Fast Isocenter Determination Using 3D Polymer Gel Dosimetry with Kilovoltage Cone-Beam CT Reading and the PolyGeVero-CT Software Package for Linac Quality Assurance in Radiotherapy
Abstract
:1. Introduction
2. Materials and Methods
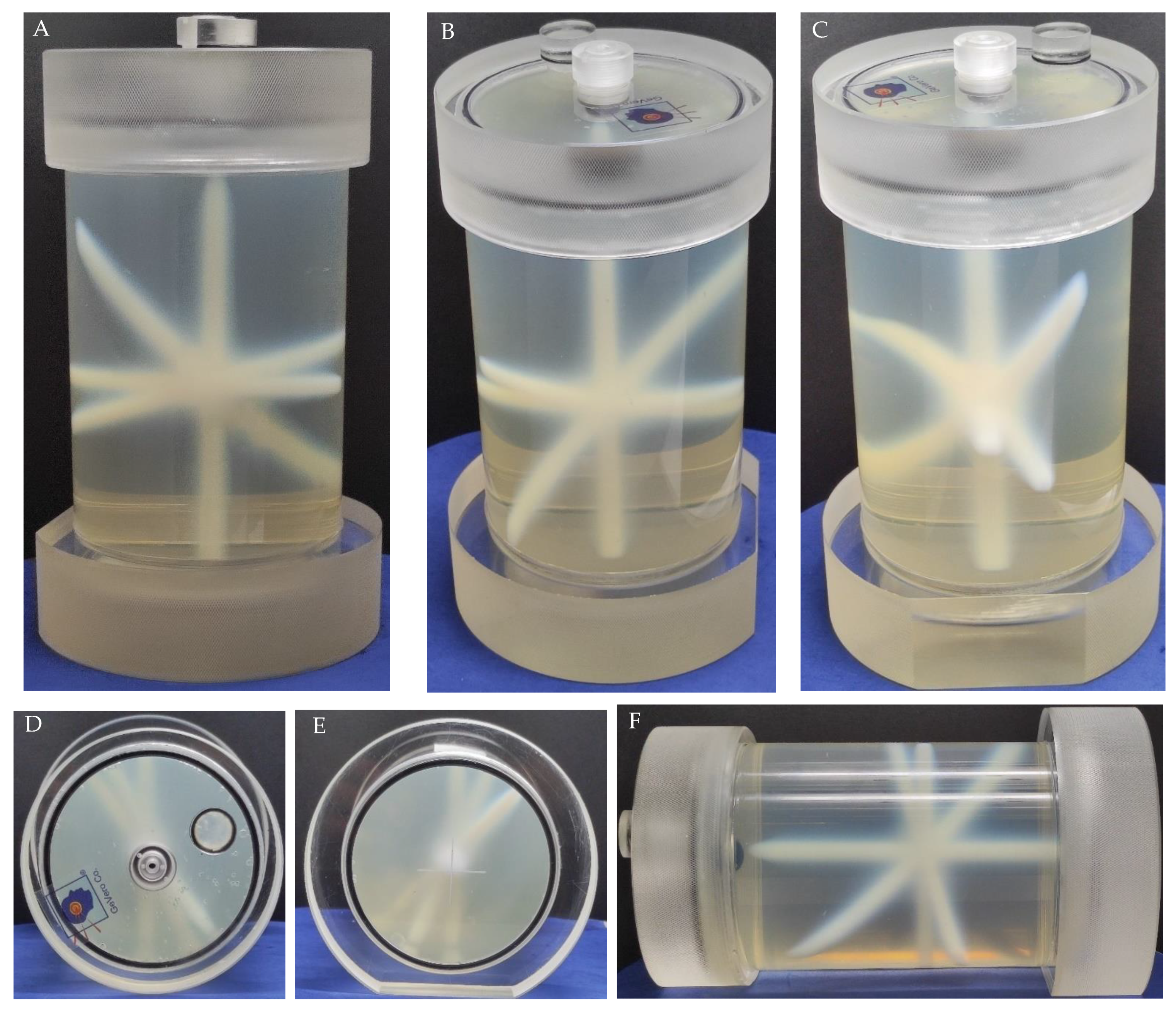
2.1. Preparation of a 3D Polymer Gel Dosimeter
2.2. Impact of Reconstruction Algorithms and Modes on Image Noise
2.3. Impact of Signal Averaging for Reconstruction Algorithms and Selected Modes on Image Quality
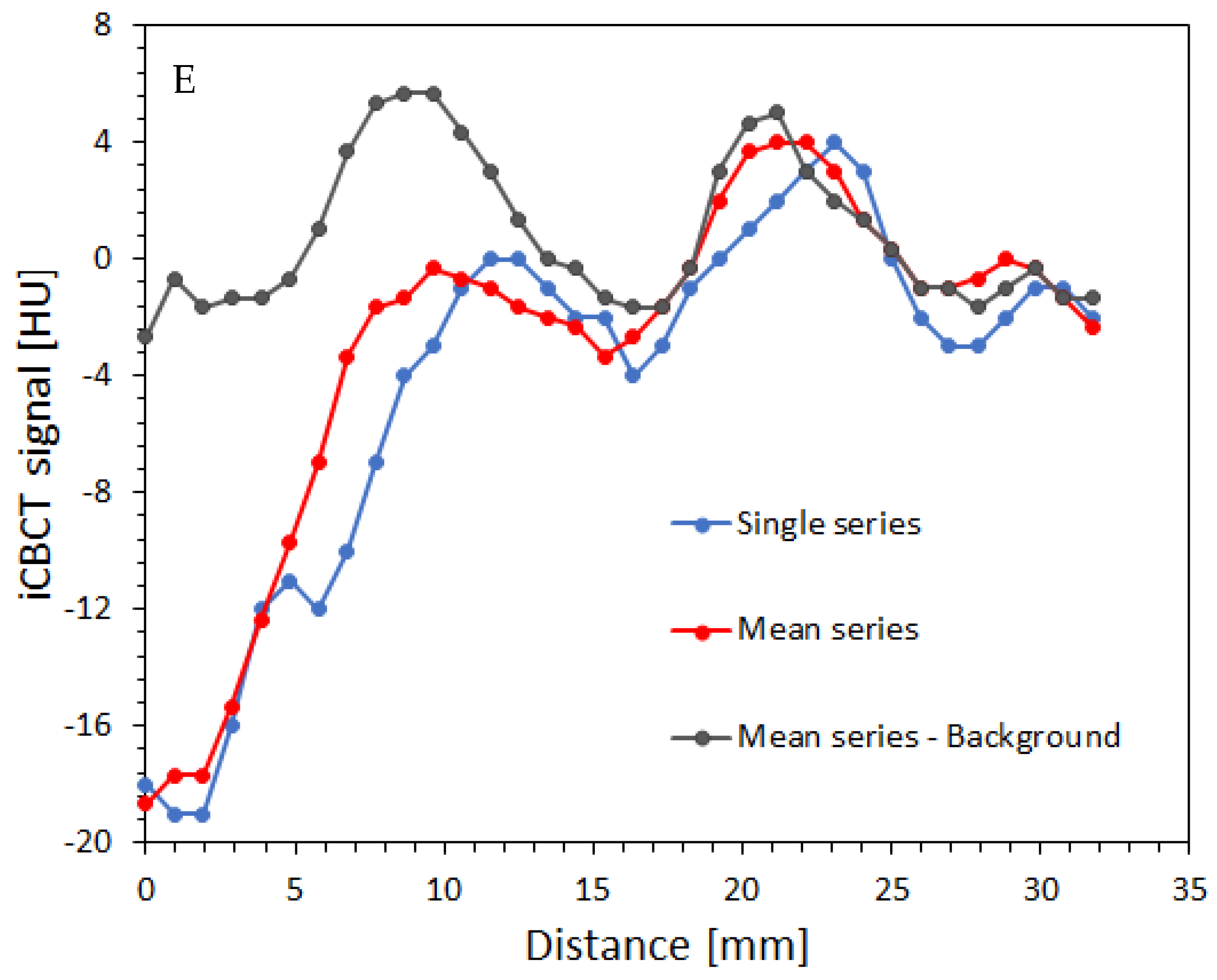
2.4. Impact of Scanning Time Interval between Consecutvie Scanings on Image Uniformity
2.5. Impact of Radiation Field Parameters
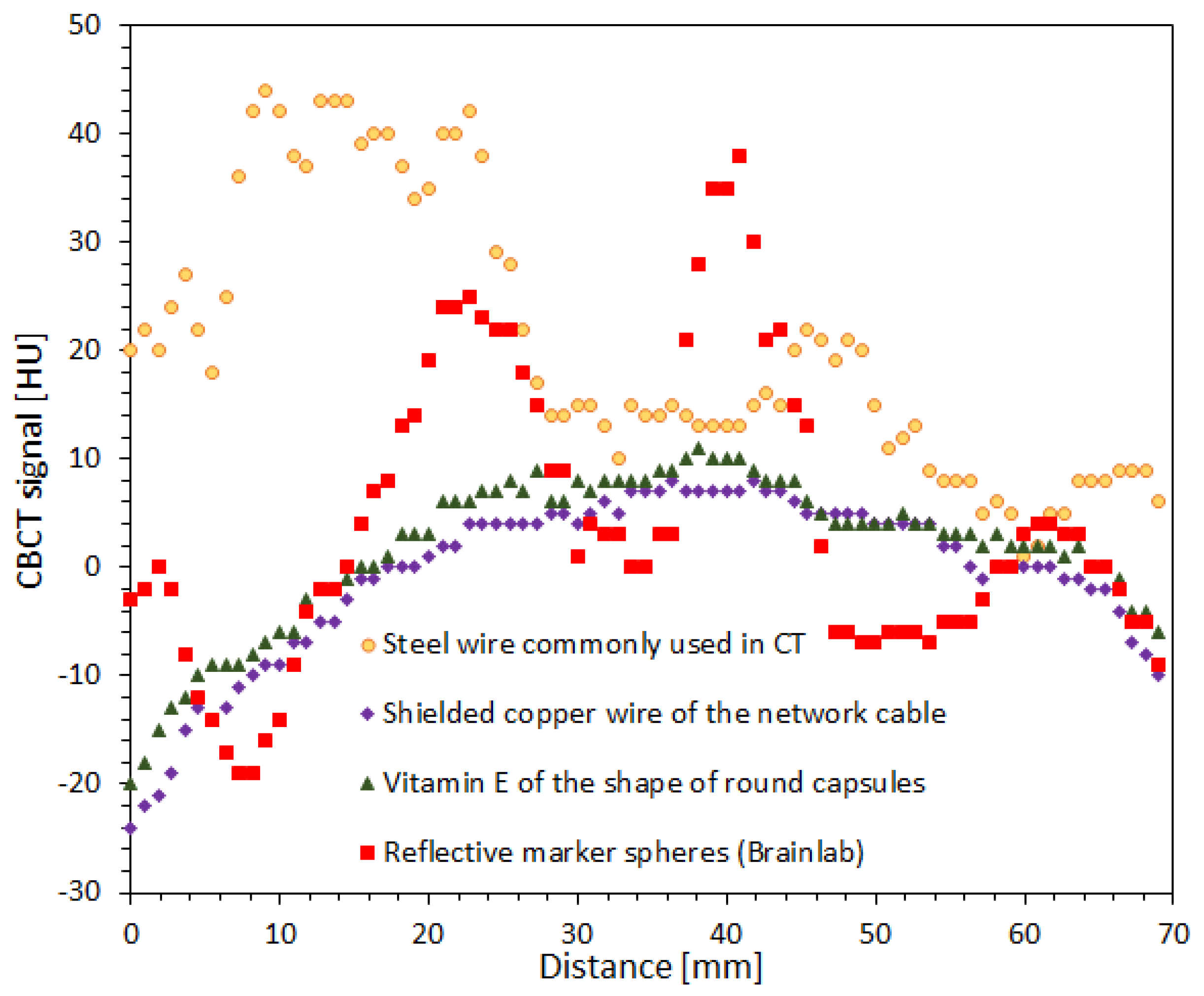
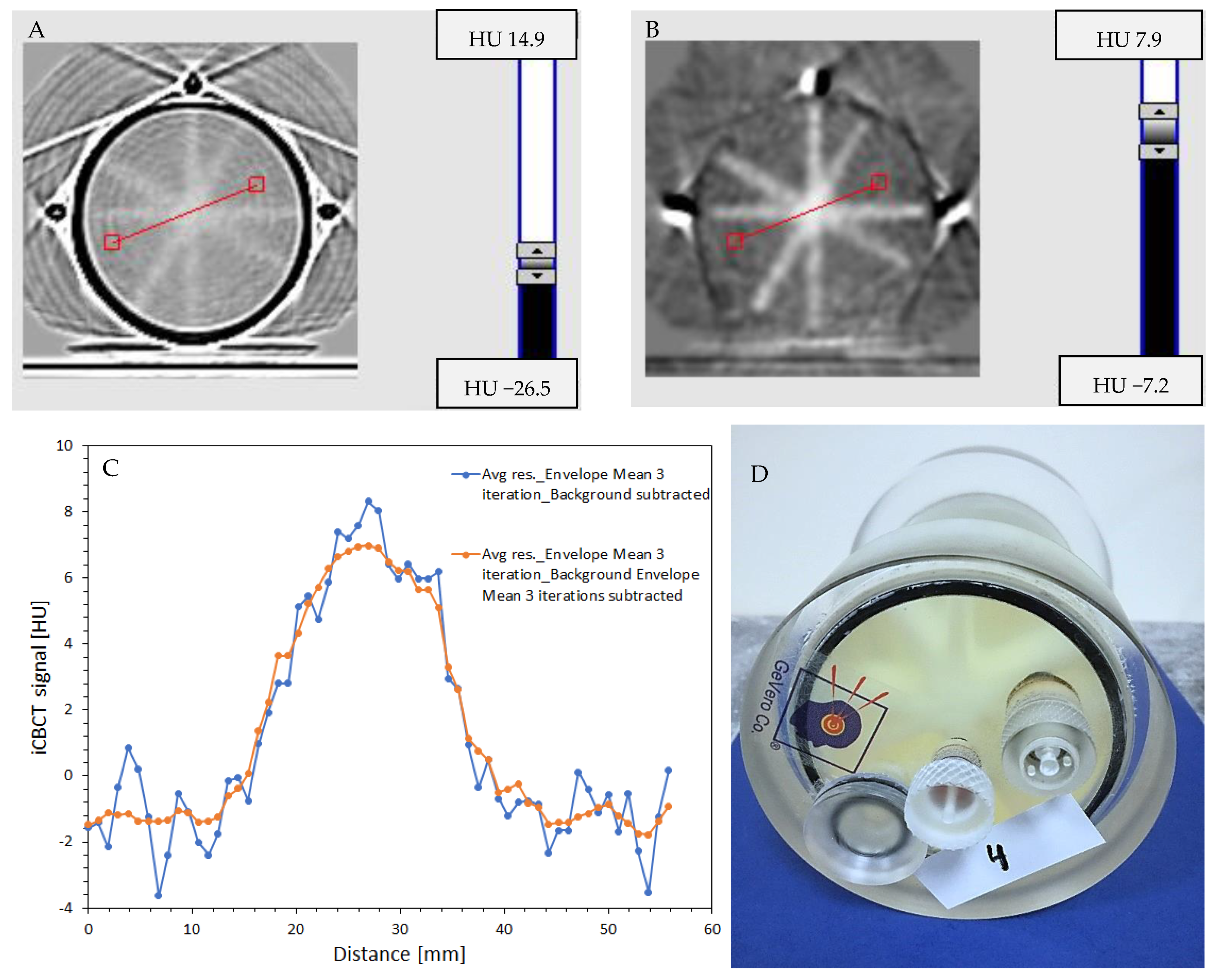
2.6. Impact of Fiducial Markers on Image Quality
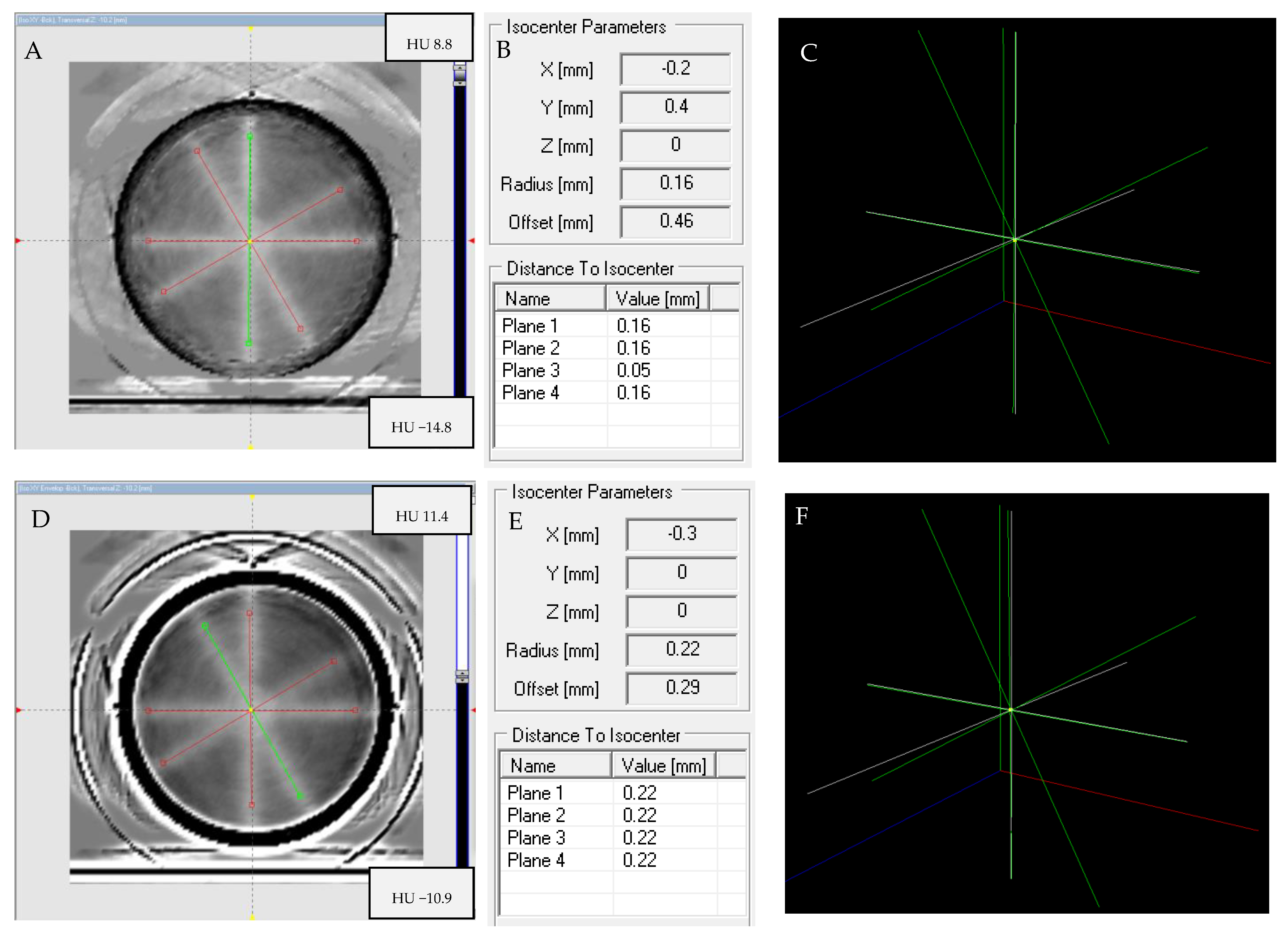
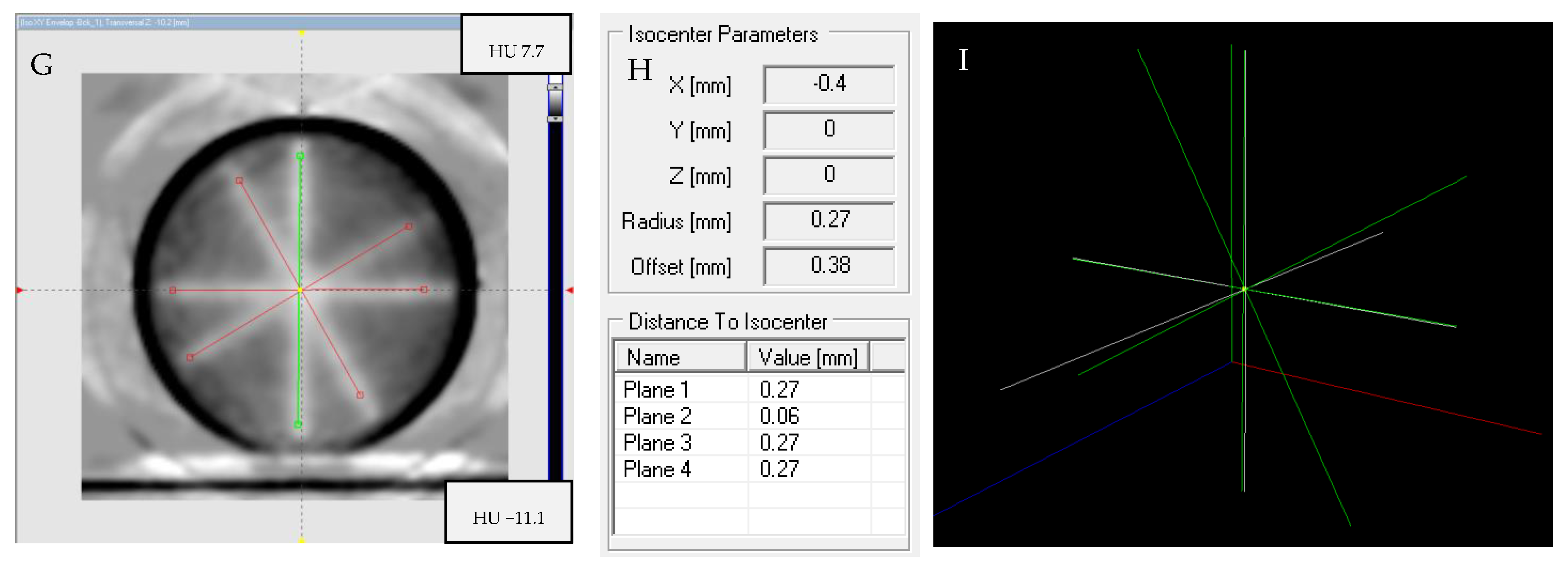
2.7. Isocenter Determination: 2D and 3D Approaches and Image Processing
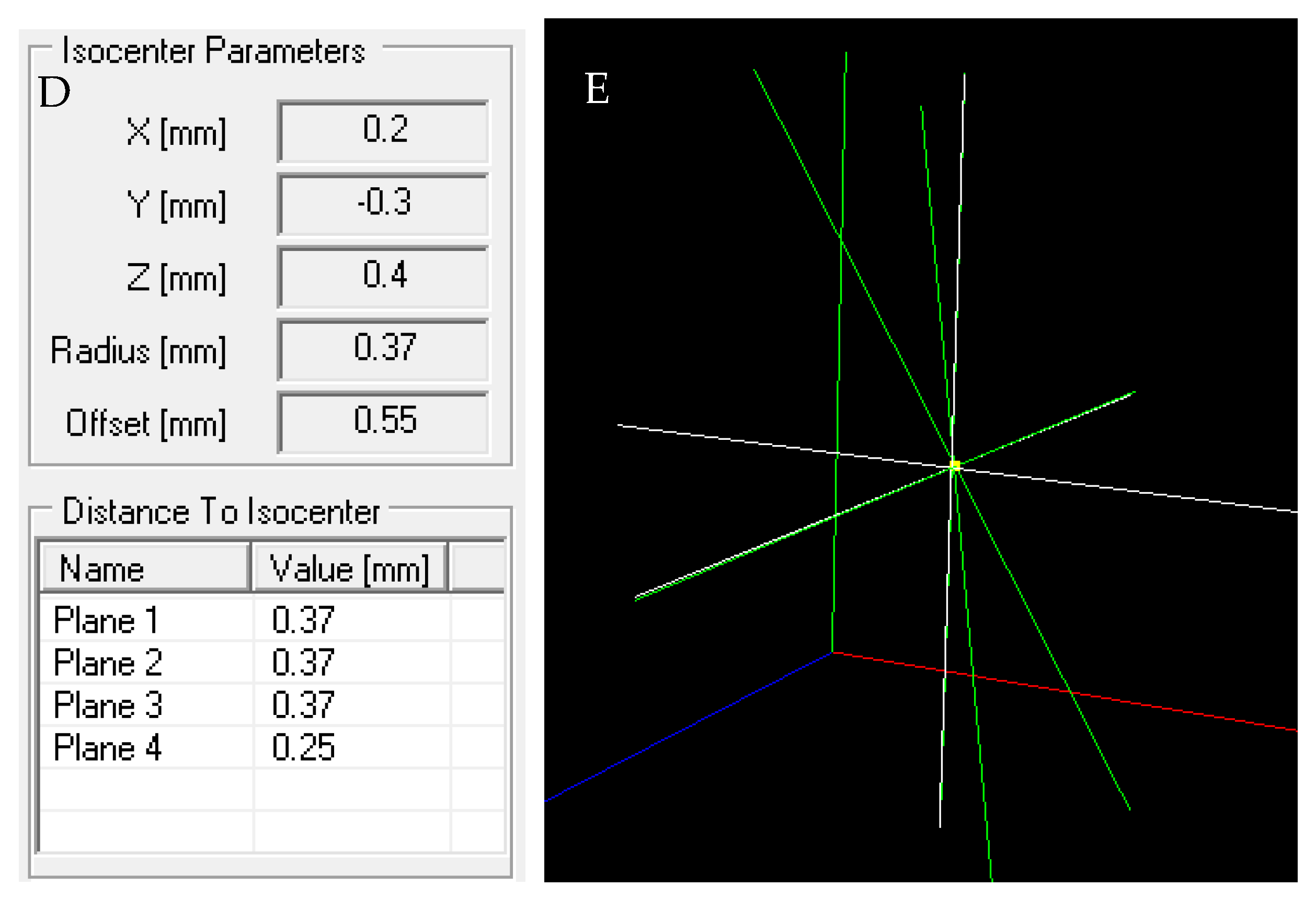
2.8. The Isocenter Data Processing
3. Results and Discussion
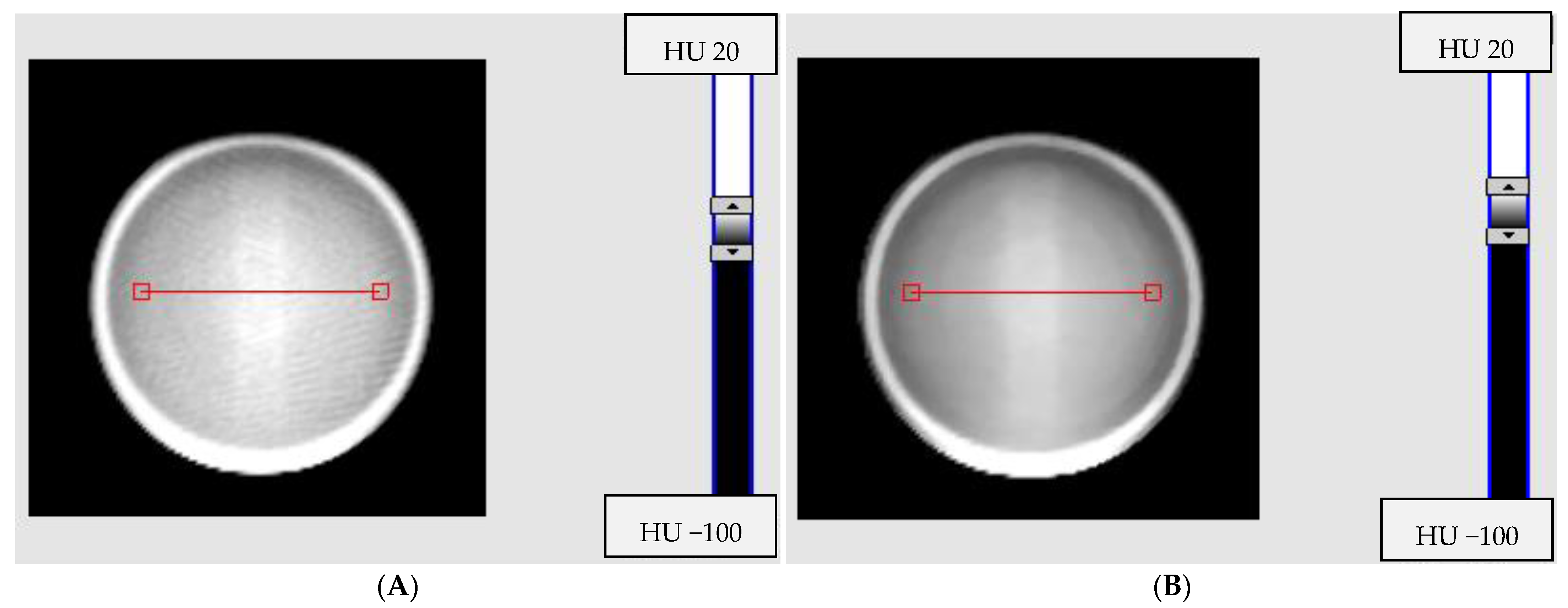
3.1. Impact of Reconstruction Algorithms and Modes on Image Noise
3.2. Impact of Signal Averaging for Reconstruction Algorithms and Selected Modes on Image Quality
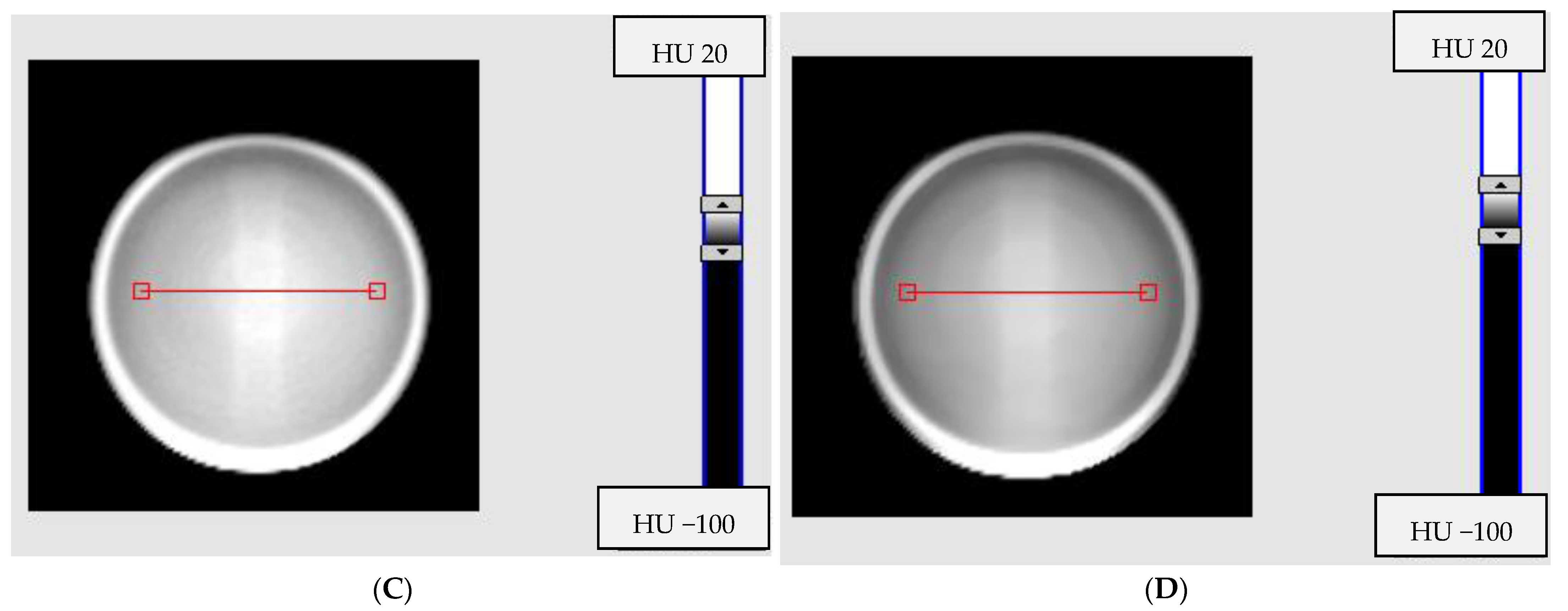
3.3. Impact of the Scanning Time Interval between Consecutvie Scanings on Image Uniformity
3.4. Impact of Radiation Field Parameters
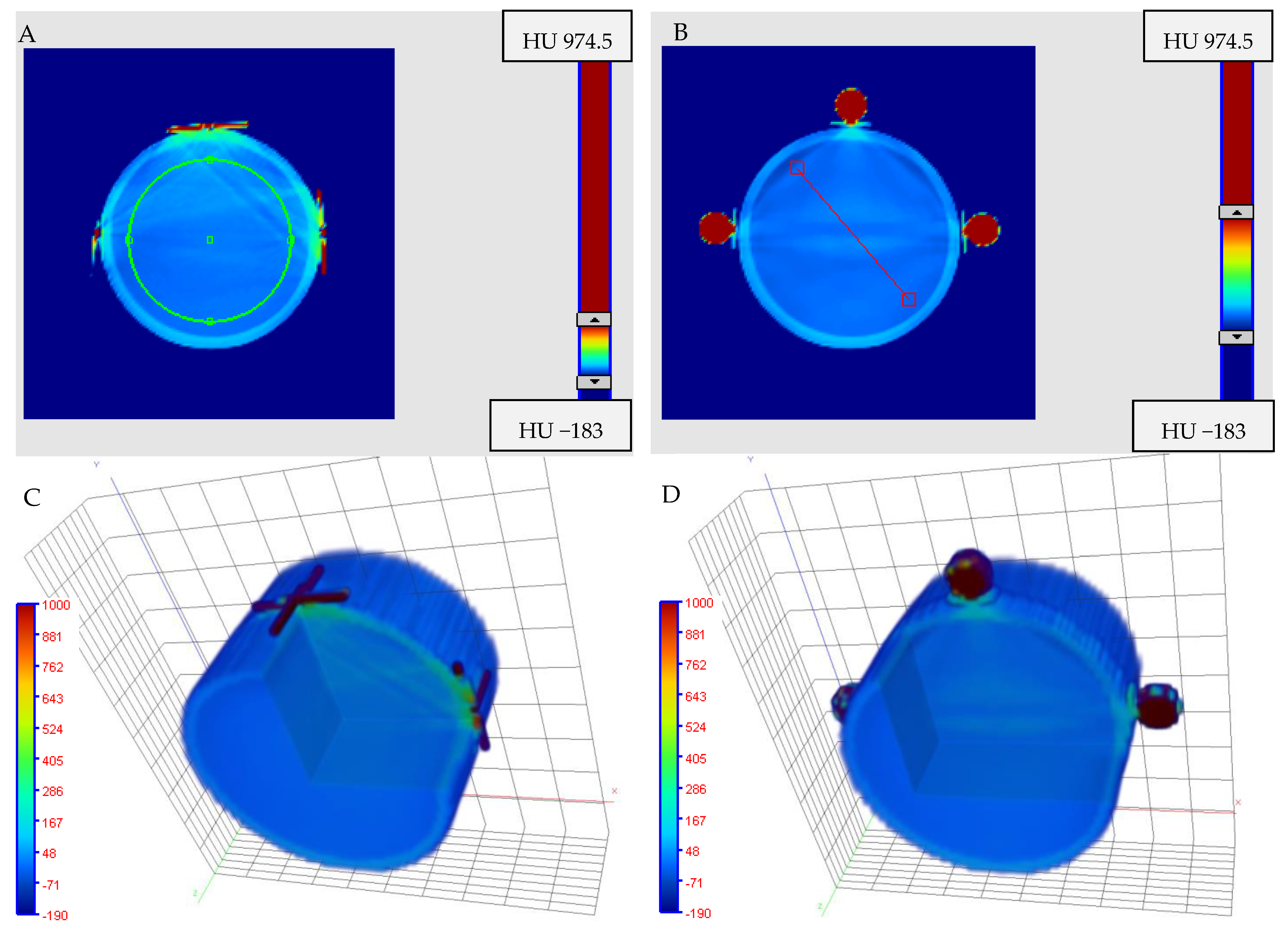
3.5. Impact of Fiducial Markers on Image Quality
3.6. Isocenter Determination: 2D and 3D Approaches and Image Processing
3.6.1. Impact of Image Processing
3.6.2. Isocenter Calculation: 2D approach
3.6.3. Isocenter Calculation: 3D Approach
3.7. Time and Effort to Determine the Isocenter
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lagendijk, J.J.W.; Raaymakers, B.W.; Raaijmakers, A.J.E.; Overweg, J.; Brown, K.J.; Kerkhof, E.M.; van der Put, R.W.; Hårdemark, B.; van Vulpen, M.; van der Heide, U.A. MRI/linac integration. Radiother. Oncol. 2008, 86, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Fallone, B.G.; Murray, B.; Rathee, S.; Stanescu, T.; Steciw, S.; Vidakovic, S.; Blosser, E.; Tymofichuk, D. First MR images obtained during megavoltage photon irradiation from a prototype integrated linac-MR system. Med. Phys. 2009, 36, 2084–2088. [Google Scholar] [CrossRef] [PubMed]
- Keall, P.J.; Barton, M.; Crozier, S. The Australian magnetic resonance imaging–linac program. Semin. Radiat. Oncol. 2014, 24, 203–206. [Google Scholar] [CrossRef]
- Wang, A.; Maslowski, A.; Messmer, P.; Lehmann, M.; Strzelecki, A.; Yu, E.; Paysan, P.; Brehm, M.; Munro, P.; Star-Lack, J.; et al. Acuros CTS: A fast, linear Boltzmann transport equation solver for computed tomography scatter—Part II: System modeling, scatter correction, and optimization. Med. Phys. 2018, 45, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Jaffray, D.A.; Siewerdsen, J.H.; Wong, J.W.; Martinez, A.A. Flat-Panel Cone-Beam Computed Tomography for image guided radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 1337–1349. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, S.-M.; Qu, T. What Do We Mean When We Talk about the Linac Isocenter? Int. J. Med. Phys. Clin. Eng. Radiat. Oncol. 2015, 4, 233–242. [Google Scholar] [CrossRef]
- Lutz, W.; Winston, K.R.; Maleki, N. A system for stereotactic radiosurgery with a linear accelerator. Int. J. Radiat. Oncol. Biol. Phys. 1988, 14, 373–381. [Google Scholar] [CrossRef]
- Treuer, H.; Hoevels, M.; Luyken, K.; Gierich, A.; Kocher, M.; Muller, R.-P.; Sturm, V. On isocentre adjustment and quality control in linear accelerator based radiosurgery with circular collimators and room lasers. Phys. Med. Biol. 2000, 45, 2331–2342. [Google Scholar] [CrossRef]
- Gonzalez, A.; Castro, I.; Martinez, J.A. A procedure to determine the radiation isocenter size in a linear accelerator. Med. Phys. 2004, 31, 1489–1493. [Google Scholar] [CrossRef]
- Depuydt, T.; Penne, R.; Verellen, D.; Hrbacek, J.; Lang, S.; Leysen, K.; Vandevondel, I.; Poels, K.; Reynders, T.; Gevaert, T.; et al. Computer-aided analysis of star shot films for high-accuracy radiation therapy treatment units. Phys. Med. Biol. 2012, 57, 2997–3011. [Google Scholar] [CrossRef]
- Fan, Q.; Zhou, S.; Lei, Y.; Li, S.; Zhang, M. A Quality assurance approach for linear accelerator mechanical isocenters with portal images. Int. J. Med. Phys. Clin. Eng. Radiat. Oncol. 2018, 7, 100–114. [Google Scholar] [CrossRef]
- Peace, T.; Subramanian, B.; Ravindran, P. An experimental study on using a diagnostic computed radiography system as a quality assurance tool in radiotherapy. Australas. Phys. Eng. Sci. Med. 2008, 31, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; van Doorn, T.; Bezak, E. The linear accelerator mechanical and radiation isocentre assessment with an electronic portal imaging device (EPID). Australas. Phys. Eng. Sci. Med. 2004, 27, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Shiu, A.; Tung, S.; Boyer, A. Verification of radiosurgery target point alignment with an electronic portal imaging device (EPID). Med. Phys. 1997, 24, 263–267. [Google Scholar] [CrossRef]
- Winey, B.; Sharp, G.; Bussière, M.A. Fast double template convolution isocenter evaluation algorithm with subpixel accuracy. Med. Phys. 2011, 38, 223–227. [Google Scholar] [CrossRef]
- Rowshanfarzad, P.; Sabet, M.; O’Connor, D.J.; Greer, P.B. Isocenter verification for linac-based stereotactic radiation therapy: Review of principles and techniques. J. Appl. Clin. Med. Phys. 2011, 12, 185–195. [Google Scholar] [CrossRef]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.B.; Oldham, M.; Schreiner, L.J. Polymer gel dosimetry. Phys. Med. Biol. 2010, 55, R1–R63. [Google Scholar] [CrossRef]
- Schreiner, L.J. True 3D chemical dosimetry (gels, plastics): Development and clinical role. J. Phys. Conf. Ser. 2015, 573, 012003. [Google Scholar] [CrossRef]
- Warman, J.M.; Luthjens, L.H.; de Haas, M.P. High-energy radiation monitoring based on radio-fluorogenic co-polymerization II: Fixed fluorescent images of collimated x-ray beams using an RFCP gel. Phys. Med. Biol. 2011, 56, 1487–1508. [Google Scholar] [CrossRef]
- Adamovics, J.; Maryanski, M. Characterization of PRESAGE™: A new 3D radiochromic solid polymer dosimeter for ionising radiation. Radiat. Protect. Dosim. 2006, 120, 107–112. [Google Scholar] [CrossRef]
- Guo, P.Y.; Adamovics, J.A.; Oldham, M. A practical three-dimensional dosimetry system for radiation therapy. Med. Phys. 2006, 33, 3962–3972. [Google Scholar] [CrossRef] [PubMed]
- Alqathami, M.; Adamovics, J.; Benning, R.; Qiao, G.; Geso, M.; Blencowe, A. Evaluation of ultra-sensitive leucomalachite dye derivatives for use in the PRESAGE® dosimeter. Radiat. Phys. Chem. 2013, 85, 204–209. [Google Scholar] [CrossRef]
- De Deene, Y.; Skyt, P.S.; Hil, R.; Booth, J.T. FlexyDos3D: A deformable anthropomorphic 3D radiation dosimeter: Radiation properties. Phys. Med. Biol. 2015, 60, 1543–1563. [Google Scholar] [CrossRef] [PubMed]
- Babic, S.; Battista, J.; Jordan, K. Radiochromic leuco dye micelle hydrogels: II. Low diffusion rate leuco crystal violet gel. Phys. Med. Biol. 2009, 54, 6791–6808. [Google Scholar] [CrossRef]
- Jordan, K.; Avvakumov, N. Radiochromic leuco dye micelle hydrogels: I. Initial investigation. Phys. Med. Biol. 2009, 54, 6773–6789. [Google Scholar] [CrossRef]
- Nasr, A.T.; Olding, T.; Schreiner, L.J.; McAuley, K.B. Evaluation of the potential for diacetylenes as reporter molecules in 3D micelle gel dosimetry. Phys. Med. Biol. 2013, 58, 787–805. [Google Scholar] [CrossRef]
- Nasr, A.T.; Alexander, K.M.; Schreiner, L.J.; McAuley, K.B. Opportunities for improving the performance of LCV micelle gel dosimeters: I. Preliminary investigation. J. Phys. Conf. Ser. 2015, 573, 012037. [Google Scholar] [CrossRef]
- Kozicki, M.; Kwiatos, K.; Kadlubowski, S.; Dudek, M. TTC-Pluronic 3D radiochromic gel dosimetry of ionising radiation. Phys. Med. Biol. 2017, 62, 5668–5690. [Google Scholar] [CrossRef]
- Kwiatos, K.; Maras, P.; Kadlubowski, S.; Stempień, Z.; Dudek, M.; Kozicki, M. Tetrazolium salts-Pluronic F-127 gels for 3D radiotherapy dosimetry. Phys. Med. Biol. 2018, 63, 095012. [Google Scholar] [CrossRef]
- Kouvati, K.; Jaszczak, M.; Papagiannis, P.; Kadlubowski, S.; Wach, R.; Maras, P.; Dudek, M.; Kozicki, M. Leuco crystal violet-Pluronic F-127 3D radiochromic gel dosimeter. Phys. Med. Biol. 2019, 64, 175017. [Google Scholar] [CrossRef]
- Jaszczak, M.; Sąsiadek, E.; Kadlubowski, S.; Dudek, M.; Kozicki, M. Preliminary study on a new 3D radiochromic KI-Pluronic F-127 gel dosimeter for radiotherapy. Radiat. Phys. Chem. 2021, 185, 109507. [Google Scholar] [CrossRef]
- Pantelis, E.; Lymperopoulou, G.; Papagiannis, P.; Sakelliou, L.; Stiliaris, S.; Sandilos, P.; Seimenis, I.; Kozicki, M.; Rosiak, J.M. Polymer gel dosimetry close to an 125I interstitial brachytherapy seed. Phys. Med. Biol. 2005, 50, 4371–4384. [Google Scholar] [CrossRef] [PubMed]
- Pappas, E.; Petrokokkinos, L.; Angelopoulos, A.; Maris, T.G.; Kozicki, M.; Dalezios, I.; Kouloulias, V. Relative output factor measurements of a 5 mm diameter radiosurgical photon beam using polymer gel dosimetry. Med. Phys. 2005, 32, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Kozicki, M.; Berg, A.; Maras, P.; Jaszczak, M.; Dudek, M. Clinical radiotherapy application of N-vinylpyrrolidone-containing 3D polymer gel dosimeters with remote external MR-reading. Phys. Medica 2020, 69, 134–146. [Google Scholar] [CrossRef]
- Dorsch, S.; Mann, P.; Lang, C.; Hearing, P.; Runz, A.; Karger, C.P. Feasibility of polymer gel-based measurements of radiation isocenter accuracy in magnetic fields. Phys. Med. Biol. 2018, 63, 11NT02. [Google Scholar] [CrossRef] [PubMed]
- Dorsch, S.; Mann, P.; Elter, A.; Runz, A.; Kluter, S.; Karger, C.P. Polymer gel-based measurements of the isocenter accuracy in an MR-LINAC. J. Phys. Conf. Ser. 2019, 1305, 012007. [Google Scholar] [CrossRef]
- Dorsch, S.; Mann, P.; Elter, A.; Runz, A.; Spindeldreier, C.K.; Klüter, S.; Karger, C.P. Measurement of isocenter alignment accuracy and image distortion of an 0.35 T MR-Linac system. Phys. Med. Biol. 2019, 64, 205011. [Google Scholar] [CrossRef]
- Pant, K.; Umeh, C.; Oldham, M.; Floyd, S.; Giles, W.; Adamson, J. Comprehensive radiation and imaging isocenter verification using NIPAM kV-CBCT dosimetry. Med. Phys. 2020, 47, 927–936. [Google Scholar] [CrossRef]
- Adamson, J.; Carroll, J.; Trager, M.; Yoon, S.W.; Kodra, J.; Maynard, E.; Hilts, M.; Oldham, M.; Jirasek, A. Delivered dose distribution visualized directly with onboard kV-CBCT: Proof of principle. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 1271–1279. [Google Scholar] [CrossRef]
- Maras, P.; Jaszczak, M.; Kozicki, M. Basic features of VIC-T dosimeter with spiral CT readout. CT scanning conditions and data processing with a new polyGeVero-CT software package. Radiat. Phys. Chem. 2021, 189, 109730. [Google Scholar] [CrossRef]
- Kozicki, M. How do monomeric components of a polymer gel dosimeter respond to ionising radiation: A steady-state radiolysis towards preparation of a 3D polymer gel dosimeter. Radiat. Phys. Chem. 2011, 80, 1419–1436. [Google Scholar] [CrossRef]
- Kozicki, M.; Jaszczak, M.; Maras, P. Features of PABIGnx 3D polymer gel as an ionising radiation dosimeter. Materials 2022, 15, 2550. [Google Scholar] [CrossRef] [PubMed]
- Kozicki, M.; Maras, P.; Karwowski, A.C. Software for 3D radiotherapy dosimetry. Validation. Phys. Med. Biol. 2014, 59, 4111–4136. [Google Scholar] [CrossRef] [PubMed]
- Kozicki, M.; Rosiak, J.M. Basic properties of a new PABIG polymer gel dosimeter for dose distribution assessment in radiotherapy treatment. In Proceedings of the Second International Symposium on Reactive Polymers in Inhomogeneous Systems, in Melts and at Interfaces, Dresden, Germany, 28 September–1 October 2003. [Google Scholar]
- Kozicki, M.; Jaszczak, M.; Dudek, M.; Maras, P. Laser Light Trapping Phenomenon in a 3D Radiotherapy Polymer Gel Dosimeter. Materials 2021, 14, 3961. [Google Scholar] [CrossRef] [PubMed]
- Halcyon Customer Release Note, Version 2.0; Varian Medical Systems, Inc.: Palo Alto, CA, USA, 2019.
- Catphan® 500 and 600 Manual; The Phantom Laboratory Incorporated: New York, NY, USA, 2006.
- Installation Product Acceptance (IPA). Varian Medical Systems; Varian: Palo Alto, CA, USA, 2021. [Google Scholar]
- Trapp, J.V. Imaging and Radiation Interactions of Polymer Gel Dosimeters. Ph.D. Thesis, School of Physical and Chemical Sciences, Queensland University of Technology, Brisbane, QLD, Australia, 2003. [Google Scholar]
- Trapp, J.V.; Bäck, S.Å.J.; Lepage, M.; Michael, G.; Baldock, C. An experimental study of the dose response of polymer gel dosimeters imaged with x-ray computed tomography. Phys. Med. Biol. 2001, 46, 2939–2951. [Google Scholar] [CrossRef]
- Klein, E.E.; Hanley, J.; Bayouth, J.; Yin, F.-F.; Simon, W.; Dresser, S.; Serago, C.; Aguirre, F.; Ma, L.; Arjomandy, B.; et al. Consultants: Sandin, C.; Holmes, T. Task Group 142 report: Quality assurance of medical accelerators. Med. Phys. 2009, 36, 4197–4212. [Google Scholar] [CrossRef]






| Mode | Reconstruction | Energy [kV] | Exposure [mAs] | CTDIVol [mGy] | DLP [mGy·cm] | Acquisition Time [s] | Scan Diameter [cm] |
|---|---|---|---|---|---|---|---|
| Image Gently | CBCT, iCBCT | 80 | 84 | 1.08 | 16.3 | 16.6 | 28.2 |
| Image Gently Large | CBCT, iCBCT | 100 | 90 | 2.38 | 35.6 | 16.6 | 38.4 |
| Head | CBCT, iCBCT | 100 | 126 | 3.33 | 49.9 | 16.6 | 28.2 |
| Head Low Dose | CBCT, iCBCT | 100 | 42 | 1.11 | 16.6 | 16.6 | 28.2 |
| Breast | CBCT | 125 | 45 | 0.90 | 13.5 | 16.6 | 49.2 |
| Thorax Fast | CBCT | 125 | 270 | 5.4 | 81 | 16.6 | 49.2 |
| Pelvis Fast | CBCT, iCBCT | 125 | 560 | 11.2 | 168 | 21.2 | 49.2 |
| Thorax | CBCT | 125 | 294 | 5.88 | 88.2 | 30.8 | 49.2 |
| Pelvis | CBCT, iCBCT | 125 | 1080 | 21.6 | 324 | 36.7 | 49.2 |
| Pelvis Large Fast | CBCT, iCBCT | 140 | 672 | 17.74 | 266.1 | 25 | 49.2 |
| Pelvis Large | CBCT, iCBCT | 140 | 1440 | 38.02 | 570.2 | 40.6 | 49.2 |
| Mode | Reconstruction | Energy [kV] | Exposure [mAs] | CTDIVol [mGy] | DLP [mGy·cm] | Fan Type | Acquisition Time [s] | Filter | Noise Suppression |
|---|---|---|---|---|---|---|---|---|---|
| Head | iCBCT | 100 | 150 | 3.17 | 67.7 | Full | 33 | S | Medium |
| Head | CBCT | 100 | 150 | 3.17 | Full | 33 | A | - | |
| Image Gently | iCBCT | 80 | 100 | 0.94 | 20.1 | Full | 33 | S | Medium |
| Image Gently | CBCT | 80 | 100 | 0.94 | Full | 33 | A | - | |
| Pelvis | iCBCT | 125 | 1080 | 15.98 | 342.1 | Half | 60 | S | Medium |
| Pelvis | iCBCT Fast | 125 | 1080 | 15.98 | Half | 60 | S | Medium | |
| Pelvis | CBCT | 125 | 1080 | 15.98 | Half | 60 | A | - | |
| Pelvis Large | iCBCT | 140 | 1688 | 36.79 | 787.3 | Half | 60 | S | Medium |
| Pelvis Large | iCBCT Fast | 140 | 1688 | 36.79 | Half | 60 | S | Medium | |
| Pelvis Large | CBCT | 140 | 1688 | 36.79 | Half | 60 | A | - | |
| Short Thorax | CBCT | 125 | 210 | 3.44 | 73.7 | Full | 23 | Sm | - |
| Spotlight | CBCT | 125 | 750 | 12.30 | 263.2 | Full | 33 | Sm | - |
| Thorax | CBCT | 125 | 270 | 4.0 | 85.5 | Half | 60 | A | - |
| Mode | Standard Deviation for VOI [HU] |
|---|---|
| Cone-beam CT (CBCT) | |
| Image Gently | 25.841 |
| Image Gently Large | 16.506 |
| Head | 18.917 |
| Head Low Dose | 24.452 |
| Pelvis | 2.984 |
| Pelvis Fast | 7.403 |
| Pelvis Large | 2.772 |
| Pelvis Large Fast | 4.570 |
| Iterative reconstruction CBCT (iCBCT) | |
| Image Gently | 9.581 |
| Image Gently Large | 4.475 |
| Head | 3.679 |
| Head Low Dose | 8.465 |
| Pelvis | 2.129 |
| Pelvis Fast | 2.703 |
| Pelvis Large | 1.585 |
| Pelvis Large Fast | 1.511 |
| Mode | Standard Deviation for VOI [HU] |
|---|---|
| Cone-beam CT (CBCT) | |
| Head | 11.867 |
| Image Gently | 17.916 |
| Pelvis Large | 4.986 |
| Pelvis | 2.743 |
| Short Thorax | 7.269 |
| Spotlight | 4.785 |
| Thorax | 3.746 |
| Iterative reconstruction CBCT (iCBCT) | |
| Head | 4.115 |
| Image Gently | 6.875 |
| Pelvis Large | 3.233 |
| Pelvis | 1.555 |
| Mode | Standard Deviation for VOI [HU] |
|---|---|
| Cone-beam CT (CBCT) | |
| Pelvis s_1 | 4.81 |
| Pelvis Large s_1 | 7.30 |
| Pelvis s_1–s_5 | 4.53 |
| Pelvis Large s_1–s_5 | 7.18 |
| Iterative reconstruction CBCT (iCBCT) | |
| Pelvis s_1 | 4.19 |
| Pelvis Large s_1 | 6.58 |
| Pelvis s_1–s_5 | 4.20 |
| Pelvis Large s_1–s_5 | 6.64 |
| Mean Value [HU] | Image Uniformity (±30 HU) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mode/Gap (i_0, i_5)/Series (s_1–s_5) | Center | Left | Top | Right | Bottom | Left (1) | Top (2) | Right (3) | Bottom (4) | Mean (1–4) | Mean (Series s_1–s_5) |
| Cone-beam CT (CBCT) | |||||||||||
| Pelvis i_0 s_1 | 11.58 | 1.89 | 3.86 | 6.36 | 2.70 | −9.69 | −7.73 | −5.23 | −8.88 | −7.88 | −7.86 |
| Pelvis i_0 s_2 | 10.61 | 3.82 | 4.11 | 2.93 | 1.83 | −6.79 | −6.50 | −7.68 | −8.78 | −7.44 | |
| Pelvis i_0 s_3 | 11.43 | 2.33 | 3.89 | 5.57 | 2.80 | −9.10 | −7.53 | −5.85 | −8.62 | −7.78 | |
| Pelvis i_0 s_4 | 11.22 | 3.45 | 3.93 | 3.00 | 1.59 | −7.76 | −7.29 | −8.21 | −9.63 | −8.22 | |
| Pelvis i_0 s_5 | 11.49 | 2.20 | 3.46 | 5.48 | 2.83 | −9.29 | −8.03 | −6.01 | −8.66 | −8.00 | |
| Pelvis i_5 s_1 | 13.42 | 3.28 | 5.41 | 7.57 | 4.01 | −10.13 | −8.01 | −5.84 | −9.40 | −8.35 | −8.02 |
| Pelvis i_5 s_2 | 12.21 | 5.29 | 5.20 | 3.71 | 2.88 | −6.92 | −7.01 | −8.50 | −9.33 | −7.94 | |
| Pelvis i_5 s_3 | 12.89 | 3.03 | 4.92 | 7.38 | 3.82 | −9.87 | −7.97 | −5.51 | −9.08 | −8.11 | |
| Pelvis i_5 s_4 | 11.72 | 4.97 | 5.24 | 3.41 | 2.63 | −6.75 | −6.49 | −8.32 | −9.10 | −7.66 | |
| Pelvis i_5 s_5 | 12.51 | 2.41 | 4.96 | 6.91 | 3.55 | −10.10 | −7.55 | −5.60 | −8.96 | −8.05 | |
| PelvisLarge i_0 s_1 | 28.42 | 7.87 | 7.16 | 5.87 | 5.30 | −20.55 | −21.27 | −22.55 | −23.12 | −21.87 | −22.33 |
| PelvisLarge i_0 s_2 | 30.88 | 6.77 | 9.49 | 11.44 | 7.46 | −24.10 | −21.39 | −19.44 | −23.42 | −22.09 | |
| PelvisLarge i_0 s_3 | 29.77 | 8.08 | 8.20 | 6.94 | 6.13 | −21.69 | −21.57 | −22.82 | −23.64 | −22.43 | |
| PelvisLarge i_0 s_4 | 30.74 | 6.86 | 8.67 | 10.31 | 6.59 | −23.88 | −22.07 | −20.43 | −24.15 | −22.63 | |
| PelvisLarge i_0 s_5 | 28.87 | 7.09 | 6.92 | 6.00 | 4.96 | −21.77 | −21.95 | −22.87 | −23.91 | −22.62 | |
| PelvisLarge i_5 s_1 | 27.00 | 3.20 | 5.31 | 7.50 | 3.57 | −23.80 | −21.69 | −19.50 | −23.43 | −22.10 | −22.10 |
| PelvisLarge i_5 s_2 | 25.58 | 3.76 | 3.99 | 2.51 | 1.86 | −21.82 | −21.59 | −23.07 | −23.71 | −22.55 | |
| PelvisLarge i_5 s_3 | 26.25 | 2.47 | 4.66 | 6.23 | 2.79 | −23.78 | −21.59 | −20.02 | −23.46 | −22.21 | |
| PelvisLarge i_5 s_4 | 24.32 | 3.39 | 3.11 | 2.04 | 0.99 | −20.93 | −21.22 | −22.29 | −23.33 | −21.94 | |
| PelvisLarge i_5 s_5 | 24.89 | 1.73 | 3.83 | 5.19 | 2.00 | −23.17 | −21.06 | −19.70 | −22.89 | −21.70 | |
| Iterative reconstruction CBCT (iCBCT) | |||||||||||
| Pelvis i_0 s_1 | −6.73 | −9.97 | −9.19 | −11.17 | −11.32 | −3.24 | −2.46 | −4.44 | −4.59 | −3.68 | −4.12 |
| Pelvis i_0 s_2 | −5.97 | −11.10 | −9.39 | −9.65 | −10.72 | −5.13 | −3.43 | −3.68 | −4.75 | −4.25 | |
| Pelvis i_0 s_3 | −7.00 | −11.00 | −10.26 | −11.84 | −12.18 | −4.00 | −3.26 | −4.84 | −5.18 | −4.32 | |
| Pelvis i_0 s_4 | −6.76 | −11.64 | −10.16 | −10.48 | −11.31 | −4.88 | −3.41 | −3.73 | −4.56 | −4.14 | |
| Pelvis i_0 s_5 | −7.61 | −11.31 | −10.72 | −12.56 | −12.61 | −3.70 | −3.11 | −4.95 | −5.00 | −4.19 | |
| Pelvis i_5 s_1 | −5.27 | −8.69 | −8.06 | −10.00 | −10.24 | −3.42 | −2.79 | −4.73 | −4.97 | −3.98 | −4.11 |
| Pelvis i_5 s_2 | −4.11 | −9.91 | −8.03 | −7.71 | −9.00 | −5.80 | −3.92 | −3.60 | −4.89 | −4.55 | |
| Pelvis i_5 s_3 | −4.88 | −8.45 | −7.76 | −9.85 | −10.10 | −3.58 | −2.88 | −4.97 | −5.22 | −4.16 | |
| Pelvis i_5 s_4 | −4.89 | −10.24 | −8.20 | −7.86 | −9.52 | −5.36 | −3.31 | −2.97 | −4.63 | −4.07 | |
| Pelvis i_5 s_5 | −5.85 | −8.98 | −8.57 | −10.20 | −10.69 | −3.13 | −2.72 | −4.36 | −4.84 | −3.76 | |
| PelvisLarge i_0 s_1 | −0.71 | −0.39 | −0.27 | −2.44 | −2.68 | 0.32 | 0.45 | −1.72 | −1.96 | −0.73 | −2.68 |
| PelvisLarge i_0 s_2 | 0.36 | −0.88 | 0.13 | −1.43 | −2.25 | −1.24 | −0.24 | −1.79 | −2.62 | −1.47 | |
| PelvisLarge i_0 s_3 | −2.03 | −2.82 | −2.33 | −4.19 | −4.92 | −0.78 | −0.29 | −2.15 | −2.89 | −1.53 | |
| PelvisLarge i_0 s_4 | −0.62 | −2.27 | −1.37 | −3.29 | −4.17 | −1.65 | −0.75 | −2.67 | −3.54 | −2.15 | |
| PelvisLarge i_0 s_5 | 17.41 | 10.01 | 11.51 | 10.38 | 7.62 | −7.39 | −5.90 | −7.03 | −9.79 | −7.53 | |
| PelvisLarge i_5 s_1 | −2.01 | −3.96 | −2.27 | −3.91 | −5.36 | −1.95 | −0.26 | −1.90 | −3.35 | −1.87 | −2.12 |
| PelvisLarge i_5 s_2 | −3.98 | −4.73 | −4.87 | −6.89 | −7.21 | −0.75 | −0.89 | −2.91 | −3.24 | −1.95 | |
| PelvisLarge i_5 s_3 | −2.49 | −5.10 | −3.40 | −5.02 | −6.42 | −2.62 | −0.91 | −2.53 | −3.93 | −2.50 | |
| PelvisLarge i_5 s_4 | −4.69 | −5.94 | −5.69 | −7.66 | −8.24 | −1.25 | −1.00 | −2.97 | −3.55 | −2.19 | |
| PelvisLarge i_5 s_5 | −3.36 | −5.42 | −3.78 | −5.80 | −6.79 | −2.07 | −0.42 | −2.44 | −3.44 | −2.09 | |
| Mean Value [HU] | Image Uniformity (±30 HU) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mode/Gap (i_0, i_5)/Series (s_1–s_5) | Center | Left | Top | Right | Bottom | Left (1) | Top (2) | Right (3) | Bottom (4) | Mean (1–4) | Mean (Series s_1–s_5 |
| Cone-beam CT (CBCT) | |||||||||||
| Pelvis i_0 s_1 | 7.52 | 1.29 | 1.16 | −1.06 | −1.73 | −6.22 | −6.35 | −8.57 | −9.25 | −7.60 | −7.41 |
| Pelvis i_0 s_2 | 7.37 | 0.22 | 1.05 | 0.69 | −1.33 | −7.15 | −6.32 | −6.68 | −8.70 | −7.21 | |
| Pelvis i_5 s_1 | 7.37 | 0.22 | 1.05 | 0.69 | −1.33 | −7.15 | −6.32 | −6.68 | −8.70 | −7.21 | −8.04 |
| Pelvis i_5 s_2 | 8.02 | 1.17 | 1.42 | −1.23 | −1.39 | −6.85 | −6.60 | −9.26 | −9.42 | −8.03 | |
| Pelvis i_5 s_3 | 8.74 | −0.09 | 0.55 | 0.58 | −1.25 | −8.83 | −8.19 | −8.16 | −9.99 | −8.79 | |
| Pelvis i_5 s_4 | 7.88 | 1.50 | 0.60 | −1.48 | −1.45 | −6.38 | −7.29 | −9.36 | −9.33 | −8.09 | |
| Pelvis i_5 s_5 | 7.95 | 0.05 | 1.00 | 0.27 | −1.72 | −7.90 | −6.95 | −7.69 | −9.68 | −8.06 | |
| PelvisLarge i_0 s_1 | 7.99 | 4.37 | 5.56 | 4.54 | 2.51 | −3.62 | −2.42 | −3.45 | −5.47 | −3.74 | −4.17 |
| PelvisLarge i_0 s_2 | 8.84 | 3.52 | 5.02 | 5.64 | 2.76 | −5.32 | −3.82 | −3.20 | −6.07 | −4.60 | |
| PelvisLarge i_5 s_1 | 10.20 | 2.51 | 2.73 | 0.46 | −0.59 | −7.69 | −7.48 | −9.75 | −10.80 | −8.93 | −8.77 |
| PelvisLarge i_5 s_2 | 9.86 | 1.29 | 2.44 | 1.65 | −0.65 | −8.58 | −7.42 | −8.21 | −10.52 | −8.68 | |
| PelvisLarge i_5 s_3 | 9.79 | 2.44 | 2.46 | 0.36 | −0.73 | −7.35 | −7.33 | −9.43 | −10.52 | −8.66 | |
| PelvisLarge i_5 s_4 | 9.92 | 1.33 | 2.31 | 1.75 | −0.79 | −8.59 | −7.61 | −8.17 | −10.71 | −8.77 | |
| PelvisLarge i_5 s_5 | 9.74 | 2.27 | 2.15 | 0.20 | −0.83 | −7.48 | −7.59 | −9.55 | −10.58 | −8.80 | |
| Iterative reconstruction CBCT (iCBCT) | |||||||||||
| Pelvis i_0 s_1 | 2.76 | −8.60 | −9.12 | −10.29 | −10.05 | −11.36 | −11.87 | −13.04 | −12.81 | −12.27 | −12.43 |
| Pelvis i_0 s_2 | 3.23 | −8.32 | −8.85 | −10.04 | −10.23 | −11.55 | −12.08 | −13.27 | −13.47 | −12.59 | |
| Pelvis i_5 s_1 | 4.25 | −8.04 | −7.77 | −8.50 | −9.48 | −12.29 | −12.03 | −12.75 | −13.73 | −12.70 | −12.42 |
| Pelvis i_5 s_2 | 3.57 | −7.29 | −7.72 | −9.59 | −9.37 | −10.86 | −11.29 | −13.16 | −12.93 | −12.06 | |
| Pelvis i_5 s_3 | 3.62 | −7.83 | −7.43 | −8.88 | −9.46 | −11.46 | −11.05 | −12.50 | −13.08 | −12.02 | |
| Pelvis i_5 s_4 | 4.06 | −7.52 | −7.94 | −9.20 | −9.16 | −11.57 | −11.99 | −13.26 | −13.21 | −12.51 | |
| Pelvis i_5 s_5 | 4.02 | −8.41 | −7.91 | −9.14 | −9.64 | −12.42 | −11.93 | −13.16 | −13.66 | −12.79 | |
| PelvisLarge i_0 s_1 | 3.71 | −3.45 | −3.29 | −3.90 | −5.02 | −7.16 | −7.00 | −7.61 | −8.73 | −7.62 | −8.07 |
| PelvisLarge i_0 s_2 | 4.69 | −3.61 | −3.27 | −3.56 | −4.88 | −8.30 | −7.96 | −8.25 | −9.57 | −8.52 | |
| PelvisLarge i_5 s_1 | 5.45 | −4.03 | −4.36 | −5.48 | −6.27 | −9.49 | −9.82 | −10.94 | −11.72 | −10.49 | −11.63 |
| PelvisLarge i_5 s_2 | 5.87 | −4.92 | −4.74 | −5.35 | −6.80 | −10.79 | −10.61 | −11.22 | −12.66 | −11.32 | |
| PelvisLarge i_5 s_3 | 6.39 | −4.76 | −4.45 | −6.03 | −6.54 | −11.14 | −10.84 | −12.41 | −12.92 | −11.83 | |
| PelvisLarge i_5 s_4 | 6.23 | −5.42 | −4.99 | −5.97 | −7.10 | −11.65 | −11.22 | −12.20 | −13.33 | −12.10 | |
| PelvisLarge i_5 s_5 | 6.33 | −4.94 | −4.75 | −6.91 | −7.66 | −11.27 | −11.08 | −13.24 | −13.98 | −12.39 | |
| Accelerator | Reconstruction Algorithm | Mode | Signal Averaging | Scanning Time Interval [min] | Radiation Field Parameters | Fiducial Markers | Image Processing |
|---|---|---|---|---|---|---|---|
| TrueBeam | iCBCT | Pelvis | iCBCT: 1 CBCT: 5 | 0 (up to 5 scans) | 2 mm MLC gap and 10,000 MU | shielded copper wire of the network cable | Image averaging (no or max from three series); Background subtraction; Filter: not obligatory |
| Halcyon | Pelvis/ Pelvis Large Fast | 0 (up to 2 scans) 5 (up to 5 scans) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maras, P.; Kozicki, M. Fast Isocenter Determination Using 3D Polymer Gel Dosimetry with Kilovoltage Cone-Beam CT Reading and the PolyGeVero-CT Software Package for Linac Quality Assurance in Radiotherapy. Materials 2022, 15, 6807. https://doi.org/10.3390/ma15196807
Maras P, Kozicki M. Fast Isocenter Determination Using 3D Polymer Gel Dosimetry with Kilovoltage Cone-Beam CT Reading and the PolyGeVero-CT Software Package for Linac Quality Assurance in Radiotherapy. Materials. 2022; 15(19):6807. https://doi.org/10.3390/ma15196807
Chicago/Turabian StyleMaras, Piotr, and Marek Kozicki. 2022. "Fast Isocenter Determination Using 3D Polymer Gel Dosimetry with Kilovoltage Cone-Beam CT Reading and the PolyGeVero-CT Software Package for Linac Quality Assurance in Radiotherapy" Materials 15, no. 19: 6807. https://doi.org/10.3390/ma15196807
APA StyleMaras, P., & Kozicki, M. (2022). Fast Isocenter Determination Using 3D Polymer Gel Dosimetry with Kilovoltage Cone-Beam CT Reading and the PolyGeVero-CT Software Package for Linac Quality Assurance in Radiotherapy. Materials, 15(19), 6807. https://doi.org/10.3390/ma15196807






