Stationary Phases for Green Liquid Chromatography
Abstract
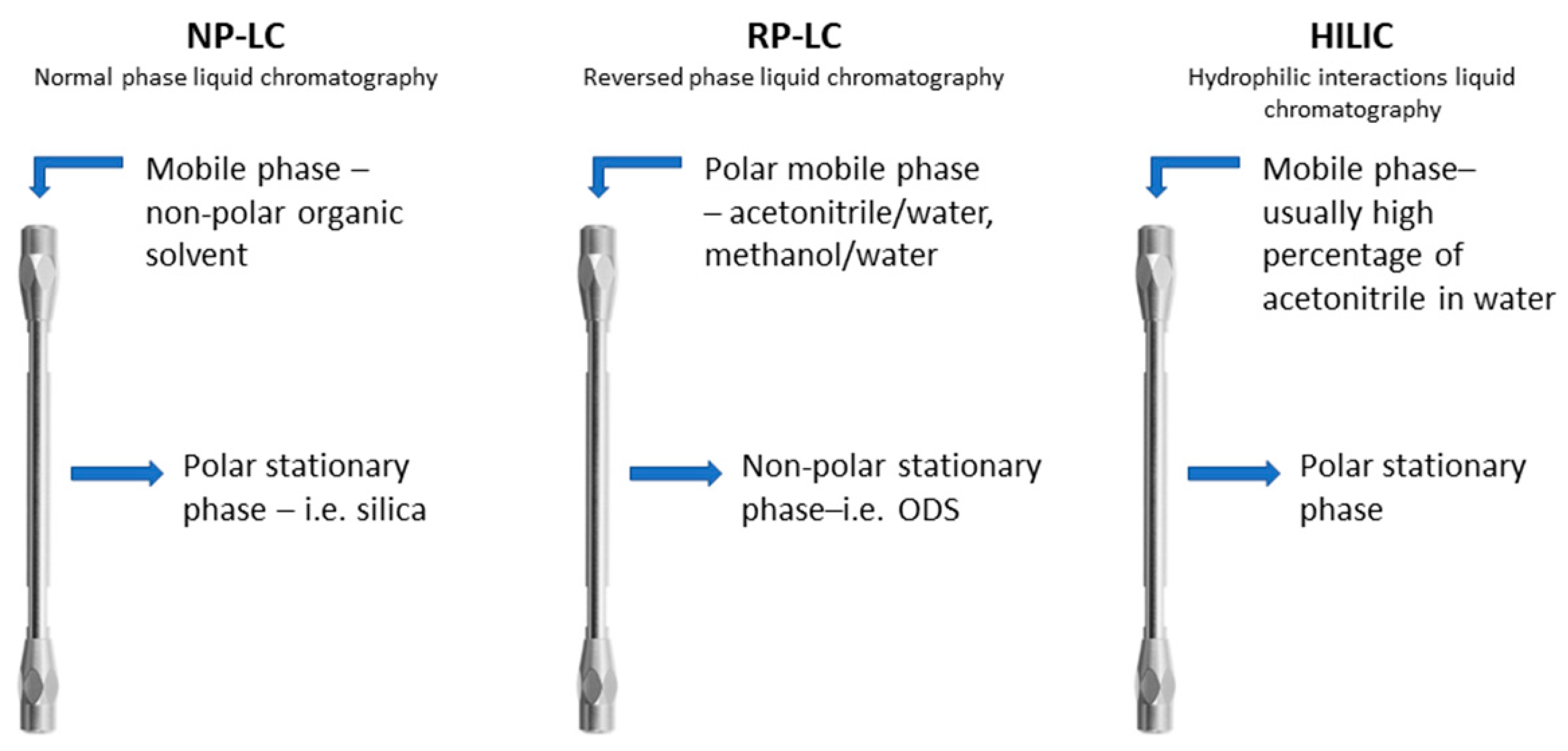
:1. Introduction
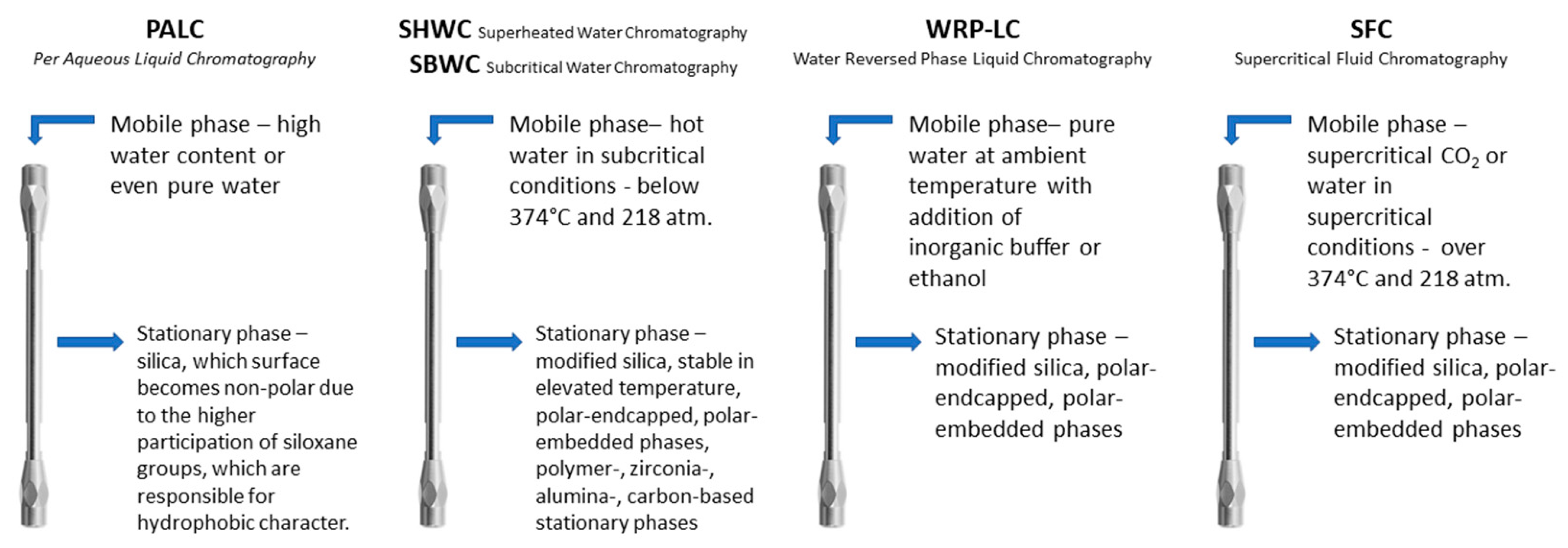
2. Purely Aqueous Conditions: Features and Requirements
3. Stationary Phases Used in Pure Water Conditions
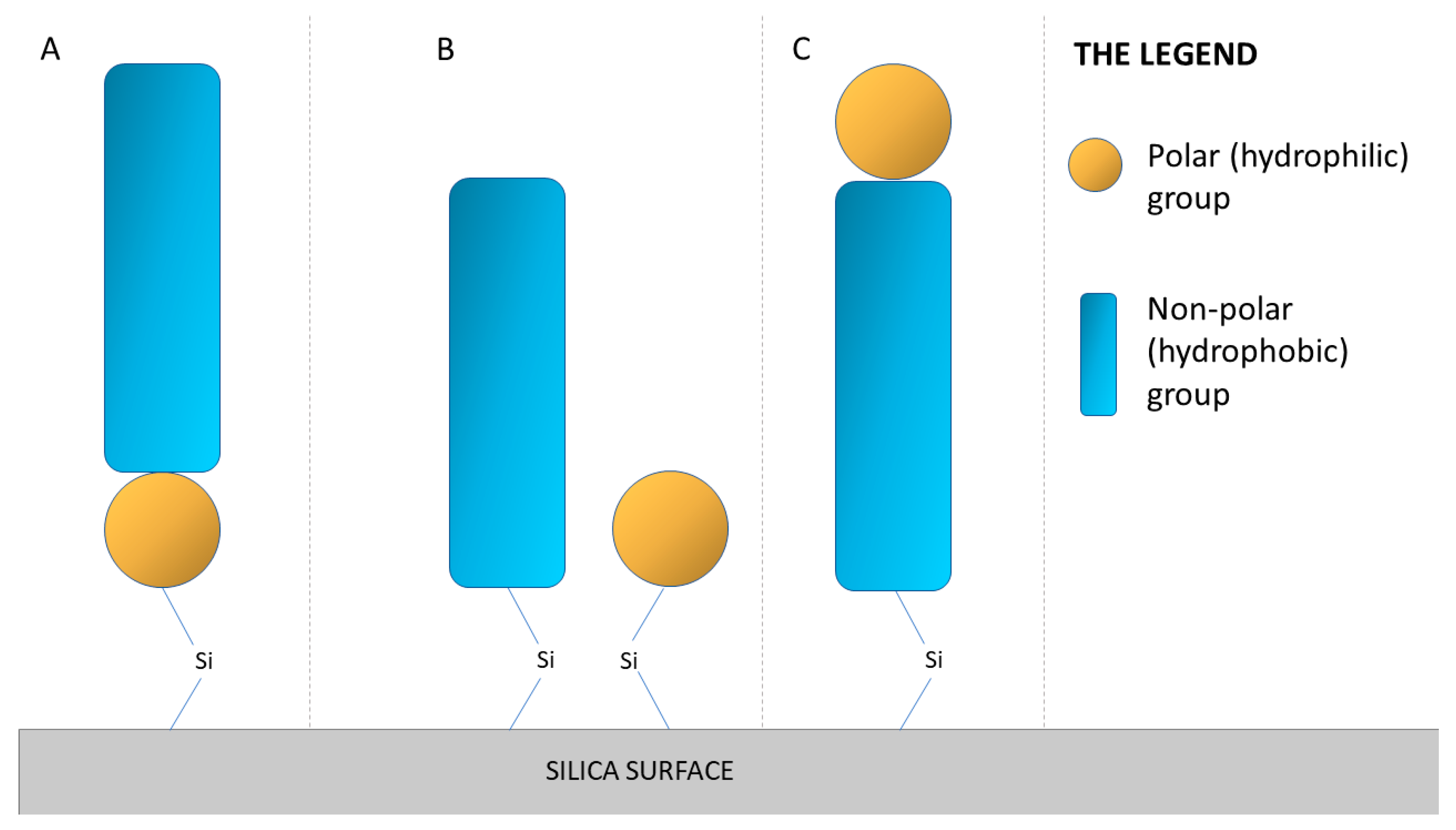
3.1. Instability of Stationary Phases—A Motivation to Search for Solutions
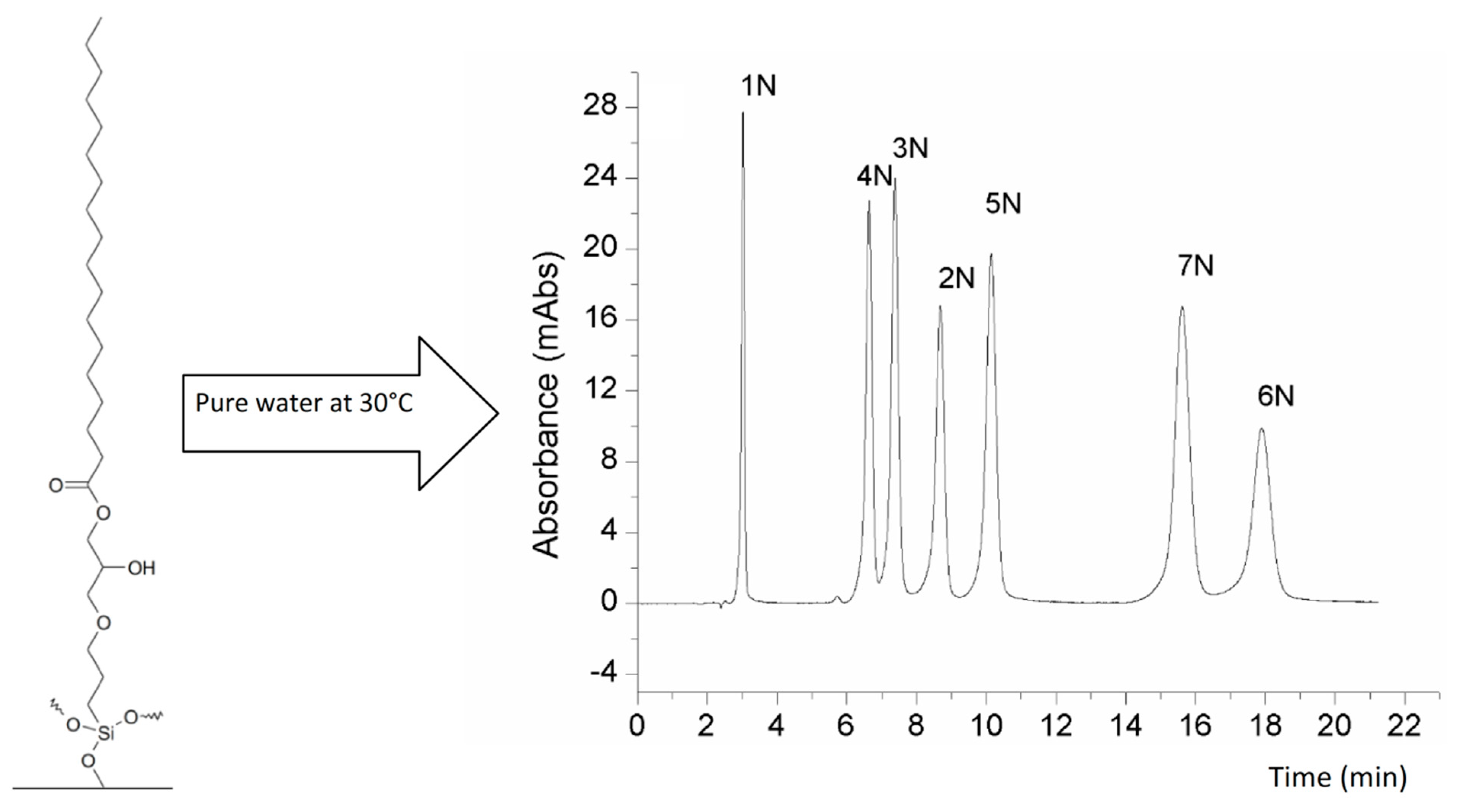
3.1.1. Silica-Based Packing Materials
| Chromatographic Column | Group of Chemical Compounds | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols | Aliphatic and Aromatic Ketones | Alkyl Benzenes/Chlorinated Benzenes/Benzene Derivatives | Anilines | Aromatic Acids | Aromatic Hydrocarbons | Barbiturates | Benzoates | Caffeine Derivatives | Carbohydrates | Chlorophenols | Diethyl Phthalates | Model Drugs | Nucleobases | Parabens | Phenols | Polychlorinated Biphenyls (PCBs) | Polycyclic Aromatic Hydrocarbons (PAHs) | Polyhydroxybenzenes | Pyridines | Steroids | Water-Soluble Vitamins | Literature | |
| Akzo Nobel Kromasil Eternity-2.5-C18 | x | x | x | x | x | x | [68] | ||||||||||||||||
| Chromatorex C-18 | x | x | x | x | [35] | ||||||||||||||||||
| Daiso (ODS-BP) | x | [77] | |||||||||||||||||||||
| Develosil C30-UG-5 | x | [78] | |||||||||||||||||||||
| Fuji Silysia Chromatorex 10 μm | x | [79] | |||||||||||||||||||||
| Hyperprep C18 | x | [80] | |||||||||||||||||||||
| Hypersil BDS C18 | x | x | [66,81,82] | ||||||||||||||||||||
| Interchim Uptisphere Strategy C18-2 and C18-3 | x | x | x | x | x | x | [68] | ||||||||||||||||
| Kromasil-C18 | x | x | [83] | ||||||||||||||||||||
| L-column ODS Chemicals Evaluations and Research Institute, Japan | x | [84] | |||||||||||||||||||||
| Nanoporous glass modified with TFPS and ethyl acetate | x | [80] | |||||||||||||||||||||
| Novapak C18 | x | x | x | [57,85] | |||||||||||||||||||
| Nucleodur-C18 | x | [83] | |||||||||||||||||||||
| Nucleosil C18 AB | x | x | x | x | [66,86] | ||||||||||||||||||
| ODS Chrompack | x | [87] | |||||||||||||||||||||
| Partisil ODS2 ES | x | x | x | [88] | |||||||||||||||||||
| Silica-silicon-based ethyl-bridged hybrid C18 5 µm | x | x | [89] | ||||||||||||||||||||
| Spherisorb octadecylsilane (ODS)-bonded silica | x | x | x | [90,91] | |||||||||||||||||||
| Spherosil XOA (200, 600, 800 mesh)-C18 | x | x | x | [92] | |||||||||||||||||||
| Supelco Ascentis Express C18 (fused-core) | x | x | x | x | x | x | [68] | ||||||||||||||||
| Xselect CSH C18 | x | x | x | x | x | x | [68] | ||||||||||||||||
| Xbridge Amide | x | x | x | x | x | x | [68] | ||||||||||||||||
| Xbridge C18 | x | x | x | [81,93,94] | |||||||||||||||||||
| XTerra MS C18 and Xterra phenyl organic/inorganic hybride | x | x | x | [83,95] | |||||||||||||||||||
| Xterra RP C8 | x | x | x | x | [93,96,97] | ||||||||||||||||||
| Xterra RP C18 | x | x | x | x | x | [83,93,97] | |||||||||||||||||
| YMC Triart C18 | x | x | x | x | x | x | [68] | ||||||||||||||||
| Zorbax RX-C-18 | x | x | x | x | x | [35] | |||||||||||||||||
| Zorbax RX-C-8 | x | x | [66,82] | ||||||||||||||||||||
| Zorbax-ODS | x | x | [98] | ||||||||||||||||||||
3.1.2. Polymer-Based Stationary Phases
| Chromatographic Column | Group of Chemical Compounds | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols | Aldehydes | Aliphatic and Aromatic Ketones | Alkyl Benzenes/Chlorinated Benzenes/Benzene Derivatives | Amino Acids | Anilines | Aromatic Acids | Barbiturates | Benzoates | Caffeine Derivatives | Carbohydrates | Carboxylic Acids | Chlorophenols | Diethyl Phthalates | Model Drugs | Nucleobases | Parabens | Phenols | Polycyclic Aromatic Hydrocarbons (PAHs) | Polyethylene Glycols | Polyhydroxybenzenes | PTH–Amino Acids | Pyridines | Sulfonamides | Water-Soluble Vitamins | Literature | |
| Aminex HPX 87-strong cationic resin | x | x | [105] | |||||||||||||||||||||||
| Oasis polymer | x | [81] | ||||||||||||||||||||||||
| P(NIPAAm-co-BMA-co-DMAPAAm) modified silica | x | [106] | ||||||||||||||||||||||||
| P(NIPAAm-co-tBAAm-co-AAc) modified silica | x | [106] | ||||||||||||||||||||||||
| PL HiPlex 8µm H | x | x | [93] | |||||||||||||||||||||||
| PLRP-S PS–DVB Polymer Laboratories | x | x | x | x | x | x | x | x | x | x | [56,60,63,81,83,85,90,93,99,100,107,108] | |||||||||||||||
| Poly(GMA-co-EDMA) particles | x | x | x | [109] | ||||||||||||||||||||||
| Polystyrene-Coated Zirconia (PS-ZrO2) | x | [54] | ||||||||||||||||||||||||
| PRP-1 Hamilton | x | x | x | x | x | x | x | x | x | x | x | x | x | [35,66,77,79,82,86,87,102,110,111] | ||||||||||||
| Showa Denko Shodex ET-RP1 4D | x | x | x | x | x | x | [68] | |||||||||||||||||||
3.1.3. Zirconia-Based Stationary Phases
| Chromatographic Column | Group of Chemical Compounds | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols | Alkyl benzenes/Chlorinated Benzenes/Benzene Derivatives | Caffeine Derivatives | Diethyl Phthalates | Flavones | Model Drugs | Phenols | Steroids | Triazole Fungicides | Literature | |
| Alumina Keystone Scientific | x | x | [86] | |||||||
| PBD-encapsulated zirconia | x | [116] | ||||||||
| ZirChrom–Carb | x | x | [81,117] | |||||||
| ZirChrom-DB-C18 | x | x | [83] | |||||||
| ZirChrom-PBD | x | x | x | x | x | x | x | x | x | [81,82,83,106,118,119,124] |
| ZirChrom-PS | x | x | [66,120] | |||||||
3.1.4. Carbon-Based Packing Materials
| Chromatographic Column | Group of Chemical Compounds | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols | Aldehydes | Aliphatic and Aromatic Ketones | Alkyl Benzenes/Chlorinated Benzenes/Benzene Derivatives | Amino Acids | Caffeine Derivatives | Carbohydrates | Carboxylic Acids | Model Drugs | Nucleobases | Peptides | Steroids | Triazine Herbicides | Morphine-Based Opiates | Literature | |
| Hypercarb 5 µm | x | x | x | x | x | x | x | [83,87,102,124,126] | |||||||
| Hypercarb PH porous graphitic carbon | x | [81] | |||||||||||||
| Porous graphitized carbon (PGC) | x | x | x | x | x | x | [125] | ||||||||
4. Stationary Phases with Integrated Polar Groups
5. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Strickland, Z.; Kapalavavi, B.; Marple, R.; Gamsky, C. Industrial application of green chromatography—I. Separation and analysis of niacinamide in skincare creams using pure water as the mobile phase. Talanta 2011, 84, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Sagandykova, G.; Szumski, M.; Buszewski, B. How much separation sciences fit in the green chemistry canoe? Curr. Opin. Green Sustain. Chem. 2021, 30, 100495. [Google Scholar] [CrossRef]
- Šatínský, D.; Brabcová, I.; Maroušková, A.; Chocholouš, P.; Solich, P. Green chromatography separation of analytes of greatly differing properties using a polyethylene glycol stationary phase and a low-toxic water-based mobile phase. Anal. Bioanal. Chem. 2013, 405, 6105–6115. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, M.; Kishida, K. Determining sulfamethazine in pork using HPLC with a 100% water mobile phase. LC-GC N. Am. 2004, 22, 1092–1096. [Google Scholar]
- Furusawa, N.; Tsumatani, E. Green HPLC with 100% Water Eluent for Analysing Melamine in, M.i.l.k. LC-GC Eur. 2012, 25, 292–298. [Google Scholar]
- Yang, Y. Subcritical water chromatography: A green approach to high-temperature liquid chromatography. J. Sep. Sci. 2007, 30, 1131–1140. [Google Scholar] [CrossRef]
- Smith, R.M. Superheated water chromatography—A green technology for the future. J. Chromatogr. A 2008, 1184, 441–455. [Google Scholar] [CrossRef]
- Pereira, A.S.; Girón, A.J.; Admasu, E.; Sandra, P. Green hydrophilic interaction chromatography using ethanol-water-carbon dioxide mixtures. J. Sep. Sci. 2010, 33, 834–837. [Google Scholar] [CrossRef]
- Dembek, M.; Bocian, S. Pure water as a mobile phase in liquid chromatography techniques. TrAC Trends Anal. Chem. 2020, 123, 115793. [Google Scholar] [CrossRef]
- Safaei, Z.; Bocian, S.; Buszewski, B. Green chromatography-carbon footprint of columns packed with core-shell materials. RSC Adv. 2014, 4, 53915–53920. [Google Scholar] [CrossRef]
- González-Ruiz, V.; León, A.G.; Olives, A.I.; Martín, M.A.; Menéndez, J.C. Eco-friendly liquid chromatographic separations based on the use of cyclodextrins as mobile phase additives. Green Chem. 2011, 13, 115–126. [Google Scholar] [CrossRef]
- Chen, K.; Lynen, F.; De Beer, M.; Hitzel, L.; Ferguson, P.; Hanna-Brown, M.; Sandra, P. Selectivity optimization in green chromatography by gradient stationary phase optimized selectivity liquid chromatography. J. Chromatogr. A 2010, 1217, 7222–7230. [Google Scholar] [CrossRef] [PubMed]
- Raynie, D. The Greening of the Chromatography Laboratory. LC-GC Eur. 2011, 24, 78–91. [Google Scholar]
- Bocian, S.; Krzemińska, K. The separations using pure water as a mobile phase in liquid chromatography using polar-embedded stationary phases. Green Chem. Lett. Rev. 2019, 12, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Sandra, P.; Vanhoenacker, G.; David, F.; Sandra, K.; Pereira, A. Green chromatography (Part 1): Introduction and liquid chromatography. LC-GC Eur. 2010, 23, 242–259. [Google Scholar]
- Bro, R. Review on multiway analysis in chemistry—2000-2005. Crit. Rev. Anal. Chem. 2006, 36, 279–293. [Google Scholar] [CrossRef]
- Anzardi, M.B.; Arancibia, J.A.; Olivieri, A.C. Processing multi-way chromatographic data for analytical calibration, classification and discrimination: A successful marriage between separation science and chemometrics. TrAC Trends Anal. Chem. 2021, 134, 116128. [Google Scholar] [CrossRef]
- Escandar, G.M.; Olivieri, A.C. Multi-way chromatographic calibration—A review. J. Chromatogr. A 2019, 1587, 2–13. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, C.; Bin, J.; Yang, M.; Kang, C. Multilinear mathematical separation in chromatography. Separations 2021, 8, 31. [Google Scholar] [CrossRef]
- Yin, X.L.; Gu, H.W.; Jalalvand, A.R.; Liu, Y.J.; Chen, Y.; Peng, T.Q. Dealing with overlapped and unaligned chromatographic peaks by second-order multivariate calibration for complex sample analysis: Fast and green quantification of eight selected preservatives in facial masks. J. Chromatogr. A 2018, 1573, 18–27. [Google Scholar] [CrossRef]
- Jupille, T.H.; Dolan, J.W.; Snyder, L.R.; Molnar, I. Two-dimensional optimization using different pairs of variables for the reversed-phase high-performance liquid chromatographic separation of a mixture of acidic compounds. J. Chromatogr. A 2002, 948, 35–41. [Google Scholar] [CrossRef]
- Galushko, S.V.; Shishkina, I.; Urtans, E.; Rotkaja, O. ChromSword®: Software for Method Development in Liquid Chromatography. In Software-Assisted Method Development In High Performance Liquid Chromatography; World Scientific Publishing Europe Ltd.: London, UK, 2018; pp. 53–94. [Google Scholar]
- Welch, C.J.; Wu, N.; Biba, M.; Hartman, R.; Brkovic, T.; Gong, X.; Helmy, R.; Schafer, W.; Cuff, J.; Pirzada, Z.; et al. Greening analytical chromatography. TrAC Trends Anal. Chem. 2010, 29, 667–680. [Google Scholar] [CrossRef]
- Coym, J.W.; Dorsey, J.G. Superheated Water Chromatography: A Brief Review of an Emerging Technique. Anal. Lett. 2004, 37, 1013–1023. [Google Scholar] [CrossRef]
- Greibrokk, T.; Andersen, T. High-temperature liquid chromatography. J. Chromatogr. A 2003, 1000, 743–755. [Google Scholar] [CrossRef]
- Heinisch, S.; Rocca, J.L. Sense and nonsense of high-temperature liquid chromatography. J. Chromatogr. A 2009, 1216, 642–658. [Google Scholar] [CrossRef]
- Nawrocki, J.; Buszewski, B. Influence of silica surface chemistry and structure on the properties, structure and coverage of alkyl-bonded phases for high-performance liquid chromatography. J. Chromatogr. A 1988, 449, 1–24. [Google Scholar] [CrossRef]
- McNeff, C.V.; Yan, B.; Stoll, D.R.; Henry, R.A. Practice and theory of high temperature liquid chromatography. J. Sep. Sci. 2007, 30, 1672–1685. [Google Scholar] [CrossRef]
- Guillarme, D.; Heinisch, S. Detection modes with high temperature liquid chromatography—A review. Sep. Purif. Rev. 2005, 34, 181–216. [Google Scholar] [CrossRef]
- Vanhoenacker, G.; Sandra, P. Elevated temperature and temperature programming in conventional liquid chromatography—Fundamentals and applications. J. Sep. Sci. 2006, 29, 1822–1835. [Google Scholar] [CrossRef]
- Dolan, J.W. Temperature selectivity in reversed-phase high performance liquid chromatography. J. Chromatogr. A 2002, 965, 195–205. [Google Scholar] [CrossRef]
- Bowermaster, J.; McNair, H.M. Temperature Programmed Microbore HPLC—Part I. J. Chromatogr. Sci. 1984, 22, 165–170. [Google Scholar] [CrossRef]
- Chen, M.H.; Horváth, C. Temperature programming and gradient elution in reversed-phase chromatography with packed capillary columns. J. Chromatogr. A 1997, 788, 51–61. [Google Scholar] [CrossRef]
- Tran, J.V.; Molander, P.; Greibrokk, T.; Lundanes, E. Temperature effects on retention in reversed phase liquid chromatography. J. Sep. Sci. 2001, 24, 930–940. [Google Scholar] [CrossRef]
- Kondo, T.; Yang, Y. Comparison of elution strength, column efficiency, and peak symmetry in subcritical water chromatography and traditional reversed-phase liquid chromatography. Anal. Chim. Acta 2003, 494, 157–166. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Yang, Y.; Miller, D.J. Extraction of Organic Pollutants from Environmental Solids with Sub- and Supercritical Water. Anal. Chem. 1994, 66, 2912–2920. [Google Scholar] [CrossRef]
- Colwell, L.F.; Hartwick, R.A. Synthesis of non-porous silica supports for HPLC from porous silica gels. J. High Resolut. Chromatogr. 1986, 9, 304–305. [Google Scholar] [CrossRef]
- Rustamov, I.; Farcas, T.; Ahmed, F.; Chan, F.; LoBrutto, R.; McNair, H.M.; Kazakevich, Y.V. Geometry of chemically modified silica. J. Chromatogr. A 2001, 913, 49–63. [Google Scholar] [CrossRef]
- Majors, R.E.; Przybyciel, M. Columns for Reversed-Phase LC Separations in Highly Aqueous Mobile Phases. LCGC Eur. 2002, 20, 584–593. [Google Scholar]
- Ruderisch, A.; Iwanek, W.; Pfeiffer, J.; Fischer, G.; Albert, K.; Schurig, V. Synthesis and characterization of a novel resorcinarene-based stationary phase bearing polar headgroups for use in reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2005, 1095, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kasturi, P.; Buszewski, B.; Jaroniec, M.; Gilpin, R.K. Reordering/resonation studies of alkylamide phases. J. Chromatogr. A 1994, 659, 261–265. [Google Scholar] [CrossRef]
- Delaurent, C.; Tomao, V.; Siouffi, A.M. Synthesis and characterization of a chemically-bonded cholesteric stationary phase for high-performance liquid chromatography. Chromatographia 1997, 45, 355–363. [Google Scholar] [CrossRef]
- O’Gara, J.E.; Alden, B.A.; Walter, T.H.; Neue, U.D.; Petersen, J.S.; Niederländer, C.L. Simple Preparation of a C8 HPLC Stationary Phase with an Internal Polar Functional Group. Anal. Chem. 1995, 67, 3809–3813. [Google Scholar] [CrossRef]
- Smith, R.M. Superheated water: The ultimate green solvent for separation science. Anal. Bioanal. Chem. 2006, 385, 419–421. [Google Scholar] [CrossRef] [Green Version]
- Foster, M.D.; Synovec, R.E. Reversed Phase Liquid Chromatography of Organic Hydrocarbons with Water as the Mobile Phase. Anal. Chem. 1996, 68, 2838–2844. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T.; Hearn, M.T.W.; Boysen, R.I. Aqueous normal-phase retention of nucleotides on silica hydride columns. J. Chromatogr. A 2009, 1216, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Pesek, J.J.; Matyska, M.T.; Fischer, S.M.; Sana, T.R. Analysis of hydrophilic metabolites by high-performance liquid chromatography-mass spectrometry using a silica hydride-based stationary phase. J. Chromatogr. A 2008, 1204, 48–55. [Google Scholar] [CrossRef]
- Dong, L.; Huang, J. Effect of Temperature on the Chromatographic Behavior of Epirubicin and its Analogues on High Purity Silica Using Reversed-Phase Solvents. Chromatographia 2007, 65, 519–526. [Google Scholar] [CrossRef]
- McCalley, D.V.; Neue, U.D. Estimation of the extent of the water-rich layer associated with the silica surface in hydrophilic interaction chromatography. J. Chromatogr. A 2008, 1192, 225–229. [Google Scholar] [CrossRef]
- Skoczylas, M.; Krzemińska, K.; Bocian, S.; Buszewski, B. Silica Gel and its Derivatization for Liquid Chromatography. Encycl. Anal. Chem. 2017, 1–39. [Google Scholar]
- Bidlingmeyer, B.A.; Del Rios, J.K.; Korpl, J. Separation of Organic Amine Compounds on Silica Gel with Reversed-Phase Eluents. Anal. Chem. 1982, 54, 442–447. [Google Scholar] [CrossRef]
- Unger, K.K. Porous Silica; Elsevier: Amsterdam, The Netherlands, 1979. [Google Scholar]
- dos Santos Pereira, A.; David, F.; Vanhoenacker, G.; Sandra, P. The acetonitrile shortage: Is reversed HILIC with water an alternative for the analysis of highly polar ionizable solutes? J. Sep. Sci. 2009, 32, 2001–2007. [Google Scholar] [CrossRef]
- Yan, B.; Zhao, J.; Brown, J.S.; Blackwell, J.; Carr, P.W. High-temperature ultrafast liquid chromatography. Anal. Chem. 2000, 72, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.M.; Burgess, R.J. Superheated water—A clean eluent for reversed-phase high-performance liquid chromatography. Anal. Commun. 1996, 33, 327–329. [Google Scholar] [CrossRef]
- Thompson, J.D.; Carr, P.W. A study of the critical criteria for analyte stability in high-temperature liquid chromatography. Anal. Chem. 2002, 74, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Chienthavorn, O.; Smith, R.M.; Saha, S.; Wilson, I.D.; Wright, B.; Taylor, S.D.; Lenz, E.M. Superheated water chromatography-nuclear magnetic resonance spectroscopy and mass spectrometry of vitamins. J. Pharm. Biomed. Anal. 2004, 36, 477–482. [Google Scholar] [CrossRef]
- Andersson, T.; Hartonen, K.; Hyötyläinen, T.; Riekkola, M.L. Stability of polycyclic aromatic hydrocarbons in pressurised hot water. Analyst 2003, 128, 150–155. [Google Scholar] [CrossRef]
- Edge, A.M.; Shillingford, S.; Smith, C.; Payne, R.; Wilson, I.D. Temperature as a variable in liquid chromatography: Development and application of a model for the separation of model drugs using water as the eluent. J. Chromatogr. A 2006, 1132, 206–210. [Google Scholar] [CrossRef]
- Sanagi, M.M.; Hong, H.S. High temperature liquid chromatography on a poly(styrene-divinylbenzene) stationary phase. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 3065–3076. [Google Scholar] [CrossRef]
- Morrison, R.D.; Dolan, J.W. Reversed-Phase LC in 100% Water. Lc Gc Eur. 2000, 13, 720–724. [Google Scholar]
- Pettersson, S.W.; Persson, B.S.; Nyström, M. General method allowing the use of 100% aqueous loading conditions in reversed-phase liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 803, 159–165. [Google Scholar] [CrossRef]
- Kiridena, W.; Poole, C.F.; Koziol, W.W. Reversed-phase chromatography on a polar endcapped octadecylsiloxane-bonded stationary phase with water as the mobile phase. Chromatographia 2003, 57, 703–707. [Google Scholar] [CrossRef]
- Walter, T.H.; Iraneta, P.; Capparella, M. Mechanism of retention loss when C8and C18HPLC columns are used with highly aqueous mobile phases. J. Chromatogr. A 2005, 1075, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Kayan, B.; Akay, S.; Yang, Y. Green Chromatographic Separation of Coumarin and Vanillins Using Subcritical Water as the Mobile Phase. J. Chromatogr. Sci. 2016, 54, 1187–1192. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Yang, Y. Studies on the long-term thermal stability of stationary phases in subcritical water chromatography. J. Chromatogr. A 2003, 989, 55–63. [Google Scholar] [CrossRef]
- Lippert, J.A.; Johnson, T.M.; Lloyd, J.B.; Smith, J.P.; Johnson, B.T.; Furlow, J.; Proctor, A.; Marin, S.J. Effects of elevated temperature and mobile phase composition on a novel C18silica column. J. Sep. Sci. 2007, 30, 1141–1149. [Google Scholar] [CrossRef]
- Haun, J.; Oeste, K.; Teutenberg, T.; Schmidt, T.C. Long-term high-temperature and pH stability assessment of modern commercially available stationary phases by using retention factor analysis. J. Chromatogr. A 2012, 1263, 99–107. [Google Scholar] [CrossRef] [PubMed]
- McCalley, D.V. Evaluation of the properties of a superficially porous silica stationary phase in hydrophilic interaction chromatography. J. Chromatogr. A 2008, 1193, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Gritti, F.; dos Santos Pereira, A.; Sandra, P.; Guiochon, G. Comparison of the adsorption mechanisms of pyridine in hydrophilic interaction chromatography and in reversed-phase aqueous liquid chromatography. J. Chromatogr. A 2009, 1216, 8496–8504. [Google Scholar] [CrossRef] [PubMed]
- Gritti, F.; dos Santos Pereira, A.; Sandra, P.; Guiochon, G. Efficiency of the same neat silica column in hydrophilic interaction chromatography and per aqueous liquid chromatography. J. Chromatogr. A 2010, 1217, 683–688. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Chen, T.; Liu, X.; Zhang, H. Covalently bonded polysaccharide-modified stationary phase for per aqueous liquid chromatography and hydrophilic interaction chromatography. J. Chromatogr. A 2011, 1218, 1503–1508. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Chen, T.; Liu, X.; Xu, Z.; Zhang, H. Carbon nanoparticles from corn stalk soot and its novel application as stationary phase of hydrophilic interaction chromatography and per aqueous liquid chromatography. Anal. Chim. Acta 2012, 726, 102–108. [Google Scholar] [CrossRef]
- Orentienë, A.; Olšauskaitë, V.; Vičkačkaitë, V.; Padarauskas, A. Retention behaviour of imidazolium ionic liquid cations on 1.7μm ethylene bridged hybrid silica column using acetonitrile-rich and water-rich mobile phases. J. Chromatogr. A 2011, 1218, 6884–6891. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.T.V.; Freire, S.M.S.C.; Duarte, R.M.B.O.; Duarte, A.C. Profiling water-soluble organic matter from urban aerosols using comprehensive two-dimensional liquid chromatography. Aerosol Sci. Technol. 2015, 49, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, Y.; Wang, G.; Chen, W.; He, P.; Wang, Q. Synthesis and characterization of a multimode stationary phase: Congo red derivatized silica in nano-flow HPLC. Analyst 2016, 141, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kondo, T.; Kennedy, T.J. HPLC separations with micro-bore columns using high-temperature water and flame ionization detection. J. Chromatogr. Sci. 2005, 43, 518–521. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Grinberg, N.; Thompson, K.C.; Wenslow, R.M.; Neue, U.D.; Morrison, D.; Walter, T.H.; O’Gara, J.E.; Wyndham, K.D. Evaluation of a C18 hybrid stationary phase using high-temperature chromatography. Anal. Chim. Acta 2005, 554, 144–151. [Google Scholar] [CrossRef]
- Yarita, T.; Nakajima, R.; Shibukawa, M. Superheated Water Chromatography of Phenols Using Poly(styrene-divinylbenzene) Packings as a Stationary Phase. Anal. Sci. 2003, 19, 269–272. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Belghazi, M.; Lagadec, A.; Miller, D.J.; Hawthorne, S.B. Elution of organic solutes from different polarity sorbents using subcritical water. J. Chromatogr. A 1998, 810, 149–159. [Google Scholar] [CrossRef]
- Wilson, I.D. Investigation of a range of stationary phases for the separation of model drugs by HPLC using superheated water as the mobile phase. Chromatographia 2000, 52, S28–S34. [Google Scholar] [CrossRef]
- Yang, Y.; Lamm, L.J.; He, P.; Kondo, T. Temperature effect on peak width and column efficiency in subcritical water chromatography. J. Chromatogr. Sci. 2002, 40, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Guillarme, D.; Heinisch, S.; Rocca, J.L. Effect of temperature in reversed phase liquid chromatography. J. Chromatogr. A 2004, 1052, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Yarita, T.; Aoyagi, Y.; Sasai, H.; Nishigaki, A.; Shinukawa, M. Separation of Parabens on a Zirconia-Based Stationary Phase in Superheated Water Chromatography. Anal. Sci. 2013, 29, 213–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.M.; Chienthavorn, O.; Wilson, I.D.; Wright, B.; Taylor, S.D. Superheated heavy water as the eluent for HPLC-NMR and HPCL-NMR-MS of model drugs. Anal. Chem. 1999, 71, 4493–4497. [Google Scholar] [CrossRef]
- Yang, Y.; Jones, A.D.; Eaton, C.D. Retention behavior of phenols, anilines, and alkylbenzenes in liquid chromatographic separations using subcritical water as the mobile phase. Anal. Chem. 1999, 71, 3808–3813. [Google Scholar] [CrossRef]
- Ingelse, B.A.; Janssen, H.G.; Cramers, C.A. HPLC-FID with superheated water as the eluent: Improved methods and instrumentation. HRC J. High Resolut. Chromatogr. 1998, 21, 613–616. [Google Scholar] [CrossRef]
- Kondo, T.; Yang, Y.; Lamm, L. Separation of polar and non-polar analytes using dimethyl sulfoxide-modified subcritical water. Anal. Chim. Acta 2002, 460, 185–191. [Google Scholar] [CrossRef]
- Fogwill, M.O.; Thurbide, K.B. Rapid column heating method for subcritical water chromatography. J. Chromatogr. A 2007, 1139, 199–205. [Google Scholar] [CrossRef]
- Smith, R.M.; Burgess, R.J. Superheated water as an eluent for reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1997, 785, 49–55. [Google Scholar] [CrossRef]
- Jones, N.; Clifford, A.A.; Bartle, K.D.; Myers, P. Chromatographic determination of solubilities in superheated water. J. Sep. Sci. 2010, 33, 3107–3109. [Google Scholar] [CrossRef]
- Quigley, W.W.C.; Ecker, S.T.; Vahey, P.G.; Synovec, R.E. Reversed phase liquid chromatography with UV absorbance and flame ionization detection using a water mobile phase and a cyano propyl stationary phase: Analysis of alcohols and chlorinated hydrocarbons. Talanta 1999, 50, 569–576. [Google Scholar] [CrossRef]
- Young, E.; Smith, R.M.; Sharp, B.L.; Bone, J.R. Liquid chromatography-flame ionisation detection using a nebuliser/spray chamber interface. Part 2. Comparison of functional group responses. J. Chromatogr. A 2012, 1236, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Smith, R.M.; Albishri, H.M.; Lin, J.M. Thermal Stability of Thiazide and Related Diuretics During Superheated Water Chromatography. Chromatographia 2010, 72, 1177–1181. [Google Scholar] [CrossRef]
- Al Khateeb, L.A.; Smith, R.M. Elevated temperature separations on hybrid stationary phases with low proportions of organic modifier in the eluent. Chromatographia 2011, 73, 743–747. [Google Scholar] [CrossRef]
- Louden, D.; Handley, A.; Taylor, S.; Sinclair, I.; Lenz, E.; Wilson, I.D. High temperature reversed-phase HPLC using deuterium oxide as a mobile phase for the separation of model pharmaceuticals with multiple on-line spectroscopic analysis (UV, IR, 1H-NMR and MS). Analyst 2001, 126, 1625–1629. [Google Scholar] [CrossRef]
- Louden, D.; Handley, A.; Lafont, R.; Taylor, S.; Sinclair, I.; Lenz, E.; Orton, T.; Wilson, I.D. HPLC analysis of ecdysteroids in plant extracts using superheated deuterium oxide with multiple on-line spectroscopic analysis (UV, IR, 1H NMR, and MS). Anal. Chem. 2002, 74, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Yang, Y. Separation of nonpolar analytes using methanol-water mixtures at elevated temperatures. Anal. Chim. Acta 2003, 485, 51–55. [Google Scholar] [CrossRef]
- Chienthavorn, O.; Smith, R.M. Buffered superheated water as an eluent for reversed-phase high performance liquid chromatography. Chromatographia 1999, 50, 485–489. [Google Scholar] [CrossRef]
- Tajuddin, R.; Smith, R.M. On-line coupled superheated water extraction (SWE) and superheated water chromatography (SWC). Analyst 2002, 127, 883–885. [Google Scholar] [CrossRef]
- Smith, R.M.; Chienthavorn, O.; Saha, S.; Wilson, I.D.; Wright, B.; Taylor, S.D. Selective deuterium exchange during superheated heavy water chromatography-nuclear magnetic resonance spectroscopy-mass spectrometry of sulfonamides. J. Chromatogr. A 2000, 886, 289–295. [Google Scholar] [CrossRef]
- Yang, Y.; Jones, A.D.; Mathis, J.A.; Francis, M.A. Flame ionization detection after splitting the water effluent in subcritical water chromatography. J. Chromatogr. A 2002, 942, 231–236. [Google Scholar] [CrossRef]
- Yang, Y. A model for temperature effect on column efficiency in high-temperature liquid chromatography. Anal. Chim. Acta 2006, 558, 7–10. [Google Scholar] [CrossRef]
- Akay, S.; Odabaşi, M.; Yang, Y.; Kayan, B. Synthesis and evaluation of NA-PHEMAH polymer for use as a new stationary phase in high-temperature liquid chromatography. Sep. Purif. Technol. 2015, 152, 1–6. [Google Scholar] [CrossRef]
- Nakajima, R.; Yarita, T.; Shibukawa, M. Analysis of alcohols by superheated water chromatography with flame ionization detection. Bunseki Kagaku 2003, 52. [Google Scholar] [CrossRef] [Green Version]
- Teutenberg, T.; Goetze, H.J.; Tuerk, J.; Ploeger, J.; Kiffmeyer, T.K.; Schmidt, K.G.; gr Kohorst, W.; Rohe, T.; Jansen, H.D.; Weber, H. Development and application of a specially designed heating system for temperature-programmed high-performance liquid chromatography using subcritical water as the mobile phase. J. Chromatogr. A 2006, 1114, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, T.M.; Poole, C.F. Solvation characteristics of pressurized hot water and its use in chromatography. Anal. Commun. 1999, 36, 71–75. [Google Scholar] [CrossRef]
- Smith, R.M.; Chienthavorn, O.; Wilson, I.D.; Wright, B. Superheated deuterium oxide reversed-phase chromatography coupled to proton nuclear magnetic resonance spectroscopy. Anal. Commun. 1998, 35, 261–263. [Google Scholar] [CrossRef]
- Srisopa, A. Preparation of monodisperse porous poly(glycidylmethacrylate-co-ethylenedimethacrylate) microspheres and their application as stationary phase for superheated water HPLC. Talanta 2016, 147, 358–363. [Google Scholar] [CrossRef]
- Miller, D.J.; Hawthorne, S.B. Subcritical Water Chromatography with Flame Ionization Detection. Anal. Chem. 1997, 69, 623–627. [Google Scholar] [CrossRef]
- Yarita, T.; Nakajima, R.; Shimada, K.; Kinugasa, S.; Shibukawa, M. Superheated water chromatography of low molecular weight polyethylene glycols with ultraviolet detection. Anal. Sci. 2005, 21, 1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawrocki, J.; Dunlap, C.; McCormick, A.; Carr, P.W.; Part, I. Chromatography using ultra-stable metal oxide-based stationary phases for HPLC. J. Chromatogr. A 2004, 1028, 1–30. [Google Scholar] [CrossRef]
- Nawrocki, J.; Rigney, M.; McCormick, A.; Carr, P.W. Chemistry of zirconia and its use in chromatography. J. Chromatogr. A 1993, 657, 229–282. [Google Scholar] [CrossRef]
- Nawrocki, J.; Dunlap, C.J.; Carr, P.W.; Blackwell, J.A. New Materials for Biotechnology: Chromatographic Stationary Phases Based on Zirconia. Biotechnol. Prog. 1994, 10, 561–573. [Google Scholar] [CrossRef]
- Dunlap, C.; McNeff, C.; Stoll, D.; Carr, P. Zirconia stationary phases for extreme separations. Anal. Chem. 2001, 73, 598A–607A. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, N.; Tang, Q.; Lippert, J.A.; Lee, M.L. Packed capillary column solvating gas chromatography using neat water mobile phase and flame ionization detection. J. Microcolumn Sep. 2001, 13, 41–47. [Google Scholar] [CrossRef]
- Scott Kephart, T.; Dasgupta, P.K. Superheated water eluent capillary liquid chromatography. Talanta 2002, 56, 977–987. [Google Scholar] [CrossRef]
- Coym, J.W.; Dorsey, J.G. Reversed-phase retention thermodynamics of pure-water mobile phases at ambient and elevated temperature. J. Chromatogr. A 2004, 1035, 23–29. [Google Scholar] [CrossRef]
- Sanagi, M.M.; See, H.H.; Ibrahim, W.A.W.; Naim, A.A. High temperature liquid chromatography of triazole fungicides on polybutadiene-coated zirconia stationary phase. J. Chromatogr. A 2004, 1059, 95–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamm, L.J.; Yang, Y. Off-line coupling of subcritical water extraction with subcritical water chromatography via a sorbent trap and thermal desorption. Anal. Chem. 2003, 75, 2237–2242. [Google Scholar] [CrossRef]
- Fields, S.M.; Ye, C.Q.; Zhang, D.D.; Branch, B.R.; Zhang, X.J.; Okafo, N. Superheated water as eluent in high-temperature high-performance liquid chromatographic separations of steroids on a polymer-coated zirconia column. J. Chromatogr. A 2001, 913, 197–204. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Carr, P.W. Fast Separations at Elevated Temperatures on Polybutadiene-Coated Zirconia Reversed-Phase Material. Anal. Chem. 1997, 69, 3884–3888. [Google Scholar] [CrossRef]
- Teutenberg, T.; Tuerk, J.; Holzhauser, M.; Kiffmeyer, T.K. Evaluation of column bleed by using an ultraviolet and a charged aerosol detector coupled to a high-temperature liquid chromatographic system. J. Chromatogr. A 2006, 1119, 197–201. [Google Scholar] [CrossRef]
- Guillarme, D.; Heinisch, S.; Gauvrit, J.Y.; Lanteri, P.; Rocca, J.L. Optimization of the coupling of high-temperature liquid chromatography and flame ionization detection: Application to the separations of alcohols. J. Chromatogr. A 2005, 1078, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Forgács, E. Retention characteristics and practical applications of carbon sorbents. J. Chromatogr. A 2002, 975, 229–243. [Google Scholar] [CrossRef]
- Tajuddin, R.; Smith, R.M. On-line coupled extraction and separation using superheated water for the analysis of triazine herbicides in spiked compost samples. J. Chromatogr. A 2005, 1084, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Krzemińska, K.; Bocian, S. The versatility of N,O-dialkylphosphoramidate stationary phase-separations in HILIC, highly aqueous RP LC conditions and purely aqueous mobile phase. Analyst 2018, 143, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Bocian, S.; Buszewski, B. Phenyl-bonded stationary phases-The influence of polar functional groups on retention and selectivity in reversed-phase liquid chromatography. J. Sep. Sci. 2014, 37, 3435–3442. [Google Scholar] [CrossRef]
- Buszewski, B.; Jandera, P.; Bocian, S.; Janas, P.; Kowalkowski, T. Separation of flavonoids on different phenyl-bonded stationary phases-the influence of polar groups in stationary phase structure. J. Chromatogr. A 2015, 1429, 198–206. [Google Scholar]
- Bocian, S.; Skoczylas, M.; Goryńska, I.; Matyska, M.; Pesek, J.; Buszewski, B. Solvation processes on phenyl-bonded stationary phases—The influence of polar functional groups. J. Sep. Sci. 2016, 39, 4369–4376. [Google Scholar] [CrossRef] [PubMed]
- Bocian, S.; Nowaczyk, A.; Buszewski, B. New alkyl-phosphate bonded stationary phases for liquid chromatographic separation of biologically active compounds. Anal. Bioanal. Chem. 2012, 404, 731–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocian, S.; Krzemińska, K.; Buszewski, B. A study of separation selectivity using embedded ester-bonded stationary phases for liquid chromatography. Analyst 2016, 141, 4340–4348. [Google Scholar] [CrossRef] [PubMed]
- Krzemińska, K.; Dembek, M.; Bocian, S. The competitiveness of solvent adsorption on polar-embedded stationary phases. J. Sep. Sci. 2018, 41, 4296–4303. [Google Scholar] [CrossRef]
- Žuvela, P.; Skoczylas, M.; Jay Liu, J.; Baczek, T.; Kaliszan, R.; Wong, M.W.; Buszewski, B. Column Characterization and Selection Systems in Reversed-Phase High-Performance Liquid Chromatography. Chem. Rev. 2019, 119, 3674–3729. [Google Scholar] [CrossRef] [PubMed]
- Studzińska, S.; Bocian, S.; Siecińska, L.; Buszewski, B. Application of phenyl-based stationary phases for the study of retention and separation of oligonucleotides. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1060, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Bocian, S.; Nowaczyk, A.; Buszewski, B. Synthesis and characterization of ester-bonded stationary phases for liquid chromatography. Talanta 2014, 131, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Walczak, J.; Pomastowski, P.; Bocian, S.; Buszewski, B. Determination of phospholipids in milk using a new phosphodiester stationary phase by liquid chromatography-matrix assisted desorption ionization mass spectrometry. J. Chromatogr. A 2016, 1432, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Bocian, S.; Buszewski, B. Synthesis and characterization of phosphodiester stationary bonded phases for liquid chromatography. Talanta 2015, 143, 35–41. [Google Scholar] [CrossRef]
- Buszewski, M.; Jaroniec, R.K.; Gilpin, B. Influence of eluent composition on retention and selectivity of alkylamide phases under reversed-phase conditions. J. Chromatogr. A 1994, 668, 293–299. [Google Scholar] [CrossRef]
- Hu, W.; Hasebe, K.; Haraguchi, H. Liquid chromatographic separation of polar organic compounds using strong anion-exchanger as the stationary phase and pure water as the mobile phase. J. Liq. Chromatogr. Relat. Technol. 1998, 21, 1387–1399. [Google Scholar] [CrossRef]
- Hu, W.; Hasebe, K.; Reynolds, D.M.; Haraguchi, H. Separation of nucleosides and their bases by reversed-phase liquid chromatography using pure water as the mobile phase. Anal. Chim. Acta 1997, 353, 143–149. [Google Scholar] [CrossRef]
- Umemura, T.; Tsunoda, K.I.; Koide, A.; Oshima, T.; Watanabe, N.; Chiba, K.; Haraguchi, H. Amphoteric surfactant-modified stationary phase for the reversed-phase high-performance liquid chromatographic separation of nucleosides and their bases by elution with water. Anal. Chim. Acta 2000, 419, 87–92. [Google Scholar] [CrossRef]
- Kanazawa, H.; Yamamoto, K.; Matsushima, Y.; Takai, N.; Kikuchi, A.; Sakurai, Y.; Okano, T. Temperature-Responsive Chromatography Using Poly(N-isopropylacrylamide)-Modified Silica. Anal. Chem. 1996, 68, 100–105. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dembek, M.; Bocian, S. Stationary Phases for Green Liquid Chromatography. Materials 2022, 15, 419. https://doi.org/10.3390/ma15020419
Dembek M, Bocian S. Stationary Phases for Green Liquid Chromatography. Materials. 2022; 15(2):419. https://doi.org/10.3390/ma15020419
Chicago/Turabian StyleDembek, Mikołaj, and Szymon Bocian. 2022. "Stationary Phases for Green Liquid Chromatography" Materials 15, no. 2: 419. https://doi.org/10.3390/ma15020419
APA StyleDembek, M., & Bocian, S. (2022). Stationary Phases for Green Liquid Chromatography. Materials, 15(2), 419. https://doi.org/10.3390/ma15020419







