Molecularly Imprinted Membrane Produced by Electrospinning for β-Caryophyllene Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
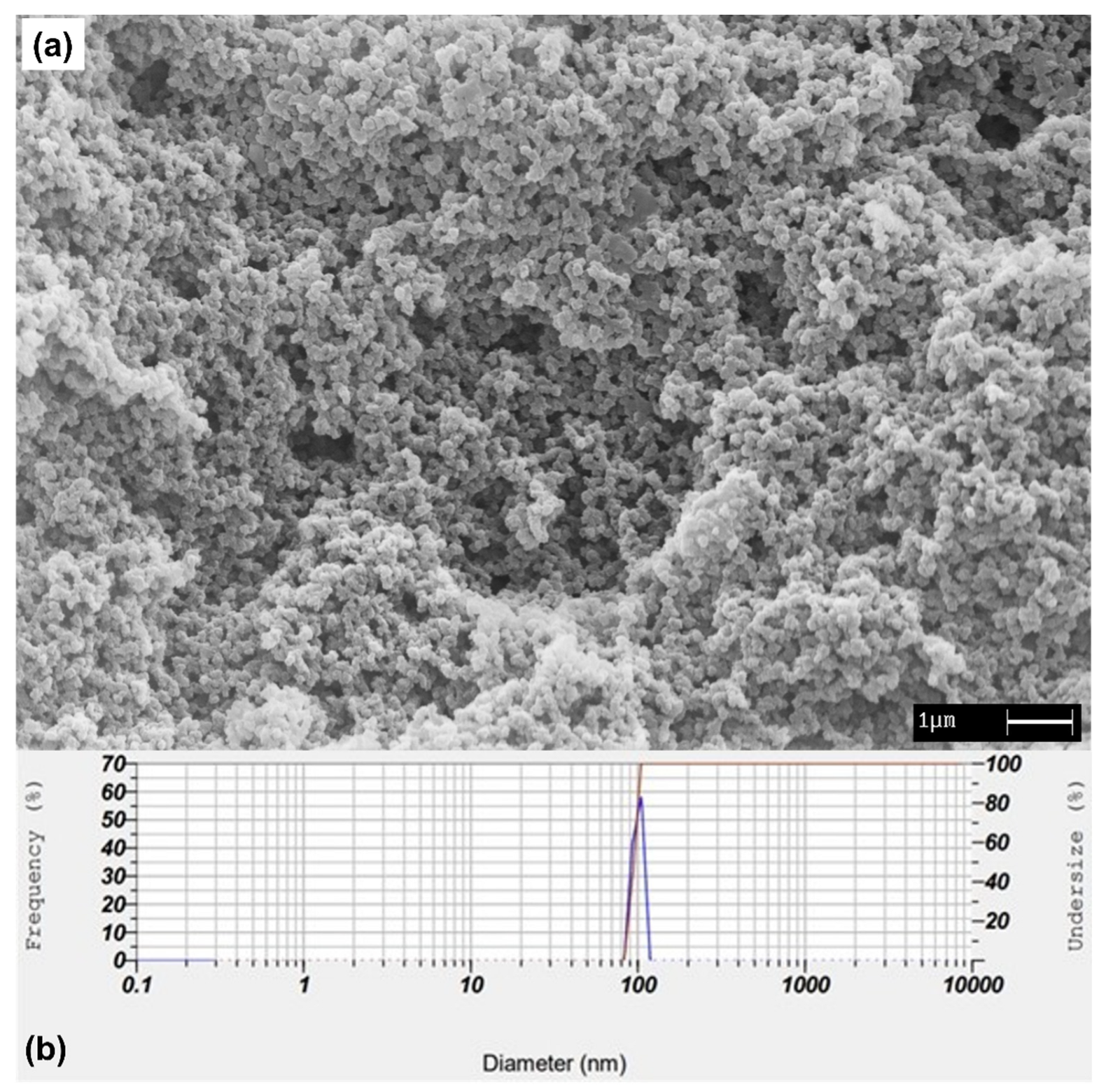
2.2. Synthesis of βCP–NP and Production of Electrospun MIM–βCP
2.3. Analysis of βCP–NP by DLS
2.4. Morphological Characterization by SEM
2.5. Analysis of βCP–NP Incorporation into PCL-Fibers
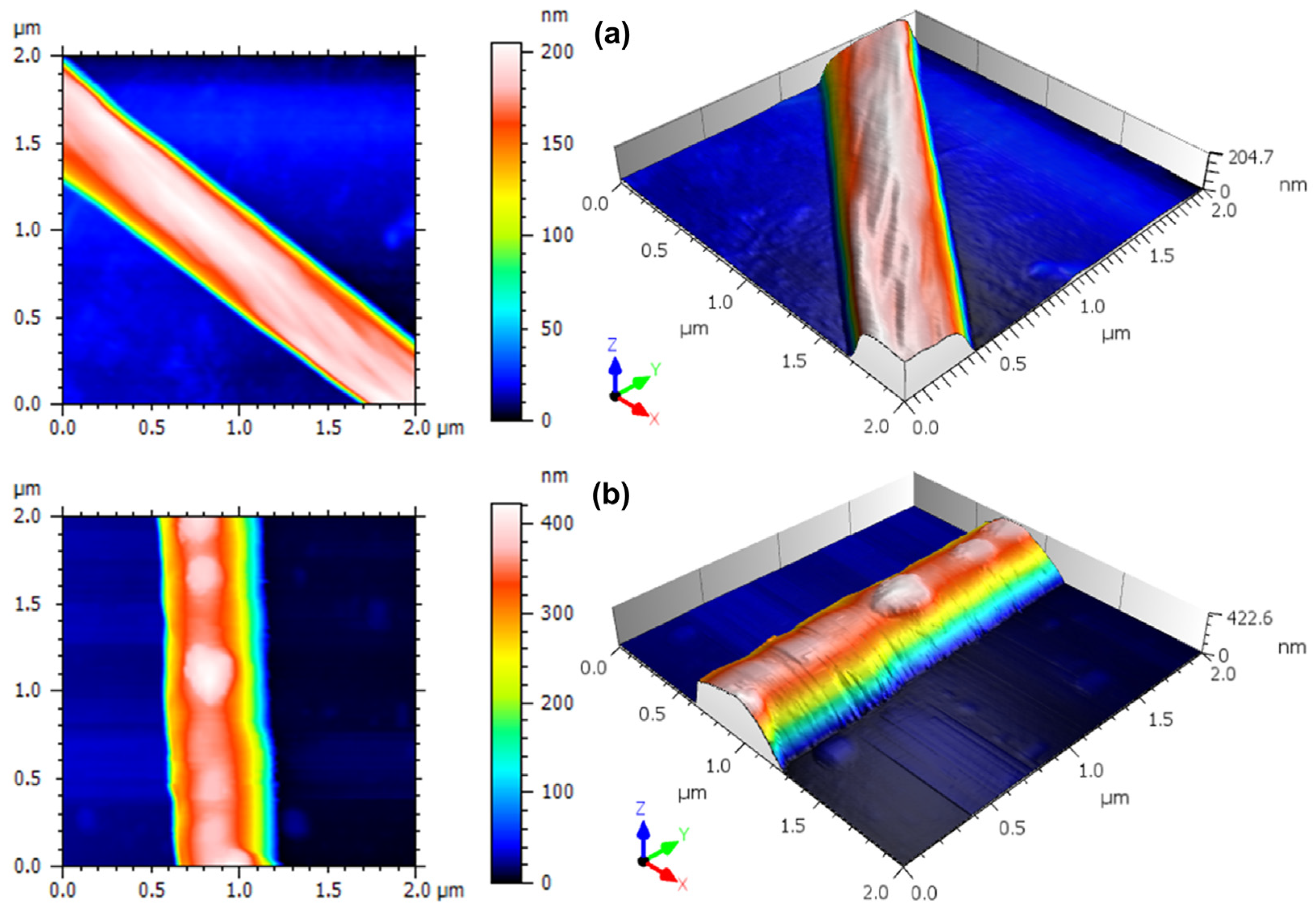
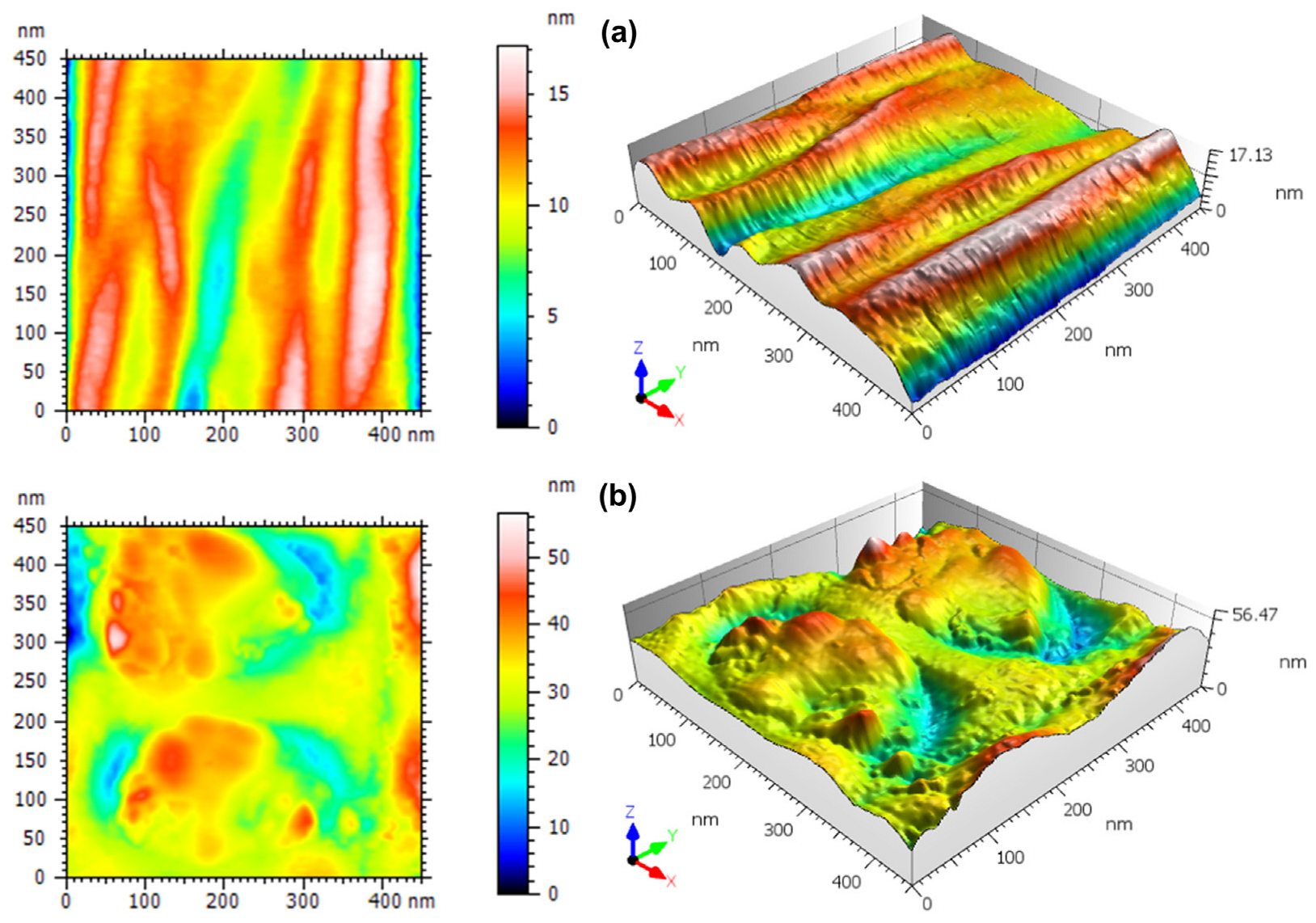
2.5.1. AFM Analysis
2.5.2. Statistical Analysis
2.5.3. XRD Spectroscopic Characterization
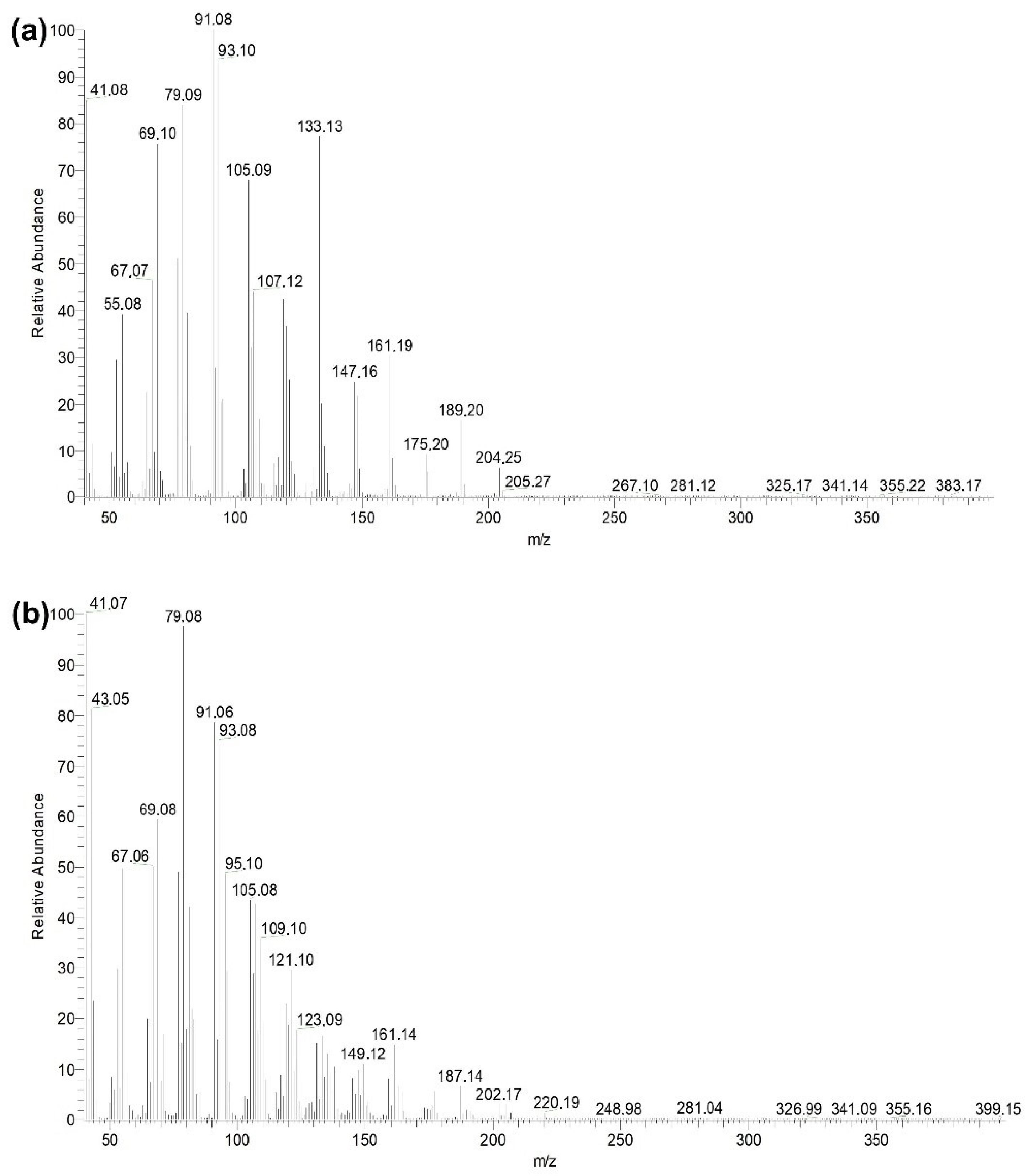
2.6. Analysis by Gas Chromatography
3. Results and Discussion
3.1. Morphology of βCP–NP and Electrospun MIM–βCP
3.2. Three-Dimensional Nanoscale Morphological Surface Analysis
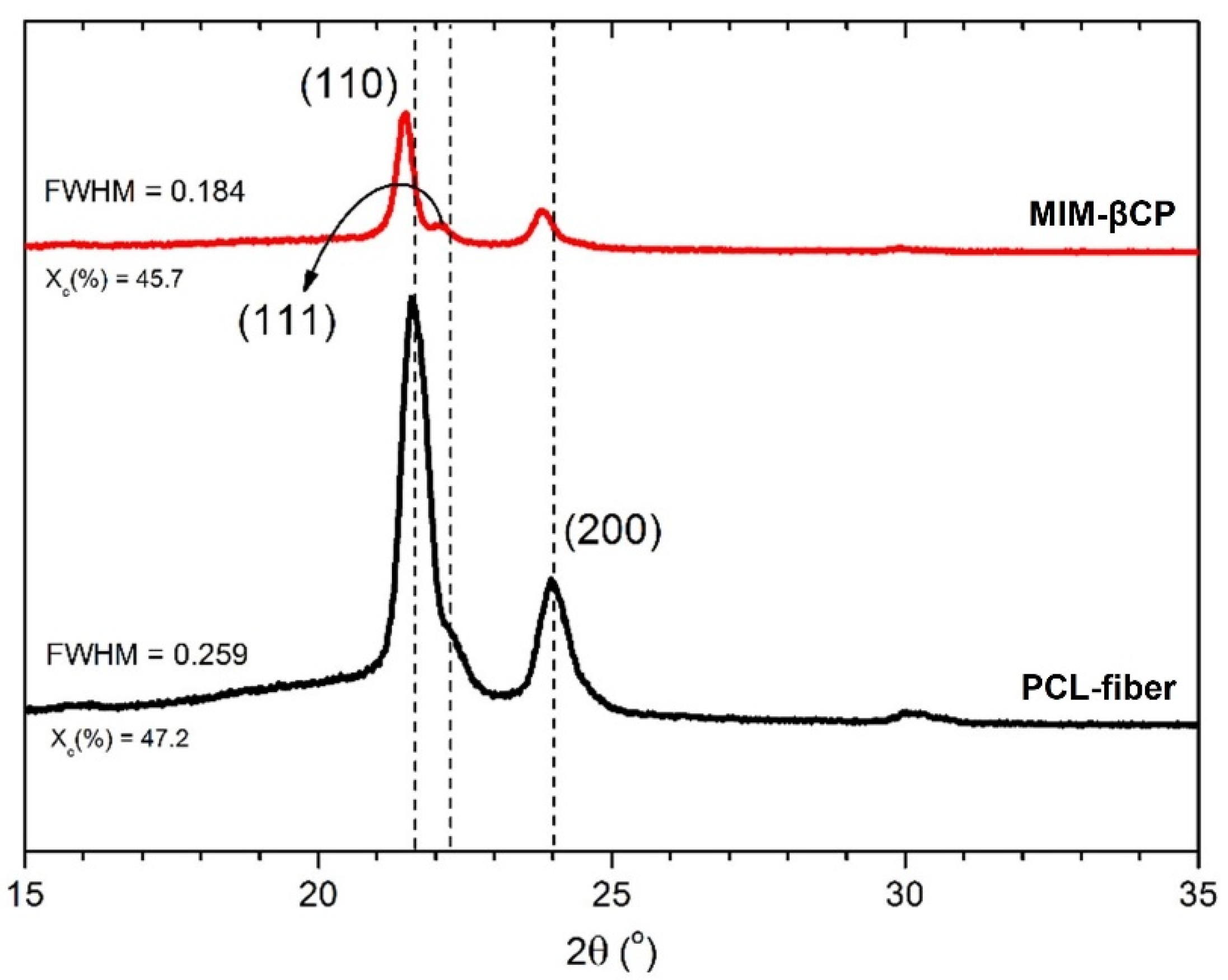
3.3. Analysis of PCL–Fiber and MIM–βCP by XRD
3.4. Calibration Curves
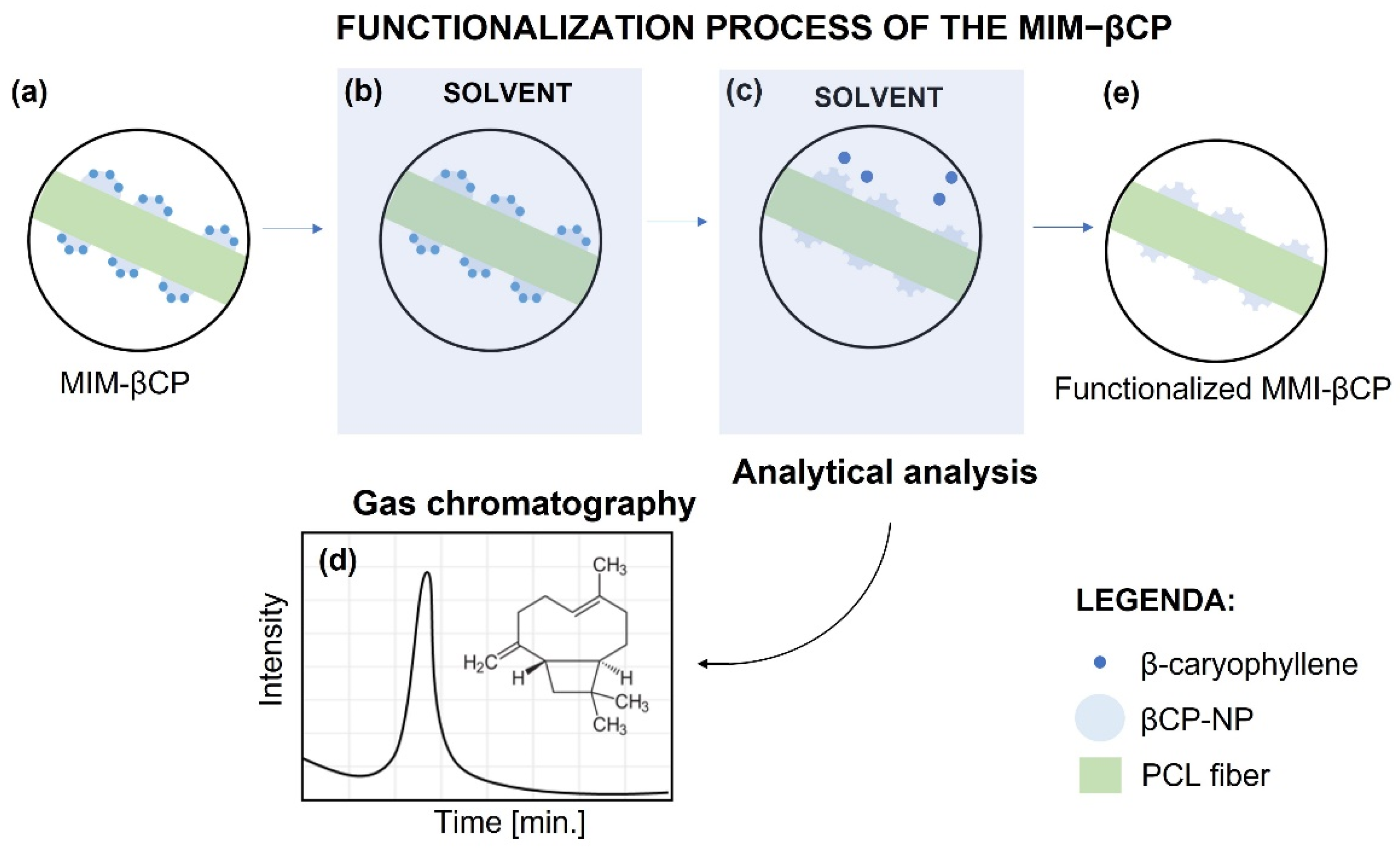
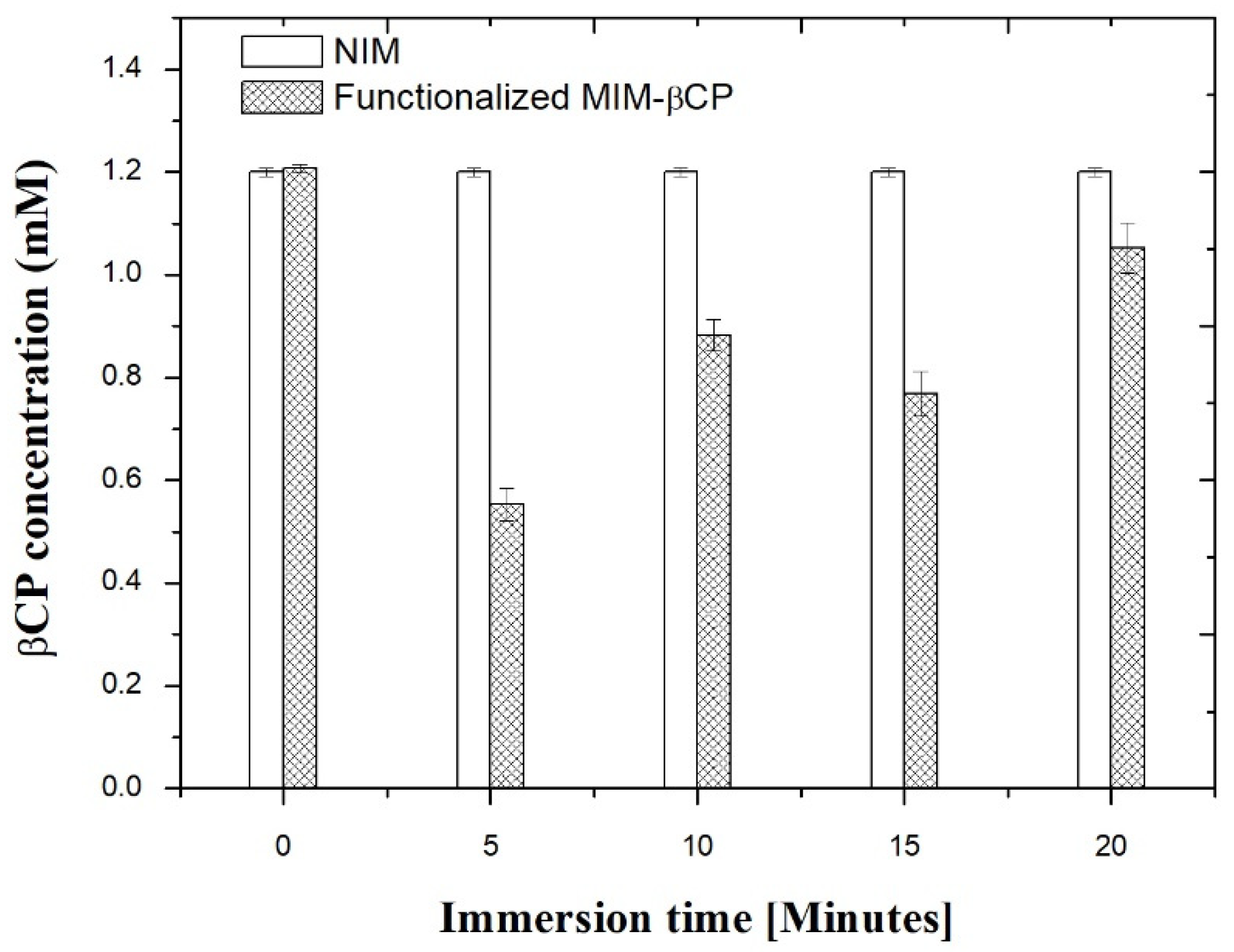
3.5. Functionalization of the MIM–βCP
3.6. βCP Extraction and Binding Capacity
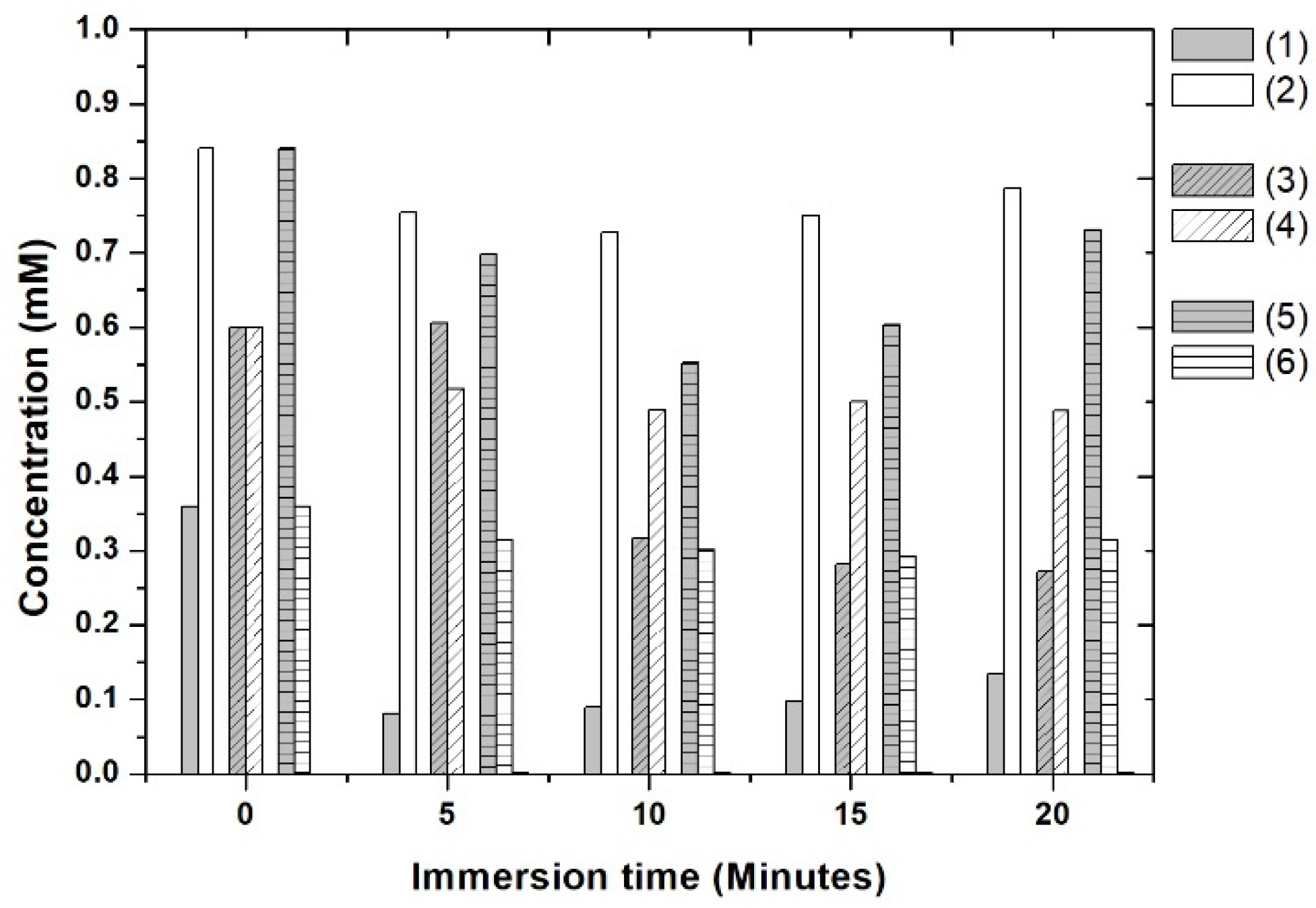
3.7. Selectivity Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular Imprinting: Perspectives and Applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, S.; Singh, S.K. Precipitation Polymerization: A Versatile Tool for Preparing Molecularly Imprinted Polymer Beads for Chromatography Applications. RSC Adv. 2016, 6, 23525–23536. [Google Scholar] [CrossRef]
- Trotta, F.; Biasizzo, M.; Caldera, F. Molecularly Imprinted Membranes. Membranes 2012, 2, 440–477. [Google Scholar] [CrossRef]
- Wang, P.; Liu, X.; Su, X.; Zhu, R. Sensitive Detection of β-Agonists in Pork Tissue with Novel Molecularly Imprinted Polymer Extraction Followed Liquid Chromatography Coupled Tandem Mass Spectrometry Detection. Food Chem. 2015, 184, 72–79. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Safitri, N.; Zulfa, A.; Neli, N.; Rahayu, D. Factors Affecting Preparation of Molecularly Imprinted Polymer and Methods on Finding Template-Monomer Interaction as the Key of Selective Properties of the Materials. Molecules 2021, 26, 5612. [Google Scholar] [CrossRef]
- Vasapollo, G.; Del Sole, R.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly Imprinted Polymers: Present and Future Prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef]
- Liu, H.; Lei, X.; Zhai, Y.; Li, L. Electrospun Nanofiber Membranes Containing Molecularly Imprinted Polymer (MIP) for Rhodamine B (RhB). Adv. Chem. Eng. Sci. 2012, 2, 266–274. [Google Scholar] [CrossRef]
- Chen, M.; Lu, J.; Gao, J.; Yu, C.; Xing, W.; Dai, J.; Meng, M.; Yan, Y.; Wu, Y. Design of Self-Cleaning Molecularly Imprinted Membrane with Antibacterial Ability for High-Selectively Separation of Ribavirin. J. Memb. Sci. 2022, 642, 119994. [Google Scholar] [CrossRef]
- Chen, R.R.; Qin, L.; Jia, M.; He, X.W.; Li, W.Y. Novel Surface-Modified Molecularly Imprinted Membrane Prepared with Iniferter for Permselective Separation of Lysozyme. J. Memb. Sci. 2010, 363, 212–220. [Google Scholar] [CrossRef]
- Fan, J.P.; Li, L.; Tian, Z.Y.; Xie, C.F.; Song, F.T.; Zhang, X.H.; Zhu, J.H. A Novel Free-Standing Flexible Molecularly Imprinted Membrane for Selective Separation of Synephrine in Methanol-Water Media. J. Memb. Sci. 2014, 467, 13–22. [Google Scholar] [CrossRef]
- Yoshimatsu, K.; Ye, L.; Lindberg, J.; Chronakis, I.S. Selective Molecular Adsorption Using Electrospun Nanofiber Affinity Membranes. Biosens. Bioelectron. 2008, 23, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Sueyoshi, Y.; Fukushima, C.; Yoshikawa, M. Molecularly Imprinted Nanofiber Membranes from Cellulose Acetate Aimed for Chiral Separation. J. Memb. Sci. 2010, 357, 90–97. [Google Scholar] [CrossRef]
- Sueyoshi, Y.; Utsunomiya, A.; Yoshikawa, M.; Robertson, G.P.; Guiver, M.D. Chiral Separation with Molecularly Imprinted Polysulfone-Aldehyde Derivatized Nanofiber Membranes. J. Memb. Sci. 2012, 401–402, 89–96. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Meng, M.; Lv, P.; Yan, M.; Wei, X.; Li, H.; Yan, Y.; Li, C. Bio-Inspired Adhesion: Fabrication of Molecularly Imprinted Nanocomposite Membranes by Developing a Hybrid Organic-Inorganic Nanoparticles Composite Structure. J. Memb. Sci. 2015, 490, 169–178. [Google Scholar] [CrossRef]
- De Middeleer, G.; Dubruel, P.; De Saeger, S. Molecularly Imprinted Polymers Immobilized on 3D Printed Scaffolds as Novel Solid Phase Extraction Sorbent for Metergoline. Anal. Chim. Acta 2017, 986, 57–70. [Google Scholar] [CrossRef]
- Faizal, C.K.M.; Kikuchi, Y.; Kobayashi, T. Molecular Imprinting Targeted for α-Tocopherol by Calix [4]Resorcarenes Derivative in Membrane Scaffold Prepared by Phase Inversion. J. Memb. Sci. 2009, 334, 110–116. [Google Scholar] [CrossRef]
- Belbruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Zaidi, S.A. Recent Developments in Molecularly Imprinted Polymer Nanofibers and Their Applications. Anal. Methods 2015, 7, 7406–7415. [Google Scholar] [CrossRef]
- Teo, W.E.; Kaur, S.; Ramakrishna, S. Electrospun Polymer Nanocomposite Fibers: Fabrication and Physical Properties. Phys. Prop. Appl. Polym. Nanocomposites 2010, 616–637. [Google Scholar] [CrossRef]
- Haider, S.; Al-zeghayer, Y.; Ali, F.A.A.; Imran, M.; Aijaz, M.O. Highly Aligned Narrow Diameter Chitosan Electrospun Nanofibers. J. Polym. Res. 2013, 20, 105. [Google Scholar] [CrossRef]
- Ruggieri, F.; D’Archivio, A.A.; Di Camillo, D.; Lozzi, L.; Maggi, M.A.; Mercorio, R.; Santucci, S. Development of Molecularly Imprinted Polymeric Nanofibers by Electrospinning and Applications to Pesticide Adsorption. J. Sep. Sci. 2015, 38, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Lu, R.; Li, Y.; Wang, Q.; Li, J.; Wang, L. Molecularly Imprinted Electrospun Nanofibers for Adsorption of 2,4-Dinitrotoluene in Water. Analyst 2018, 143, 3466–3471. [Google Scholar] [CrossRef] [PubMed]
- De Assis, I.M.; de Moraes, M.O.S.; da Conceição, R.C.; Romaguera-Barcelay, Y.; de Souza, R.F.B.; Larrudé, D.R.G.; Rocco, M.L.M.; Brito, W.R. Novel Electrochemical Sensor Based on Molecularly Imprinted Polymer for Selective Recognition of Sesquiterpene β-Caryophyllene. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2019, 217, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, D.; Levy, R.; Carroll, B. Toxicological Evaluation of β-Caryophyllene Oil: Subchronic Toxicity in Rats. Int. J. Toxicol. 2016, 35, 558–567. [Google Scholar] [CrossRef]
- Meeran, M.F.N.; Al Taee, H.; Azimullah, S.; Tariq, S.; Adeghate, E.; Ojha, S. β-Caryophyllene, a Natural Bicyclic Sesquiterpene Attenuates Doxorubicin-Induced Chronic Cardiotoxicity via Activation of Myocardial Cannabinoid Type-2 (CB2) Receptors in Rats. Chem. Biol. Interact. 2019, 304, 158–167. [Google Scholar] [CrossRef]
- Shen, Q.; Jiang, Y.; Guo, S.; Huang, L.; Xie, H.; Li, L. One-Step Electrospinning Membranes with Gradual-Transition Wettability Gradient for Directional Fluid Transport. J. Memb. Sci. 2022, 644, 120091. [Google Scholar] [CrossRef]
- Moraes Segundo, J.D.D.P.D.; Oneide Silva de Moraes, M.; Brito, W.R.; d’Ávila, M.A. Incorporation of Molecularly Imprinted Polymer Nanoparticles in Electrospun Polycaprolactone Fibers. Mater. Lett. 2020, 275, 128088. [Google Scholar] [CrossRef]
- Matos, R.S.; Pinto, E.P.; Ramos, G.Q.; da Fonseca de Albuquerque, M.D.; da Fonseca Filho, H.D. Stereometric Characterization of Kefir Microbial Films Associated with Maytenus Rigida Extract. Microsc. Res. Tech. 2020, 83, 1401–1410. [Google Scholar] [CrossRef]
- Matos, R.S.; Pinheiro, B.S.; Souza, I.S.; Paes de Castro, R.R.; Ramos, G.Q.; Pinto, E.P.; Silva, R.S.; da Fonseca Filho, H.D. 3D Micromorphology Evaluation of Kefir Microbial Films Loaded with Extract of Amazon Rainforest Fruit Cupuaçu. Micron 2021, 142, 102996. [Google Scholar] [CrossRef]
- Ramos, G.Q.; da Costa, Í.C.; Maia da Costa, M.E.H.; Pinto, E.P.; Matos, R.S.; da Fonseca Filho, H.D. Stereometric Analysis of Amazon Rainforest Anacardium occidentale L. Leaves. Planta 2021, 253, 6. [Google Scholar] [CrossRef]
- Nezafat, N.B.; Ghoranneviss, M.; Elahi, S.M.; Shafiekhani, A.; Ghorannevis, Z.; Solaymani, S. Topographic Characterization of Canine Teeth Using Atomic Force Microscopy Images in Nano-Scale. Int. Nano Lett. 2019, 9, 311–315. [Google Scholar] [CrossRef]
- Da Conceição, W.S.; Ţălu, Ş.; Matos, R.S.; Ramos, G.Q.; Zayas, F.G.; da Fonseca Filho, H.D. Stereometric Characterization of Dinizia Excelsa Ducke Wood from Amazon Rainforest Using Atomic Force Microscopy. Microsc. Res. Tech. 2021, 84, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next Generation of Scientific Image Data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.R.; Banerjee, S.; Bonin, K.; Guthold, M. Determining the Mechanical Properties of Electrospun Poly-ε-Caprolactone (PCL) Nanofibers Using AFM and a Novel Fiber Anchoring Technique. Mater. Sci. Eng. C 2016, 59, 203–212. [Google Scholar] [CrossRef] [PubMed]
- ISO Geometrical Product Specifications (GPS)—Surface Texture: Areal—Part 2: Terms, Definitions and Surface Texture Parameters. Available online: https://www.iso.org/standard/42785.html (accessed on 20 July 2019).
- Blateyron, F. Characterization of Areal Surface Texture; Leach, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-36457-0. [Google Scholar]
- Franco, L.A.; Sinatora, A. 3D Surface Parameters (ISO 25178-2): Actual Meaning of Spk and Its Relationship to Vmp. Precis. Eng. 2015, 40, 106–111. [Google Scholar] [CrossRef]
- Kiran, A.S.K.; Kumar, T.S.S.; Sanghavi, R.; Doble, M.; Ramakrishna, S. Antibacterial and Bioactive Surface Modifications of Titanium Implants by PCL/TiO2 Nanocomposite Coatings. Nanomaterials 2018, 8, 860. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carroll Stream, IL, USA, 2007. [Google Scholar]
- Esfandyari-Manesh, M.; Javanbakht, M.; Shahmoradi, E.; Dinarvand, R.; Atyabi, F. The Control of Morphological and Size Properties of Carbamazepine-Imprinted Microspheres and Nanospheres under Different Synthesis Conditions. J. Mater. Res. 2013, 28, 2677–2686. [Google Scholar] [CrossRef]
- Cui, H.Y.; Wu, J.; Li, C.Z.; Lin, L. Anti-Listeria Effects of Chitosan-Coated Nisin-Silica Liposome on Cheddar Cheese. J. Dairy Sci. 2016, 99, 8598–8606. [Google Scholar] [CrossRef] [PubMed]
- Ghobeira, R.; Asadian, M.; Vercruysse, C.; Declercq, H.; De Geyter, N.; Morent, R. Wide-Ranging Diameter Scale of Random and Highly Aligned PCL Fibers Electrospun Using Controlled Working Parameters. Polymer 2018, 157, 19–31. [Google Scholar] [CrossRef]
- Da Costa, T.C.; Matos, R.S.; De Azevedo, S.G.; Costa, C.A.R.; Sanches, E.A.; Da Fonseca Filho, H.D. Microscopy-Based Infrared Spectroscopy as a Tool to Evaluate the Influence of Essential Oil on the Surface of Loaded Bilayered-Nanoparticles. Nanotechnology 2021, 32, 345703. [Google Scholar] [CrossRef]
- De Oliveira, L.M.; Matos, R.S.; Campelo, P.H.; Sanches, E.A.; da Fonseca Filho, H.D. Evaluation of the Nanoscale Surface Applied to Biodegradable Nanoparticles Containing Allium Sativum Essential Oil. Mater. Lett. 2020, 275, 128111. [Google Scholar] [CrossRef]
- Matos, R.S.; Ramos, G.Q.; da Fonseca Filho, H.D.; Ţălu, Ş. Advanced Micromorphology Study of Microbial Films Grown on Kefir Loaded with Açaí Extract. Micron 2020, 137, 102912. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.M.D.; Matos, R.S.; Ara, J.D.; Henrique, P.; Felix, C. Three-Dimensional Nanoscale Morphological Surface Analysis of Polymeric Particles Containing Allium sativum Essential Oil. Materials 2022, 15, 2635. [Google Scholar] [CrossRef] [PubMed]
- Gandarilla, A.M.D.; Matos, R.S.; Barcelay, Y.R.; da Fonseca Filho, H.D.; Brito, W.R. Molecularly Imprinted Polymer on Indium Tin Oxide Substrate for Bovine Serum Albumin Determination. J. Polym. Res. 2022, 29, 166. [Google Scholar] [CrossRef]
- Jeon, H.J.; Lee, H.; Kim, G.H. Fabrication and Characterization of Nanoscale-Roughened PCL/Collagen Micro/Nanofibers Treated with Plasma for Tissue Regeneration. J. Mater. Chem. B 2015, 3, 3279–3287. [Google Scholar] [CrossRef]
- Rahman, M.M.; Asiri, A.M. Nanofiber Research—Reaching New Heights [Internet]; IntechOpen: London, UK, 2016; ISBN 9789535125297. [Google Scholar]
- Liu, Y.; Zhou, G.; Liu, Z.; Guo, M.; Jiang, X.; Taskin, M.B.; Zhang, Z.; Liu, J.; Tang, J.; Bai, R.; et al. Mussel Inspired Polynorepinephrine Functionalized Electrospun Polycaprolactone Microfibers for Muscle Regeneration. Sci. Rep. 2017, 7, 8197. [Google Scholar] [CrossRef]
- Whitehouse, D.J. Handbook of Surface and Nanometrology, 1st ed.; Taylor & Francis: New York, NY, USA, 2002; ISBN 9780429145544. [Google Scholar]
- Yu, N.; Polycarpou, A.A. Contact of Rough Surfaces with Asymmetric Distribution of Asperity Heights. J. Tribol. 2002, 124, 367–376. [Google Scholar] [CrossRef]
- Tayebi, N.; Polycarpou, A.A. Modeling the Effect of Skewness and Kurtosis on the Static Friction Coefficient of Rough Surfaces. Tribol. Int. 2004, 37, 491–505. [Google Scholar] [CrossRef]
- Leach, R. Characterisation of Areal Surface Texture; Springer: Berlin/Heidelberg, Germany, 2013; Volume 9783642364, ISBN 9783642364587. [Google Scholar]
- de Menezes, B.R.C.; Montanheiro, T.L.D.A.; Sampaio, A.D.G.; Koga-Ito, C.Y.; Thim, G.P.; Montagna, L.S. PCL/β-AgVO3 Nanocomposites Obtained by Solvent Casting as Potential Antimicrobial Biomaterials. J. Appl. Polym. Sci. 2021, 138, 50130. [Google Scholar] [CrossRef]
- Cobos, M.; Ramos, J.R.; Guzmán, D.J.; Fernández, M.D.; Fernández, M.J. PCL/POSS Nanocomposites: Effect of POSS Derivative and Preparation Method on Morphology and Properties. Polymers 2019, 11, 33. [Google Scholar] [CrossRef]
- Gómez-Lizárraga, K.K.; Flores-Morales, C.; Del Prado-Audelo, M.L.; Álvarez-Pérez, M.A.; Piña-Barba, M.C.; Escobedo, C. Polycaprolactone- and Polycaprolactone/Ceramic-Based 3D-Bioplotted Porous Scaffolds for Bone Regeneration: A Comparative Study. Mater. Sci. Eng. C 2017, 79, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Wang, B.; Zhang, P. The Effect of Crystallization Behavior on High Conductivity, Enhanced Mechanism and Thermal Stability of Poly(ε-Caprolactone)/Multi-Walled Carbon Nanotube Composites. J. Dispers. Sci. Technol. 2019, 40, 94–102. [Google Scholar] [CrossRef]
- Monteiro, M.S.S.B.; Lunz, J.; Sebastião, P.J.; Tavares, M.I.B. Evaluation of Nevirapine Release Kinetics from Polycaprolactone Hybrids. Mater. Sci. Appl. 2016, 07, 680–701. [Google Scholar] [CrossRef]
- Lucca, L.G.; de Matos, S.P.; Borille, B.T.; Dias, D.d.O.; Teixeira, H.F.; Veiga, V.F.; Limberger, R.P.; Koester, L.S. Determination of β-Caryophyllene Skin Permeation/Retention from Crude Copaiba Oil (Copaifera Multijuga Hayne) and Respective Oil-Based Nanoemulsion Using a Novel HS-GC/MS Method. J. Pharm. Biomed. Anal. 2015, 104, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Scorrano, S.; Mergola, L.; Di Bello, M.P.; Lazzoi, M.R.; Vasapollo, G.; Del Sole, R. Molecularly Imprinted Composite Membranes for Selective Detection of 2-Deoxyadenosine in Urine Samples. Int. J. Mol. Sci. 2015, 16, 13746–13759. [Google Scholar] [CrossRef]


| Parameter | Unit | PCL | PCL + NP |
|---|---|---|---|
| Sq | [nm] | 4.1 ± 0.7 | 7.2 ± 1.8 |
| Ssk | [-] | −0.8 ± 0.3 | −0.1 ± 0.1 |
| Sku * | [-] | 5.0 ± 1.7 | 4.6 ± 1.3 |
| Sp | [nm] | 8.9 ± 2.6 | 26.2 ± 5.1 |
| Sv | [nm] | 21.4 ± 8.9 | 41.1 ± 7.5 |
| Sz | [nm] | 30.3 ± 11.4 | 67.3 ± 10.0 |
| Time | Binary Solution (30/70) | Binary Solution (50/50) | Binary Solution (70/30) | |||
|---|---|---|---|---|---|---|
| Min. | βCP | CP–Oxide | βCP | CP–Oxide | βCP | CP–Oxide |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 77% | 10% | 0 | 14% | 17% | 13% |
| 10 | 75% | 13% | 47% | 18% | 34% | 16% |
| 15 | 73% | 10% | 53% | 17% | 28% | 19% |
| 20 | 63% | 6% | 55% | 19% | 13% | 13% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraes Segundo, J.d.D.P.d.; Moraes, M.O.S.d.; Brito, W.R.; Matos, R.S.; Salerno, M.; Barcelay, Y.R.; Segala, K.; Fonseca Filho, H.D.d.; d’Ávila, M.A. Molecularly Imprinted Membrane Produced by Electrospinning for β-Caryophyllene Extraction. Materials 2022, 15, 7275. https://doi.org/10.3390/ma15207275
Moraes Segundo JdDPd, Moraes MOSd, Brito WR, Matos RS, Salerno M, Barcelay YR, Segala K, Fonseca Filho HDd, d’Ávila MA. Molecularly Imprinted Membrane Produced by Electrospinning for β-Caryophyllene Extraction. Materials. 2022; 15(20):7275. https://doi.org/10.3390/ma15207275
Chicago/Turabian StyleMoraes Segundo, João de Deus Pereira de, Maria Oneide Silva de Moraes, Walter Ricardo Brito, Robert S. Matos, Marco Salerno, Yonny Romaguera Barcelay, Karen Segala, Henrique Duarte da Fonseca Filho, and Marcos Akira d’Ávila. 2022. "Molecularly Imprinted Membrane Produced by Electrospinning for β-Caryophyllene Extraction" Materials 15, no. 20: 7275. https://doi.org/10.3390/ma15207275
APA StyleMoraes Segundo, J. d. D. P. d., Moraes, M. O. S. d., Brito, W. R., Matos, R. S., Salerno, M., Barcelay, Y. R., Segala, K., Fonseca Filho, H. D. d., & d’Ávila, M. A. (2022). Molecularly Imprinted Membrane Produced by Electrospinning for β-Caryophyllene Extraction. Materials, 15(20), 7275. https://doi.org/10.3390/ma15207275












