Statistical Analysis and Optimization of the Brilliant Red HE-3B Dye Biosorption onto a Biosorbent Based on Residual Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Batch Biosorption Method
2.2.2. Biosorption Process Modeling Using Computer-Assisted Statistical Analysis
3. Results and Discussion
3.1. Biosorption Process Performance
3.2. Experimental Modeling Using Computer-Assisted Statistical Analysis
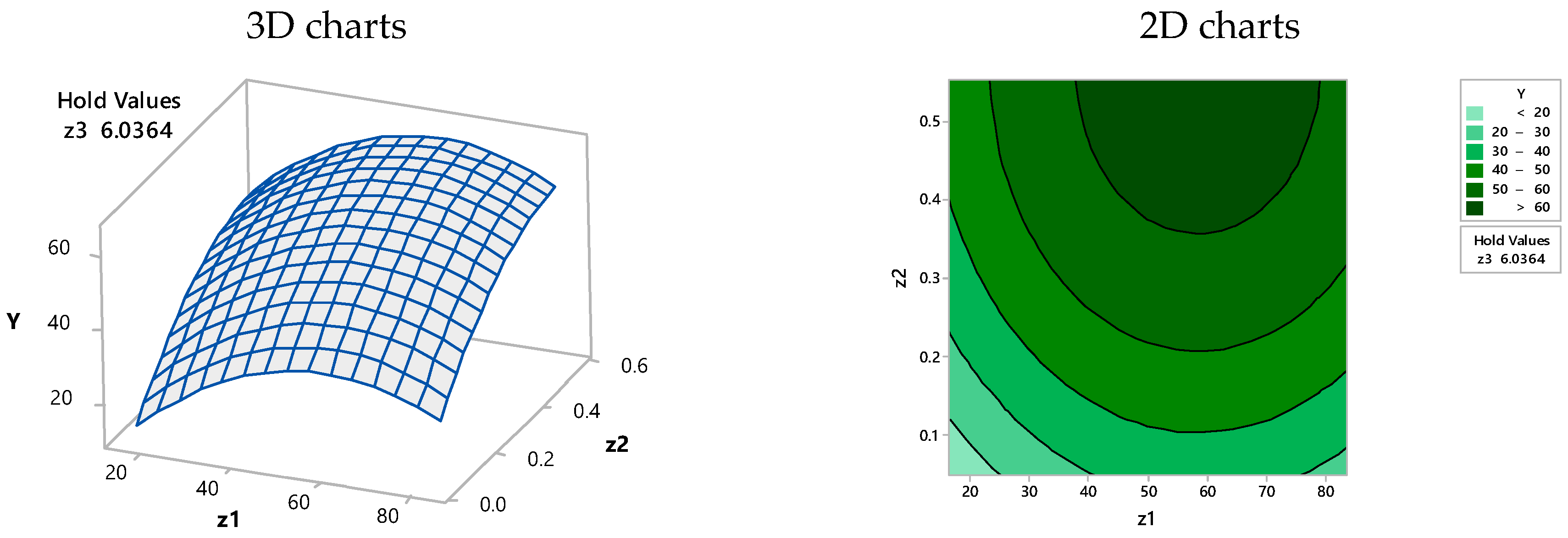
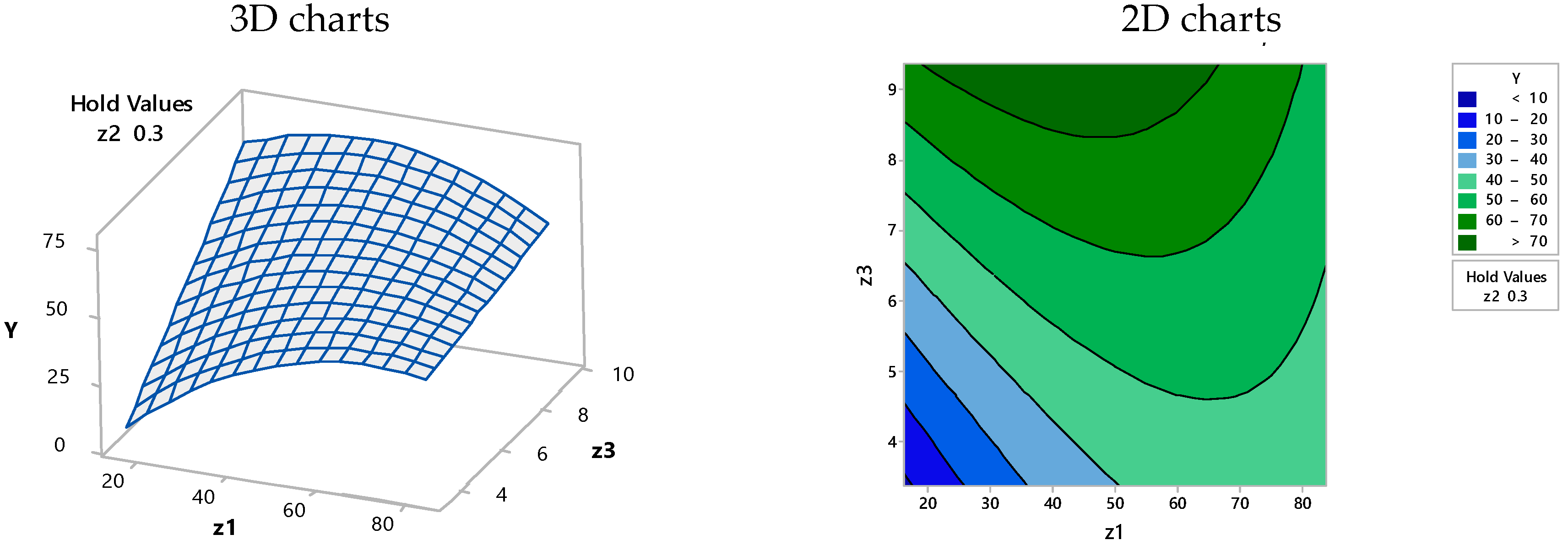
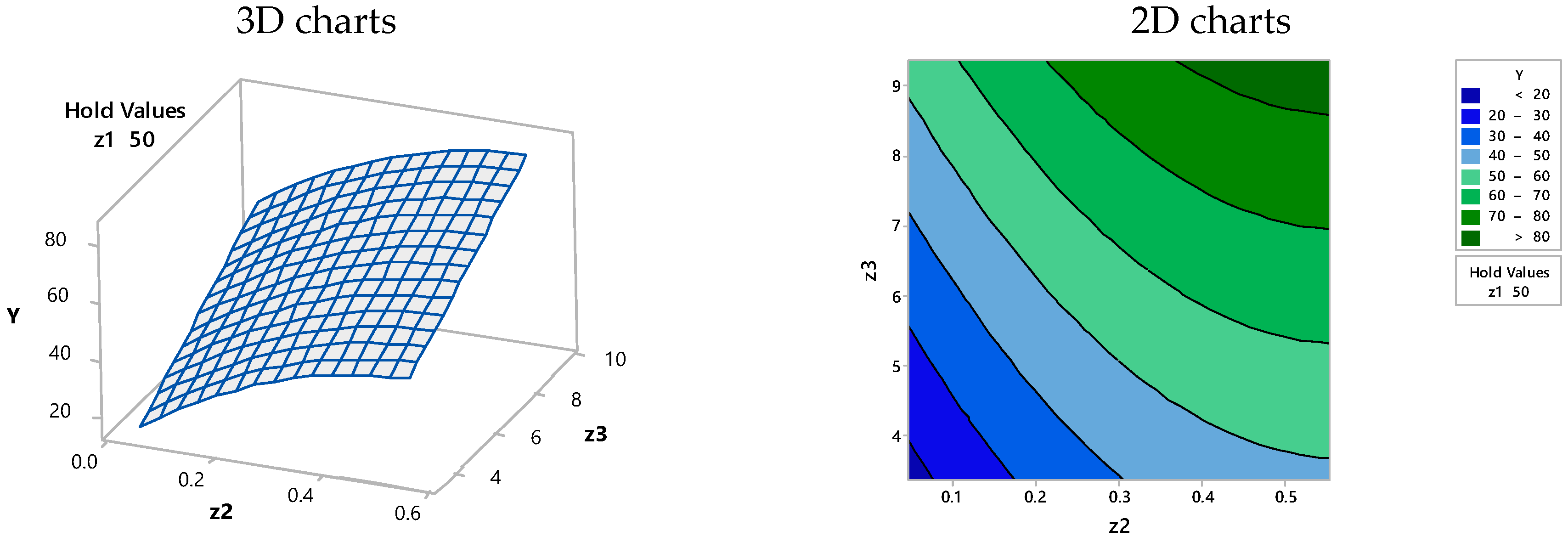
3.3. Optimization of the Proposed Model Using Computer-Assisted Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaharia, C.; Suteu, D. Textile Organic Dyes–Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents—A Critical Overview. In Organic Pollutants—Ten Years after the Stockholm Convention: Environmental and Analytical Update; Puzyn, T., Mostrag-Szlichtyng, A., Eds.; InTech Open Science/Open minds: Rijeka, Croatia, 2012; pp. 55–86. Available online: https://www.intechopen.com (accessed on 23 July 2022).
- Qi, X.; Tong, X.; Pan, W.; Zeng, Q.; You, S.; Shen, J. Recent advances in polysaccharide-based adsorbents for wastewater treatment. J. Clean. Prod. 2021, 315, 128221. [Google Scholar] [CrossRef]
- Aravind, J.; Kamaraj, M.; Muthukumaran, P.; Thirumurugan, A.; Ramachandran, K.K. Plant polysaccharides-based adsorbents. In Natural Polymers-Based Green Adsorbents for Water Treatment; Kalia, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 53–72. ISBN 9780128205419. [Google Scholar] [CrossRef]
- Blaga, A.C.; Zaharia, C.; Suteu, D. Polysaccharides as Support for Microbial Biomass-Based Adsorbents with Applications in Removal of Heavy Metals and Dyes. Polymers 2021, 13, 2893. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J.; Chang, S.; Chung, W. Bacterial Biosorbents, an Efficient Heavy Metals Green Clean-Up Strategy: Prospects, Challenges, and Opportunities. Microorganisms 2022, 10, 610. [Google Scholar] [CrossRef]
- Morales-Barrera, L.; Cristiani-Urbina, E. Equilibrium Biosorption of Zn2+ and Ni2+ Ions from Monometallic and Bimetallic Solutions by Crab Shell Biomass. Processes 2022, 10, 886. [Google Scholar] [CrossRef]
- Madeła, M.; Skuza, M. Towards a Circular Economy: Analysis of the Use of Biowaste as Biosorbent for the Removal of Heavy Metals. Energies 2021, 14, 5427. [Google Scholar] [CrossRef]
- Blagojev, N.; Vasić, V.; Kukić, D.; Šćiban, M.; Prodanović, J.; Bera, O. Modelling and efficiency evaluation of the continuous biosorption of Cu(II) and Cr(VI) from water by agricultural waste materials. J. Environ. Manag. 2021, 281, 111876. [Google Scholar] [CrossRef]
- Zaharia, C. Application of waste materials as ‘low cost’ sorbents for industrial effluent treatment. A comparative overview. Int. J. Mater. Prod. Technol. 2015, 50, 196–220. [Google Scholar] [CrossRef]
- Suteu, D.; Zaharia, C.; Blaga, A.C. Biosorption-current bioprocess for wastewater treatment (chapter 10). In Current Topics, Concepts and Research Priorities in Environmental Chemistry; Zaharia, C., Ed.; Publishing House of the ‘Al.I. Cuza’ University: Iasi, Romania, 2012; Volume I, pp. 221–244. [Google Scholar]
- Hussein, K.A.; Hassan, D.H.; Joo, J.H. Potential capacity of Beauveria bassiana and Matarhizium anisopliae in the biosorption of Cd2+ and Pb2+. J. Gen. Appl. Microbiol. 2011, 56, 347–355. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Darwish, H.; Alharthi, S.; Al-Zaban, M.I.; Noureldeen, A.; Hassan, S.H.A. Process optimization and modeling of Cd 2+ biosorption onto the free and immobilized Turbinaria ornata using Box-Behnken experimental design. Sci. Rep. 2022, 12, 3256. [Google Scholar] [CrossRef]
- Fertu, D.I.; Dragoi, E.N.; Bulgariu, L.; Curteanu, S.; Gavrilescu, M. Modeling the Biosorption Process of Heavy Metal Ions on Soybean-Based Low-Cost Biosorbents Using Artificial Neural Networks. Processes 2022, 10, 603. [Google Scholar] [CrossRef]
- Al-Zaban, M.I.; Alharbi, N.K.; Albarakaty, F.M.; Alharthi, S.; Hassan, S.H.A.; Fawzy, M.A. Experimental Modeling Investigations on the Biosorption of Methyl Violet 2B Dye by the Brown Seaweed Cystoseira tamariscifolia. Sustainability 2022, 14, 5285. [Google Scholar] [CrossRef]
- Beyan, S.M.; Prabhu, S.V.; Ambio, T.A.; Gomadurai, C. A Statistical Modeling and Optimization for Cr(VI) Adsorption from Aqueous Media via Teff Straw-Based Activated Carbon: Isotherm, Kinetics, and Thermodynamic Studies. Adsorpt. Sci. Technol. 2022, 2022, 7998069. [Google Scholar] [CrossRef]
- Jaikumar, V.; Ramamurthi, V. Statistical Analysis and Optimization of Acid Dye Biosorption by Brewery Waste Biomass Using Response Surface Methodology. Mod. Appl. Sci. 2009, 3, 71–84. [Google Scholar] [CrossRef]
- El-Ahwany, A.M.D. Statistical analysis and optimization of copper biosorption capability by Oenococcus oeni PSU-1African. J. Biotechnol. 2012, 11, 4225–4233. [Google Scholar] [CrossRef]
- Alharbi, N.K.; Al-Zaban, H.I.; Alborakaty, F.M.; Abdelwahab, S.F.; Hassan, S.H.; Fawzy, M.A. Kinetic, isotherm and thermodynamic aspects of Zn2+ biosorption by Spirulina platensis: Optimization of process variables of response surface methodology. Life 2022, 12, 585. [Google Scholar] [CrossRef]
- Maurya, R.; Ghosh, T.; Paliwal, C.; Shrivastav, A.; Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S. Biosorption of Methylene Blue by De-Oiled Algal Biomass: Equilibrium, Kinetics and Artificial Neural Network Modelling. PLoS ONE 2014, 9, e109545. [Google Scholar] [CrossRef] [PubMed]
- Suteu, D.; Blaga, A.C.; Zaharia, C.; Cimpoesu, R.; Puițel, A.C.; Tataru-Farmus, R.-E.; Tanasă, A.M. Polysaccharides Used in Biosorbents Preparation for Organic Dyes Retaining from Aqueous Media. Polymers 2022, 14, 588. [Google Scholar] [CrossRef]
- Zaharia, C.; Suteu, D. Empirical Modeling and Optimization by Active Central Composite Rotatable Design: Brilliant Red HE-3B Dye Biosorption onto Residual Yeast Biomass-Based Biosorbents. Appl. Sci. 2022, 12, 6377. [Google Scholar] [CrossRef]
- Box, G.E.P.; Hunter, J.S. Multi-Factor Experimental Designs for Exploring Response Surfaces. Ann. Math. Stat. 1957, 28, 195–241. [Google Scholar] [CrossRef]
- Box, G.E.P.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions. J. R. Stat. Soc. Ser. B 1951, 13, 1–45. [Google Scholar] [CrossRef]
- Puițel, A.C.; Suditu, G.D.; Danu, M.; Ailiesei, G.L.; Nechita, M.T. An Experimental Study on the Hot Alkali Extraction of Xylan-Based Hemicelluloses from Wheat Straw and Corn Stalks and Optimization Methods. Polymers 2022, 14, 1662. [Google Scholar] [CrossRef]
- Nechita, M.T.; Suditu, G.D.; Puițel, A.C.; Drăgoi, E.N. Differential evolution-based optimization of corn stalks black liquor decolorization using active carbon and TiO2/UV. Sci. Rep. 2021, 11, 18481. [Google Scholar] [CrossRef]
- Tripathi, V.K.; Ambekar, S. Optimization and Analysis of Wear Rate of CFRP-NanoZno/Nanoclay Hybrid Composites Using RSM. J. Bio-Tribo-Corros. 2019, 5, 96. [Google Scholar] [CrossRef]
- Meiabadi, M.S.S.M.; Kazerooni, A.; Moradi, M.; Torkamany, M.J. Laser assisted joining of St12 to polycarbonate:Experimental study and numerical simulation. Optik 2020, 208, 164151. [Google Scholar] [CrossRef]
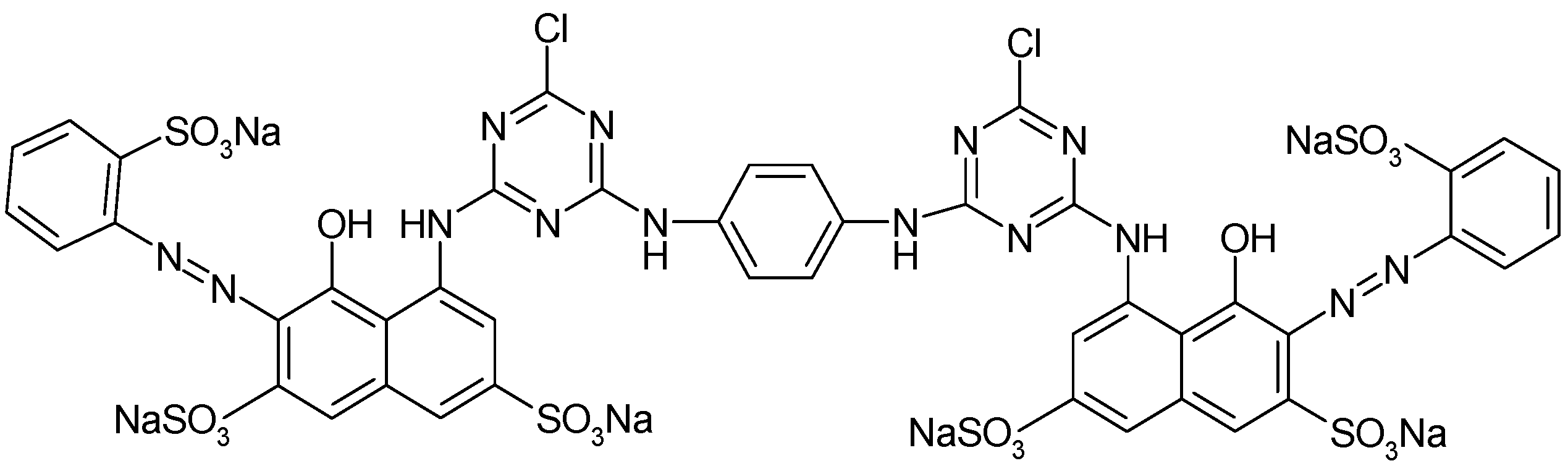

| Exp. No. | Z1, (g/L) | Z2, (mg/L) | Z3, (h) | Yei (%) |
|---|---|---|---|---|
| 1 | 6 | 30 | 4 | 13.62 |
| 2 | 18 | 30 | 4 | 34.79 |
| 3 | 6 | 70 | 4 | 33.35 |
| 4 | 18 | 70 | 4 | 48.49 |
| 5 | 6 | 30 | 8 | 52.63 |
| 6 | 18 | 30 | 8 | 72.49 |
| 7 | 6 | 70 | 8 | 47.06 |
| 8 | 18 | 70 | 8 | 74.18 |
| 9 | 1.908 | 50 | 6 | 36.68 |
| 10 | 22.09 | 50 | 6 | 63.32 |
| 11 | 12 | 16.36 | 6 | 34.65 |
| 12 | 12 | 83.64 | 6 | 51.91 |
| 13 | 12 | 50 | 2.64 | 44.94 |
| 14 | 12 | 50 | 9.36 | 69.66 |
| 15 | 12 | 50 | 6 | 56.74 |
| 16 | 12 | 50 | 6 | 55.38 |
| 17 | 0.60 | 50 | 6 | 56.82 |
| 18 | 0.60 | 50 | 6 | 58.11 |
| 19 | 0.60 | 50 | 6 | 54.90 |
| 20 | 0.60 | 50 | 6 | 56.50 |
| Variable | Symbol | Coding Level Values | ||||
|---|---|---|---|---|---|---|
| −α | −1 (Lower Level) | 0 (Base Level) | +1 (Higher Level) | +α | ||
| Residual immobilized biomass, (g/L) | Z1 | 1.91 | 6 | 12 | 18 | 22.09 |
| Dye concentration, (mg/L) | Z2 | 16.36 | 30 | 50 | 70 | 83.64 |
| Biosorption time, (h) | Z3 | 2.64 | 4 | 6 | 8 | 9.36 |
| Model | S | R-sq | R-sq(adj) | PRESS | R-sq(pred) |
|---|---|---|---|---|---|
| Linear | 7.10238 | 80.72% | 77.10% | 1318.14 | 68.51% |
| Full quadratic | 4.00812 | 96.16% | 92.71% | 1079.82 | 74.21% |
| Cubic | 1.14226 | 99.84% | 99.41% | - | - |
| Full quadratic simplified | 3.99308 | 95.05% | 92.76% | 609.094 | 85.45% |
| Source | DF | Seq SS | Contribution | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|---|---|
| Model | 6 | 3978.92 | 95.05% | 3978.92 | 663.15 | 41.59 | 0.000 |
| Linear | 3 | 3379.10 | 80.72% | 3233.23 | 1077.74 | 67.59 | 0.000 |
| Z1 | 1 | 251.25 | 6.00% | 153.01 | 153.01 | 9.60 | 0.008 |
| Z2 | 1 | 1201.35 | 28.70% | 1201.35 | 1201.35 | 75.35 | 0.000 |
| Z3 | 1 | 1926.49 | 46.02% | 1878.87 | 1878.87 | 117.84 | 0.000 |
| Square | 2 | 425.88 | 10.17% | 425.88 | 212.94 | 13.36 | 0.001 |
| Z1×Z1 | 1 | 328.29 | 7.84% | 358.76 | 358.76 | 22.50 | 0.000 |
| Z2×Z2 | 1 | 97.59 | 2.33% | 97.59 | 97.59 | 6.12 | 0.028 |
| 2-Way Interaction | 1 | 173.94 | 4.15% | 173.94 | 173.94 | 10.91 | 0.006 |
| Z1×Z3 | 1 | 173.94 | 4.15% | 173.94 | 173.94 | 10.91 | 0.006 |
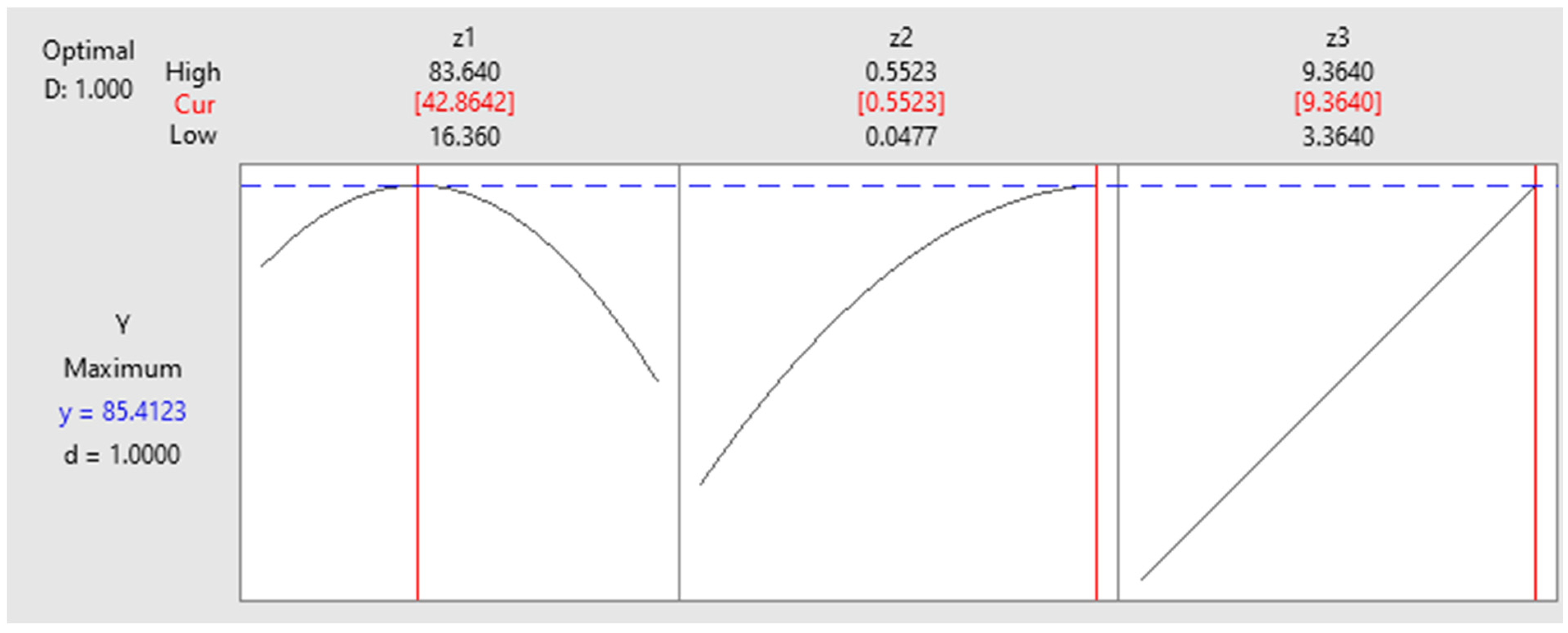
| Solution | Z1 (g/L) | Z2/100 (mg/L) | Z3 (h) | Y Fit (%) | Composite Desirability |
|---|---|---|---|---|---|
| 1 | 42.86 | 0.55 | 9.36 | 85.41 | 1.00000 |
| 2 | 50.00 | 0.30 | 9.01 | 74.18 | 1.00000 |
| 3 | 29.99 | 0.29 | 9.36 | 74.18 | 1.00000 |
| 4 | 19.23 | 0.55 | 8.92 | 74.17 | 0.99981 |
| 5 | 24.67 | 0.55 | 9.36 | 72.85 | 0.97775 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suditu, G.D.; Blaga, A.C.; Tataru-Farmus, R.-E.; Zaharia, C.; Suteu, D. Statistical Analysis and Optimization of the Brilliant Red HE-3B Dye Biosorption onto a Biosorbent Based on Residual Biomass. Materials 2022, 15, 7180. https://doi.org/10.3390/ma15207180
Suditu GD, Blaga AC, Tataru-Farmus R-E, Zaharia C, Suteu D. Statistical Analysis and Optimization of the Brilliant Red HE-3B Dye Biosorption onto a Biosorbent Based on Residual Biomass. Materials. 2022; 15(20):7180. https://doi.org/10.3390/ma15207180
Chicago/Turabian StyleSuditu, Gabriel Dan, Alexandra Cristina Blaga, Ramona-Elena Tataru-Farmus, Carmen Zaharia, and Daniela Suteu. 2022. "Statistical Analysis and Optimization of the Brilliant Red HE-3B Dye Biosorption onto a Biosorbent Based on Residual Biomass" Materials 15, no. 20: 7180. https://doi.org/10.3390/ma15207180
APA StyleSuditu, G. D., Blaga, A. C., Tataru-Farmus, R.-E., Zaharia, C., & Suteu, D. (2022). Statistical Analysis and Optimization of the Brilliant Red HE-3B Dye Biosorption onto a Biosorbent Based on Residual Biomass. Materials, 15(20), 7180. https://doi.org/10.3390/ma15207180










