Characterization of the Native Oxide Shell of Copper Metal Powder Spherical Particles
Abstract
1. Introduction
2. Material and Experimental Work
2.1. Cu Metal Powder Specifications
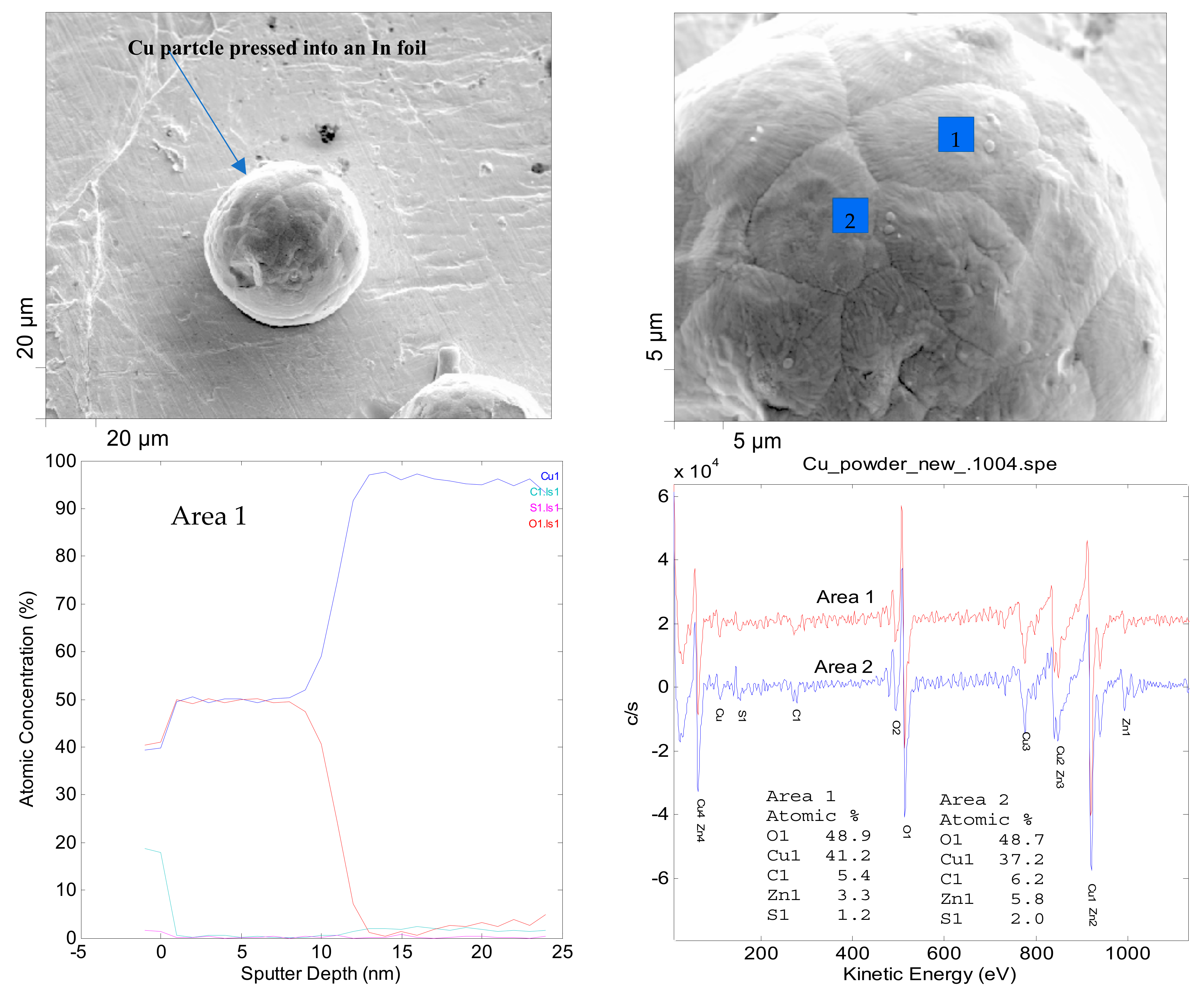
2.2. Characterizations of As-Received Cu Powder
2.3. Auger Electron Spectroscopy
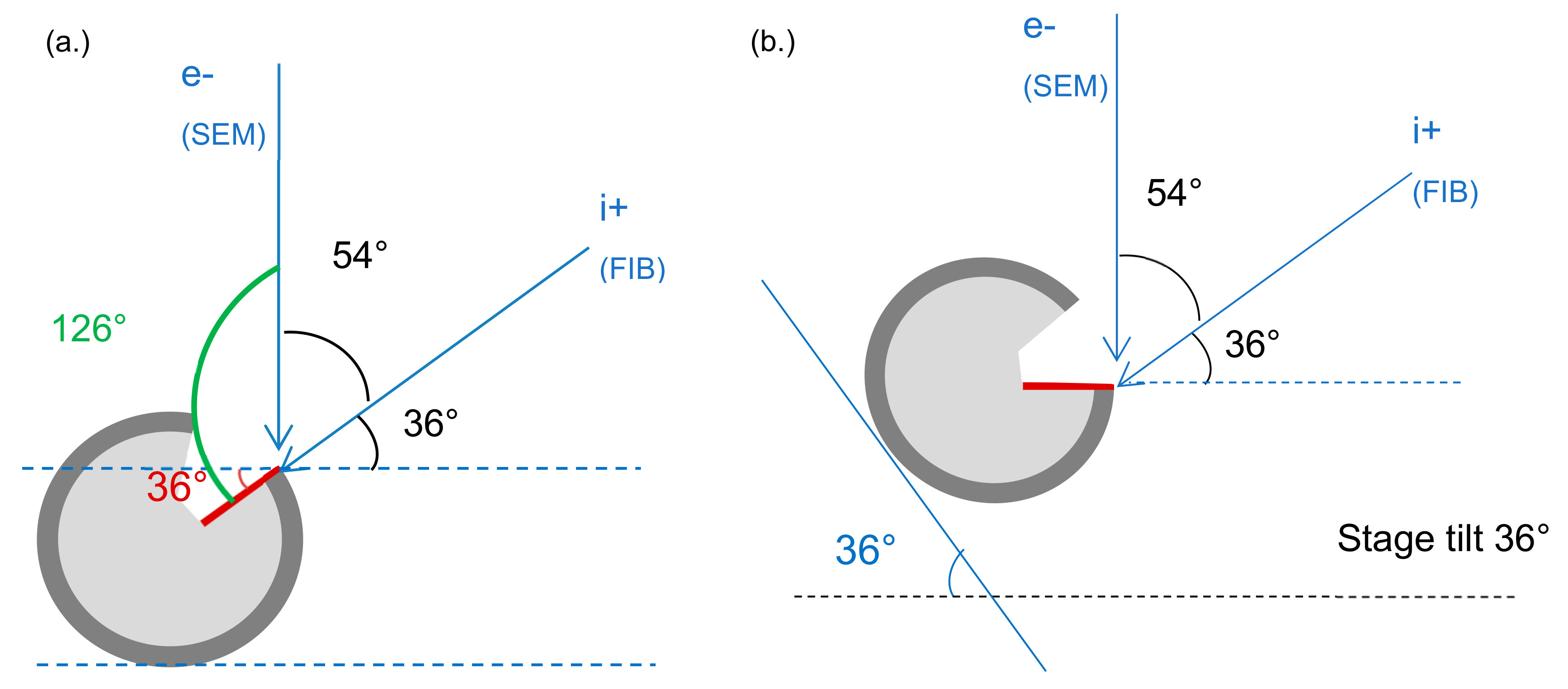
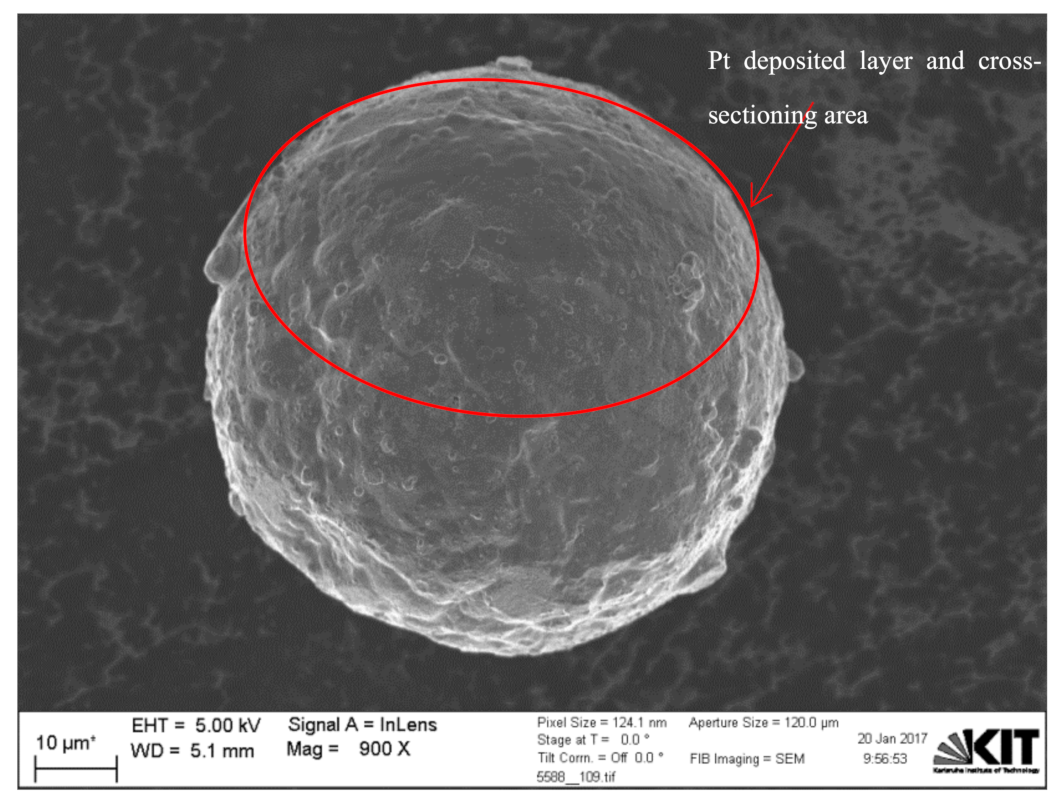
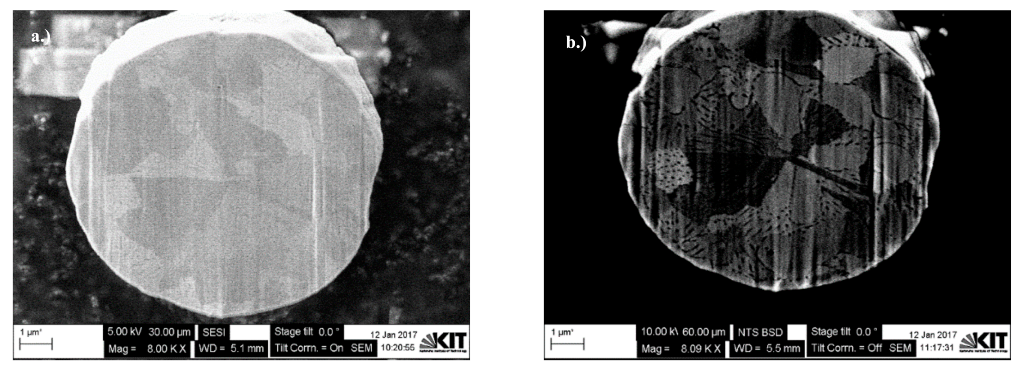
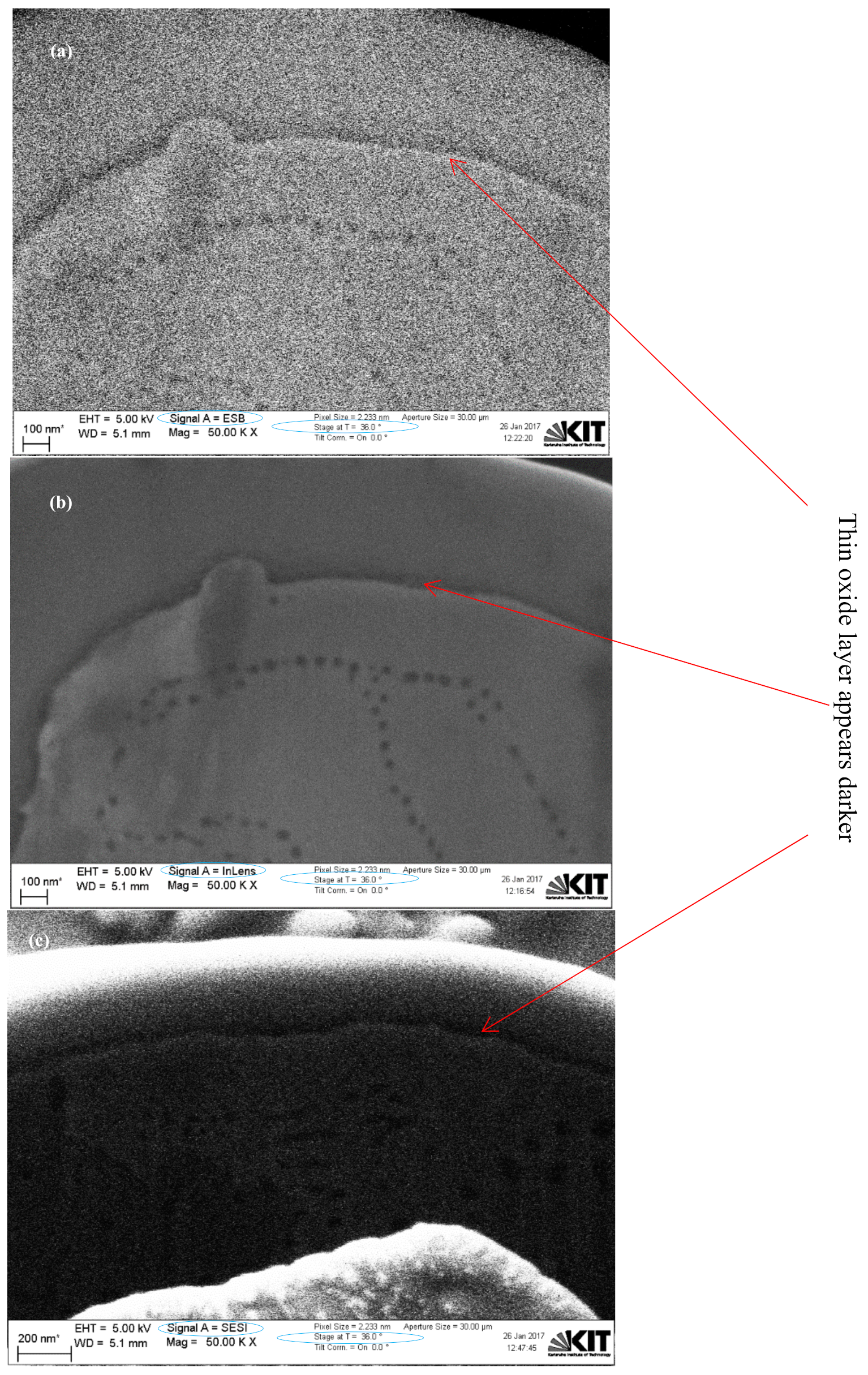
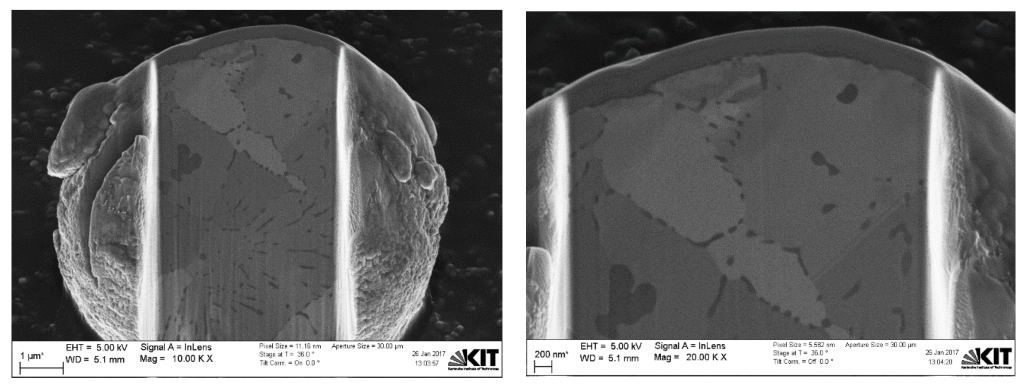
2.4. Dual-Beam SEM-FIB Characterization
3. Results and Discussions
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Clark, D.E.; Folz, D.C.; Folgar, C.E.; Mahmoud, M.M. Microwave Solutions for Ceramic Engineers; The American Ceramic Society: Westerville, OH, USA, 2006. [Google Scholar]
- Binner, J. Microwave processing of materials: London, 12 April 1991. Mater. Des. 1991, 12, 231. [Google Scholar] [CrossRef][Green Version]
- Sutton, W.H. Microwave Processing of Ceramic Materials. Am. Ceram. Soc. Bull. 1989, 68, 376–386. [Google Scholar]
- Clark, D.E.; Sutton, W.H. Microwave processing of materials. Annu. Rev. Mater. Sci. 1996, 26, 299–331. [Google Scholar] [CrossRef]
- Ge, L.; Liu, X.; Feng, H.; Jiang, H.; Zhou, T.; Chu, H.; Zhang, Y.; Xu, C.; Wang, Z. The interaction between microwave and coal: A discussion on the state-of-the-art. Fuel 2022, 314, 123140. [Google Scholar] [CrossRef]
- Zheng, H.; Hao, X.; Zhang, S.; Omran, M.; Chen, G.; Chen, J.; Gao, L. Modeling of process and analysis of drying characteristics for natural TiO2 under microwave heating. Chem. Eng. Process. Process Intensif. 2022, 174, 108900. [Google Scholar] [CrossRef]
- Shojae, K.; Khoshandam, B. The experimental and modeling study on a new process of iron/cobalt alloy production using indirect reducing agent of glycerol under microwave heating. Chem. Eng. Process. Process Intensif. 2022, 171, 108765. [Google Scholar] [CrossRef]
- Ke, C.; Liu, T.; Zhang, Y.; Xiong, Q. Energy absorption performances of silicon carbide particles during microwave heating process. Chem. Eng. Process. -Process Intensif. 2022, 172, 108796. [Google Scholar] [CrossRef]
- Ramos, P.A.V.; Albuquerque, D.M.S.; Pereira, J.C.F. Numerical simulation and optimization of the ceramic pigments production process using microwave heating. Chem. Eng. Process. Process Intensif. 2021, 169, 108567. [Google Scholar] [CrossRef]
- Hu, T.; Kou, L.; Yang, L.; Zhou, B.; Zhou, J. Micro-structural evolution of high aluminium fly ash enhanced by microwave heating to accelerate activation reaction process. Powder Technol. 2021, 377, 739–747. [Google Scholar] [CrossRef]
- Gupta, P.; Chaudhary, S.; Kumar, V.; Kumar, S.; Sahu, R. Investigation and characterization of brass joint fabricated by microwave hybrid heating process. Mater. Today: Proc. 2021, 47, 3973–3978. [Google Scholar] [CrossRef]
- Sharma, A.; Sehgal, S.; Goyal, D. Effects of process parameters in joining of Inconel-625 alloy through microwave hybrid heating. Mater. Today: Proc. 2020, 28, 1323–1327. [Google Scholar] [CrossRef]
- Staciwa, P.; Sibera, D.; Pełech, I.; Narkiewicz, U.; Łojkowski, W.; Dąbrowska, S.; Cormia, R. Effect of microwave assisted solvothermal process parameters on carbon dioxide adsorption properties of microporous carbon materials. Microporous Mesoporous Mater. 2021, 314, 110829. [Google Scholar] [CrossRef]
- Loharkar, P.K.; Ingle, A.; Jhavar, S. Parametric review of microwave-based materials processing and its applications. J. Mater. Res. Technol. 2019, 8, 3306–3326. [Google Scholar] [CrossRef]
- Taurino, R.; Karamanov, A.; Rosa, R.; Karamanova, E.; Barbieri, L.; Atanasova-Vladimirova, S.; Avdeev, G.; Leonelli, C. New ceramic materials from MSWI bottom ash obtained by an innovative microwave-assisted sintering process. J. Eur. Ceram. Soc. 2017, 37, 323–331. [Google Scholar] [CrossRef]
- Mishra, R.R.; Sharma, A.K. Microwave–material interaction phenomena: Heating mechanisms, challenges and opportunities in material processing. Compos. Part A Appl. Sci. Manuf. 2016, 81, 78–97. [Google Scholar] [CrossRef]
- Buttress, A.; Jones, A.; Kingman, S. Microwave processing of cement and concrete materials–towards an industrial reality? Cem. Concr. Res. 2015, 68, 112–123. [Google Scholar] [CrossRef]
- Fukushima, J.; Kashimura, K.; Takayama, S.; Sato, M.; Sano, S.; Hayashi, Y.; Takizawa, H. In-situ kinetic study on non-thermal reduction reaction of CuO during microwave heating. Mater. Lett. 2013, 91, 252–254. [Google Scholar] [CrossRef]
- Roy, R.; Agrawal, D.; Cheng, J.; Gedevanlshvili, S. Full sintering of powdered-metal bodies in a microwave field. Nature 1999, 399, 668–670. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Chou, T.W. Microwave processing: Fundamentals and applications. Compos. Part A Appl. Sci. Manuf. 1999, 30, 1055–1071. [Google Scholar] [CrossRef]
- Peng, Z.; Hwang, J.-Y. Microwave-assisted metallurgy. Int. Mater. Rev. 2015, 60, 30–63. [Google Scholar] [CrossRef]
- Bhatt, S.C.; Ghetiya, N.D. Review on effect of tooling parameters on microwave processing of metallic materials with special emphasis on melting/casting application. Int. J. Met. 2022, 5, 58–73. [Google Scholar] [CrossRef]
- Tayier, W.; Janasekaran, S.; Tai, V.C. Microwave hybrid heating (MHH) of Ni-based alloy powder on Ni and steel-based metals –A review on fundamentals and parameters. Int. J. Lightweight Mater. Manuf. 2022, 5, 58–73. [Google Scholar] [CrossRef]
- Rawat, S.; Samyal, R.; Bedi, R.; Kumar Bagha, A. A comparative study of interface material through selective microwave hybrid heating for joining metal plates. Mater. Today: Proc. 2022, 65, 3117–3125. [Google Scholar] [CrossRef]
- El Khaled, D.; Novas, N.; Gazquez, J.A.; Manzano-Agugliaro, F. Microwave dielectric heating: Applications on metals processing. Renew. Sustain. Energy Rev. 2018, 82, 2880–2892. [Google Scholar] [CrossRef]
- Crane, C.A.; Pantoya, M.L.; Weeks, B.L.; Saed, M. The effects of particle size on microwave heating of metal and metal oxide powders. Powder Technol. 2014, 256, 113–117. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; Link, G.; Thumm, M. The role of the native oxide shell on the microwave sintering of copper metal powder compacts. J. Alloy. Compd. 2015, 627, 231–237. [Google Scholar] [CrossRef]
- Ro¨diger, K.; Dreyer, K.; Gerdes, T.; Willert-Porada, M. Microwave sintering of hardmetals. Int. J. Refract. Met. Hard Mater. 1998, 16, 409–416. [Google Scholar] [CrossRef]
- Sarkar, S.; Jana, P.K.; Chaudhuri, B.K.; Sakata, H. Copper (II) oxide as a giant dielectric material. Appl. Phys. Lett. 2006, 89, 212905. [Google Scholar] [CrossRef]
- Volkovskaya, I.I.; Semenov, V.E. Scattering and Absorption of Electromagnetic Waves in the Case of Excitation of Dipole Modes of a Spherical Particle. Radiophys. Quantum Electron. 2021, 64, 38–53. [Google Scholar] [CrossRef]
- Zhai, D.; Zhang, F.; Wei, C.; Shang, X.; Chen, J.; Liu, M.; Peng, J. Dynamic absorption efficiency of paracetamol powder in microwave drying. High Temp Mater Process. 2019, 38, 715–725. [Google Scholar] [CrossRef]
- Han, Z.; Xu, L.; Guo, S.; Wu, Q.; Zhang, L.; Liu, J. Study on the microwave sintering characteristics of spherical tin-silver alloy powder. Mater. Res. Express 2019, 6, 1265k7. [Google Scholar] [CrossRef]
- Rybakov, K.I.; Volkovskaya, I.I. Electromagnetic field effects in the microwave sintering of electrically conductive powders. Ceram. Int. 2019, 45, 9567–9572. [Google Scholar] [CrossRef]
- Volkovskaya, I.I.; Semenov, V.E.; Rybakov, K.I. Effective High-Frequency Permeability of Compacted Metal Powders. Radiophys. Quantum Electron. 2018, 60, 797–807. [Google Scholar] [CrossRef]
- Rybakov, K.I.; Semenov, V.E.; Volkovskaya, I.I. Microwave Heating of Metal Power Clusters. Tech. Phys. 2018, 63, 45–50. [Google Scholar] [CrossRef]
- Rybakov, K.I.; Buyanova, M.N. Microwave resonant sintering of powder metals. Scr. Mater. 2018, 149, 108–111. [Google Scholar] [CrossRef]
- Volkovskaya, I.I.; Semenov, V.E.; Rybakov, K.I. Effective magnetic permeability of compacted metal powders at microwave frequencies. In Proceedings of the 10th International Workshop 2017 “Strong Microwaves and Terahertz Waves: Sources and Applications”, Nizhny Novgorod, Russia, 17–22 July 2017. [Google Scholar]
- Rybakov, K.I.; Semenov, V.E. Effective Microwave Dielectric Properties of Ensembles of Spherical Metal Particles. IEEE Trans. Microw. Theory Tech. 2017, 65, 1479–1487. [Google Scholar] [CrossRef]
- Ertugrul, O.; Park, H.S.; Onel, K.; Willert-Porada, M. Effect of particle size and heating rate in microwave sintering of 316L stainless steel. Powder Technol. 2014, 253, 703–709. [Google Scholar] [CrossRef]
- Rybakov, K.I.; Semenov, V.E.; Egorov, S.V.; Eremeev, A.G.; Plotnikov, I.V.; Bykov, Y.V. Microwave heating of conductive powder materials. J. Appl. Phys. 2006, 99, 023506. [Google Scholar] [CrossRef]
- Mathieu, H.J.; Datta, M.; Landolt, D. Thickness of natural oxide films determined by AES and XPS with/without sputtering. J. Vac. Sci. Technol. A 1985, 3, 331–335. [Google Scholar] [CrossRef]
- Nirschl, H.; Guo, X. Characterisation of structured and functionalised particles by small-angle X-ray scattering (SAXS). Chem. Eng. Res. Des. 2018, 136, 431–446. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, Y.; Peng, X.; Zhong, K.; Lin, Y.; Huang, Z. The Phase Evolution and Physical Properties of Binary Copper Oxide Thin Films Prepared by Reactive Magnetron Sputtering. Materials 2018, 11, 1253. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, M.; Ren, S.; Ling, H.; Hang, T.; Hu, A.-m.; Li, M. The influence of non-uniform copper oxide layer on tin whisker growth and tin whisker growth behavior in SnAg microbumps with small diameter. Mater. Lett. 2020, 258, 126773. [Google Scholar] [CrossRef]
- Musztyfaga-Staszuk, M.; Janicki, D.; Gawlińska-Nęcek, K.; Socha, R.; Putynkowski, G.; Panek, P. Copper Oxides on a Cu Sheet Substrate Made by Laser Technique. Materials 2020, 13, 3794. [Google Scholar] [CrossRef] [PubMed]
- Swanson, H.E.; McMurdie, H.F.; Morris, M.C.; Evans, E.H. Standard X-ray diffraction powder patterns. Natl. Bur. Stand. U.S. Circ. 1953, 359, 1. [Google Scholar]
- Cullity, B.D.; Stock, S.R.; Stock, S.R. Elements of X-Ray Diffraction; Prentice Hall: Hoboken, NJ, USA, 2001. [Google Scholar]
- Castle, J.E. Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy; Briggs, D., Seah, M.P., Eds.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 1983; 533. [Google Scholar] [CrossRef]
- Neelisetty, K.K.; Mu, X.; Gutsch, S.; Vahl, A.; Molinari, A.; von Seggern, F.; Hansen, M.; Scherer, T.; Zacharias, M.; Kienle, L.; et al. Electron Beam Effects on Oxide Thin Films—Structure and Electrical Property Correlations. Microsc. Microanal. 2019, 25, 592–600. [Google Scholar] [CrossRef]







| Type | Particle Size | wt% |
|---|---|---|
| Cu spherical less than 149 µm 99.95% Stock Nr. 11 070 | less than 44 µm | 53.8% |
| greater than 44 µm–less than 74 µm | 24.0% | |
| greater than 74 µm–less than 149 µm | 21.9% | |
| greater than 149 µm | 0.3% | |
| Cu spherical APS 10 µm 99.9% Stock Nr. 42 689 | less than 7.39 µm | 10% |
| less than 9.78 µm | 50% | |
| less than 14.05 µm | 90% |
| Detection Limit (wt. %) | Cu Powder with 10 µm APS | Cu Powder with Less Than 149 µm | ||
|---|---|---|---|---|
| Oxygen Content Mean (wt.%) | SD | Oxygen Content Mean (wt.%) | SD | |
| 0.006 | 0.573 | 0.012 | 0.578 | 0.037 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, M.M. Characterization of the Native Oxide Shell of Copper Metal Powder Spherical Particles. Materials 2022, 15, 7236. https://doi.org/10.3390/ma15207236
Mahmoud MM. Characterization of the Native Oxide Shell of Copper Metal Powder Spherical Particles. Materials. 2022; 15(20):7236. https://doi.org/10.3390/ma15207236
Chicago/Turabian StyleMahmoud, Morsi M. 2022. "Characterization of the Native Oxide Shell of Copper Metal Powder Spherical Particles" Materials 15, no. 20: 7236. https://doi.org/10.3390/ma15207236
APA StyleMahmoud, M. M. (2022). Characterization of the Native Oxide Shell of Copper Metal Powder Spherical Particles. Materials, 15(20), 7236. https://doi.org/10.3390/ma15207236






