbarzadeh, A. Improving piezoelectric and pyroelectric properties of electrospun PVDF nanofibers using nanofillers for energy harvesting application. Polym. Adv. Technol. 2019, 30, 279–291. [Google Scholar] [CrossRef]
- Tseghai, G.B.; Mengistie, D.A.; Malengier, B.; Fante, K.A.; Van Langenhove, L. PEDOT: PSS-based conductive textiles and their applications. Sensors 2020, 20, 1881. [Google Scholar] [CrossRef]
- Li, X.; Lin, Z.H.; Cheng, G.; Wen, X.; Liu, Y.; Niu, S.; Wang, Z.L. 3D fiber-based hybrid nanogenerator for energy harvesting and as a self-powered pressure sensor. ACS Nano 2014, 8, 10674–10681. [Google Scholar] [CrossRef]
- Aileni, R.M.; Chiriac, L.; Sandulache, I. Conductive membranes for sensors. In Proceedings of the 4th International Conference on Emerging Technologies in Materials Engineering, Bucharest, Romania, 4–5 November 2021; ISSN 2602-0416. [Google Scholar]





| Sample No. | PVDF | PEG | PVP | PVA | GO | Ni | Cu1 | Cu2 | Rs * (Ω) | M ** (g/m2) | δ *** (mm) | Pv **** | Pa ***** (L/m2/s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | - | - | - | - | - | - | - | - | 1012 | 415 | 1.06 | - | 33.76 |
| 2 | x | - | - | - | x | - | - | - | 1012 | 460.4 | 1.52 | 28.5 | 95.2 |
| 3 | x | - | - | - | - | x | - | - | 103 | 593.6 | 1.144 | 26.7 | 45.9 |
| 4 | x | - | - | - | - | - | x | - | 1010 | 748 | 1.096 | 30.7 | 24.42 |
| 5 | x | - | x | - | - | - | - | x | 109 | 824.4 | 1.474 | 25.3 | 3.332 |
| 6 | - | x | x | x | - | - | - | x | 106 | 997.6 | 1.168 | - | 10.86 |
| 7 | - | x | x | - | - | - | - | x | 106 | 1042 | 1.044 | - | 1.38 |
| 8 | - | x | x | - | - | - | x | - | 107 | 1262 | 1.06 | - | 2.066 |
| 9 | - | x | x | - | x | - | - | - | 107 | 940.4 | 1.008 | - | 2.076 |
| 10 | - | x | x | - | - | - | x | - | 107 | 1222 | 1.006 | - | 2.024 |
| 11 | - | - | x | - | - | x | - | - | 1010 | 717.6 | 1.732 | 24.1 | 27.44 |
| 12 | - | - | x | - | - | - | x | - | 1011 | 608 | 1.322 | 24.1 | 36.74 |
| 13 | - | - | x | - | - | - | - | x | 1010 | 784.8 | 1.898 | 26 | 19.68 |
| 14 | - | x | x | - | - | - | x | - | 106 | 1187.2 | 1.034 | - | 1.184 |
| Sample No. | PVDF | GO | Ni | Cu1 | Cu2 | Rs1 * (Ω) | Rs2 ** (Ω) | T *** (° C) | Conductive Effect |
|---|---|---|---|---|---|---|---|---|---|
| T1 | x | x | - | - | - | 1012 | 109 | 19.2 | antistatic |
| T2 | x | - | x | - | - | 103 | 109 | 19.1 | antistatic |
| T3 | x | - | - | x | - | 1010 | 109 | 19.1 | antistatic |
| T4 | x | - | - | - | x | 109 | 109 | 19.4 | antistatic |
| Sample No. | Initial | Acid Perspiration Treatment | Alkaline Perspiration Treatment |
|---|---|---|---|
| 1 |  | - | - |
| 2 |  |  |  |
| 3 |  |  |  |
| 4 |  |  |  |
| 5 |  | 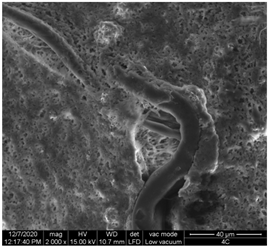 |  |
| Sample No. | Initial | After Coating | Contact Angle View | Contact Angle Value [°] |
|---|---|---|---|---|
| 1 |  | - | 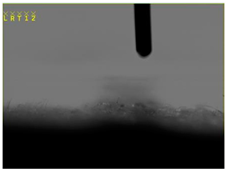 | 0 |
| 2 |  |  |  | 131.5 |
| 3 |  |  | 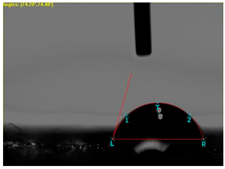 | 74.2 |
| 4 |  | 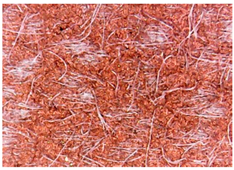 | 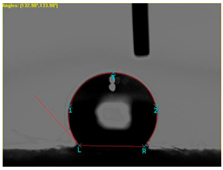 | 132.9 |
| 5 |  | 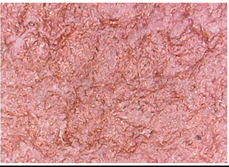 | 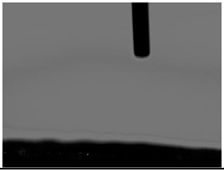 | 0 |
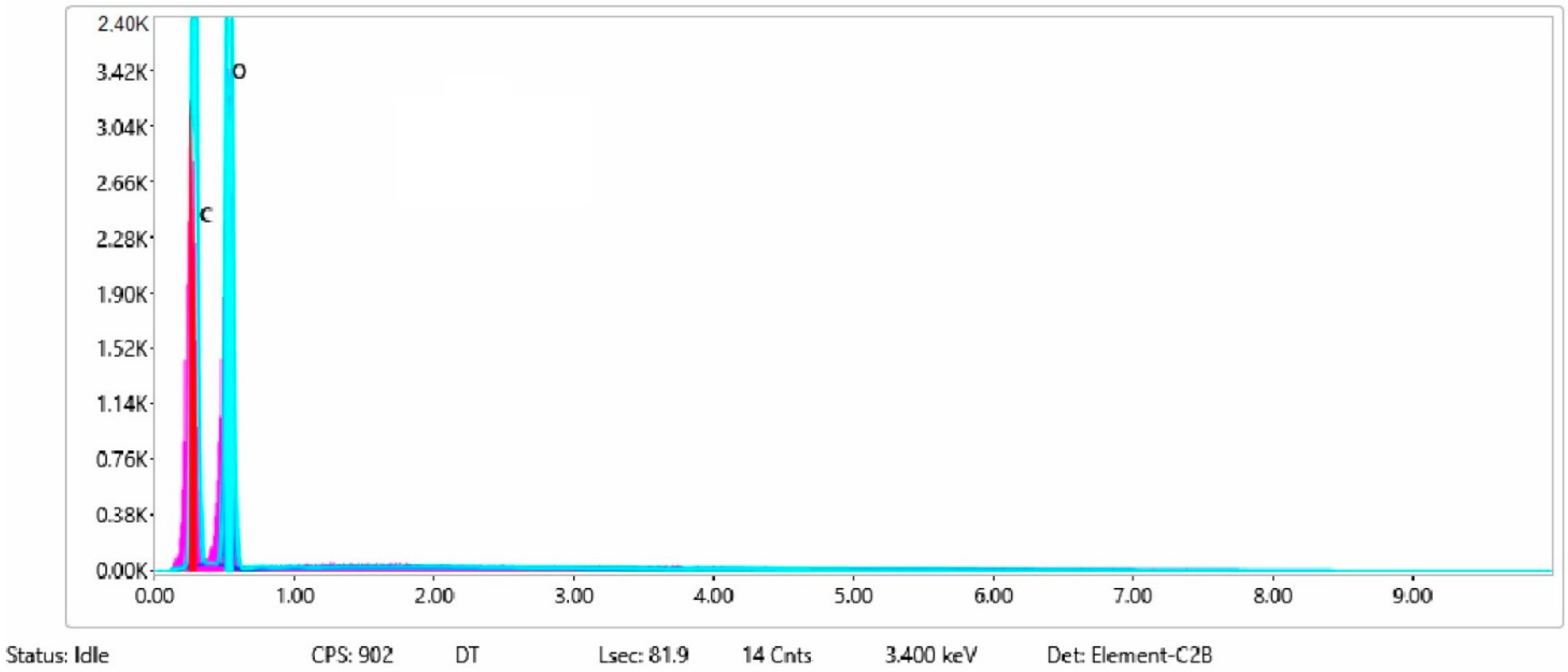
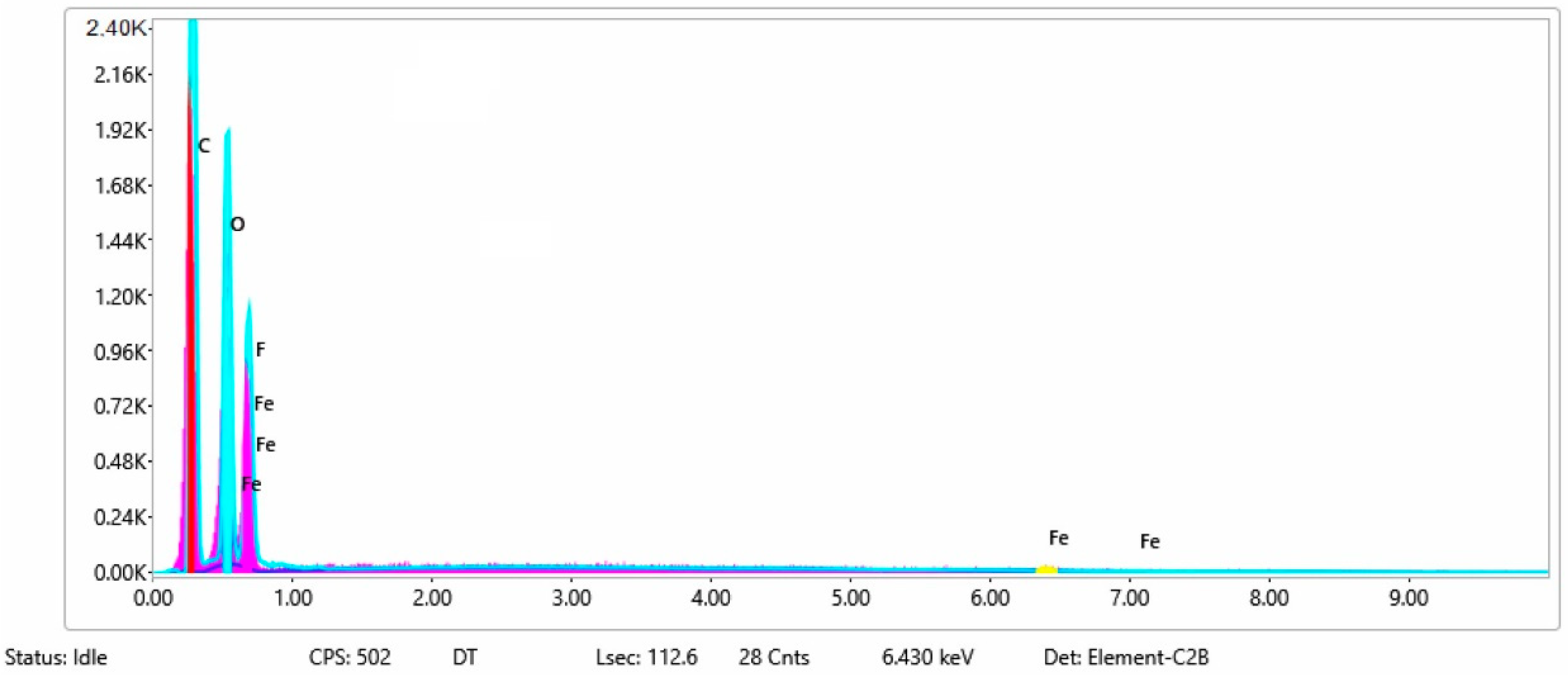
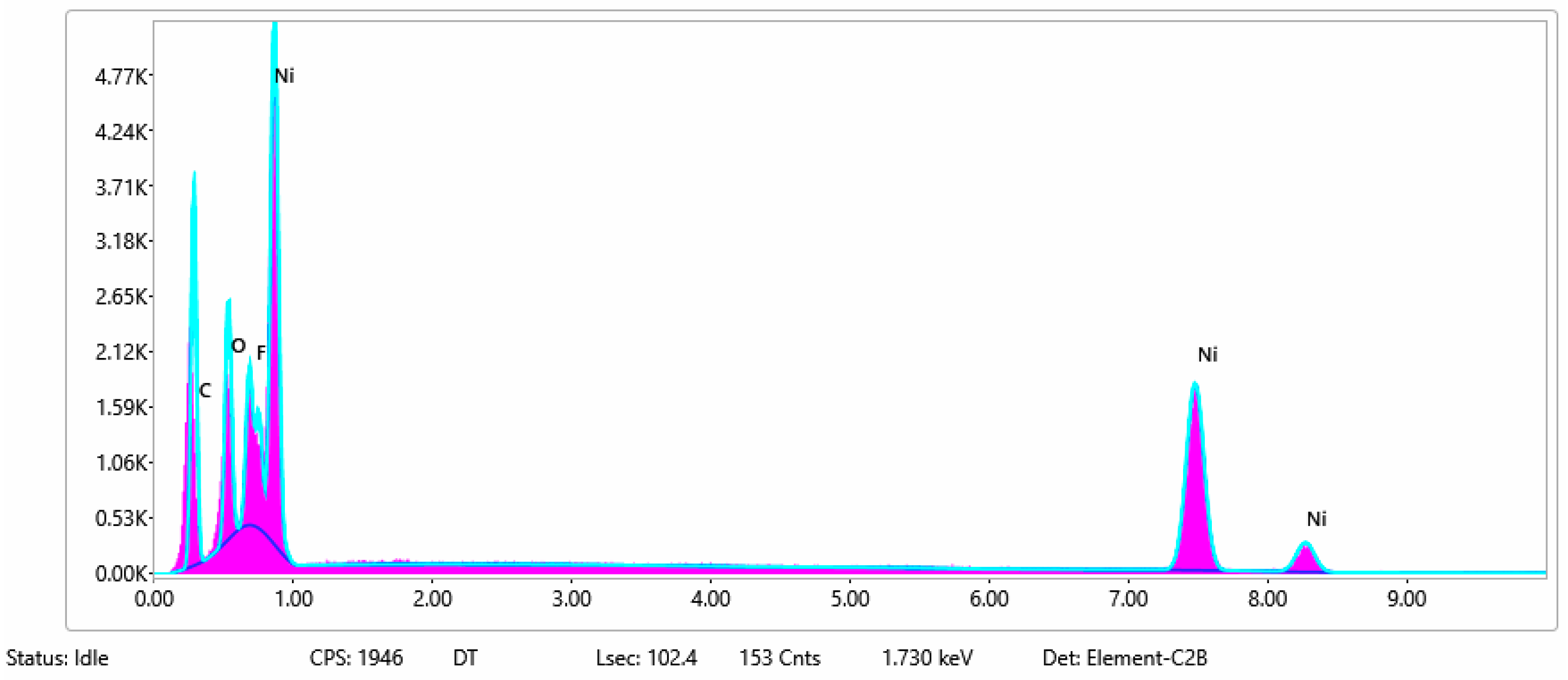
| Sample No. | C | O | F | Ni | Na | P | Cu | Si | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 41 | 59 | |||||||
| 2 | 39 | 34 | 25 | 2 | |||||
| 2a | 32 | 35 | 32 | ||||||
| 2b | 33 | 32 | 35 | ||||||
| 3 | 16 | 19 | 22 | 43 | |||||
| 3a | 25 | 33 | 15 | 27 | |||||
| 3b | 26 | 34 | 21 | 18 | 2 | ||||
| 4 | 9 | 15 | 10 | 66 | |||||
| 4a | 15 | 30 | 9 | 3 | 43 | ||||
| 4b | 15 | 26 | 12 | 3 | 40 | 3 | |||
| 5 | 18 | 6 | 71 | 5 | |||||
| 5a | 17 | 9 | 71 | 2 | 2 | ||||
| 5b | 15 | 10 | 70 | 2 | 3 |
| No. | Electrode Material | Rs | C | Fabrication Technique | Sensor Type | Reference |
|---|---|---|---|---|---|---|
| 1 | PVDF membrane-based nanofibers | - | - | Electrospinning | Potentiometer | [1] |
| 2 | PVDF-MWCNT | - | - | Electrospinning | Piezoelectric sensor | [2] |
| 3 | ZnO/PVDF nanofiber membrane | - | - | Electrospinning | Pressure sensor | [3] |
| 4 | PVDF/Graphene Membrane | - | - | Electrospinning | Humidity sensor | [4] |
| 5 | - | - | - | - | - | [5] |
| 6 | - | - | - | - | - | [6] |
| 7 | PVDF-based Ni membrane | 103 Ω | - | Coating (scrapping) | - | Actual work |
| 8 | Graphene | 350/2100 Ω | - | CVD | Strain sensor | [7] |
| 9 | Graphene oxide reduced | 103 Ω | - | [8] | ||
| 10 | Graphene/Ni | - | CVD | [8] | ||
| 11 | Ag fabric | 465 kΩ | - | Intarsia knitting | Electrode for transcutaneous electrical nerve stimulation | [36] |
| 12 | Ag printed fabric | 194–953 Ω | - | Digital printing with inks-based Ag | Electrode for muscular electrostimulation | [37] |
| 13 | Ag nonwoven | 10.22 Ω | - | Nonwoven | Electrode for electrostimulation | [38] |
| 14 | AgNO3 coated fabric | 19 Ω | - | Knit coated | Electrode for electrotherapy | [39] |
| 15 | Ag/PA knitted | 426 MΩ | - | Knitted electrodes based on silver-plated polyamide (Ag/PA) yarns | Electrode for biomedical monitoring | [40,41] |
| 16 | Woven-based conductive core-spun yarns | - | - | Woven electrode | Electronic Fabric Artificial Skin | [42,43,44] |
| 17 | Fiber–Bragg Gratings-based electrode | - | - | Woven electrodes | Pulse/Temperature Monitoring | [45] |
| 18 | Woven-based conductive yarns | - | - | Woven structure | Electrodes for triboelectric nanogenerator | [46] |
| 19 | Energy harvesting insole-based PVDF nanofibers | - | - | Nonwoven | Electrodes for triboelectric nanogenerator | [47] |
| 20 | PEDOT: PSS coated fabric | - | - | Coating | Electrodes for triboelectric nanogenerator | [48] |
| 21 | Fiber-based hybrid nanogenerator (FBHNG) | - | - | FBHNG-based piezoelectric nanogenerator (PENG) and triboelectric nanogenerator (TENG) | Electrodes for energy harvesting | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aileni, R.M.; Chiriac, L. Conductive Membranes Based on Cotton Fabric Coated with Polymers for Electrode Applications. Materials 2022, 15, 7286. https://doi.org/10.3390/ma15207286
Aileni RM, Chiriac L. Conductive Membranes Based on Cotton Fabric Coated with Polymers for Electrode Applications. Materials. 2022; 15(20):7286. https://doi.org/10.3390/ma15207286
Chicago/Turabian StyleAileni, Raluca Maria, and Laura Chiriac. 2022. "Conductive Membranes Based on Cotton Fabric Coated with Polymers for Electrode Applications" Materials 15, no. 20: 7286. https://doi.org/10.3390/ma15207286
APA StyleAileni, R. M., & Chiriac, L. (2022). Conductive Membranes Based on Cotton Fabric Coated with Polymers for Electrode Applications. Materials, 15(20), 7286. https://doi.org/10.3390/ma15207286








