Spectroscopic and Physicochemical Investigations of Azomethines with Triphenylamine Core towards Optoelectronics
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Measurements
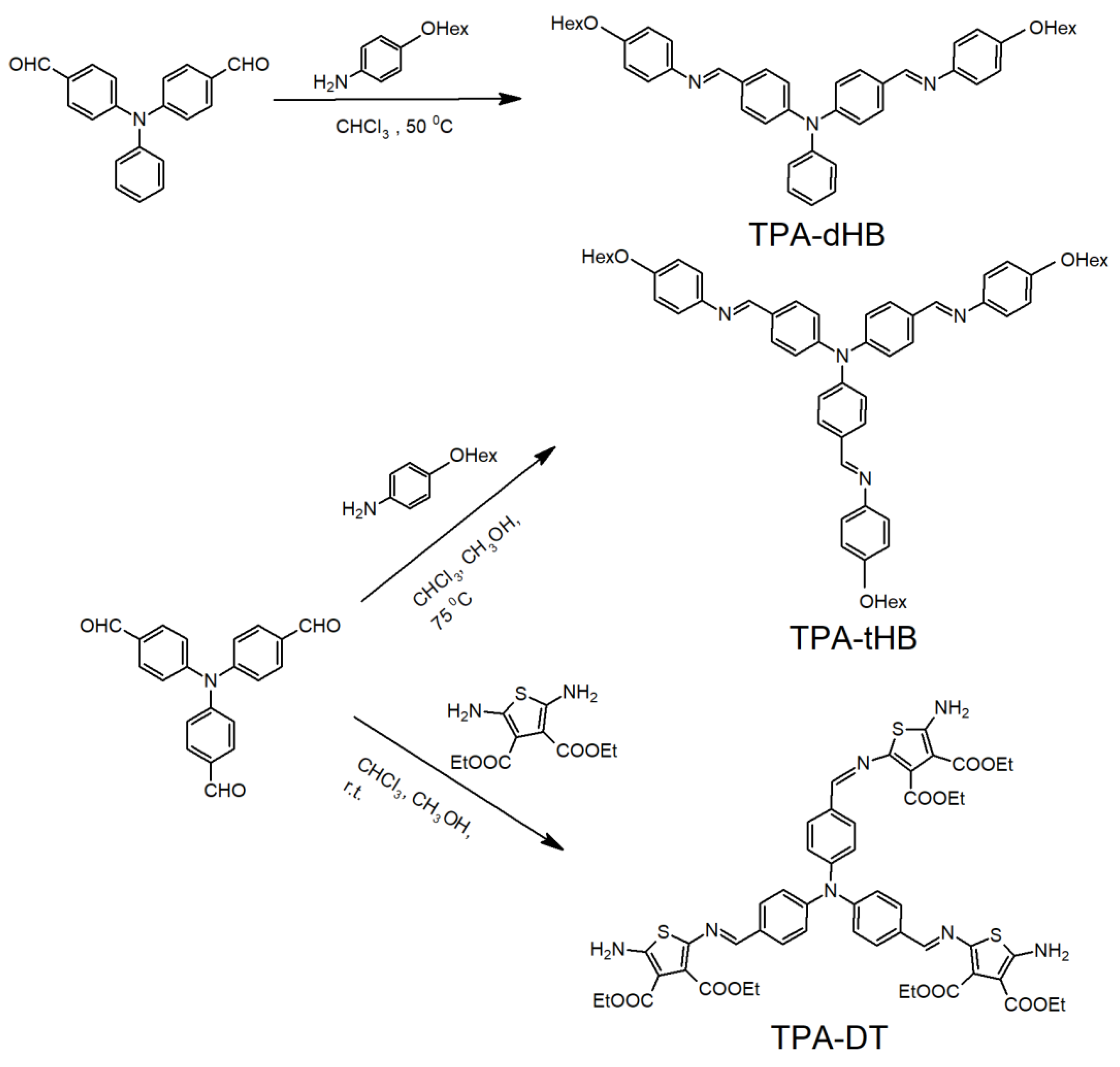
2.3. Synthesis of Triphenylamine Based Azomethines
2.3.1. Synthesis of TPA-DT
2.3.2. Synthesis of TPA-tHB
2.3.3. Synthesis of TPA-dHB
3. Results and Discussion
3.1. Structural Characterization
3.2. Thermal Properties
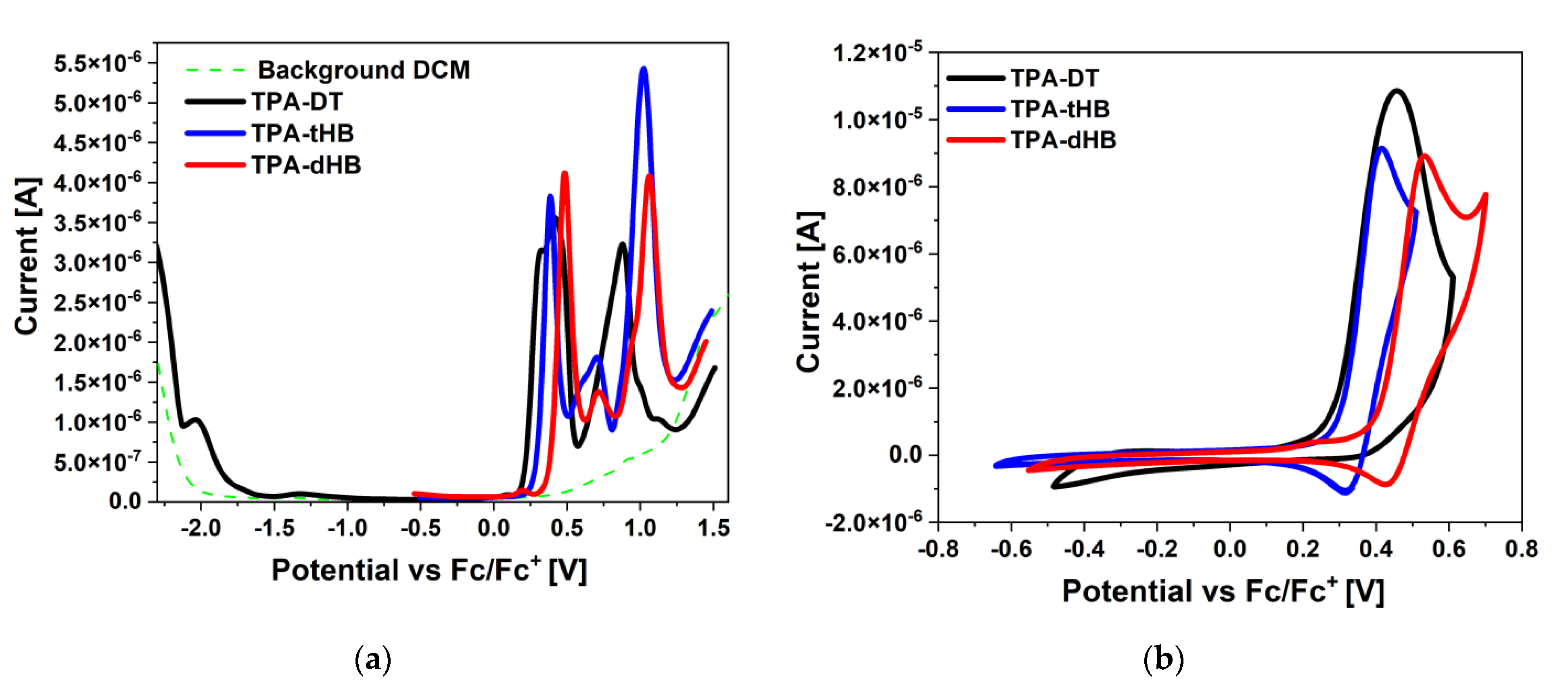
3.3. Redox Properties
3.4. DFT Calculations
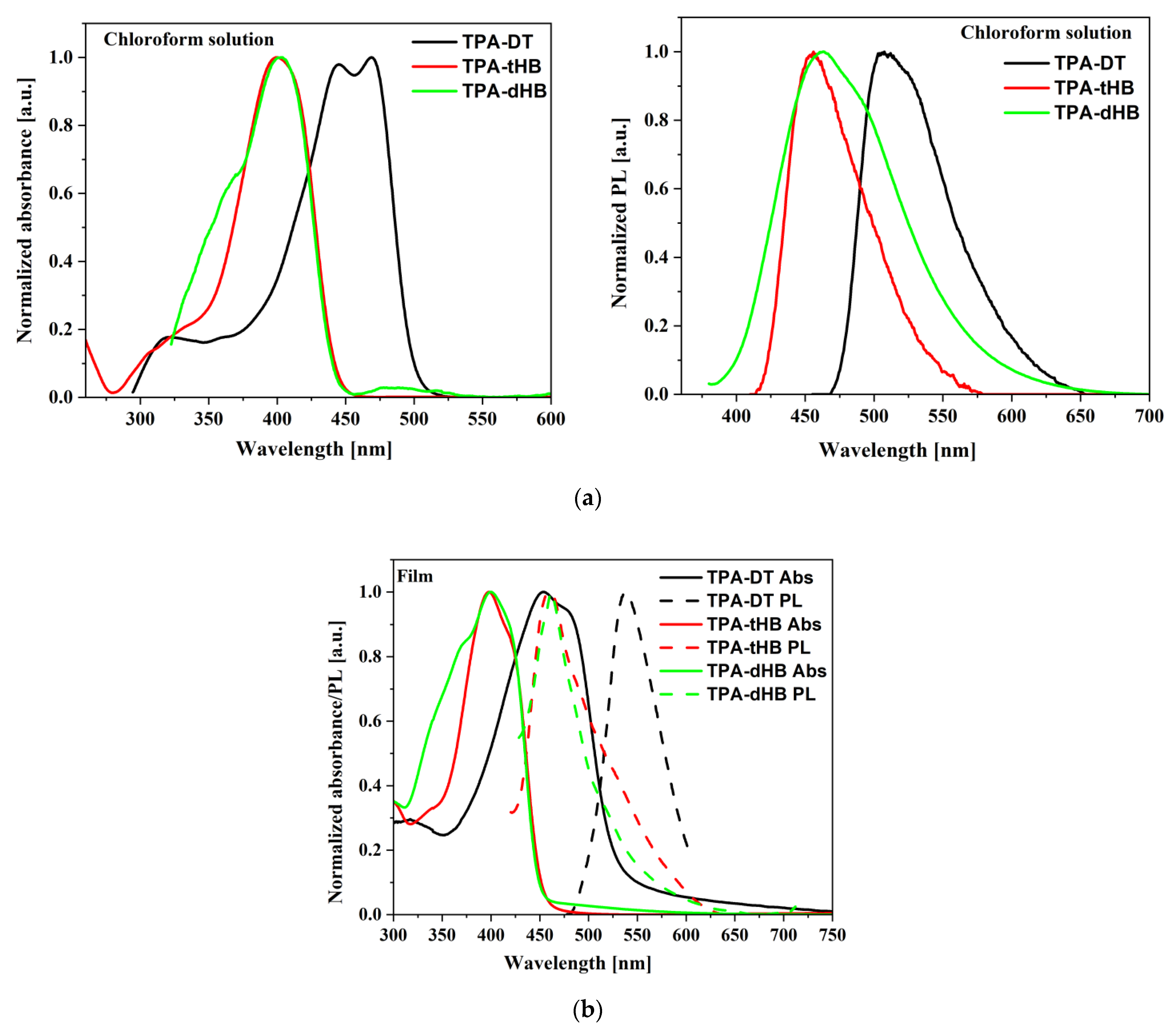
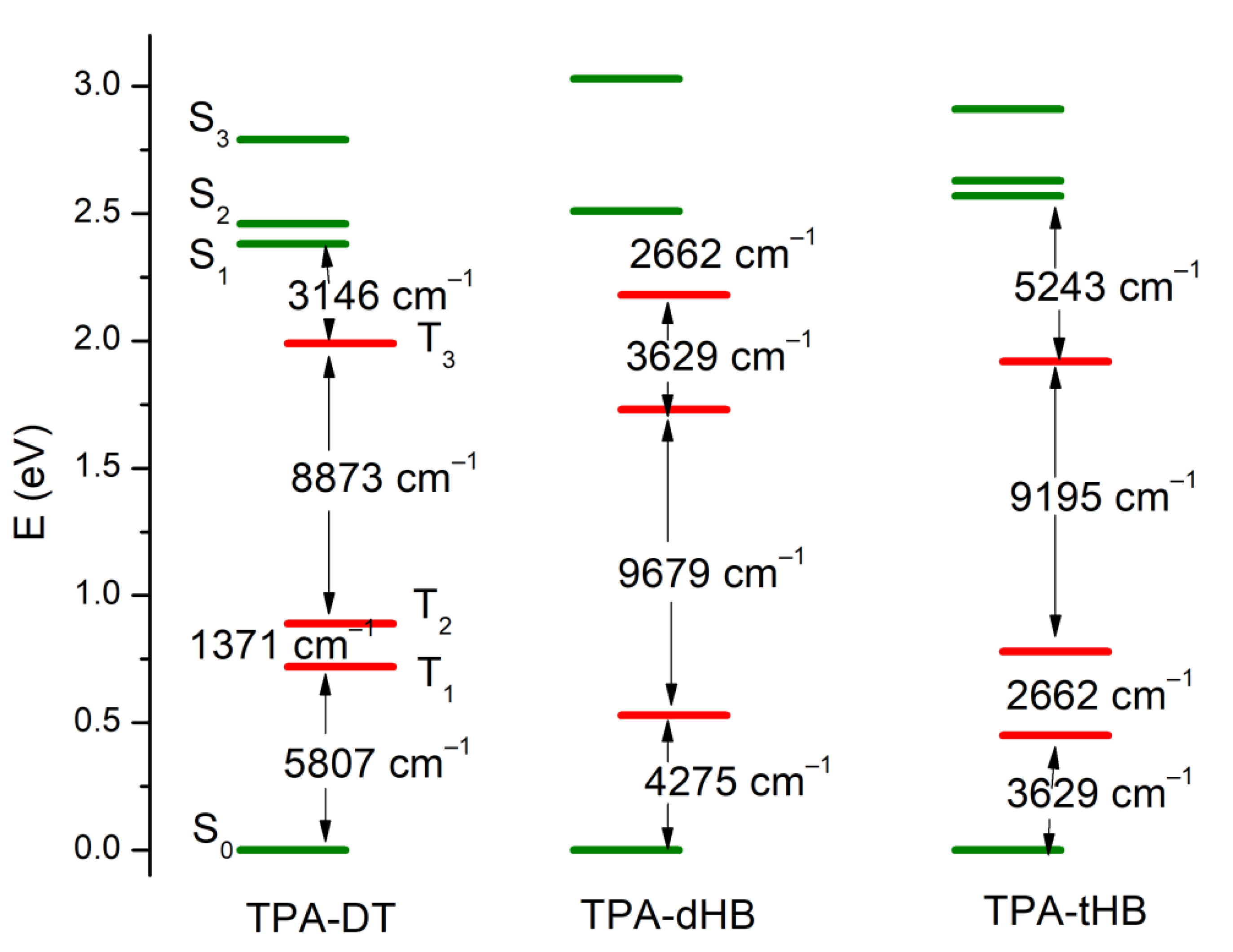
3.5. Photophysical Properties
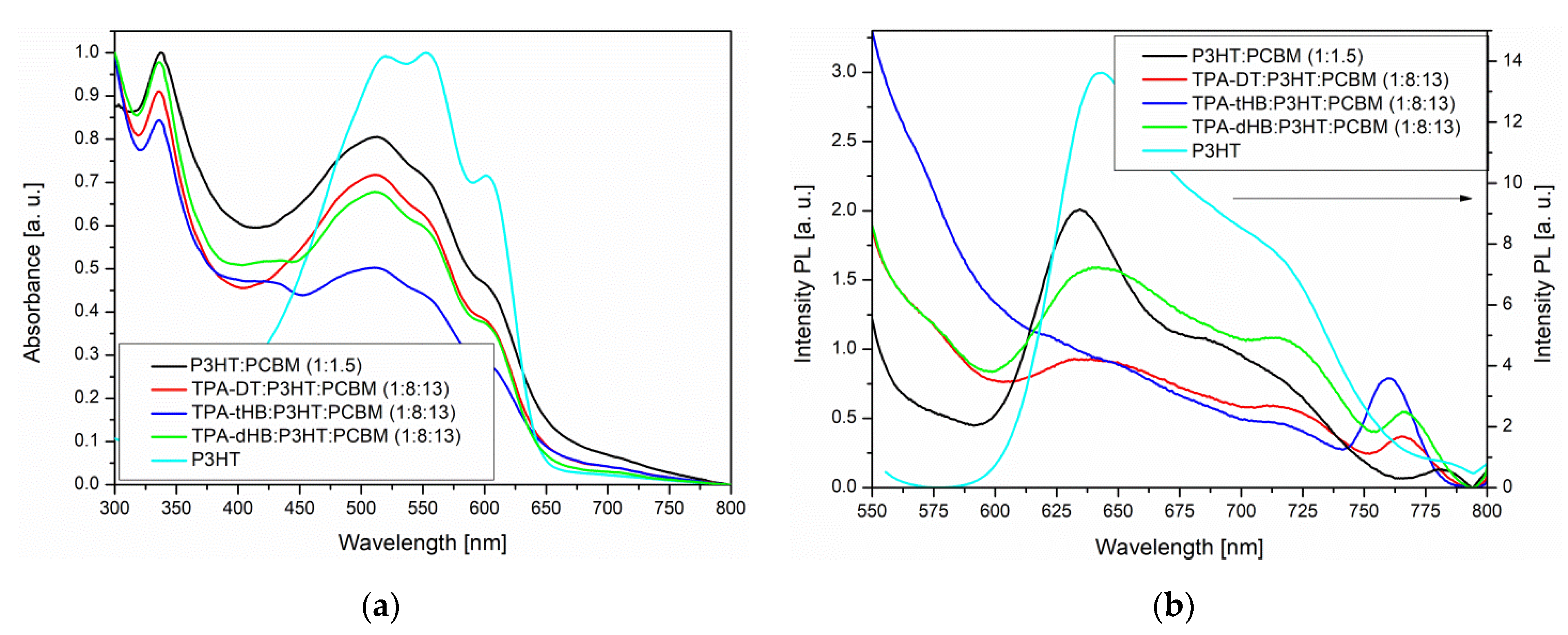
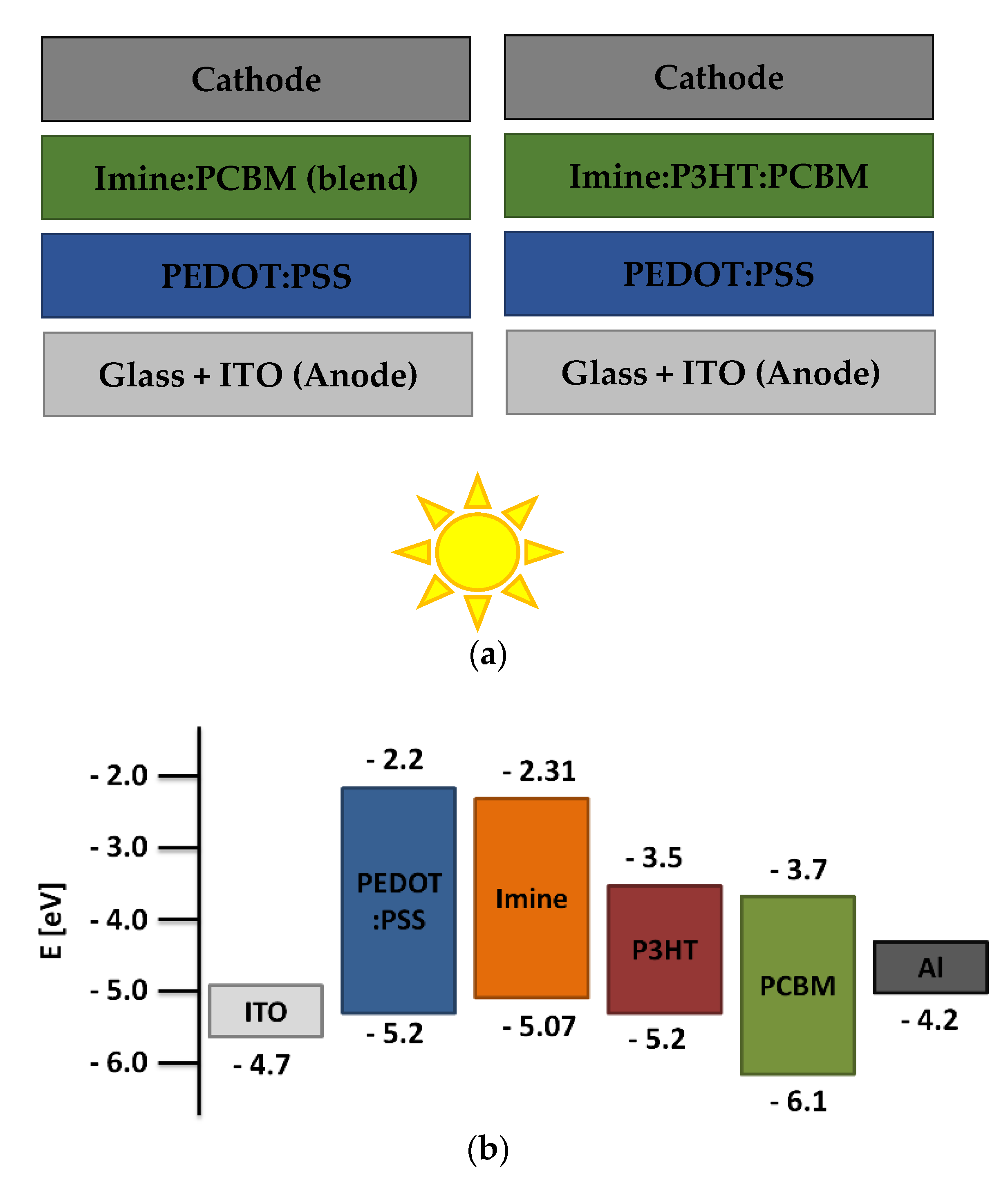
3.6. Photovoltaic Tests
4. Conclusions
- (a)
- Substitution of TPA with amino-thiophene-3,4-dicarboxylic acid diethyl ester let to obtained thermally induced amorphous material with high Tg, and on the other hand it resulted in a decrease in thermal stability compared to azomethine with hexyloxyphenyl structures;
- (b)
- The imine with three hexyloxyphenyl units undergoes oxidation slightly easier, but in the case of azomethines with such substituent reduction was not observed;
- (c)
- Replacement of hexyloxyphenyl groups with amino-thiophene-3,4-dicarboxylic acid diethyl ester units leading to the wide absorption window;
- (d)
- Addition of the synthesized imines to the P3HT:PCBM blend caused emission quenching, thus rationalizing testing them as donors in BHJ solar cells;
- (e)
- The best donor activity showed imine with thiophene rings, and the devices based on its blend with P3HT and PCBM showed the highest JSC of 8.20 mAcm−2, which results in the best of PCE.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiang, C.K.; Fincher, C., Jr.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; Gau, S.C.; MacDiarmid, A.G. Electrical conductivity in doped polyacetylene. Phys. Rev. Lett. 1977, 39, 1098. [Google Scholar] [CrossRef]
- McGehee, M.; Miller, E.K.; Moses, D.; Heeger, A.J. Twenty years of conducting polymers: From fundamental science to applications. Adv. Synth. Met. Twenty Years Prog. Sci. Technol. 1999, 98–205. [Google Scholar]
- Fujita, M.; Ishihara, K.; Ueno, T.; Asano, T.; Noda, S.; Ohata, H.; Tsuji, T.; Nakada, H.; Shimoji, N. Optical and electrical characteristics of organic light-emitting diodes with two-dimensional photonic crystals in organic/electrode layers. Jpn. J. Appl. Phys. 2005, 44, 3669. [Google Scholar] [CrossRef]
- Braun, D. Semiconducting polymer LEDs. Mater. Today 2002, 5, 32–39. [Google Scholar] [CrossRef]
- He, C.; He, Q.; Yi, Y.; Wu, G.; Bai, F.; Shuai, Z.; Li, Y. Improving the efficiency of solution processable organic photovoltaic devices by a star-shaped molecular geometry. J. Mater. Chem. 2008, 18, 4085–4090. [Google Scholar] [CrossRef]
- Bronstein, H.; Chen, Z.; Ashraf, R.S.; Zhang, W.; Du, J.; Durrant, J.R.; Shakya Tuladhar, P.; Song, K.; Watkins, S.E.; Geerts, Y. Thieno [3, 2-b] thiophene—diketopyrrolopyrrole-containing polymers for high-performance organic field-effect transistors and organic photovoltaic devices. J. Am. Chem. Soc. 2011, 133, 3272–3275. [Google Scholar] [CrossRef]
- Bundgaard, E.; Krebs, F.C. Low band gap polymers for organic photovoltaics. Sol. Energy Mater. Sol. Cells 2007, 91, 954–985. [Google Scholar] [CrossRef]
- Dufresne, S.; Skene, W. Optoelectronic property tailoring of conjugated heterocyclic azomethines–the effect of pyrrole, thiophene and furans. J. Phys. Org. Chem. 2012, 25, 211–221. [Google Scholar] [CrossRef]
- Kiriy, N.; Bocharova, V.; Kiriy, A.; Stamm, M.; Krebs, F.C.; Adler, H.-J. Designing thiophene-based azomethine oligomers with tailored properties: Self-assembly and charge carrier mobility. Chem. Mater. 2004, 16, 4765–4771. [Google Scholar] [CrossRef]
- Iwan, A.; Mazurak, Z.; Kaczmarczyk, B.; Jarzabek, B.; Sek, D. Synthesis and characterization of polyketanils with 3, 8-diamino-6-phenylphenanthridine moieties exhibiting light emitting properties: Molecular and supramolecular engineering concept. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 69, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Palewicz, M.; Iwan, A.; Sibinski, M.; Sikora, A.; Mazurek, B. Organic photovoltaic devices based on polyazomethine and fullerene. Energy Procedia 2011, 3, 84–91. [Google Scholar] [CrossRef][Green Version]
- Iwan, A.; Palewicz, M.; Chuchmała, A.; Gorecki, L.; Sikora, A.; Mazurek, B.; Pasciak, G. Opto (electrical) properties of new aromatic polyazomethines with fluorene moieties in the main chain for polymeric photovoltaic devices. Synth. Met. 2012, 162, 143–153. [Google Scholar] [CrossRef]
- Hindson, J.C.; Ulgut, B.; Friend, R.H.; Greenham, N.C.; Norder, B.; Kotlewski, A.; Dingemans, T.J. All-aromatic liquid crystal triphenylamine-based poly (azomethine) s as hole transport materials for opto-electronic applications. J. Mater. Chem. 2010, 20, 937–944. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Chen, J.; Bai, F.-Q.; Zhang, H.-X. Theoretical investigation of triphenylamine-based sensitizers with different π-spacers for DSSC. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Cekaviciute, M.; Simokaitiene, J.; Jankauskas, V.; Raisys, S.; Kazlauskas, K.; Jursenas, S.; Grazulevicius, J. Structure–Properties Relationship of Phenylethenyl-Substituted Triphenylamines. J. Phys. Chem. C 2013, 117, 7973–7980. [Google Scholar] [CrossRef]
- Kato, S.-I.; Matsuoka, T.; Suzuki, S.; Asano, M.S.; Yoshihara, T.; Tobita, S.; Matsumoto, T.; Kitamura, C. Synthesis, structures, and properties of neutral and radical cationic S, C, C-bridged triphenylamines. Org. Lett. 2019, 22, 734–738. [Google Scholar] [CrossRef]
- Narayanaswamy, K.; Swetha, T.; Kapil, G.; Pandey, S.S.; Hayase, S.; Singh, S.P. Simple metal-free dyes derived from triphenylamine for DSSC: A comparative study of two different anchoring group. Electrochim. Acta 2015, 169, 256–263. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Li, X.; Zhu, L.; Meng, Q.; Xiao, Y.; Li, D. Novel hole transporting materials with a linear π-conjugated structure for highly efficient perovskite solar cells. Chem. Commun. 2014, 50, 5829–5832. [Google Scholar] [CrossRef] [PubMed]
- Cias, P.; Slugovc, C.; Gescheidt, G. Hole transport in triphenylamine based OLED devices: From theoretical modeling to properties prediction. J. Phys. Chem. A 2011, 115, 14519–14525. [Google Scholar] [CrossRef] [PubMed]
- Lumpi, D.; Holzer, B.; Bintinger, J.; Horkel, E.; Waid, S.; Wanzenböck, H.D.; Marchetti-Deschmann, M.; Hametner, C.; Bertagnolli, E.; Kymissis, I. Substituted triphenylamines as building blocks for star shaped organic electronic materials. New J. Chem. 2015, 39, 1840–1851. [Google Scholar] [CrossRef]
- Gudeika, D.; Bundulis, A.; Mihailovs, I.; Volyniuk, D.; Rutkis, M.; Grazulevicius, J.V. Donor and acceptor substituted triphenylamines exhibiting bipolar charge-transporting and NLO properties. Dye. Pigment. 2017, 140, 431–440. [Google Scholar] [CrossRef]
- Malinauskas, T.; Tomkute-Luksiene, D.; Daskeviciene, M.; Jankauskas, V.; Juska, G.; Gaidelis, V.; Arlauskas, K.; Getautis, V. One small step in synthesis, a big leap in charge mobility: Diphenylethenyl substituted triphenylamines. Chem. Commun. 2011, 47, 7770–7772. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.D.; Hu, H.; Feron, K.; Manzhos, S.; Wang, H.; Lam, Y.M.; Sonar, P. Thienylvinylenethienyl and naphthalene core substituted with triphenylamines—Highly efficient hole transporting materials and their comparative study for inverted perovskite solar cells. Sol. RRL 2017, 1, 1700105. [Google Scholar] [CrossRef]
- Berson, S.; Cecioni, S.; Billon, M.; Kervella, Y.; de Bettignies, R.; Bailly, S.; Guillerez, S. Effect of carbonitrile and hexyloxy substituents on alternated copolymer of polythiophene–Performances in photovoltaic cells. Sol. Energy Mater. Sol. Cells 2010, 94, 699–708. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Jewłoszewicz, B.; Iwan, A.; Dysz, K.; Przybyl, W.; Januszko, A.; Marzec, M.; Cichy, K.; Świerczek, K.; Kavan, L. Selected Electrochemical Properties of 4, 4’-((1E, 1’E)-((1, 2, 4-thiadiazole-3, 5-diyl) bis (azaneylylidene)) bis (methaneylylidene)) bis (N, N-di-p-tolylaniline) towards Perovskite Solar Cells with 14.4% Efficiency. Materials 2020, 13, 2440. [Google Scholar] [CrossRef] [PubMed]
- Korzec, M.; Kotowicz, S.; Pająk, A.K.; Schab-Balcerzak, E. Symmetrical and asymmetrical imino-naphthalimides in perovskite solar cells. Opto-Electron. Rev. 2021, 4, 175–180. [Google Scholar]
- Nitschke, P.; Jarząbek, B.; Damaceanu, M.-D.; Bejan, A.-E.; Chaber, P. Spectroscopic and electrochemical properties of thiophene-phenylene based Shiff-bases with alkoxy side groups, towards photovoltaic applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119242. [Google Scholar] [CrossRef] [PubMed]
- Damaceanu, M.-D.; Constantin, C.-P.; Bejan, A.-E.; Mihaila, M.; Kusko, M.; Diaconu, C.; Mihalache, I.; Pascu, R. Heteroatom-mediated performance of dye-sensitized solar cells based on T-shaped molecules. Dye. Pigment. 2019, 166, 15–31. [Google Scholar] [CrossRef]
- Bourgeaux, M.; Vomscheid, S.; Skene, W. Optimized Synthesis and Simple Purification of 2, 5-Diamino-thiophene-3, 4-dicarboxylic Acid Diethyl Ester. Synth. Commun. 2007, 37, 3551–3558. [Google Scholar] [CrossRef]
- Bujak, P.; Kulszewicz-Bajer, I.; Zagorska, M.; Maurel, V.; Wielgus, I.; Pron, A. Polymers for electronics and spintronics. Chem. Soc. Rev. 2013, 42, 8895–8999. [Google Scholar] [CrossRef]
- Data, P.; Pander, P.; Zassowski, P.; Mimaite, V.; Karon, K.; Lapkowski, M.; Grazulevicius, J.; Slepski, P.; Darowicki, K. Electrochemically induced synthesis of triphenylamine-based polyhydrazones. Electrochim. Acta 2017, 230, 10–21. [Google Scholar] [CrossRef]
- Bourgeaux, M.; Skene, W. A highly conjugated p-and n-type polythiophenoazomethine: Synthesis, spectroscopic, and electrochemical investigation. Macromolecules 2007, 6, 1792–1795. [Google Scholar] [CrossRef]
- Pająk, A.K.; Kotowicz, S.; Gnida, P.; Małecki, J.G.; Ciemięga, A.; Łuczak, A.; Jung, J.; Schab-Balcerzak, E. Synthesis and Characterization of New Conjugated Azomethines End-Capped with Amino-thiophene-3,4-dicarboxylic Acid Diethyl Ester. Int. J. Mol. Sci. 2022, 23, 8160. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-J.; Liou, G.-S. Novel blue and red electrochromic poly (azomethine ether) s based on electroactive triphenylamine moieties. Org. Electron. 2010, 11, 299–310. [Google Scholar] [CrossRef]
- Li, Y.; Sonar, P.; Murphy, L.; Hong, W. High mobility diketopyrrolopyrrole (DPP)-based organic semiconductor materials for organic thin film transistors and photovoltaics. Energy Environ. Sci. 2013, 6, 1684–1710. [Google Scholar] [CrossRef]
- Wałęsa-Chorab, M.; Tremblay, M.H.; Skene, W.G. Hydrogen-Bond and Supramolecular-Contact Mediated Fluorescence Enhancement of Electrochromic Azomethines. Chem.–A Eur. J. 2016, 22, 11382–11393. [Google Scholar] [CrossRef]
- Más-Montoya, M.; Janssen, R.A. The effect of H-and J-aggregation on the photophysical and photovoltaic properties of small thiophene–pyridine–DPP molecules for bulk-heterojunction solar cells. Adv. Funct. Mater. 2017, 27, 1605779. [Google Scholar] [CrossRef]
- Pajak, A.K.; Gnida, P.; Kotowicz, S.; Malecki, J.G.; Libera, M.; Bednarczyk, K.; Schab-Balcerzak, E. New thiophene imines acting as hole transporting materials in photovoltaic devices. Energy Fuels 2020, 34, 10160–10169. [Google Scholar] [CrossRef]
- Bourgeaux, M.; Guarìn, S.A.P.; Skene, W. Photophysical, crystallographic, and electrochemical characterization of novel conjugated thiopheno azomethines. J. Mater. Chem. 2007, 17, 972–979. [Google Scholar] [CrossRef]
- Kaim, A.; Piotrowski, P.; Zarębska, K.; Bogdanowicz, K.A.; Przybył, W.; Kwak, A.; Skompska, M.; Gnida, P.; Schab-Balcerzak, E.; Iwan, A. Thermal imaging and deep optical and electrochemical study of C70 fullerene derivatives with thiophene, pyrrolidine or indene moieties along with electropolymerization with thiophene substituted imine: Blends with P3HT and PTB7. Electrochim. Acta 2022, 426, 140741. [Google Scholar] [CrossRef]
- Nismy, N.A.; Jayawardena, K.I.; Adikaari, A.D.T.; Silva, S.R.P. Photoluminescence Quenching in Carbon Nanotube-Polymer/Fullerene Films: Carbon Nanotubes as Exciton Dissociation Centres in Organic Photovoltaics. Adv. Mater. 2011, 23, 3796–3800. [Google Scholar] [CrossRef] [PubMed]
- Samah, A.M.; Sharif, M.A.; Al-Esseili, R.; Al-Wahish, M.A.; Hodali, H.A.; Müller-Buschbaum, P.; Schmidt-Mende, L.; Al-Hussein, M. Photovoltaic cells based on ternary P3HT:PCBM:Ruthenium(II) complex bearing 8-(diphenylphosphino)quinoline active layer. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126685. [Google Scholar] [CrossRef]
- von Hauff, E.; Parisi, J.; Dyakonov, V. Field effect measurements on charge carrier mobiliries in various polymer-fullerene blend compositions. Thin Solid Film. 2006, 511, 506–511. [Google Scholar] [CrossRef]
- Cook, S.; Katoh, R.; Furube, A. Ultrafast studies of charge generation in PCBM:P3HT blend films following excitation of the fullerene PCBM. J. Phys. Chem. C 2009, 113, 2547–2552. [Google Scholar] [CrossRef]
- Burak, K.; Fakher, A.R.K.; Ahmed, S.A.-A.; Hussain, A.B. Morphological, structural, optical, and photovoltaic cell of copolymer P3HT:ICBA and P3HT:PCBM. Opt.—Int. J. Light Electron Opt. 2020, 204, 164153. [Google Scholar] [CrossRef]
- Chirvase, D.; Parisi, J.; Hummelen, J.C.; Dyakonov, V. Influence of nanomorphology on the photovoltaic action of polymer-fullerene composites. Nanotechnology 2004, 15, 1317. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Jewloszewicz, B.; Dysz, K.; Przybyl, W.; Dylong, A.; Mech, W.; Korona, K.P.; Skompska, M.; Kaim, A.; Kamińska, M. Electrochemical and optical studies of new symmetrical and unsymmetrical imines with thiazole and thiophene moieties. Electrochim. Acta 2020, 332, 135476. [Google Scholar] [CrossRef]
- Iwan, A.; Boharewicz, B.; Tazbir, I.; Malinowski, M.; Filapek, M.; Kłąb, T.; Luszczynska, B.; Glowacki, I.; Korona, K.P.; Kaminska, M. New environmentally friendly polyazomethines with thiophene rings for polymer solar cells. Sol. Energy 2015, 117, 246–259. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Qin, C.; Yang, X.; Yasuda, T.; Islam, A.; Zhang, K.; Peng, W.; Chen, W.; Han, L. A dopant-free hole-transporting material for efficient and stable perovskite solar cells. Energy Environ. Sci. 2014, 7, 2963–2967. [Google Scholar] [CrossRef]
| Code | DSC | TGA | |||
|---|---|---|---|---|---|
| Tm [°C] | Tg [°C] | T5 [°C] | T10 [°C] | Tmax [°C] | |
| TPA-DT | 172 | 147 | 277 | 312 | 197, 293, 395 |
| TPA-tHB | 102 | - | 393 | 409 | 432 |
| TPA-dHB | 79 | - | 380 | 400 | 439 |
| Code | Method | Ered 1 | Ered 1(onset) | Eox 1 | Eox 1(onset) | Eox 2 | Eox 3 | ELUMO | LUMO a | EHOMO | HOMO a | Eg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [V] | [V] | [V] | [V] | [V] | [V] | [eV] | [eV] | [eV] | [eV] | [eV] | ||
| TPA-DT | CV | −2.11 | −1.84 | 0.45 | 0.26 | 0.90 | nd | −3.26 | −2.31 | −5.36 | −5.07 | 2.10 |
| DPV | −2.03 | −1.78 | 0.40 | 0.20 | 0.88 | nd | −3.32 | −5.30 | 1.98 | |||
| TPA-tHB | CV | nd | nd | 0.42 | 0.29 | 0.72 | 1.07 | −2.62 c | −2.09 | −5.39 | −5.34 | 2.77 b |
| DPV | nd | nd | 0.38 | 0.28 | 0.71 | 1.02 | −2.61 c | −5.38 | ||||
| TPA-dHB | CV | nd | nd | 0.53 | 0.40 | 0.76 | 1.22 | −2.68 c | −2.13 | −5.50 | −5.35 | 2.82 b |
| DPV | nd | nd | 0.49 | 0.35 | 0.70 | 1.06 | −2.63 c | −5.45 |
| Code | Medium | UV–Vis | PL | ||
|---|---|---|---|---|---|
| λmax (nm), (b ε·104) | λem (nm) | Stokes Shifts (cm−1) | Φ (%) | ||
| TPA-DT | a C6H5Cl | 287 (0.7), 317 sh (0.4), 447 (3.1), 471 (3.2) | - | - | - |
| a CHCl3 | 321 sh (1.8), 445 (11), 469 (10.9) | 503 | 2591 | 2.12 | |
| a CH2Cl2 | 250 (1.0), 320 sh (0.6), 445 (2.7), 466 (2.6) | 515 | 2979 | 0.36 | |
| Film | 317, 455, 483 sh | 532 | 3675 | 0.22 | |
| imine:PCBM | 339, 450 sh | 400 | 4498 | - | |
| imine:P3HT:PCBM | 333, 435 sh, 511, 556, 604 | 380 | 3714 | - | |
| TPA-tHB | a C6H5Cl | 288 (1.2), 400 (3.6) | - | - | - |
| a CHCl3 | 324 (2.1), 330 sh, 400 (11.4) | 454 | 2974 | 0.06 | |
| a CH2Cl2 | 242 (1.1), 295 (0.6), 336 sh, 398 (3.5) | 455 | 3148 | 0.50 | |
| Film | 339 sh, 398, 422 sh | 460 | 3386 | 0.84 | |
| imine:PCBM | 334, 401 | 390 | 4299 | - | |
| imine:P3HT:PCBM | 336, 430 sh, 506, 509, 555 sh, 605 sh | 393 | 4316 | - | |
| TPA-dHB | a C6H5Cl | 289 (1.9), 367 sh (1.6), 400 (1.1) | - | - | - |
| a CHCl3 | 364 sh (4.6), 401 (6.7), 478 sh (0.4) | 460 | 3199 | 0.10 | |
| a CH2Cl2 | 248 (0.6), 278 (0.5), 364 sh (1.2), 401 (2.0) | 456 | 3008 | 0.04 | |
| Film | 370 sh, 400 | 460 | 3261 | 2.22 | |
| imine:PCBM | 334, 405 | 449 | 3624 | - | |
| imine:P3HT:PCBM | 335, 512, 556 sh, 605 sh | 388 | 7668 | - | |
| P3HT | Donor:PCBM | 336, 502, 561 sh, 607 sh | 387 | 3922 | - |
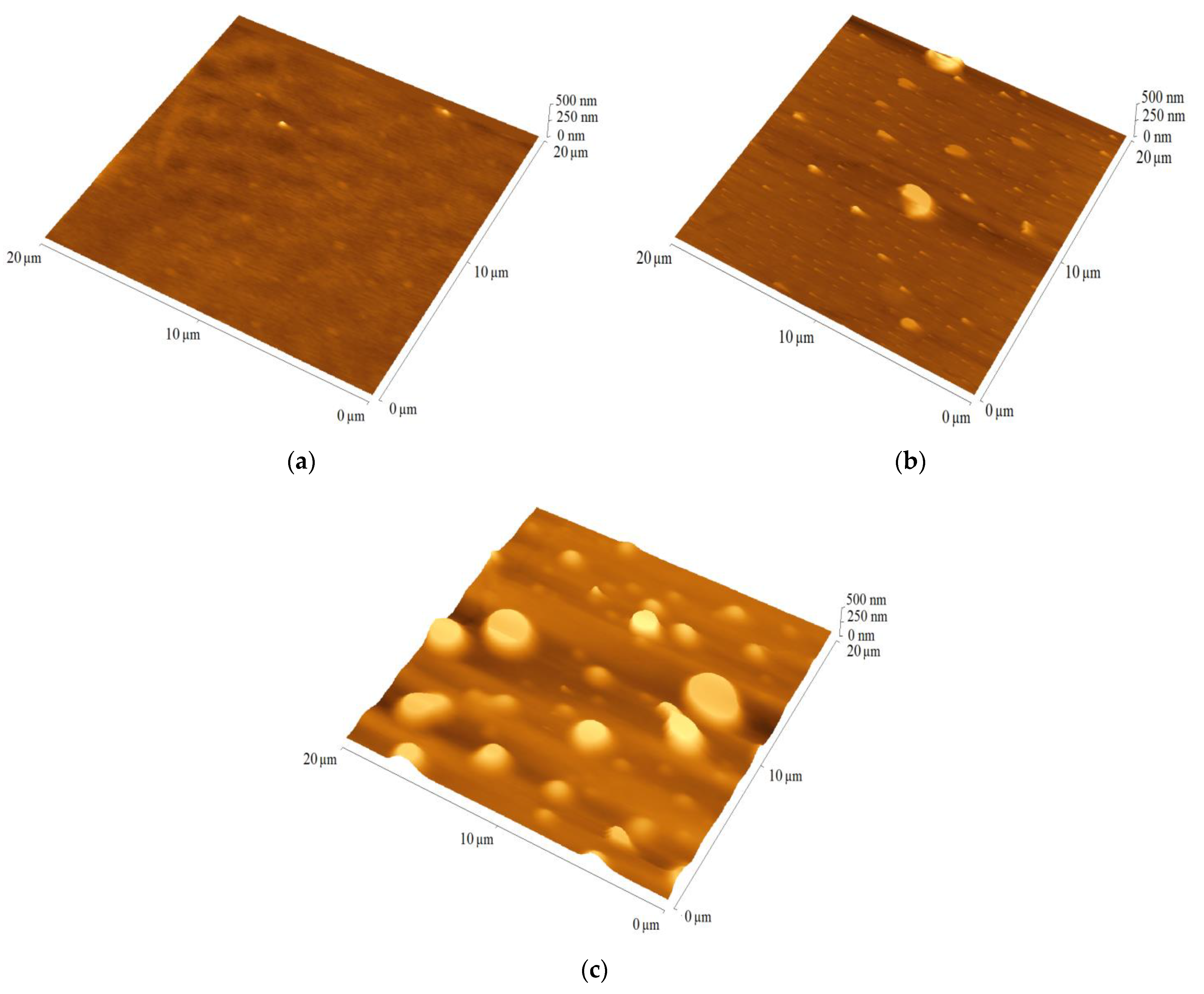
| Active Layer Structure | Voc [mV] | Jsc [mA cm−2] | FF [–] | PCE [%] | d [nm] | RMS [nm] |
|---|---|---|---|---|---|---|
| TPA-DT:PC60BM (1:1.5) | 616 (±10) | 0.90 (±0.05) | 0.31 (±0.01) | 0.18 (±0.02) | 40 | 7 |
| TPA-tHB:PC60BM (1:1.5) | 457 (±4) | 0.72 (±0.04) | 0.28 (±0.01) | 0.09 (±0.03) | 45 | 7 |
| TPA-dHB:PC60BM (1:1.5) | 503 (±15) | 0.67 (±0.03) | 0.16 (±0.01) | 0.05 (±0.02) | 55 | 6 |
| TPA-DT:P3HT:PC60BM (1:8:13) | 416 (±9) | 8.20 (±0.13) | 0.30 (±0.01) | 1.05 (±0.05) | 80 | 5 |
| TPA-tHB:P3HT:PC60BM (1:8:13) | 475 (±12) | 1.10 (±0.15) | 0.16 (±0.01) | 0.15 (±0.02) | 85 | 7 |
| TPA-dHB:P3HT:PC60BM (1:8:13) | 339 (±5) | 5.01 (±0.20) | 0.30 (±0.01) | 0.51 (±0.11) | 85 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, M.F.; Gnida, P.; Kotowicz, S.; Małecki, J.G.; Siwy, M.; Nitschke, P.; Schab-Balcerzak, E. Spectroscopic and Physicochemical Investigations of Azomethines with Triphenylamine Core towards Optoelectronics. Materials 2022, 15, 7197. https://doi.org/10.3390/ma15207197
Amin MF, Gnida P, Kotowicz S, Małecki JG, Siwy M, Nitschke P, Schab-Balcerzak E. Spectroscopic and Physicochemical Investigations of Azomethines with Triphenylamine Core towards Optoelectronics. Materials. 2022; 15(20):7197. https://doi.org/10.3390/ma15207197
Chicago/Turabian StyleAmin, Muhammad Faisal, Paweł Gnida, Sonia Kotowicz, Jan Grzegorz Małecki, Mariola Siwy, Paweł Nitschke, and Ewa Schab-Balcerzak. 2022. "Spectroscopic and Physicochemical Investigations of Azomethines with Triphenylamine Core towards Optoelectronics" Materials 15, no. 20: 7197. https://doi.org/10.3390/ma15207197
APA StyleAmin, M. F., Gnida, P., Kotowicz, S., Małecki, J. G., Siwy, M., Nitschke, P., & Schab-Balcerzak, E. (2022). Spectroscopic and Physicochemical Investigations of Azomethines with Triphenylamine Core towards Optoelectronics. Materials, 15(20), 7197. https://doi.org/10.3390/ma15207197






