1. Introduction
In the last few decades, numerous drug delivery systems such as polymersomes, liposomes, nanoparticles, micelles, and hydrogels have been formulated for the delivery or controlled release of cytotoxic or low bioavailable therapeutic agents [
1]. Specifically, hydrogels, which are cross-linked and three-dimensional network configurations of hydrophilic polymer chains and can engross a huge volume of water/biological fluids or can encapsulate therapeutic molecules in their internal networks, have received great attention in drug delivery. Regardless of their benefits, traditional hydrogels require a surgical procedure for their implantation to a malignant site. To address this problem, injectable hydrogels which can be employed by nominally invasive means and can be molded into any shape have received great interest in drug delivery. These injectable hydrogels not only mimic extremely hydrated 3D microenvironment of native extracellular matrix, but can also deliver therapeutic molecules to diseased sites. The injectable hydrogels could be engineered by synthetic or natural polymers using sol-gel phase transition [
2], in-situ polymerization [
3], and physical or chemical cross-linking strategies [
4].
From a bio-imitation perspective, an ideal injectable hydrogel should be fast absorbent, i.e., can absorb a large amount of water and should possess interlocked pores to facilitate proficient supply of oxygen, nutrients, growth factor/therapeutic compounds and/or waste diffusion, mimicking the native tissue-environment [
5]. Previous reports have shown that highly porous hydrogels have several benefits over their non-porous counterparts [
6]. Natural polymer-based porous hydrogels could be developed by using various additives and approaches. For instance, porous scaffolds derived from gelatin were developed by an emulsion template method [
7]. For this purpose, gelatin droplets were initially engineered by emulsification, and subsequently stabilized by genipin cross-linkers, generating microspheres, which were later employed as a template for the development of gelatin-based porous hydrogels. It was demonstrated that hydrogels possessed macro-porous and interlocked networks and that the pore-sizes could be programmed by changing the diameters of the microspheres. However, the genipin cross-linker could comprise the biocompatibility of the resulting porous materials. Porogen leaching is another method to carve porosity in hydrogels, which implicates mixing of inorganic salts (e.g., sodium chloride, sugar crystals, etc.) with a polymer solution prior to casting into a mold [
8]. The hydrogels are then lyophilized and the salt particles are rinsed out to generate porosity in the hydrogels. Nevertheless, large difference in pore sizes, absence of interconnectivity, and uneven pore geometry have restricted the use of this technique. Lyophilization has also been widely exploited to form porous hydrogels [
9]. Nevertheless, poor control over pore-sizes and low structure stability of the resulting porous hydrogels renders the lyophilization technique less practical.
Recently, porous hydrogels prepared through an in-situ bubble generating procedure were developed from hyaluronic acid (HA) and cystamine via carbodiimide chemistry [
10]. Specifically, 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) and N-hydroxy-succinimide (NHS) released carbon dioxide gas as a by-product during the coupling reaction, generating pores in the hydrogel networks. This approach is facile and simple, the EDC and NHS coupling agents, however, might induce unwanted cytotoxicities, and are not considered safe for injectable drug delivery systems. It is noteworthy that most of the above-mentioned approaches either employ cytotoxic reagents or use destructive techniques which are not suitable for injectable hydrogels, and omits any chance of injecting them into the diseased site. Therefore, porous and injectable hydrogels possessing better biocompatibility are highly needed for biomedical applications.
Various polymeric materials can be used to make porous and injectable hydrogels. Hyaluronic acid (HA), which is a natural linear polysaccharide composed of alternating units of the repeating disaccharide, β-1,4-D-glucuronic acid and β-1,3-N-acetyl-D-glucosamine, have received much attention in drug delivery due to its exceptional cytocompatibility and biodegradability [
11,
12,
13,
14]. In spite of a number of advantages, HA has feeble mechanical properties and a quick degradation profile [
15]. The mechanical strength and degradation profiles of HA have been improved by covalent cross-linkers, such as glutaraldehyde, genipin, and EDC, previously. Although these cross-linkers have produced stable hydrogels, their high cytotoxicities have been reported [
16].
Recently, click chemistry has been considered as a promising approach to engineer covalently cross-linked hydrogels [
17,
18]. In particular, the inverse electron demand Diels-Alder (IEDDA) reaction between norbornene (Nb) functional groups and tetrazine (Tz) functional groups has been regarded as a highly biocompatible approach for the synthesis of hydrogels [
19]. The IEDDA reaction possesses high chemo-selectivity and ultrafast kinetics [
20], and can be performed in catalyst-free environment at mild conditions. Interestingly, the sole by-product of this reaction is nitrogen gas, which is non-toxic, making it suitable for injectable drug delivery applications [
21,
22,
23]. In addition, the liberated nitrogen gas can generate pores in the hydrogels [
20], and this porous structure can modulate the drug encapsulation and release performance of the resulting hydrogels [
24]. Furthermore, depending upon the amount of Tz and/or cross-linkers, the gelation times of hydrogels could be adjusted to few seconds to several minutes, making these materials injectable.
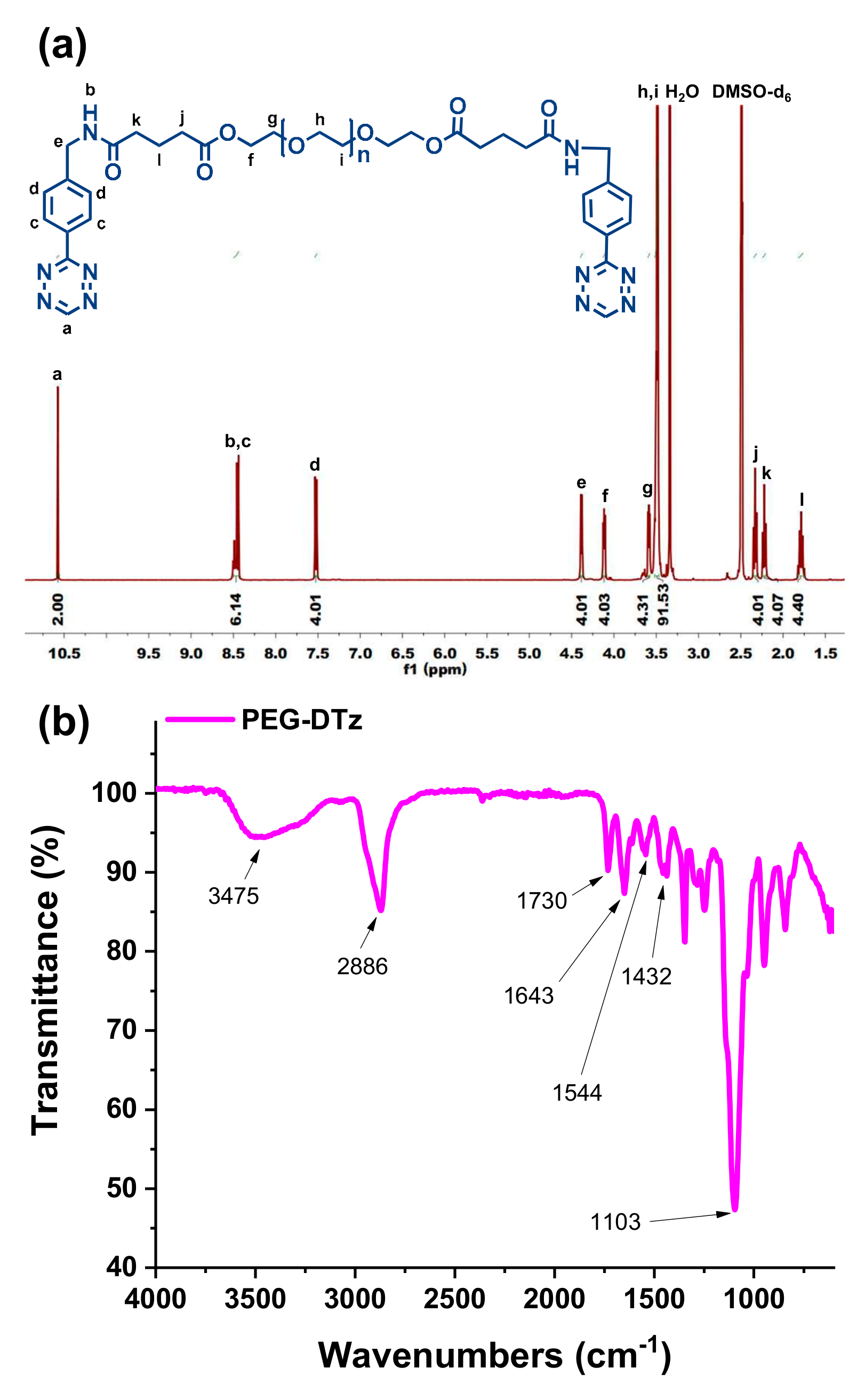
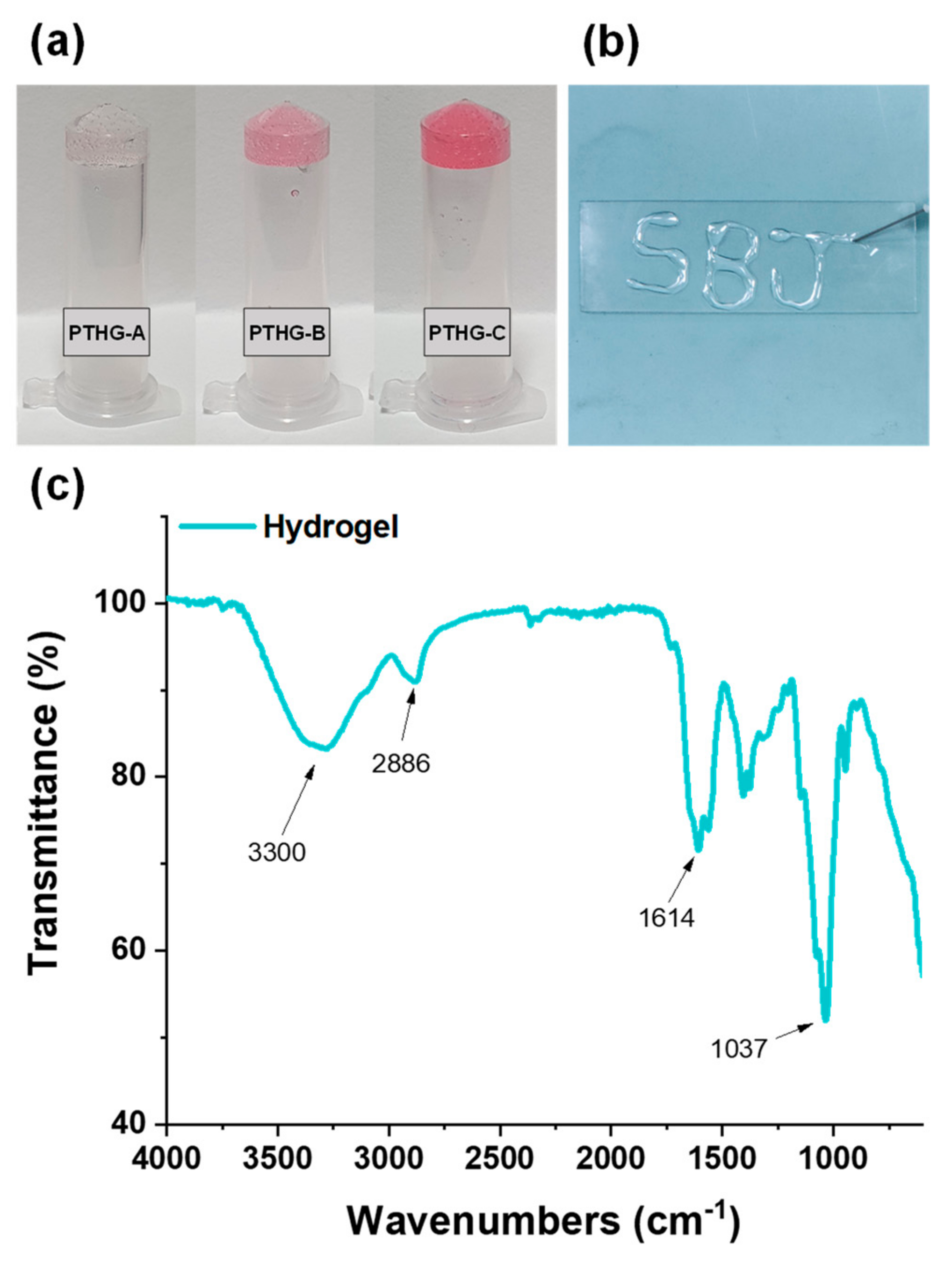
In this study, highly biocompatible and fast absorbent injectable hydrogels were developed by using Nb-functionalized HA (HA-Nb) and a water-soluble cross-linker (polyethylene glycol-ditetrazine, PEG-DTz) possessing Tz functional groups on both ends of PEG. The PEG-Tz was synthesized by a simple carbodiimide reaction (
Scheme 1a). Since PEG-DTz and HA-Nb (
Scheme 1b) are highly water-soluble precursors and the IEDDA click reaction is highly bioorthogonal, it was possible to produce hydrogels with excellent biocompatibility. Three types of hydrogels were developed by changing the mol. ratios of the cross-linker (Nb:Tz = 10:10, 10:5, 10:2.5). The hydrogels formed fast (sol-gel time < 100 s), offering a possibility to use them as injectable drug delivery systems (as illustrated in
Scheme 1c). Nitrogen gas liberated during the IEDDA reaction resulted in a highly porous structure. The porous structure could regulate the drug release from the hydrogels as illustrated in
Scheme 1d. Experimental data of the prepared hydrogels showed rapid swelling owing to the existence of PEG chains and highly porous architectures of the hydrogels. Curcumin (a hydrophobic bioactive drug derived from turmeric) was used as a model therapeutic molecule to assess the drug loading and release behavior of the hydrogels. The hydrogels were able to encapsulate a high amount of curcumin (~99%) and released it in a temporal pattern. The PEG-DTz cross-linker, HA-Nb and the resulting hydrogels showed no cytotoxicity in HEK-293 cells, exhibiting excellent biocompatibility of these hydrogels. These fast absorbent and injectable hydrogels with excellent biocompatibility could be promising drug carriers for injectable drug delivery applications.
2. Materials and Methods
2.1. Materials
Polyethylene glycol (OH-PEG-OH, Mn = 1000), 4-(dimethylamino)pyridine (DMAP, 99%), sodium chloride (NaCl, 99%), NHS (98%), triethylamine (TEA, 99%) were purchased from Sigma Aldrich (Waltham, M, USA). EDC.HCl (99%) and 5-Norbornene-2-methylamine (Nb-NH2, 98%), were acquired from Tokyo Chemical Industry (TCI, Tokyo, Japan). Acetone, dichloromethane (DCM), diethyl ether, and ethyl acetate, were obtained from Duksan Pure Chemicals (Siheung, Korea). Sodium hyaluronate (HA, Mw ~800 kDa) was purchased from Bioland Korea (Chungbuk, Korea). 4- (Aminomethyl)benzonitrile hydrochloride (98%) was purchased from Matrix Scientific. Curcumin (95%) was purchased from Alfa Aesar (Haverhill, MA, USA).
2.2. Measurements
1H NMR analyses were carried out using a JEOL NMR spectrometer (JNM ECZ-400, JEOL, Akishima-shi, Japan). Fourier transform infrared (FTIR) spectra were obtained using an Agilent Cary640-FTIR. The surface and cross-sectional structure of hydrogels was observed by using a low vacuum scanning electron microscope (SEM, JEOL, Gatan, JSM-6490LV, Pleasanton, CA, USA). The UV-vis spectra were recorded on an Optizen POP UV-vis spectrophotometer. The rheology of hydrogels was investigated using a Discovery HR-2 hybrid rheometer (TA instrument) mounted on a flat steel plate.
2.3. Methods
2.3.1. Synthesis and Characterization of Precursors
Synthesis of (4-(Cyano) Benzylamino)-5-Oxopentanoic Acid
The (4-(cyano) benzylamino)-5-oxopentanoic acid was obtained following a previously published protocol after minor changes [
25]. Briefly, 4- (aminomethyl) benzonitrile hydrochloride (2 g, 11.86 mmol) was mixed with 100 mL of extra pure acetonitrile in a two-neck round bottom flask (RBF). After purging with nitrogen for 30 min, TEA (5 mL, 35.58 mmol) was introduced int the RBF. After stirring for another 30 min, glutaric anhydride (1.48 g, 13.04 mmol) was dispersed in acetonitrile and then was incorporated with the mixture. The reaction was refluxed at 85 °C for 24 h. After evaporating the solvent by a rotary evaporator, solid crystals were suspended in 100 mL of DI water. Next, 1N HCl was introduced to the mixture to adjust the pH to 3 and then it was extracted 3 times with 100 mL of ethyl acetate. The fractions of the ethyl acetate were combined and washed again with water (100 mL) and brine (100 mL). The mixture was treated with anhydrous MgSO
4 to remove the water residues and was filtered. Finally, the ethyl acetate was evaporated using a rotary evaporator to obtain a white solid (91% yield). The structure of (4-(cyano) benzylamino)-5-oxopentanoic acid was confirmed by
1H NMR (
Supplementary Materials Figure S1a) and
13C NMR (
Supplementary Materials Figure S1b). (
1H NMR, 400 MHz, DMSO-d
6, δ): 12.05 (s, 1H), 8.44 (t, J = 5.9 Hz, 1H), 7.78 (d, J = 8.5 Hz, 2H), 7.42 (d, J = 8.6 Hz, 2H), 4.33 (d, J = 6.0 Hz, 2H), 2.26–2.15 (m, 4H), 1.79–1.69 (m, 2H).
Synthesis of Tetrazine(benzylamino)-5-Oxopentanoic Acid (Tetrazine-COOH)
The Tetrazine-COOH) was obtained by following a previously published protocol after minor changes [
25]. Briefly, (4-cyanobenzylamino)-5-oxopentanoic acid (1 g, 4.08 mmol) and formamidine acetate salt (2.12 g, 20.39 mmol) were mixed in RBF. After adding zinc triflate (0.074 g, 0.20 mmol) into the mixture, anhydrous hydrazine (3.26 mL, 102 mmol) was incorporated dropwise. After vigorous gas release, the resulting viscous slurry was stirred overnight at room temperature (RT). Next, sodium nitrite (2.82 g, 40.80 mmol) was dissolved in 5 mL of DI H
2O and was incorporated with the reaction solution. Next, the RBF was moved to an ice bath (0 °C) and 1N HCl was carefully introduced into the solution dropwise (at this stage vigorous gas release was noted). When around 150 mL of 1N HCl was consumed and the pH approached to 3, the solution turned into bright pink and the generation of gas stopped. Subsequently, the suspension was extracted with 100 mL DCM (3 times) and the organic phase was washed with brine (3 × 100 mL). After drying with anhydrous MgSO
4 and filtering, DCM was evaporated by using a rotary evaporator. Finally, the solid was purified by recrystallization in cold isopropyl alcohol to obtain Tetrazine-COOH as a bright pink solid (53% yield). The structure of Tetrazine-COOH was confirmed by
1H NMR (
Supplementary Materials Figure S2a) and
13C NMR (
Supplementary Materials Figure S2b). (
1H NMR, 400 MHz, DMSO-d
6, δ): 12.06 (s, 1H), 10.58 (s, 1H), 8.52–8.40 (m, 3H), 7.53 (d, J = 8.6 Hz, 2H), 4.40 (d, J = 5.9 Hz, 2H), 2.28–2.17 (m, 4H), 1.77 (p, J = 7.3 Hz, 2H).
Synthesis of PEG-DTz
Typically, tetrazine-COOH (198.86 mg, 0.66 mmol) was mixed with a solvent mixture containing 2 mL of DMSO and 10 mL of DCM in a RBF, and then the resulting mixture was degassed under stirred at 0 °C. Next, DMAP (91.62 mg, 0.75 mmol) was dissolved in 10 mL of DCM and was introduced while maintaining an inert environment. Next, EDC.HCl was mixed with 10 mL of DCM and then was introduced dropwise into the reaction flask. The solution was stirred at RT for 1 h to activate carboxylate functionality. Afterwards, PEG (300 mg, 0.3 mmol) was mixed with 2 mL of DCM and then was incorporated with reaction mixture. The contents were then stirred under nitrogen at RT for 48 h and then mixed with 70 mL of DCM. The diluted mixture was washed 3 times with DI H2O (125 mL) and once with brine (100 mL). The DCM phase was dried with anhydrous MgSO4 and filtered with a filter paper, and then DCM was evaporated by using a rotary evaporator. Finally, the product was precipitated in 40 mL of diethyl ether. The resulting crystals were filtered and dried in under low pressure for 1 d to acquire PEG-DTz as a pink solid. (67.13% yield), 1H NMR (400 MHz, DMSO-D6) δ = 10.58 (s, 2 H), 8.52–8.40 (m, 6 H), 7.53 (d, 4 H), 4.40 (d, 4H), 4.13 (t, 4 H), 3.68 (t, 4 H), 3.51 (s, 91 H), 2.34 (t, 4 H), 2.23 (t, 4 H), 1.80 (t, 4 H).
Synthesis of HA-Nb
HA-Nb was synthesized following a previous protocol [
21]. Briefly, HA (2000 mg, 0.0025 mmol) was mixed with 200 mL of DI water until a homogenous solution was obtained. Next, EDC.HCl (475.40 mg, 2.48 mmol) and NHS (427.84 mg, 3.72 mmol) were incorporated with the suspension and stirred for 1 h. After adding Nb-NH2 (458 mg, 3.72 mmol, dissolved in 5 mL of DMSO) to the solution, the mixture was stirred for 24 h at RT. Subsequent, NaCl (1.5 g) was mixed with 5 mL of DI water and incorporated with the mixture. After stirring for 10 min, the solution was precipitated in an excess of acetone. After filtering, the sponge-like solid product was dried under reduced pressure. Next, the solid was re-dispersed in 200 mL of DI water and shifted to a dialysis bag (MWCO = 14 kDa) and dialyzed in DI water for 2 d. After freezing the mixture, it was lyophilized to acquire the dry HA-Nb with 18.55% degree of substitution (measured by
1H NMR). The structure of the HA-Nb was further characterized by FTIR analysis.
2.3.2. Preparation of Hydrogels
The cross-linked hydrogels based on the IEDDA click reaction were developed by a one-step mixing of the PEG-DTz cross-linker and HA-Nb. Hydrogels with 3 cross-linking conformations (as presented in
Table 1) were prepared to acquire the hydrogels with varying physicochemical properties. Briefly, 200 μL of a 2% HA-Nb solution (prepared by dissolving HA-Nb in DI water) and 35 μL of a PEG-DTz solution (in DI water) having various concentration were gently mixed in an Eppendorf for 15 s by means of a vortex. The solution mixture was instantly transferred to a syringe (25G needle) to test the injectability.
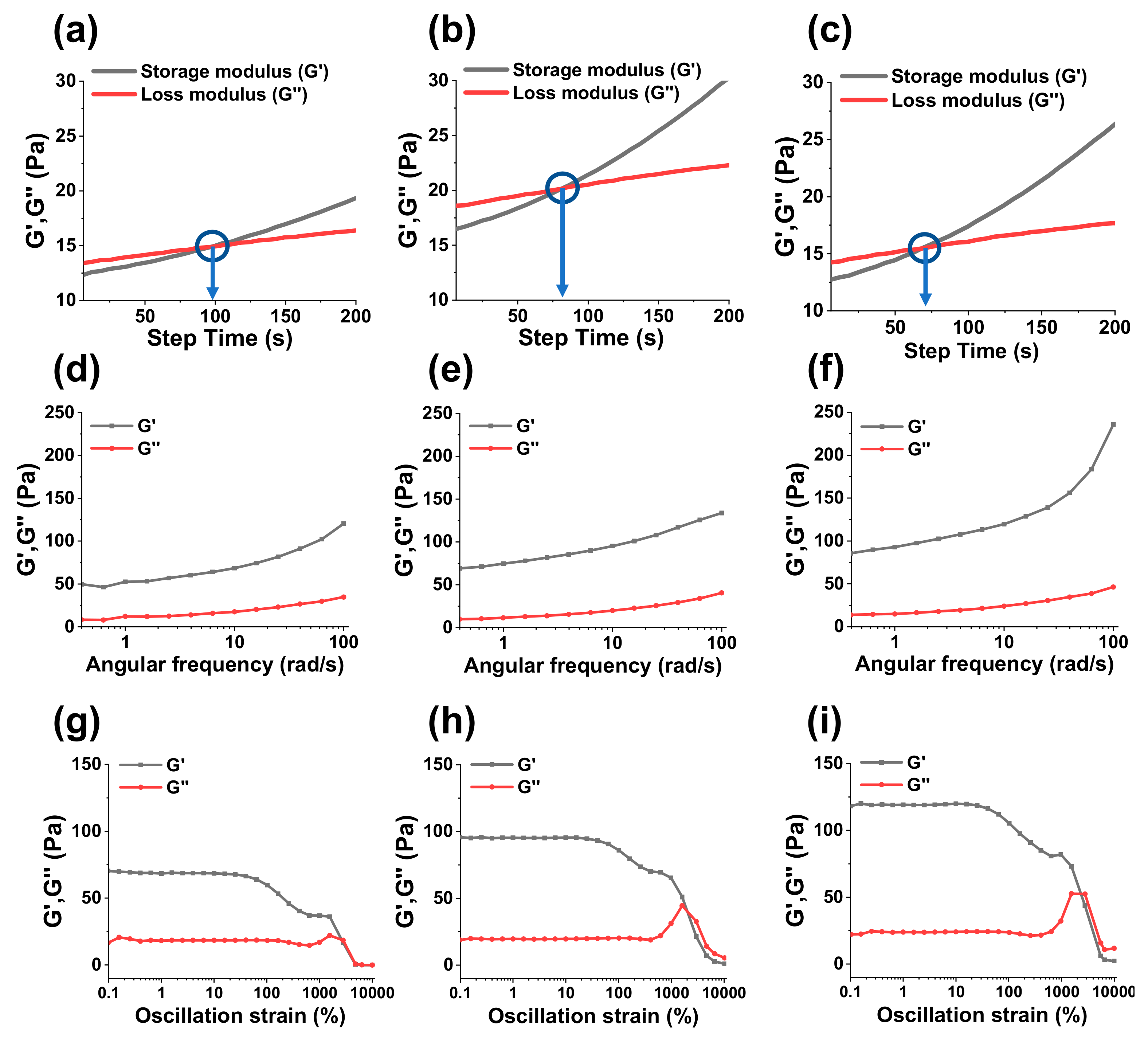
2.3.3. Rheological Properties of Hydrogels
The viscoelastic behaviors of hydrogels were assessed by a rheometer. Specifically, sol-gel transformation times (gelation times) were calculated by measuring the storage modulus G“ and loss modulus (G”) of hydrogels as function of step-time. The sol-gel transformation time could be considered when G’ and G” intersect each other. Similarly, an angle frequency sweep test and amplitude sweep test were carried out to assess the mechanical strength and shear thinning properties of hydrogels.
2.3.4. Swelling Studies
To measure swelling ratios, hydrogels were lyophilized and weighed before immersing them in 3 mL of PBS (pH = 7.4). After pre-determined times, hydrogels were taken out from the swelling medium and were carefully treated with a tissue paper to eliminate extra water presented on the upper surface of hydrogels before measuring their weights. The non-lyophilized hydrogels were also assessed for their swelling behavior. The swelling ratios of hydrogels were measured by using the following equation:
where, M
s is the mass of the swollen hydrogels and M
d is the mass of the dried hydrogels.
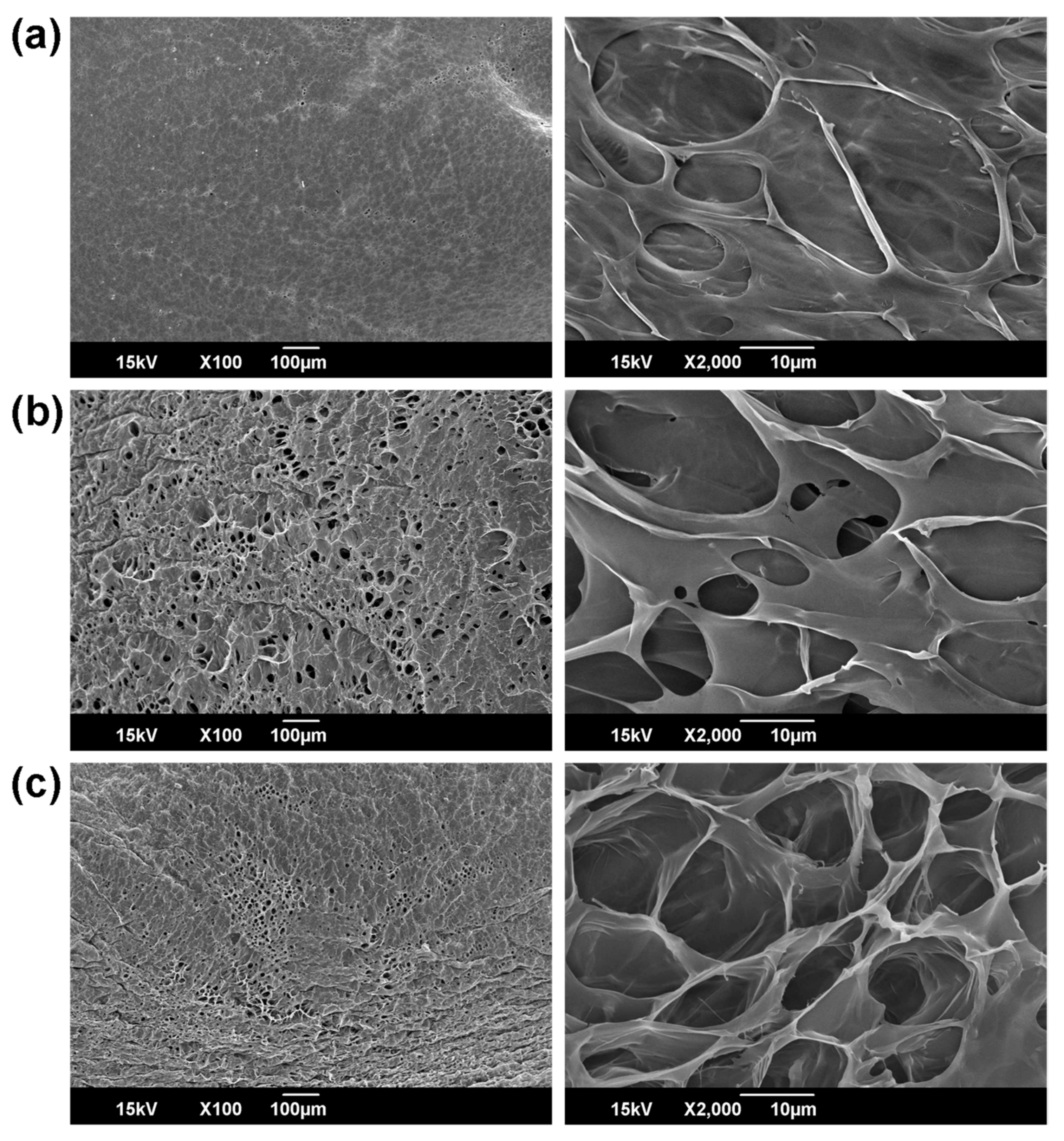
2.3.5. Morphology of Hydrogels
To investigate the surface and internal morphologies, hydrogels were frozen in liquefied nitrogen for 5 min, and the stored at −80 °C for 1 d before being lyophilized for 3 d. The freeze-dried hydrogels were again immersed in liquid nitrogen and were cut vertically, and then their surfaces and cross-sections were observed by using a SEM.
2.3.6. Drug Loading and Release Studies
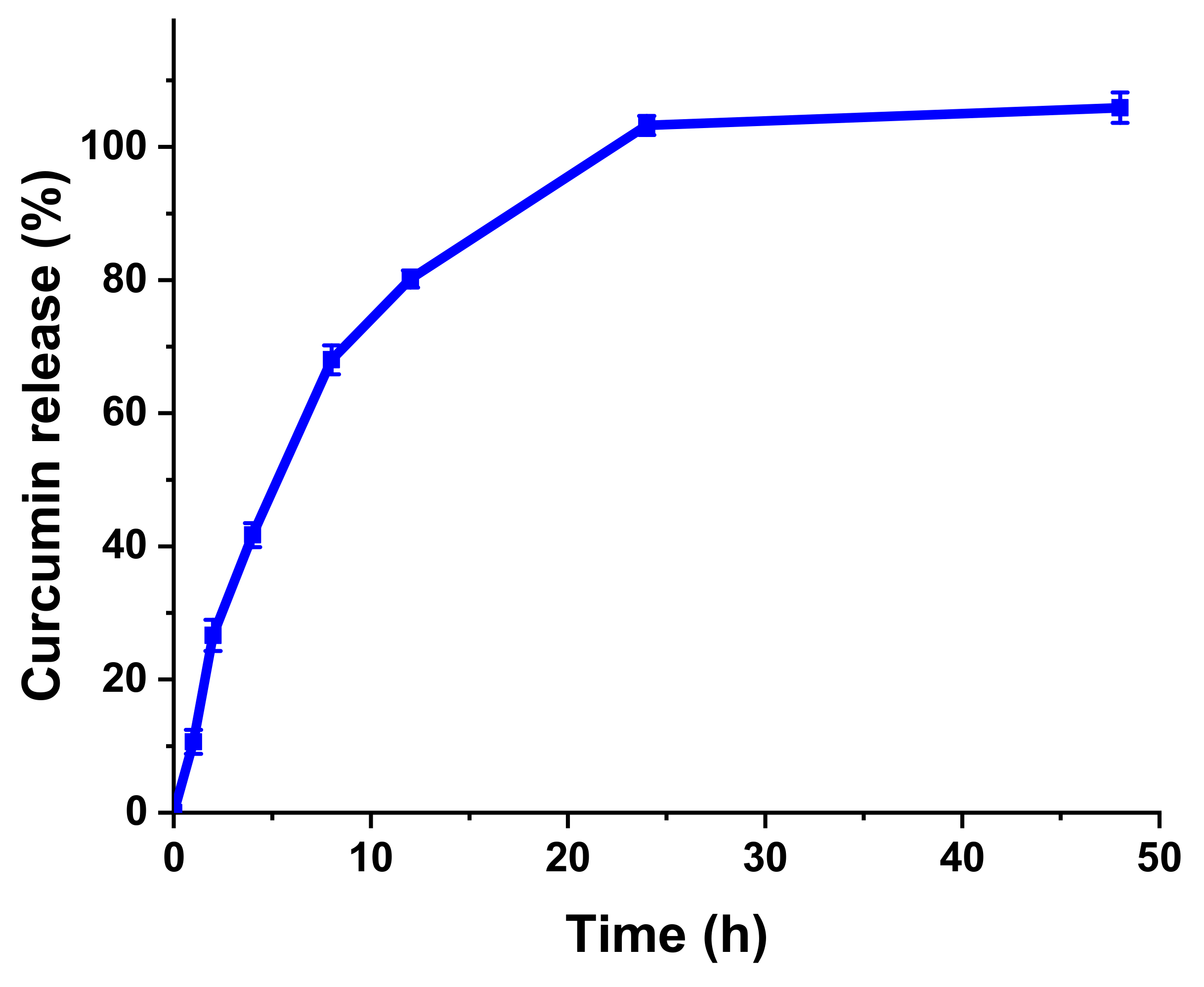
To prepare drug-loaded hydrogels, 1 mg/mL curcumin solution was incorporated with solid HA-Nb to obtain 2% solution of the polymer, followed by stirring until a homogenous mixture was obtained. Next, 200 μL of the curcumin-HA-Nb solution was mixed with 35 μL of a PEG-DTz solution (dissolved in DI water at various compositions ranging from 10 mg/mL, 20 mg/mL, and 40 mg/mL) in an Eppendorf tube. Then a vortex was used to gently mix the suspension for 15 s before being incubated in dark for 12 h at RT. Next, the curcumin-loaded hydrogels were rinsed with 3 mL of 20% solution of ethyl alcohol in DI water to remove un-loaded or surface-adhering curcumin. The absorptions of rinsing solutions were noted using a UV-Vis spectrophotometer (430 nm wavelength) to measure the drug loading efficiencies (DLE%) via a standard curve of curcumin. Curcumin loading efficiency was measured by using the following equation:
The in vitro drug release evaluations of curcumin-loaded hydrogels were carried out by immersing them in a release medium (35 mL) containing 20% ethanol and 80% DI water. The samples were placed in a shaking incubator at 37 °C under moderate shaking (50 rpm). At a specific time-interval, 2 mL solution from the release medium was taken and analyzed by a UV-Vis spectrophotometer to measure the amount of the released curcumin. After measuring the absorbance at 430 nm wavelength, the withdrawn sample was placed back into the respective vial to maintain a constant volume.
2.3.7. In Vitro Cytocompatibility of Precursors and Hydrogels
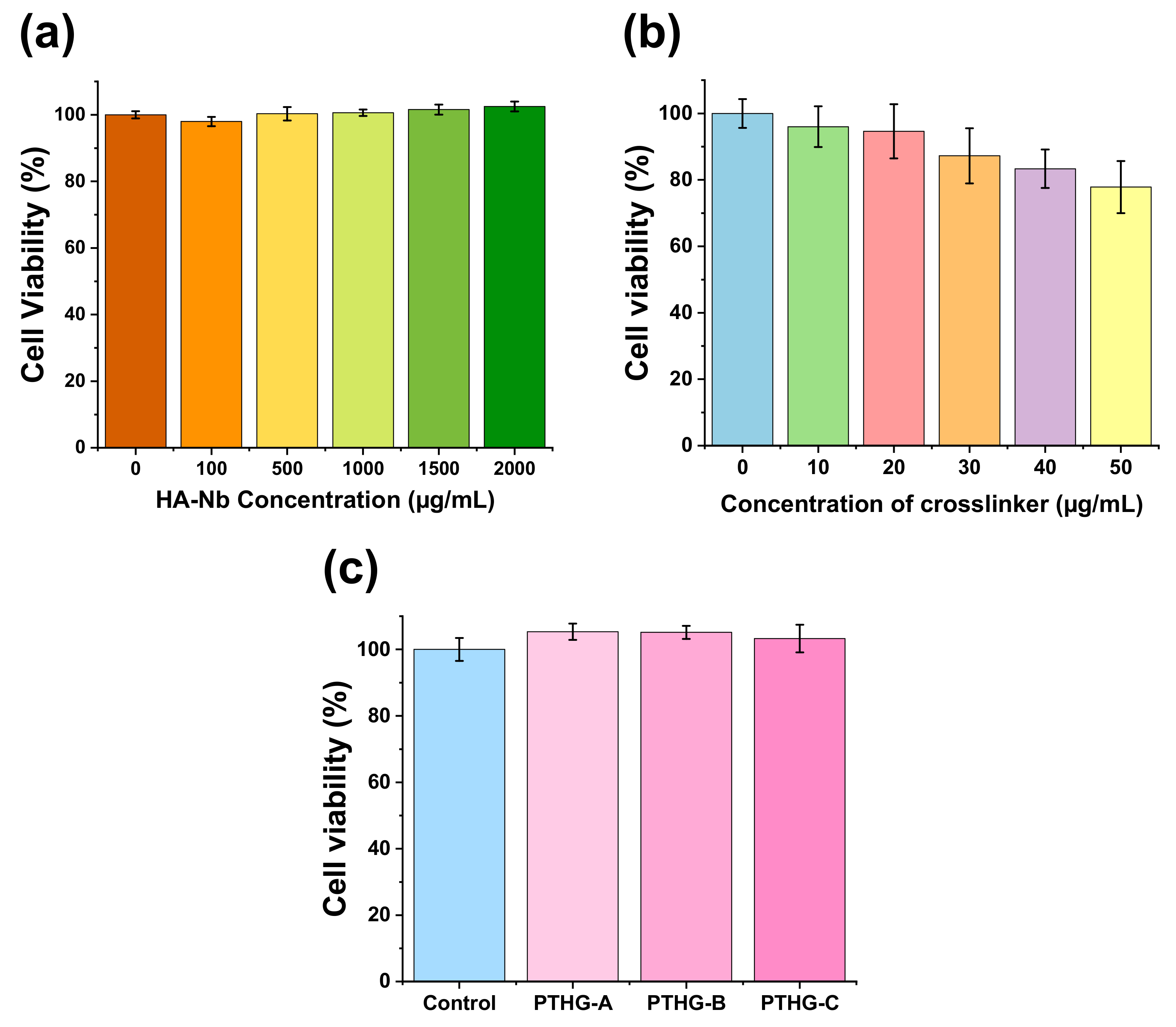
Cell compatibilities of HA-Nb, PEG-DTz, and the resulting hydrogels were studied in human embryonic kidney cells (HEK-293). Briefly, cells were seeded in a 48-well plate using Dulbecco’s Modified Eagle Medium (DMEM) along with 10% fetal bovine serum (FBS) and 1% Antibiotic-Antimycotic solution (Gibco™) at cell seeding densities of 10,000 cells/well, 37 °C, and 5% CO2 for 24 h. Next, the cell-medium was removed and exchanged with a fresh cell-medium containing different amounts of HA-Nb (0, 100, 500, 1000, 1500 and 2000 μg/mL) or PEG-DTz (0, 10, 20, 30, 40, and 50 μg/mL). After incubation for 24 h, cells were rinsed with 2 × 100 μL PBS, and were treated with 10 μL of a WST assay solution (EZ-cytox, Seoul, Korea). The absorbance of the resulting purple solution (formazan) was calculated using a microplate reader (wavelength 450 nm). Similarly, the cell compatibilities of the blank hydrogels were evaluated using hydrogel extracts.