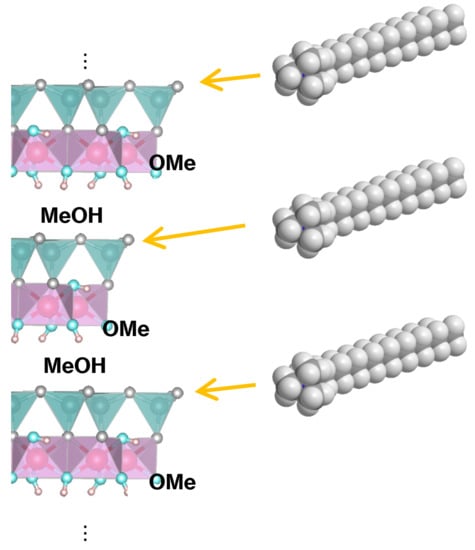
Regioselective Approach to Characterizing Increased Edge Availability in Layered Crystal Materials following Layer Expansion: Reaction of Kaolinite with Octadecyltrimethylammonium Salts
Abstract
:1. Introduction
2. Materials and Methods
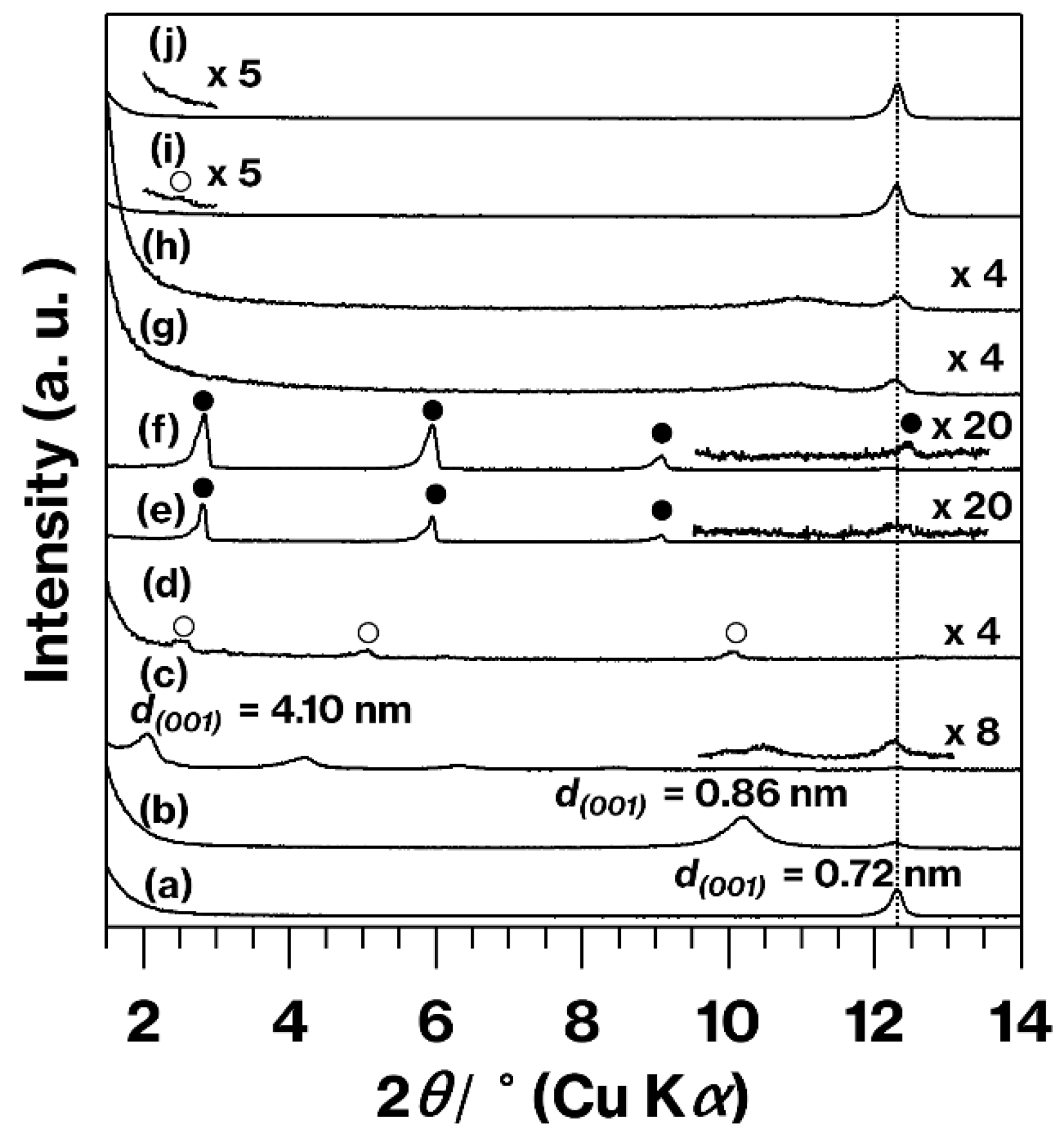
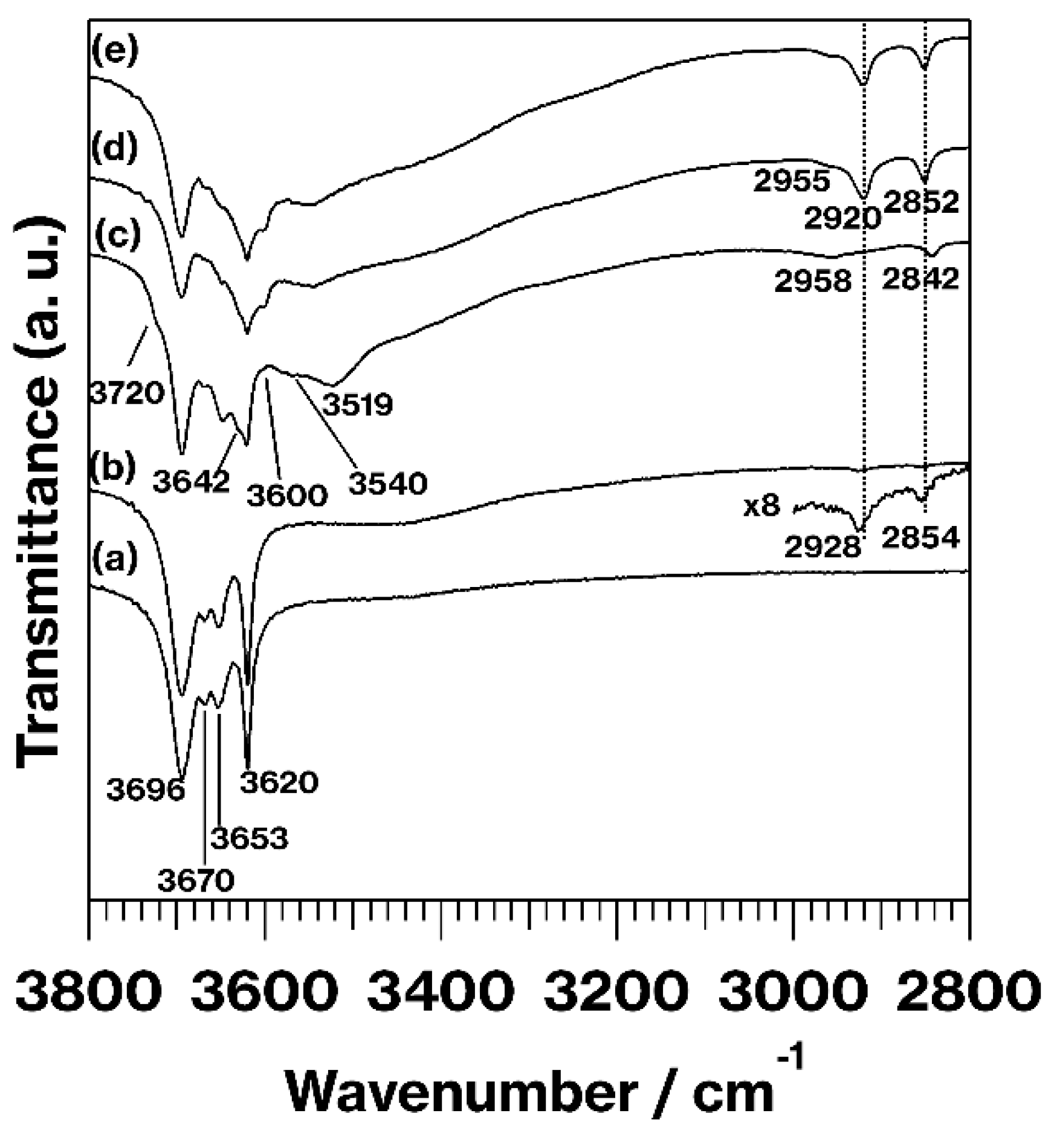
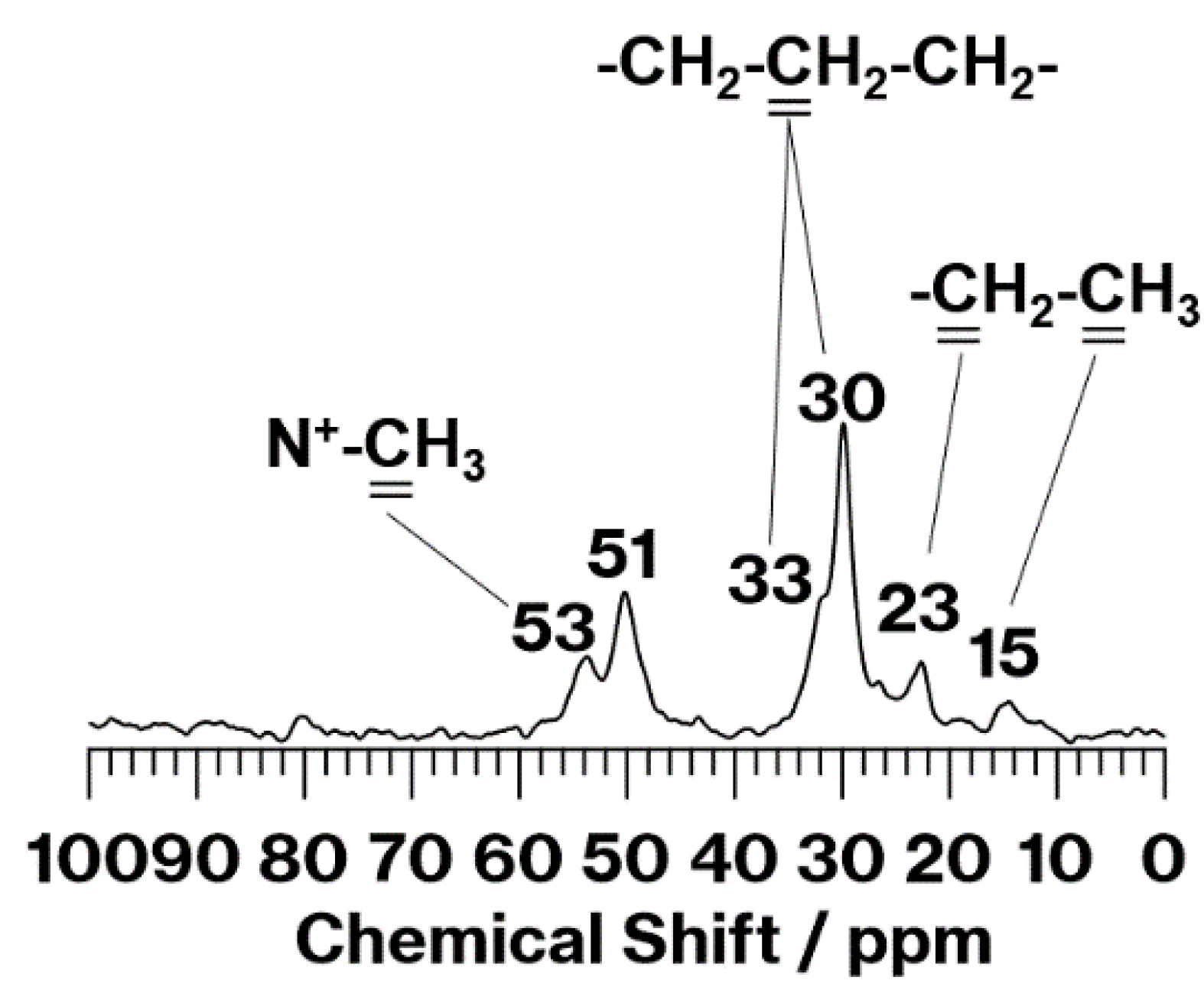
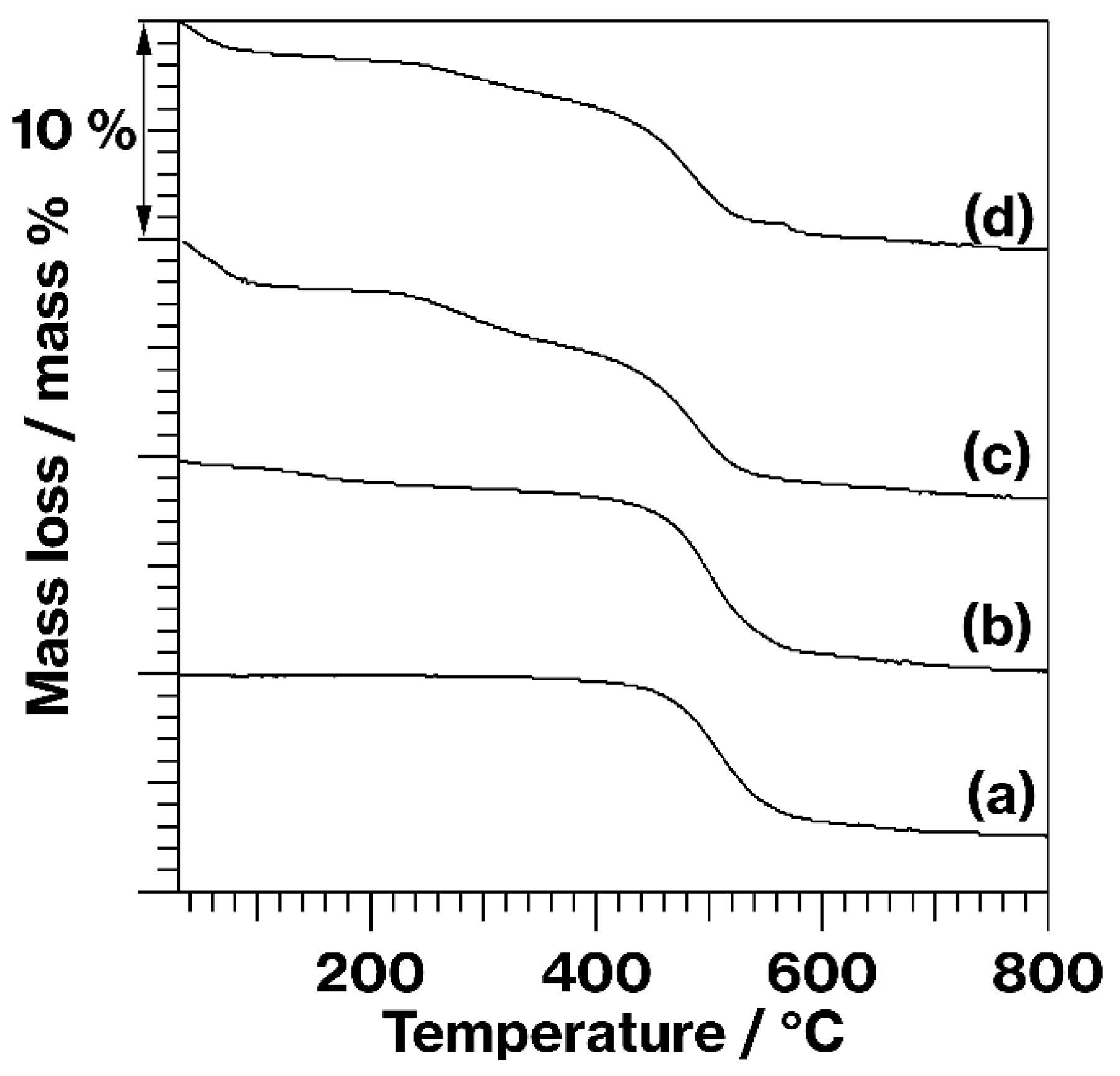
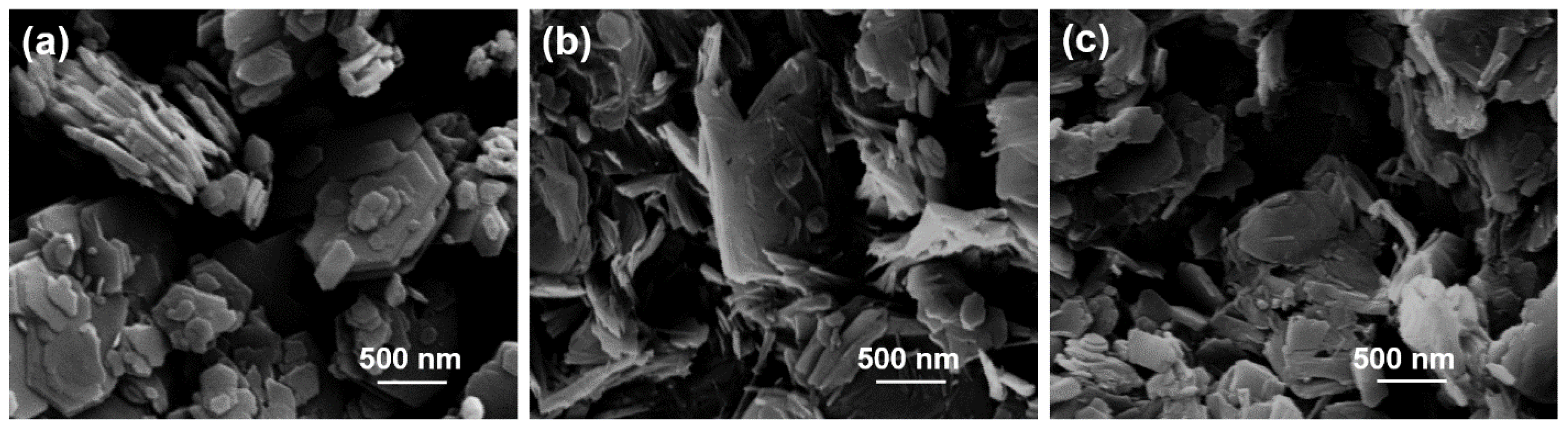
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoonheydt, R.A.; Johnston, C.T. Surface and Interface Chemistry Minerals. In Developments in Clay Science Volume 5 A Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Oxford, UK, 2013; pp. 145–148. [Google Scholar]
- Lagaly, G.; Dékány, I. Colloid Clay Science. In Developments in Clay Science Volume 5A Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Oxford, UK, 2013; pp. 243–249. [Google Scholar]
- Wang, Y.; Yan, D.; El Hankari, S.; Zou, Y.; Wang, S. Recent Progress on Layered Double Hydroxides and Their Derivatives for Electrocatalytic Water Splitting. Adv. Sci. 2018, 5, 1800064. [Google Scholar] [CrossRef]
- Gangadharan, D.T.; Ma, D. Searching for Stability at Lower Dimensions: Current Trends and Future Prospects of Layered Perovskite Solar Cells. Energy Environ. Sci. 2019, 12, 2860–2889. [Google Scholar] [CrossRef]
- Yuan, S.; Pang, S.-Y.; Hao, J. 2D Transition Metal Dichalcogenides, Carbides, Nitrides, and Their Applications in Supercapacitors and Electrocatalytic Hydrogen Evolution Reactions. Appl. Phys. Rev. 2020, 7, 021304. [Google Scholar] [CrossRef]
- Li, S.; Mu, B.; Wang, X.; Wang, A. Recent Researches on Natural Pigments Stabilized by Clay Minerals: A Review. Dye. Pigment. 2021, 190, 109322. [Google Scholar] [CrossRef]
- Whittingham, M.S.; Jacobsen, A.J. Intercalation Chemistry; Elsevier: Oxford, UK, 1982; pp. 1–585. [Google Scholar]
- Ogawa, M.; Kuroda, K. Photofunctions of Intercalation Compounds. Chem. Rev. 1995, 95, 399–438. [Google Scholar] [CrossRef]
- Ogawa, M.; Saito, K.; Sohmiya, M. A Controlled Spatial Distribution of Functional Units in the Two Dimensional Nanospace of Layered Silicates and Titanates. Dalton Trans. 2014, 43, 10340–10354. [Google Scholar] [CrossRef] [PubMed]
- Lagaly, G.; Ogawa, M.; Dékány, I. Clay Mineral-Organic Interactions, In Developments in Clay Science Volume 5A Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Oxford, UK, 2013; pp. 435–445. [Google Scholar]
- Nakao, A.; Funakawa, S.; Kosaki, T. Cation Exchange Capacity Derived from Tetrahedral Layer Charges Increase with Decreasing Hydroxyl-Al Polymers in 2:1 Phyllosilicates through Soil Acidification in Acidic Forest Soils (Dystrudepts) in Kinki-District, Japan. Clay Sci. 2013, 17, 75–82. [Google Scholar]
- Tunney, J.J.; Detellier, C. Chemically Modified Kaolinite. Grafting of Methoxy Groups on the Interlamellar Aluminol Surface of Kaolinite. J. Mater. Chem. 1996, 6, 1679–1685. [Google Scholar] [CrossRef]
- Komori, Y.; Endo, H.; Takenawa, R.; Hayashi, S.; Sugahara, Y.; Kuorda, K. Modification of the Interlayer Surface of Kaolinite with Methoxy Groups. Langmuir 2000, 16, 5506–5508. [Google Scholar] [CrossRef]
- Constanzo, P.M.; Giese, R.F., Jr.; Lipicicas, M. Static and Dynamic Structure of Water in Hydrated Kaolinites. Ⅰ. The Static Structure. Clay Clay Miner. 1984, 32, 419–428. [Google Scholar] [CrossRef]
- Constanzo, P.M.; Giese, R.F., Jr. Dehydration of Synthetic Hydrated Kaolinites: A Model for the Dehydration of Halloysite (10Å). Clay Clay Miner. 1985, 5, 415–423. [Google Scholar] [CrossRef]
- Sugahara, Y.; Satokawa, S.; Yoshioka, K.-I.; Kuorda, K.; Kato, C. Kaolinite-Pyridine Intercalation Compound Derived from Hydrated Kaolinite. Clays Clay Miner. 1989, 37, 143–150. [Google Scholar] [CrossRef]
- Costanzo, P.M.; Giese, R.F. Ordered and Disordered Organic Intercalates of 8.4-Å, Synthetically Hydrated Kaolinite. Clays Clay Miner. 1990, 38, 160–170. [Google Scholar] [CrossRef]
- Kuroda, Y.; Ito, K.; Itabashi, K.; Kuroda, K. One-Step Exfoliation of Kaolinites and Their Transformation into Nanoscrolls. Langmuir 2011, 27, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A. A Secret of Chinese Porcelain Manufacture. Angew. Chem. Int. Ed. 1963, 12, 697–703. [Google Scholar] [CrossRef]
- Itageki, T.; Kuroda, K. Organic Modification of the Interlayer Surface of Kaolinite with Propanediols by Transesterification. J. Mater. Chem. 2003, 13, 1064–1068. [Google Scholar]
- Vaia, R.A.; Teukolsky, R.K.; Giannelis, E.P. Interlayer Structure and Molecular Environment of Alkylammonium Layered Silicates. Chem. Mater. 1994, 6, 1017–1022. [Google Scholar] [CrossRef]
- Horváth, E.; Kristóf, J.; Frost, R.L. Vibrational Spectroscopy of Intercalated Kaolinites. Part I. Appl. Spectrosc. Rev. 2010, 45, 130–147. [Google Scholar] [CrossRef] [Green Version]
- Machida, S.; Idota, N.; Sugahara, Y. Interlayer Grafting of Kaolinite Using Trimethylphosphate. Dalton. Trans. 2019, 48, 11663–11673. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.A.; Ploetze, M.; Skrabal, P. Structure and Properties of Alkylammonium Monolayers Self-Assembled on Montmorillonite Platelets. J. Phys. Chem. B 2004, 108, 2580–2588. [Google Scholar] [CrossRef]
- Osman, M.A. Organo-Vermiculites: Synthesis, Structure and Properties. Platelike Nanoparticles with High Aspect Ratio. J. Mater. Chem. 2006, 16, 3007–3013. [Google Scholar] [CrossRef]
- Machida, S.; Guégan, R.; Sugahara, Y. Preparation and Comparative Stability of a Kaolinite-Tetrabutylphosphonium Bromide Intercalation Compound for Heat and Solvent Treatments. Langmuir 2019, 35, 13553–13561. [Google Scholar] [CrossRef]
- Matusik, J.; Kłapyta, Z. Characterization of Kaolinite Intercalation Compounds with Benzylalkylammonium Chlorides using XRD, TGA/DTA and CHNS Elemental Analysis. Appl. Clay Sci. 2013, 83–84, 433–440. [Google Scholar] [CrossRef]
- Tunney, J.J.; Detellier, C. Preparation and Characterization of Two Distinct Ethylene Glycol Derivatives of Kaolinite. Clays Clay Miner. 1994, 42, 552–560. [Google Scholar] [CrossRef]
- Gardolinski, J.E.F.C.; Lagaly, G. Grafted Organic Derivatives of Kaolinite: Ⅱ. Intercalation of Primary n-Alkylamines and Delamination. Clay Miner. 2005, 40, 547–556. [Google Scholar] [CrossRef]
- Wada, N.; Raythatha, R.; Minomura, S. Pressure Effects on Water-Intercalated Kaolinite. Solid State Commun. 1987, 63, 783–786. [Google Scholar] [CrossRef]
- Dedzo, G.K.; Detellier, C. Characterization and Applications of Kaolinite Robustly Grafted by an Ionic Liquid with Naphthyl Functionality. Materials 2017, 10, 1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machida, S.; Katsumata, K.-i.; Yasumori, A. Regioselective Approach to Characterizing Increased Edge Availability in Layered Crystal Materials following Layer Expansion: Reaction of Kaolinite with Octadecyltrimethylammonium Salts. Materials 2022, 15, 588. https://doi.org/10.3390/ma15020588
Machida S, Katsumata K-i, Yasumori A. Regioselective Approach to Characterizing Increased Edge Availability in Layered Crystal Materials following Layer Expansion: Reaction of Kaolinite with Octadecyltrimethylammonium Salts. Materials. 2022; 15(2):588. https://doi.org/10.3390/ma15020588
Chicago/Turabian StyleMachida, Shingo, Ken-ichi Katsumata, and Atsuo Yasumori. 2022. "Regioselective Approach to Characterizing Increased Edge Availability in Layered Crystal Materials following Layer Expansion: Reaction of Kaolinite with Octadecyltrimethylammonium Salts" Materials 15, no. 2: 588. https://doi.org/10.3390/ma15020588
APA StyleMachida, S., Katsumata, K.-i., & Yasumori, A. (2022). Regioselective Approach to Characterizing Increased Edge Availability in Layered Crystal Materials following Layer Expansion: Reaction of Kaolinite with Octadecyltrimethylammonium Salts. Materials, 15(2), 588. https://doi.org/10.3390/ma15020588