Properties of CuFeS2/TiO2 Nanocomposite Prepared by Mechanochemical Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
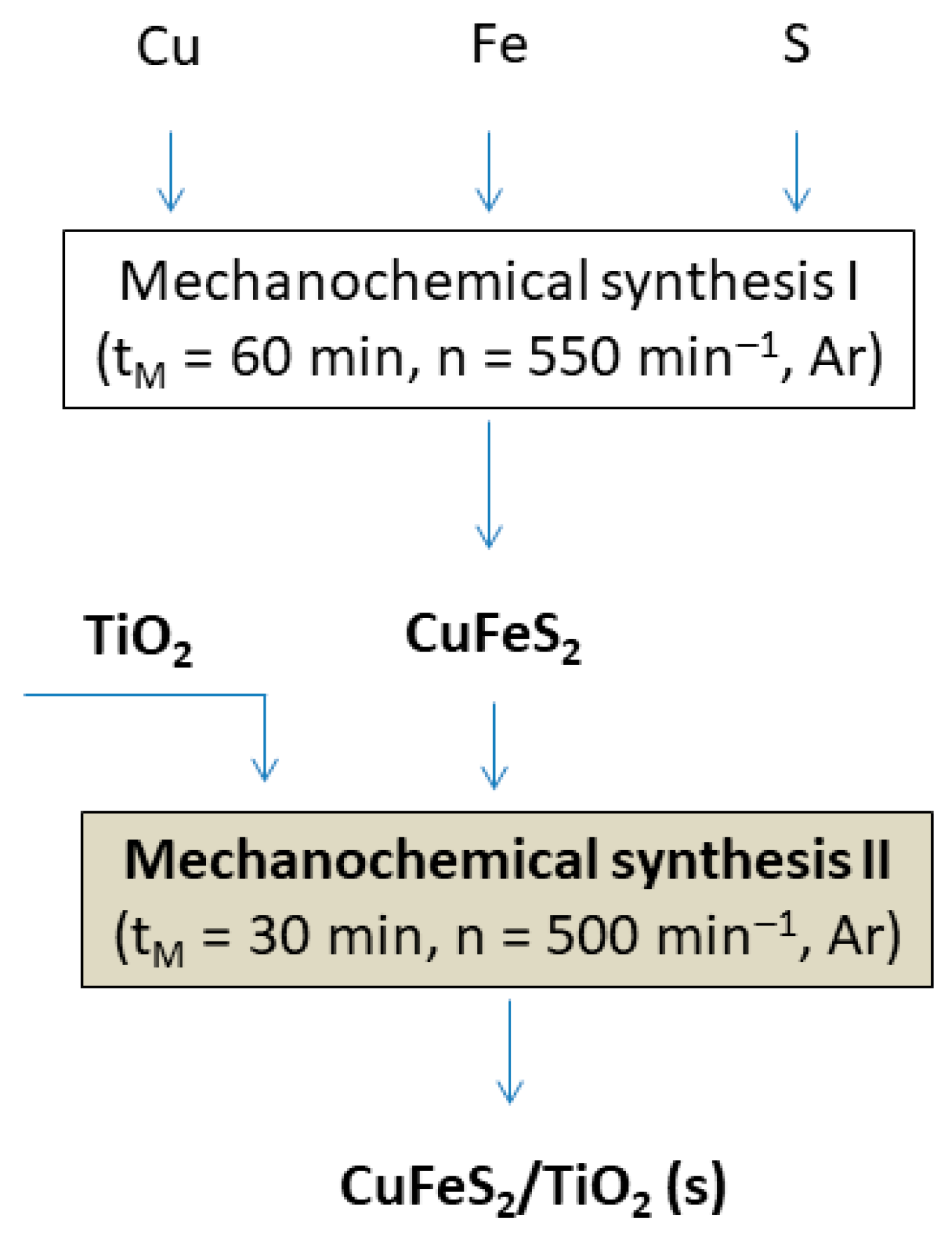
2.2. Mechanochemical Synthesis of CuFeS2/TiO2 Nanocomposite
2.3. Characterization Methods
3. Results and Discussion
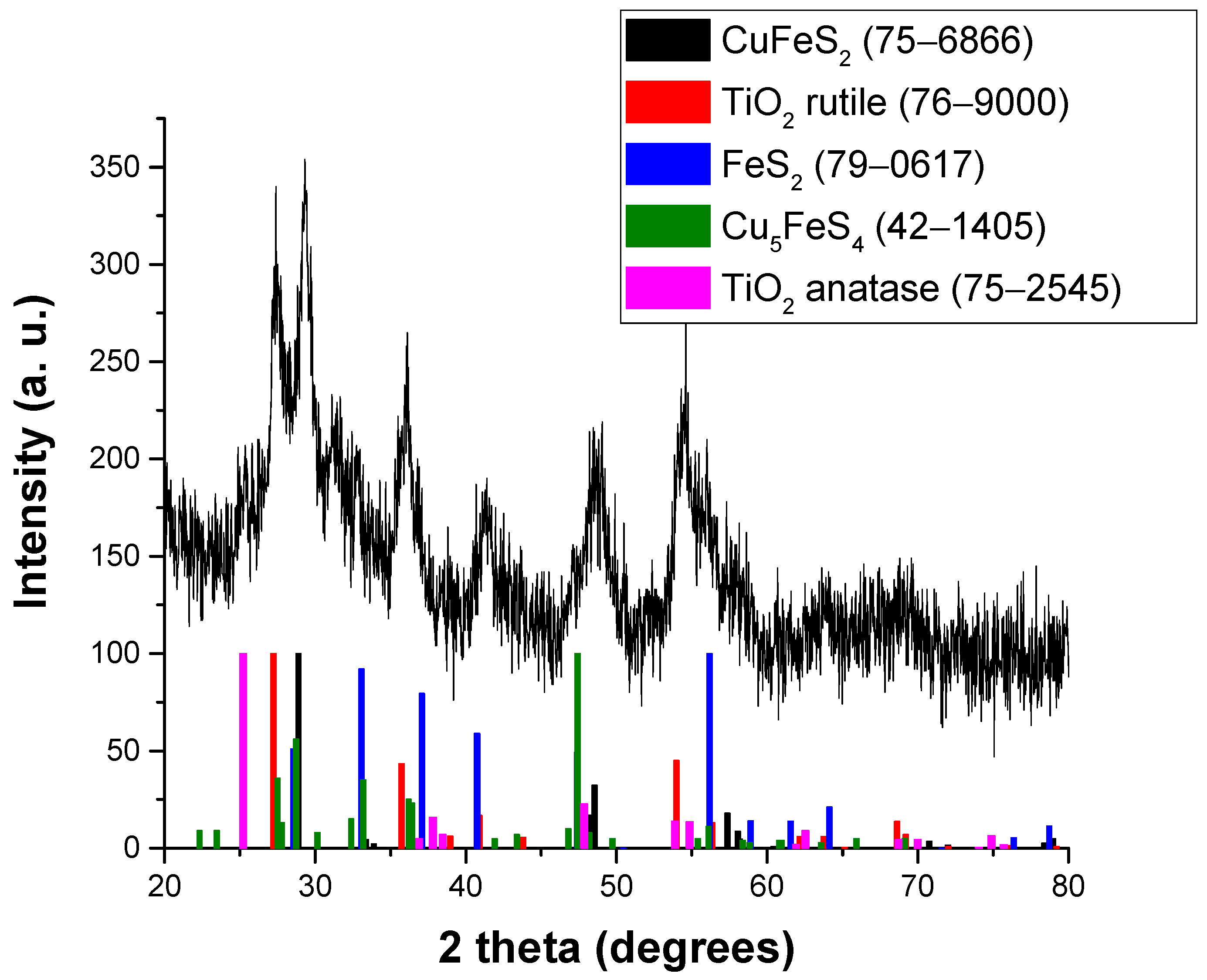
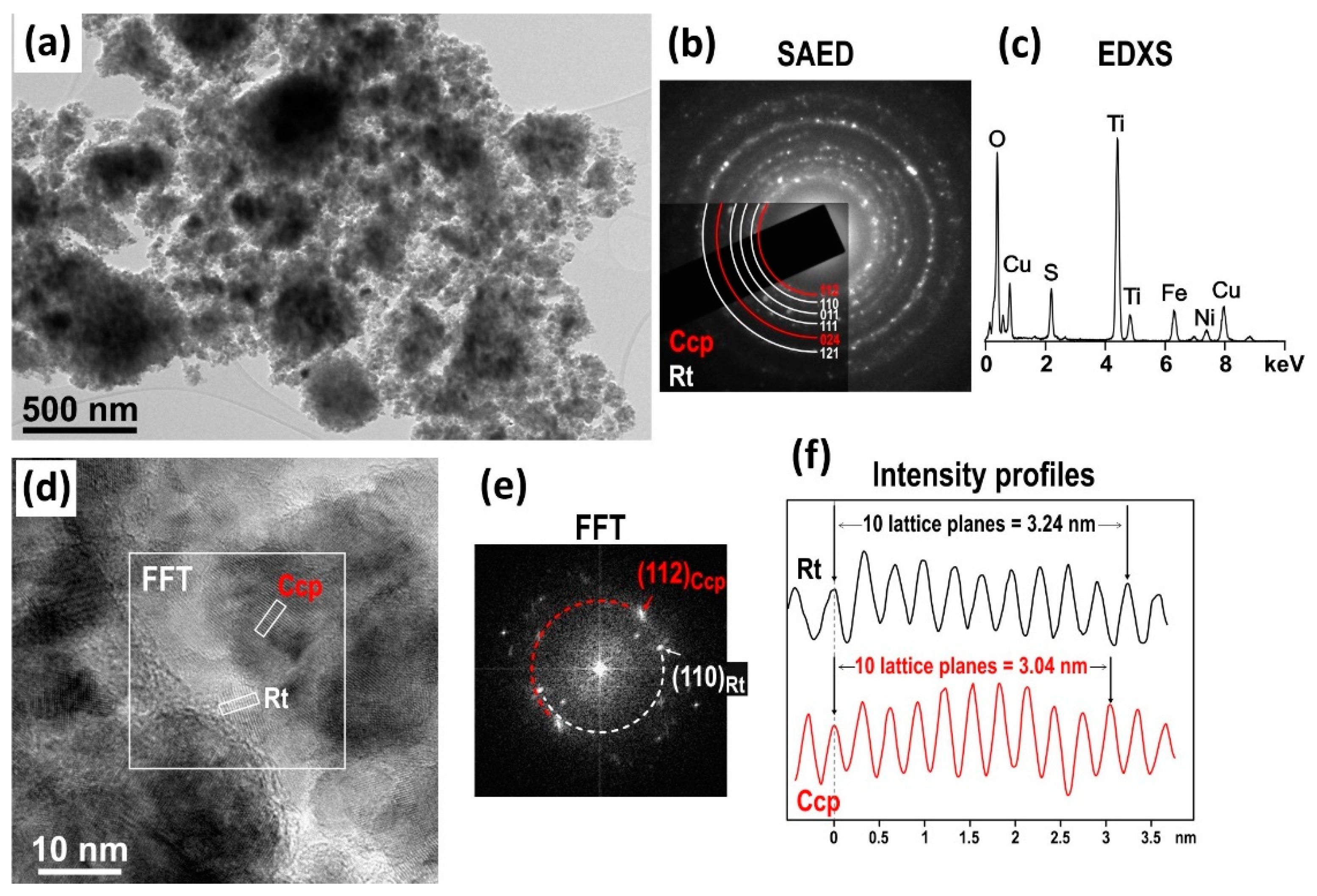
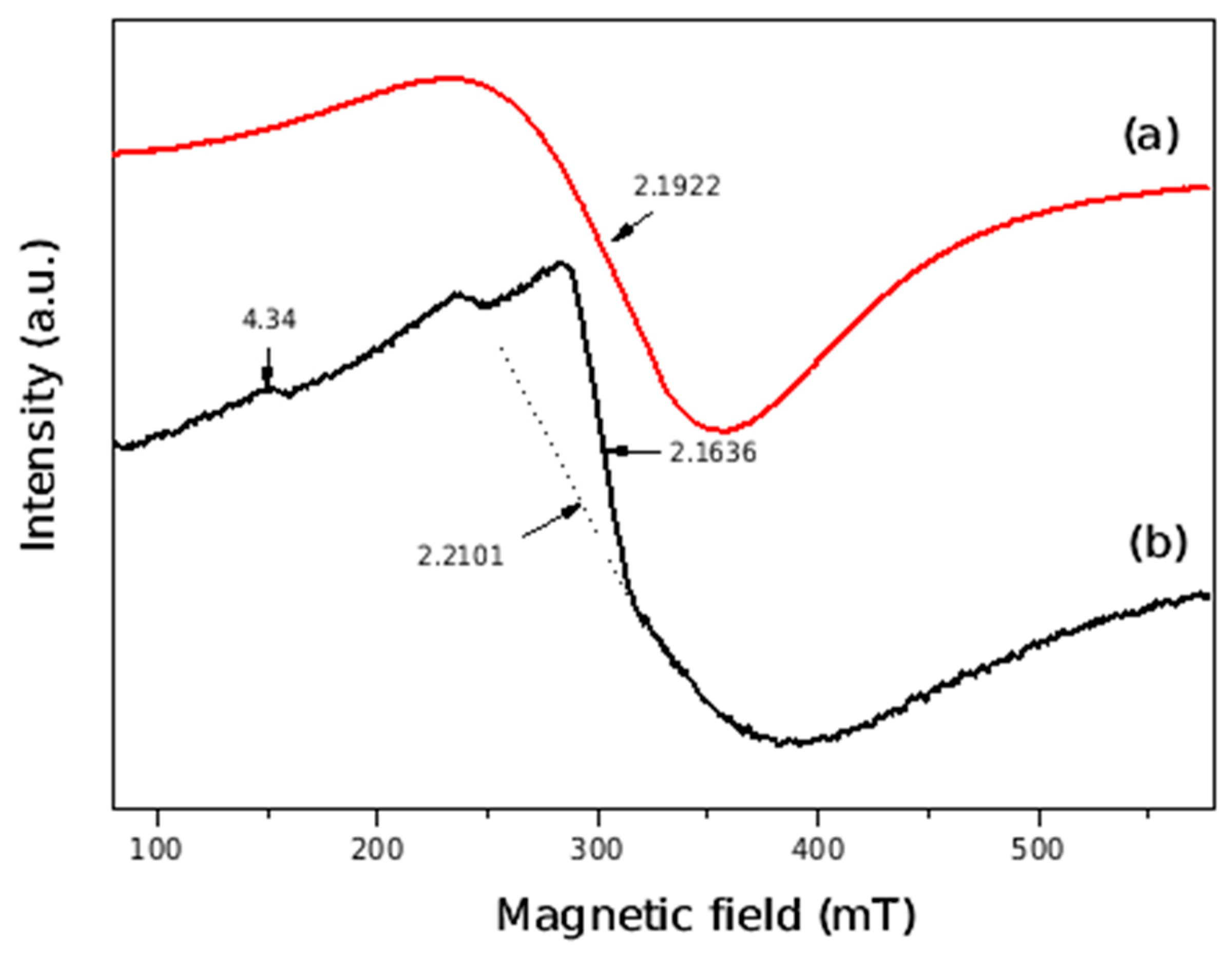
3.1. Structural and Microstructural Characterization
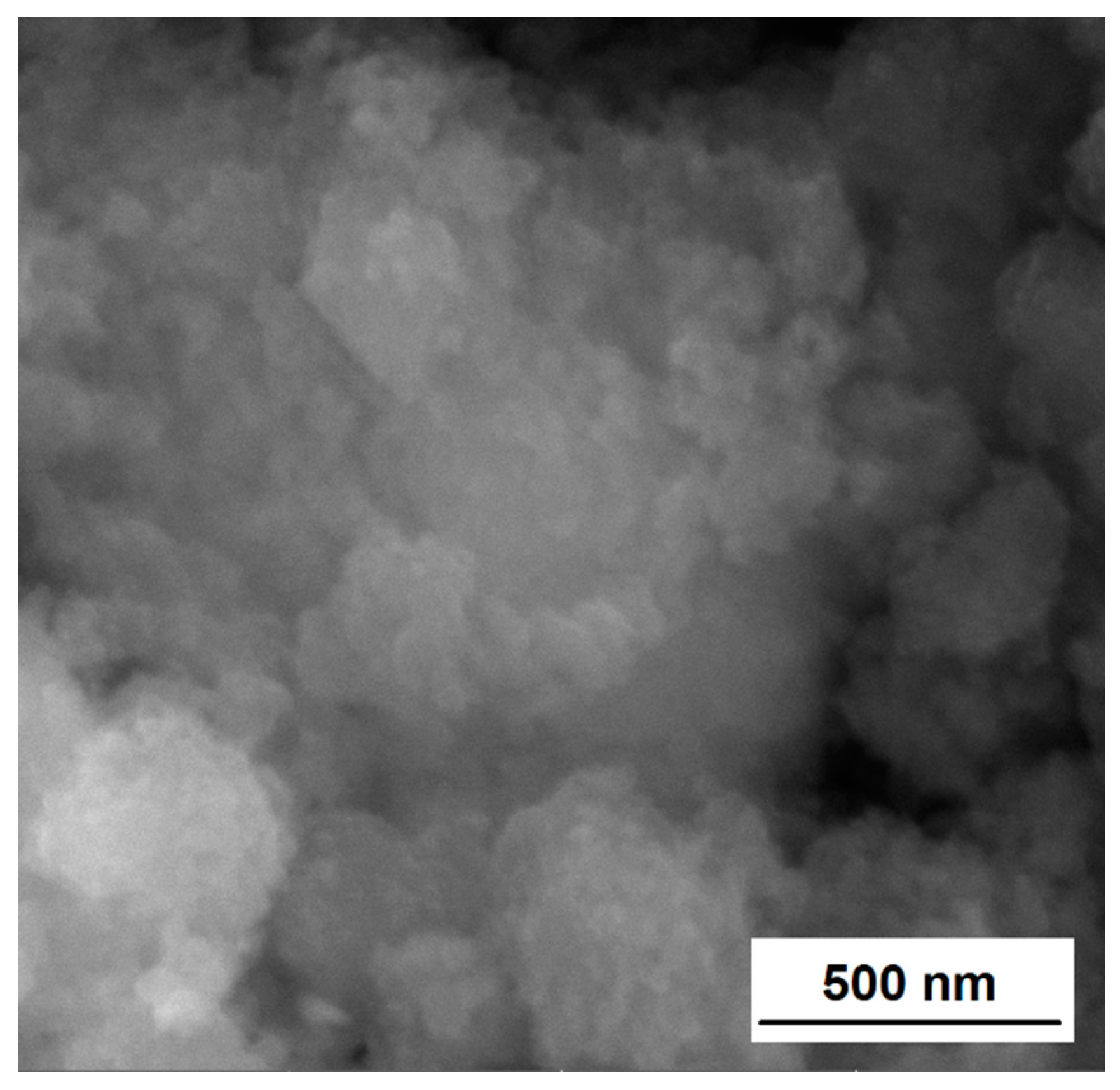
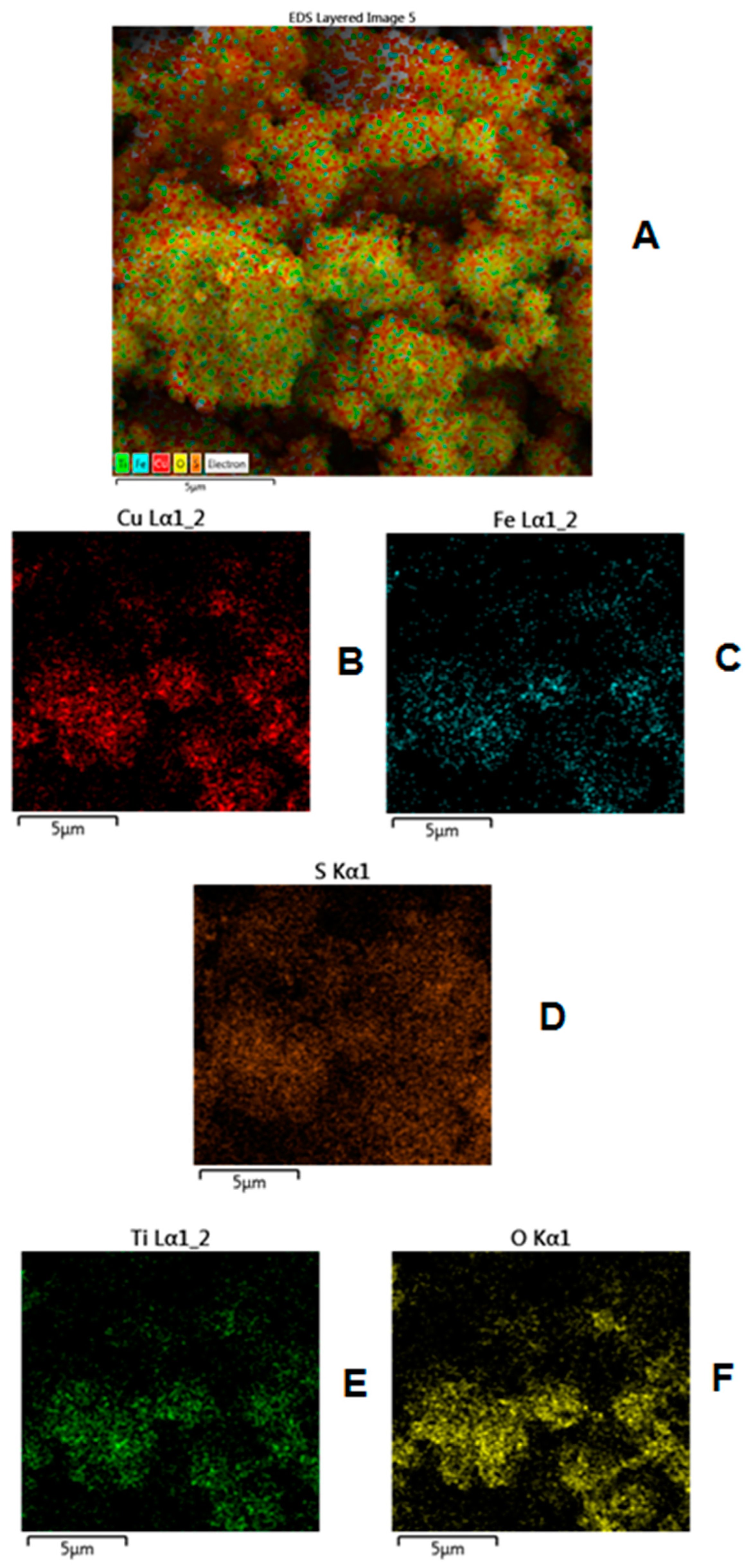
3.2. Surface and Morphological Characterization
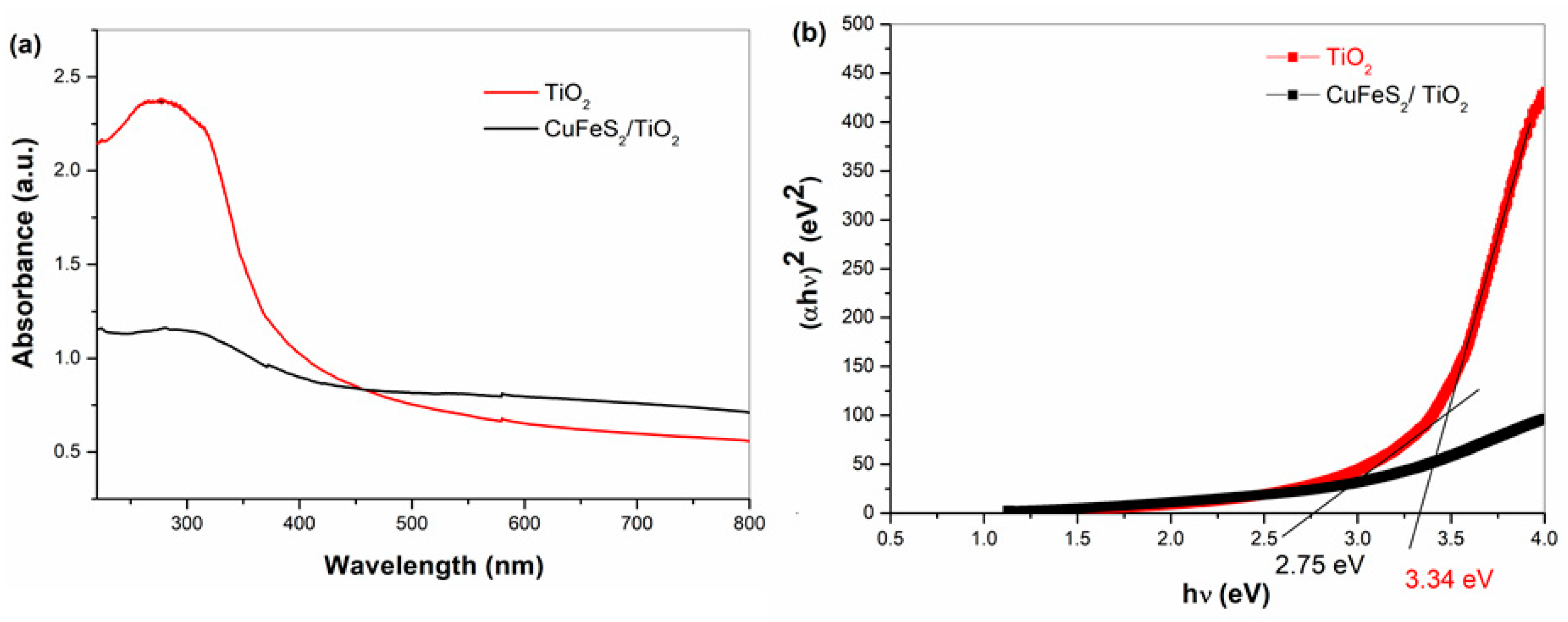
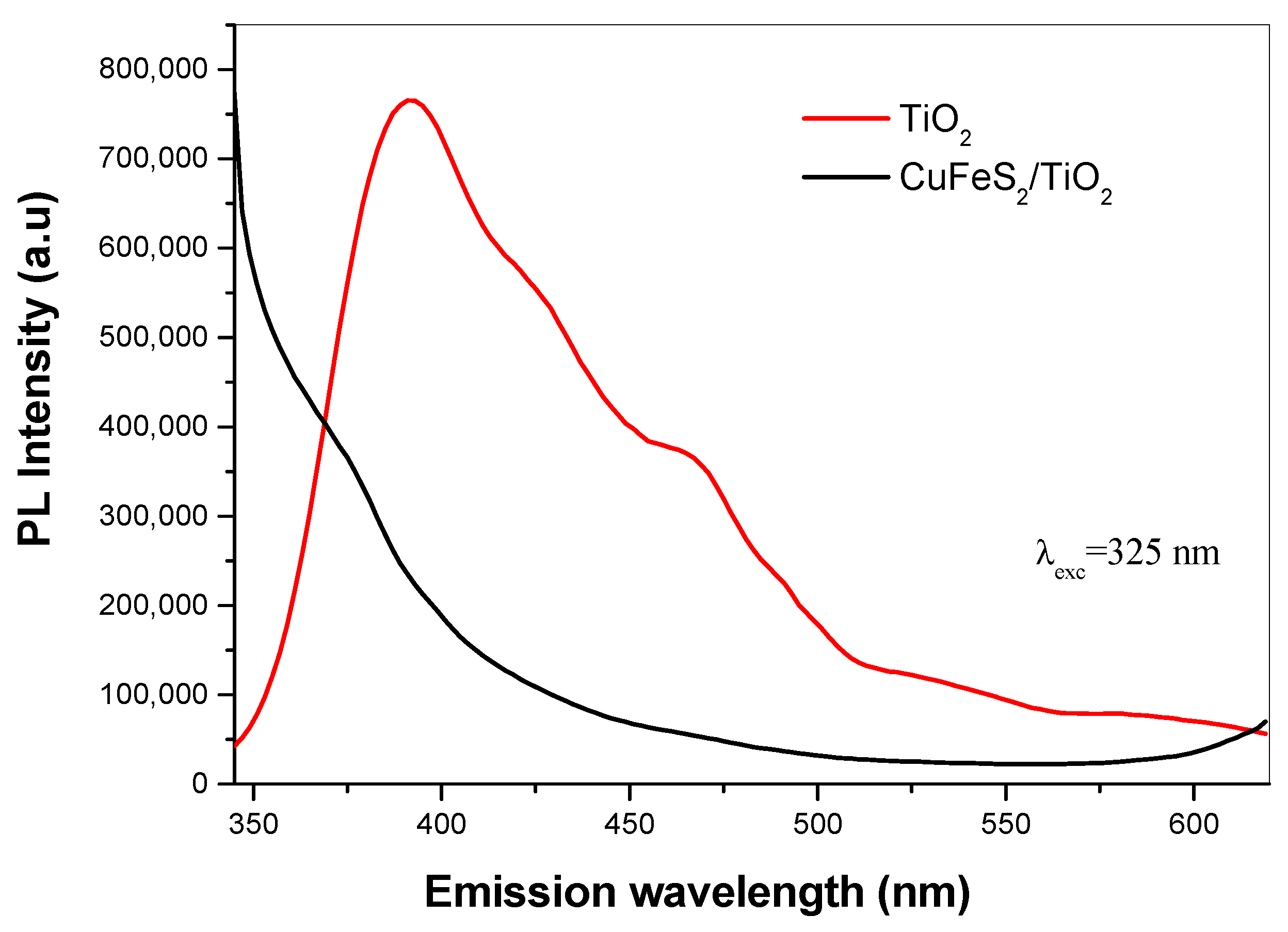
3.3. Optical Properties
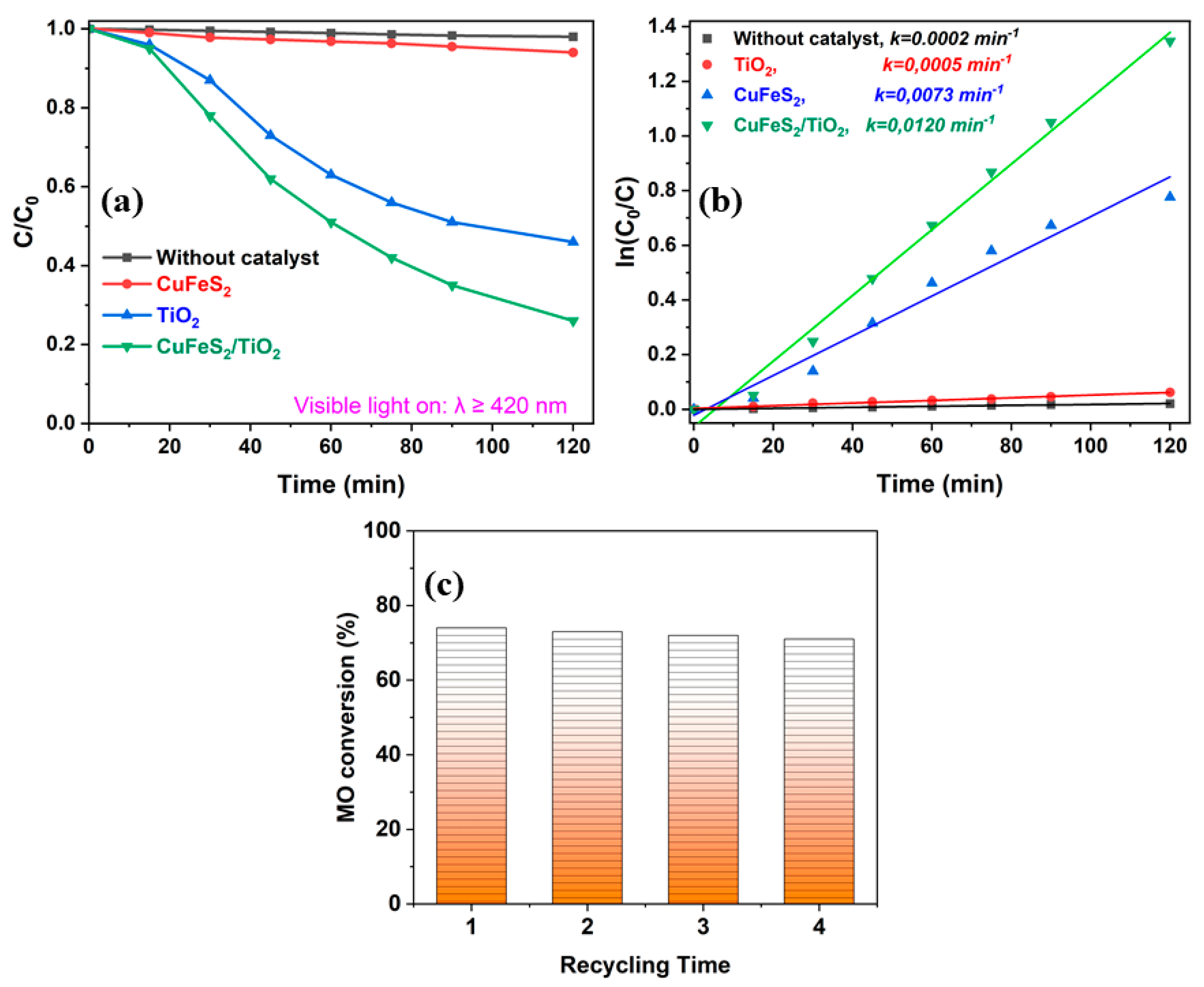
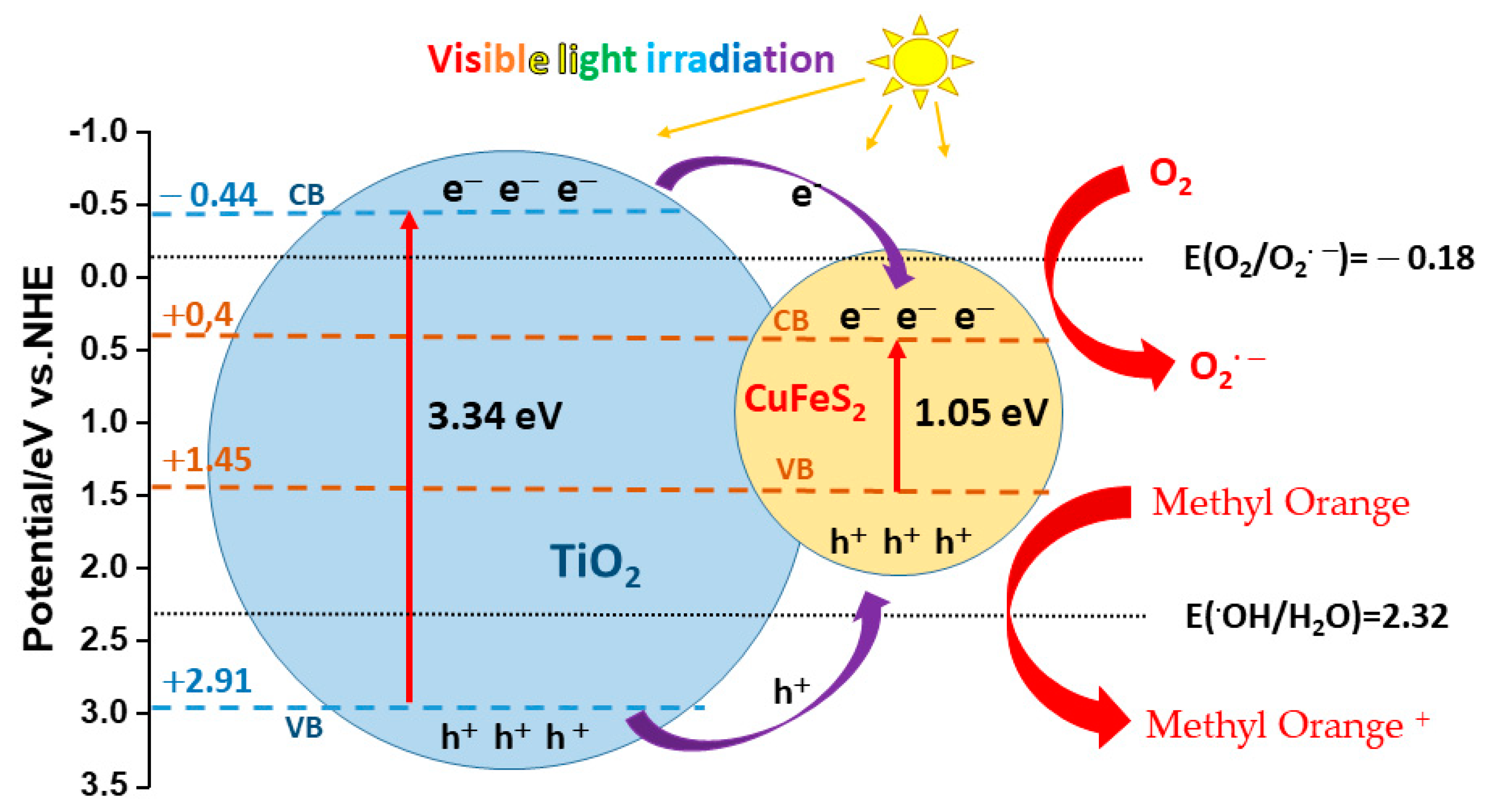
3.4. Photocatalytic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nunes, D.; Pimentel, A.; Branquinho, R.; Fortunato, E.; Martins, R. Metal Oxide-Based Photocatalytic Paper: A Green Alternative for Environmental Remediation. Catalysts 2021, 11, 504. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Santos, L.; Barquinha, P.; Fortunato, E.; Martins, R. Photocatalytic TiO2 Nanorod Spheres and Arrays Compatible with Flexible Applications. Catalysts 2017, 7, 60. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Qiao, J.L.; Zhou, J.; Gao, S.M. Fabrication of CuInSe2 quantum dots sensitized TiO2 nanotube arrays for enhancing visible light photoelectrochemical performance. Electrochim. Acta 2015, 167, 470–475. [Google Scholar] [CrossRef]
- Sun, W.T.; Yu, Y.; Pan, H.Y.; Gao, X.F.; Chen, Q.; Peng, L.M. CdS quantum dots sensitized TiO2 nanotube-array photoelectrodes. J. Am. Chem. Soc. 2008, 130, 1124–1125. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.D.; Cho, S.B.; Ghosh, T.; Zhu, L.; Choi, J.G.; Park, C.Y.; Oh, W.C. Photocatalytic Performance of ZnS and TiO2 Supported on AC Under Visible Light Irradiation. Korean J. Mater. Res. 2012, 22, 91–96. [Google Scholar] [CrossRef][Green Version]
- Yue, W.J.; Pan, Y.W.; Xie, Z.W.; Yang, X.; Hu, L.L.; Hong, L.R.; Tong, Y.F.; Cheng, Q.Q. Different depositing amount of CuInS2 on TiO2 nanoarrays for polymer/CuInS2-TiO2 solar cells. Mater. Sci. Semicond. Process. 2015, 40, 257–261. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Wang, Y.H.A.; Bao, N.Z.; Gupta, A. Shape-controlled synthesis of semiconducting CuFeS2 nanocrystals. Solid State Sci. 2010, 12, 387–390. [Google Scholar] [CrossRef]
- Kang, S.; Kwak, B.S.; Park, M.; Jeong, K.M.; Park, S.M.; Kang, M. Synthesis of Core@shell Structured CuFeS2@TiO2 Magnetic Nanomaterial and Its Application for Hydrogen Production by Methanol Aqueous Solution Photosplitting. Bull. Korean Chem. Soc. 2014, 35, 2813–2817. [Google Scholar] [CrossRef][Green Version]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.F.M.; Torresi, R.M.; Emmerling, F.; Camargo, P.H.C. Challenges and opportunities in the bottom-up mechanochemical synthesis of noble metal nanoparticles. J. Mater. Chem. A 2020, 8, 16114–16141. [Google Scholar] [CrossRef]
- Balaz, P.; Hegedus, M.; Achimovicova, M.; Balaz, M.; Tesinsky, M.; Dutkova, E.; Kanuchova, M.; Briancin, J. Semi-industrial Green Mechanochemical Syntheses of Solar Cell Absorbers Based on Quaternary Sulfides. Acs Acs Sustain. Chem. Eng. 2018, 6, 2132–2141. [Google Scholar] [CrossRef]
- Crawford, D.E.; Casaban, J. Recent Developments in Mechanochemical Materials Synthesis by Extrusion. Adv. Mater. 2016, 28, 5747–5754. [Google Scholar] [CrossRef]
- Kostova, N.; Dutkova, E. Mechanochemical synthesis and properties of ZnS/TiO2 composites. Bulg. Chem. Commun. 2016, 48, 161–166. [Google Scholar]
- Kostova, N.G.; Dutkova, E.; Eliyas, A.; Stoyanova-Eliyas, E.; Fabian, M.; Balaz, P. Mechanochemical synthesis, characterization, and photocatalytic activity of CdS/TiO2 composites in air purification. Bulg. Chem. Commun. 2015, 47, 87–93. [Google Scholar]
- Dutkova, E.; Bujnakova, Z.; Kovac, J.; Skorvanek, I.; Sayagues, M.J.; Zorkovska, A.; Kovac, J.; Balaz, P. Mechanochemical synthesis, structural, magnetic, optical and electrooptical properties of CuFeS2 nanoparticles. Adv. Powder Technol. 2018, 29, 1820–1826. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C plus. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Evans, J.S.O. Advanced Input Files & Parametric Quantitative Analysis Using Topas. Mater. Sci. Forum 2010, 651, 1–9. [Google Scholar] [CrossRef]
- Žák, T.; Jirásková, Y. CONFIT: Mössbauer spectra fitting program. Surf. Interface Anal. 2006, 38, 710–714. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Ghosh, B.; Samanta, L.K. Mechanosynthesis of nanocrystalline CuFeS2 chalcopyrite. Phys. E-Low-Dimens. Syst. Nanostruct. 2006, 33, 144–146. [Google Scholar] [CrossRef]
- Balaz, P.; Balaz, M.; Dutkova, E.; Hegedus, M.; Rajnak, M.; Knizek, K.; Hejtmanek, J.; Navratil, J.; Achimovicova, M.; Briancin, J. Magnetization as an Effective Tool for Kinetics Evaluation in Mechanochemical Synthesis of Chalcopyrite CuFeS2. Acta Phys. Pol., A 2020, 137, 647–649. [Google Scholar] [CrossRef]
- Levinsky, P.; Hejtmanek, J.; Knizek, K.; Pashchenko, M.; Navratil, J.; Masschelein, P.; Dutkova, E.; Balaz, P. Nanograined n- and p-Type Chalcopyrite CuFeS2 Prepared by Mechanochemical Synthesis and Sintered by SPS. Acta Phys. Pol. A 2020, 137, 904–907. [Google Scholar] [CrossRef]
- Bagheri, M.; Mahjoub, A.R.; Mehri, B. Enhanced photocatalytic degradation of congo red by solvothermally synthesized CuInSe2-ZnO nanocomposites. Rsc Adv. 2014, 4, 21757–21764. [Google Scholar] [CrossRef]
- Li, K.L.L. Cysteine modified anatase TiO2 hollow microspheres with enhanced visible-light-driven photocatalytic activity. J. Mol. Catal. A 2012, 356, 78–84. [Google Scholar]
- Xu, J.; Li, L.; Yan, Y.; Wang, H.; Wang, X.; Fu, X.; Li, G. Synthesis and photoluminescence of well-dispersible anatase TiO2 nanoparticles. J. Colloids Interface Sci. 2008, 318, 29–34. [Google Scholar] [CrossRef]
- Jing, L.Q.; Qu, Y.C.; Wang, B.Q.; Li, S.D.; Jiang, B.J.; Yang, L.B.; Fu, W.; Fu, H.G.; Sun, J.Z. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Pecchi, G.; Reyes, P.; Lopez, T.; Gomez, R.; Moreno, A.; Fierro, J.L.G.; Martinez-Arias, A. Catalytic combustion of methane on Fe-TiO2 catalysts prepared by sol-gel method. J. Sol-Gel Sci. Technol. 2003, 27, 205–214. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Mossin, S.; Riisager, A.; Fehrmann, R. Heteropoly acid promoted Cu and Fe catalysts for the selective catalytic reduction of NO with ammonia. Catal. Today 2011, 176, 292–297. [Google Scholar] [CrossRef]
- Vaughan, D.J.; Tossell, J.A. Magnetic Transitions Observed in Sulfide Minerals at Elevated Pressures and Their Geophysical Significance. Science 1973, 179, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Ok, H.N.; Baek, K.S.; Choi, E.J. Mossbauer Study of Antiferromagnetic Cufes2-Xsex. Phys. Rev. B 1994, 50, 10327–10330. [Google Scholar] [CrossRef] [PubMed]
- Waanders, F.B.; Silva, L.F.O.; Saikia, B.K. The use of Mossbauer spectroscopy in environmental research. Hyperfine Interact. 2017, 238, 52. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Tatykayev, B.; Chouchene, B.; Balan, L.; Gries, T.; Medjahdi, G.; Girot, E.; Uralbekov, B.; Schneider, R. Heterostructured g-CN/TiO(2)Photocatalysts Prepared by Thermolysis of g-CN/MIL-125(Ti) Composites for Efficient Pollutant Degradation and Hydrogen Production. Nanomaterials 2020, 10, 1387. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Cai, W.M.; Cai, J.; Zhou, B.X.; Chai, X.Y.; Wu, Y.H. Efficient photocatalytic degradation of phenol over Co3O4/BiVO4 composite under visible light irradiation. J. Phys. Chem. B 2006, 110, 20211–20216. [Google Scholar] [CrossRef] [PubMed]
- Vahidshad, Y.; Mirkazemi, S.M.; Tahir, M.N.; Ghasemzadeh, R.; Tremel, W. Synthesis of CuFeS2 Nanoparticles by One-pot Facile Method. J. Nanostruct. 2017, 7, 284–291. [Google Scholar]

| Sample | Components | δ, mm/s | Δ (2ε), mm/s | Bhf, T | Γexp, mm/s | G, % |
|---|---|---|---|---|---|---|
| CuFeS2 | Sx1 Sx2 Db | 0.25 0.25 0.34 | −0.01 −0.05 0.56 | 35.5 31.8 - | 0.32 0.82 0.34 | 47 36 17 |
| CuFeS2/TiO2 | Sx Db | 0.30 0.35 | −0.03 0.60 | 32.9 - | 0.88 0.44 | 24 76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutkova, E.; Baláž, M.; Daneu, N.; Tatykayev, B.; Karakirova, Y.; Velinov, N.; Kostova, N.; Briančin, J.; Baláž, P. Properties of CuFeS2/TiO2 Nanocomposite Prepared by Mechanochemical Synthesis. Materials 2022, 15, 6913. https://doi.org/10.3390/ma15196913
Dutkova E, Baláž M, Daneu N, Tatykayev B, Karakirova Y, Velinov N, Kostova N, Briančin J, Baláž P. Properties of CuFeS2/TiO2 Nanocomposite Prepared by Mechanochemical Synthesis. Materials. 2022; 15(19):6913. https://doi.org/10.3390/ma15196913
Chicago/Turabian StyleDutkova, Erika, Matej Baláž, Nina Daneu, Batukhan Tatykayev, Yordanka Karakirova, Nikolay Velinov, Nina Kostova, Jaroslav Briančin, and Peter Baláž. 2022. "Properties of CuFeS2/TiO2 Nanocomposite Prepared by Mechanochemical Synthesis" Materials 15, no. 19: 6913. https://doi.org/10.3390/ma15196913
APA StyleDutkova, E., Baláž, M., Daneu, N., Tatykayev, B., Karakirova, Y., Velinov, N., Kostova, N., Briančin, J., & Baláž, P. (2022). Properties of CuFeS2/TiO2 Nanocomposite Prepared by Mechanochemical Synthesis. Materials, 15(19), 6913. https://doi.org/10.3390/ma15196913









