Homogenous Cr and C Doped 3D Self-Supporting NiO Cellular Nanospheres for Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Experiment
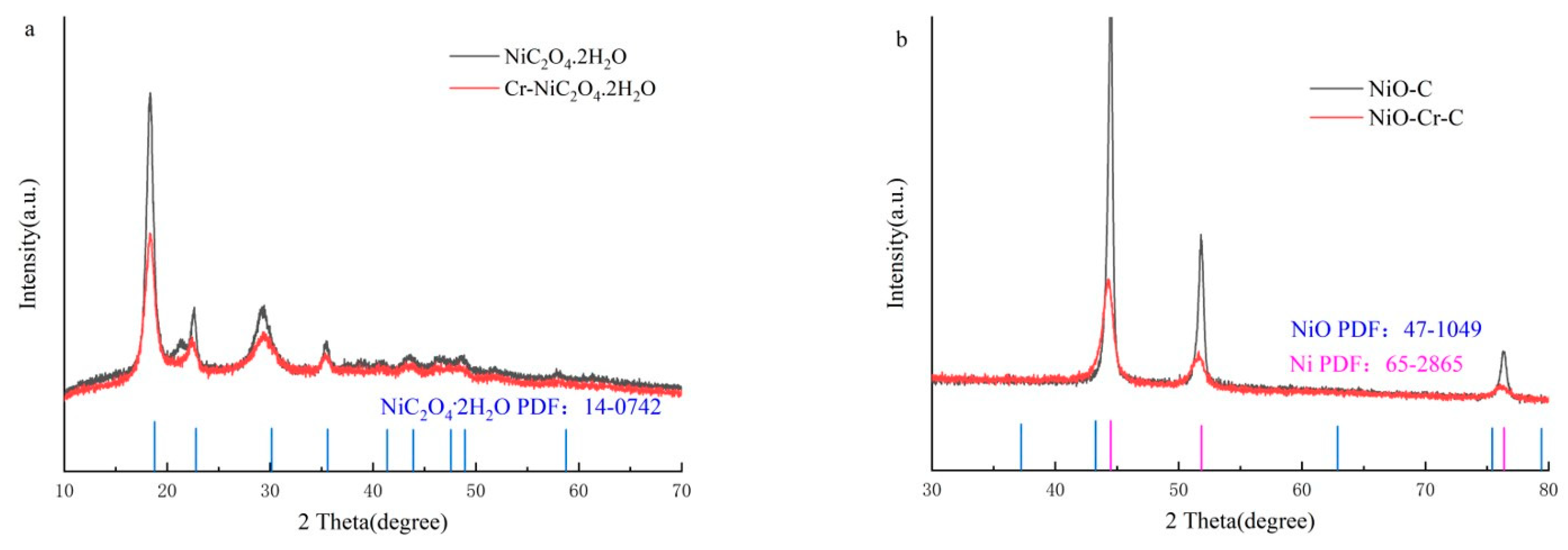
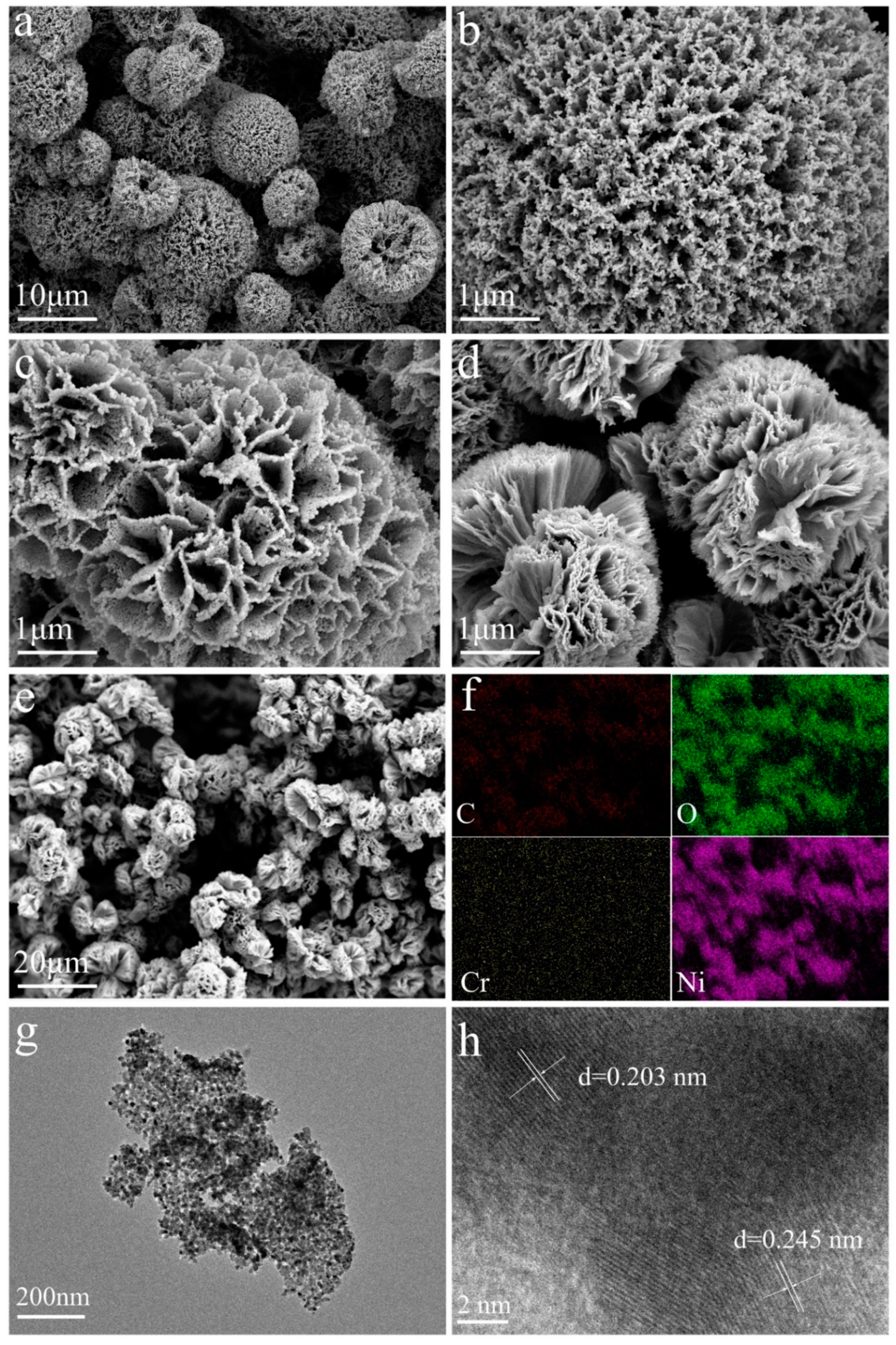
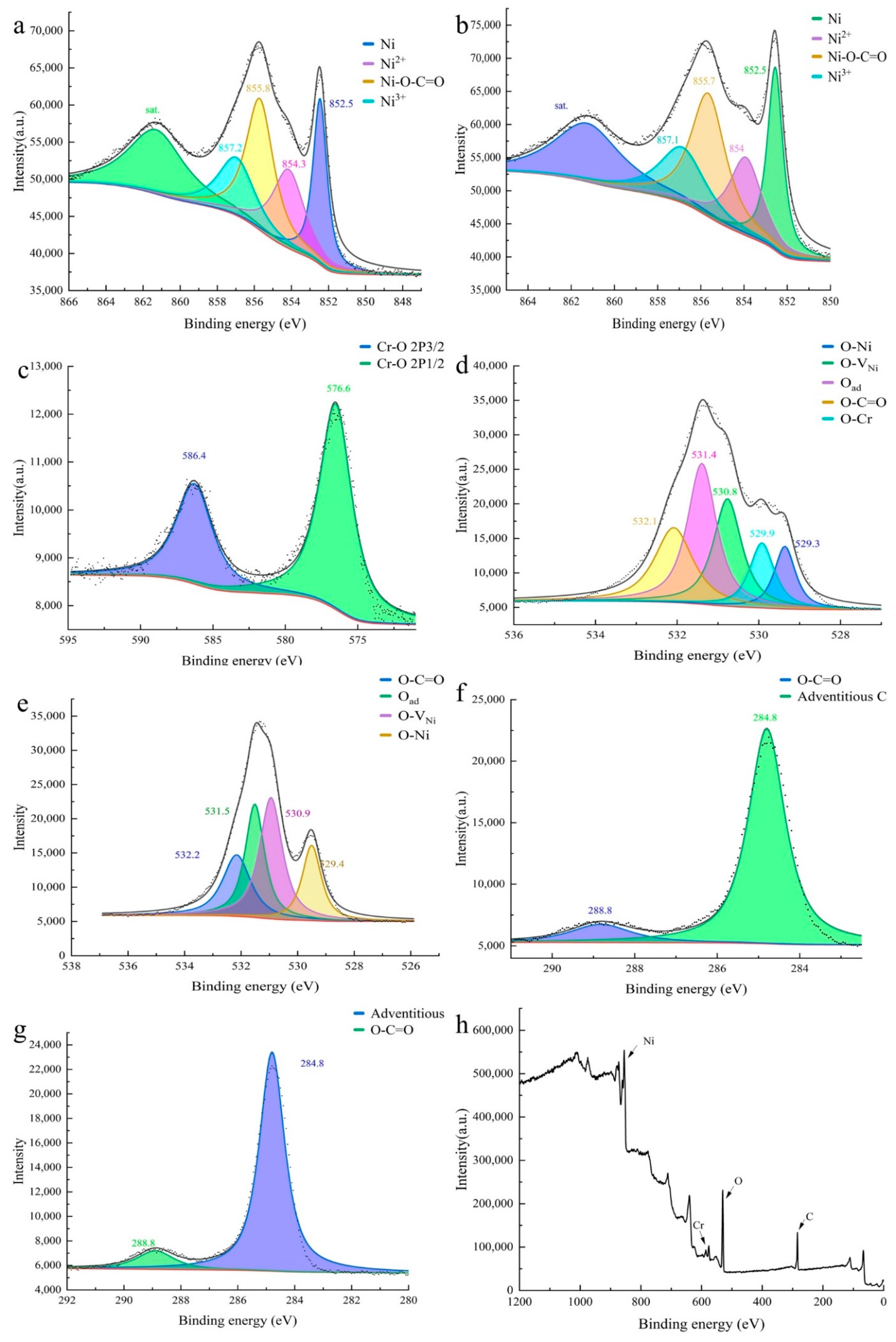
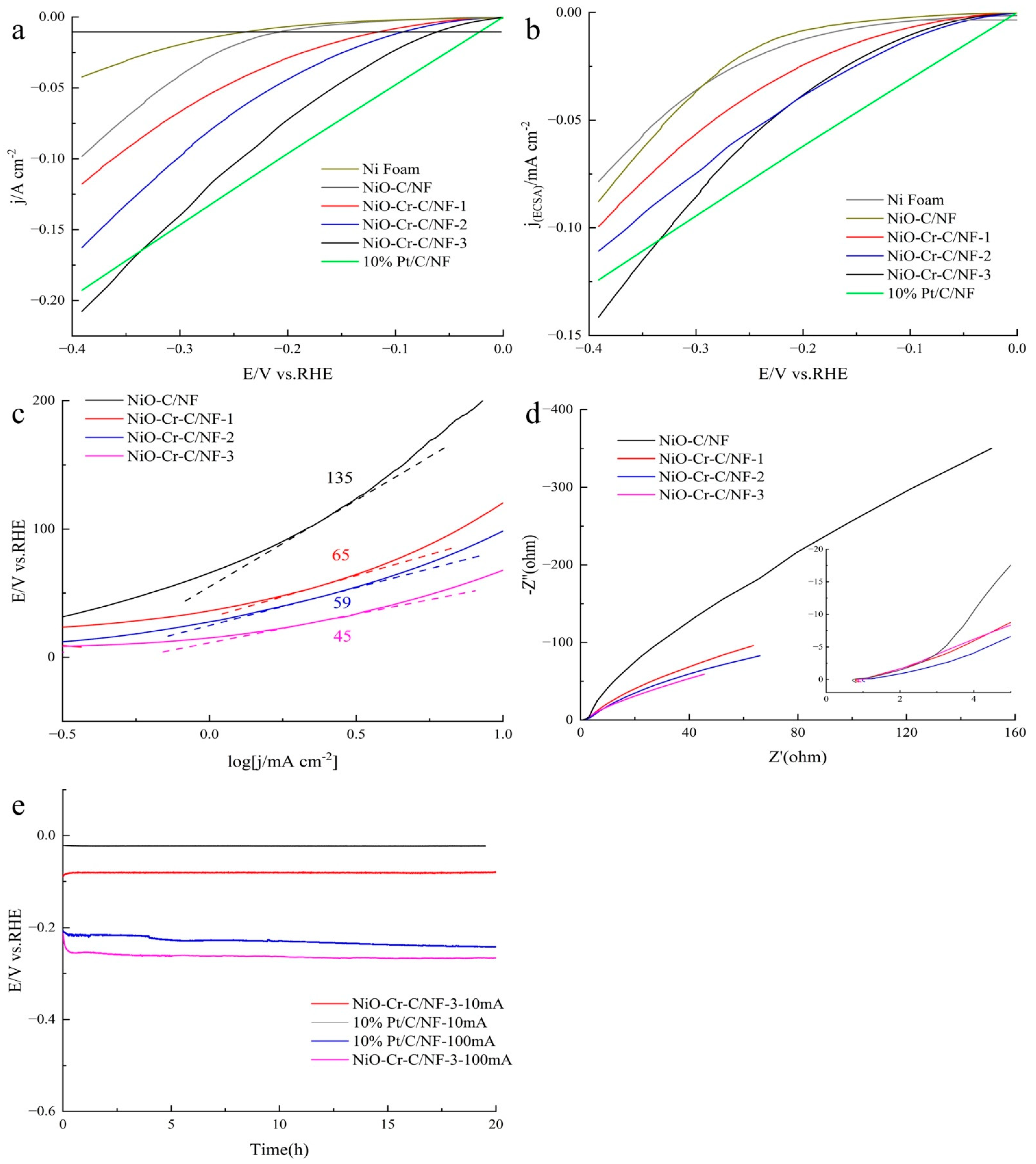
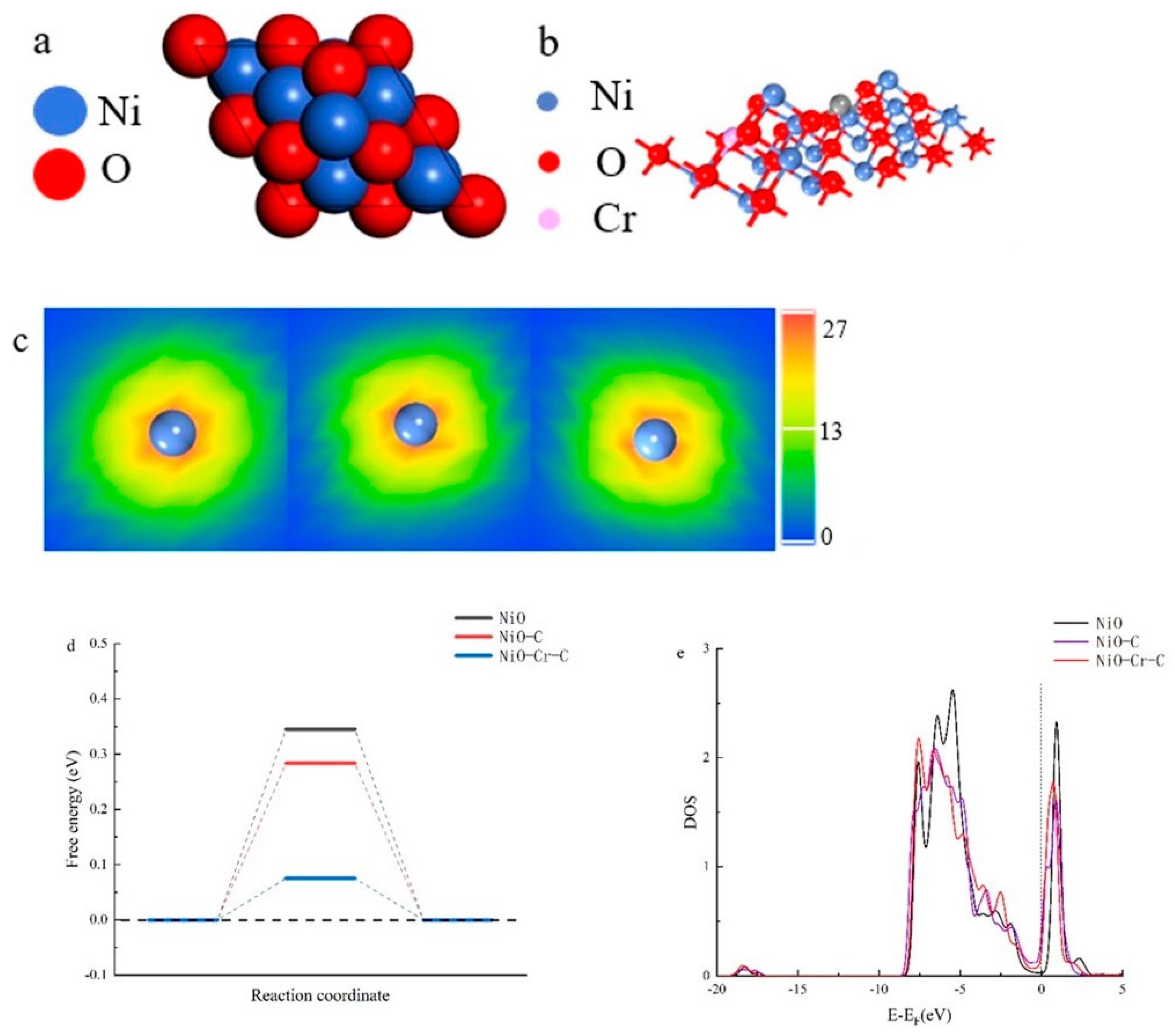
3. Result
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Wang, P.; Shao, Q.; Huang, X. Metallic nanostructures with low dimensionality for electrochemical water splitting. Chem. Soc. Rev. 2020, 49, 3072–3106. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Liu, Y.; Qian, Q. Partially exposed RuP2 surface in hybrid structure endows its bifunctionality for hydrazine oxidation and hydrogen evolution catalysis. Sci. Adv. 2020, 6, 4197–4207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lu, X.; Wu, Z.; Luan, D.; Lou, X. Engineering Platinum-Cobalt Nano-alloys in Porous Nitrogen-Doped Carbon Nanotubes for Highly Efficient Electrocatalytic Hydrogen Evolution. Angew. Chem. 2021, 133, 19216–19221. [Google Scholar] [CrossRef]
- Liang, R.; Song, L.; Lu, J.; Xu, W.; Ding, C.; Jia, Y. Palladium-Catalyzed Enantioselective Heteroarenyne Cycloisomerization Reaction. Angew. Chem. 2021, 133, 7488–7493. [Google Scholar] [CrossRef]
- Huang, L.; Bismuto, A.; Rath, S.A.; Trapp, N.; Morandi, B. Ruthenium-Catalyzed Dehydrogenation through an Intermolecular Hydrogen Atom Transfer Mechanism. Angew. Chem. 2021, 133, 7366–7372. [Google Scholar] [CrossRef]
- Song, Q.; Wang, W.; Lu, K. Three-dimensional hydrophobic porous organic polymers confined Pd nanoclusters for phase-transfer catalytic hydrogenation of nitroarenes in water. Chem. Eng. J. 2021, 415, 128856–128867. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Y.; Montoya, J.; Hochfilzer, D.; Cao, A.; Kibsgaard, J.; Chorkendorff, I.; Nørskov, J. Origins of the Instability of Nonprecious Hydrogen Evolution Reaction Catalysts at Open-Circuit Potential. ACS Energy Lett. 2021, 6, 2268–2274. [Google Scholar] [CrossRef]
- Hughes, J.P.; Clipsham, J.; Chavushoglu, H.; Rowley-Neale, S.J.; Banks, C.E. Polymer electrolyte electrolysis: A review of the activity and stability of non-precious metal hydrogen evolution reaction and oxygen evolution reaction catalysts. Renew. Sustain. Energy Rev. 2021, 139, 110709. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Bisoi, S.R.; Huang, Y.; Tsai, D.; Lee, C.-P. 2D-Layered Non-Precious Electrocatalysts for Hydrogen Evolution Reaction: Fundamentals to Applications. Catalysts 2021, 11, 689. [Google Scholar] [CrossRef]
- Ding, M.; Ao, W.; Xu, H.; Chen, W. Facile construction of dual heterojunction CoO@TiO2/MXene hybrid with efficient and stable catalytic activity for phenol degradation with peroxymonosulfate under visible light irradiation. J. Hazard. Mater. 2021, 420, 126686–126696. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Kim, K.; Kim, M.; Kim, J. Synergistic metal-oxide interaction for efficient self-reconstruction of cobalt oxide as highly active water oxidation electrocatalyst. J. Catal. 2021, 404, 80–88. [Google Scholar] [CrossRef]
- Khan, L.U.; Jabeen, N.; Jabbar, I. Investigating Local Structure of Ion-Implanted (Ni2+) and Thermally Annealed Rock Salt CoO Film by EXAFS Simulation Using Evolutionary Algorithm. ACS Appl. Energy Mater. 2021, 4, 2049–2055. [Google Scholar] [CrossRef]
- Ando, F.; Gunji, T.; Tanabe, T. Enhancement of the Oxygen Reduction Reaction Activity of Pt by Tuning Its d -Band Center via Transition Metal Oxide Support Interactions. ACS Catal. 2021, 8, 9317–9332. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Chen, Y. An enhanced activity of Pt/CeO2/CNT triple junction interface catalyst prepared by atomic layer deposition for oxygen reduction reaction. Chem. Phys. Lett. 2020, 755, 137793–137804. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, B.; Li, L.; Yang, S. Phosphorus and Yttrium Co-doped Co(OH)F Nanoarray as Highly Efficient and Bifunctional Electrocatalysts for Overall Water Splitting. Small 2019, 15, 1904105–1904114. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, M.; Yan, D.; Mao, J.; Liu, H.; Hu, W.; Du, X.-W.; Ling, T.; Qiao, S.-Z. Engineering oxygen vacancy on NiO nanorod arrays for alkaline hydrogen evolution. Nano Energy 2018, 43, 103–109. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, H.; Sun, L. Highly Active Three-dimensional NiFe/Cu2O Nanowires/Cu Foam Electrode for Water Oxidation. ChemSusChem 2017, 10, 1475–1481. [Google Scholar]
- Gong, M.; Zhou, W.; Tsai, M.; Zhou, J.; Guan, M.; Lin, M.; Zhang, B.; Hu, Y.; Wang, D.-Y.; Yang, J.; et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 5695–5701. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Miao, R.; Suib, S.L. Mesoporous MoO3−x Material as an Efficient Electrocatalyst for Hydrogen Evolution Reactions. Adv. Energy Mater. 2016, 6, 1600528–1600539. [Google Scholar] [CrossRef]
- Yan, X.; Tian, L.; Chen, X. Crystalline/amorphous Ni/NiO core/shell nanosheets as highly active electrocatalysts for hydrogen evolution react. J. Power Source 2015, 300, 336–343. [Google Scholar] [CrossRef]
- Kou, T.; Chen, M.; Wu, F.; Smart, T.J.; Wang, S.; Wu, Y.; Zhang, Y.; Li, S.; Lall, S.; Zhang, Z.; et al. Carbon doping switching on the hydrogen adsorption activity of NiO for hydrogen evolution reaction. Nat. Commun. 2020, 11, 590. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mao, S.; Wei, Z.; Wang, H.; Liu, Z.; Chen, Y. The dominating role of NiO on the interface of Ni/NiO for enhanced hydrogen evolution reaction. ACS Appl. Mater. Interface 2017, 9, 7139–7151. [Google Scholar] [CrossRef] [PubMed]
- Arias, A. The Release of Hydrogen on Ball Milling Chromium in Water; National Aeronautics and Space Administration: Washington, DC, USA, 1968; pp. 1–23. [Google Scholar]
- Jiang, L.; Ji, S.-L.; Xue, H.-G.; Suen, N.-T. HER activity of MxNi1−x (M = Cr, Mo and W; x ≈ 0.2) alloy in acid and alkaline media. Int. J. Hydrog. Energy 2020, 45, 17533–17539. [Google Scholar] [CrossRef]
- Jakšić, M.M. Electrocatalysis of hydrogen evolution in the light of the brewer-engel theory for bonding in metals and intermetallic phases. Electrochim. Acta 1984, 29, 1539–1550. [Google Scholar] [CrossRef]
- Szeleszczuk, Ł.; Pisklak, D.M.; Zielińska-Pisklak, M. Can we predict the structure and stability of molecular crystals under increased pressure? First-principles study of glycine phase transitions. J. Comput. Chem. 2018, 39, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Xavier, N.F.; da Silva, A.M.; Bauerfeldt, G.F. Supercell calculations of the geometry and lattice energy of α-glycine crystal. J. Mol. Modeling 2019, 25, 244–255. [Google Scholar] [CrossRef]
- Mei, A.; Luo, X. The structural, electronic and optical properties of γ-glycine under pressure: A first principles study. RSC Adv. 2019, 9, 3877–3883. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, A.; Hafner, J.; Kresse, G. Molecular adsorption on the surface of strongly correlated transition-metal oxides: A case study for CO/NiO (100). Phys. Rev. B 2004, 69, 75413. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Mcintyre, N.S.; Cook, M.G. X-ray photoelectron studies on some oxides and hydroxides of cobalt, nickel, and copper, 1975. Anal. Chem. 1975, 47, 2208–2213. [Google Scholar] [CrossRef]
- Roustila, A.; Severac, C.; Chene, J.; Percheron-Guegan, A. Hydrogen effects on the electronic and microstructural properties of Ce, Ni, and CeNi2 intermetallic compound. Surf. Sci. 1994, 311, 33–44. [Google Scholar] [CrossRef]
- Klein, J.C.; Hercules, D.M. Surface Characterization of Model Urushibara Catalysts. J. Catal. 1983, 82, 424. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Massimo, T. X-ray photoelectron spectra of defective nickel oxide. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1988, 84, 3501–3510. [Google Scholar]
- Shalvoy, R.B.; Reucroft, P.J.; Davis, B.H. Characterization of coprecipitated nickel on silica methanation catalysts by X-ray photoelectron spectroscopy. J. Catal. 1979, 56, 336–348. [Google Scholar] [CrossRef]
- Mansour, A.N. Characterization of NiO by XPS. Surf. Sci. Spectra 1994, 3, 231. [Google Scholar] [CrossRef]
- Dube, C.E.; Workie, B.; Kounaves, S.P.; Robbat, A., Jr.; Aksu, M.L.; Davies, G. Electrodeposition of Metal Alloy and Mixed Oxide Films Using a Single-Precursor Tetranuclear Copper-Nickel Complex. J. Electrochem. Soc. 1995, 142, 3357. [Google Scholar] [CrossRef]
- Tsutsumi, T.; Ikemoto, I.; Namikawa, T.; Kuroda, H. X-ray photoelectron spectrum of Cr2O5. Bull. Chem. Soc. Jpn. 1981, 54, 913–914. [Google Scholar] [CrossRef]
- Agostinelli, E.; Battistoni, C.; Fiorani, D.; Mattogno, G. An XPS study of the electronic structure of the ZnxCd1−xCr2(X = S, Se) spinel system. J. Phys. Chem. Solids 1989, 50, 269–272. [Google Scholar] [CrossRef]
- Howng, W.-Y.; Thorn, R.J. Investigation of the electronic structure of La1−x (M2+)xCrO3, Cr2O3 and La2O3 by X-ray photoelectron spectroscopy. J. Phys. Chem. Solids 1980, 41, 75–81. [Google Scholar] [CrossRef]
- Allen, G.C.; Curtis, M.T.; Hooper, A.J.; Tucker, P.M. X-ray photoelectron spectroscopy of chromium–oxygen systems. J. Chem. Soc. Dalton Trans. 1973, 13, 1675–1683. [Google Scholar] [CrossRef]
- Ikemoto, I.; Ishii, K.; Kinoshita, S.; Kuroda, H.; Franco, M.A.A.; Thomas, J.M. X-ray photoelectron spectroscopic studies of CrO2 and some related chromium compounds. J. Solid State Chem. 1976, 17, 425–430. [Google Scholar] [CrossRef]
- Dickinson, T.; Povey, A.F.; Sherwood, P.M.A. X-ray photoelectron spectroscopic studies of oxide films on platinum and gold electrodes. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1975, 71, 298–311. [Google Scholar] [CrossRef]
- Maxwell, A.J.; Bruhwiler, P.A.; Nilsson, A.; Martensson, N.; Rudolf, P. Photoemission, autoionization, and X-ray-absorption spectroscopy of ultrathin-film on Au(110). Phys. Rev. B 1994, 49, 10717–10725. [Google Scholar] [CrossRef]
- Xie, Y.; Sherwood, P.M.A. Highly oriented pyrolytic graphite by core level and valence band XPS. Surf. Sci. Spectra 1992, 1, 253–258. [Google Scholar] [CrossRef]
- Taylor, J.A.; Lancaster, G.M.; Rabalais, J.W. Surface alteration of graphite, graphite monofluoride and teflon by interaction with Ar+ and Xe+ beams. Appl. Surf. Sci. 1978, 1, 503–514. [Google Scholar] [CrossRef]
- Battiato, S.; Bruno, L.; Terrasi, A.; Mirabella, S. Superior performances of electroless-deposited Ni–P films decorated with an ultralow content of Pt for water-splitting reactions. ACS Appl. Energy Mater. 2022, 5, 2391–2399. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Zhang, D.; Qin, Y.; Xiong, J.; Lai, J.; Wang, L. Systematic engineering on Ni-Based nanocatalysts effectively promote hydrogen evolution reaction. Small 2022, 18, 2108072. [Google Scholar] [CrossRef]
- Sha, W.; Song, Y.; Liu, P.; Wang, J.; Xu, B.; Feng, X.; Guo, J. Constructing multiple heterostructures on nickel oxide using rare-earth oxide and nickel as efficient bifunctional electrocatalysts for overall water splitting. ChemCatChem 2022, 14, e202101975. [Google Scholar] [CrossRef]
- Pan, S.; Yu, X.; Ling, Y.; Yang, Z. Stable and efficient hydrogen evolution reaction catalyzed by NiO-Rh2P heterostructure electrocatalyst. Catal. Commun. 2022, 163, 106404. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, H.; Zhu, S.; Liang, Y.; Wu, S.; Li, Z.; Cui, Z.; Chang, C.; Yang, X.; Inoue, A. Highly efficient and self-standing nanoporous NiO/Al3Ni2 electrocatalyst for hydrogen evolution reaction. ACS Appl. Energy Mater. 2019, 2, 7913–7922. [Google Scholar] [CrossRef]
- Khan, S.B.; Asiri, A.M. Copper oxide doped composite nanospheres decorated graphite pencil toward efficient hydrogen evolution electrocatalysis. J. Mol. Liq. 2021, 335, 116084. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kumar, V.; Choi, G.J.; Ryu, J.W.; Mane, S.M.; Shin, J.C.; Gwag, J.S. Hexagonal NiO nanosheets on Ni-foam as an electrocatalyst for high-performance water splitting application. Mater. Lett. 2022, 324, 132740. [Google Scholar] [CrossRef]
- Yuan, Z.; Yao, X.; Zhang, G.; Fu, N.; Liu, Y.; Ye, F. Regulating the heterostructure of metal/oxide toward the enhanced hydrogen evolution reaction. ACS Appl. Energy Mater. 2022, 5, 5644–5651. [Google Scholar] [CrossRef]
- Franz, T.; Zabloudil, J.; Mittendorfer, F.; Gragnaniello, L.; Parteder, G.; Allegretti, F.; Surnev, S.; Netzer, F.P. Deformed Surface Oxides: Uncommon Structure of a (6×1) NiO Surface Oxide on Rh(111). J. Phys. Chem. Lett. 2012, 3, 186–190. [Google Scholar] [CrossRef]
- Ventrice, C.A., Jr.; Bertrams, T.; Hannemann, H.; Brodde, A.; Neddermeyer, H. Stable reconstruction of the polar (111) surface of NiO on Au(111). Phys. Rev. B 1994, 49, 5773–5776. [Google Scholar] [CrossRef]
- Rohr, F.; Wirth, K.; Libuda, J.; Cappus, D.; Bäumer, M.; Freund, H.-J. Hydroxy1 driven reconstruction of the polar NiO(111) surface. Surf. Sci. 1994, 315, 977–982. [Google Scholar] [CrossRef]
- Kitakatsu, N.; Maurice, V.; Marcus, P. Local decomposition of NiO ultra-thin films formed on Ni(111). Surf. Sci. 1998, 441, 215–230. [Google Scholar] [CrossRef]
- Barbier, A.; Mocuta, C.; Kuhlenbeck, H.; Peters, K.F. Atomic Structure of the Polar NiO(111)- p(2×2) Surface. Phys. Rev. Lett. 2000, 84, 2897–2901. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, B.-Y. Stability of the polar NiO (111) surface. J. Chem. Phys. 2008, 128, 124703. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D. Reconstruction of NaCl surfaces from a dipolar solution to the Madelung problem. Phys. Rev. Lett. 1992, 68, 3315. [Google Scholar] [CrossRef]
- Finocchi, F.; Barbier, A.; Jupille, J.; Noguera, C. Stability of rocksalt (111) polar surfaces: Beyond the octupole. Phys. Rev. Lett. 2004, 92, 136101. [Google Scholar] [CrossRef] [PubMed]
- Erdman, N.; Warschkow, O.; Ellis, D.E.; Marks, L.D. Solution of the p(2×2) NiO surface structure using direct methods. Surf. Sci. 2000, 470, 1–14. [Google Scholar] [CrossRef]
| Catalysts | Electrolyte | j (mA∙cm−2) | η (mV) | References |
|---|---|---|---|---|
| 5-Pt/Ni–P/NF | 1 M KOH | 10 | 22 | [50] |
| Ir-Ni/NiO@CNT | 1 M KOH | 10 | 24.6 | [51] |
| Er2O3/Ni-NiO | 1 M KOH | 10 | 39 | [52] |
| NiO-Rh2P | 1 M KOH | 10 | 46 | [53] |
| NiO/Al3Ni2 | 1 M KOH | 10 | 66 | [54] |
| NiO-Cr-C/NF-3 | 1 M KOH | 10 | 69 | This work |
| CuO-NiO/CN@GP | 1 M KOH | 10 | 76.2 | [55] |
| NiOx@BCNTs | 1 M KOH | 10 | 79 | [22] |
| NiO nanosheets | 1 M KOH | 10 | 83 | [56] |
| Ni/NiO-cp | 1 M KOH | 10 | 124 | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Z.; Li, C.; Wang, L.; Kang, M.; Wang, W.; Tang, M.; Li, G.; Feng, Z.; Yan, Z. Homogenous Cr and C Doped 3D Self-Supporting NiO Cellular Nanospheres for Hydrogen Evolution Reaction. Materials 2022, 15, 7120. https://doi.org/10.3390/ma15207120
Tan Z, Li C, Wang L, Kang M, Wang W, Tang M, Li G, Feng Z, Yan Z. Homogenous Cr and C Doped 3D Self-Supporting NiO Cellular Nanospheres for Hydrogen Evolution Reaction. Materials. 2022; 15(20):7120. https://doi.org/10.3390/ma15207120
Chicago/Turabian StyleTan, Zhaojun, Chuanbin Li, Lijun Wang, Mingjie Kang, Wen Wang, Mingqi Tang, Gang Li, Zaiqiang Feng, and Zhenwei Yan. 2022. "Homogenous Cr and C Doped 3D Self-Supporting NiO Cellular Nanospheres for Hydrogen Evolution Reaction" Materials 15, no. 20: 7120. https://doi.org/10.3390/ma15207120
APA StyleTan, Z., Li, C., Wang, L., Kang, M., Wang, W., Tang, M., Li, G., Feng, Z., & Yan, Z. (2022). Homogenous Cr and C Doped 3D Self-Supporting NiO Cellular Nanospheres for Hydrogen Evolution Reaction. Materials, 15(20), 7120. https://doi.org/10.3390/ma15207120





