Comparison of CO2 Separation Efficiency from Flue Gases Based on Commonly Used Methods and Materials
Abstract
:1. Introduction
1.1. Absorption
1.2. Adsorption
1.3. Membrane Separation
2. Experimental Results and Discussion
2.1. Absorption in a Packed Column
2.2. Adsorption in a Packed Column
2.3. Membrane Separation
2.4. Comparison of Process Parameters for the Investigated Methods
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Nomenclature
| barrer | non-SI unit of gas permeability; 1 barrer = 3.35 × 10−16 (mol m s−1 Pa−1 m−2) |
| D | membrane diffusion coefficient, m2 s−1 |
| l | membrane thickness, m |
| N | molar flux, kmol m−2 s−1 |
| P | permeability, kmol m−1 s−1 Pa−1 |
| p | pressure difference, Pa |
| s | solubility, kmol m−3 Pa−1 |
| S | sorption capacity, kg CO2 kg−1 sorbbent |
| Vg | gas flow rate, L/h |
| α | ideal selectivity |
| ρ | density, kg m−3 |
| Subscripts | |
| CO2 | carbon dioxide |
| i | CO2, N2 |
| in | inlet |
| N2 | nitrogen |
| out | outlet |
References
- Cozier, M. Recent developments in carbon capture utilisation and storage. Greenh. Gases Sci. Technol. 2019, 9, 613–616. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- European Commision. 2020 Climate & Energy Package. 2019. Available online: https://ec.europa.eu/clima/policies/strategies/2020_en (accessed on 1 November 2021).
- European Commision. The EU Emissions Trading System (EU ETS). 2019. Available online: https://ec.europa.eu/clima/sites/clima/files/factsheet_ets_en.pdf (accessed on 1 November 2021).
- Ramstein, C.; Dominioni, G.; Ettehad, S.; Lam, L.; Quant, M.; Zhang, J.; Mark, L.; Nierop, S.; Berg, T.; Leuschner, P.; et al. State and Trends of Carbon Pricing 2019; World Bank Group: Washington, DC, USA, 2019. [Google Scholar]
- Fouret, F. What Are the Effects of the Price Increase for the CO2 Allowances on the EU ETS Market? Beyond Ratings. 2019. Available online: https://beyond-ratings.com/publications/what-effects-priceincrease-co2-allowances-eu-ets-market (accessed on 1 November 2021).
- Yoro, K.O.; Daramola, M.O. Chapter 1—CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Rahimpour, M.R., Farsi, M., Makarem, M.A., Eds.; Woodhead Publishing: Shaxton, UK, 2020; pp. 3–28. [Google Scholar]
- Mac Dowell, N.; Fennell, P.S.; Shah, N.; Maitland, G.C. The role of CO2 capture and utilization in mitigating climate change. Nat. Clim. Change 2017, 7, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Atlaskin, A.A.; Kryuchkov, S.S.; Yanbikov, N.R.; Smorodin, K.A.; Petukhov, A.N.; Trubyanov, M.M.; Vorotyntsev, V.M.; Vorotyntsev, I.V. Comprehensive experimental study of acid gases removal process by membrane-assisted gas absorption using imidazolium ionic liquids solutions absorbent. Sep. Purif. Technol. 2020, 239, 116578. [Google Scholar] [CrossRef]
- Davison, J. Performance and costs of power plants with capture and storage of CO2. Energy 2007, 32, 1163–1176. [Google Scholar] [CrossRef]
- Gulzar, A.; Gulzar, A.; Ansari, M.B.; He, F.; Gai, S.; Yang, P. Carbon dioxide utilization: A paradigm shift with CO2 economy. Chem. Eng. J. Adv. 2020, 3, 100013. [Google Scholar] [CrossRef]
- Kanniche, M.; Gros-Bonnivard, R.; Jaud, P.; Valle-Marcos, J.; Amann, J.M.; Bouallou, C. Pre-combustion, post-combustion and oxy-combustion in thermal power plant for CO2 capture. Appl. Therm. Eng. 2010, 30, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Klingberg, P.; Wilkner, K.; Schlüter, M.; Grünauer, J.; Shishatskiy, S. Separation of Carbon Dioxide from Real Power Plant Flue Gases by Gas Permeation Using a Supported Ionic Liquid Membrane: An Investigation of Membrane Stability. Membranes 2019, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Sasikumar, B.; Arthanareeswaran, G.; Ismail, A. Recent progress in ionic liquid membranes for gas separation. J. Mol. Liq. 2018, 266, 330–341. [Google Scholar] [CrossRef]
- Martin-Gil, V.; Ahmad, M.Z.; Castro-Muñoz, R.; Fila, V. Economic Framework of Membrane Technologies for Natural Gas Applications. Sep. Purif. Rev. 2018, 48, 298–324. [Google Scholar] [CrossRef]
- Fatima, S.S.; Borhan, A.; Ayoub, M.; Ghani, N.A. Development and progress of functionalized silica-based adsorbents for CO2 capture. J. Mol. Liq. 2021, 338, 116913. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Coleman, C. A policy analysis of the driving factors behind carbon capture and storage facilities. LSU J. Energy L. Resour. 2018, 6, 557. [Google Scholar]
- Budzianowski, W. Explorative analysis of advanced solvent processes for energy efficient carbon dioxide capture by gas–liquid absorption. Int. J. Greenh. Gas Control 2016, 49, 108–120. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, Y.; Yuan, Y.; Gao, J.; Wang, S.; Zhuo, Y.; Chen, C. Study on corrosion in CO2 chemical absorption process using amine solution. Energy Procedia 2011, 4, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhang, X.; Zhang, X.; Dong, H.; Zhang, S. Thermodynamic Modeling and Assessment of Ionic Liquid-Based CO2 Capture Processes. Ind. Eng. Chem. Res. 2014, 53, 11805–11817. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Joel, A.S.; Ramshaw, C.; Eimer, D.; Musa, N.M. Process intensification for post-combustion CO2 capture with chemical absorption: A critical review. Appl. Energy 2015, 158, 275–291. [Google Scholar] [CrossRef]
- Ramdin, M.; de Loos, T.W.; Vlugt, T.J. State-of-the-Art of CO2 Capture with Ionic Liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Lucquiaud, M.; Liang, X.; Errey, O.; Chalmers, H.; Gibbins, J. Addressing Technology Uncertainties in Power Plants with Post-Combustion Capture. Energy Procedia 2013, 37, 2359–2368. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.; Brodal, E. Optimization of the Energy Consumption of a Carbon Capture and Sequestration Related Carbon Dioxide Compression Processes. Energies 2019, 12, 1603. [Google Scholar] [CrossRef] [Green Version]
- Luis, P. Use of monoethanolamine (MEA) for CO2 capture in a global scenario: Consequences and alternatives. Desalination 2016, 380, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Cousins, A.; Yu, H.; Feron, P.; Tade, M.; Luo, W.; Chen, J. Systematic study of aqueous monoethanolamine-based CO2 capture process: Model development and process improvement. Energy Sci. Eng. 2016, 4, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wang, S.; Niu, H.; Gao, S. Process and Integration Optimization of Post-Combustion CO2 Capture System in a Coal Power Plant. Energy Procedia 2018, 154, 86–93. [Google Scholar] [CrossRef]
- De Guido, G.; Compagnoni, M.; Pellegrini, L.A.; Rossetti, I. Mature versus emerging technologies for CO2 capture in power plants: Key open issues in post-combustion amine scrubbing and in chemical looping combustion. Front. Chem. Sci. Eng. 2018, 12, 315–325. [Google Scholar] [CrossRef]
- Budinis, S.; Krevor, S.; Mac Dowell, N.; Brandon, N.; Hawkes, A. An assessment of CCS costs, barriers and potential. Energy Strat. Rev. 2018, 22, 61–81. [Google Scholar] [CrossRef]
- Nakao, S.I.; Yogo, K.; Goto, K.; Kai, T.; Yamada, H. Advanced CO2 Capture Technologies: Absorption, Adsorption, and Membrane Separation Methods; Springer: New York, NY, USA, 2019. [Google Scholar]
- Sarmad, S.; Mikkola, J.P.; Ji, X. CO2 Capture with Ionic Liquids (ILs) and Deep Eutectic Solvents (DESs): A New Generation of Sorbents. ChemSusChem 2017, 10, 324–352. [Google Scholar] [CrossRef]
- Haghbakhsh, R.; Raeissi, S.; Parvaneh, K.; Shariati, A. The friction theory for modeling the viscosities of deep eutectic solvents using the CPA and PC-SAFT equations of state. J. Mol. Liq. 2018, 249, 554–561. [Google Scholar] [CrossRef]
- Haghbakhsh, R.; Parvaneh, K.; Raeissi, S.; Shariati, A. A general viscosity model for deep eutectic solvents: The free volume theory coupled with association equations of state. Fluid Phase Equilibria 2018, 470, 193–202. [Google Scholar] [CrossRef]
- Blatha, J.; Deublerb, N.; Hirtha, T.; Schiestelb, T. Chemisorption of carbon dioxide in imidazolium based ionic liquids with carboxylic anions. Chem. Eng. J. 2012, 181–182, 152–158. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Shukla, S.K.; Khokarale, S.G.; Bui, T.Q.; Mikkola, J.-P.T. Ionic Liquids: Potential Materials for Carbon Dioxide Capture and Utilization. Front. Mater. 2019, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Sarmad, S.; Mikkola, J.-P.; Ji, X. Development of Low-Cost Deep Eutectic Solvents for CO2 Capture. Energy Procedia 2017, 142, 3320–3325. [Google Scholar] [CrossRef]
- Florindo, C.; Lima, F.; Ribeiro, B.D.; Marrucho, I.M. Deep eutectic solvents: Overcoming 21st century challenges. Curr. Opin. Green Sustain. Chem. 2019, 18, 31–36. [Google Scholar] [CrossRef]
- Alkhatib, I.I.I.; Ferreira, M.L.; Alba, C.G.; Bahamon, D.; Llovell, F.; Pereiro, A.B.; Araújo, J.M.M.; Abu-Zahra, M.R.; Vega, L.F. Screening of Ionic Liquids and Deep Eutectic Solvents for Physical CO2 Absorption by Soft-SAFT Using Key Performance Indicators. J. Chem. Eng. Data 2020, 65, 5844–5861. [Google Scholar] [CrossRef]
- Strazisar, B.R.; Anderson, A.R.R.; White, C.M. Degradation Pathways for Monoethanolamine in a CO2 Capture Facility. Energy Fuels 2003, 17, 1034–1039. [Google Scholar] [CrossRef]
- Dubois, L.; Thomas, D. Screening of Aqueous Amine-Based Solvents for Postcombustion CO2 Capture by Chemical Absorption. Chem. Eng. Technol. 2012, 35, 513–524. [Google Scholar] [CrossRef]
- Bara, J.E. Ionic liquids for post combustion CO2 capture. In Absorption Based Post-Combustion Capture of Carbon Dioxide; Feron, P.H.M., Ed.; Woodhed Publishing: Duxford, UK, 2016. [Google Scholar]
- Yokozeki, A.; Shiflett, M.B.; Junk, C.P.; Grieco, L.M.; Foo, T. Physical and Chemical Absorptions of Carbon Dioxide in Room-Temperature Ionic Liquids. J. Phys. Chem. B 2008, 112, 16654–16663. [Google Scholar] [CrossRef] [PubMed]
- Gurau, G.; Rodríguez, H.; Kelley, S.P.; Janiczek, P.; Kalb, R.S.; Rogers, R.D. Demonstration of Chemisorption of Carbon Dioxide in 1,3-Dialkylimidazolium Acetate Ionic Liquids. Angew. Chem. Int. Ed. 2011, 50, 12024–12026. [Google Scholar] [CrossRef] [PubMed]
- Janiczek, P.; Kalb, R.S.; Thonhauser, G.; Gamse, T. Carbon dioxide absorption in a technical-scale-plant utilizing an imidazoliumbased ionic liquid. Sep. Purif. Technol. 2012, 97, 20–25. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Yokozeki, A. Phase behavior of carbon dioxide in ionic liquids: [Emim][Acetate], [Emim][Trifluoroacetate], and [Emim][Acetate] [Emim][Tri- fluoroacetate] mixtures. J. Chem. Eng. Data 2009, 54, 108–114. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Niehaus, A.M.S.; Elliott, B.A.; Yokozeki, A. Phase Behavior of N2O and CO2 in Room-Temperature Ionic Liquids [bmim][Tf2N], [bmim][BF4], [bmim][N(CN)2], [bmim][Ac], [eam][NO3], and [bmim][SCN]. Int. J. Thermophys. 2012, 33, 412–436. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Dong, K.; Zhang, Y.; Shen, Y.; Lv, X. Supported Absorption of CO2 by Tetrabutylphosphonium Amino Acid Ionic Liquids. Chem.-A Eur. J. 2006, 12, 4021–4026. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room Temperature Ionic Liquids from 20 Natural Amino Acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Lu, X.; Zhou, Q.; Fan, W.; Zhang, X. Dual Amino-Functionalised Phosphonium Ionic Liquids for CO2 Capture. Chem.-A Eur. J. 2009, 15, 3003–3011. [Google Scholar] [CrossRef] [PubMed]
- Shiflett, M.B.; Drew, D.W.; Cantini, R.A.; Yokozeki, A. Carbon Dioxide Capture Using Ionic Liquid 1-Butyl-3-methylimidazolium Acetate. Energy Fuels 2010, 24, 5781–5789. [Google Scholar] [CrossRef]
- Khonkaen, K.; Siemanond, K.; Henni, A. Simulation of Carbon Dioxide Capture Using Ionic Liquid 1-Ethyl-3-methylimidazolium Acetate. In Computer Aided Chemical Engineering, Proceedings of the 24th European Symposium on Computer Aided Process Engineering, Budapest, Hungary, 15–18 June 2014; Elsevier: Amsterdam, The Netherlands, 2014; Volume 33, pp. 1045–1050. [Google Scholar]
- Bhown, A.S.; Freeman, B.C. Analysis and Status of Post-Combustion Carbon Dioxide Capture Technologies. Environ. Sci. Technol. 2011, 45, 8624–8632. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, J.; Mumford, K.; Stevens, G.W.; Fei, W.; Wang, Y. Recent advances in carbon dioxide capture and utilization with amines and ionic liquids. Green Chem. Eng. 2020, 1, 16–32. [Google Scholar] [CrossRef]
- Yoro, K.O.; Daramola, M.O.; Sekoai, P.T.; Armah, E.K.; Wilson, U.N. Advances and emerging techniques for energy recovery during absorptive CO2 capture: A review of process and non-process integration-based strategies. Renew. Sustain. Energy Rev. 2021, 147, 111241. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Pevida, C.; Plaza, M.G.; Arias, B.; Fermoso, J.; Rubiera, F.; Pis, J.J. Surface modification of activated carbons for CO2 capture. Appl. Surf. Sci. 2008, 254, 7165–7172. [Google Scholar] [CrossRef] [Green Version]
- El Gamal, M.; Mousa, H.A.; El-Naas, M.H.; Zacharia, R.; Judd, S. Bio-regeneration of activated carbon: A comprehensive review. Sep. Purif. Technol. 2018, 197, 345–359. [Google Scholar] [CrossRef]
- Rehman, A.; Park, M.; Park, S.-J. Current Progress on the Surface Chemical Modification of Carbonaceous Materials. Coatings 2019, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Zhu, J.; Wang, H.; Zhou, M.; Zhang, S. Surface Functionalization of Activated Carbon with Phosphonium Ionic Liquid for CO2 Adsorption. Coatings 2019, 9, 590. [Google Scholar] [CrossRef] [Green Version]
- Ramli, A.; Ahmed, S.; Yusup, S. Adsorption behavior of Si-MCM-41 for CO2: Effect of pressure and temperature on adsorption. Chem. Eng. Trans. 2014, 39, 271–276. [Google Scholar]
- Satyapal, S.; Filburn, T.; Trela, J.; Strange, J. Performance and Properties of a Solid Amine Sorbent for Carbon Dioxide Removal in Space Life Support Applications. Energy Fuels 2001, 15, 250–255. [Google Scholar] [CrossRef]
- Hiremath, V.; Jadhav, A.H.; Lee, H.; Kwon, S.; Gil Seo, J. Highly reversible CO2 capture using amino acid functionalized ionic liquids immobilized on mesoporous silica. Chem. Eng. J. 2016, 287, 602–617. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, E.; Li, Y.; Bernards, M.; He, Y.; Shi, Y. CO2 capture with polyamine-based protic ionic liquid functionalized mesoporous silica. J. CO2 Util. 2019, 34, 606–615. [Google Scholar] [CrossRef]
- Girish, C.R. Various Impregnation Methods Used for the Surface Modification of the Adsorbent: A Review. Int. J. Eng. Technol. 2018, 7, 330–334. [Google Scholar] [CrossRef] [Green Version]
- Cecilia, J.; Vilarrasa-García, E.; García-Sancho, C.; Saboya, R.; Azevedo, D.; Cavalcante, C.; Rodríguez-Castellón, E. Functionalization of hollow silica microspheres by impregnation or grafted of amine groups for the CO2 capture. Int. J. Greenh. Gas Control. 2016, 52, 344–356. [Google Scholar] [CrossRef]
- Shankar, K.; Kandasamy, P. Carbon dioxide separation using α -alumina ceramic tube supported cellulose triacetate-tributyl phosphate composite membrane. Greenh. Gases Sci. Technol. 2019, 9, 287–305. [Google Scholar] [CrossRef]
- Kárászová, M.; Zach, B.; Petrusová, Z.; Červenka, V.; Bobák, M.; Šyc, M.; Izák, P. Post-combustion carbon capture by membrane separation, Review. Sep. Purif. Technol. 2020, 238, 116448. [Google Scholar] [CrossRef]
- Yan, X.; Anguille, S.; Bendahan, M.; Moulin, P. Ionic liquids combined with membrane separation processes: A review. Sep. Purif. Technol. 2019, 222, 230–253. [Google Scholar] [CrossRef]
- Vakharia, V.; Salim, W.; Wu, D.; Han, Y.; Chen, Y.; Zhao, L.; Ho, W.W. Scale-up of amine-containing thin-film composite membranes for CO2 capture from flue gas. J. Membr. Sci. 2018, 555, 379–387. [Google Scholar] [CrossRef]
- AlQaheem, Y.; Alomair, A.; Vinoba, M.; Pérez, A. Polymeric Gas-Separation Membranes for Petroleum Refining. Int. J. Polym. Sci. 2017, 2017, 1–19. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Qiao, Z.; Wu, H.; Dong, S.; Zhao, S.; Wang, J. Post-combustion CO2 capture with membrane process: Practical membrane performance and appropriate pressure. J. Membr. Sci. 2019, 581, 195–213. [Google Scholar] [CrossRef]
- Roussanaly, S.; Anantharaman, R.; Lindqvist, K.; Hagen, B. A new approach to the identification of high-potential materials for cost-efficient membrane-based post-combustion CO2 capture. Sustain. Energy Fuels 2018, 2, 1225–1243. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Jia, H.; Yang, N.; Wang, Q.; Yang, G.; Zhang, M.; Xu, S.; Zang, Y.; Ma, L.; Jiang, P.; et al. High Efficiency Gas Permeability Membranes from Ethyl Cellulose Grafted with Ionic Liquids. Polymers 2019, 11, 1900. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, G.; Houde, A.A.; Stern, S.A. Poly(etherurethane) and poly(ether urethane urea) membranes with high H2S/CH4 selectivity. J. Membr. Sci. 1997, 135, 99–106. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Ismail, A.; Khulbe, K.; Matsuura, T. Gas Separation Membranes: Polymeric and Inorganic; Springer: Heidelberg, Germany, 2015. [Google Scholar]
- Amooghin, A.E.; Omidkhah, M.; Kargari, A. Enhanced CO2 transport properties of membranes by embedding nano-porous zeolite particles into Matrimid®5218 matrix. RSC Adv. 2015, 5, 8552–8565. [Google Scholar] [CrossRef]
- Liu, S.; Wang, R.; Liu, Y.; Chng, M.; Chung, N.T.-S. The physical and gas permeation properties of 6FDA-durene/2,6-diaminotoluene copolyimides. Polymers 2001, 42, 8847–8855. [Google Scholar] [CrossRef]
- Scholes, C.A.; Chen, G.Q.; Lu, H.T.; Kentish, S.E. Crosslinked PEG and PEBAX Membranes for Concurrent Permeation of Water and Carbon Dioxide. Membranes 2016, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amooghin, A.E.; Sanaeepur, H.; Pedram, M.Z.; Omidkhah, M.; Kargari, A. New advances in polymeric membranes for CO2 separation. In Polymer Science: Research Advances, Practical Applications and Educational Aspects; Vilas, A.M., Martin, A.S., Eds.; Formatex Research Center S.L.: Badajoz, Spain, 2016; pp. 354–368. ISBN 978-8494213489. [Google Scholar]
- Salih, A.A.; Yi, C.; Peng, H.; Yang, B.; Yin, L.; Wang, W. Interfacially polymerized polyetheramine thin film composite membranes with PDMS inter-layer for CO2 separation. J. Membr. Sci. 2014, 472, 110–118. [Google Scholar] [CrossRef]
- Pian, C.; Shen, J.; Liu, G.; Liu, Z.; Jin, W. Ceramic hollow fiber-supported PDMS composite membranes for oxygen enrichment from air. Asia-Pac. J. Chem. Eng. 2016, 11, 460–466. [Google Scholar] [CrossRef]
- Mark, J.E.; Allcock, H.R.; West, R. Inorganic Polymers, 2nd ed.; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Sadrzadeh, M.; Shahidi, K.; Mohammadi, T. Synthesis and gas permeation properties of a single layer PDMS membrane. J. Appl. Polym. Sci. 2010, 117, 33–48. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Fuoco, A. Porous liquids—Future for CO2 capture and separation? Curr. Res. Green Sustain. Chem. 2021, 4, 100070. [Google Scholar] [CrossRef]
- Camper, D.; Bara, J.E.; Gin, D.L.; Noble, R.D. Room-Temperature Ionic Liquid−Amine Solutions: Tunable Solvents for Efficient and Reversible Capture of CO2. Ind. Eng. Chem. Res. 2008, 47, 8496–8498. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Y.; Lu, X.; Ji, X. Energy consumption analysis for CO2 separation using imidazolium-based ionic liquids. Appl. Energy 2014, 136, 325–335. [Google Scholar] [CrossRef]
- Chakma, A. CO2 capture processes—Opportunities for improved energy efficiencies. Energy Convers. Manag. 1997, 38, S51–S56. [Google Scholar] [CrossRef]
- Cserjési, P.; Nemestothy, N.; Bélafi-Bakó, K. Gas separation properties of supported liquid membranes prepared with unconventional ionic liquids. J. Membr. Sci. 2010, 349, 6–11. [Google Scholar] [CrossRef]
- Santos, E.; Albo, J.; Irabien, A. Acetate based Supported Ionic Liquid Membranes (SILMs) for CO2 separation: Influence of the temperature. J. Membr. Sci. 2014, 452, 277–283. [Google Scholar] [CrossRef]
- Bara, J.; Carlisle, T.K.; Gabriel, C.J.; Camper, D.; Finotello, A.; Gin, D.; Noble, R.D. Guide to CO2 Separations in Imidazolium-Based Room-Temperature Ionic Liquids. Ind. Eng. Chem. Res. 2009, 48, 2739–2751. [Google Scholar] [CrossRef]
- Albo, J.; Yoshioka, T.; Tsuru, T. Porous Al2O3/TiO2 tubes in combination with 1-ethyl-3-methylimidazolium acetate ionic liquid for CO2/N2 separation. Sep. Purif. Technol. 2014, 122, 440–448. [Google Scholar] [CrossRef]
- Sánchez Fuentes, C.E.; Guzmán-Lucero, D.; Torres-Rodriguez, M.; Likhanova, N.V.; Navarrete Bolaños, J.; Olivares-Xometl, O.; Lijanova, I.V. CO2/N2 separation using alumina supported membranes based on new functionalized ionic liquids. Sep. Purif. Technol. 2017, 182, 59–68. [Google Scholar] [CrossRef]
- Khraisheh, M.; Zadeh, K.M.; Alkhouzaam, A.; Turki, D.; Hassan, M.K.; Al Momani, F.; Zaidi, S.M.J. Characterization of polysulfone/diisopropylamine 1-alkyl-3-methylimidazolium ionic liquid membranes: High pressure gas separation applications. Greenh. Gases Sci. Technol. 2020, 10, 795–808. [Google Scholar] [CrossRef]
- Karousos, D.S.; Labropoulos, A.I.; Tzialla, O.; Papadokostaki, K.; Gjoka, M.; Stefanopoulos, K.L.; Beltsios, K.G.; Iliev, B.; Schubert, T.J.S.; Romanos, G.E. Effect of a cyclic heating process on the CO2/N2 separation performance and structure of a ceramic nanoporous membrane supporting the ionic liquid 1-methyl-3-octylimidazolium tricyanomethanide. Sep. Purif. Technol. 2018, 200, 11–22. [Google Scholar] [CrossRef]
- Ziobrowski, Z.; Rotkegel, A. The influence of water content in imidazolium based ILs on carbon dioxide removal efficiency. Sep. Purif. Technol. 2017, 179, 412–419. [Google Scholar] [CrossRef]
- Ziobrowski, Z.; Krupiczka, R.; Rotkegel, A. Carbon dioxide absorption in a packed column using imidazolium based ionic liquids and MEA solution. Int. J. Greenh. Gas Control. 2016, 47, 8–16. [Google Scholar] [CrossRef]
- Ziobrowski, Z.; Rotkegel, A. Feasibility study of CO2/N2 separation intensification on supported ionic liquid membranes by commonly used impregnation methods. Greenh. Gases Sci. Technol. 2021, 11, 297–312. [Google Scholar] [CrossRef]
- Luis, P.; Neves, L.; Afonso, C.A.M.; Coelhoso, I.; Crespo, J.; Garea, A.; Irabien, A. Facilitated transport of CO2 and SO2 through Supported Ionic Liquid Membranes (SILMs). Desalination 2009, 245, 485–493. [Google Scholar] [CrossRef]
- Freire, M.G.; Teles, A.R.R.; Rocha, M.A.A.; Schröder, B.; Neves, C.M.S.S.; Carvalho, P.J.; Evtuguin, D.V.; Santos, L.M.N.B.F.; Coutinho, J.A.P. Thermophysical Characterization of Ionic Liquids Able To Dissolve Biomass. J. Chem. Eng. Data 2011, 56, 4813–4822. [Google Scholar] [CrossRef]
- Kreiter, R.; Overbeek, J.P.; Correia, L.A.; Vente, J.F. Pressure resistance of thin ionic liquid membranes using tailored ceramic supports. J. Membr. Sci. 2011, 370, 175–178. [Google Scholar] [CrossRef] [Green Version]
- Close, J.J.; Farmer, K.; Moganty, S.S.; Baltus, R.E. CO2/N2 separations using nanoporous alumina-supported ionic liquid membranes: Effect of the support on separation performance. J. Membr. Sci. 2012, 390–391, 201–210. [Google Scholar] [CrossRef]
- Barghi, S.; Adibi, M.; Rashtchian, D. An experimental study on permeability, diffusivity, and selectivity of CO2 and CH4 through [bmim][PF6] ionic liquid supported on an alumina membrane: Investigation of temperature fluctuations effects. J. Membr. Sci. 2010, 362, 346–352. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Wang, J.; Luo, J.; Feng, S.; Li, H.; Wan, Y.; Zhang, X. Recent development of ionic liquid membranes. Green Energy Environ. 2016, 1, 43–61. [Google Scholar] [CrossRef] [Green Version]
- Tomé, L.C.; Marrucho, I.M. Ionic liquid-based materials: A platform to design engineered CO2 separation membranes. Chem. Soc. Rev. 2016, 45, 2785–2824. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, R.; Afonso, C.A.M.; Reis, M.A.; Crespo, J.G. Supported liquid membranes using ionic liquids: Study of stability and transport mechanisms. J. Membr. Sci. 2004, 242, 197–209. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.J.; de los Rios, A.P.; Tomás-Alonso, F.; Palacios, J.M.; Villora, G. Preparation of supported ionic liquid membranes: Influence of the ionic liquid immobilization method on their operational stability. J. Membr. Sci. 2009, 341, 172–177. [Google Scholar] [CrossRef]
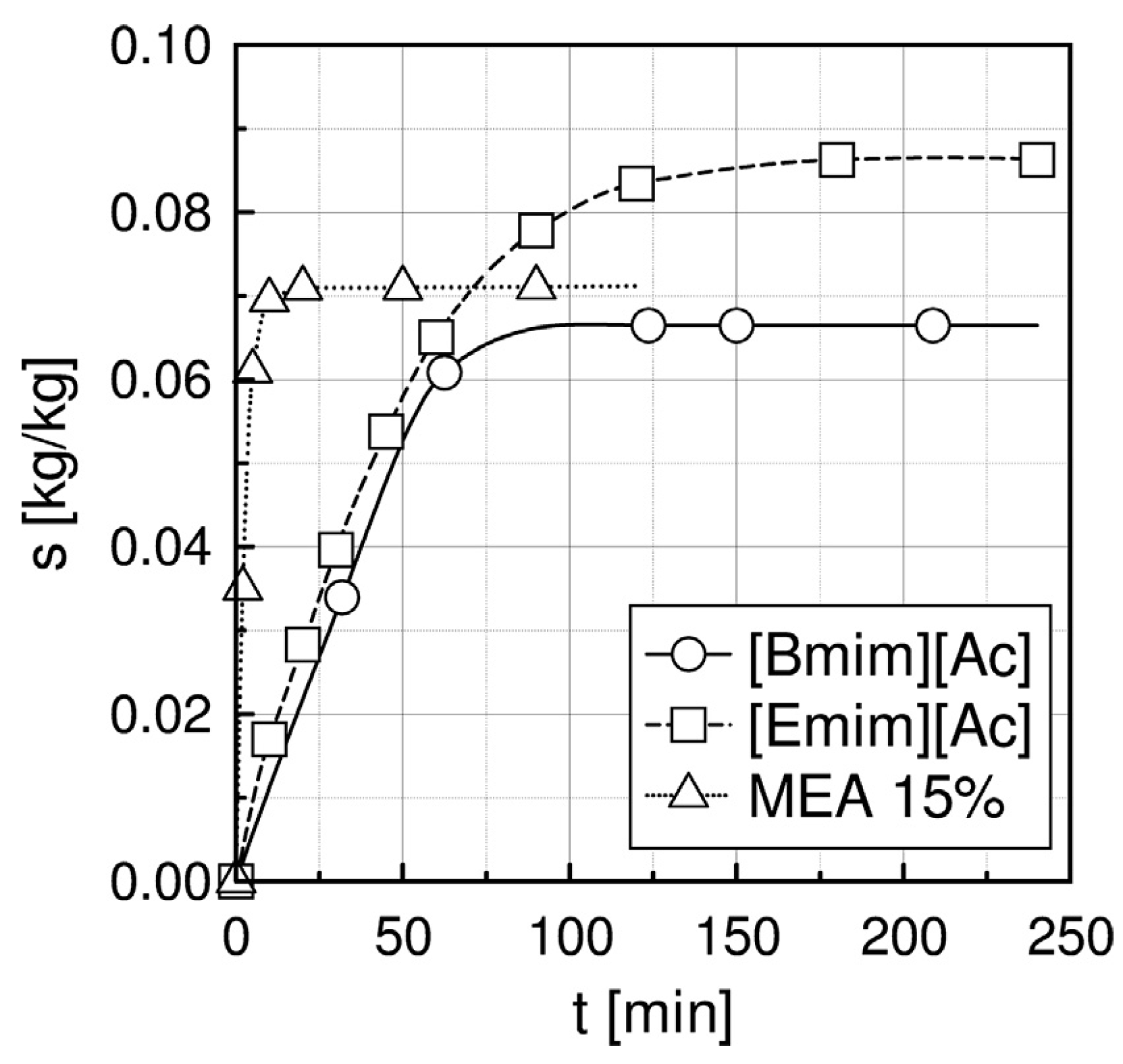
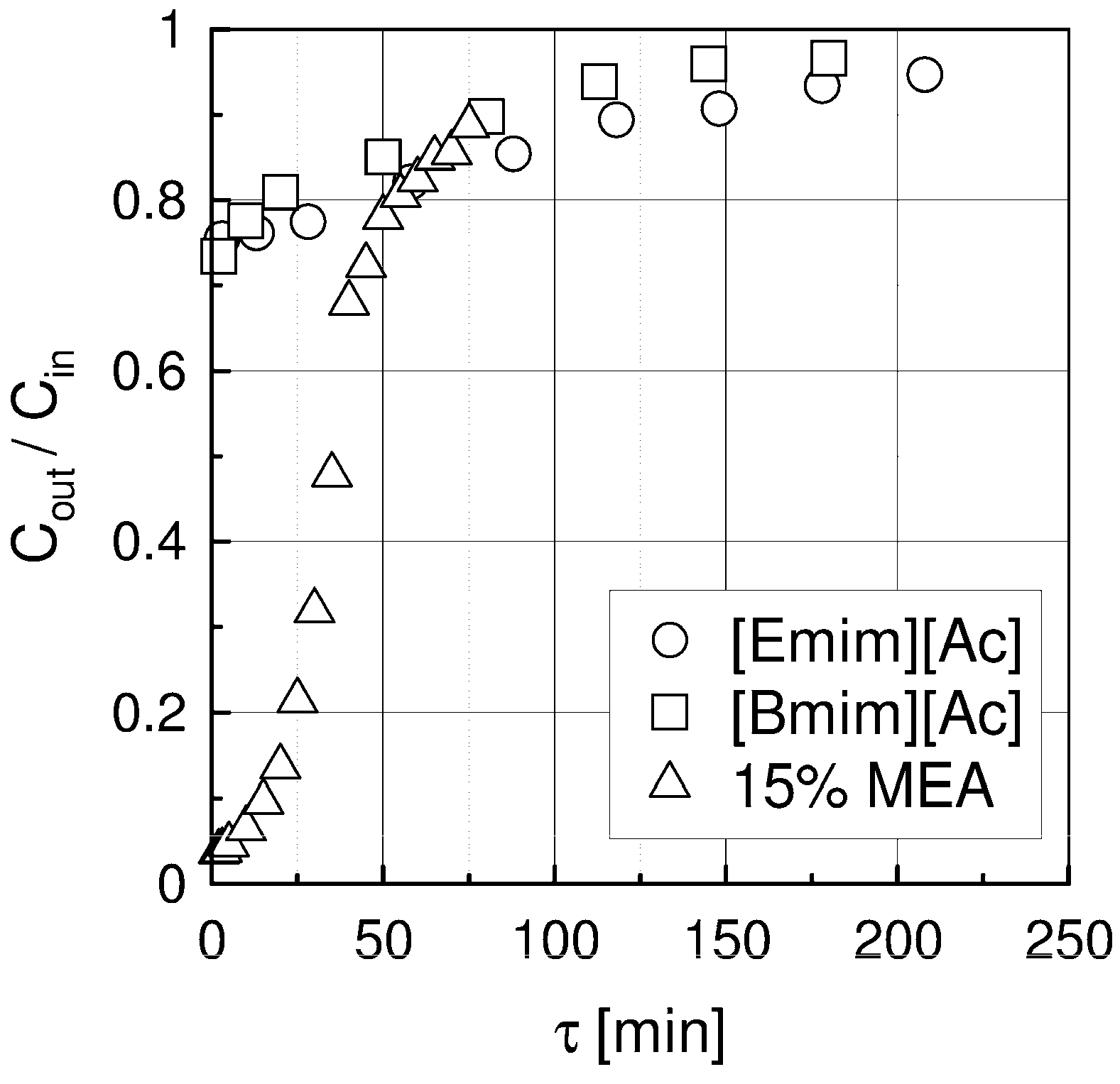
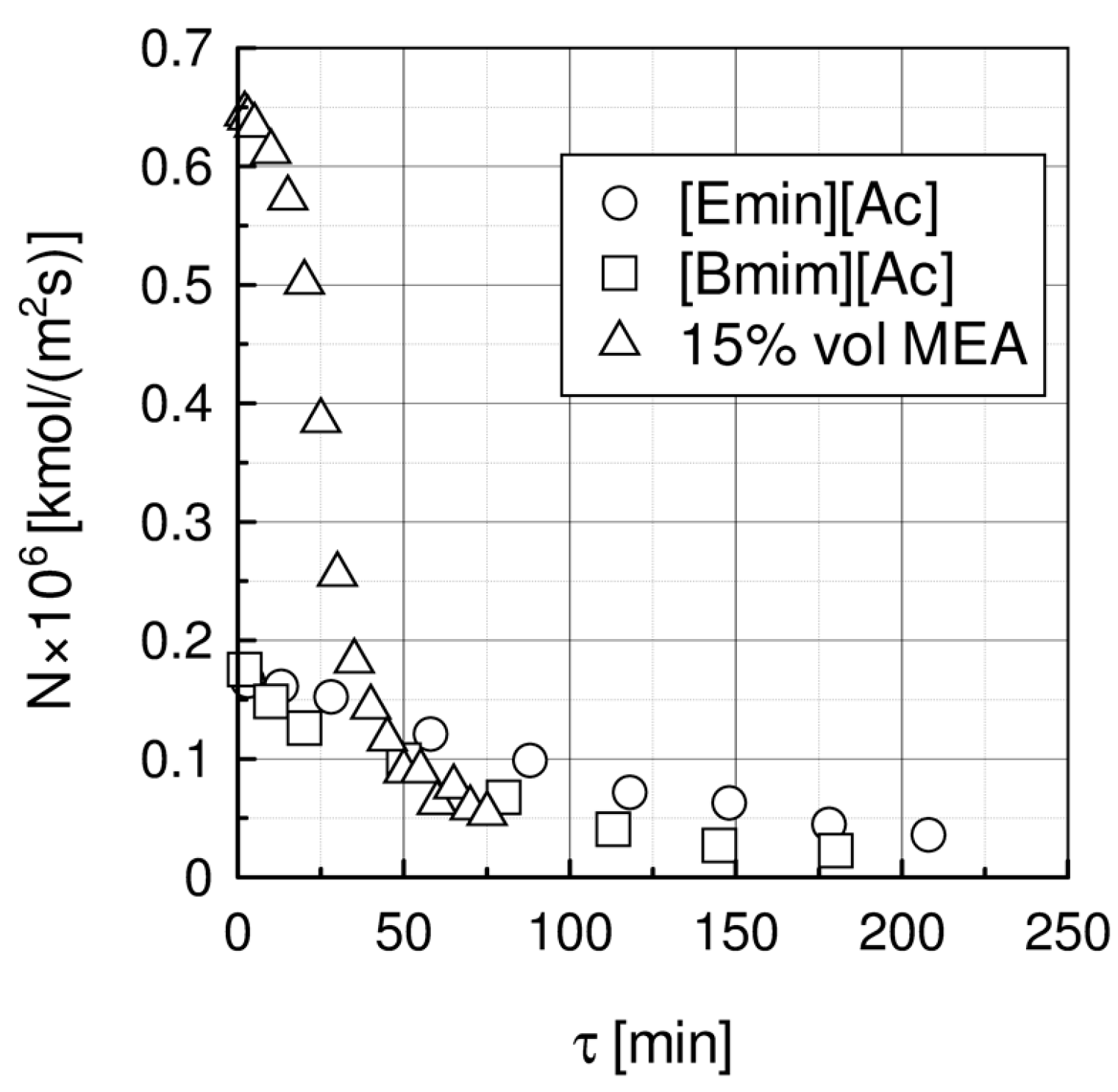
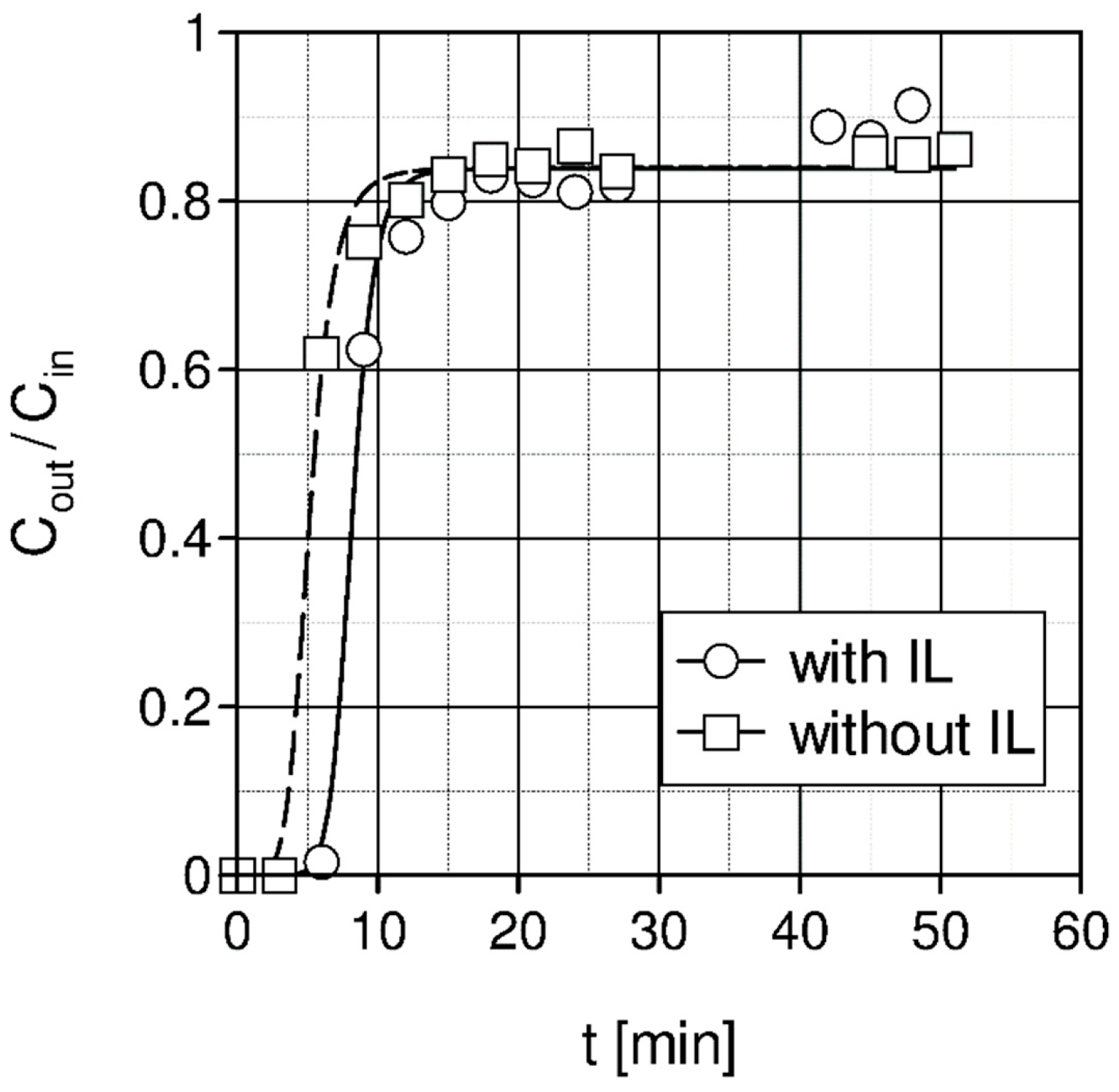
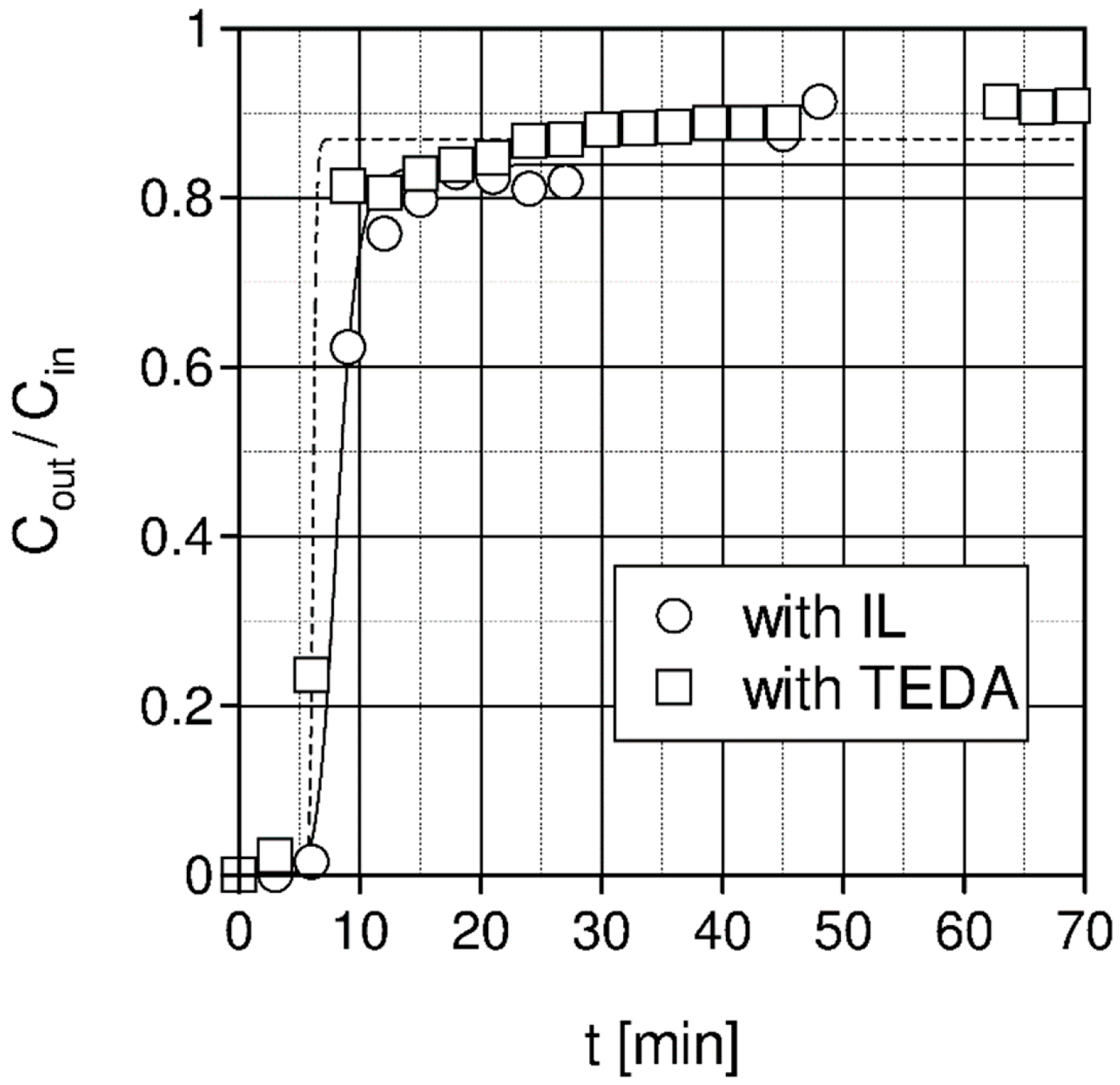
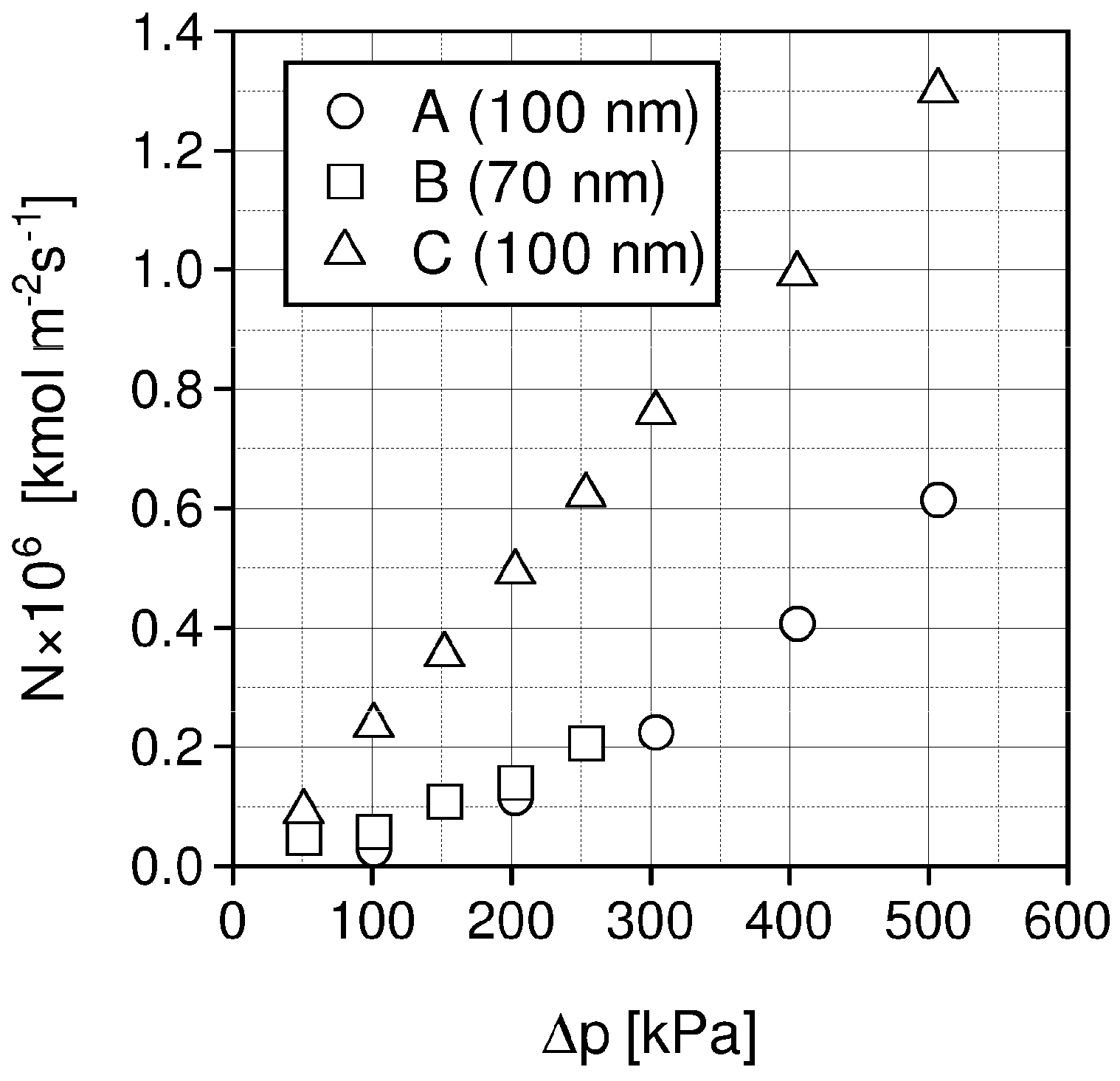
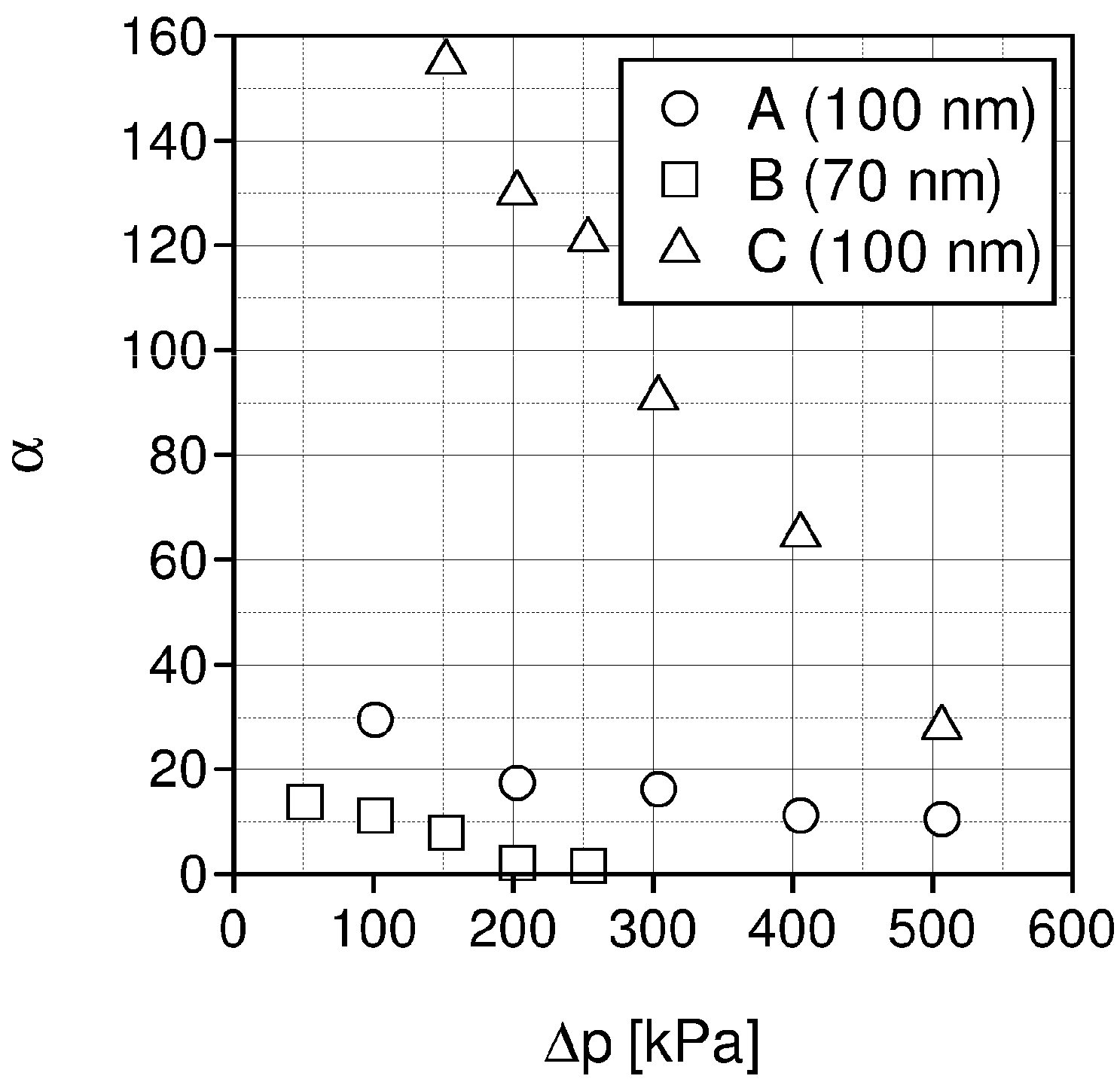
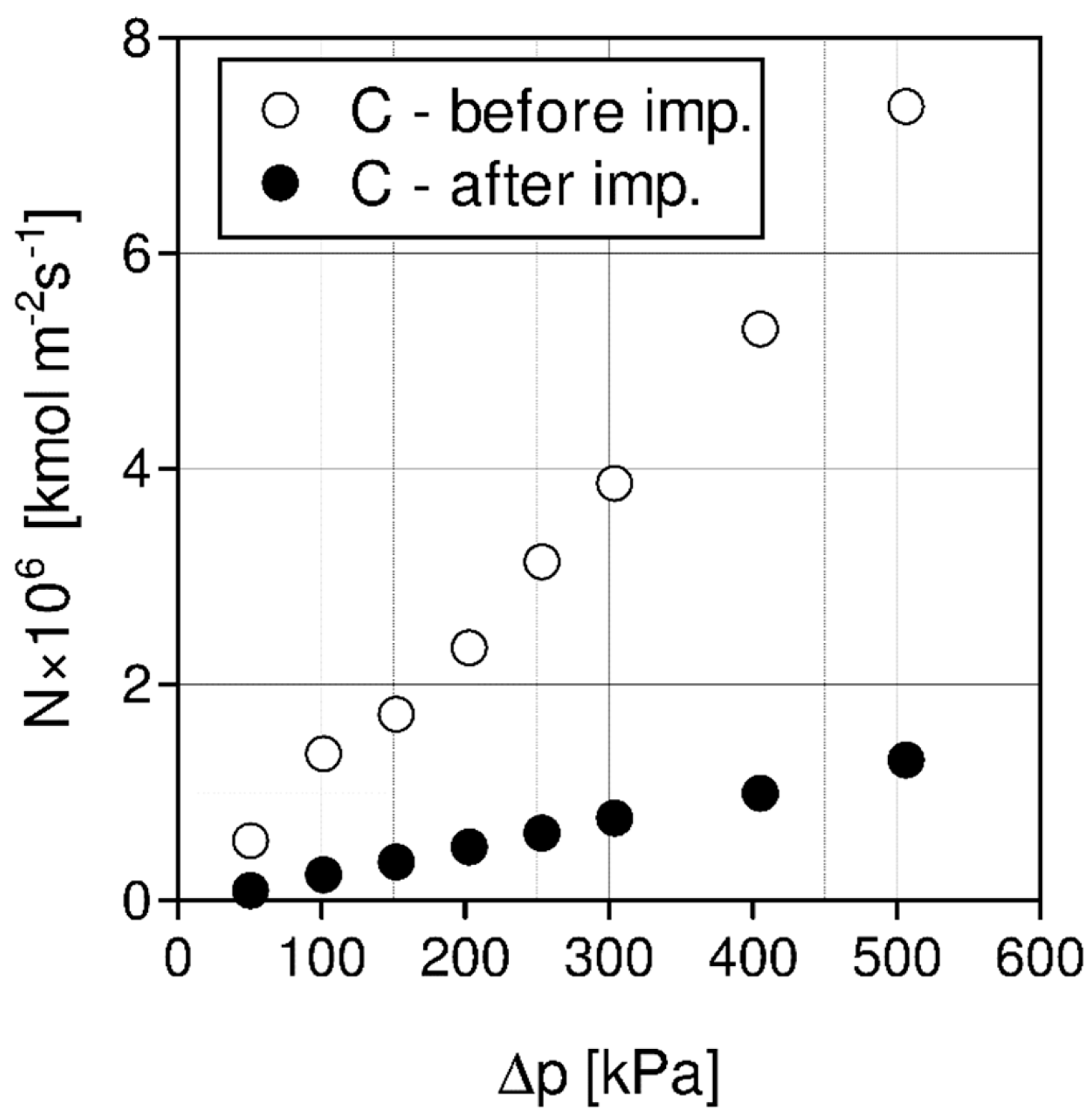
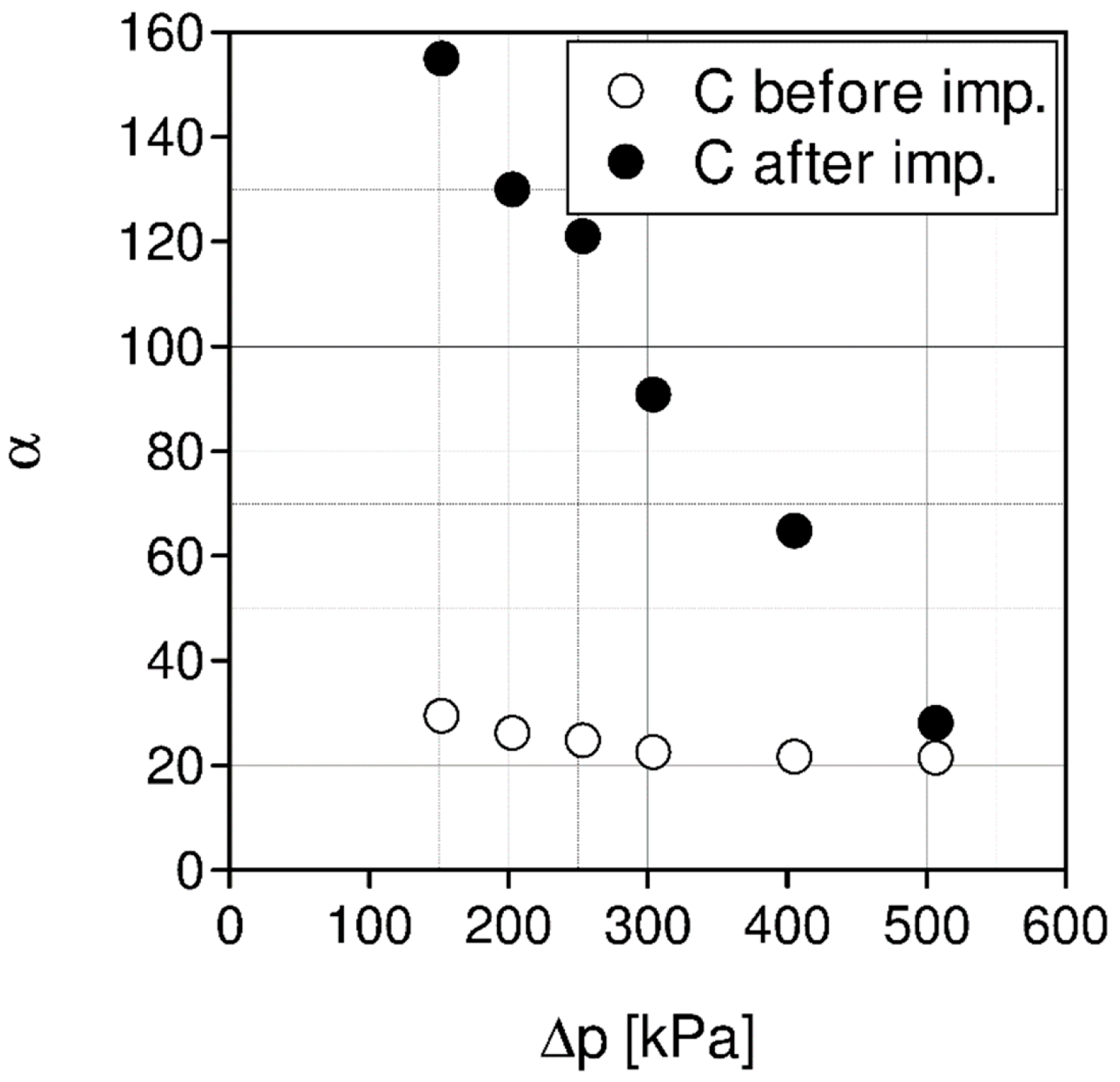
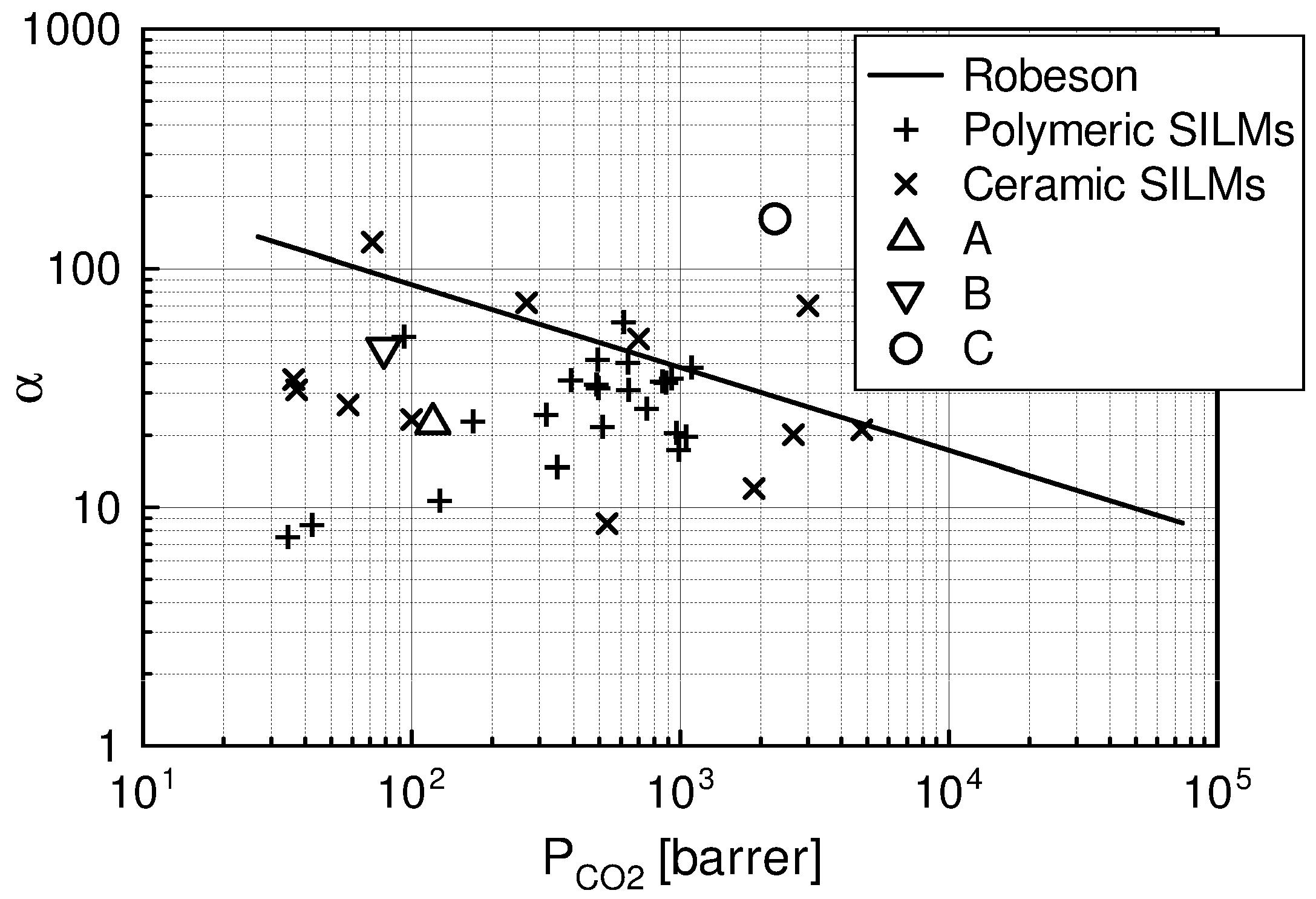
| Liquid | ρ kg/m3 | η × 103 Pa s | DCO2 × 1010 m2 s−1 | kL × 108 kmol/(m2 s) | kG × 108 kmol/(m2 s) | Nexp,τ = 0 × 106 kmol/(km2 s) | S kg/kg | Price *) €/kg |
|---|---|---|---|---|---|---|---|---|
| [Emim][Ac] | 1.025 | 66.98 | 4.21 | 9.90 | 3.08 | 0.166 | 0.053 | 350 |
| [Bmim][Ac] | 1.050 | 145.3 | 2.51 | 6.04 | 3.08 | 0.177 | 0.043 | 460 |
| 15% MEA | 0.999 | 0.938 | 22.4 | 204 | 3.08 | 0.668 | 0.049 | 20 |
| Liquid | M kg/kmol | ρ kg/m3 | η × 103 Pa s | CO2 Solubility % mol. | DCO2 × 1010 m2 s−1 | Price *) €/kg |
|---|---|---|---|---|---|---|
| [Emim][Ac] | 170.21 | 1.025 | 66.98 | 26.7 | 4.21 | 350 |
| [Bmim][Ac] | 198.26 | 1.050 | 145.3 | 19.4 | 2.51 | 460 |
| [Emim][BF4] | 197.97 | 1.27 | 34.0 | 2.0 | 5.95 | 620 |
| [Emim][Tf2N] | 391.31 | 1.52 | 32.6 | 3.0 | 5.6 | 690 |
| Investigated Methods with [Emim][Ac] | Cin (CO2% vol.) | Gas Flow, V (l3/h) | P (atm) | N·106 (kmol m−2 s−1) | S (kg CO2/kg Sorbent)/α | Regeneration Step |
|---|---|---|---|---|---|---|
| Absorption in pure liquid | 100 | 36 | near atmospheric | - | s = 0.086 | thermal t = 95 °C and vacuum |
| Absorption in packed column | 15 | 138 | near atmospheric | 0.166 | s = 0.053 | thermal t = 90 °C with N2 |
| Adsorption in packed column | 12 | 750 | near atmospheric | 0.111 | s = 0.013 | thermal t = 120 °C |
| Membrane separation | 12 | - | 2–6 | 0.025–0.1 | α = 10–136 | n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziobrowski, Z.; Rotkegel, A. Comparison of CO2 Separation Efficiency from Flue Gases Based on Commonly Used Methods and Materials. Materials 2022, 15, 460. https://doi.org/10.3390/ma15020460
Ziobrowski Z, Rotkegel A. Comparison of CO2 Separation Efficiency from Flue Gases Based on Commonly Used Methods and Materials. Materials. 2022; 15(2):460. https://doi.org/10.3390/ma15020460
Chicago/Turabian StyleZiobrowski, Zenon, and Adam Rotkegel. 2022. "Comparison of CO2 Separation Efficiency from Flue Gases Based on Commonly Used Methods and Materials" Materials 15, no. 2: 460. https://doi.org/10.3390/ma15020460
APA StyleZiobrowski, Z., & Rotkegel, A. (2022). Comparison of CO2 Separation Efficiency from Flue Gases Based on Commonly Used Methods and Materials. Materials, 15(2), 460. https://doi.org/10.3390/ma15020460






