Dynamic Polarization Behaviors of Equimolar CoCrFeNi High-Entropy Alloy Compared with 304 Stainless Steel in 0.5 M H2SO4 Aerated Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Electrochemical Measurements
2.3. Component Analyses of the Passivation Films
2.4. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
2.5. First-Principles Calculations
3. Result and Discussion
3.1. Electrochemical Corrosion Behaviors Analysis
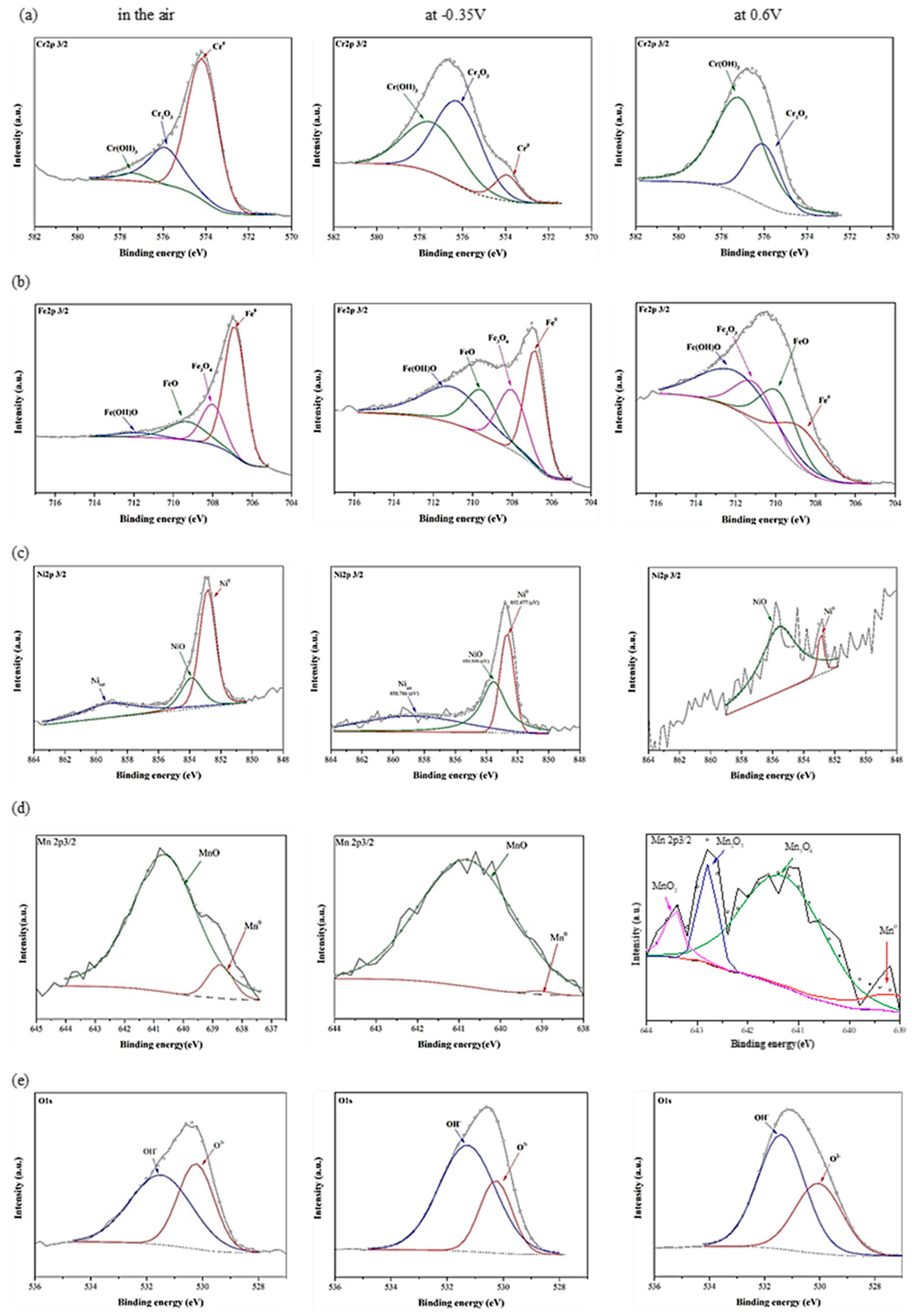
3.2. Characteristics of the Passivation Films by X-ray Photoelectron Spectroscopy
3.3. The Concentration of Dissolved Elements
3.4. First-Principles Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, J.-W.; Chen, S.K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Gao, M.C. Chapter 11: Design of High-Entropy Alloys. In High-Entropy Alloys: Fundamentals and Applications; Gao, M.C., Yeh, J.-W., Liaw, P.K., Zhang, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Gao, M.C.; Zhang, B.; Guo, S.M.; Qiao, J.W.; Hawk, J.A. High-Entropy Alloys in Hexagonal Close-Packed Structure. Metall. Mater. Trans. A 2016, 47, 3322–3332. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Woodward, C. Effect of aluminum on the microstructure and properties of two refractory high-entropy alloys. Acta Mater. 2014, 68, 214–228. [Google Scholar] [CrossRef]
- Zhang, Z.; Mao, M.M.; Wang, J.; Gludovatz, B.; Zhang, Z.; Mao, S.X.; George, E.; Yu, Q.; Ritchie, R.O. Nanoscale origins of the damage tolerance of the high-entropy alloy CrMnFeCoNi. Nat. Commun. 2015, 6, 10143. [Google Scholar] [CrossRef]
- Hemphill, M.; Yuan, T.; Wang, G.; Yeh, J.; Tsai, C.; Chuang, A.; Liaw, P. Fatigue behavior of Al0.5CoCrCuFeNi high entropy alloys. Acta Mater. 2012, 60, 5723–5734. [Google Scholar] [CrossRef]
- Wang, W.-R.; Wang, W.-L.; Yeh, J.-W. Phases, microstructure and mechanical properties of AlxCoCrFeNi high-entropy alloys at elevated temperatures. J. Alloys Compd. 2014, 589, 143–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.; Cheng, Y.; Liaw, P.K. High-entropy Alloys with High Saturation Magnetization, Electrical Resistivity and Malleability. Surf. Sci. Rep. 2013, 3, 1455. [Google Scholar] [CrossRef]
- Chuang, M.-H.; Tsai, M.-H.; Wang, W.-R.; Lin, S.-J.; Yeh, J.-W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- King, P.F.; Uhlig, H.H. Passivity in the iron-chromium binary alloys. J. Phys. Chem. 1959, 63, 2026–2032. [Google Scholar] [CrossRef]
- Aronowitz, G.; Hackerman, N. The Passivity of Iron-Chromium Alloys. J. Electrochem. Soc. 1963, 110, 633. [Google Scholar] [CrossRef]
- McBee, C.; Kruger, J. Nature of passive films on iron-chromium alloys. Electrochim. Acta 1972, 17, 1337–1341. [Google Scholar] [CrossRef]
- Holliday, J.E.; Frankenthal, R.P. Characterization of Passivating Films on Fe-Cr Alloys by Soft X-Ray Spectroscopy. J. Electrochem. Soc. 1972, 119, 1190. [Google Scholar] [CrossRef]
- Asami, K.; Hashimoto, K.; Shimodaira, S. An ESCA study of the Fe2+/Fe3+ ratio in passive films on iron-chromium alloys. Corros. Sci. 1976, 16, 387–391. [Google Scholar] [CrossRef]
- Asami, K.; Hashimoto, K.; Shimodaira, S. Changes in the surface compositions of FeCr alloys caused by heating in a high vacuum. Corros. Sci. 1978, 18, 125–137. [Google Scholar] [CrossRef]
- Leygraf, C.; Hultquist, G.; Olefjord, I.; Elfström, B.-O.; Knyazheva, V.; Plaskeyev, A.; Kolotyrkin, Y. Selective dissolution and surface enrichment of alloy components of passivated Fe18Cr and Fe18Cr3Mo single crystals. Corros. Sci. 1979, 19, 343–357. [Google Scholar] [CrossRef]
- Ogawa, H.; Omata, H.; Itoh, I.; Okada, H. Auger Electron Spectroscopic and Electrochemical Analysis of the Effect of Alloying Elements on the Passivation Behavior of Stainless Steels. Corrosion 2013, 34, 52–60. [Google Scholar] [CrossRef]
- Sugimoto, K.; Matsuda, S. Passive and transpassive films on Fe-Cr alloys in acid and neutral solutions. Mater. Sci. Eng. 1980, 42, 181–189. [Google Scholar] [CrossRef]
- Revesz, A.G.; Kruger, J. Passivity of metals. In Corrosion Monographs Series; Frankenthal, R.P., Kruger, J., Eds.; The Electrochemical Society: Pennington, NJ, USA, 1978; p. 131. [Google Scholar]
- Hamada, E.; Yamada, K.; Nagoshi, M.; Makiishi, N.; Sato, K.; Ishii, T.; Fukuda, K.; Ishikawa, S.; Ujiro, T. Direct imaging of native passive film on stainless steel by aberration corrected STEM. Corros. Sci. 2010, 52, 3851–3854. [Google Scholar] [CrossRef]
- Marcus, P.; Maurice, V. Atomic level characterization in corrosion studies. Philos. Trans. A Math. Phys. Eng. Sci. 2017, 375, 20160414. [Google Scholar] [CrossRef] [PubMed]
- Muangtong, P.; Rodchanarowan, A.; Chaysuwan, D.; Chanlek, N.; Goodall, R. The corrosion behaviour of CoCrFeNi-x (x = Cu, Al, Sn) high entropy alloy systems in chloride solution. Corros. Sci. 2020, 172, 108740. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Liaw, P.K.; Li, G.; Liu, R. Effect of Nb content on thermal stability, mechanical and corrosion behaviors of hypoeutectic CoCrFeNiNbχ high-entropy alloys. J. Mater. Res. 2018, 33, 3276–3286. [Google Scholar] [CrossRef]
- Shang, X.-L.; Wang, Z.-J.; Wu, Q.-F.; Wang, J.-C.; Li, J.-J.; Yu, J.-K. Effect of Mo Addition on Corrosion Behavior of High-Entropy Alloys CoCrFeNiMox in Aqueous Environments. Acta Metall. Sin. Engl. 2019, 32, 41–51. [Google Scholar] [CrossRef]
- Kao, Y.-F.; Lee, T.-D.; Chen, S.-K.; Chang, Y.-S. Electrochemical passive properties of AlxCoCrFeNi (x = 0, 0.25, 0.50, 1.00) alloys in sulfuric acids. Corros. Sci. 2010, 52, 1026–1034. [Google Scholar] [CrossRef]
- Yen, C.-C.; Lu, H.-N.; Tsai, M.-H.; Wu, B.-W.; Lo, Y.-C.; Wang, C.-C.; Chang, S.-Y.; Yen, S.-K. Corrosion mechanism of annealed equiatomic AlCoCrFeNi tri-phase high-entropy alloy in 0.5 M H2SO4 aerated aqueous solution. Corros. Sci. 2019, 157, 462–471. [Google Scholar] [CrossRef]
- Luo, H.; Li, Z.; Mingers, A.M.; Raabe, D. Corrosion behavior of an equiatomic CoCrFeMnNi high-entropy alloy compared with 304 stainless steel in sulfuric acid solution. Corros. Sci. 2018, 134, 131–139. [Google Scholar] [CrossRef]
- Yang, H.; Shang, X.; Wang, L.; Wang, Z.; Wang, J.; Lin, X. Effect of Constituent Elements on the Corrosion Resistance of Single-Phase CoCrFeNi High-Entropy Alloys in NaCl Solution. Acta Metall. Sin. Engl. 2018, 54, 905–910. [Google Scholar] [CrossRef]
- Gong, P.; Wang, D.; Zhang, C.; Wang, Y.; Jamili-Shirvan, Z.; Yao, K.; Wang, X. Corrosion behavior of TiZrHfBeCu(Ni) high-entropy bulk metallic glasses in 3.5 wt.% NaCl. npj Mater. Degrad. 2022, 6, 77. [Google Scholar] [CrossRef]
- Xing, B.; Ding, Q.; Jin, B.; Zuo, X.; Zhang, N.; Yin, S. Corrosion Resistance and Passivation Behavior of CoCrFeNi-TiAl High-Entropy Alloy Coatings in Acidic Solutions. J. Therm. Spray Technol. 2022, 31, 1673–1682. [Google Scholar] [CrossRef]
- Obot, I.; Macdonald, D.; Gasem, Z. Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: An overview. Corros. Sci. 2015, 99, 1–30. [Google Scholar] [CrossRef]
- Nie, J.; Ao, L.; Zu, X.; Gao, H. First-principles study of O2 adsorption on the α-U (001) surface. J. Phys. Chem. Solids 2014, 75, 130–135. [Google Scholar] [CrossRef]
- Baseggio, O.; Romeo, M.; Fronzoni, G.; Stener, M. The near-edge X-ray-absorption fine-structure of O2 chemisorbed on Ag (110) surface studied by density functional theory. Surf. Sci. 2013, 616, 178–185. [Google Scholar] [CrossRef]
- Ritchie, R.O. The conflicts between strength and toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef]
- van de Walle, A.; Tiwary, P.; de Jong, M.; Olmsted, D.; Asta, M.; Dick, A.; Shin, D.; Wang, Y.; Chen, L.-Q.; Liu, Z.-K. Efficient stochastic generation of special quasirandom structures. Calphad 2013, 42, 13–18. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Sillen, L.G.; Martell, A.E. Stability Constants of Metal-Ion Complexes; Special Publ. No. 17; Lange’s Handbook; The Chemical Society: London, UK, 1964; pp. 8–11. [Google Scholar]
- Chen, Y.Y.; Hong, U.T.; Shih, H.C.; Yeh, J.W.; Duval, T. Electrochemical kinetics of the high entropy alloys in aqueous environments—A comparison with type 304 stainless steel. Corros. Sci. 2005, 47, 2679–2699. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solution; Pergamon Press: London, UK, 1974; pp. 256–461. [Google Scholar]
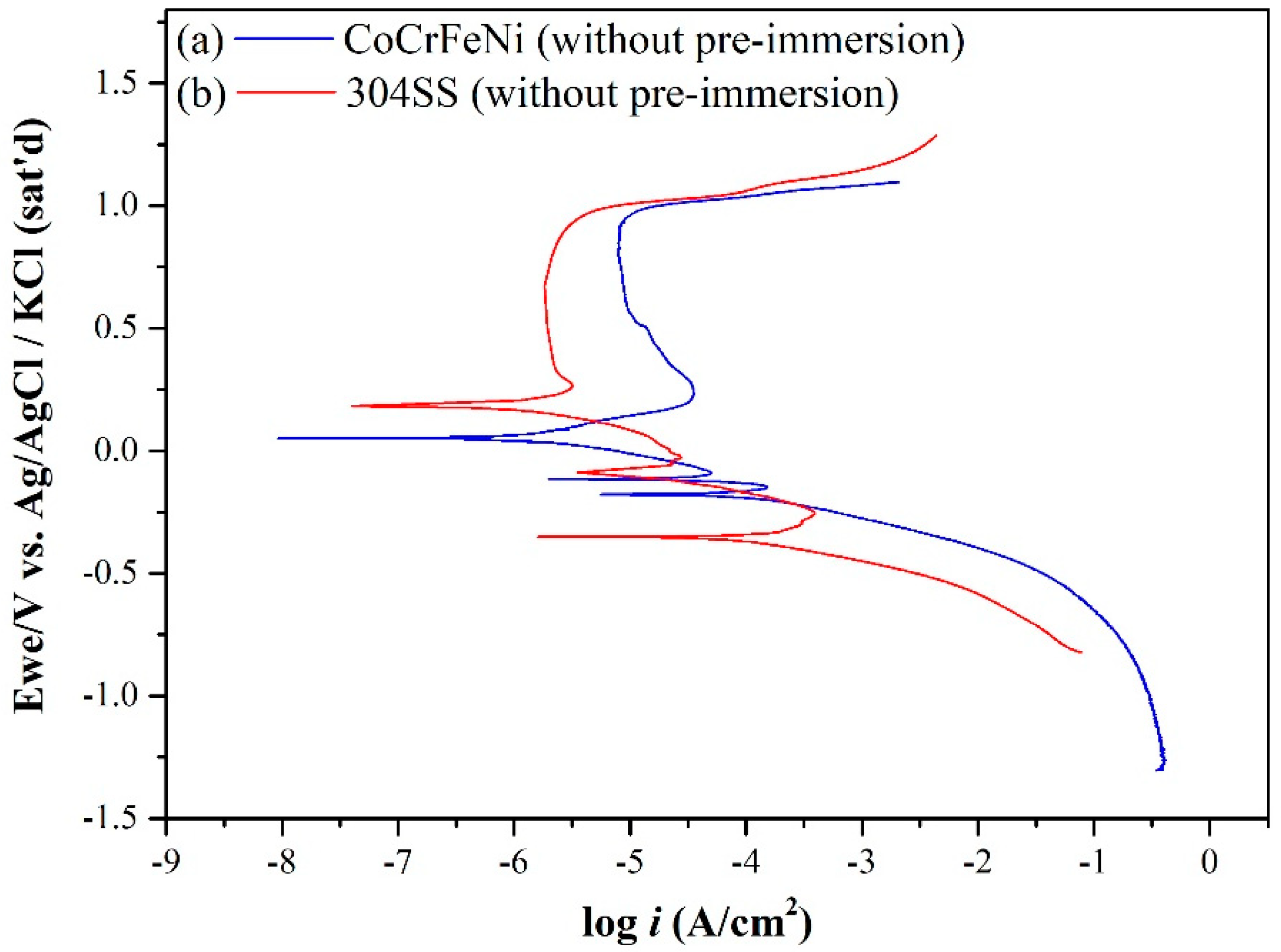
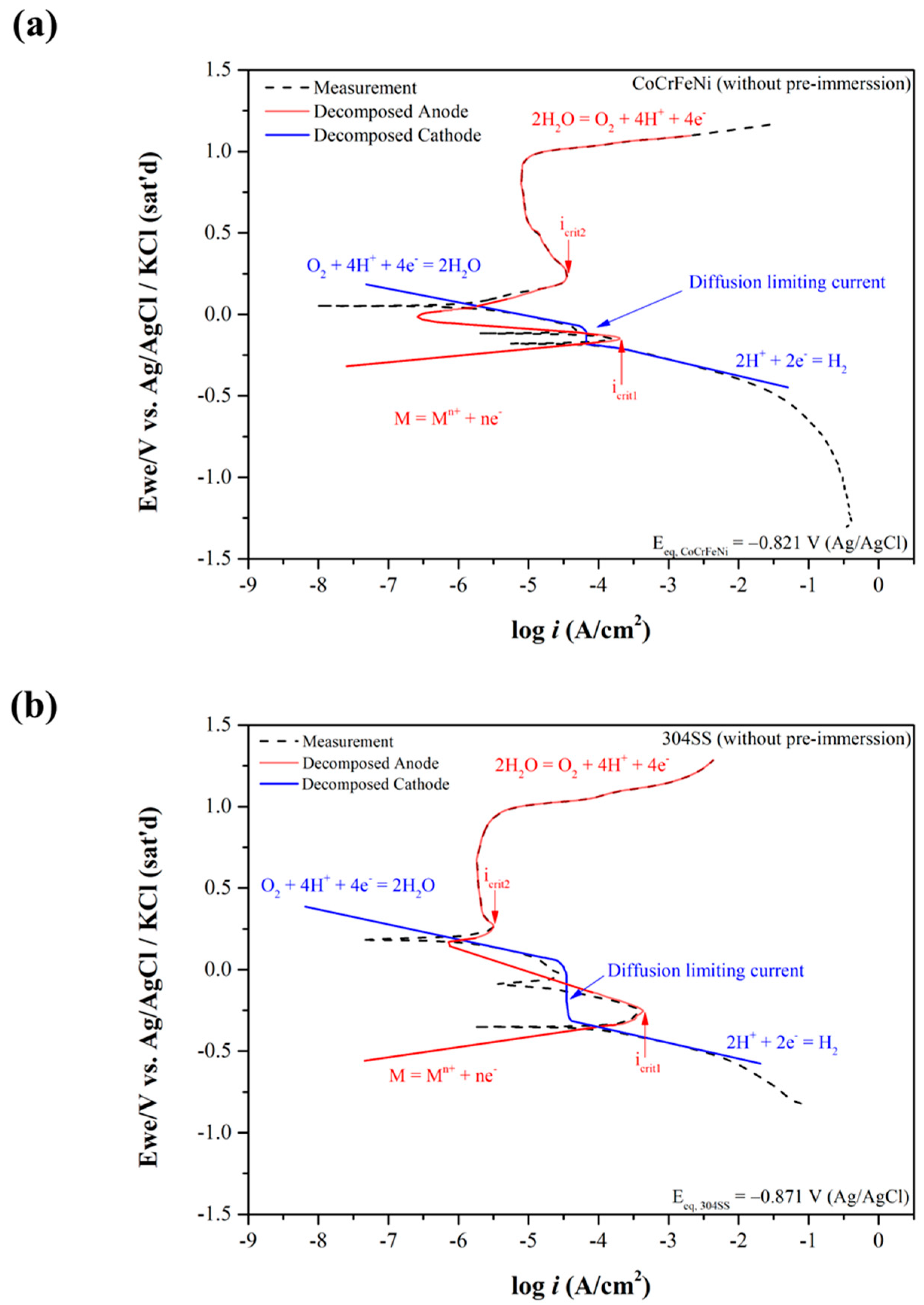
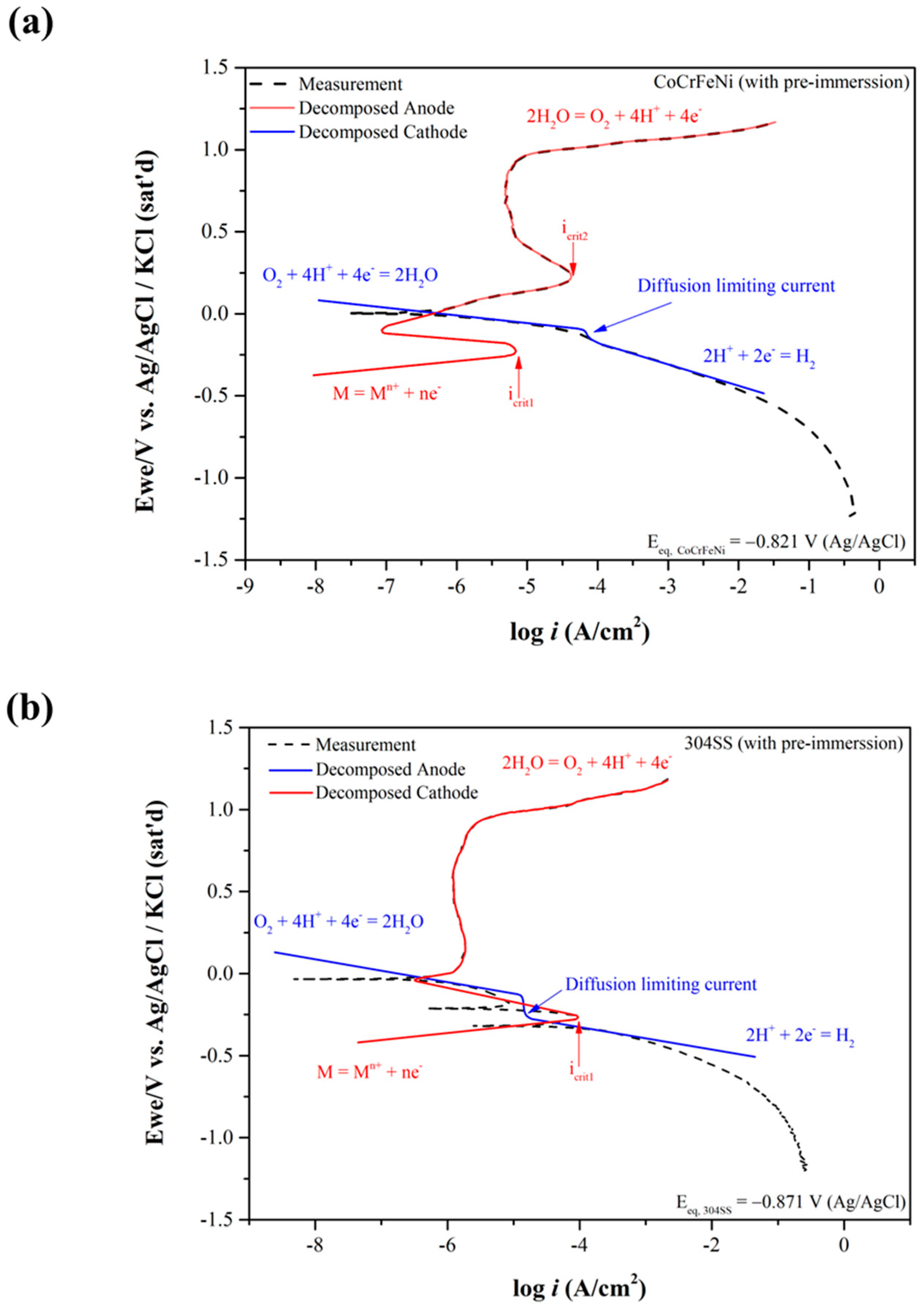



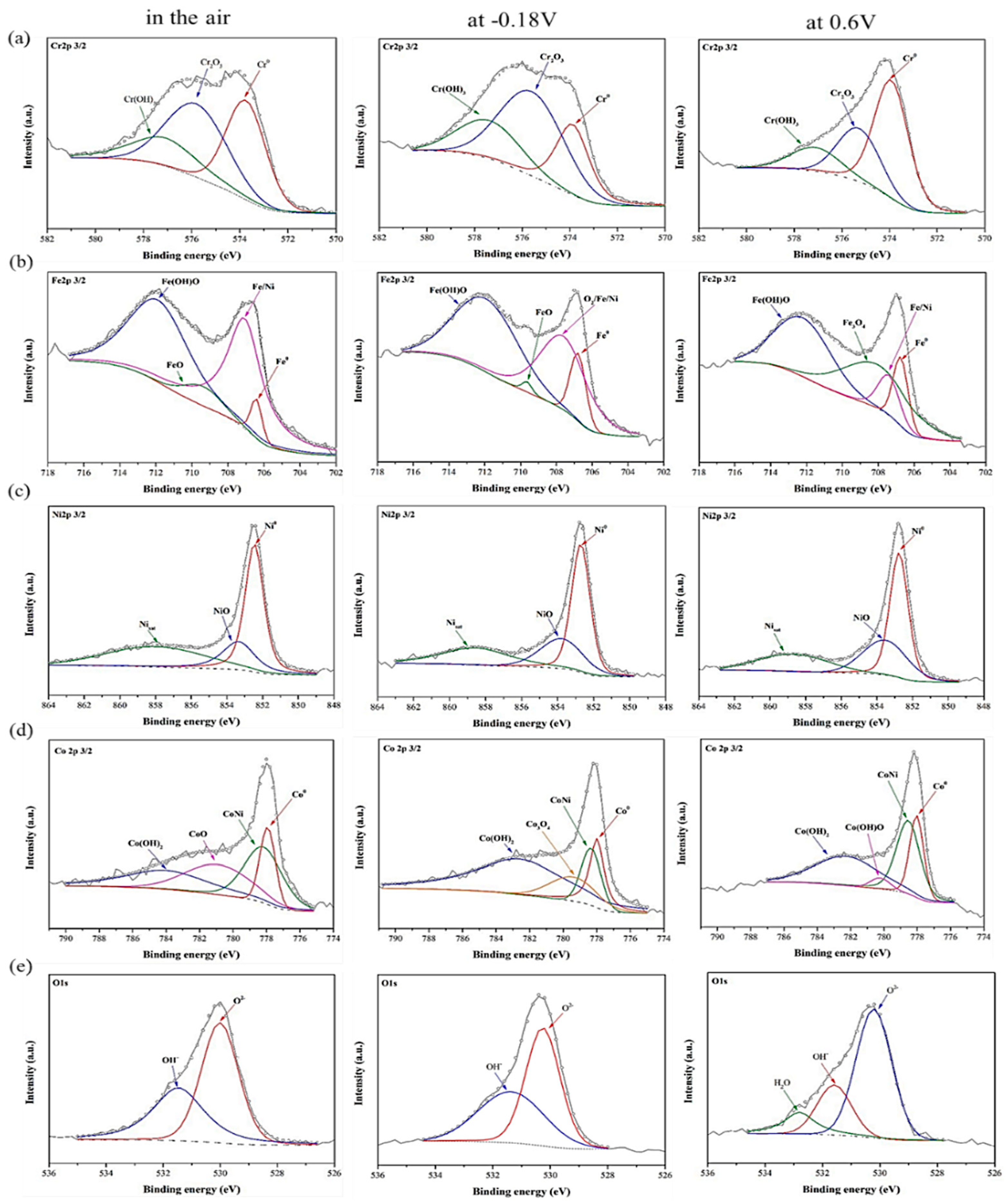


| Elements | Co | Cr | Fe | Ni | Mn | Si |
|---|---|---|---|---|---|---|
| 304SS | - | 20.65 | 68.65 | 7.50 | 2.12 | 1.08 |
| CoCrFeNi HEA | 24.36 | 26.85 | 25.18 | 23.61 | - |
| CoCrFeNi HEA (without Immersion) | CoCrFeNi HEA (Immersion for 30 min) | 304SS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ecorr (mV) | 1st | 2nd | 3rd | 5 | 1st | 2nd | 3rd | ||
| −179 | −116 | 52 | −354 | −87.9 | 183.2 | ||||
| icorr (μA/cm2) | 1st | 2nd | 3rd | 1.4 | 1st | 2nd | 3rd | ||
| 50.2 | 16.2 | 2.14 | 143.5 | 8.4 | 2.3 | ||||
| Etrans (mV) | 982 | 963 | 981 | ||||||
| ipass (μA/cm2) | 7.94 | 4.8 | 1.83 | ||||||
| icrit (μA/cm2) | 1st | 2nd | 42.7 | 1st | 2nd | ||||
| 152.8 | 35.5 | 390.8 | 3.2 | ||||||
| Metal-Metal hydroxides | |
| = −0.305 V (Ag/AgCl) | |
| = −0.359 V (Ag/AgCl) | |
| Metal-Metal ion | |
| Co = Co2+ + 2e− | = −0.457VSHE |
| Cr = Cr3+ + 3e− | = −0.864VSHE |
| Fe = Fe2+ + 2e− | = −0.627VSHE |
| Ni = Ni2+ + 2e− | = −0.430VSHE |
| Mn = Mn2+ + 2e− | = −1.356VSHE |
| Si + 3H2O = H2SiO3 + 4H+ + 4e− | = −1.096VSHE |
| = −0.675 VSHE = −0.831 V (Ag/AgCl) | |
| = −0.694 VSHE = −0.900 V (Ag/AgCl) | |
| Compound | ksp | Compound | ksp |
|---|---|---|---|
| Co(OH)2 | 3 10−16 | Fe(OH)2 | 2 10−15 |
| Co(OH)3 | 1 10−43 | Fe(OH)3 | 6 10−38 |
| Cr(OH)3 | 7 10−31 | Ni(OH)2 | 2 10−16 |
| Mn(OH)2 | 2 10−13 | Mn(OH)3 | 1 10−36 |
| 304SS | ||||
|---|---|---|---|---|
| Cr | Fe | Ni | Mn | |
| Ecorr1 | 4.3 × 102 | 5.6 × 103 | 8.0 × 102 | 5.9 × 103 |
| 0.25 V | 3.7 × 104 | 1.6 × 105 | 1.6 × 104 | 1.2 × 104 |
| 0.6 V | 5.0 × 104 | 2.1 × 105 | 2.0 × 104 | 1.4 × 104 |
| Increment from Eoc1 to 0.25 V | 3.6 × 104 | 1.6 × 105 | 1.5 × 104 | 5.8 × 103 |
| Increase ratio from Eoc1 to 0.25 V | 85 | 28 | 19 | 0.99 |
| Increment from 0.25 V to 0.6 V | 1.3 × 103 | 4.6 × 104 | 3.7 × 103 | 2.0 × 103 |
| Increase ratio from 0.25 V to 0.6 V | 0.35 | 0.28 | 0.23 | 0.18 |
| CoCrFeNi HEA | ||||
| Cr | Fe | Ni | Co | |
| Ecorr1 | 3 × 102 | 8.3 × 102 | 3.2 × 102 | 9.1 × 10 |
| 0.25 V | 5.2 × 102 | 3.8 × 103 | 9.9 × 102 | 6.9 × 102 |
| 0.6 V | 6.9 × 102 | 7.3 × 103 | 6.4 × 103 | 6.0 × 103 |
| Increment from Eoc1 to 0.25 V | 2.2 × 102 | 3 × 103 | 6.8 × 102 | 6.0 × 102 |
| Increase ratio from Eoc1 to 0.25 V | 0.72 | 3.57 | 2.14 | 6.57 |
| Increment 2 from 0.25 V to 0.6 V | 1.7 × 102 | 3.5 × 103 | 5.4 × 103 | 5.3 × 103 |
| Increase ratio from 0.25 V to 0.6 V | 0.33 | 0.93 | 5.46 | 7.76 |
| Facets of 304SS (Cr27Fe99Ni13Mn3Si2) | ||
|---|---|---|
| FCC (100) (144 + 18 OH) | 1.7982 | 0.2866 |
| FCC (110) (144 + 12 OH) | 1.9843 | 0.2229 |
| FCC (111) (144 + 16 OH) | 1.4342 | 0.2631 |
| Average | 1.7389 | 0.2575 |
| Facets of HEA (Co36Cr36Fe36Ni36) | (eV/OH) | (eV/Å2) |
| FCC (100) (144 + 18 OH) | 1.4242 | 0.2273 |
| FCC (110) (144 + 12 OH) | 1.6362 | 0.1845 |
| FCC (111) (144 + 16 OH) | 1.1862 | 0.2189 |
| Average | 1.4156 | 0.2103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, C.-C.; Tsai, T.-L.; Wu, B.-W.; Lo, Y.-C.; Tsai, M.-H.; Yen, S.-K. Dynamic Polarization Behaviors of Equimolar CoCrFeNi High-Entropy Alloy Compared with 304 Stainless Steel in 0.5 M H2SO4 Aerated Aqueous Solution. Materials 2022, 15, 6976. https://doi.org/10.3390/ma15196976
Yen C-C, Tsai T-L, Wu B-W, Lo Y-C, Tsai M-H, Yen S-K. Dynamic Polarization Behaviors of Equimolar CoCrFeNi High-Entropy Alloy Compared with 304 Stainless Steel in 0.5 M H2SO4 Aerated Aqueous Solution. Materials. 2022; 15(19):6976. https://doi.org/10.3390/ma15196976
Chicago/Turabian StyleYen, Chao-Chun, Ting-Lun Tsai, Bo-Wei Wu, Yu-Chieh Lo, Ming-Hung Tsai, and Shiow-Kang Yen. 2022. "Dynamic Polarization Behaviors of Equimolar CoCrFeNi High-Entropy Alloy Compared with 304 Stainless Steel in 0.5 M H2SO4 Aerated Aqueous Solution" Materials 15, no. 19: 6976. https://doi.org/10.3390/ma15196976
APA StyleYen, C.-C., Tsai, T.-L., Wu, B.-W., Lo, Y.-C., Tsai, M.-H., & Yen, S.-K. (2022). Dynamic Polarization Behaviors of Equimolar CoCrFeNi High-Entropy Alloy Compared with 304 Stainless Steel in 0.5 M H2SO4 Aerated Aqueous Solution. Materials, 15(19), 6976. https://doi.org/10.3390/ma15196976






