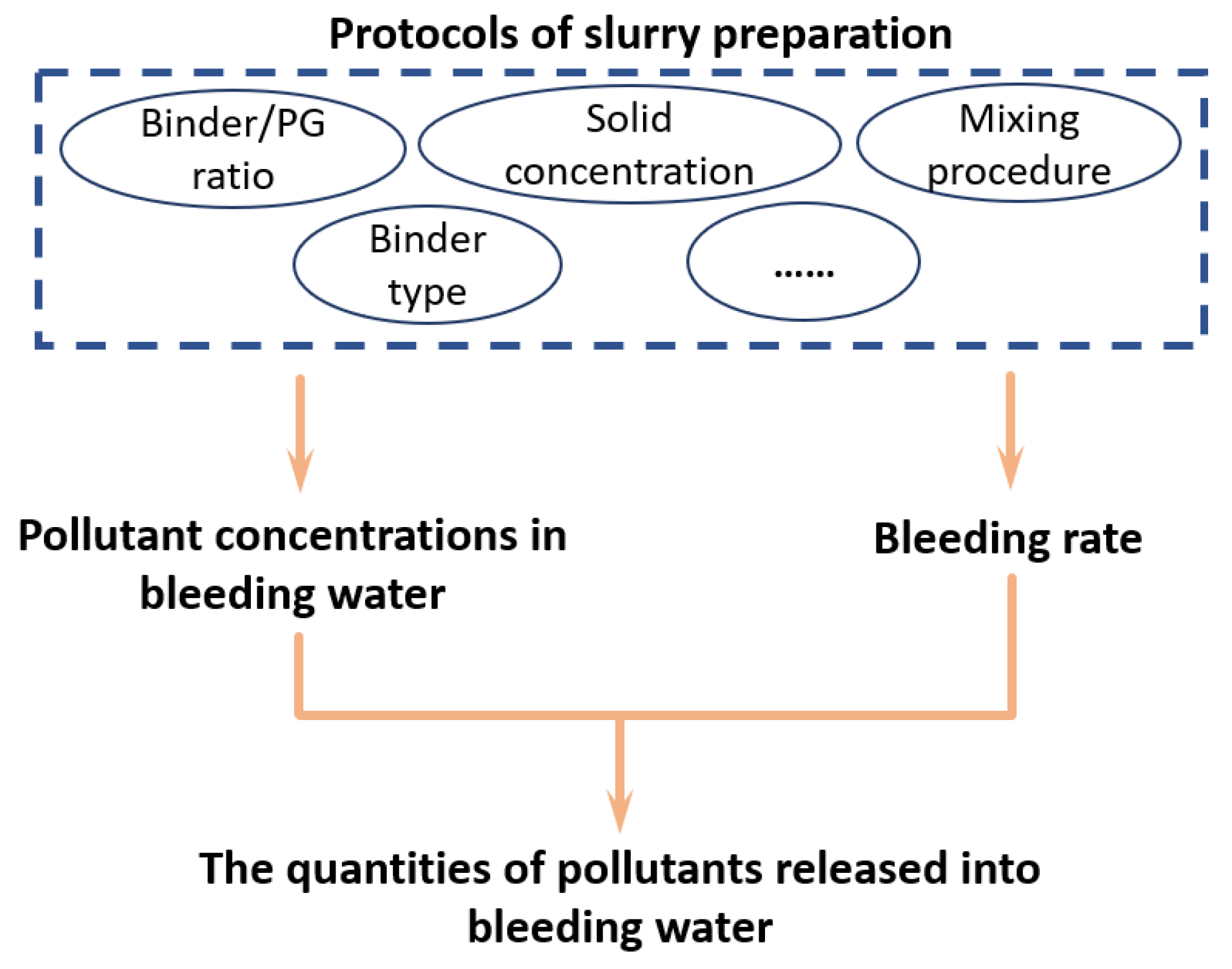
The Release of Pollutants through the Bleeding of Cemented Phosphogypsum Backfill: Link to Protocols for Slurry Preparation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
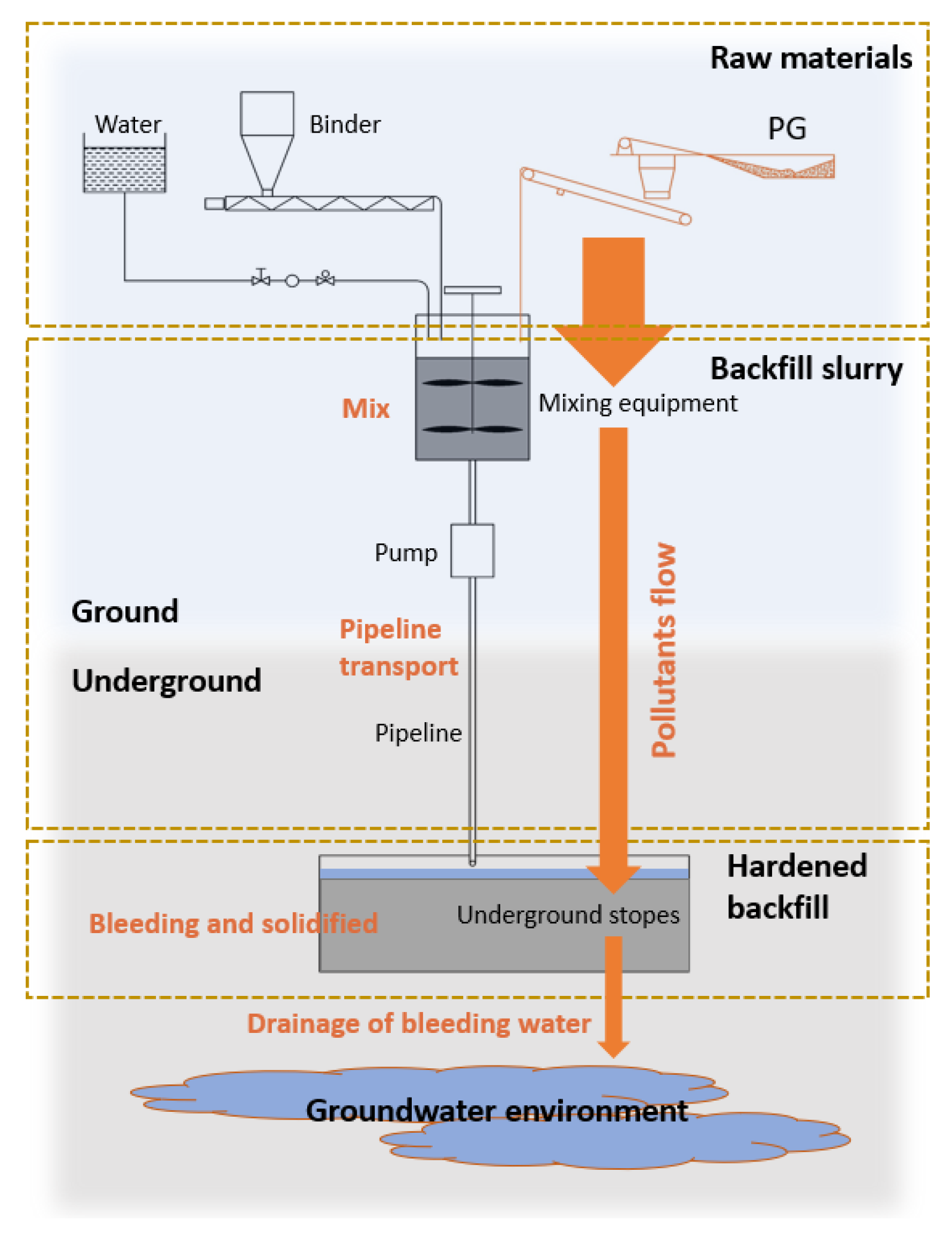
2.2.1. Preparation of Backfill Slurry
2.2.2. Toxic Leaching Test
2.2.3. Bleeding Rate
2.2.4. Sampling and Chemical Analyses of Bleeding Water
2.2.5. Quantities of Pollutants Released Per Ton of Solid in Backfill
3. Results
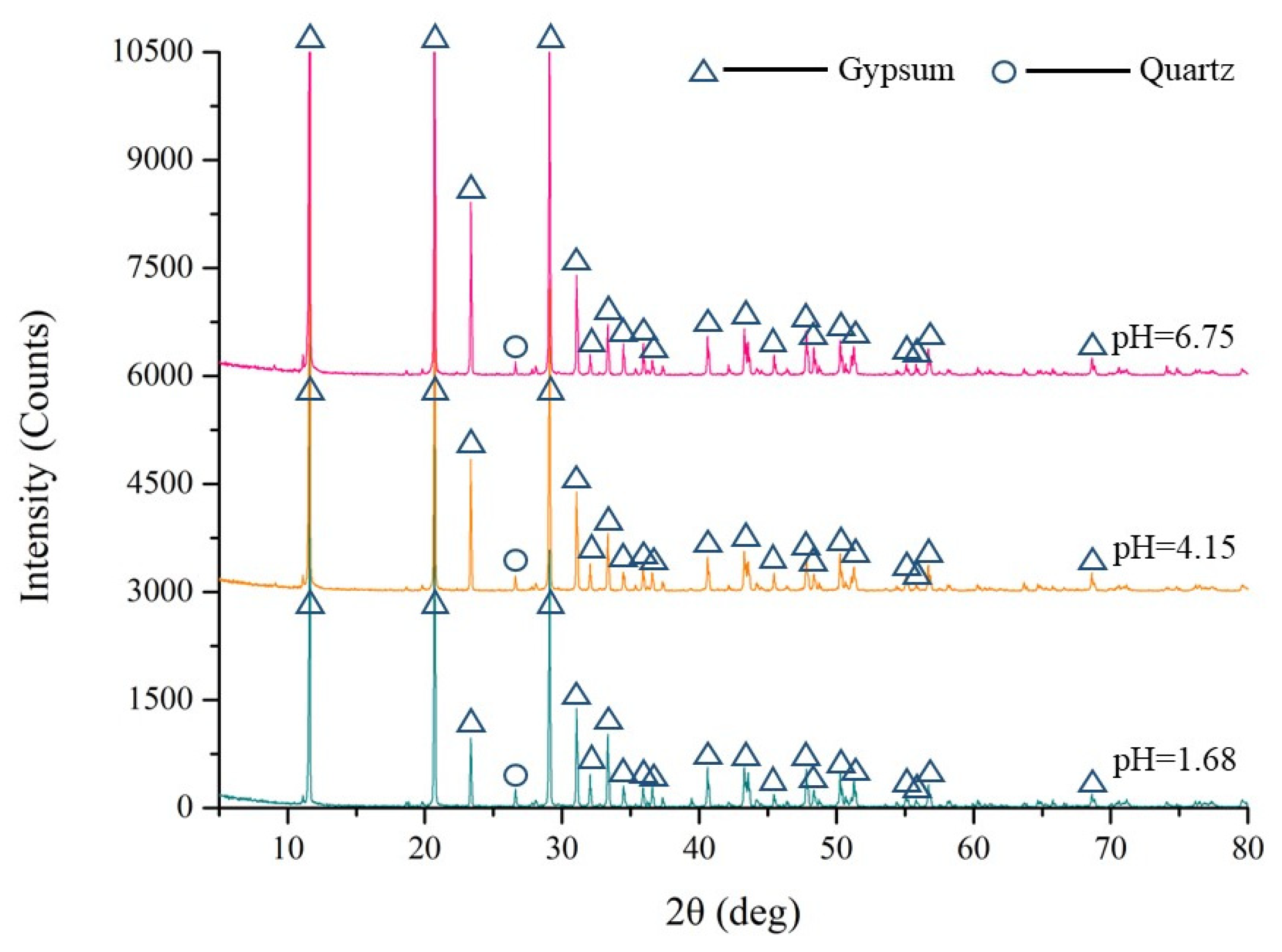
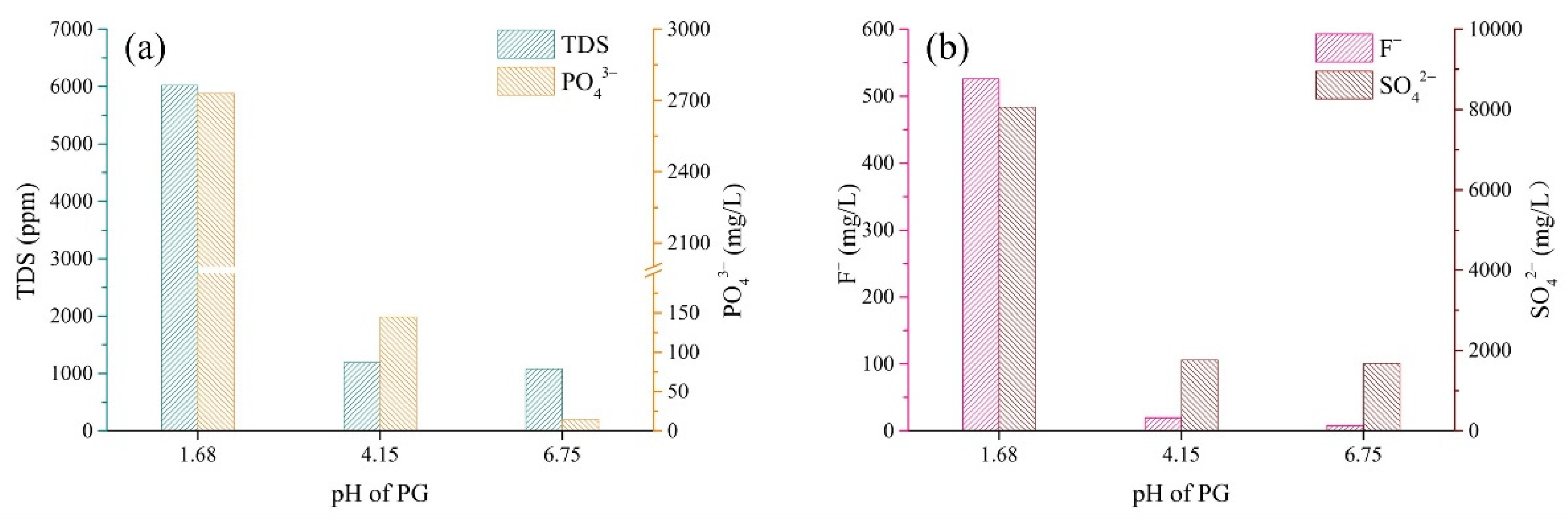
3.1. Chemical Properties of PG
3.2. Binder/PG Ratio
- (1)
- pH and bleeding rate of backfill slurry
- (2)
- Pollutants in bleeding water
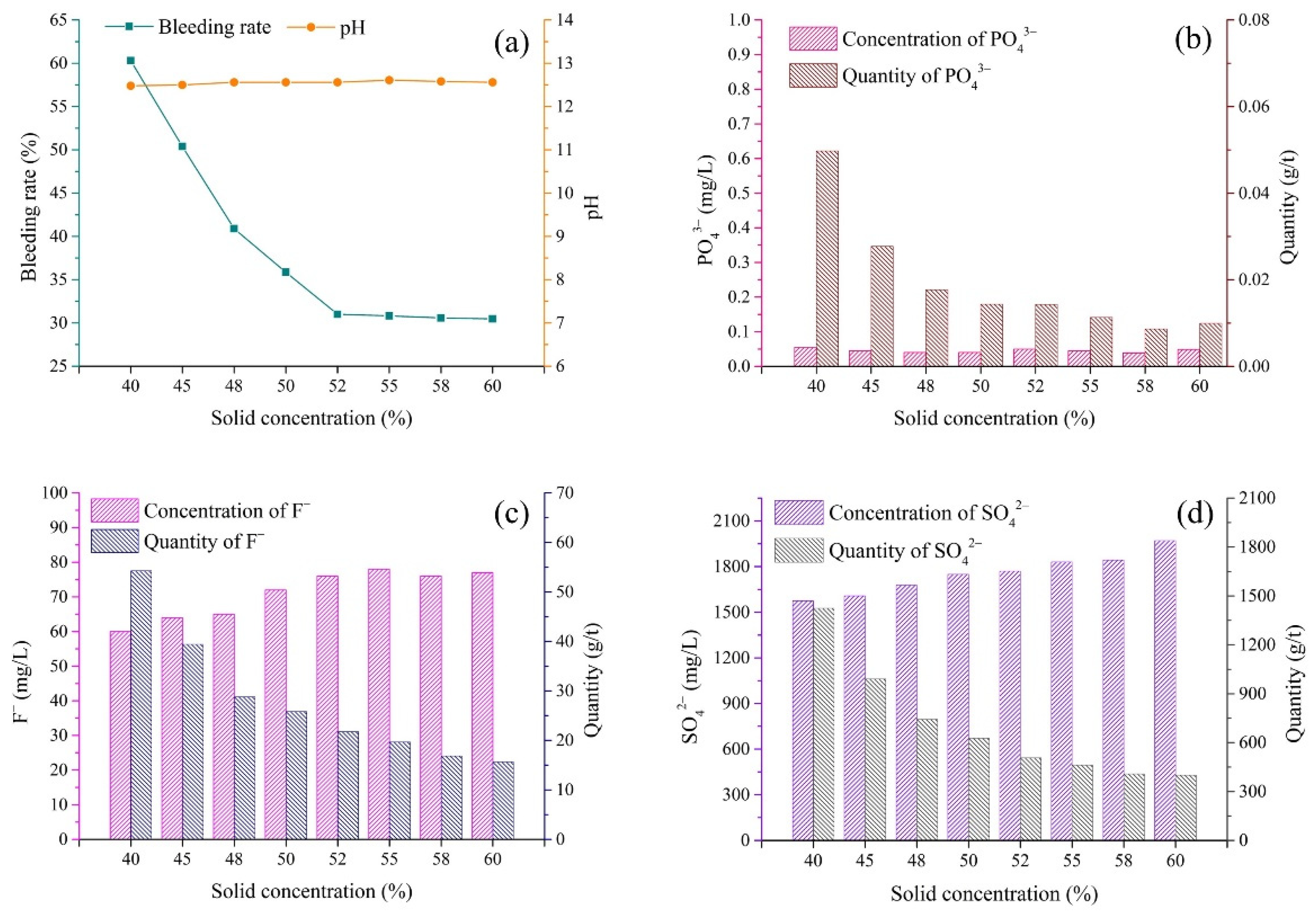
3.3. Solid Concentration
- (1)
- pH and bleeding rate of backfill slurry
- (2)
- Pollutants in bleeding water
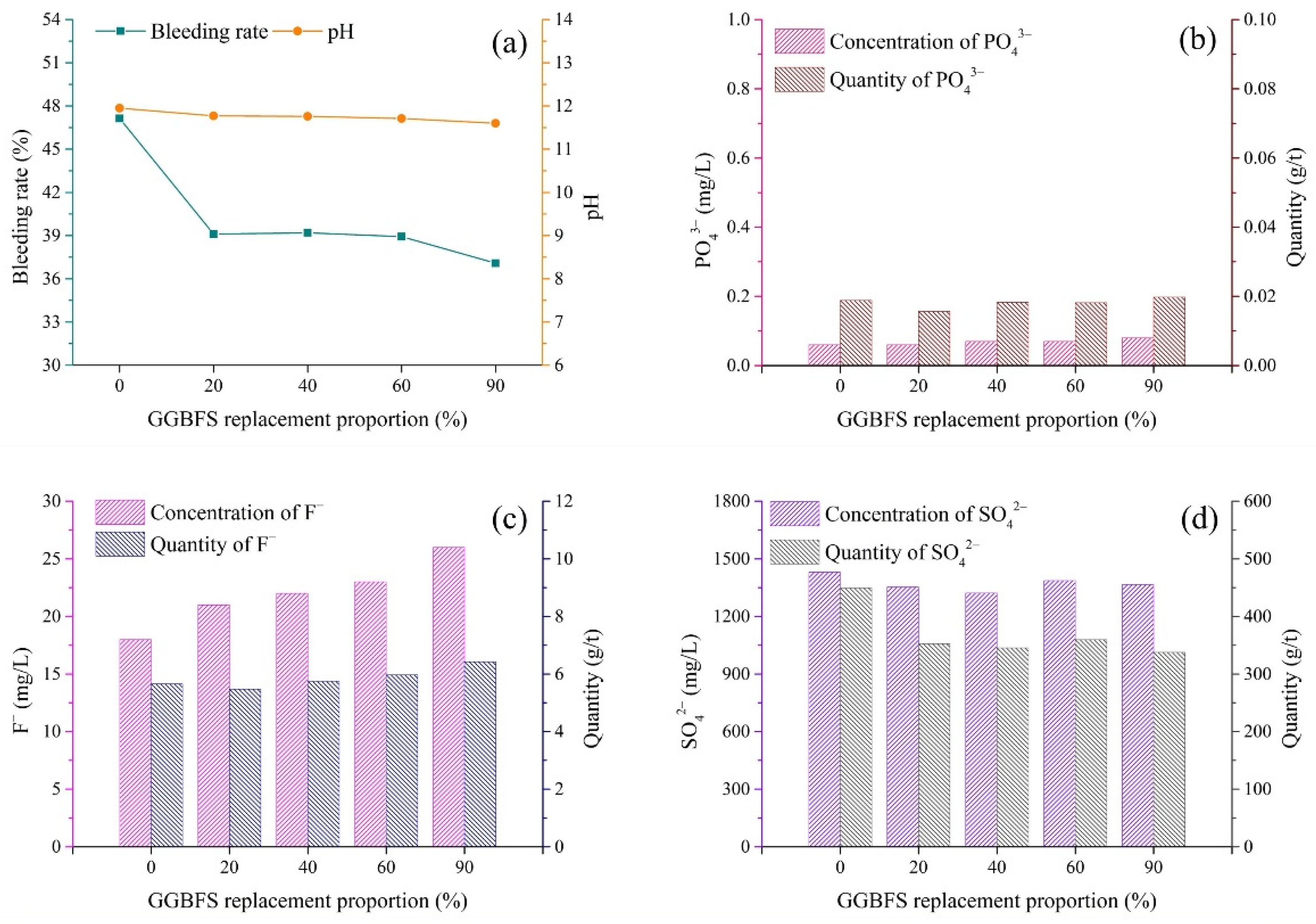
3.4. Binder Type
- (1)
- pH and bleeding rate of backfill slurry
- (2)
- Pollutants in bleeding water
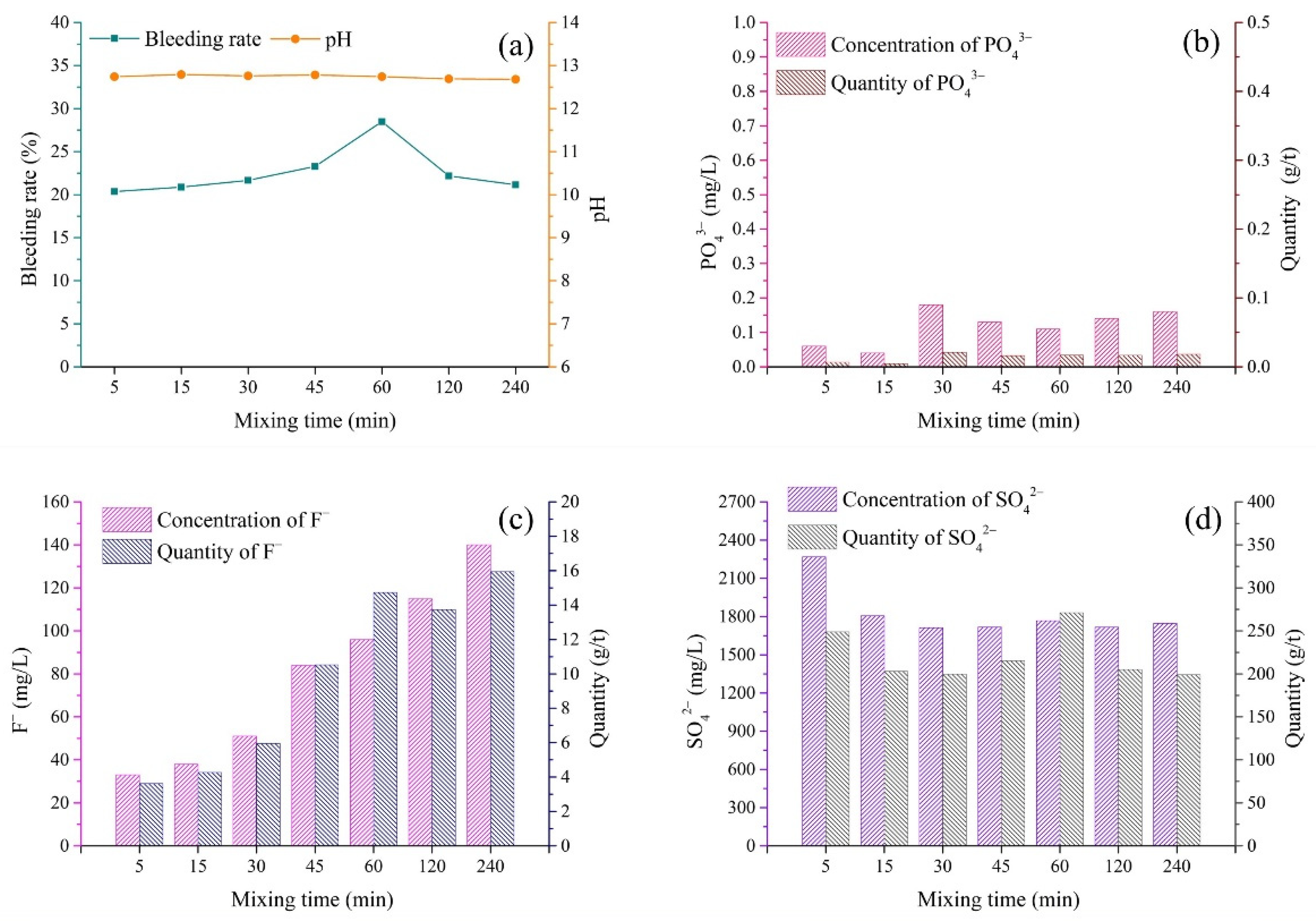
3.5. Mixing Procedure
- (1)
- pH and bleeding rate of backfill slurry
- (2)
- Pollutants in bleeding water
4. Discussion
4.1. Release of Pollutants from Backfill Slurry into the Bleeding Water
4.2. Effect of Preparation Protocols on Chemical Properties of Bleeding Water
4.3. Effect of Preparation Protocols on Bleeding Rate of Backfill Slurry
5. Conclusions
- (1)
- The bleeding water from cemented PG backfill contained PO43−, F− and SO42−, which pose a risk of damage to the surrounding environment. The high pollutant concentrations and the high bleeding rate resulted in higher quantities of pollutants being released from the backfill slurry;
- (2)
- The pollutant concentrations in the bleeding water could be minimized via proper protocols for slurry preparation, such as increasing the binder/PG ratio. A greater amount of more binder efficiently transformed PO43−, F− and SO42− into undissolved forms. The dilution effect induced by the low solid concentration also helped to reduce the pollutant concentrations;
- (3)
- The poor water retention and high initial water content of the backfill slurry increased the bleeding rate. Introducing more fine raw materials into the backfill slurry, such as binder and GGBFS, could bond more surface layer water and increase the water retention. The pH of the backfill slurry is also negatively related to water retention;
- (4)
- Cemented PG backfill could solidify/stabilize PO43− in the bleeding water well when the pH value was higher than 11. However, the concentration of F− in the bleeding water always exceeded the limit value of the national standard (10 mg/L); therefore, further studies are needed for the S/S of F−.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Li, X.; Yao, J.; Gong, F.; Li, X.; Du, K.; Tao, M.; Huang, L.; Du, S. Experimental investigation of rock breakage by a conical pick and its application to non-explosive mechanized mining in deep hard rock. Int. J. Rock Mech. Min. Sci. 2019, 122, 104063. [Google Scholar] [CrossRef]
- Li, X.; Du, J.; Gao, L.; He, S.; Gan, L.; Sun, C.; Shi, Y. Immobilization of phosphogypsum for cemented paste backfill and its environmental effect. J. Clean. Prod. 2017, 156, 137–146. [Google Scholar] [CrossRef]
- Fall, M.; Benzaazoua, M.; Saa, E.G. Mix proportioning of underground cemented tailings backfill. Tunn. Undergr. Space Tech. 2008, 23, 80–90. [Google Scholar] [CrossRef]
- Huynh, L.; Beattie, D.A.; Fornasiero, D.; Ralston, J. Effect of polyphosphate and naphthalene sulfonate formaldehyde condensate on the rheological properties of dewatered tailings and cemented paste backfill. Miner. Eng. 2006, 19, 28–36. [Google Scholar] [CrossRef]
- Jiang, G.; Wu, A.; Wang, Y.; Li, J. The rheological behavior of paste prepared from hemihydrate phosphogypsum and tailing. Constr. Build. Mater. 2019, 229, 116870. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, A.; Tang, M.; Liu, X. The filling role of pozzolanic material. Cem. Concr. Res. 1996, 26, 943–947. [Google Scholar] [CrossRef]
- Koohestani, B. Effect of saline admixtures on mechanical and microstructural properties of cementitious matrices containing tailings. Constr. Build. Mater. 2017, 156, 1019–1027. [Google Scholar] [CrossRef]
- Wei, H.; Xiao, B.; Gao, Q. Flow properties analysis and identification of a fly ash-waste rock mixed backfilling slurry. Minerals 2021, 11, 576. [Google Scholar] [CrossRef]
- Xiao, B.; Wen, Z.; Wu, F.; Li, L.; Yang, Z.; Gao, Q. A simple L-shape pipe flow test for practical rheological properties of backfill slurry: A case study. Powder Technol. 2019, 356, 1008–1015. [Google Scholar] [CrossRef]
- Wang, S.; Sun, L.; Huang, L.; Li, X.; Shi, Y.; Yao, J.; Du, S. Non-explosive mining and waste utilization for achieving green mining in underground hard rock mine in China. Trans. Nonferr. Metal. Soc. China 2019, 29, 1914–1928. [Google Scholar] [CrossRef]
- Yang, X. Development of Phosphogypsum—Based Early Strength Cementitious Materials and Resource Utilization of Waste; University of Science and Technology Beijing: Beijing, China, 2017. (In Chinese) [Google Scholar]
- Jiang, J. The Application Research of Phosphogypsum-Based Cementing Filling Marerials in Huang Mailing Mine; Wuhan University of Technology: Wuhan, China, 2016. (In Chinese) [Google Scholar]
- Bisone, S.; Gautier, M.; Chatain, V.; Blanc, D. Spatial distribution and leaching behavior of pollutants from phosphogypsum stocked in a gypstack: Geochemical characterization and modeling. J. Environ. Manag. 2017, 193, 567–575. [Google Scholar] [CrossRef]
- Pérez-López, R.; Macías, F.; Cánovas, C.R.; Sarmiento, A.M.; Pérez-Moreno, S.M. Pollutant flows from a phosphogypsum disposal area to an estuarine environment: An insight from geochemical signatures. Sci. Total Environ. 2016, 553, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.M. Potential use of phosphogypsum in alkali-activated fly ash under the effects of elevated temperatures and thermal shock cycles. J. Clean. Prod. 2015, 87, 717–725. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2022. 2022; p. 125. Available online: https://pubs.usgs.gov/periodicals/mcs2022/mcs2022.pdf (accessed on 29 August 2022).
- Ye, X. The using current situation, form analysis and measures of phosphogypsum utilization in China in 2017. Sulphuric Acid Ind. 2018, 8, 1–4. (In Chinese) [Google Scholar]
- Rusch, K.A.; Malone, R.F. Final Report: Searching for Optimum Composition of Phosphogypsum: Fly ash: Cement Composites for Oyster Culch Materials. 2004. Available online: https://cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.abstractDetail/abstract_id/5886/report/F (accessed on 29 August 2022).
- Essabir, H.; Nekhlaoui, S.; Bensalah, M.O.; Rodrigue, D.; Bouhfid, R.; Qaiss, A.E.K. Phosphogypsum Waste Used as Reinforcing Fillers in Polypropylene Based Composites: Structural, Mechanical and Thermal Properties. J. Polym. Environ. 2017, 25, 658–666. [Google Scholar] [CrossRef]
- Hentati, O.; Abrantes, N.; Caetano, A.L.; Bouguerra, S.; Goncalves, F.; Roembke, J.; Pereira, R. Phosphogypsum as a soil fertilizer: Ecotoxicity of amended soil and elutriates to bacteria, invertebrates, algae and plants. J. Hazard. Mater. 2015, 294, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Rosales, J.; Perez, S.M.; Cabrera, M.; Gazquez, M.J.; Bolivar, J.P.; de Brito, J.; Agrela, F. Treated phosphogypsum as an alternative set regulator and mineral addition in cement production. J. Clean. Prod. 2020, 244, 118752. [Google Scholar] [CrossRef]
- Singh, M. Treating waste phosphogypsum for cement and plaster manufacture. Cem. Concr. Res. 2002, 32, 1033–1038. [Google Scholar] [CrossRef]
- Degirmenci, N.; Okucu, A.; Turabi, A. Application of phosphogypsum in soil stabilization. Build. Environ. 2007, 42, 3393–3398. [Google Scholar] [CrossRef]
- Ennaciri, Y.; Zdah, I.; El Alaoui-Belghiti, H.; Bettach, M. Characterization and purification of waste phosphogypsum to make it suitable for use in the plaster and the cement industry. Chem. Eng. Commun. 2020, 207, 382–392. [Google Scholar] [CrossRef]
- Diwa, R.R.; Tabora, E.U.; Palattao, B.L.; Haneklaus, N.H.; Vargas, E.P.; Reyes, R.Y.; Ramirez, J.D. Evaluating radiation risks and resource opportunities associated with phosphogypsum in the Philippines. J. Radioanal. Nucl. Chem. 2022, 331, 967–974. [Google Scholar] [CrossRef]
- Gijbels, K.; Pontikes, Y.; Samyn, P.; Schreurs, S.; Schroeyers, W. Effect of NaOH content on hydration, mineralogy, porosity and strength in alkali/sulfate-activated binders from ground granulated blast furnace slag and phosphogypsum. Cem. Concr. Res. 2020, 132, 106054. [Google Scholar] [CrossRef]
- Tayibi, H.; Choura, M.; Lopez, F.A.; Alguacil, F.J.; Lopez-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef]
- Shi, Y.; Gan, L.; Li, X.; He, S.; Sun, C.; Gao, L. Dynamics of metals in backfill of a phosphate mine of Guiyang, China using a three-step sequential extraction technique. Chemosphere 2018, 192, 354–361. [Google Scholar] [CrossRef]
- Zhou, S.; Li, X.; Zhou, Y.; Min, C.; Shi, Y. Effect of phosphorus on the properties of phosphogypsum-based cemented backfill. J. Hazard. Mater. 2020, 399, 122993. [Google Scholar] [CrossRef] [PubMed]
- Min, C.; Shi, Y.; Liu, Z. Properties of cemented phosphogypsum (PG) backfill in case of partially substitution of composite Portland cement by ground granulated blast furnace slag. Constr. Build. Mater. 2021, 305, 124786. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, B.; Li, X.; Li, D.; Dong, L. Experimental study on backfilling mine goafs with chemical waste phosphogypsum. Geofluids 2019, 2019, 9218916. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Zhu, Q.; Zhou, S.; Min, C.; Shi, Y. Slurry preparation effects on the cemented phosphogypsum backfill through an orthogonal experiment. Minerals 2019, 9, 31. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, G.; Qi, T.; Guo, Y.; Du, X. Evaluation of static segregation of cemented gangue-fly ash backfill material using electrical resistivity method. Measurement 2020, 154, 107483. [Google Scholar] [CrossRef]
- Yao, Y.; Sun, H. A novel silica alumina-based backfill material composed of coal refuse and fly ash. J. Hazard. Mater. 2012, 213, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, P.; Feng, G.; Qi, T.; Liu, G.; Ren, A. Performance of coal gangue-based cemented backfill material modified by water-reducing agents. Adv. Mater. Sci. Eng. 2020, 2020, 2302895. [Google Scholar] [CrossRef]
- Yao, Z. Technoligical Study and Reliability Analysis of Yellow Phosphorus Slag and Phosphgysum Backfill in Kaiyang Mine; Central South University: Changsha, China, 2009. (In Chinese) [Google Scholar]
- Min, C.; Li, X.; He, S.; Zhou, S.; Zhou, Y.; Yang, S.; Shi, Y. Effect of mixing time on the properties of phosphogypsum-based cemented backfill. Constr. Build. Mater. 2019, 210, 564–573. [Google Scholar] [CrossRef]
- HJ 557-2010; Solid Waste—Extraction Procedure for Leaching Toxicity—Horizontal Vibration Method. China Environmental Science Press: Beijing, China, 2010.
- GB/T 50080-2016; Standard for Test Method of Performance on Ordinary Fresh Concrete. China Architecture & Building Press: Beijing, China, 2016.
- GB 8978-1996; Integrated Wastewater Discharge Standard. China Environmental Science Press: Beijing, China, 1996.
- Song, S.; Sohn, D.; Jennings, H.M.; Mason, T.O. Hydration of alkali-activated ground granulated blast furnace slag. J. Mater. Sci. 2000, 35, 249–257. [Google Scholar] [CrossRef]
- Yao, H.; Liu, D. Utilization of low activity CFBC ash in cemented paste backfill containing phosphate tailings. Min. Metall. Explor. 2021, 38, 2485–2492. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, J.; Liu, J.; Li, F.; Pang, Z. Investigation of hydraulic-mechanical properties of paste backfill containing coal gangue-fly ash and its application in an underground coal mine. Energies 2017, 10, 1309. [Google Scholar] [CrossRef]
- Simon, D.; Grabinsky, M. Apparent yield stress measurement in cemented paste backfill. Int. J. Min. Reclam. Environ. 2013, 27, 231–256. [Google Scholar] [CrossRef]
- Elakneswaran, Y.; Nawa, T.; Kurumisawa, K. Zeta potential study of paste blends with slag. Cem. Concr. Res. 2009, 31, 72–76. [Google Scholar] [CrossRef]
- Mikanovic, N.; Jolicoeur, C. Influence of superplasticizers on the rheology and stability of limestone and cement pastes. Cem. Concr. Res. 2008, 38, 907–919. [Google Scholar] [CrossRef]
- Mehdipour, I.; Razzaghi, M.S.; Amini, K.; Shekarchi, M. Effect of mineral admixtures on fluidity and stability of self-consolidating mortar subjected to prolonged mixing time. Constr. Build. Mater. 2013, 40, 1029–1037. [Google Scholar] [CrossRef]
- Yang, L.; Qiu, J.; Jiang, H.; Hu, S.; Li, H.; Li, S. Use of Cemented Super-Fine Unclassified Tailings Backfill for Control of Subsidence. Minerals 2017, 7, 11. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Q.; Qi, C.; Fourie, A.; Xiao, C. Recycling phosphogypsum and construction demolition waste for cemented paste backfill and its environmental impact. J. Clean. Prod. 2018, 186, 418–429. [Google Scholar] [CrossRef]
- Niroshan, N.; Sivakugan, N.; Veenstra, R.L. Flow Characteristics of Cemented Paste Backfill. Geotech. Geol. Eng. 2018, 36, 2261–2272. [Google Scholar] [CrossRef]
- Kesimal, A.; Yilmaz, E.; Ercikdi, B. Evaluation of paste backfill mixtures consisting of sulphide-rich mill tailings and varying cement contents. Cem. Concr. Res. 2004, 34, 1817–1822. [Google Scholar] [CrossRef]
- Cao, S.; Song, W.; Yilmaz, E. Influence of structural factors on uniaxial compressive strength of cemented tailings backfill. Constr. Build. Mater. 2018, 174, 190–201. [Google Scholar] [CrossRef]
- Fall, M.; Benzaazoua, M.; Ouellet, S. Experimental characterization of the influence of tailings fineness and density on the quality of cemented paste backfill. Miner. Eng. 2005, 18, 41–44. [Google Scholar] [CrossRef]

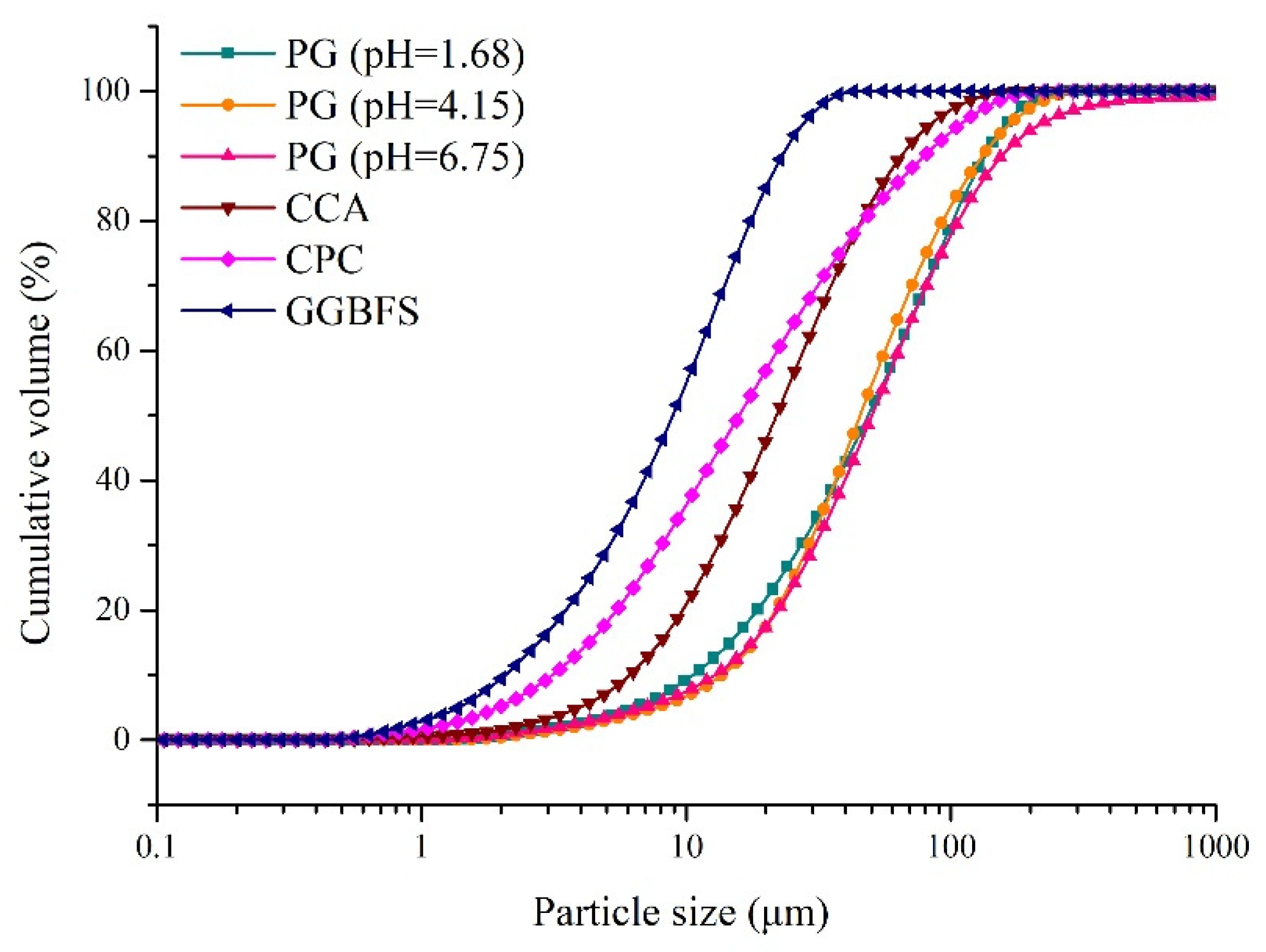
| Sample | D10 (μm) | D30 (μm) | D60 (μm) | Cu | Cc |
|---|---|---|---|---|---|
| PG (pH = 1.68) | 10.53 | 27.40 | 62.91 | 6.08 | 1.13 |
| PG (pH = 4.15) | 13.65 | 29.02 | 56.38 | 4.13 | 1.09 |
| PG (pH = 6.75) | 13.56 | 33.15 | 62.76 | 4.63 | 1.29 |
| CCA | 6.12 | 13.26 | 27.74 | 4.53 | 1.04 |
| CPC | 3.33 | 8.14 | 22.60 | 6.79 | 0.88 |
| GGBFS | 2.00 | 5.55 | 11.94 | 5.97 | 1.29 |
| Chemical Component | PG (pH = 1.68) (%) | PG (pH = 4.15) (%) | PG (pH = 6.75) (%) |
|---|---|---|---|
| SO3 | 44.76 | 42.19 | 42.05 |
| CaO | 34.93 | 34.04 | 34.01 |
| SiO2 | 3.23 | 2.73 | 2.31 |
| P2O5 | 2.61 | 0.80 | 0.65 |
| F | 0.81 | 0.43 | 0.35 |
| Fe2O3 | 0.28 | 0.25 | 0.27 |
| Ba | 0.17 | 0.17 | 0.09 |
| MgO | 0.14 | 0.04 | 0.05 |
| Na2O | 0.13 | 0.03 | - |
| K2O | 0.10 | 0.08 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, C.; Shi, Y.; Zhou, Y.; Liu, Z. The Release of Pollutants through the Bleeding of Cemented Phosphogypsum Backfill: Link to Protocols for Slurry Preparation. Materials 2022, 15, 7126. https://doi.org/10.3390/ma15207126
Min C, Shi Y, Zhou Y, Liu Z. The Release of Pollutants through the Bleeding of Cemented Phosphogypsum Backfill: Link to Protocols for Slurry Preparation. Materials. 2022; 15(20):7126. https://doi.org/10.3390/ma15207126
Chicago/Turabian StyleMin, Chendi, Ying Shi, Yanan Zhou, and Zhixiang Liu. 2022. "The Release of Pollutants through the Bleeding of Cemented Phosphogypsum Backfill: Link to Protocols for Slurry Preparation" Materials 15, no. 20: 7126. https://doi.org/10.3390/ma15207126
APA StyleMin, C., Shi, Y., Zhou, Y., & Liu, Z. (2022). The Release of Pollutants through the Bleeding of Cemented Phosphogypsum Backfill: Link to Protocols for Slurry Preparation. Materials, 15(20), 7126. https://doi.org/10.3390/ma15207126