Citric Acid in the Passivation of Titanium Dental Implants: Corrosion Resistance and Bactericide Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- Control. As-received material.
- HCl: The discs were immersed in a solution of hydrochloric acid (HCl) 20% (v) for 40 s at room temperature (HCl group). This type of passivation is the very common in the implants and prosthesis.
- Citric acid 20% 10′. The discs were immersed in a solution of citric acid 20% (v) for 10 min at room temperature.
- Citric acid 20% 20′. The discs were immersed in a solution of citric acid 20% (v) for 10 min at room temperature.
- Citric acid 20% 30′. The discs were immersed in a solution of citric acid 20% (v) for 10 min at room temperature.
- Citric acid 40% 10′. The discs were immersed in a solution of citric acid 40% (v) for 10 min at room temperature.
2.2. Methods
2.2.1. Confocal Laser Scanning Microscopy (CLSM)
2.2.2. Contact Angle and Surface Free Energy
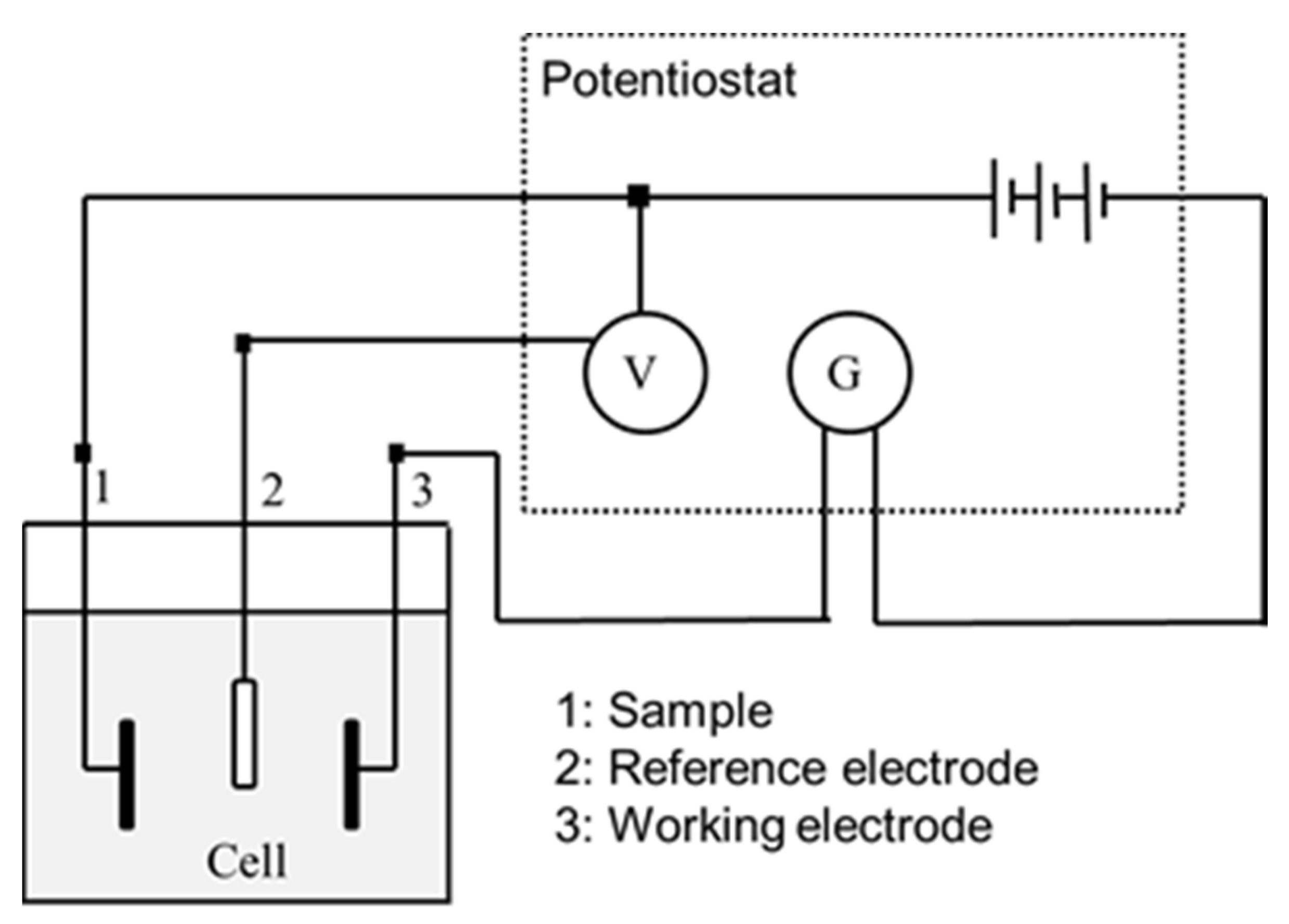
2.2.3. Electrochemical Measurements
- -
- icorr (μA/cm2)/corrosion current density.
- -
- Ecorr (mV)/Corrosion potential: value at which the current density changes from cathodic to anodic.
- -
- Erep (mV)/Repassivation potential: potential at which the passive layer regenerates.
- -
- Ep (mV)/Pitting potential: value at which pitting corrosion may occur.
- -
- ip (μA/cm2)/passivation current density.
- -
- irep (μA/cm2)/repassivation current density.
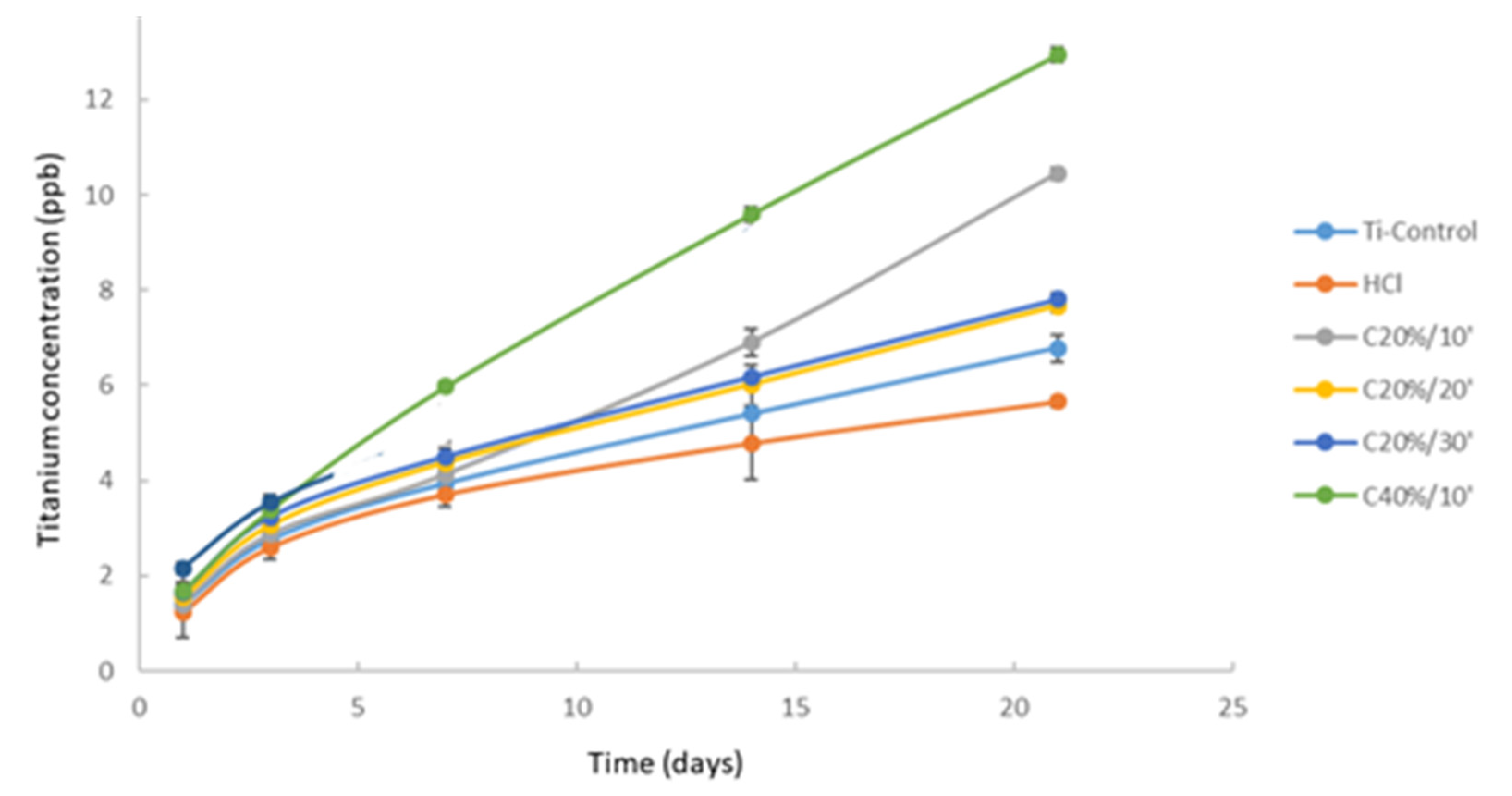
2.2.4. Ion Release
2.2.5. Bacterial Strains and Culture Conditions
2.2.6. Statistical Analysis
2.2.7. Ethical Approval
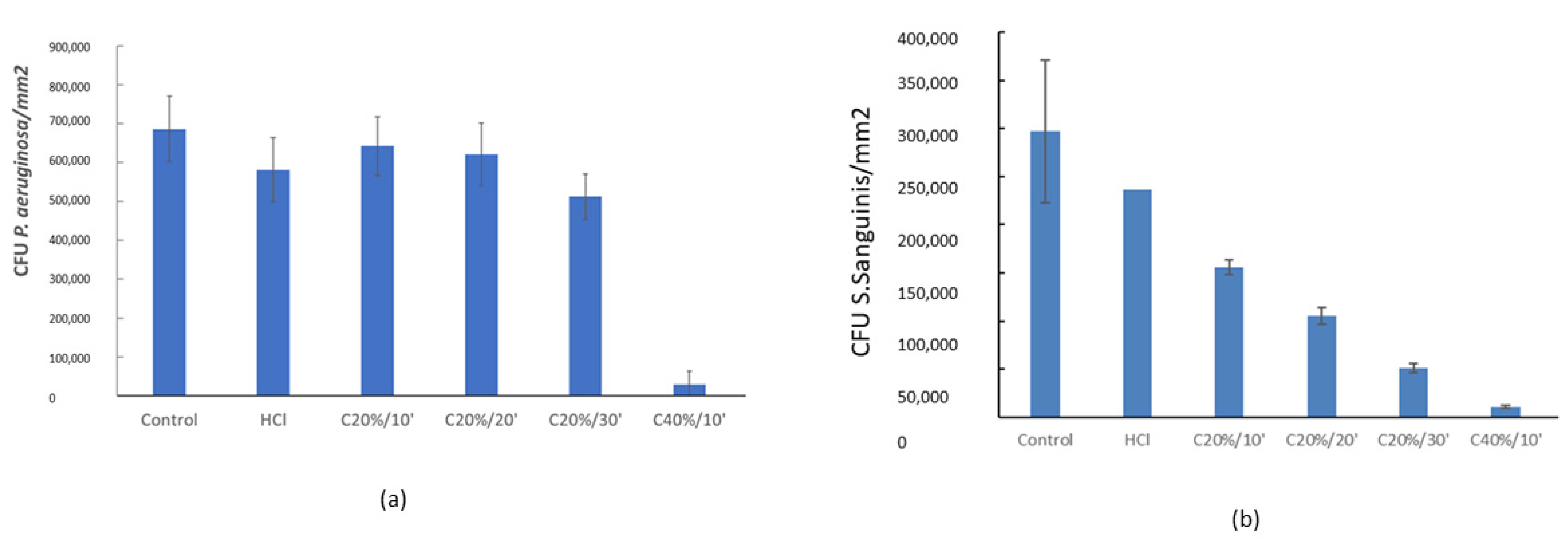
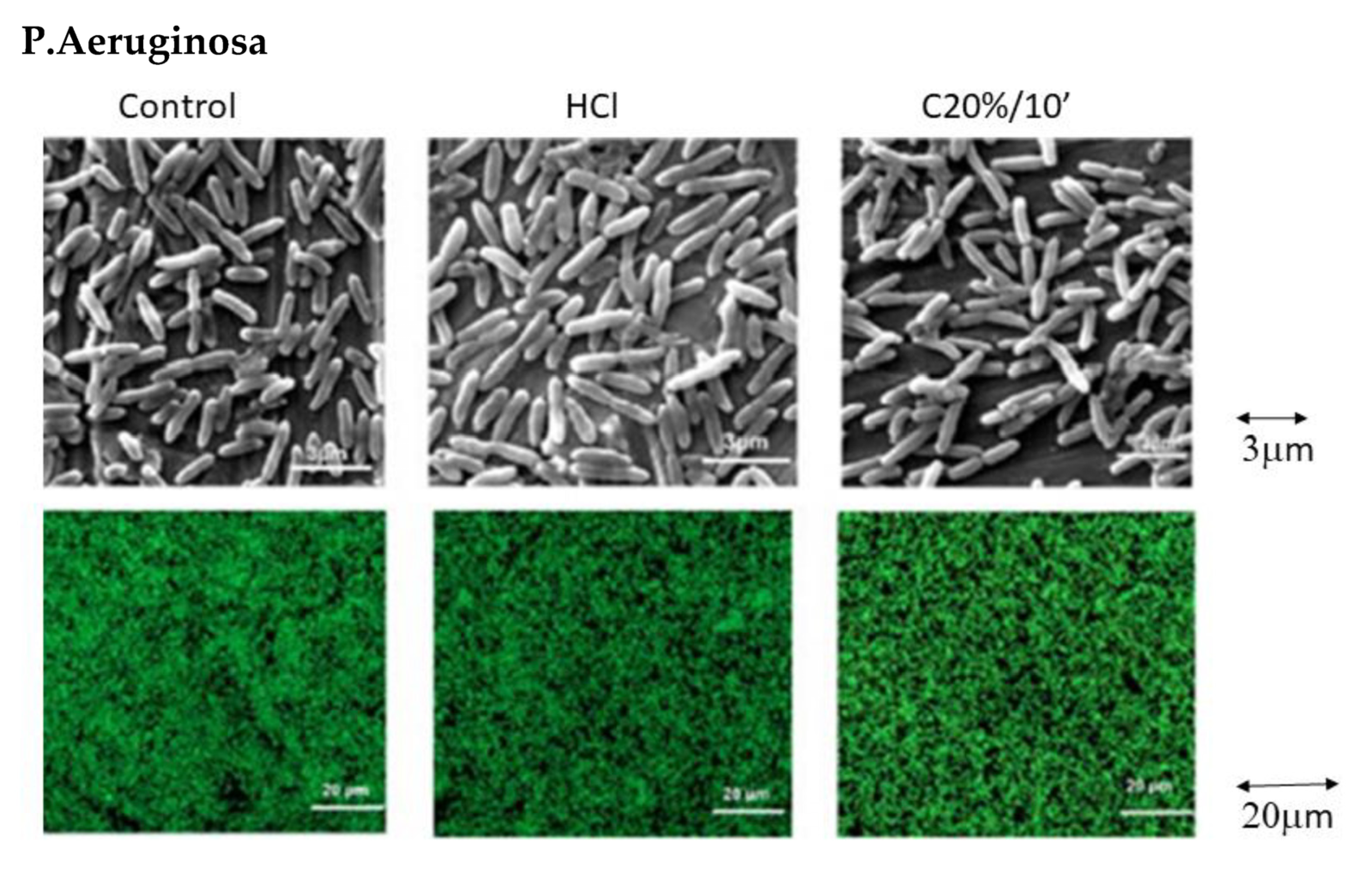
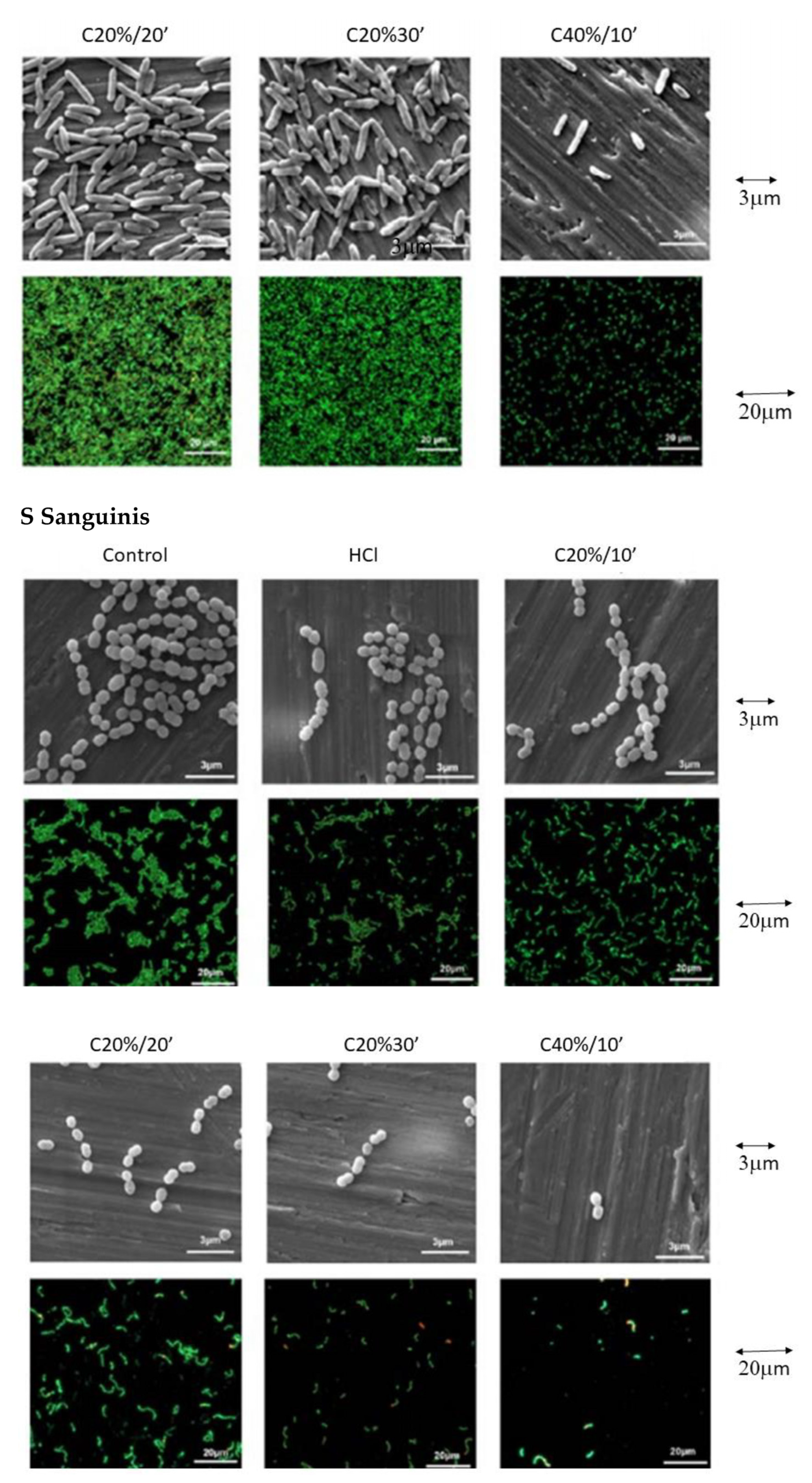
3. Results
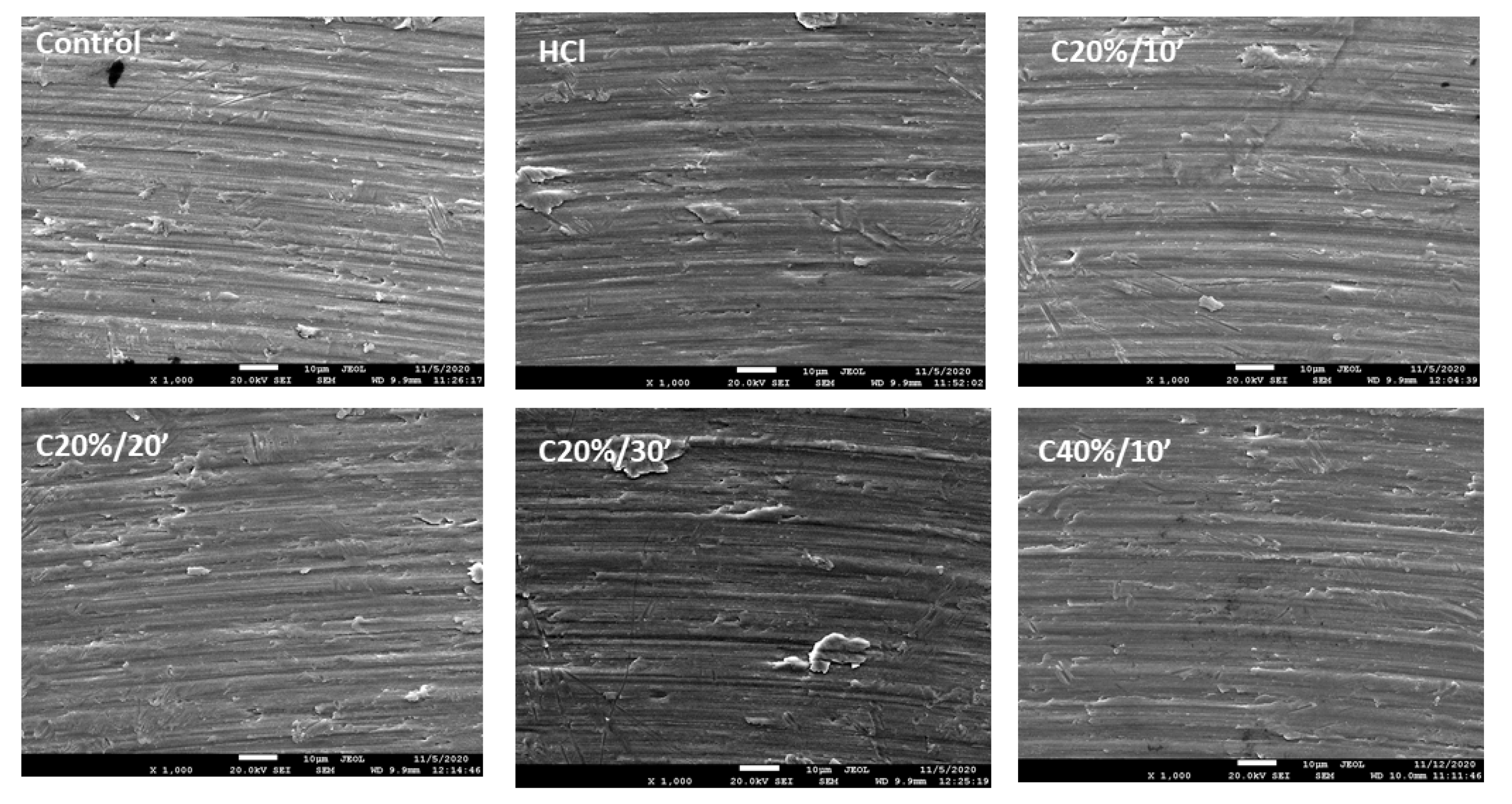
3.1. Surface Characterization
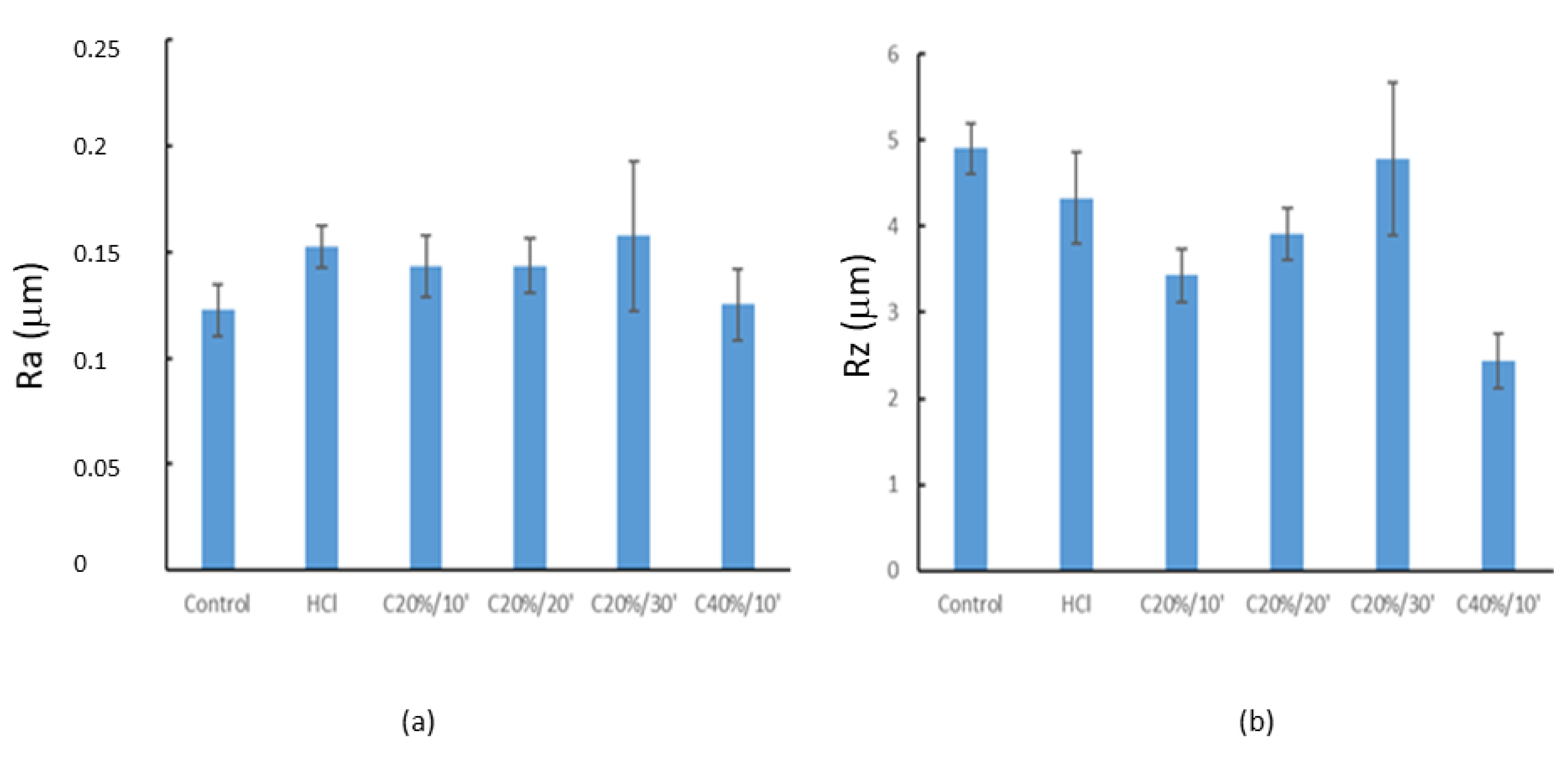
3.1.1. Roughness
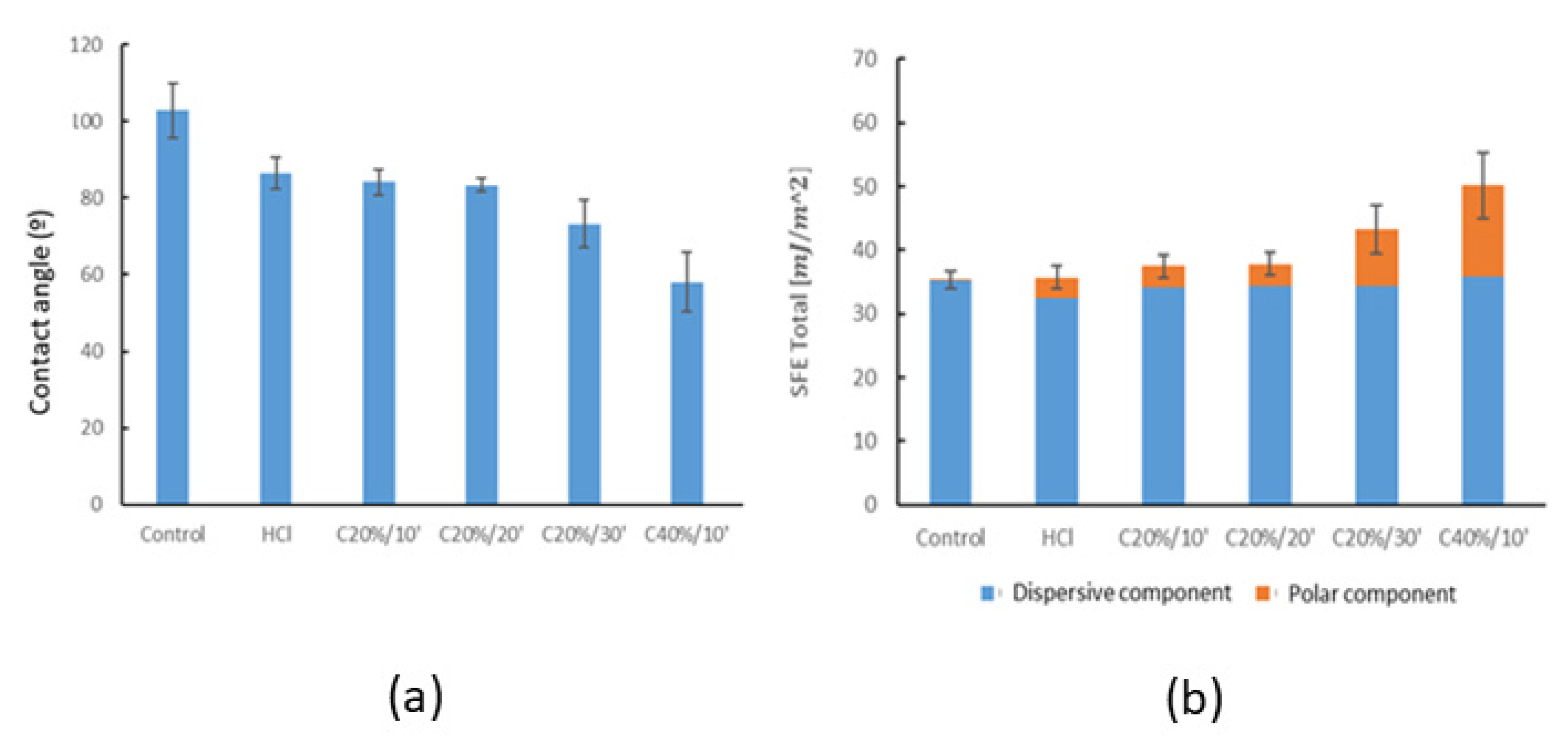
3.1.2. Wettability
3.2. Corrosion Behaviour
3.3. Bacterial Adhesion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [PubMed]
- Meltzer, A.M. Primary stability and initial bone-to-implant contact: The effects on immediate placement and restoration of dental implants. J. Implant. Reconstruct. Dent. 2009, 1, 35–41. [Google Scholar]
- Prasad, D.K.; Swaminathan, A.A.; Prasad, D.A. Current trends in surface textures of implants. J. Dent. Implant. 2016, 6, 85–91. [Google Scholar]
- Anil, S.; Anand, P.S.; Alghamdi, H.; Janse, J.A. Dental Implant Surface Enhancement and Osseointegration. Implant. Dent.Rapidly Evol. Pract. 2011, 83–108. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [Green Version]
- Triplett, R.G.; Frohberg, U.; Sykaras, N.; Woody, R.D. Implant materials, design, and surface topographies: Their influence on osseointegration of dental implants. J. Long-Term Eff. Med. Implant. 2003, 13, 485–501. [Google Scholar]
- El-Banna, A.; Bissa, M.W.; Khurshid, Z.; Zohaib, S.; Asiri, F.Y.I.; Zafar, M.S. Surface modification techniques of dental implants. Dent. Implant. 2020, 49–68. [Google Scholar] [CrossRef]
- Elias, C.N. Factors Affecting the Success of Dental Implants Internet. Available online: https://www.intechopen.com/chapters/18426 (accessed on 1 December 2021).
- Wheelis, S.E.; Gindri, I.M.; Valderrama, P.; Wilson, T.G., Jr.; Huang, J.; Rodrigues, D.C. Effects of decontamination solutions on the surface of titanium: Investigation of surface morphology, composition, and roughness. Clin. Oral Impl. Res. 2015, 27, 329–340. [Google Scholar]
- Bagno, A.; Di Bello, C. Surface treatments and roughness properties of Ti-based biomaterials. J. Mater. Sci. Mater. Med. 2004, 15, 939–945. [Google Scholar]
- Pegueroles, M.; Aparicio, C.; Bosio, M.; Engel, E.; Gil, F.J.; Planell, J.A.; Altankov, G. Spatial Organization of Osteoblast Fibronectin-Matrix on Titanium Surface—Effects of Roughness, Chemical Heterogeneity, and Surface Free Energy. Acta Biomater. 2010, 6, 291–301. [Google Scholar] [PubMed]
- Kasemo, B.; Gold, J. Implant Surfaces and Interface Processes. Adv. Dent. Res. 1999, 13, 8–20. [Google Scholar]
- Variola, F.; Lauria, A.; Nanci, A.; Rosei, F. Influence of Treatment Conditions on the Chemical Oxidative Activity of H2SO4/H2O2Mixtures for Modulating the Topography of Titanium. Adv. Eng. Mater. 2009, 11, B227–B234. [Google Scholar]
- Variola, F.; Francis-Zalzal, S.; Leduc, A.; Barbeau, J.; Nanci, A. Oxidative nanopatterning of titanium generates mesoporous surfaces with antimicrobial properties. Int. J. Nanomed. 2014, 9, 2319–2325. [Google Scholar]
- Brunette, D.M.; Chehroudi, B. The effects of the surface topography of micromachined titanium substrata on cell behavior in vitro and in vivo. J. Biomech. Eng. 1999, 121, 49–57. [Google Scholar] [PubMed]
- Yoshinari, M.; Matsuzaka, K.; Inoue, T.; Oda, Y.; Shimono, M. Effects of multigrooved surfaces on fibroblast behavior. Biomed. Mater. Res. 2003, 65, 359–368. [Google Scholar]
- Barfeie, A.; Wilson, J.; Rees, J. Implant surface characteristics and their effect on osseointegration. Br. Dent. J. 2015, 218, E9. [Google Scholar] [CrossRef]
- Liu, Y.; Rath, B.; Tingart, M.; Eschweiler, J. Role of implants surface modification on osseointegration: A systematic review. J. Biomed. Mater. Res. Part A 2019, 108, 470–484. [Google Scholar] [CrossRef] [Green Version]
- Sykaras, N.; Iacopino, A.M.; Marker, V.A.; Triplett, R.G.; Woody, R.D. Implant materials, designs, and surface topographies: Their effect on osseointegration. A literature review. Int. J. Oral Maxillofac. Implant. 2000, 15, 675–690. [Google Scholar]
- Gupta, A.; Dhanraj, M.; Sivagami, G. Implant surface modification: Review of literature. Internet J. Dent. Sci. 2008, 7, 7. [Google Scholar]
- Gupta, A.; Dhanraj, M.; Sivagami, G. Status of surface treatment in endosseous implant: A literary overview. Indian J. Dent. Res. 2010, 21, 433–438. [Google Scholar]
- Hanawa, T. Surface treatment and modification of metals to add biofunction. Dent. Mater. J. 2017, 36, 533–538. [Google Scholar] [CrossRef]
- Padrós, R.; Giner-Tarrida, L.; Herrero-Climent, M.; Punset, M.; Gil, F.J. Corrosion Resistance and Ion Release of Dental Prosthesis of CoCr Obtained by CAD-CAM Milling, Casting and Laser Sintering. Metals 2020, 10, 827. [Google Scholar] [CrossRef]
- Denizoğlu, S.; Duymuş, Z.Y.; Akyalçin, Ş. Evaluation of Ion Release from Two Base-Metal Alloys at Various pH Levels. J. Int. Med. Res. 2004, 32, 33–38. [Google Scholar]
- Benatti, O.F.M.; Miranda, W.G.; Muench, A. In vitro and in vivo corrosion evaluation of nickel-chromium and copper-aluminum-based alloys. J. Prosthet. Dent. 2000, 84, 360–363. [Google Scholar]
- Gil, F.J.; Rodríguez, D.; Planell, J.A.; Cortada, M.; Giner, L.; Costa, S. Galvanic corrosion behaviour of Titanium implants coupled to dental alloys. J. Mater. Sci. Mater. Med. 2000, 11, 287–293. [Google Scholar]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; Fitzgerald, M.G.; Fulton, R.S.; et al. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar]
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaumann, S.; Staufenbiel, I.; Scherer, R.; Schilhabel, M.; Winkel, A.; Stumpp, S.; Eberhard, J.; Stiesch, M. Pyrosequencing of supra- and subgingival biofilms from inflamed peri-implant and periodontal sites. BMC Oral Health 2014, 14, 157. [Google Scholar] [CrossRef] [Green Version]
- Lindhe, J.; Meyle, J. Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35, 282–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zitzmann, N.U.; Berglundh, T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008, 35, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.A. Peri-implant diseases: Diagnosis and risk indicators. J. Clin. Periodontol. 2008, 35, 292–304. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant Dent. Relat. Res. 2019, 21 (Suppl. 1), 4–7. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira-Neto, O.B.; Lemos, C.A.A.; Barbosa, F.T.; De Sousa-Rodrigues, C.F.; Camello De Lima, F.J. Immediate dental implants placed into infected sites present a higher risk of failure than immediate dental implants placed into non-infected sites: Systematic review and meta-analysis. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e518–e528. [Google Scholar] [CrossRef] [PubMed]
- French, D.; Grandin, H.M.; Ofec, R. Retrospective cohort study of 4591 dental implants: Analysis of risk indicators for bone loss and prevalence of peri-implant mucositis and peri-implantitis. J. Periodontol. 2019, 90, 691–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; van der Mei, H.C.; Ren, Y.; Busscher, H.J. Keratinocytes protect soft-tissue integration of dental implant materials against bacterial challenges in a 3D-tissue infection model. Acta Biomater. 2019, 96, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, D.; von Holy, A. Bacterial biofilms within the clinical setting: What healthcare professionals should know. J. Hosp. Infect. 2006, 64, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Donelli, G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol. Med. Microbiol. 2010, 59, 227–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buxadera-Palomero, J.; Godoy-Gallardo, M.; Molmeneu, M.; Punset, M.; Gil, F.J. Antibacterial Properties of Triethoxysilylpropyl Succinic Anhydride Silane (TESPSA) on Titanium Dental Implants. Polymers 2020, 12, 773. [Google Scholar] [CrossRef] [Green Version]
- Sabri, N.A.; Schmitt, H.; Van der Zaan, B.; Gerritsen, H.W.; Zuidema, T.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Prevalence of antibiotics and antibiotic resistance genes in a wastewater effluent-receiving river in the Netherlands. J. Environ. Chem. Eng. 2020, 8, 102245. [Google Scholar] [CrossRef]
- Gao, P.; Munir, M.; Xagoraraki, I. Correlation of tetracycline and sulfonamide antibiotics with corresponding resistance genes and resistant bacteria in a conventional municipal wastewater treatment plant. Sci. Total Environ. 2012, 421–422, 173–183. [Google Scholar] [CrossRef]
- O’Neill, J. Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist. 2016, 3, 1–16. [Google Scholar]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Doundoulakis, J.H. Surface analysis of titanium after sterilization: Role in implant-tissue interface and bioadhesion. J. Prosthet. Dent. 1987, 58, 471–478. [Google Scholar]
- Colnot, C.; Romero, D.; Huang, S.; Rahman, J.; Currey, J.; Nanci, A.; Brunski, J.; Helms, J. Molecular analysis of healing at a bone-implant interface. J. Dent. Res. 2007, 86, 862–867. [Google Scholar] [PubMed] [Green Version]
- Albrektsson, T.; Dahlin, C.; Jemt, T.; Sennerby, L.; Turri, A.; Wennerberg, A. Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clin. Implant Dent. Relat. Res. 2014, 16, 155–165. [Google Scholar] [PubMed]
- Att, W.; Hori, N.; Iwasa, F.; Yamada, M.; Ueno, T.; Ogawa, T. The effect of UV-photofunctionalization on the time-related bioactivity of titanium and chromium-cobalt alloys. Biomaterials 2009, 30, 4268–4276. [Google Scholar]
- Morra, M.; Cassinelli, C.; Bruzzone, G.; Carpi, A.; Santi, G.D.; Giardino, R.; Fini, M. Surface chemistry effects of topographic modification of titanium dental implant surfaces: 1. Surface analysis. Int. J. Oral Maxillofac Implant. 2003, 18, 40–45. [Google Scholar]
- Clean Implant Foundation CIF GmbH, Berlin, Germany. Available online: http://www.cleanimplant.com (accessed on 20 July 2017).
- Souza, J.G.; Cordeiro, J.M.; Lima, C.V.; Barão, V.A. Citric acid reduces oral biofilm and influences the electrochemical behavior of Titanium: An in situ and in vitro study. J. Periodontol. 2018, 90, 149–158. [Google Scholar] [PubMed]
- Bollenl, C.M.L.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar]
- Castner, D.G.; Ratner, B.D. Biomedical surface science: Foundations to frontiers. Surf. Sci. 2002, 500, 28–60. [Google Scholar]
- Htet, M.; Madi, M.; Zakaria, O.; Miyahara, T.; Xin, W.; Lin, Z.; Kasugai, S. Decontamination of anodized implant surface with different modalities for peri-implantitis treatment: Lasers and mechanical debridement with citric acid. J. Periodontol. 2016, 87, 953–961. [Google Scholar]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91B, 470–480. [Google Scholar]
- Subramani, K.; Jung, R.E.; Molenberg, A. Hammerle CH Biofilm on dental implants: A review of the literature. Int. J. Oral Maxillofac Implant. 2009, 24, 616–626. [Google Scholar]
- Duncan, W.J.; Lee, M.H.; Bae, T.S.; Lee, S.J.; Gay, J.; Loch, C. Anodisation increases integration of unloaded titanium implants in sheep mandible. BioMed Res. Int. 2015, 15, 857969. [Google Scholar]
- Aparicio, C.; Rodriguez, D.; Gil, F.J. Variation of roughness and adhesion strength of deposited apatite layers on titanium dental implants. Mater. Sci. Eng. C 2011, 31, 320–324. [Google Scholar]
- Violant, D.; Galofré, M.; Nart, J.; Teles, R.P. In vitro evaluation of a multispecies oral biofilm on different implant surfaces. Biomed. Mater. 2014, 9, 035007. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1961, 13, 1741–1747. [Google Scholar]
- Liu, Y.; Zhao, Q. Influence of surface energy of modified surfaces on bacterial adhesion, Department of Mechanical Engineering, University of Dundee, Dundee, DD1 4HN, UK. Biophys. Chem. 2005, 117, 39–46. [Google Scholar]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Fernández-Calderón, M.C.; Pérez-Giraldo, C.; Manero, J.M.; Albericio, F.; Gil, F.J.; Rodríguez, D. Covalent immobilization of hLf1-11 peptide on a titanium surface reduces bacterial adhesion and biofilm formation. Acta Biomater. 2014, 10, 3522–3534. [Google Scholar] [PubMed]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Yu, K.; Manero, J.M.; Gil, F.J.; Kizhakkedathu, J.N.; Rodriguez, D. Antibacterial properties of hLf1–11 peptide onto titanium surfaces: A comparison study between silanization and surface initiated polymerization. Biomacromolecules 2014, 16, 483–496. [Google Scholar]
- ASTM-E3-11; Standard Guide for Preparation of Metallographic Specimens; ASTM International: West Conshohocken, PA, USA, 2017.
- Technical Report No. ASTM G5-14e1; Standard reference test method for making potentiostatic and potentiodynamic anodic polarization measurements; ASTM International: West Conshohocken, PA, USA, 2014.
- ISO 10993-5:2009. Biological evaluation of medical devices. In Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneve, Switzerland, 2009. [Google Scholar]
- ASTM G-102-89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements; ASTM International: West Conshohocken, PA, USA, 2010.
- Rodrigues, D.; Valderrama, P.; Wilson, T.; Palmer, K.; Thomas, A.; Sridhar, S.; Sadhwani, C. Titanium Corrosion Mechanisms in the Oral Environment: A Retrieval Study. Materials 2013, 6, 5258–5274. [Google Scholar] [PubMed] [Green Version]
- Godoy-Gallardo, M.; Manzanares-Céspedes, M.C.; Sevilla, P.; Nart, J.; Manzanares, N.; Manero, J.M.; Gil, F.J.; Boyd, S.K.; Rodríguez, D. Evaluation of bone loss in antibacterial coated dental implants: An experimental study in dogs. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 538–545. [Google Scholar]
- Gil, F.J.; Rodriguez, A.; Espinar, E.; Llamas, J.M.; Padulles, E.; Juarez, A. Effect of the oral bacteria on the mechanical behavior of titanium dental implants. Int. J. Oral Maxillofac Impl. 2012, 27, 64–68. [Google Scholar]
- Mombelli, A.; van Oosten, M.A.; Schurch, E.; Land, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar]
- Godoy-Gallardo, M.; Wang, Z.; Shen, Y.; Manero, J.M.; Gil, F.J.; Rodriguez, D.; Haapasalo, M. Antibacterial coatings on titanium surfaces: A comparison study between in vitro single-species and multispecies biofilm. ACS Appl. Mater. Interfaces 2015, 7, 5992–6001. [Google Scholar]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar]
- Heitz-Mayfield, L.J.; Lang, N.P. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol 2000 2010, 53, 167–181. [Google Scholar]
- Hoyos, M.; Velasco, F.; Ginebra, M.P.; Manero, J.M.; Gil, F.J.; Mas-Moruno, C. Regenerating bone via multifunctional coatings: The blending of cell integration and bacterial inhibition properties on the Surface of biomaterials. ACS Appl. Mater. Interfaces 2019, 11, 36449–36457. [Google Scholar]
- Pegueroles, M.; Tonda-Turo, C.; Planell, J.A.; Gil, F.J.; Aparicio, C. Adsorption of fibronectin, fibrinogen and albumin on TiO2: A kinetics, structural changes, and competition study. J. R. Soc. Interface Biointerfaces 2012, 7, 13. [Google Scholar]
- Godoy-Gallardo, M.; Guillem-Marti, J.; Sevilla, P.; Manero, J.M.; Gil, F.J.; Rodriguez, D. Anhydride-functional silane immobilized onto titanium surfaces induces osteoblast cell differentiation and reduces bacterial adhesion and biofilm formation. Biointerphases 2016, 59, 524–532. [Google Scholar]
- Grivet, M.; Morrier, J.J.; Benay, G.; Barsotti, O. Effect of hydrophobicity on in vitro streptococcal adhesion to dental alloys. J. Mater. Sci. Mater. Med. 2000, 11, 637–642. [Google Scholar]
- Lukina, E.; Laka, A.; Kollerov, M.; Sampiev, M.; Mason, P.; Wagstaff, P.; Noordeen, H.; Weng Yoon, W.; Blunn, G. Metal concentrations in the blood and tissues after implantation of titanium growth guidance sliding instrumentation. Spine J. 2016, 16, 381–388. [Google Scholar]
- Sarmiento-González, A.; Encinar, J.R.; Marchante-Gayón, J.M. Titanium levels in the organs and blood of rats with a titanium implant, in the absence of wear, as determined by double-focusing ICP-MS. Anal. Bioanal. Chem. 2009, 393, 335. [Google Scholar] [CrossRef]
- Zabielska, J.; Kunicka-Styczynska, A.; Otlewska, A. Adhesive and hydrophobic properties of Pseudomonas aeruginosa and Pseudomonas cedrina associated with cosmetics. Institute of fermentation Technology and Microbiology, Faculty of Biotechnology and food science, Lodz University of Technology, Wolczanska, Lodz, Poland. Ecol. Quest. 2017, 28, 41–46. [Google Scholar]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar]
- Punset, M.; Villarrasa, J.; Nart, J.; Manero, J.M.; Bosch, B.; Padrós, R.; Perez, R.A.; Gil, F.J. Citric Acid Passivation of Titanium Dental Implants for Minimizing Bacterial Colonization Impact. Coatings 2021, 11, 214. [Google Scholar] [CrossRef]
| Chemical Product | Composition (mM) |
|---|---|
| K2HPO4 | 0.44 |
| KCl | 5.4 |
| CaCl2 | 1.3 |
| Na2HPO4 | 0.25 |
| NaCl | 137 |
| NaHCO3 | 4.2 |
| MgSO4 | 1.0 |
| C6H12O6 | 5.5 |
| Parameter | Control | HCl | C20/10’ | C20/20’ | C20%/30’ | C40%/10’ |
|---|---|---|---|---|---|---|
| Ra | 0.12 ± 0.01 | 0.15 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.16 ± 0.03 | 0.12 ± 0.02 |
| Rz | 4.90 ± 0.30 | 4.33±0.53 | 3.43 ± 0.31 | 3.91 ± 0.31 | 4.78 ± 0.88 | 2.43 ± 0.31 |
| Sample | CA (o) | SFE (mJ/m2) | |||
|---|---|---|---|---|---|
| WA | DIIO | ϒ | ϒD | ϒP | |
| Control | 102.77± 7.00 | 48.40 ± 2.32 | 35.28 ± 1.35 | 35.15 ± 1.28 | 0.12 ± 0.12 |
| HCl | 86.38 ± 4.12 | 53.34 ± 0.92 | 35.70 ± 1.60 | 32.39 ± 0.52 | 3.31 ± 1.28 |
| C20%/10′ | 84.06 ± 3.26 | 50.22 ± 1.34 | 37.46 ± 1.27 | 34.14 ± 0.75 | 3.31 ± 1.05 |
| C20%/20′ | 83.43 ± 1.89 | 49.88 ± 1.99 | 37.82 ± 1.20 | 34.26 ± 1.23 | 3.56 ± 0.61 |
| C20%/30′ | 73.26 ± 6.28 | 52.72 ± 2.99 | 41.77 ± 2.82 | 34.27 ± 1.69 | 9.03 ± 2.07 |
| C40%/10′ | 58.05 ± 7.67 | 47.02 ± 1.63 | 50.14 ± 3.87 | 35.91 ± 0.88 | 14.22 ± 4.29 |
| Parameter/Sample | Control | HCl | C20/10’ | C20/20’ | C20%/30’ | C40%/10’ |
|---|---|---|---|---|---|---|
| EOCP (mV) | −196 ± 1 | −195 ± 11 | −223 ± 0 | −165 ± 0 | −141 ± 22 | −210 ± 13 |
| Sample/Parameter | Ecorr (mV) | Icorr (µA/cm2) | Rp (MΩ/cm2) | Vc (µm/year) |
|---|---|---|---|---|
| Control | −196 ± 14 | 0.027 ± 0.008 | 2.428 ± 0.390 | 0.233 ± 0.066 |
| HCl | −536 ± 39 | 0.020 ± 0.005 | 2.479 ± 0.083 | 0.176 ± 0.048 |
| C20/10’ | −401 ± 42 | 0.031 ± 0.005 | 1.866 ± 0.010 | 0.268 ± 0.043 |
| C20/20’ | −471 ± 81 | 0.025 ± 0.001 | 2.797 ± 0.306 | 0.223 ± 0.001 |
| C20%/30’ | −470 ± 24 | 0.018 ± 0.002 | 3.566 ± 0.699 | 0.159 ± 0.020 |
| C40%/10’ | −429 ± 21 | 0.024 ± 0.008 | 2.845 ± 0.770 | 0.214 ± 0.071 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdeguer, P.; Gil, J.; Punset, M.; Manero, J.M.; Nart, J.; Vilarrasa, J.; Ruperez, E. Citric Acid in the Passivation of Titanium Dental Implants: Corrosion Resistance and Bactericide Behavior. Materials 2022, 15, 545. https://doi.org/10.3390/ma15020545
Verdeguer P, Gil J, Punset M, Manero JM, Nart J, Vilarrasa J, Ruperez E. Citric Acid in the Passivation of Titanium Dental Implants: Corrosion Resistance and Bactericide Behavior. Materials. 2022; 15(2):545. https://doi.org/10.3390/ma15020545
Chicago/Turabian StyleVerdeguer, Pablo, Javier Gil, Miquel Punset, José María Manero, José Nart, Javi Vilarrasa, and Elisa Ruperez. 2022. "Citric Acid in the Passivation of Titanium Dental Implants: Corrosion Resistance and Bactericide Behavior" Materials 15, no. 2: 545. https://doi.org/10.3390/ma15020545
APA StyleVerdeguer, P., Gil, J., Punset, M., Manero, J. M., Nart, J., Vilarrasa, J., & Ruperez, E. (2022). Citric Acid in the Passivation of Titanium Dental Implants: Corrosion Resistance and Bactericide Behavior. Materials, 15(2), 545. https://doi.org/10.3390/ma15020545








