An Orthogonal Experimental Study on the Preparation of Cr Coatings on Long-Size Zr Alloy Tubes by Arc Ion Plating
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Preparation
2.3. Characterization
2.4. Orthogonal Analysis
2.4.1. Range Analysis
2.4.2. Variance Analysis
3. Results
3.1. Appearance
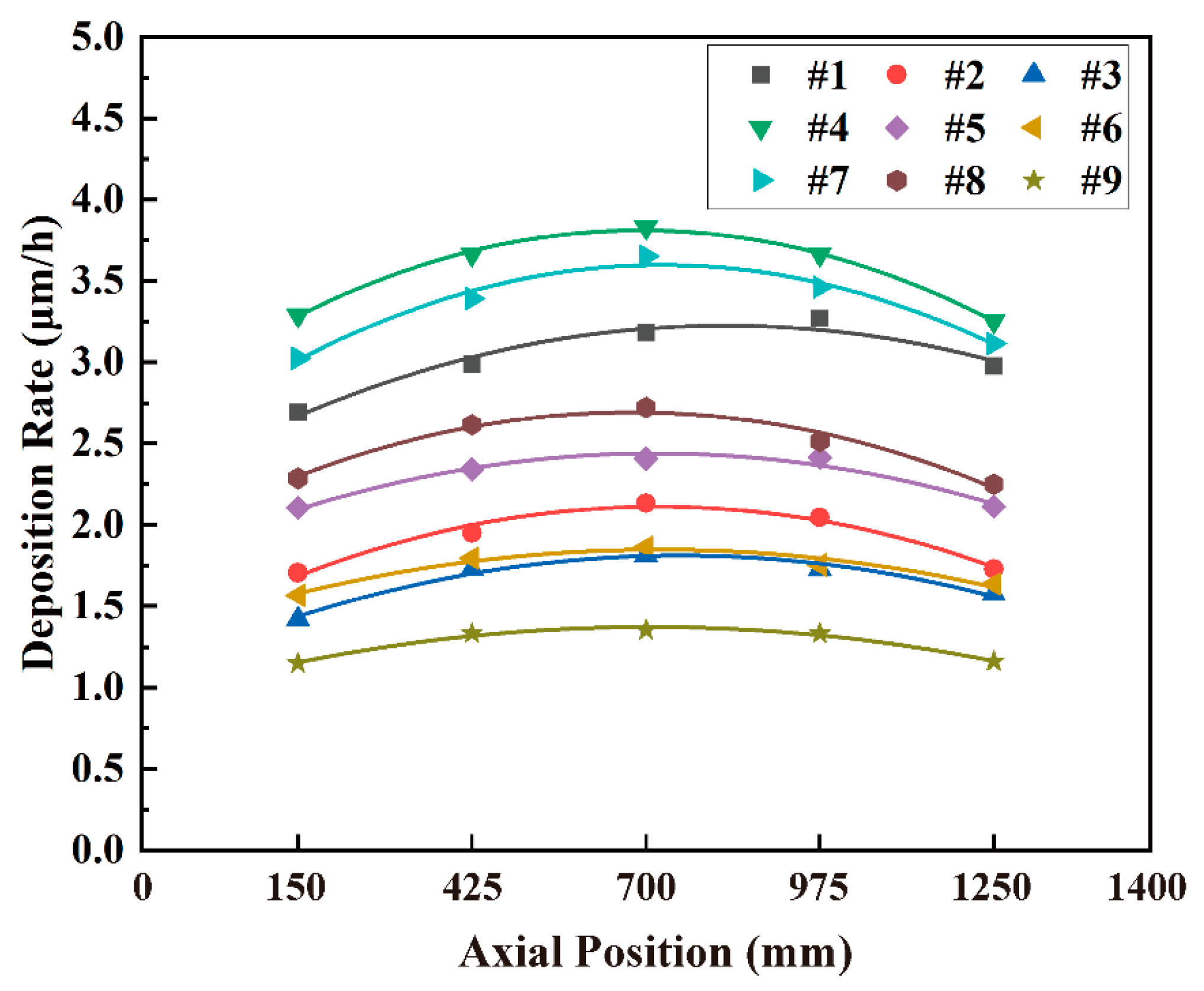
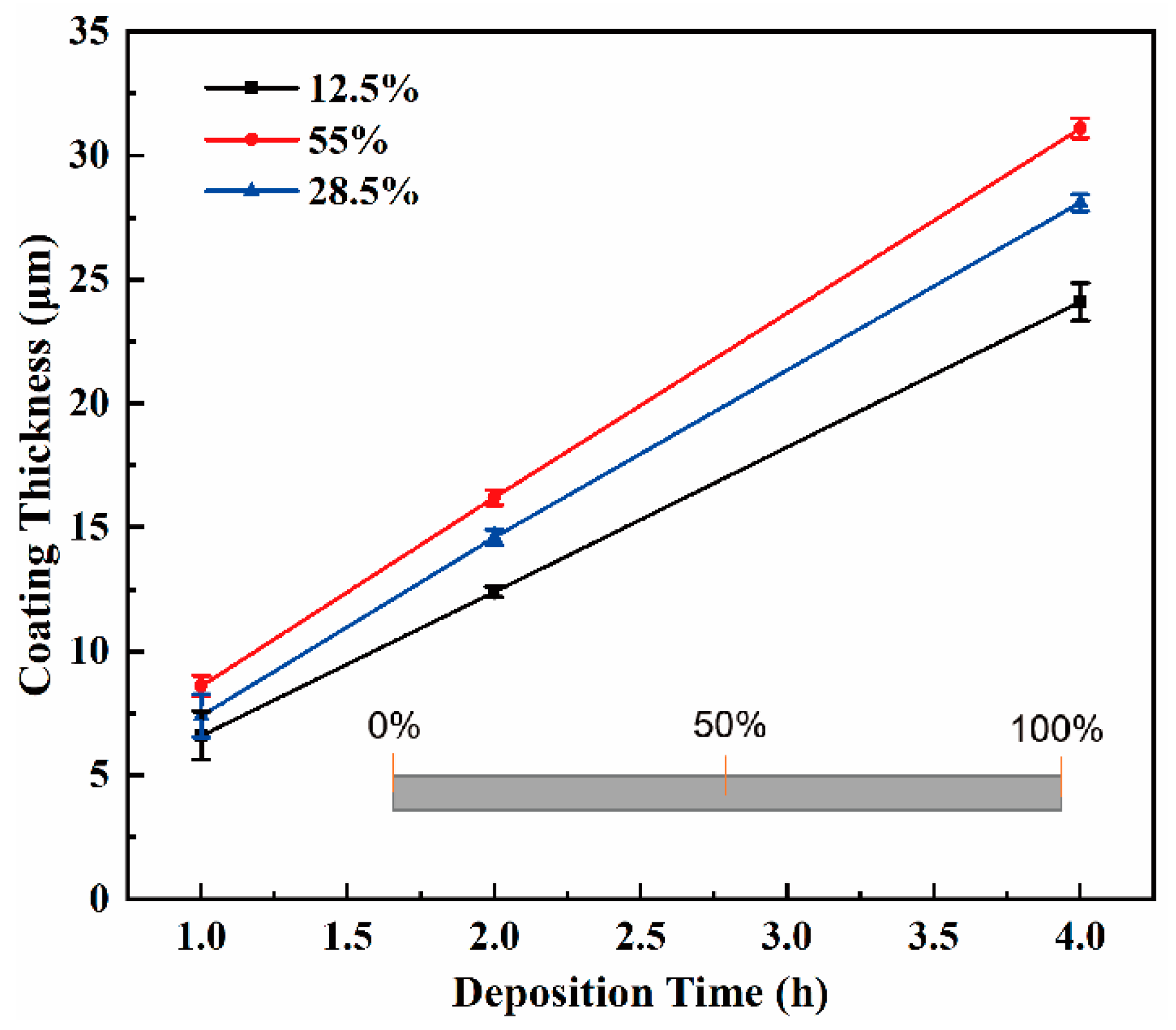
3.2. Coating Thickness and Deposition Rate
3.3. Micro-Morphology and Roughness of Coating Surface
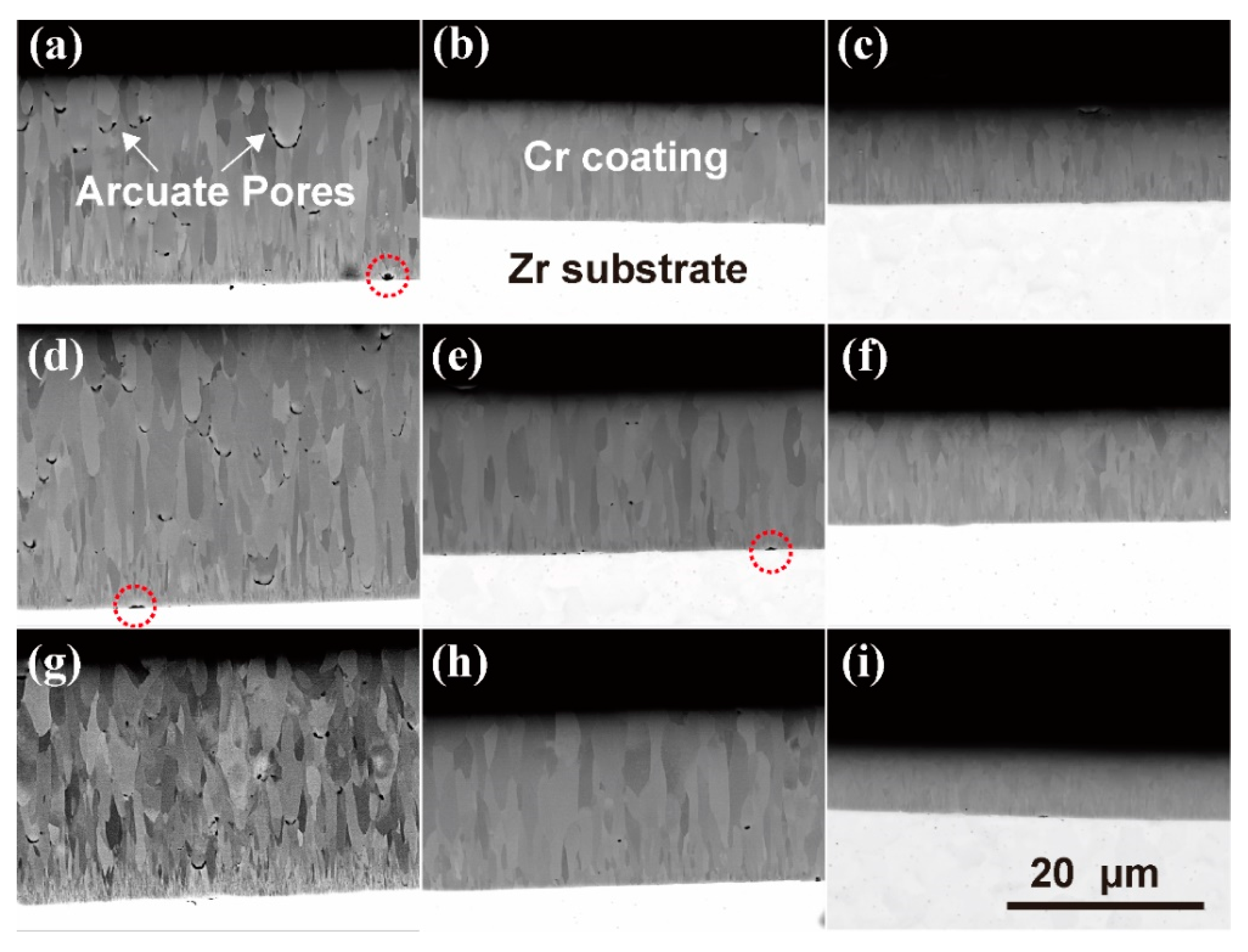
3.4. Micro-Morphology of Coating Cross-Sections and Internal Defects
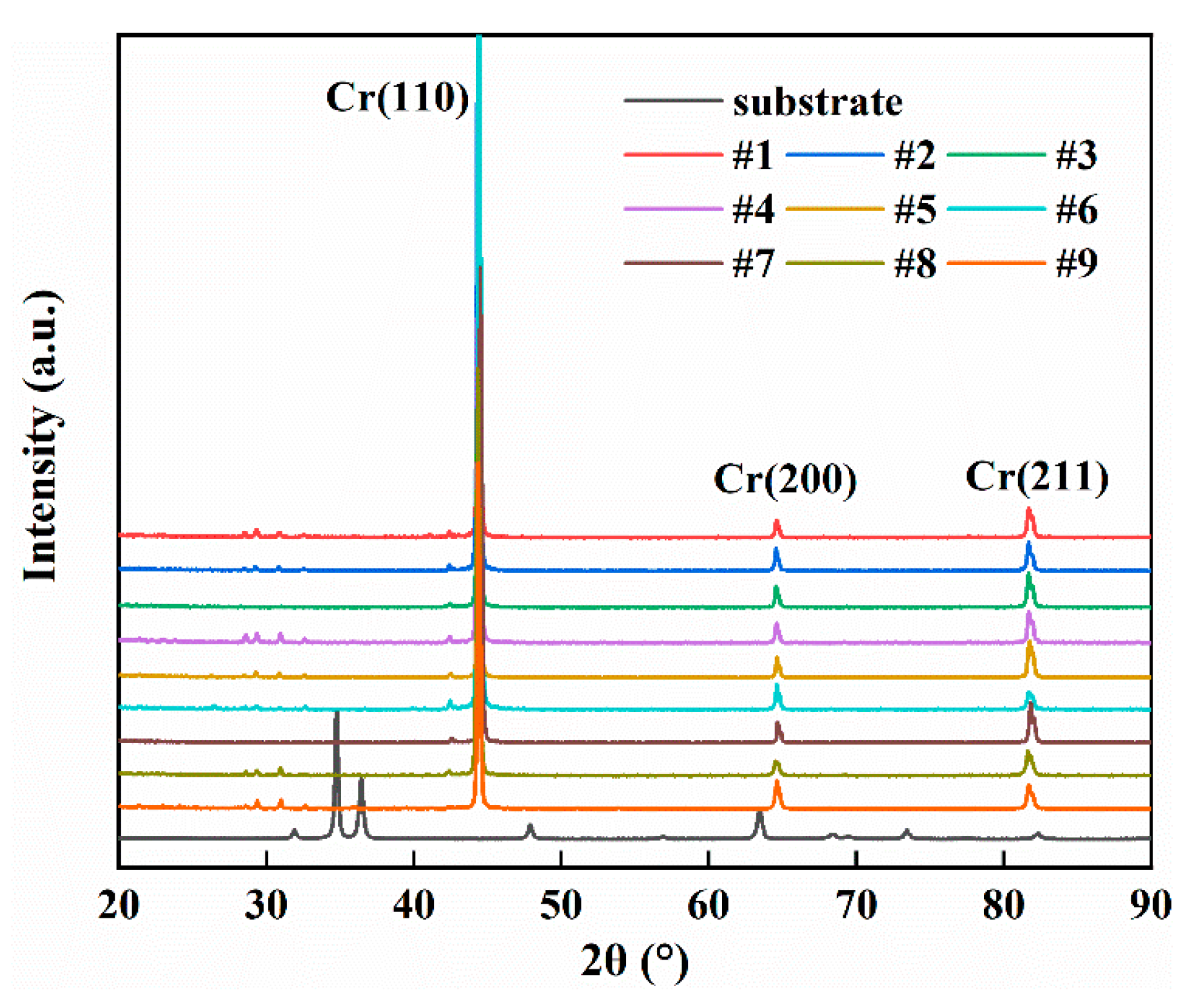
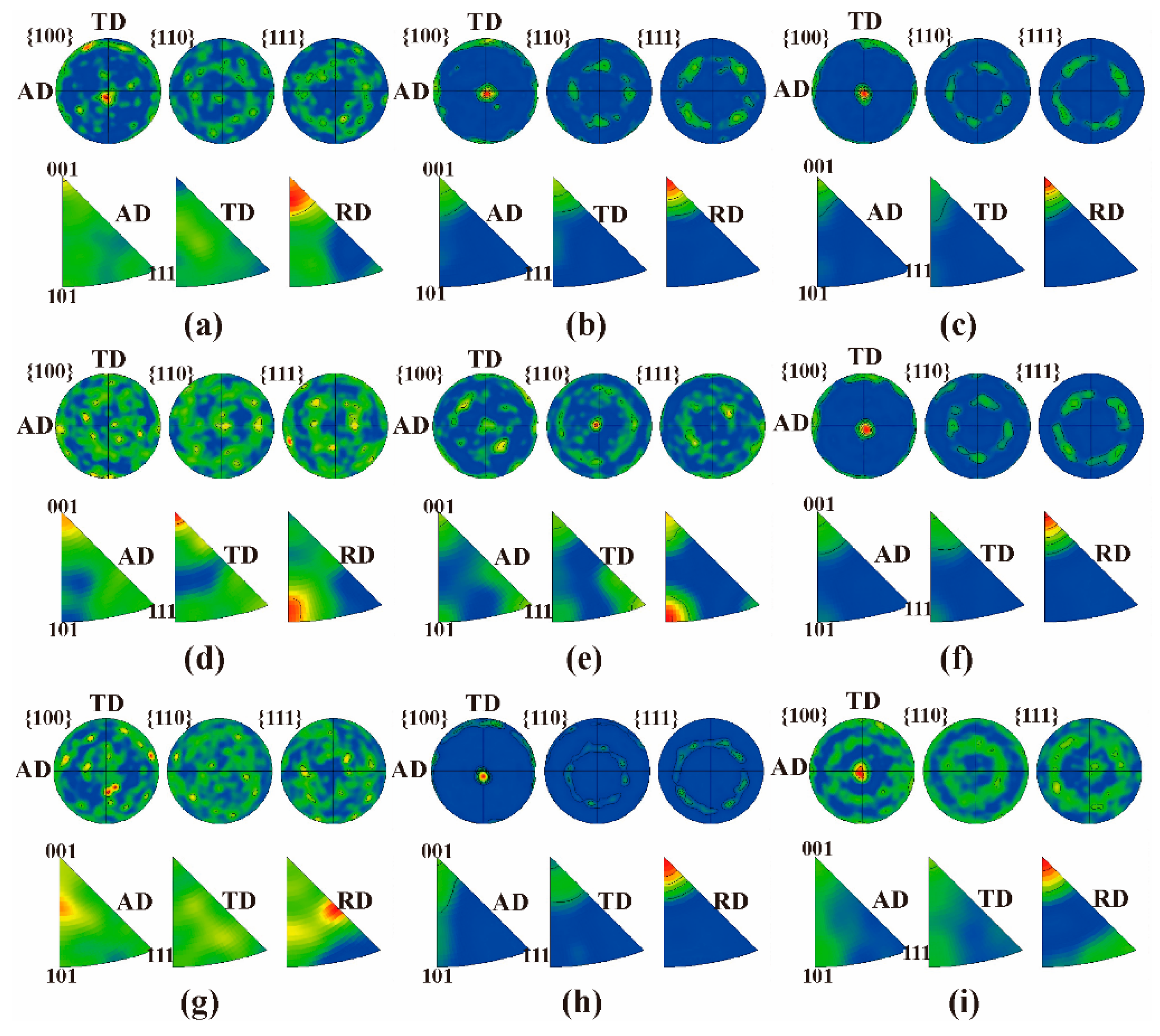
3.5. Crystal Structure and Preferred Orientation of Cr Coatings
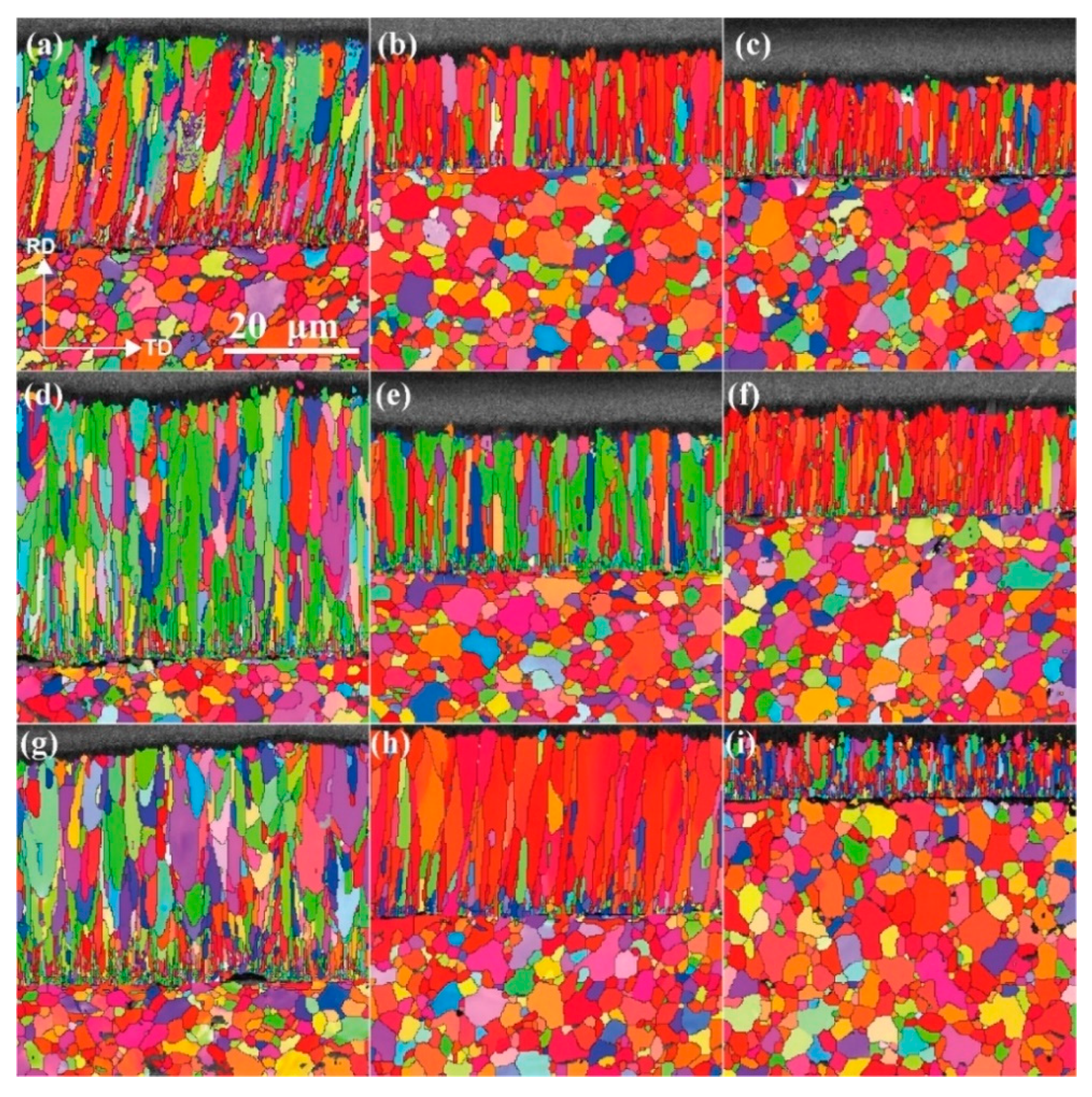
3.6. Grain Structure of the Cr Coatings
4. Discussion
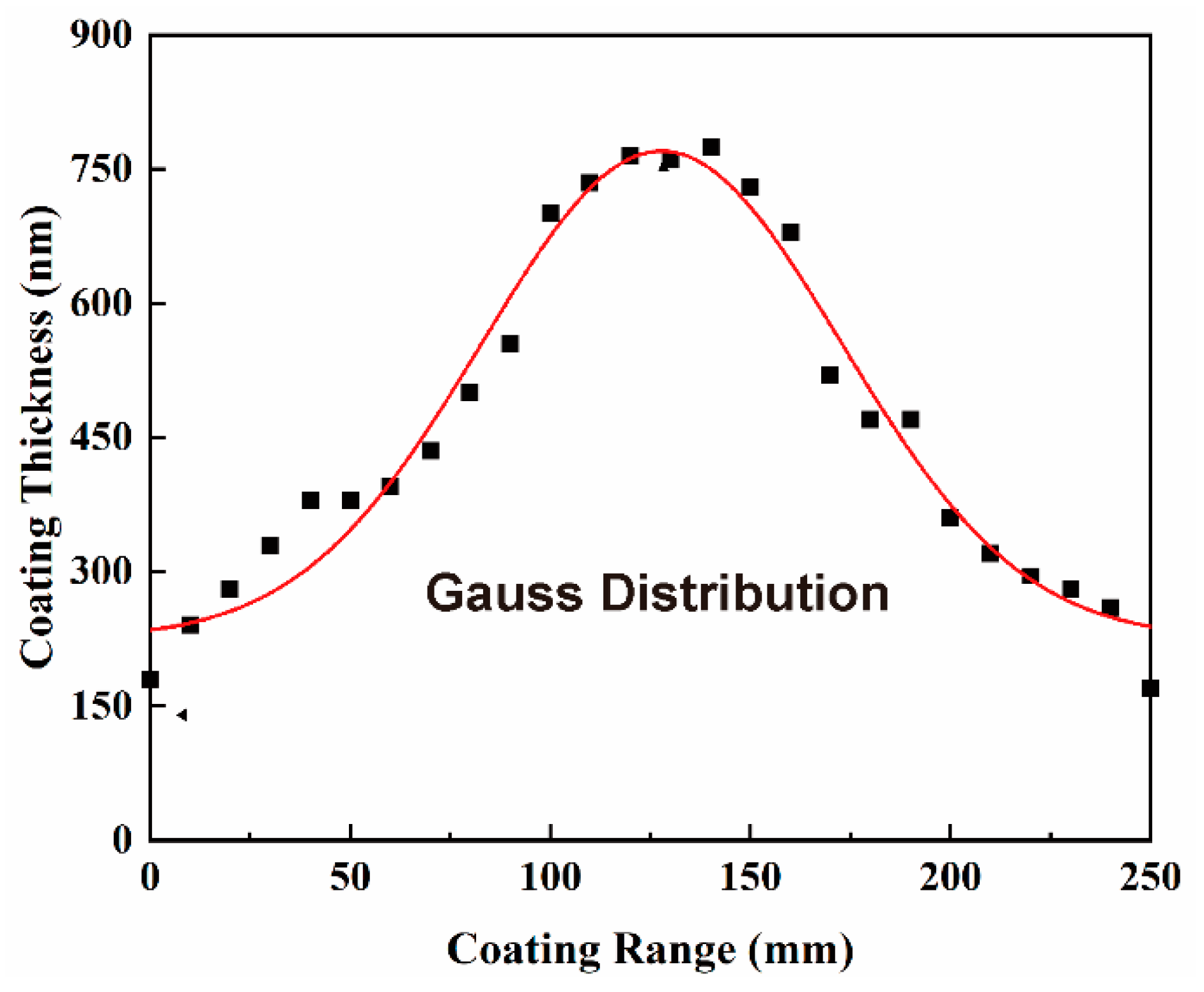
4.1. Axial Distribution of Coating Thickness
4.2. Effect of Process Parameters on Deposition Rate
4.3. Effect of Process Parameters on Droplet Particles and Surface Roughness
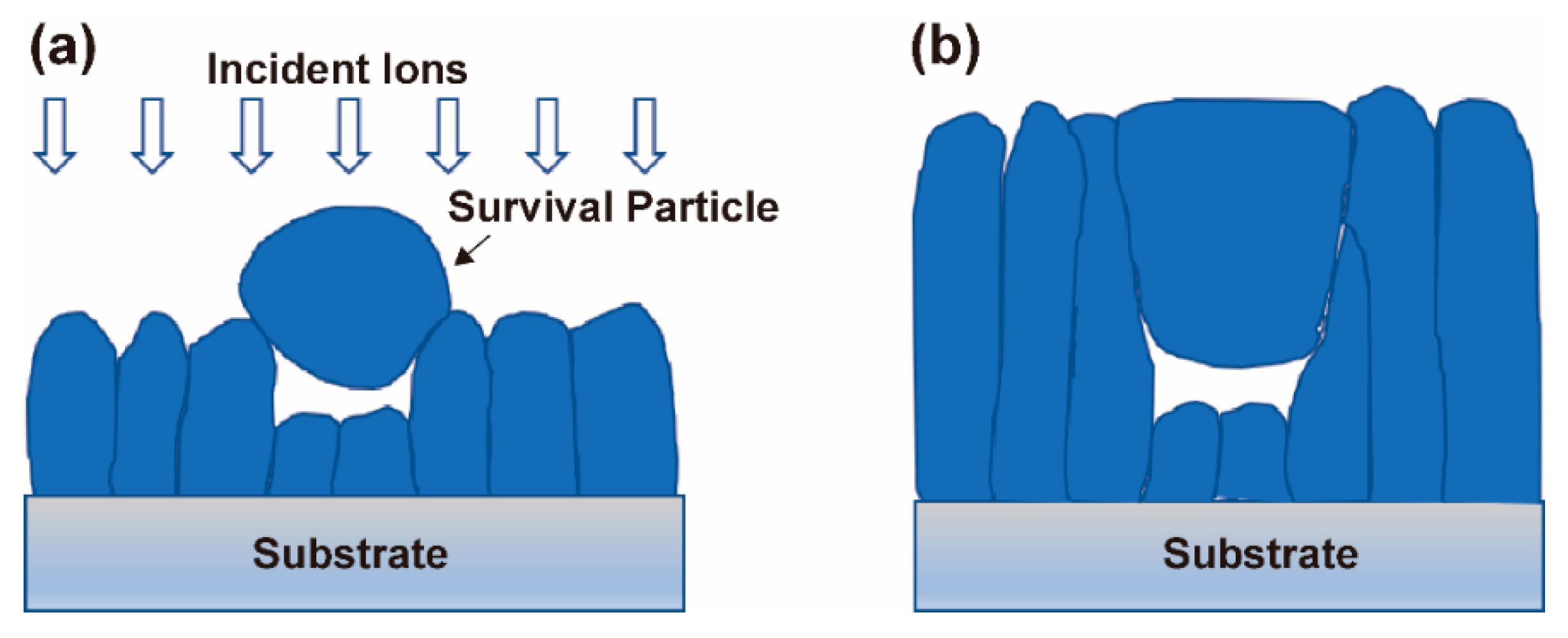
4.4. Formation Mechanism of Arcuate Pores
4.5. Effect of Process Parameters on Crystallographic Orientation
4.6. Effect of Process Parameters on Grain Structure
5. Conclusions
- (1)
- The axial distribution of the coating thickness decreasing from the central part to both ends of the cladding tube is attributed to the “ion shrinkage” phenomenon induced by a bias field. This coating uniformity can be improved by overlapping the effective deposition zones of arc sources but is seldom carried out by the processing parameters.
- (2)
- The deposition rate of the Cr coating is mainly dependent on the arc current, the increase of which will lead to a monotonic increase in the coating deposition rate by increasing the incident ion flux.
- (3)
- The number and size of droplet particles are greatly influenced by the arc current. Particularly, decreasing the arc current can effectively inhibit the droplet emission of the arc source, thus reducing the surface roughness and also improving the compactness of the Cr coatings with less formation of pores induced by droplet particles embedded in the coatings.
- (4)
- The change of process parameters will significantly affect the growth of the high-index plane of (211) rather than that of the low-index plane of (110), even though the preferred orientation of the latter is prevailing in the Cr coatings. An enhancement of the growth of the (211) plane can be achieved via a decrease in the gas pressure that reduces the collision between ions and their energy depletion.
- (5)
- The key to controlling the grain structure of the Cr coating is changing the crystal nucleation rate. The simplest way is to increase the negative bias voltage to create more nucleate sites, inhibiting the grain growth to coarse columnar crystals. Moreover, coating thickening contributes to the columnar growth of grains.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Orthogonal Analysis Method
Appendix A.1. Range Analysis
| No. | A | B | C | D | Index |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | x1 |
| 2 | 1 | 2 | 2 | 2 | x2 |
| 3 | 1 | 3 | 3 | 3 | x3 |
| 4 | 2 | 1 | 2 | 3 | x4 |
| 5 | 2 | 2 | 3 | 1 | x5 |
| 6 | 2 | 3 | 1 | 2 | x6 |
| 7 | 3 | 1 | 3 | 2 | x7 |
| 8 | 3 | 2 | 1 | 3 | x8 |
| 9 | 3 | 3 | 2 | 1 | x9 |
| K1j | K11 = x1 + x2 + x3 | K12 = x1 + x4 + x7 | K13 = x1 + x6 + x8 | K14 = x1 + x5 + x9 | |
| K2j | K21 = x4 + x5 + x6 | K22 = x2 + x5 + x8 | K23 = x2 + x4 + x9 | K24 = x2 + x6 + x7 | |
| K3j | K31 = x7 + x8 + x9 | K32 = x3 + x6 + x9 | K33 = x3 + x5 + x7 | K34 = x3 + x4 + x8 | |
| K1j | K11 / 3 | K12 / 3 | K13 / 3 | K14 / 3 | |
| K2j | K21 / 3 | K22 / 3 | K23 / 3 | K24 / 3 | |
| K3j | K31 / 3 | K32 / 3 | K33 / 3 | K34 / 3 | |
| Rj | (max − min) (K11, K21, K31) | (max − min) (K12, K22, K32) | (max − min) (K13, K23, K33) | (max − min) (K14, K24, K34) |
Appendix A.2. Variance Analysis
| No. | A | B | C | D | Index |
|---|---|---|---|---|---|
| … | … | … | … | … | … |
| K1j2 | K112 | K122 | K132 | K142 | |
| K2j2 | K212 | K222 | K232 | K242 | |
| K3j2 | K312 | K322 | K332 | K342 | |
| SSj | SS1 | SS2 | SS3 | SS4 |
| Variance Source | F | Table Value | Significance Level | |||
|---|---|---|---|---|---|---|
| A | A | |||||
| B△ | ||||||
| C | C | |||||
| Error | ||||||
| Error△ | ||||||
| Sum | 8 |
References
- Rebak, R.B. Accident-Tolerant Materials for Light Water Reactor Fuels; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-817504-0. [Google Scholar]
- Terrani, K.A. Accident Tolerant Fuel Cladding Development: Promise, Status, and Challenges. J. Nucl. Mater. 2018, 501, 13–30. [Google Scholar] [CrossRef]
- Karoutas, Z.; Brown, J.; Atwood, A.; Hallstadius, L.; Lahoda, E.; Ray, S.; Bradfute, J. The Maturing of Nuclear Fuel: Past to Accident Tolerant Fuel. Prog. Nucl. Energ. 2018, 102, 68–78. [Google Scholar] [CrossRef]
- Pint, B.A.; Terrani, K.A.; Yamamoto, Y.; Snead, L.L. Material Selection for Accident Tolerant Fuel Cladding. Metall. Mater. Trans. E 2015, 2, 190–196. [Google Scholar] [CrossRef]
- Pint, B.A.; Terrani, K.A.; Brady, M.P.; Cheng, T.; Keiser, J.R. High Temperature Oxidation of Fuel Cladding Candidate Materials in Steam–Hydrogen Environments. J. Nucl. Mater. 2013, 440, 420–427. [Google Scholar] [CrossRef]
- Tao, P.; Liu, W.; Wang, Y. Fabrication of Two-Layer SiC Nanowire Cladding Tube with High Thermal Conductivity. J. Eur. Ceram. Soc. 2020, 40, 3399–3405. [Google Scholar] [CrossRef]
- Kumara, C.; Wang, R.; Lu, R.Y.; Deck, C.; Gazza, J.; Qu, J. Grid-to-Rod Fretting Wear Study of SiC/SiC Composite Accident-Tolerant Fuel Claddings Using an Autoclave Fretting Bench Test. Wear 2022, 488–489, 204172. [Google Scholar] [CrossRef]
- Sakamoto, K.; Miura, Y.; Ukai, S.; Oono, N.H.; Kimura, A.; Yamaji, A.; Kusagaya, K.; Takano, S.; Kondo, T.; Ikegawa, T.; et al. Development of Accident Tolerant FeCrAl-ODS Fuel Cladding for BWRs in Japan. J. Nucl. Mater. 2021, 557, 153276. [Google Scholar] [CrossRef]
- Dryepondt, S.; Unocic, K.A.; Hoelzer, D.T.; Massey, C.P.; Pint, B.A. Development of Low-Cr ODS FeCrAl Alloys for Accident-Tolerant Fuel Cladding. J. Nucl. Mater. 2018, 501, 59–71. [Google Scholar] [CrossRef]
- Cheng, B.; Kim, Y.-J.; Chou, P. Improving Accident Tolerance of Nuclear Fuel with Coated Mo-Alloy Cladding. Nucl. Eng. Technol. 2016, 48, 16–25. [Google Scholar] [CrossRef]
- Koh, H.C.; Hosemann, P.; Glaeser, A.M.; Cionea, C. Compatibility Studies on Mo–Coating Systems for Nuclear Fuel Cladding Applications. J. Nucl. Mater. 2017, 496, 367–378. [Google Scholar] [CrossRef]
- Gonzalez-Julian, J. Processing of MAX Phases: From Synthesis to Applications. J. Am. Ceram. Soc. 2021, 104, 659–690. [Google Scholar] [CrossRef]
- Galvin, T.; Hyatt, N.C.; Rainforth, W.M.; Reaney, I.M.; Shepherd, D. Slipcasting of MAX Phase Tubes for Nuclear Fuel Cladding Applications. Nucl. Mater. Energ. 2020, 22, 100725. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Zhang, R. Application and Development Progress of Cr-Based Surface Coating in Nuclear Fuel Elements: II. Current Status and Shortcomings of Performance Studies. Coatings 2020, 10, 835. [Google Scholar] [CrossRef]
- Yang, J.; Steinbrück, M.; Tang, C.; Große, M.; Liu, J.; Zhang, J.; Yun, D.; Wang, S. Review on Chromium Coated Zirconium Alloy Accident Tolerant Fuel Cladding. J. Alloys Compd. 2022, 895, 162450. [Google Scholar] [CrossRef]
- Brachet, J.-C.; Idarraga-Trujillo, I.; Flem, M.L.; Saux, M.L.; Vandenberghe, V.; Urvoy, S.; Rouesne, E.; Guilbert, T.; Toffolon-Masclet, C.; Tupin, M.; et al. Early Studies on Cr-Coated Zircaloy-4 as Enhanced Accident Tolerant Nuclear Fuel Claddings for Light Water Reactors. J. Nucl. Mater. 2019, 517, 268–285. [Google Scholar] [CrossRef]
- Ma, X.-F.; Wu, Y.-W.; Tan, J.; Meng, C.-Y.; Yang, L.; Dang, W.-A.; He, X.-J. Evaluation of Corrosion and Oxidation Behaviors of TiAlCrN Coatings for Nuclear Fuel Cladding. Surf. Coat. Technol. 2019, 358, 521–530. [Google Scholar] [CrossRef]
- Van Nieuwenhove, R.; Andersson, V.; Balak, J.; Oberländer, B. In-Pile Testing of CrN, TiAlN, and AlCrN Coatings on Zircaloy Cladding in the Halden Reactor. In Zirconium in the Nuclear Industry: 18th International Symposium; Comstock, R.J., Motta, A.T., Eds.; ASTM International: West Conshohocken, PA, USA, 2018; pp. 965–982. ISBN 978-0-8031-7641-6. [Google Scholar]
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partezana, J.M.; Wolfe, D.E. Multilayer (TiN, TiAlN) Ceramic Coatings for Nuclear Fuel Cladding. J. Nucl. Mater. 2016, 478, 236–244. [Google Scholar] [CrossRef]
- Gigax, J.G.; Kennas, M.; Kim, H.; Maier, B.R.; Yeom, H.; Johnson, G.O.; Sridharan, K.; Shao, L. Interface Reactions and Mechanical Properties of FeCrAl-Coated Zircaloy-4. J. Nucl. Mater. 2019, 519, 57–63. [Google Scholar] [CrossRef]
- Yeom, H.; Maier, B.; Johnson, G.; Dabney, T.; Walters, J.; Sridharan, K. Development of Cold Spray Process for Oxidation-Resistant FeCrAl and Mo Diffusion Barrier Coatings on Optimized ZIRLOTM. J. Nucl. Mater. 2018, 507, 306–315. [Google Scholar] [CrossRef]
- Park, D.J.; Kim, H.G.; Jung, Y.I.; Park, J.H.; Yang, J.H.; Koo, Y.H. Microstructure and Mechanical Behavior of Zr Substrates Coated with FeCrAl and Mo by Cold-Spraying. J. Nucl. Mater. 2018, 504, 261–266. [Google Scholar] [CrossRef]
- Ougier, M.; Michau, A.; Lomello, F.; Schuster, F.; Maskrot, H.; Schlegel, M.L. High-Temperature Oxidation Behavior of HiPIMS as-Deposited Cr–Al–C and Annealed Cr2AlC Coatings on Zr-Based Alloy. J. Nucl. Mater. 2020, 528, 151855. [Google Scholar] [CrossRef]
- Imtyazuddin, M.; Mir, A.H.; Tunes, M.A.; Vishnyakov, V.M. Radiation Resistance and Mechanical Properties of Magnetron-Sputtered Cr2AlC Thin Films. J. Nucl. Mater. 2019, 526, 151742. [Google Scholar] [CrossRef]
- Tang, C.; Steinbrueck, M.; Stueber, M.; Grosse, M.; Yu, X.; Ulrich, S.; Seifert, H.J. Deposition, Characterization and High-Temperature Steam Oxidation Behavior of Single-Phase Ti2AlC-Coated Zircaloy-4. Corros. Sci. 2018, 135, 87–98. [Google Scholar] [CrossRef]
- Fazi, A.; Stiller, K.; Andrén, H.-O.; Thuvander, M. Cold Sprayed Cr-Coating on Optimized ZIRLOTM Claddings: The Cr/Zr Interface and Its Microstructural and Chemical Evolution after Autoclave Corrosion Testing. J. Nucl. Mater. 2022, 560, 153505. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, R.; Yang, H.; Liu, H.; Qiu, S.; Wang, Y.; Du, P.; He, K.; Hu, X.; Dong, C. Microstructure, Corrosion Resistance and Oxidation Behavior of Cr-Coatings on Zircaloy-4 Prepared by Vacuum Arc Plasma Deposition. Corros. Sci. 2019, 158, 108077. [Google Scholar] [CrossRef]
- Bischoff, J.; Delafoy, C.; Vauglin, C.; Barberis, P.; Roubeyrie, C.; Perche, D.; Duthoo, D.; Schuster, F.; Brachet, J.-C.; Schweitzer, E.W.; et al. AREVA NP’s Enhanced Accident-Tolerant Fuel Developments: Focus on Cr-Coated M5 Cladding. Nucl. Eng. Technol. 2018, 50, 223–228. [Google Scholar] [CrossRef]
- Yeom, H.; Maier, B.; Johnson, G.; Dabney, T.; Lenling, M.; Sridharan, K. High Temperature Oxidation and Microstructural Evolution of Cold Spray Chromium Coatings on Zircaloy-4 in Steam Environments. J. Nucl. Mater. 2019, 526, 151737. [Google Scholar] [CrossRef]
- Brachet, J.-C.; Rouesne, E.; Ribis, J.; Guilbert, T.; Urvoy, S.; Nony, G.; Toffolon-Masclet, C.; Le Saux, M.; Chaabane, N.; Palancher, H.; et al. High Temperature Steam Oxidation of Chromium-Coated Zirconium-Based Alloys: Kinetics and Process. Corros. Sci. 2020, 167, 108537. [Google Scholar] [CrossRef]
- Ma, H.-B.; Yan, J.; Zhao, Y.-H.; Liu, T.; Ren, Q.-S.; Liao, Y.-H.; Zuo, J.-D.; Liu, G.; Yao, M.-Y. Oxidation Behavior of Cr-Coated Zirconium Alloy Cladding in High-Temperature Steam above 1200 °C. Npj Mater. Degrad. 2021, 5, 7. [Google Scholar] [CrossRef]
- Ma, H.-B.; Zhao, Y.-H.; Liu, Y.; Zhu, J.-T.; Yan, J.; Liu, T.; Ren, Q.-S.; Liao, Y.-H.; Liu, G.; Lin, X.-D.; et al. Self-Healing Behavior of Cr-Coated Zr Alloy Cladding in High Temperature Steam Oxidation Process. J. Nucl. Mater. 2022, 558, 153327. [Google Scholar] [CrossRef]
- Ribis, J.; Wu, A.; Guillou, R.; Brachet, J.-C.; Baumier, C.; Gentils, A.; Loyer-Prost, M. Radiation-Induced Sharpening in Cr-Coated Zirconium Alloy. Materials 2022, 15, 2322. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Chen, H.; Zhan, C.; Du, P.; Zhang, R.; Wang, Y.; Wei, T.; Yang, H.; Zou, Y.; Yang, J. Formation of Voids in the Cr Coatings with (110)-Preferred Orientation Prepared by Arc Ion Plating under an Au+ Irradiation of 20 Dpa. Surf. Coat. Technol. 2021, 425, 127750. [Google Scholar] [CrossRef]
- Huang, M.; Li, Y.; Ran, G.; Yang, Z.; Wang, P. Cr-Coated Zr-4 Alloy Prepared by Electroplating and Its in Situ He+ Irradiation Behavior. J. Nucl. Mater. 2020, 538, 152240. [Google Scholar] [CrossRef]
- Jiang, L.; Xiu, P.; Yan, Y.; Lu, C.; Huang, M.; Liu, T.; Ye, C.; Sun, H.; Shu, R.; Wang, L. Effects of Ion Irradiation on Chromium Coatings of Various Thicknesses on a Zirconium Alloy. J. Nucl. Mater. 2019, 526, 151740. [Google Scholar] [CrossRef]
- Kim, H.-G.; Yang, J.-H.; Kim, W.-J.; Koo, Y.-H. Development Status of Accident-Tolerant Fuel for Light Water Reactors in Korea. Nucl. Eng. Technol. 2016, 48, 1–15. [Google Scholar] [CrossRef]
- Kim, H.-G.; Kim, I.-H.; Jung, Y.-I.; Park, D.-J.; Park, J.-Y.; Koo, Y.-H. Adhesion Property and High-Temperature Oxidation Behavior of Cr-Coated Zircaloy-4 Cladding Tube Prepared by 3D Laser Coating. J. Nucl. Mater. 2015, 465, 531–539. [Google Scholar] [CrossRef]
- Ševeček, M.; Gurgen, A.; Seshadri, A.; Che, Y.; Wagih, M.; Phillips, B.; Champagne, V.; Shirvan, K. Development of Cr Cold Spray–Coated Fuel Cladding with Enhanced Accident Tolerance. Nucl. Eng. Technol. 2018, 50, 229–236. [Google Scholar] [CrossRef]
- Maier, B.; Yeom, H.; Johnson, G.; Dabney, T.; Walters, J.; Xu, P.; Romero, J.; Shah, H.; Sridharan, K. Development of Cold Spray Chromium Coatings for Improved Accident Tolerant Zirconium-Alloy Cladding. J. Nucl. Mater. 2019, 519, 247–254. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Zhang, R. Application and Development Progress of Cr-Based Surface Coatings in Nuclear Fuel Element: I. Selection, Preparation, and Characteristics of Coating Materials. Coatings 2020, 10, 808. [Google Scholar] [CrossRef]
- Ko, J.; Kim, J.W.; Min, H.W.; Kim, Y.; Yoon, Y.S. Review of Manufacturing Technologies for Coated Accident Tolerant Fuel Cladding. J. Nucl. Mater. 2022, 561, 153562. [Google Scholar] [CrossRef]
- Maier, B.R.; Yeom, H.; Johnson, G.; Dabney, T.; Hu, J.; Baldo, P.; Li, M.; Sridharan, K. In Situ TEM Investigation of Irradiation-Induced Defect Formation in Cold Spray Cr Coatings for Accident Tolerant Fuel Applications. J. Nucl. Mater. 2018, 512, 320–323. [Google Scholar] [CrossRef]
- Li, Q.; Song, P.; Zhang, R.; Li, Z.; Wang, Y.; Du, P.; Lu, J. Oxidation Behavior and Cr-Zr Diffusion of Cr Coatings Prepared by Atmospheric Plasma Spraying on Zircaloy-4 Cladding in Steam at 1300 °C. Corros. Sci. 2022, 203, 110378. [Google Scholar] [CrossRef]
- Huang, H.; Qiu, C.; Chen, Y.; Hu, L.; Liu, Y.; Li, H. High temperature oxidation resistance of magnetron sputtering and multi-arc ion plating Cr films on zirconium alloy. China Surf. Eng. 2018, 31, 51–58. (In Chinese) [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Sidelev, D.V.; Syrtanov, M.S.; Tang, C.; Steinbrück, M. Oxidation Kinetics of Cr-Coated Zirconium Alloy: Effect of Coating Thickness and Microstructure. Corros. Sci. 2020, 175, 108883. [Google Scholar] [CrossRef]
- He, X.; Tian, Z.; Shi, B.; Xu, X.; Meng, C.; Dang, W.; Tan, J.; Ma, X. Effect of Gas Pressure and Bias Potential on Oxidation Resistance of Cr Coatings. Ann. Nucl. Energy 2019, 132, 243–248. [Google Scholar] [CrossRef]
- Chen, Q.S.; Liu, C.H.; Zhang, R.Q.; Yang, H.Y.; Wei, T.G.; Wang, Y.; Li, Z.; He, L.X.; Wang, J.; Wang, L.; et al. Microstructure and High-Temperature Steam Oxidation Properties of Thick Cr Coatings Prepared by Magnetron Sputtering for Accident Tolerant Fuel Claddings: The Role of Bias in the Deposition Process. Corros. Sci. 2020, 165, 108378. [Google Scholar] [CrossRef]
- Meng, Y.; Zeng, S.; Teng, Z.; Han, X.; Zhang, H. Control of the Preferential Orientation Cr Coatings Deposited on Zircaloy Substrates and Study of Their Oxidation Behavior. Thin Solid Film. 2021, 730, 138699. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Sidelev, D.V.; Pushilina, N.S.; Yang, J.; Tang, C.; Steinbrueck, M. Influence of Coating Parameters on Oxidation Behavior of Cr-Coated Zirconium Alloy for Accident Tolerant Fuel Claddings. Corros. Sci. 2022, 203, 110359. [Google Scholar] [CrossRef]
- Huang, J.; Zou, S.; Xiao, W.; Yang, C.; Tang, D.; Yu, H.; Zhang, L.; Zhang, K. Influences of Arc Current on Microstructure of Cr Coating for Zr-4 Alloy Prepared by Multi-Arc Ion Plating via EBSD. Mater. Charact. 2021, 178, 111211. [Google Scholar] [CrossRef]
- Zeng, X.; Qiu, C.; Zhang, W.; Wang, H.; Wang, X.; Liu, Y.; Li, H. Effect of Negative Bias on Droplet Deposition and Interfacial Adhesion of Chromium Coating. Vac. Sci. Technol. 2017, 37, 177–181. (In Chinese) [Google Scholar] [CrossRef]
- Zeng, X.; Huang, H.; Zhang, W.; Wang, H.; Liu, Z.; Qiu, C. Effect of Arc Current on Microstructure and Mechanical Properties of Cr Coatings Deposited by Multi-Arc Ion Plating. Rare Met. Cem. Carbides 2017, 45, 53–62. (In Chinese) [Google Scholar] [CrossRef]
- Wang, H.; Qiu, C.; Zeng, X.; Zhang, W.; Wang, X.; Liu, Y.; Li, H. Effect of Temperature on Morphology and Corrosion Resistance of Pure Cr Coating. Surf. Technol. 2017, 46, 192–196. (In Chinese) [Google Scholar] [CrossRef]
- Yang, H. Material Test Design, 1st ed.; Electronic Industries Press: Beijing, China, 2020; ISBN 978-7-121-14150-8. (In Chinese) [Google Scholar]
- Zhao, S. A Study on Deposition Process in Cathodic Arc Ion Plating through Computer Silmulation. Master’s Thesis, Shenyang University, Shenyang, China, 2007. (In Chinese). [Google Scholar]
- Gong, C. Study of Droplets and Its Effects on Performance of TiN Films Prepared by Arc Ion Plating. Ph.D. Thesis, Central South University, Changsha, China, 2013. (In Chinese). [Google Scholar]
- Mattox, D.M. Surface Effects on the Growth, Adhesion and Properties of Reactively Deposited Hard Coatings. Surf. Coat. Technol. 1996, 81, 8–16. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Sidelev, D.V.; Rombaeva, M.; Syrtanov, M.S.; Bleykher, G.A. Chromium Coatings Deposited by Cooled and Hot Target Magnetron Sputtering for Accident Tolerant Nuclear Fuel Claddings. Surf. Coat. Technol. 2020, 389, 125618. [Google Scholar] [CrossRef]
- Umretiya, R.V.; Elward, B.; Lee, D.; Anderson, M.; Rebak, R.B.; Rojas, J.V. Mechanical and Chemical Properties of PVD and Cold Spray Cr-Coatings on Zircaloy-4. J. Nucl. Mater. 2020, 541, 152420. [Google Scholar] [CrossRef]
- Wang, S.; Hong, L. The Differentiation about the Formulae of Texture of Electroplating Deposit by Using XRD. J. Huaqiao Univ. Nat. Sci. 2008, 29, 476–478. (In Chinese) [Google Scholar] [CrossRef]
- Li, H.; Jiang, B.; Cao, Z. Influence of Duty Cycle on Microstructures of Magnetron Sputtered Pure Cr Coatings. Vac. Sci. Technol. 2010, 30, 133–137. (In Chinese) [Google Scholar] [CrossRef]
- Hu, M.; Shen, M.; Liu, Z.; Guo, C.; Li, Q.; Zhu, S. Self-Ion Bombarded Cr Films: Crystallographic Orientation and Oxidation Behaviour. Corros. Sci. 2018, 143, 212–220. [Google Scholar] [CrossRef]
- Ribis, J.; Wu, A.; Brachet, J.-C.; Barcelo, F.; Arnal, B. Atomic-Scale Interface Structure of a Cr-Coated Zircaloy-4 Material. J. Mater. Sci. 2018, 53, 9879–9895. [Google Scholar] [CrossRef]
- Krejčí, J.; Kabátová, J.; Manoch, F.; Kočí, J.; Cvrček, L.; Málek, J.; Krum, S.; Šutta, P.; Bublíková, P.; Halodová, P.; et al. Development and Testing of Multicomponent Fuel Cladding with Enhanced Accidental Performance. Nucl. Eng. Technol. 2020, 52, 597–609. [Google Scholar] [CrossRef]
- Jüttner, B. Cathode Spots of Electric Arcs. J. Phys. D. 2001, 34, R103–R123. [Google Scholar] [CrossRef]
- Cooke, K.E.; Hamsphire, J.; Southall, W.; Teer, D.G. The Industrial Application of Pulsed DC Bias Power Supplies in Closed Field Unbalanced Magnetron Sputter Ion Plating. Surf. Coat. Technol. 2004, 177–178, 789–794. [Google Scholar] [CrossRef]
- Hideo, S. Plasma Electronic Engineering, 1st ed.; Science Press: Beijing, China, 2002; ISBN 7-03-009986-9. (In Chinese) [Google Scholar]
- Daalder, J.E. A Cathode Spot Model and Its Energy Balance for Metal Vapour Arcs. J. Phys. D 1978, 11, 1667–1682. [Google Scholar] [CrossRef]
- Martin, P. Review of the Filtered Vacuum Arc Process and Materials Deposition. Thin Solid Films 2001, 394, 1–14. [Google Scholar] [CrossRef]
- Boxman, R.L.; Goldsmith, S. Macroparticle Contamination in Cathodic Arc Coatings: Generation, Transport and Control. Surf. Coat. Technol. 1992, 52, 39–50. [Google Scholar] [CrossRef]
- Anders, A. Approaches to Rid Cathodic Arc Plasmas of Macro- and Nanoparticles: A Review. Surf. Coat. Technol. 1999, 120–121, 319–330. [Google Scholar] [CrossRef]
- Huang, M.; Lin, G.; Zhao, Y.; Sun, C.; Wen, L.; Dong, C. Macro-Particle Reduction Mechanism in Biased Arc Ion Plating of TiN. Surf. Coat. Technol. 2003, 176, 109–114. [Google Scholar] [CrossRef]
- Keidar, M.; Beilis, I.; Boxman, R.L.; Goldsmith, S. Macroparticle Interaction with a Substrate in Cathodic Vacuum Arc Deposition. Surf. Coat. Technol. 1996, 86–87, 415–420. [Google Scholar] [CrossRef]
- Mattox, D.M.; Kominiak, G.J. Structure Modification by Ion Bombardment during Deposition. J. Vac. Sci. Technol. 1972, 9, 528–532. [Google Scholar] [CrossRef]
- Messier, R.; Giri, A.P.; Roy, R.A. Revised Structure Zone Model for Thin Film Physical Structure. J. Vac. Sci. Technol. A 1984, 2, 500–503. [Google Scholar] [CrossRef]
- Yang, B. Thin Film Physics and Technology, 1st ed.; University of Electronic Science and Technology of China Press: Chengdu, China, 1994; ISBN 978-7-81016-749-9. (In Chinese) [Google Scholar]
- Hu, G.; Cai, X.; Rong, Y. Fundamentals of Materials Science, 3rd ed.; Shanghai Jiao Tong University Press: Shanghai, China, 2010; ISBN 978-7-313-02480-0. (In Chinese) [Google Scholar]
- Zhou, J.; Liu, Z.; Wang, W. Brief Introduction of Townsend Discharge. J. Dalian Univ. 2003, 24, 16–18. (In Chinese) [Google Scholar]


| Test No. | Heating Temperature (°C) | Arc Current (A) | Gas Pressure (Pa) | Negative Bias Voltage (V) |
|---|---|---|---|---|
| 1 | 350 | 150 | 1.6 | −160 |
| 2 | 350 | 120 | 1.2 | −120 |
| 3 | 350 | 90 | 0.8 | −80 |
| 4 | 300 | 150 | 1.2 | −80 |
| 5 | 300 | 120 | 0.8 | −160 |
| 6 | 300 | 90 | 1.6 | −120 |
| 7 | 250 | 150 | 0.8 | −120 |
| 8 | 250 | 120 | 1.6 | −80 |
| 9 | 250 | 90 | 1.2 | −160 |
| Circum. | a | b | c | d | e | RAD | ||
|---|---|---|---|---|---|---|---|---|
| Axial | ||||||||
| 1 | 24.78 | 21.36 | 23.26 | 23.06 | 22.3 | 23 | 3.91% | |
| 2 | 27.05 | 24.86 | 26.86 | 26.29 | 25.34 | 26.1 | 3.01% | |
| 3 | 27.91 | 26.39 | 27.25 | 27.24 | 27.15 | 27.2 | 1.23% | |
| 4 | 27.06 | 24.34 | 25.73 | 24.88 | 24.78 | 25.4 | 3.27% | |
| 5 | 23.91 | 22.03 | 22.78 | 22.5 | 22.21 | 22.7 | 2.32% | |
| 26.1 | 23.8 | 25.2 | 24.8 | 24.4 | 24.9 | 2.54% | ||
| RAD | 4.34% | 6.84% | 4.29% | 5.81% | 6.12% | 5.45% | ||
| Factor Level | Temperature (°C) | Arc Current (A) | Gas Pressure (Pa) | Bias Voltage (V) |
|---|---|---|---|---|
| k1j | 2.196 | 3.297 | 2.408 | 2.188 |
| k2j | 2.513 | 2.222 | 2.239 | 2.321 |
| k3j | 2.357 | 1.547 | 2.419 | 2.557 |
| Rj | 0.317 | 1.750 | 0.180 | 0.369 |
| SSj | 0.151069 | 4.673750 | 0.061105 | 0.209813 |
| Variance Source | F | Table Value | Significance Level | |||
|---|---|---|---|---|---|---|
| Arc current | 4.673750 | 2 | 2.336875 | 76.49 | F0.013(2,2) = 75.92 | α ~ 0.013 |
| Bias voltage | 0.209813 | 2 | 0.104906 | 3.43 | F0.226(2,2) = 3.42 | α ~ 0.226 |
| Temperature | 0.151069 | 2 | 0.075534 | 2.47 | F0.288(2,2) = 2.47 | α ~ 0.288 |
| Error | 0.061105 | 2 | 0.030552 | |||
| Sum | 0.1791 | 8 |
| Sample | Number | Diameter (μm) | Total Area (%) | |
|---|---|---|---|---|
| Ave. | Max. | |||
| 1 | 4717 | 1.00 | 8.96 | 20.00 |
| 2 | 3033 | 0.82 | 2.84 | 5.28 |
| 3 | 1079 | 0.95 | 3.74 | 2.57 |
| 4 | 4457 | 1.16 | 4.62 | 15.60 |
| 5 | 2107 | 0.96 | 4.59 | 3.84 |
| 6 | 739 | 0.93 | 2.96 | 1.29 |
| 7 | 4478 | 1.16 | 4.39 | 15.81 |
| 8 | 594 | 0.87 | 2.27 | 1.18 |
| 9 | 908 | 0.35 | 4.13 | 1.47 |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| TC110 | 1.803 | 1.812 | 1.461 | 1.911 | 1.134 | 2.136 | 1.599 | 1.797 | 1.407 |
| TC200 | 0.567 | 0.654 | 0.747 | 0.537 | 0.864 | 0.588 | 0.615 | 0.570 | 1.026 |
| TC211 | 0.630 | 0.537 | 0.792 | 0.552 | 1.005 | 0.276 | 0.786 | 0.633 | 0.567 |
| No. | Average | Maximum | ||
|---|---|---|---|---|
| 1 | 1.548 | 3.302 | 7.471 | 14.225 |
| 2 | 1.795 | 4.353 | 6.189 | 18.211 |
| 3 | 1.527 | 4.370 | 4.882 | 17.193 |
| 4 | 1.709 | 4.178 | 8.801 | 20.793 |
| 5 | 1.713 | 4.031 | 6.926 | 18.438 |
| 6 | 1.708 | 4.297 | 4.814 | 19.061 |
| 7 | 1.589 | 3.519 | 8.705 | 16.091 |
| 8 | 2.044 | 4.111 | 11.890 | 22.843 |
| 9 | 1.094 | 3.197 | 3.215 | 12.426 |
| mean | 1.636 | 3.929 | 6.988 | 17.698 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Ma, Z.; Wang, Y.; Wei, T.; Yang, H.; Du, P.; Wang, X.; Zhang, R. An Orthogonal Experimental Study on the Preparation of Cr Coatings on Long-Size Zr Alloy Tubes by Arc Ion Plating. Materials 2022, 15, 7177. https://doi.org/10.3390/ma15207177
Chen H, Ma Z, Wang Y, Wei T, Yang H, Du P, Wang X, Zhang R. An Orthogonal Experimental Study on the Preparation of Cr Coatings on Long-Size Zr Alloy Tubes by Arc Ion Plating. Materials. 2022; 15(20):7177. https://doi.org/10.3390/ma15207177
Chicago/Turabian StyleChen, Huan, Zhaodandan Ma, Yu Wang, Tianguo Wei, Hongyan Yang, Peinan Du, Xiaomin Wang, and Ruiqian Zhang. 2022. "An Orthogonal Experimental Study on the Preparation of Cr Coatings on Long-Size Zr Alloy Tubes by Arc Ion Plating" Materials 15, no. 20: 7177. https://doi.org/10.3390/ma15207177
APA StyleChen, H., Ma, Z., Wang, Y., Wei, T., Yang, H., Du, P., Wang, X., & Zhang, R. (2022). An Orthogonal Experimental Study on the Preparation of Cr Coatings on Long-Size Zr Alloy Tubes by Arc Ion Plating. Materials, 15(20), 7177. https://doi.org/10.3390/ma15207177







