Research Progress and Key Issues of Hydrodebenzylation of Hexabenzylhexaazaisowurtzitane (HBIW) in the Synthesis of High Energy Density Material Hexanitrohexaazaisowurtzitane (HNIW)
Abstract
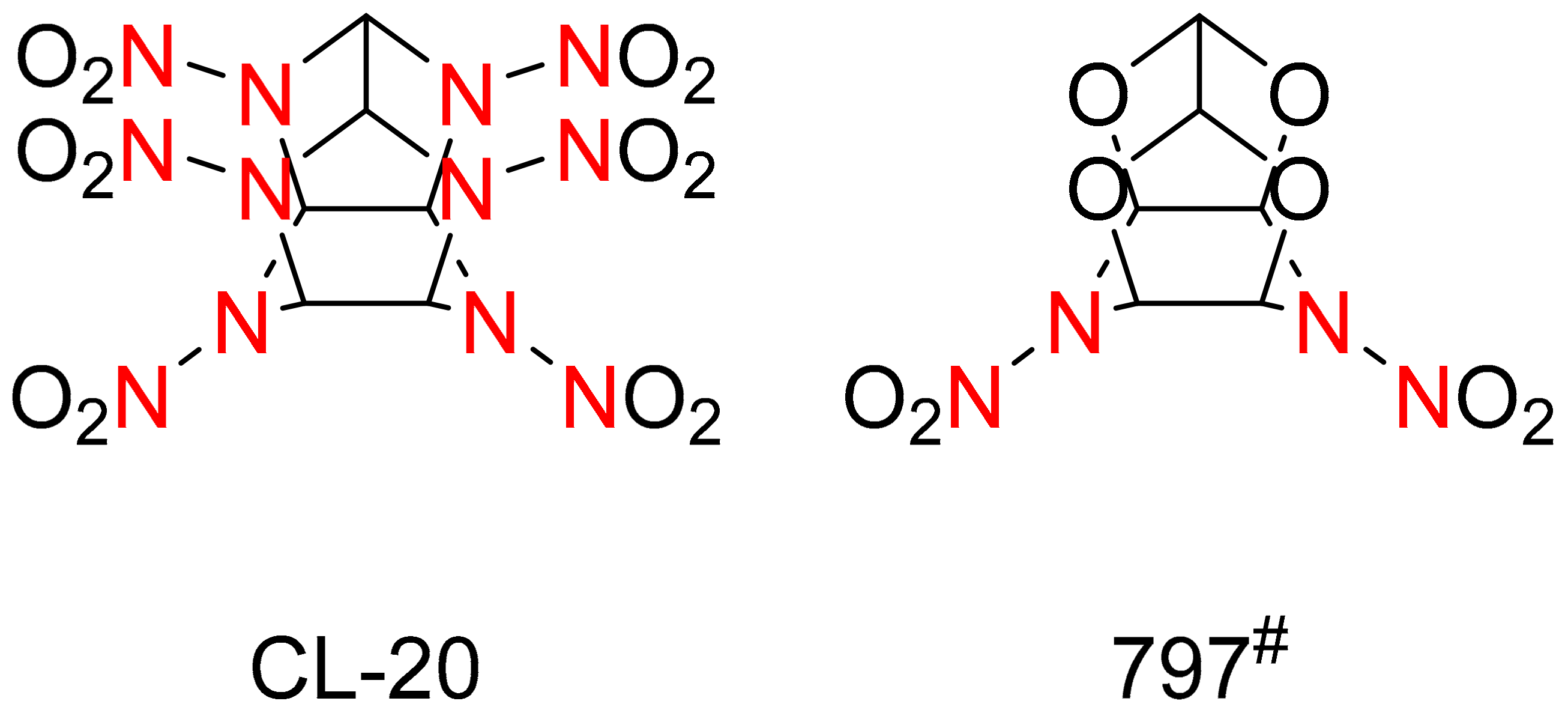
:1. The Development and Performances of the Explosives
2. Synthesis of HNIW
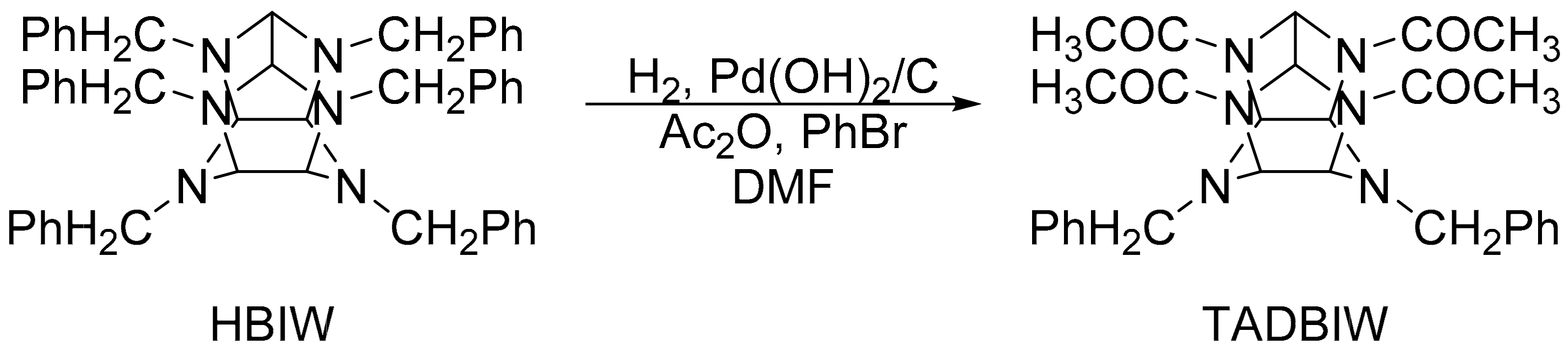
2.1. Typical Synthesis Method
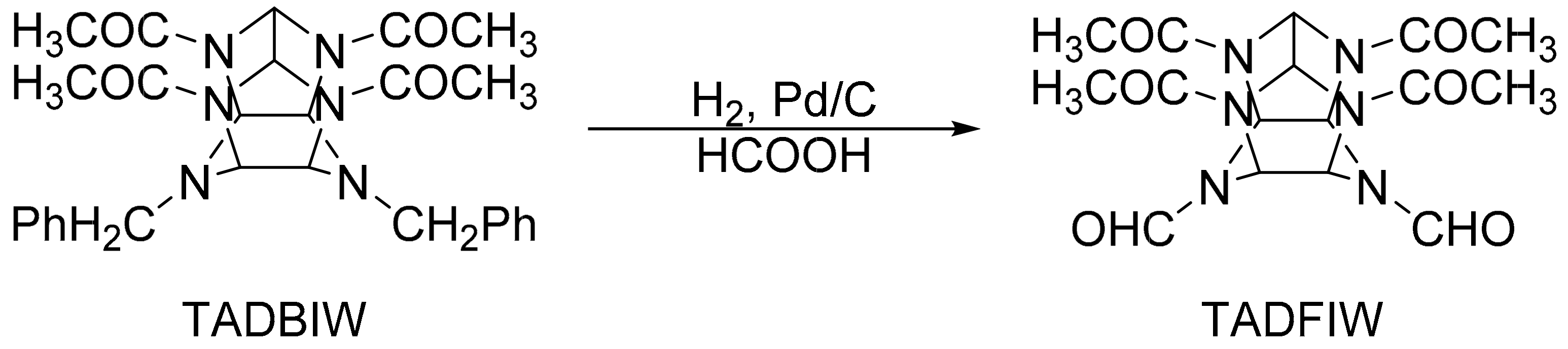
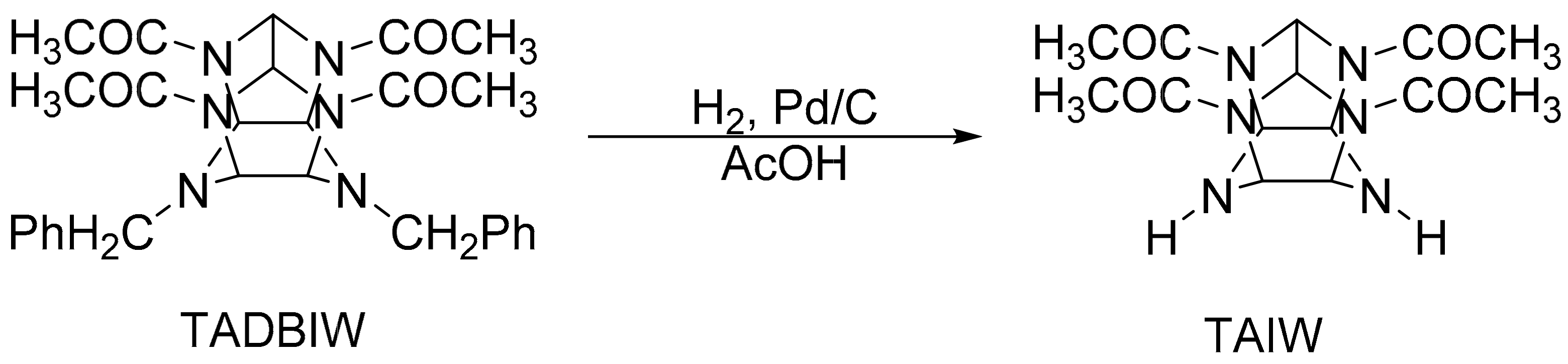
2.1.1. Synthesis of TADBIW
2.1.2. Synthesis of TADFIW
2.1.3. Synthesis of TAIW
2.2. Other Synthesis Methods
2.2.1. Non-Hydrodebenzylation-Oxidation Debenzylation of HBIW
2.2.2. Non-HBIW Route—Synthesis of Other Iso-Woodsane Precursors
2.2.3. High-Energy Materials with Non-Cage Structures—Three-Dimensional MOFs with Nitrogen-Rich Elements
3. Problems in HNIW Engineering Manufacturing
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| HBIW | hexabenzylhexaazaisowurtzitane |
| HFIW | hexafurfurylhexaazaisowurtzitane |
| HAIW | hexaacetylhexaazaisowurtzitane |
| HEDM | high energy density materials |
| HNIW | hexanitrohexaazaisowurtzitane |
| TADBIW | tetraacetyldibenzylhex-aazaisowurtzitane |
| TADFIW | tetraacetyldif-ormylhexazoisowoody |
| TAIW | tetraacetylhexazoisowoody |
| NG | nitroglycerine |
| TNT | trinitrotoluene |
| RDX | 1,3,5-trinitroperhydro-1,3,5-triazine |
| HMX | octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine |
References
- Qing, L. Study on the Morphology and Particle Size Control of CL-20; Beijing Institute of Technology: Beijing, China, 2016. [Google Scholar]
- Bayat, Y.; Malmir, S.; Hajighasemali, F.; Dehghani, H. Reductive Debenzylation of Hexabenzylhexaazaisowurtzitane using Multi-walled Carbon Nanotube-supported Palladium Catalysts: An Optimization Approach. Cent. Eur. J. Energ. Mat. 2015, 12, 439–458. [Google Scholar]
- Explosives Subject of BIT: Aspiring to the Highest Peak of Explosives in the World. Available online: http://edu.sina.com.cn/gaokao/2016-04-19/doc-ifxriqqx3013740.shtml (accessed on 19 April 2016).
- Yu, Y.; Guan, X. Studies on the Synthesis of Hexanitrohexaazaisowurtzitane. Chin. J. Energ. Mater. 1999, 7, 1–4. [Google Scholar]
- Chen, F.; Duan, B.; Yu, Y. Research Reports Compilation of Chinese Ministry of Armament Industry; No. 214. Research Institute (1978–1980); Chinese Ministry of Armament Industry: Bengbu, China, 1983.
- Zhang, M.; Liu, S.; Li, L.; Li, X.; Huang, H.; Yin, J.; Shao, X.; Yang, J. Effect of Carbon Supports on Pd Catalyst for Hydrogenation Debenzylation of Hexabenzylhexaazaisowurtzitane (HBIW). J. Energ. Mater. 2017, 35, 251–264. [Google Scholar] [CrossRef]
- Pang, S.; Shen, F.; Lyu, P.; Dong, K.; Zhang, Y.; Sun, C.; Song, J.; Zhao, X. Research Progress in Synthesis of Hexanitrohexaazaisowurtzitane. Acta Armamentarii 2014, 35, 725–732. [Google Scholar]
- Ou, Y.; Chen, B.; Jia, H.; Pan, Z.; Xu, Y. Structural Identification of Hexanitrohexaazaisowurtzitane. Chin. J. Energ. Mater. 1995, 3, 1–8. [Google Scholar]
- Nielsen, A.T. Synthesis of caged nitramine explosives. In Proceedings of the JANNAF Joint Army Navy NASA Air Force Propulsion Meeting, San Diego, CA, USA, 15–17 December 1987. [Google Scholar]
- Maksimowski, P.; Golofit, T.; Tomaszewski, W. Palladium Catalyst in the HBIW Hydrodebenzylation Reaction. Deactivation and Spent Catalyst Regeneration Procedure. Cent. Eur. J. Energ. Mat. 2016, 13, 333–348. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Liu, S. Application of Palladium Bimetallic Catalyst in HBIW Catalytic Hydrolysis. China Patent CN 106,946,894 A, 14 July 2017. [Google Scholar]
- Lou, D.; Wang, H.; Liu, S.; Li, L.; Zhao, W.; Chen, X.; Wang, J.; Li, X.; Wu, P.; Yang, J. PdFe bimetallic catalysts for debenzylation of hexabenzylhexaazaisowurtzitane (HBIW) and tetraacetyldibenzylhexaazaisowurtzitane (TADBIW). Catal. Commun. 2018, 109, 28–32. [Google Scholar] [CrossRef]
- Ou, Y.; Xu, Y.; Chen, J.; Chen, B.; Zheng, F.; Jia, H.; Wang, C. Synthesis of Cage Heterocyclic Compounds with High Strain. Chem. J. Chin. Univ. 1999, 20, 561–564. [Google Scholar]
- Gong, X.; Sun, C.; Pang, S.; Zhang, J.; Li, Y.; Zhao, X. Research Progress in Study of Isowurtzitane Derivatives. Chin. J. Org. Chem. 2012, 32, 486–496. [Google Scholar] [CrossRef]
- Ou, Y.; Liu, J.; Wang, Y.; Meng, Z. Synthesis and Industrial Production of Five Hydrogenolysis-debenzylation Compounds from Hexabenzylhexaazaisowurtzitane. Chin. J. Org. Chem. 2005, 68, 731–735. [Google Scholar]
- Liu, S.; Ji, F.; Li, X.; Pan, X.; Chen, S.; Wang, X.; Zhang, Y.; Men, Y. Stick-like mesoporous titania loaded Pd as highly active and cost effective catalysts for hydrodebenzylation of hexabenzylhexaazaisowurtzitane (HBIW). Mol. Catal. 2019, 477, 110556. [Google Scholar] [CrossRef]
- Zhang, M. Preparation and Performances of Palladium Carbon Catalysts Supported by Different Type Carbon Materials; Liaocheng University: Liaocheng, China, 2016. [Google Scholar]
- Fotouhi-Far, F.; Bashiri, H.; Hamadanian, M.; Keshavarz, M.H. Increment of activity of Pd(OH)(2)/C catalyst in order to improve the yield of high performance 2,4,6,8,10,12-hexanitrohexaazaisowurtzitane (HNIW). Inorg. Nano-Met. Chem. 2017, 47, 1489–1494. [Google Scholar] [CrossRef]
- Qiu, W.; Jiang, J.; Sun, C.; Pang, S.; Liu, H.; Zi, X.; Zhang, G.; He, H. Preparation and characterization of highly active hydrodebenzyl Pd(OH)2/C catalyst. In Proceedings of the 9th Chinese National Annual Conference on Industrial Catalytic Technology and Application, Xiamen, China, 1 August 2012; pp. 197–199. [Google Scholar]
- Bayat, Y.; Ebrahimi, H.; Fotouhi-Far, F. Optimization of Reductive Debenzylation of Hexabenzylhexaazaisowurtzitane (the Key Step for Synthesis of HNIW) Using Response Surface Methodology. Org. Process Res. Dev. 2012, 16, 1733–1738. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, H.; Dong, K.; Sun, C.; Pang, S.; Bai, G.; Zi, X.; Zhang, G.; He, H. Preparation of Pd(OH)2/C Catalyst for Hydrogenolytic Debenzylation of Hexabenzylhexaazaisowurtzitane. Chin. J. Energ. Mater. 2014, 22, 441–446. [Google Scholar]
- Wardle, R.B.; Edwards, W.W. Hydrogenolysis of 2,4,6,8,10,12-Hexabenzyl-2,4,6,8,10,12-Hexaazatetracyclo [5.5.0.05,9.03,11] Dodecane: World Intellectual Property Organization. US Patent US 5,739,325 A, 14 April 1998. [Google Scholar]
- Chen, L.; Fang, T.; Guo, X.; Zhao, X. Investigation on Hydrogrnolysis of Hexabenzylhexaazaisowurtzitane. Chin. J. Explos. Propell. 2002, 25, 29–30. [Google Scholar]
- Han, W.; Ou, Y.; Zhang, X.; Huang, X.; Mou, W.; Gao, Y. Hydrogenolysis of Triacetyltribenzylhexaazaisowurtzitane and Tetraacetyldibenzylhexaazaisowurtzitane. Chin. J. Energ. Mater. 2008, 16, 153–155. [Google Scholar]
- Liu, J.; Wang, J.; Han, W.; Lu, L. Study on Hydrogenolysis of HBIW and Crystal Structures of the Reaction Products. Chin. J. Energ. Mater. 2003, 11, 4–7. [Google Scholar]
- Ou, Y.; Jia, H.; Chen, B.; Fan, G.; Xu, Y.; Pan, Z.; Wang, C. Synthesis and Crystal Structure of Hexanitrohexazaisowoodane. Sci. China Ser. B 1999, 1, 39–46. [Google Scholar]
- Chen, J.; Zheng, F.; Ou, Y.; Chen, B.; Wang, Z.; Li, B. Study on the Kinetics of Hydrogenolysis Debenzylation of 2,4,6,8,10,12 Hexabenzyl 2,4,6,8,10, 12 Hexaazaisowurtzitane(HBIW). Chin. J. Beijing Inst. Technol. 1999, 9, 133–136. [Google Scholar]
- Han, W.; Ou, Y.; Liu, J.; Chen, B. Synthesis and Crystal Structure of Triacetyltribenzyl-hexaazaisowurtzitane (TATBIW•0.5H2O). Chin. J. Org. Chem. 2005, 25, 114–119. [Google Scholar]
- Bellamy, A. Reductive debenzylation of hexabenzylhexaazaisowurtzitane. Tetrahedron 1995, 51, 4711–4715. [Google Scholar] [CrossRef]
- Duddu, R.; Dave, P.R. Process and Compositions for Nitration of N-Nitric Acid at Elevated Temperatures to Form HNIW and Recovery of Gamma HNIW with High Yields and Purities and Crystallizations to Recover Epsilon HNIW Crystals. US Patent US 6,160,113 A, 12 December 2000. [Google Scholar]
- Simpson, R.L.; Lee, R.S.; Tillotson, T.M.; Hrubesh, L.W.; Swansiger, R.W.; Fox, G.A. Process for Preparing Energetic Materials. US Patent US 8,075,716 B1, 13 December 2011. [Google Scholar]
- Lü, L.; Ou, Y.; Wang, J. Nitrosolysis-debenzylation to Tetraacetyldibenzylhexaazaisowurtzitane. Chin. J. Explos. Propell. 2003, 26, 41–43. [Google Scholar]
- Lü, L.; Ou, Y.; Wang, J. Synthesis and Crystal Strucutre of Tetraacetyldinitroso-hexaazaisowurtzitane (TADNIW•H2O). Chin. J. Org. Chem. 2005, 4, 399–404. [Google Scholar]
- Bayat, Y.; Hajimirsadeghi, S.S.; Pourmortazavi, S.M. Statistical Optimization of Reaction Parameters for the Synthesis of 2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane. Org. Process Res. Dev. 2011, 15, 810–816. [Google Scholar] [CrossRef]
- Stierstorfer, J.; Klaptke, T.M. High Energy Materials. Propellants, Explosives and Pyrotechnics. By Jai Prakash Agrawal. Angew. Chem. Int. Edit. 2010, 36, 6253. [Google Scholar] [CrossRef]
- Latypov, N.V.; Wellmar, U.; Goede, P.; Bellamy, A.J. Synthesis and Scale-Up of 2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane from 2,6,8,12-Tetraacetyl-4,10-dibenzyl-2,4,6,8,10,12-hexaazaisowurtzitane (HNIW, CL-20). Org. Process Res. Dev. 2000, 4, 156–158. [Google Scholar] [CrossRef]
- Bayat, Y.; Mokhtari, J. Preparation of 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane from 2,6,8,12-tetraacetyl 2,4,6,8,12-hexaazaisowurtzitane using Various Nitrating Agents. Def. Sci. J. 2011, 61, 171–173. [Google Scholar]
- Mandal, A.K.; Pant, C.S.; Kasar, S.M.; Soman, T. Process Optimization for Synthesis of CL-20. J. Energ. Mater. 2009, 27, 231–246. [Google Scholar] [CrossRef]
- Koskin, A.P.; Simakova, I.L.; Parmon, V.N. Reductive debenzylation of hexabenzylhexaazaisowurtzitane—The key step of the synthesis of polycyclic nitramine hexanitrohexaazaisowurtzitane. Russ. Chem. Bull. 2007, 56, 2370–2375. [Google Scholar] [CrossRef]
- Maksimowski, P.; Fabijańska, A.; Adamiak, J. Tetraaeetyl-dibenzyl-hexaazaisowurtzitane nitrosation—Studies on scale-up synthesis of HNIW. Propell. Explos. Pyrot. 2010, 35, 353–358. [Google Scholar] [CrossRef]
- Bazaki, H.; Kawabe, S.; Miya, H.; Kodama, T. Synthesis and sensitivity of hexanitrohexaazaisowurzitane (HNIW). Propell. Explos. Pyrot. 1999, 23, 333–336. [Google Scholar] [CrossRef]
- Ou, Y.; Xu, Y.; Chen, B.; Liu, L.; Wang, C. Synthesis of Hexanitrohexaazaisowurtzitane from Tetraacetyldiformylhexaazaisowurtzitane. Chin. J. Org. Chem. 2000, 20, 556–559. [Google Scholar]
- Zhao, X.; Fang, T.; Sun, C. Research and Development of HNIW synthesis. Acta Armamentarii 2004, 25, 354–358. [Google Scholar]
- Zhao, X.; Shi, N. The Crystal Structure of ε-hexanitrohexazazozygrotane. Chin. J. Chem. 1995, 23, 2158–2160. [Google Scholar]
- Wang, C.; Ou, Y.; Chen, B. Synthesis of Hexaacetylhexaazaisowurtzitane. Chin. J. Chem. World 2000, 9, 462–464. [Google Scholar]
- Wang, C.; Ou, Y.; Chen, B. Synthesis and crystal structure of hexaacetylhexaazaisowurtzitane. Chin. J. Chem. Online 2000, 3, 44. [Google Scholar] [CrossRef]
- Liu, J.; Ou, Y.; Han, W.; Chen, B. Hydrogenolysis Debenzylation of TADBIW in Propionic Acid and n-Butyric Acid. Chin. J. Explos. Propell. 2004, 27, 10–12. [Google Scholar]
- Sanderson, A.J.; Warner, K.; Wardle, R.B. Process for Making 2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-Hexaazatetracyclo [5.5.0.05,903,11] Dodecane. US Patent US 6,391,130 B1, 15 February 2000. [Google Scholar]
- Bayat, Y.; Mokhtari, J.; Farhadian, N.; Bayat, M. Heteropolyacids: An Efficient Catalyst for Synthesis of CL-20. J. Energ. Mater. 2012, 30, 124–134. [Google Scholar] [CrossRef]
- Surapaneni, R.; Damavarapu, R. Process improvements in CL-20 manufacture. In Proceedings of the 31st International Annual Conference “ICT on Energetic Materials”, Karlsruhe, Germany, 27–30 June 2000; pp. 108/1–108/4. [Google Scholar]
- Chen, S.; Qiu, W.; Yu, Y. Oxidation Debenzylation and Acetylation of Hexabenzylhexaazaisowurtzitane. Chin. J. Explos. Propell. 2000, 23, 11–12. [Google Scholar]
- Qiu, W.; Chen, S.; Yu, Y. The Structure of 2,4,8,10-tetrabenzyl-6,12-dibenzoyl-2,4,6,8,10,12-hexaazatetracyclo [5.5.0.05,9.03,11] dodecane. Chin. J. Explos. Propell. 2001, 24, 62–63. [Google Scholar]
- Pang, S.; Yu, Y.; Zhao, X. Separation and Identification of the Nitrosation Products from Monoacetyltribenzoyldibenzylhexaazaisowutzitane. Chin. J. Energ. Mater. 2002, 10, 1–3. [Google Scholar]
- Pang, S.; Yu, Y.; Zhao, X. Phase Transfer Catalyze the Oxidation of Hexabenzylhexaazaisowutzitane. Acta Armamentarii 2002, 23, 276–278. [Google Scholar]
- Pang, S.; Yu, Y.; Zhao, X. Nitrosation of the Oxidation Products of Hexabenzylhexaazaisowurtzitane. Chin. J. Explos. Propell. 2002, 25, 27–28. [Google Scholar]
- Liu, J.; Chen, S.; Yu, T.; Zhao, X. Study on Debenzylation of TADBIW by Oxidation. Chin. J. Energ. Mater. 2002, 10, 145–147. [Google Scholar]
- Liu, J.; Chen, S.; Yu, Y.; Zhao, X. Nitrosation of the Oxidation Products of Hexabenzylhexaazaisowurtzitane. Chin. J. Explos. Propell. 2003, 26, 60–61. [Google Scholar]
- Liu, J.; Chen, S.; Yu, Y.; Zhao, X. Oxidation of Derivatives of Hexaazaisowurtzitane. Chin. J. Explos. Propell. 2003, 26, 5–7. [Google Scholar]
- Pang, S.; Yu, Y.; Zhao, X. Nitration of Tetraacetyldibenzylhexaazaisowutzitane with Phase Transfer Catalysts. Chin. J. Energ. Mater. 2003, 11, 222–223. [Google Scholar]
- Pang, S.; Yu, Y. The Hydrolysis-nitration of Hexabenzylhexaazaisowurtzitane’s Oxidation Products. Chin. J. Energ. Mater. 2004, 12, 41–43. [Google Scholar]
- Pang, S.; Yu, Y.; Zhao, X. The Synthesis of Hexanitrohexaazaisowutzitane by Oxidation. Chin. J. Explos. Propell. 2004, 27, 9–11. [Google Scholar]
- Pang, S.; Yu, Y.; Zhao, X. A Novel Synthetic Route to Hexanitrohexaazaisowurtzitane. Propell. Explos. Pyrot. 2005, 30, 442–444. [Google Scholar] [CrossRef]
- Gore, G.M.; Sivabalan, R.; Nair, U.N.; Saikia, A.; Venugopalan, S.; Gandhe, B.R. Synthesis of CL-20: By oxidative debenzylation with cerium (IV) ammonium nitrate (CAN). Indian J. Chem. 2007, 46B, 505–508. [Google Scholar] [CrossRef]
- Sysolyatin, S.V.; Lobanova, A.A.; Chernikova, Y.T.; Sakovich, G.V. Methods of synthesis and properties of hexanitrohexaazaisowurtzitane. Russ. Chem. Rev. 2005, 74, 757–764. [Google Scholar] [CrossRef]
- Cagnon, G.; Eck, G.; Herve, G.; Jacob, G. Process for the 2-Stage Synthesis of Hexanitrohexaazaisowurtzitane Starting from a Primary Amine. US Patent US 7,279,572 B2, 9 October 2007. [Google Scholar]
- Chapman, R.D.; Hollins, R.A. Benzylamine-Free, Heavy-Metal-Free Synthesis of CL-20 via Hexa(1-propenyl)hexaazaisowurtzitane. J. Energ. Mater. 2008, 26, 246–273. [Google Scholar] [CrossRef]
- Chapman, R.D. Processes for Preparing Eartain Hexaazaisowurtzitanes and Their Use in Preparing Hexanitrohexaazaisowurtzitane. US Patent US 8,268,993 B1, 18 September 2012. [Google Scholar]
- Li, X.; Sun, C.; Zhao, X.; Song, J. Synthesis of Hexaallylhexaazaisowurtzitane. Chin. J. Energ. Mater. 2007, 15, 490–491. [Google Scholar]
- Dong, K.; Wang, Y.; Gong, X.; Zhang, J.; Sun, C.; Pang, S. Formyl azido substituted nitro hexaazaisowurtzitane—Synthesis, characterization and energetic properties. New J. Chem. 2013, 37, 3685–3691. [Google Scholar] [CrossRef]
- Hou, A. The Synthesis and Its Technology of the Hexanitrohexanzaisowurtzitane; Southeast University: Nanjing, China, 2007. [Google Scholar]
- Han, W.; Ou, Y.; Chen, B. Studies of Different Supported Palladium Catalysts for Debenzylation of the Caged Tertiary Amine. Chin. J. Fine Chem. 2004, 21, 819–822. [Google Scholar]
- Chen, H.; Chen, S.; Hu, F.; Li, L.; Yang, R. Optical Spectral Verification of a Harmful Impurity to Palladium Catalyst. Chin. J. Spectrosc. Spect. Anal. 2007, 27, 469–472. [Google Scholar]
- Chen, H.; Chen, S.; Li, L.; Shi, Y.; Yang, R.; Xiang, Y. Identification and Forming Process of Main Impurity in HBIW. Acta Armamentarii 2007, 28, 1179–1182. [Google Scholar]
- Fotouhi-Far, F.; Bashiri, H.; Hamadanian, M. Study of Deactivation of Pd(OH)(2)/C Catalyst in Reductive Debenzylation of Hexabenzylhexaazaisowurtzitane. Propell. Explos. Pyrot. 2017, 42, 213–219. [Google Scholar] [CrossRef]
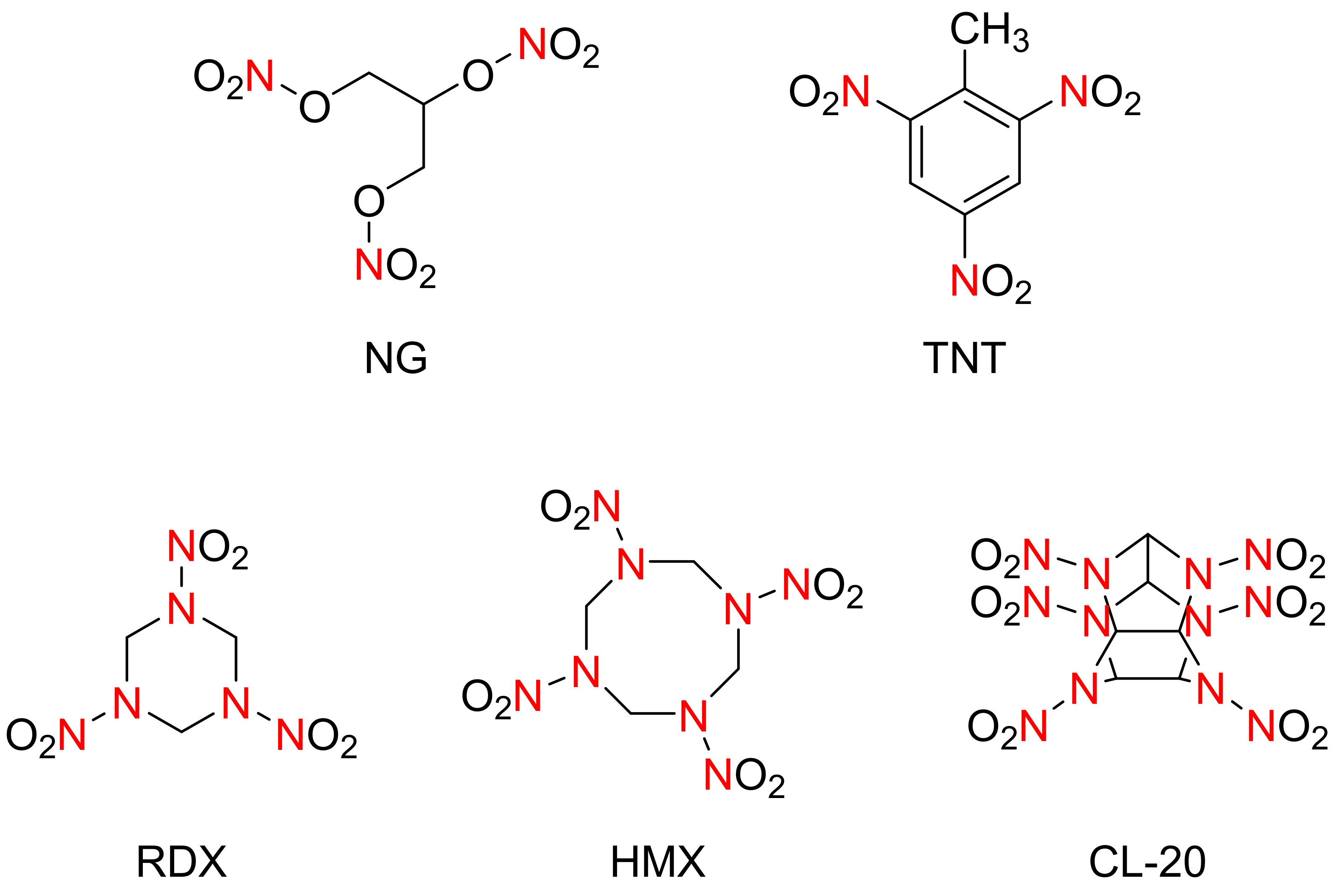

| ITEM | TNT | RDX | HMX | CL-20 |
|---|---|---|---|---|
| Density/g·cm−3 | 1.65 | 1.82 | 1.90 | 2.04 |
| Relative density/g·cm−3 | 91.64 | 91.77 | 91.88 | - |
| Oxygen balance/% | −74.0 | −21.6 | −21.6 | −10.95 |
| Standard enthalpy of formation/kJ·mol−1 | −45.4 | 92.6 | 104.8 | 416.28 |
| Detonation velocity/km·s−1 | 6.9 | 8.6 | 9.0 | 9.6 ** |
| Detonation pressure/GPa | 19 | 34 | 39 | 43 ** |
| Critical capacity/cm3·g−1 | 738 | 903 | 886 | 827 ** |
| Friction sensitivity */N | 353 | 120 | 120 | 54 |
| Impact sensitivity/N·m | 15 | 7.4 | 7.4 | 4 |
| Flash point/°C | 300 | 230 | 287 | 228 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Zhu, R.; Shi, T.; Wang, Y.; Niu, X.; Zhang, Y.; Zhu, J.; Li, W.; Hu, W.; Xu, R. Research Progress and Key Issues of Hydrodebenzylation of Hexabenzylhexaazaisowurtzitane (HBIW) in the Synthesis of High Energy Density Material Hexanitrohexaazaisowurtzitane (HNIW). Materials 2022, 15, 409. https://doi.org/10.3390/ma15020409
Tang X, Zhu R, Shi T, Wang Y, Niu X, Zhang Y, Zhu J, Li W, Hu W, Xu R. Research Progress and Key Issues of Hydrodebenzylation of Hexabenzylhexaazaisowurtzitane (HBIW) in the Synthesis of High Energy Density Material Hexanitrohexaazaisowurtzitane (HNIW). Materials. 2022; 15(2):409. https://doi.org/10.3390/ma15020409
Chicago/Turabian StyleTang, Xiaofei, Rui Zhu, Tianjing Shi, Yu Wang, Xiaochen Niu, Yao Zhang, Junchen Zhu, Wei Li, Wanpeng Hu, and Ruoqian Xu. 2022. "Research Progress and Key Issues of Hydrodebenzylation of Hexabenzylhexaazaisowurtzitane (HBIW) in the Synthesis of High Energy Density Material Hexanitrohexaazaisowurtzitane (HNIW)" Materials 15, no. 2: 409. https://doi.org/10.3390/ma15020409
APA StyleTang, X., Zhu, R., Shi, T., Wang, Y., Niu, X., Zhang, Y., Zhu, J., Li, W., Hu, W., & Xu, R. (2022). Research Progress and Key Issues of Hydrodebenzylation of Hexabenzylhexaazaisowurtzitane (HBIW) in the Synthesis of High Energy Density Material Hexanitrohexaazaisowurtzitane (HNIW). Materials, 15(2), 409. https://doi.org/10.3390/ma15020409





