Hydrogen Permeation Behavior of Zirconium Nitride Film on Zirconium Hydride
Abstract
:1. Introduction
2. Materials and Methods
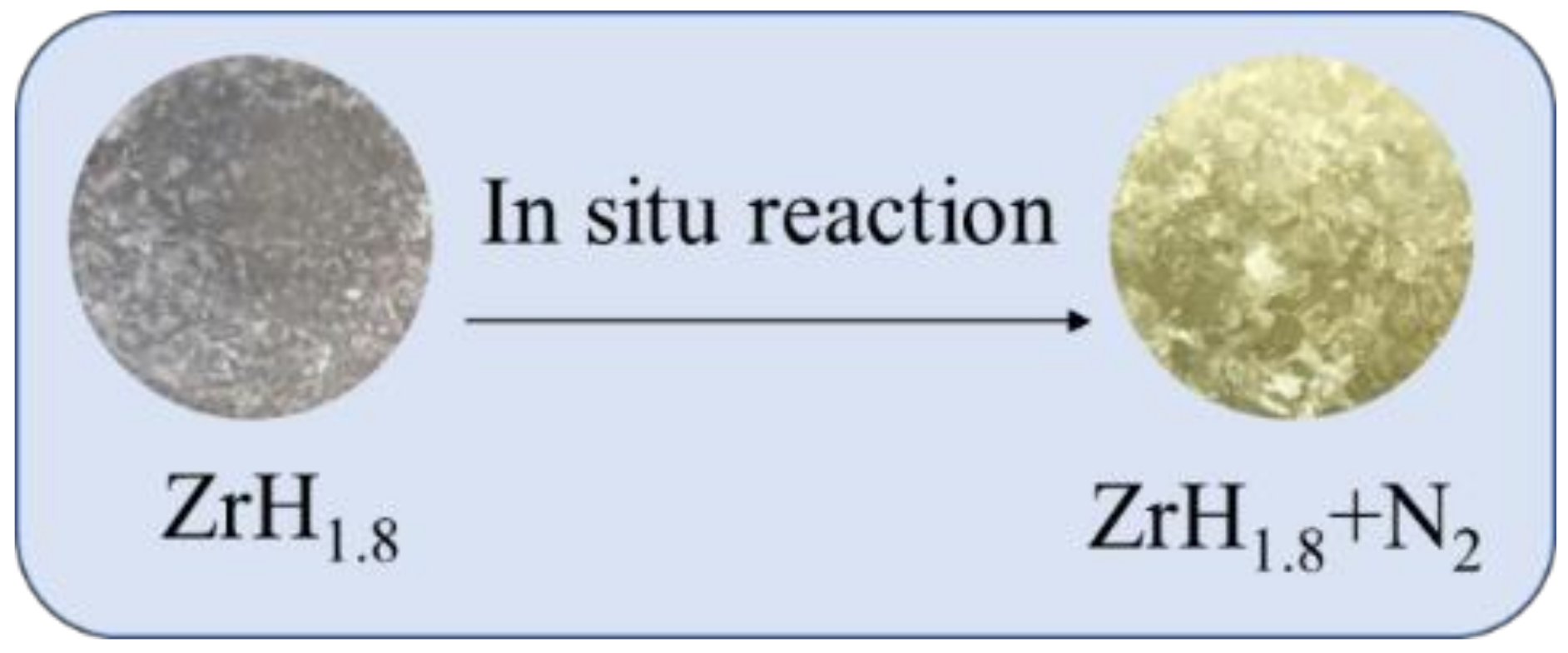
2.1. Preparation of Hydrogen Permeation Barrier
2.2. Characterization of Composition, Morphology and Structure
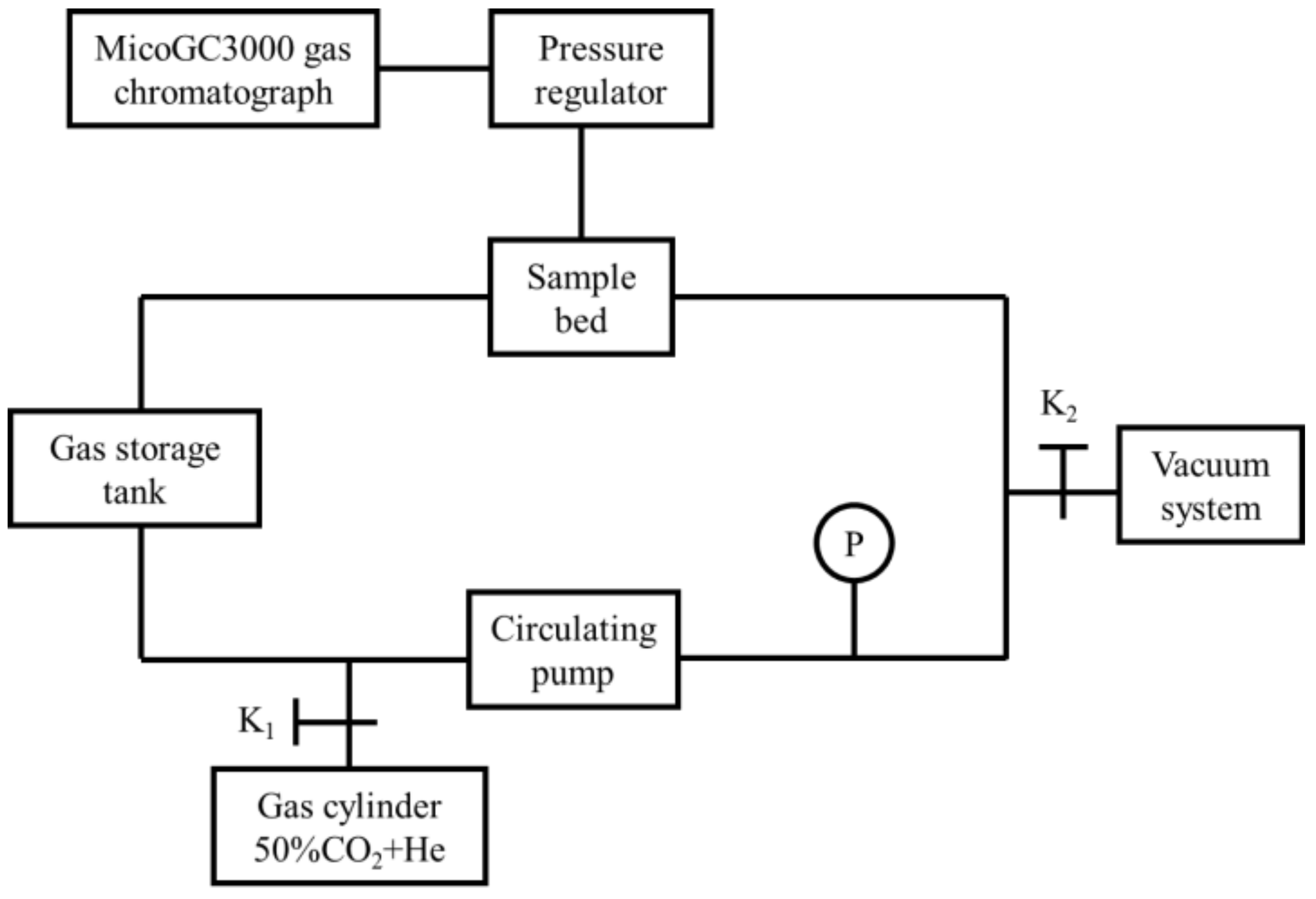
2.3. Characterization of Hydrogen Permeation Behavior
2.3.1. Permeation Reduction Factor
2.3.2. Vacuum Thermal Dehydrogenation
2.3.3. Hydrogen Permeation Rate
3. Results and Discussion
3.1. Morphology
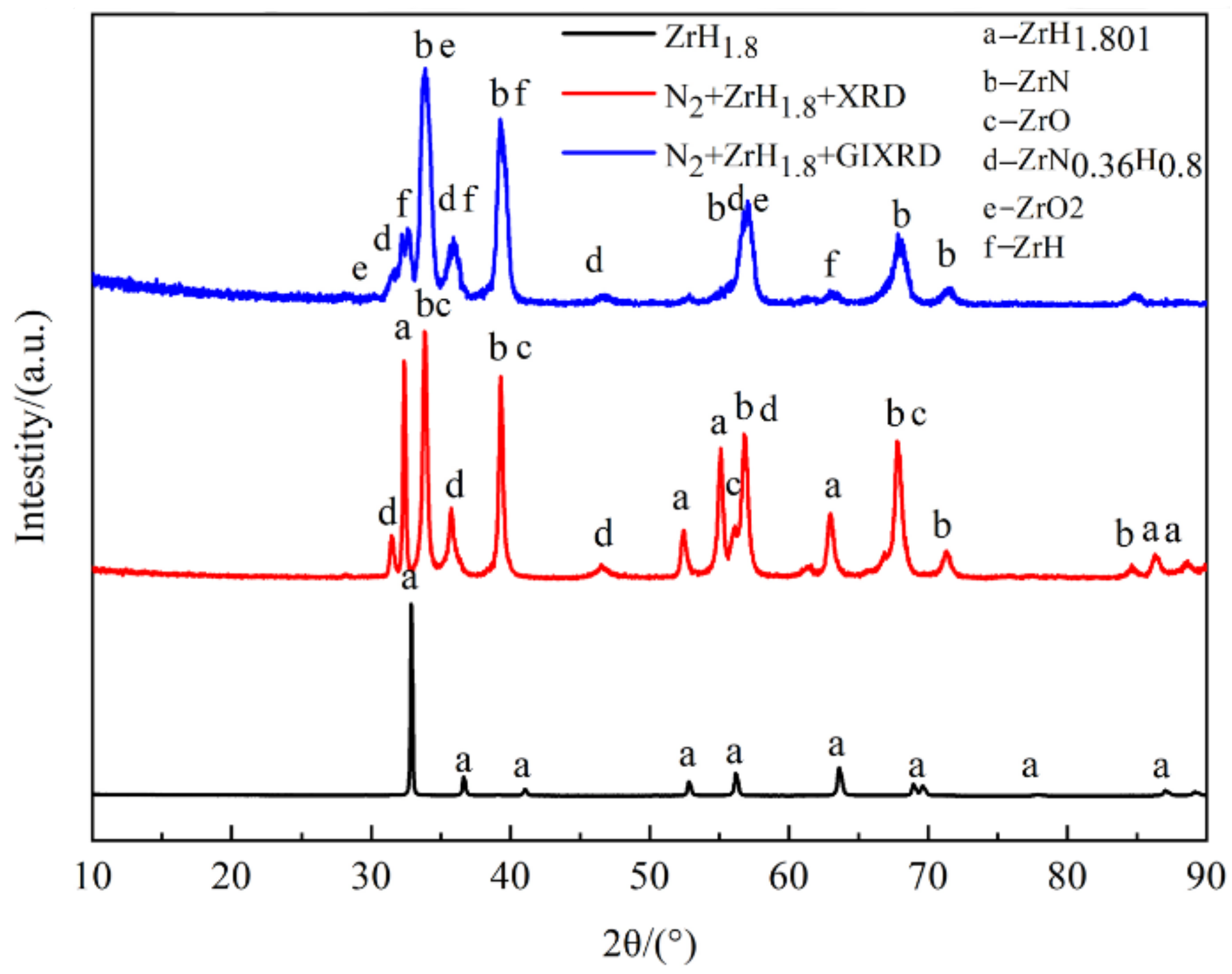
3.2. Phase Structure
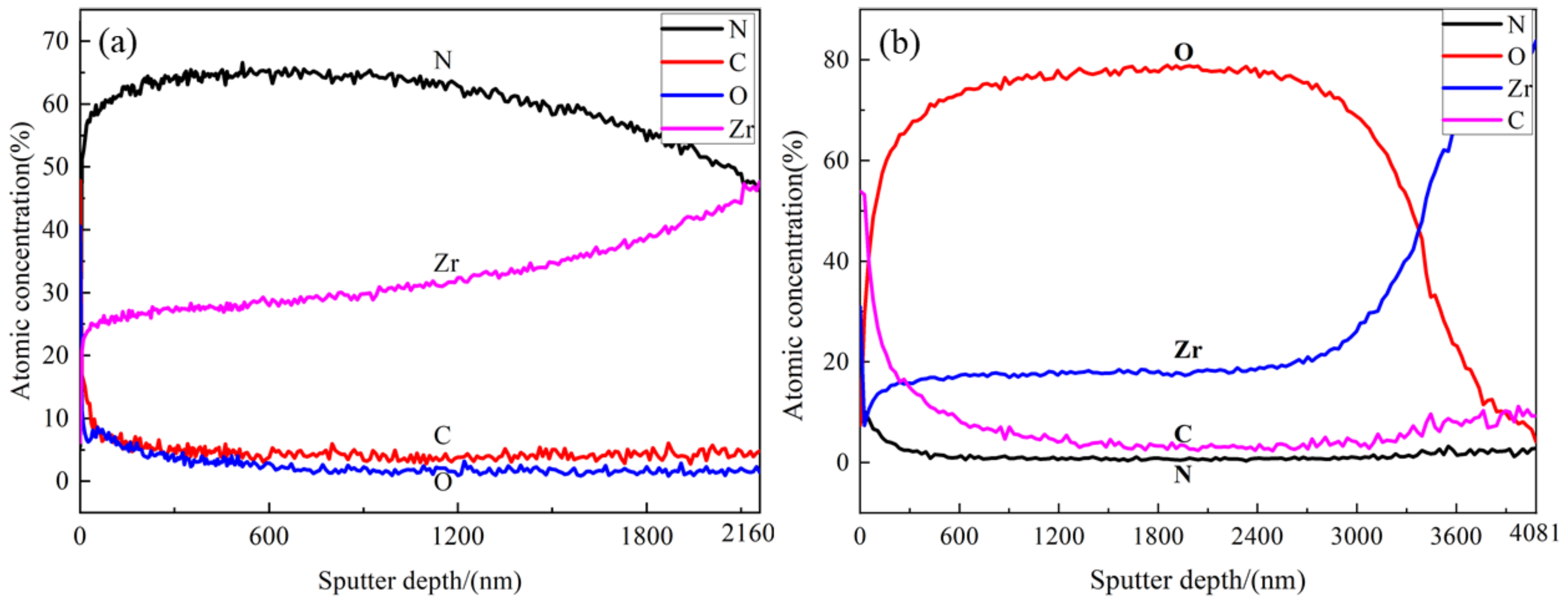
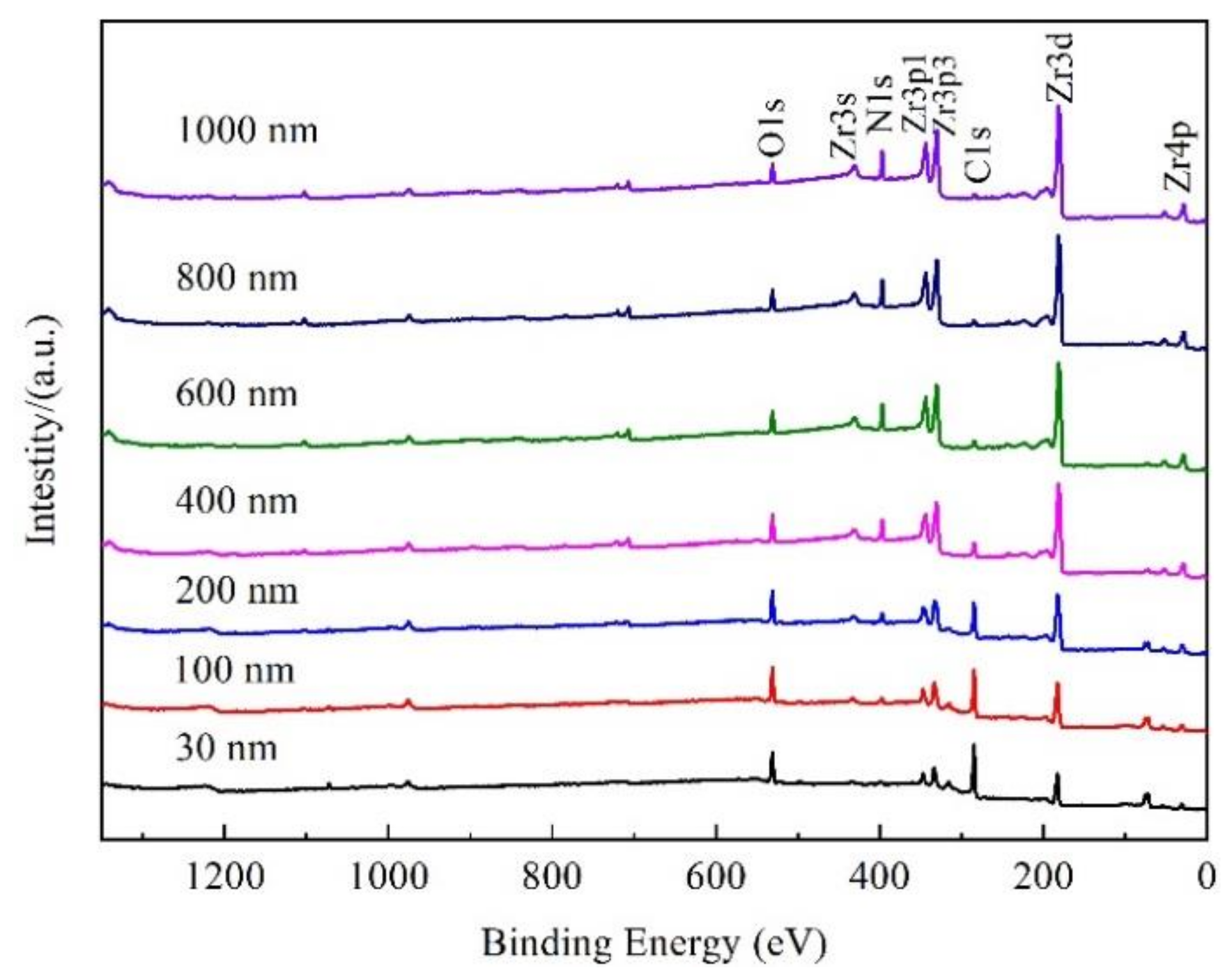
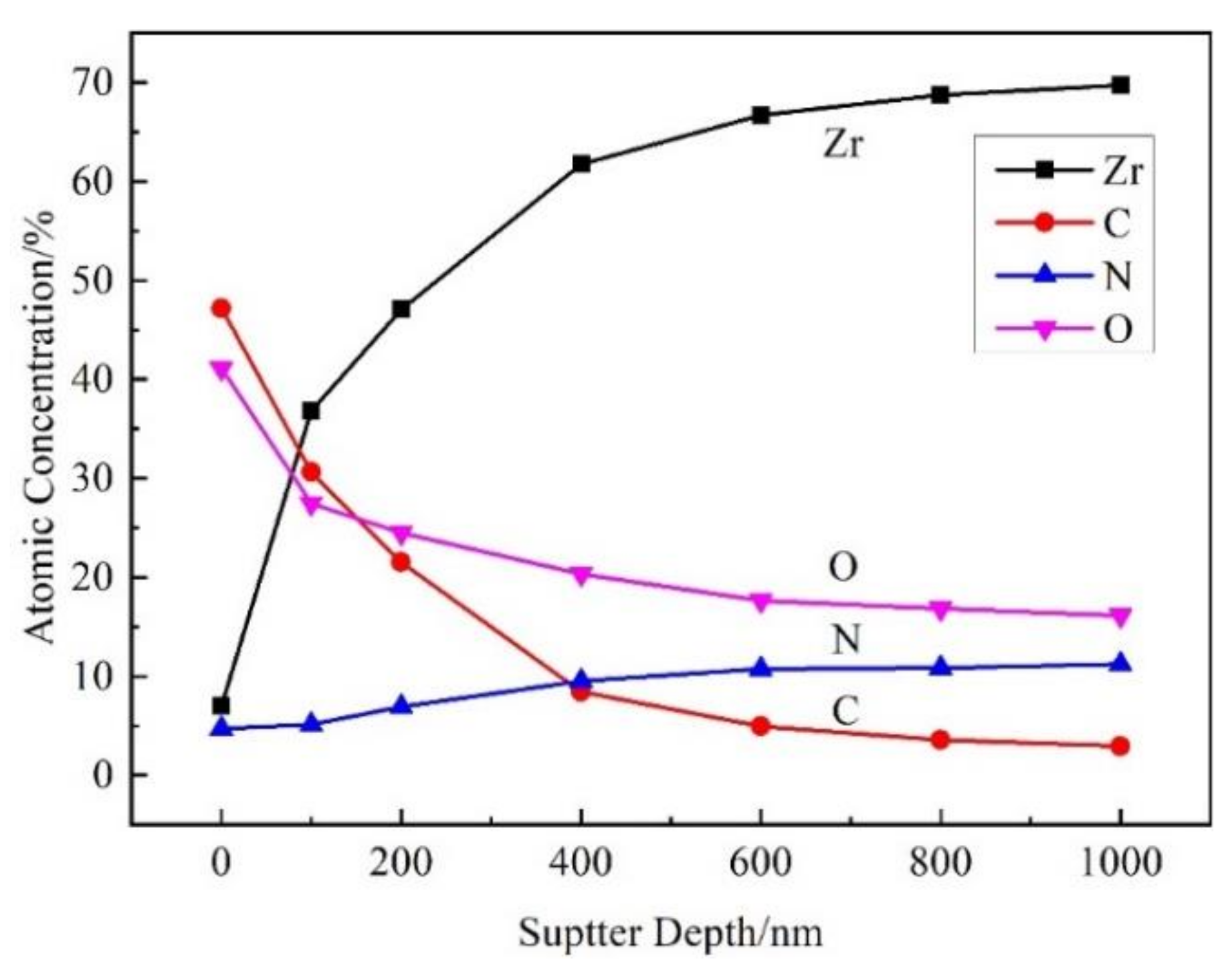
3.3. Element Distribution
3.4. Valence State
3.5. Hydrogen Permeation Behavior
3.5.1. Permeation Reduction Factor
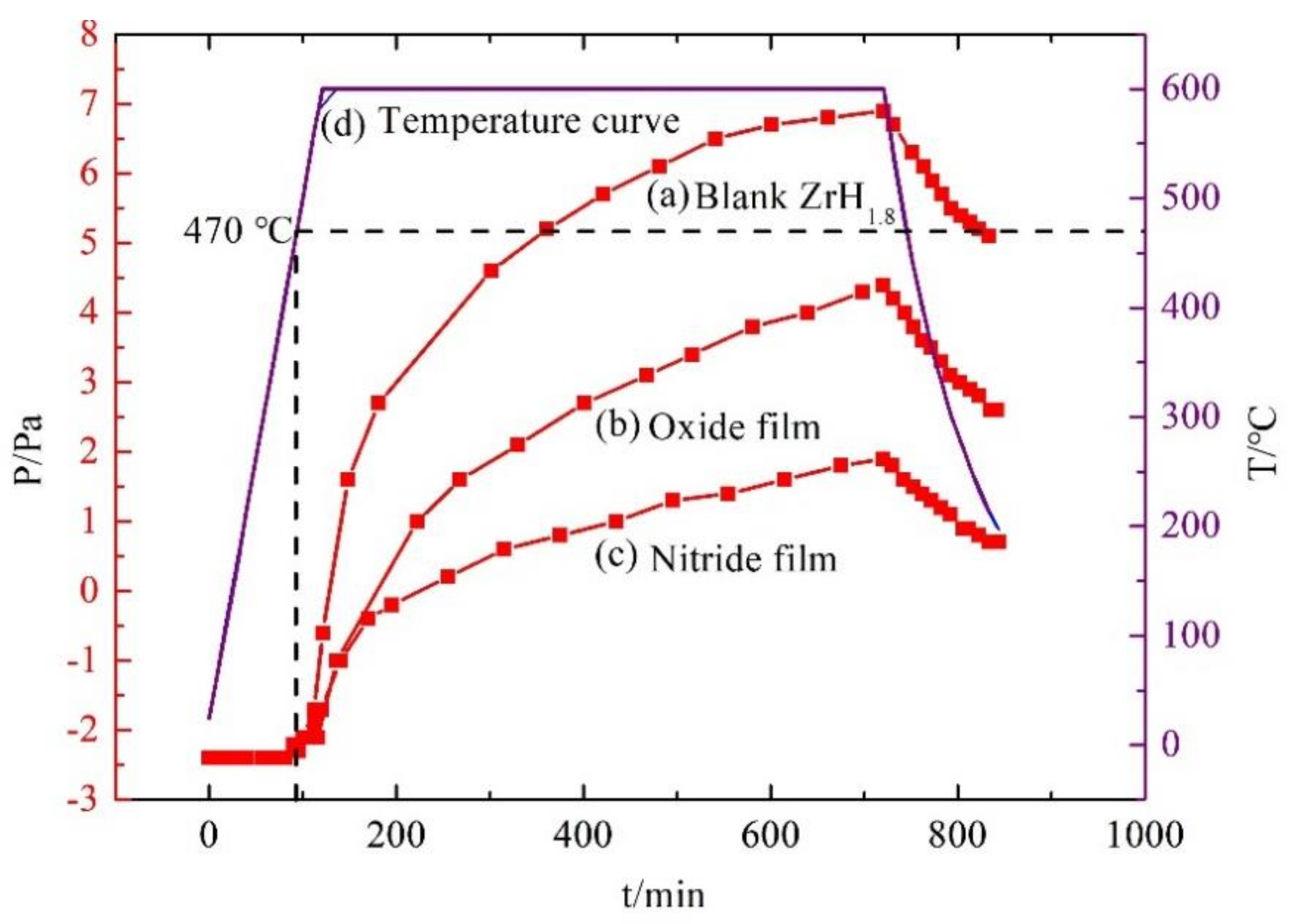
3.5.2. Vacuum Thermal Dehydrogenation
3.5.3. Hydrogen Permeation Rate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murakami, T. A historical review and analysis on the selection of nuclear reactor types and implications to development programs for advanced reactors; A Japanese study. Energy Rep. 2021, 7, 3428–3436. [Google Scholar] [CrossRef]
- Yang, Q.F.; Lu, H.L. Research and application of space nuclear reactor power supply. Spacecraft Eng. 1995, 4, 11–21. [Google Scholar]
- Bennett, G.L. A look at the Soviet space nuclear power program. In Proceedings of the 24th Intersociety Energy Conversion Engineering Conference, Washington, DC, USA, 6–11 August 1989; pp. 1187–1194. [Google Scholar]
- Chernikov, A.S.; Syasin, V.A.; Kostin, V.M.; Boiko, E.B. Influence of hydrogen content on the strength and the presence of defects in zirconium hydride. J. Alloys Compd. 2002, 330–332, 393–395. [Google Scholar] [CrossRef]
- Konashi, K.; Ikeshoji, T.; Kawazoe, Y.; Matsui, H. A molecular dynamics study of thermal conductivity of zirconium hydride. J. Alloys Compd. 2003, 356–357, 279–282. [Google Scholar] [CrossRef]
- Tsuchiya, B.; Teshigawara, M.; Konashi, K.; Yamawaki, M. Thermal diffusivity and electrical resistivity of zirconium hydride. J. Alloys Compd. 2002, 330, 357–360. [Google Scholar] [CrossRef]
- Tsuchiya, B.; Huang, J.; Konashi, K.; Teshigawara, K.; Yamawaki, M. Thermophysical properties of zirconium hydride and uranium–zirconium hydride. J. Nucl. Mater. 2001, 289, 329–333. [Google Scholar] [CrossRef]
- Ma, M.W.; Liang, L.; Tang, B.H.; Xiang, W.; Wang, Y.; Cheng, Y.L.; Tan, X.H. Decomposition kinetics study of zirconium hydride by interrupted thermal desorption spectroscopy. J. Alloys Compd. 2015, 645, 217–220. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Lv, W.L.; Mo, L.B.; Duan, D.W.; Gu, H.W.; Ding, F.Z.; Tang, T.; Luo, D.L.; Cao, J.L. Stability of Y2O3 hydrogen isotope permeation barriers in hydrogen at high temperatures. Int. J. Hydrog. Energy 2013, 38, 4266–4271. [Google Scholar] [CrossRef]
- He, D.; Li, S.; Liu, X.P.; Zhang, C.; Yu, Q.H.; Wang, S.M.; Jiang, L.J. Preparation of Cr2O3 film by MOCVD as hydrogen permeation barrier. Fusion Eng. Des. 2014, 89, 35–39. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Xiang, Q.Y.; Yan, K.; Yao, W.Q.; Cao, J.L. Study on influence factors of permeation reduction factor of Al2O3 hydrogen isotopes permeation barriers. Int. J. Hydrog. Energy 2016, 41, 4326–4331. [Google Scholar] [CrossRef]
- Wu, Y.P.; Zhu, S.F.; Zhang, Y.P.; Liu, T.W.; Rao, Y.C.; Luo, L.Z.; Wang, Q.G. The adhesion strength and deuterium permeation property of SiC films synthesized by magnetron sputtering. Int. J. Hydrog. Energy 2016, 41, 10827–10832. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Xiang, Q.Y.; Cao, J.L. Performances of AlN coatings as hydrogen isotopes permeation barriers. Fusion Eng. Des. 2016, 102, 94–98. [Google Scholar] [CrossRef]
- Nemanič, V.; McGuiness, P.; Daneu, N.; Zajec, B.; Siketić, Z.; Waldhauser, W. Hydrogen permeation through silicon nitride films. J. Alloys Compd. 2012, 539, 184–189. [Google Scholar] [CrossRef]
- Tamura, M.; Noma, M.; Yamashita, M. Characteristic change of hydrogen permeation in stainless steel plate by BN coating. Surf. Coat. Technol. 2014, 260, 148–154. [Google Scholar] [CrossRef]
- Bazzanella, N.; Checchetto, R.; Miotello, A.; Patton, B.; Kale, A.N.; Kothari, D.C. High temperature efficient deuterium permeation and oxidation (Al,Ti)N barriers deposited on stainless steel. Appl. Phys. Lett. 2002, 81, 3762–3764. [Google Scholar] [CrossRef]
- Mcguiness, P.J.; Čekada, M.; Nemanič, V.; Zajec, B.; Recnik, A. Hydrogen permeation through TiAlN-coated Eurofer '97 steel. Surf. Coat. Technol. 2011, 205, 2709–2713. [Google Scholar] [CrossRef]
- Chen, W.D.; Wang, L.J.; Wang, J.W.; Yan, S.F. Oxidation behavior of zirconium hydride in O2 and CO2. Rare Met. Mater. Eng. 2008, 37, 1970–1972. [Google Scholar]
- Wu, M.; Chen, Y.; Peng, J.Q.; Yan, G.Q.; Sun, Y.P.; Zhang, J.D.; Zhang, S.L.; Wang, L.J. Hydrogen permeation resistance and characterization of Si-Al and Si-Zr composite sol oxide coating on surface of zirconium hydride. Rare Met. 2017, 36, 55–60. [Google Scholar] [CrossRef]
- Wu, M.; Peng, J.Q.; Yan, G.Q.; Chen, Y.; He, Z.Y.; Xu, Z.G.; Wang, L.J. Preparation and properties of composite hydrogen permeation barrier on ZrH1.8 by sol–gel technique. Surf. Coat. Technol. 2018, 352, 159–165. [Google Scholar] [CrossRef]
- Yan, G.Q.; Chen, Y.; Wu, M.; Peng, J.Q.; Wang, L.J. AES/XPS analysis of in-situ reaction layer between urea and zirconium hydride at high temperature. Rare Met. Mater. Eng. 2016, 45, 3302–3305. [Google Scholar]
- Qi, S.; Ma, Z.H.; Yan, G.Q.; Wang, Z.H.; Wang, L.J. Hydrogen permeation rate of coating zirconium hydride moderator-a prediction model. Int. J. Hydrog. Energy 2021, 45, 14710–14719. [Google Scholar] [CrossRef]
- Zhou, D.P.; Peng, H.; Zhu, L.; Guo, H.B.; Gong, S.K. Microstructure, hardness and corrosion behaviour of Ti/TiN multilayer coatings produced by plasma activated EB-PVD. Surf. Coat. Technol. 2014, 258, 102–107. [Google Scholar] [CrossRef]
- Cui, Z.Q.; Qin, Y.C. Metallurgy and Heat Treatment; China Machine Press: Beijing, China, 2007; pp. 299–302. [Google Scholar]
- Zhang, H.; Qin, H.F.; Ren, Z.C.; Zhao, J.Y.; Hou, X.N.; Doll, G.L.; Donga, Y.L.; Ye, C. Low-temperature nitriding of nanocrystalline Inconel 718 alloy. Surf. Coat. Technol. 2017, 330, 10–16. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Ali, H.; Karatas, C.; Al-Sharafi, A. Laser gas assisted nitriding and characterization of tungsten surface. Opt. Laser Technol. 2018, 107, 274–280. [Google Scholar] [CrossRef]
- Bai, S.; Yuan, X.M.; Yan, G.Q.; Liu, Z.X.; Yang, H.Q.; Wang, L.J. Hydrogen permeation rate and stability of in-situ oxide layer on surface of ZrH1.85 at 600 °C. Rare Met. 2020, 44, 41–47. [Google Scholar]
- Niyomsoan, S.; Grant, W.; Olson, D.L.; Mishral, B. Variation of color in titanium and zirconium nitride decorative thin films. Thin Solid Film. 2002, 415, 187–194. [Google Scholar] [CrossRef]
- Xin, Z.Y.; Yin, X.R.; Ling, Y.H.; Zhang, Z.J.; Liu, X.; Liang, H.; Deng, X.K. Hydrogen permeation behavior and mechanism of Cr2O3/Al2O3 bipolar oxide film under plasma discharging environment. Int. J. Hydrog. Energy. 2017, 42, 20869–20878. [Google Scholar] [CrossRef]
- Shchukarev, A.V.; Korolkov, D.V. XPS Study of group IA carbonates. Cent. Eur. J. Chem. 2004, 2, 347–362. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.S.; Qin, L.J.; Chang, Y.; Zhao, P. Preparation of hydrogen permeation barrier layer on zirconium hydride surface by CO2 reaction method. Surf. Technol. 2005, 2, 32–34. [Google Scholar]
- Muneshwar, T.; Cadien, K. Comparing XPS on bare and capped ZrN films grown by plasma enhanced ALD: Effect of ambient oxidation. Appl. Surf. Sci. 2018, 435, 367–376. [Google Scholar] [CrossRef]
- Tunes, M.A.; Silva, C.M.; Edmondson, P.D. Site specific dependencies of hydrogen concentrations in zirconium hydrides. Scr. Mater. 2019, 158, 136–140. [Google Scholar] [CrossRef]
- Woo, O.T.; Carpenter, G.J.C. EELS characterization of zirconium hydrides. Microsc. Microanal. Microstruct. 1992, 3, 35–44. [Google Scholar]




| Element | Fe | Hf | Nb | O | N |
|---|---|---|---|---|---|
| Content/ωt% | 0.003 | 0.0052 | 0.96 | 0.0056 | 0.0043 |
| Sample | Film | Initial Thicknesses |
|---|---|---|
| ZrH1.8 with nitride film | ZrN | 1.6 μm |
| ZrH1.8 with oxide film | ZrO2 | 2 μm |
| Sample | Phase | |||
|---|---|---|---|---|
| Blank ZrH1.8 | ZrH1.801 | |||
| XRD of film | ZrH1.801 | ZrN | ZrN0.36H0.8 | ZrO |
| GIXRD of film | ZrH | ZrN | ZrN0.36H0.8 | ZrO2 |
| Elements | h/nm | Peak1/eV | Peak2/eV | Peak3/eV | Peak4/eV |
|---|---|---|---|---|---|
| C1s | 30 | 284.78 | 285.67 | - | - |
| 200 | 284.80 | 285.42 | - | - | |
| 600 | 284.82 | 282.12 | - | - | |
| 1000 | 284.82 | 282.17 | - | - | |
| Zr3d | 30 | 179.84 | 182.70 | 184.85 | 185.36 |
| 200 | 179.94 | 180.27 | 182.89 | 185.52 | |
| 600 | 179.55 | 179.92 | 182.24 | 184.84 | |
| 1000 | 179.77 | 181.14 | 182.23 | 184.60 | |
| O1s | 30 | 530.71 | 531.83 | - | - |
| 200 | 531.24 | 532.64 | - | - | |
| 600 | 530.82 | 532.32 | - | - | |
| 1000 | 530.84 | 532.09 | 534.66 | - | |
| N1s | 30 | 398.67 | - | - | - |
| 200 | 397.53 | 399.42 | - | - | |
| 600 | 396.90 | 397.51 | 399.27 | - | |
| 1000 | 396.92 | 397.51 | 398.82 | - |
| Samples | m1/g | m2/g | Δm/g | Δm/S g·m−2 | PRF | Average PRF | Standard Deviation |
|---|---|---|---|---|---|---|---|
| ZrH1.8 | 5.833 | 5.825 | 0.008 | 3.7230 | - | - | - |
| 20.0762 | 20.0625 | 0.0137 | |||||
| 20.0358 | 20.0195 | 0.0163 | |||||
| Oxide film | 5.4483 | 5.4447 | 0.0036 | 1.1983 | 3.1069 | 3.4811 | 0.4900 |
| 19.7596 | 19.7555 | 0.0041 | 1.1770 | 3.1631 | |||
| 19.6229 | 19.6198 | 0.0031 | 0.8921 | 4.1733 | |||
| Nitride film | 5.3744 | 5.3735 | 0.0009 | 0.2962 | 12.5692 | 9.3970 | 2.3157 |
| 19.1102 | 19.1087 | 0.0015 | 0.4372 | 8.5156 | |||
| 19.1605 | 19.1587 | 0.0018 | 0.5239 | 7.1063 |
| Nitride Film | Oxide Film | ||||
|---|---|---|---|---|---|
| t/day | Va/kg·m−2·s−1 | σ1 | t/day | Va/kg·m−2·s−1 | σ2 |
| 0.003 | 4.309 × 10−7 | 1.67867 × 10−8 | 0.003 | 4.220 × 10−7 | 2.78229 × 10−9 |
| 0.907 | 2.364 × 10−9 | 2.82329 × 10−11 | 0.625 | 1.072 × 10−8 | 2.09711 × 10−10 |
| 1.820 | 5.344 × 10−10 | 3.29286 × 10−11 | 1.509 | 3.424 × 10−9 | 1.38303 × 10−10 |
| 2.841 | 1.596 × 10−9 | 2.22545 × 10−11 | 2.575 | 2.167 × 10−9 | 6.02349 × 10−11 |
| 3.843 | 1.354 × 10−9 | 7.21309 × 10−11 | 3.707 | 1.608 × 10−9 | 1.38992 × 10−11 |
| 4.862 | 8.778 × 10−10 | 3.29768 × 10−11 | 4.598 | 1.265 × 10−9 | 4.48827 × 10−11 |
| 5.855 | 1.201 × 10−9 | 9.16133 × 10−12 | 5.640 | 1.260 × 10−9 | 1.38900 × 10−11 |
| 6.843 | 5.707 × 10−10 | 3.71908 × 10−11 | 6.470 | 6.866 × 10−10 | 4.30914 × 10−11 |
| Film | Va/kg·m−2·s−1 | R2 | 1 Year | 2 Year | 3 Year |
|---|---|---|---|---|---|
| Nitride | 0.99998 | 3.179% | 3.228% | 3.253% | |
| Oxide | 1 | 7.069% | 7.259% | 7.360% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Yan, G.; Zhang, J.; Ma, Z.; Wang, L.; Guo, Z.; Zhang, S.; Wu, Y. Hydrogen Permeation Behavior of Zirconium Nitride Film on Zirconium Hydride. Materials 2022, 15, 550. https://doi.org/10.3390/ma15020550
Wang W, Yan G, Zhang J, Ma Z, Wang L, Guo Z, Zhang S, Wu Y. Hydrogen Permeation Behavior of Zirconium Nitride Film on Zirconium Hydride. Materials. 2022; 15(2):550. https://doi.org/10.3390/ma15020550
Chicago/Turabian StyleWang, Wenke, Guoqing Yan, Jiandong Zhang, Zhaohui Ma, Lijun Wang, Zhancheng Guo, Shunli Zhang, and Yanke Wu. 2022. "Hydrogen Permeation Behavior of Zirconium Nitride Film on Zirconium Hydride" Materials 15, no. 2: 550. https://doi.org/10.3390/ma15020550
APA StyleWang, W., Yan, G., Zhang, J., Ma, Z., Wang, L., Guo, Z., Zhang, S., & Wu, Y. (2022). Hydrogen Permeation Behavior of Zirconium Nitride Film on Zirconium Hydride. Materials, 15(2), 550. https://doi.org/10.3390/ma15020550





