Hydrodesulfurization of Dibenzothiophene over Ni-Mo-W Sulfide Catalysts Supported on Sol-Gel Al2O3-CeO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Support Preparation
2.2. Catalyst Preparation
2.3. Catalyst Characterization
2.4. Activity Tests
3. Results
3.1. Characterization of Supports
3.2. Characterization of the Calcined Catalysts
3.3. Catalyst Activity
3.4. Characterization of Used Catalysts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Topsøe, H.; Clausen, B.S.; Massoth, F.E. Hydrotreating Catalysts; Springer Verlag: Berlin/Heidelberg, Germany, 1996; p. 458. [Google Scholar]
- Raybaud, P.; Toulhoat, H. Catalysis by Transition Metal Sulphides: From Theory to Industrial Application; Editions Technip: Paris, France, 2013; p. 832. [Google Scholar]
- Navarro, R.; Pawelec, B.; Fierro, J.L.G.; Vasudevan, P.T. Dibenzothiophene hydrodesulfurization on silica-alumina-supported transition metal sulfide catalysts. Appl. Catal. A Gen. 1996, 148, 23–40. [Google Scholar] [CrossRef]
- Tavizón-Pozos, J.A.; Suárez-Torello, V.A.; de los Reyes, J.A.; Guevara-Lara, A.; Pawelec, B.; Fierro, J.L.G.; Vrinat, M.; Geantet, C. Deep hydrodesulfurization of dibenzothiophene over NiW sulfide catalysts supported on sol-gel titania-alumina. Top. Catal. 2016, 59, 241–251. [Google Scholar] [CrossRef]
- Huirache-Acuña, R.; Navarro Yerga, R.M.; Pawelec, B. Hydrodesulfurization on supported CoMoS2 catalysts ex ammonium tetrathiomolybdate: Effects of support morphology and al modification method. Top. Catal. 2022, 1–14. [Google Scholar] [CrossRef]
- Singh, R.; Kunzru, D.; Sivakumar, S. Enhanced catalytic activity of ultrasmall NiMoW trimetallic nanocatalyst for hydrodesulfurization of fuels. Fuel 2021, 288, 119603. [Google Scholar] [CrossRef]
- Absi-Halabi, M.; Stanislaus, A.; Al-Dolama, K. Performance comparison of alumina-supported Ni–Mo, Ni–W and Ni–Mo–W catalysts in hydrotreating vacuum residue. Fuel 1998, 7, 787–790. [Google Scholar] [CrossRef]
- Huirache-Acuña, R.; Zepeda, T.A.; Vázquez, P.J.; Rivera-Muñoz, E.M.; Maya-Yescas, R.; Pawelec, B.; Alonso-Núñez, G. The use of inorganic Al-HMS as a support for NiMoW sulfide HDS catalysts. Inorg. Chim. Acta 2021, 524, 120450. [Google Scholar] [CrossRef]
- Gallegos-Hernández, A.Y.; Martínez-Rosales, M.; Rico, J.L.; Avalos-Borja, M. Improvement in the hydrodesulfurization of dibenzothiophene over supported NiMoW catalysts. Reac. Kinet. Mech. Cat. 2021, 132, 317–330. [Google Scholar] [CrossRef]
- Mendoza-Nieto, J.A.; Vizueth-Montes de Oca, A.; Calzada, L.A.; Klimova, T.E. Trimetallic NiMoW and CoMoW catalysts supported on SBA-15 modified with titania or zirconia for deep hydrodesulfurization. Catal. Today 2021, 360, 78–89. [Google Scholar] [CrossRef]
- Guzmán, M.A.; Huirache-Acuña, R.; Loricera, C.V.; Hernández, J.R.; Díaz de León, J.N.; de los Reyes, J.A.; Pawelec, B. Removal of refractory S-containing compounds from liquid fuels over P-loaded NiMoW/SBA-16 sulfide catalysts. Fuel 2013, 103, 321–333. [Google Scholar] [CrossRef]
- Gómez-Orozco, S.Y.; Huirache-Acuña, R.; Pawelec, B.; Fierro, J.L.G.; Rivera-Muñoz, E.M.; Lara-Romero, J.; Alonso-Nuñez, G. Characterizations and HDS performances of sulfided NiMoW catalysts supported on mesoporous titania-modified SBA-15. Catal. Today 2018, 305, 152–161. [Google Scholar] [CrossRef]
- Vázquez-Salas, P.J.; Huirache-Acuña, R.; Zepeda, T.A.; Alonso-Núñez, G.; Maya-Yescas, R. Enhancement of dibnzothiophene hydrodesulfurization via hydrogenation route on NiMoW catalyst supported on HMS modified with Ti. Catal. Today 2018, 305, 65–74. [Google Scholar] [CrossRef]
- Huirache-Acuña, R.; Rivera-Muñoz, E.M.; Pawelec, B.; Ostrooumov, M.; Maya-Yescas, R.; Rico, J.L. The use of a natural Mexican zeolite as support of NiMoW sulphide hydrotreating catalysts. Catal. Today 2014, 220–222, 301–309. [Google Scholar] [CrossRef]
- Stuart, N.M.; Sohlberg, K. The Microstructure of γ-Alumina. Energies 2021, 14, 6472. [Google Scholar] [CrossRef]
- Levin, I.; Brandon, D. Metastable Alumina Polymorphs: Crystal Structures and Transition Sequences. J. Am. Ceram. Soc. 1998, 81, 1995–2012. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. γ-Alumina as a support for catalysts: A review of fundamental aspects. Eur. J. Inorg. Chem. 2005, 2005, 3393–3403. [Google Scholar] [CrossRef]
- Prins, R. On the structure of γ-Al2O3. J. Catal. 2020, 392, 336–346. [Google Scholar] [CrossRef]
- Feng, K.; An, J.; Ren, W.; Lei, X.; Li, G.; Rong, D.; Wen, X. The synthesis of urchin-like γ-Al2O3 hierarchical microspheres from Al foils and its rapid adsorption ability towards Congo red. Mater. Res. Express 2020, 7, 035011. [Google Scholar] [CrossRef]
- Abello, M.C.; Velasco, A.P.; Ferretti, O.A.; Fierro, J.L.G. A Monte Carlo approach to describe the reduction behavior of bideimensional MoOx structures grown on an alumina substrate. Lat. Am. Appl. Res. 2007, 37, 307–313. [Google Scholar]
- Ozawa, M.; Kimura, M. Effect of cerium addition on the thermal stability of gamma alumina support. J. Mater. Sci. Lett. 1990, 9, 291–293. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R. Catalytic Hydroprocessing of Chemical Models for Bio-oil. Energy Fuels 2009, 23, 631–637. [Google Scholar] [CrossRef]
- Kordouli, E.; Pawelec, B.; Kordulis, C.; Lycourghiotis, A.; Fierro, J.L.G. Hydrodeoxygenation of phenol on bifunctional Ni-based catalysts: Effects of Mo promotion and support. Appl. Catal. B Environ. 2018, 238, 147–160. [Google Scholar] [CrossRef]
- Tang, S.; Ji, L.; Lin, J.; Zeng, H.C.; Tan, K.L.; Li, K. CO2 Reforming of Methane to Synthesis Gas over Sol–Gel-made Ni/γ-Al2O3 Catalysts from Organometallic Precursors. J. Catal. 2000, 194, 424–430. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, G.; Sheng, S.; Yang, W. Deactivation studies over NiO/γ-Al2O3 catalysts for partial oxidation of methane to syngas. Catal. Today 2000, 63, 517–522. [Google Scholar] [CrossRef]
- Kim, J.-H.; Suh, D.J.; Park, T.-J.; Kim, K.-L. Effect of metal particle size on coking during CO2 reforming of CH4 over Ni–alumina aerogel catalysts. Appl. Catal. A 2000, 197, 191–200. [Google Scholar] [CrossRef]
- Osorio-Vargas, P.; Campos, C.H.; Navarro, R.M.; Fierro, J.L.G.; Reyes, P. Improved ethanol steam reforming on Rh/Al2O3 catalysts doped with CeO2 or/and La2O3: Influence in reaction pathways including coke formation. Appl. Catal. A Gen. 2015, 505, 159–172. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q. Role of CeO2 in NiMoW/CeO2-Al2O3 catalysts for carbon dioxide reforming of methane. Appl. Catal. B Environ. 1998, 19, 267–277. [Google Scholar] [CrossRef]
- Damyanova, S.; Perez, C.A.; Schmal, M.; Bueno, J.M.C. Characterization of ceria-coated alumina carrier. Appl. Catal. A Gen. 2002, 234, 271–282. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Palcheva, R.; Karakirova, Y.; Capel-Sanchez, M.C.; Tyuliev, G.; Gaigneaux, E.; Fierro, J.L.G. Structure and surface properties of ceria-modified Ni-based catalysts for hydrogen production. Appl. Catal. B Environ. 2018, 225, 340–353. [Google Scholar] [CrossRef]
- Pawelec, B.; Guil-López, R.; Mota, N.; Fierro, J.L.G.; Navarro Yerga, R.M. Catalysts for the conversion of CO2 to low molecular weight olefins–A review. Materials 2021, 14, 6952. [Google Scholar] [CrossRef]
- Mota, N.; Ordoñez, E.M.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Direct synthesis of dimethyl ether from CO2: Recent advances in bifunctional/hybrid catalytic systems. Catalysts 2021, 11, 411. [Google Scholar] [CrossRef]
- Ciesielski, R.; Shtyka, O.; Zakrzewski, M.; Kubicki, J.; Maniukiewicz, W.; Kedziora, A.; Maniecki, T.P. Mechanistic studies of methanol synthesis reaction over Cu and Pd–Cu catalysts. Kinet. Catal. 2020, 61, 623–630. [Google Scholar] [CrossRef]
- Fan, X.; Yao, M.; Liu, F.; Wang, X.; Cao, J. Effect of preparation methods on physicochemical properties of Al2O3-CeO2 and its catalytic performance of CO2 hydrogenation to methanol. J. Synth. Cryst. 2021, 50, 1745–1755 and 1795. [Google Scholar]
- Wu, Y.; Lin, J.; Ma, G.; Xu, Y.; Zhang, J.; Samart, C.; Ding, M. Ni nanocatalysts supported on mesoporous Al2O3-CeO2 for CO2 methanation at low temperature. RSC Adv. 2020, 10, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Li, J.; Long, J.; Yang, X.; Miao, P. Promotion effect of cerium on Mo/Al2O3 catalyst for methanation. Appl. Catal. A Gen. 2020, 598, 117559. [Google Scholar] [CrossRef]
- Cheng, D.G.; Chong, M.; Chen, F.; Zhan, X. XPS Characterization of CeO2 Catalyst for Hydrogenation of Benzoic Acid to Benzaldehyde. Catal. Lett. 2008, 120, 82–85. [Google Scholar] [CrossRef]
- Vilé, G.; Bridier, B.; Wichert, J.; Pérez-Ramírez, J. Ceria in hydrogenation catalysis: High selectivity in the conversion of alkynes to olefins. Angew. Chem. Int. Ed. 2012, 124, 8748–8751. [Google Scholar] [CrossRef]
- García-Melchor, M.; Bellarosa, L.; López, N. Unique reaction path in heterogeneous catalysis: The concerted semi-hydrogenation of propyne to propene on CeO2. ACS Catal. 2014, 4, 4015–4020. [Google Scholar] [CrossRef]
- Carrasco, J.; Vilé, G.; Fernández-Torre, D.; Pérez, R.; Pérez-Ramírez, J.; Ganduglia-Pirovano, M.V. Molecular-level understanding of CeO2 as a catalyst for partial alkyne hydrogenation. J. Phys. Chem. C 2014, 118, 5352–5360. [Google Scholar] [CrossRef]
- Manríquez, M.E.; Hernández-Pichardo, M.L.; Barrera, M.C.; Ramírez-López, R.; Castro, L.V. Enhanced catalytic activity on the naphthalene hydrogenation reaction over Pt-Pd/Al2O3-CeO2 catalysts. Rev. Mex. Ing. Quim. 2018, 17, 913–925. [Google Scholar] [CrossRef]
- Miciukiewicz, J.; Laniecki, M.; Domka, F. Thiophene hydrodesulfurization over modified alumina-supported molybdenum sulfide catalysts. Catal. Lett. 1998, 51, 65–68. [Google Scholar] [CrossRef]
- Jirátová, K.; Spojakina, A.; Kaluža, L.; Palcheva, R.; Balabánová, J.; Tyuliev, G. Hydrodesulfurization activities of NiMo catalysts supported on mechanochemically prepared Al-Ce mixed oxides. Cuihua Xuebao/Chinese J. Catal. 2016, 37, 258–267. [Google Scholar] [CrossRef]
- Lu, X.; Luo, L.; Cheng, X. Effect of ceria on noble metal catalysts for hydrodesulfurization. Spec. Petrochem. 2008, 25, 34–38. [Google Scholar]
- Alonso-Pérez, M.O.; Pawelec, B.; Zepeda, T.A.; Alonso-Núñez, G.; Nava, R.; Navarro, R.M.; Huirache-Acuña, R. Effect of the titanium incorporation method on the morphology and HDS activity of supported ternary Ni-Mo-W/SBA-16 catalysts. Micropor. Mesopor. Mater. 2021, 312, 110779. [Google Scholar] [CrossRef]
- Kokka, A.; Petala, A.; Panagiotopoulou, P. Support Effects on the Activity of Ni Catalysts for the Propane Steam Reforming Reaction. Nanomaterials 2021, 11, 1948. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zeng, T.; Cheng, Z.; Yuan, W. Preparation of a catalyst for selective hydrogenation of pyrolysis gasoline. Ind. Eng. Chem. Res. 2010, 49, 11112–11118. [Google Scholar] [CrossRef]
- Mendoza-Nieto, J.A.; Vera-Vallejo, O.; Escobar-Alarcón, L.; Solis-Casados, D.; Klimova, T. Development of new trimetallic NiMoW catalysts supported on SBA-15 for deep hydrodesulfurization. Fuel 2013, 110, 268–277. [Google Scholar] [CrossRef]
- Bêche, E.; Charvin, P.; Perarnau, D.; Abanades, S.; Flamant, G. Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (CexTiyOz). Surf. Interface Anal. 2008, 40, 264–267. [Google Scholar] [CrossRef]
- Ghule, A.V.; Ghule, K.; Punde, T.; Liu, J.-Y.; Tzing, S.-H.; Chang, J.-Y.; Chang, H.; Ling, Y.-C. In situ monitoring of NiO-Al2O3 nanoparticles synthesis by thermo-Raman spectroscopy. Mater. Chem. Phys. 2010, 119, 86–92. [Google Scholar] [CrossRef]
- NIST Standard Reference Database Number 69. Available online: https://webbook.nist.gov/chemistry/ (accessed on 30 June 2022).
- Wallin, M.; Grönbeck, H.; Lloyd Spetz, A.; Skoglundh, M. Vibrational study of ammonia adsorption on Pt/SiO2. Appl. Surf. Sci. 2004, 235, 487–500. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Li, R.; Yabe, S.; Yamashita, M.; Momose, S.; Yoshida, S.; Yin, S.; Sato, T. UV-shielding properties of zinc oxide-doped ceria fine powders derived via soft solution chemical routes. Mater. Chem. Phys. 2002, 75, 39–44. [Google Scholar] [CrossRef]
- Li, X.; Zhou, F.; Wang, A.; Wang, L.; Hu, Y. Influence of templates on the overgrowth of MCM-41 over HY and hydrodesulfurization performance of the supported Ni-Mo catalysts. Ind. Eng. Chem. Res. 2009, 48, 2870–2877. [Google Scholar] [CrossRef]
- Raikwar, D.; Munagala, M.; Majumdar, S.; Shee, D. Hydrodeoxygenation of guaiacol over Mo, W and Ta modified supported nickel catalysts. Catal. Today 2019, 325, 117–130. [Google Scholar] [CrossRef]
- Tauc, J.; Abeles, F. (Eds.) The Optical Properties of Solids; North-Holland Publishing Company: Amsterdam, The Netherlands, 1972. [Google Scholar]
- Wang, Y.; Herron, N. Nanometer-sized semiconductor clusters: Materials synthesis, quantum size effects, and photophysical properties. J. Phys. Chem. 1991, 95, 525–532. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, C.W.; Kim, M.; Oh, J.H.; Song, S.A.; Jang, S.C.; Yoon, C.W.; Han, J.; Yoon, S.P.; Nam, S.W. Preparation of CoMo/Al2O3, CoMo/CeO2 and CoMo/TiO2 catalysts using ultrasonic spray pyrolysis for the hydrodesulfurization of 4,6-dimethyldibenzothiophene for fuell cell applications. Int. J. Hydrogen Energy 2016, 41, 18846–18857. [Google Scholar] [CrossRef]
- Lauritsen, J.V.; Kibsgaard, J.; Olesen, G.H.; Moses, P.G.; Hinneman, B.; Helveg, S.; Nørskov, J.K.; Clausen, B.S.; Topsøe, H.; Lægsgaard, E.; et al. Location and coordination of promoter atoms in Co- and Ni-promoted MoS2-based hydrotreating catalysts. J. Catal. 2007, 249, 220–233. [Google Scholar] [CrossRef]
- Zhang, B.S.; Yi, Y.J.; Zhang, W.; Liang, C.H.; Su, D.S. Electron microscopy investigation of the microstructure of unsupported Ni-Mo-W sulfide. Mater. Charact. 2011, 62, 684690. [Google Scholar] [CrossRef]
- Datye, A.K.; Srinivasan, S.; Allard, L.F.; Peden, C.H.F.; Brenner, J.R.; Thompson, L.T. Oxide supported MoS2 catalysts of unusual morphology. J. Catal. 1996, 158, 205–216. [Google Scholar] [CrossRef]
- Rangarajan, S.; Mavrikakis, M. On the prefered active sites of promoted MoS2 for hydrodesulfurization with minimal organonitrogen inhibition. ACS Catal. 2017, 7, 501–509. [Google Scholar] [CrossRef]
- Nyberg, N.; Nørskov, J.K.; Clausen, B.S.; Topsøe, H.; Lægsgaard, E.; Besenbacher, F. Hydrodesulfurization reaction pathways on MoS2 nanoclusters revealed by scanning tunneling microscopy. J. Catal. 2004, 224, 94–106. [Google Scholar]
- Topsøe, H.; Hinnemann, B.; Nørskov, J.K.; Lauritsen, J.V.; Besenbacher, F.; Hansen, P.L.; Hytoft, G.; Egeberg, R.G.; Knudsen, K.G. The role of reaction pathways and support interactions in the development of high activity hydrotreating catalysts. Catal. Today 2005, 107–108, 12–22. [Google Scholar] [CrossRef]
- Lauritsen, J.V.; Nyberg, M.; Vang, R.T.; Bollinger, M.V.; Clausen, B.S.; Topsøe, H.; Jacobsen, K.W.; Lægsgaard, E.; Nørskov, J.K.; Besenbacher, F. Chemistry of one-dimensional metallic edge states in MoS2 nanoclusters. Nanotechnology 2003, 14, 385–389. [Google Scholar] [CrossRef]
- Nogueira, A.; Znaiguia, R.; Uzio, D.; Afanasiev, P.; Berhault, G. Curved nanostructures of unsupported and Al2O3-supported MoS2 catalysts - Synthesis and HDS catalytic properties. Appl. Catal. A Gen. 2012, 429–430, 92–105. [Google Scholar] [CrossRef]
- Phan, B.; Ha, Q.L.M.; Le, N.P.; Ngo, P.T.; Nguyen, T.H.; Dang, T.T.; Nguyen, L.H.; Nguyen, D.A.; Luu, L.C. Infuence of various supports, gamma-Al2O3, CeO2, and SBA-15 on HDO performance of NiMo catalyst. Catal. Lett. 2015, 145, 662–667. [Google Scholar] [CrossRef]
- Fierro, J.L.G. Hydrogenation of carbon oxide on metal oxides. In Metal Oxides: Chemistry and Applications, 1st ed.; Fierro, J.L.G., Ed.; CRC Press Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 569–594. [Google Scholar]
- Li, Z.; Werner, K.; Qian, K.; You, R.; Płucienik, A.; Jia, A.; Wu, L.; Zhang, L.; Pan, H.; Kuhlenbeck, H.; et al. Oxidation of reduced ceria by incorporation of hydrogen. Angew. Chem. Int. Ed. 2019, 58, 14686–14693. [Google Scholar] [CrossRef]
- Binet, C.; Daturi, M.; Lavalley, J.-C. IR study of polycrystalline ceria properties in oxidized and reduced states. Catal. Today 1999, 50, 207–225. [Google Scholar] [CrossRef]
- Chen, H.-T.; Choi, Y.M.; Liu, M.; Lin, M.C. A theoretical study of surface reduction mechanisms of CeO2(111) and (110) by H2. ChemPhysChem 2007, 8, 849–855. [Google Scholar] [CrossRef]
- Fernández-Torre, D.; Carrasco, J.; Ganduglia-Pirovano, M.V.; Pérez, R. Hydrogen activation, diffusion, and clustering on CeO2(111): A DFT+U study. J. Chem. Phys. 2014, 141, 014703. [Google Scholar] [CrossRef]
- Farag, H.; El-Hendawy, A.; Kishida, M. Kinetic modeling of the influence of H2S on dibenzothiophene hydrodesulfurization in a batch system over nano-MoS2. Adv. Chem. Eng. Sci. 2020, 10, 135–148. [Google Scholar] [CrossRef]
- Sun, Y.; Prins, R. Mechanistic studies and kinetics of the hydrodesulfurization of dibenzothiophene on Co–MoS2 /γ-Al2O3. J. Catal. 2009, 267, 193–201. [Google Scholar] [CrossRef]
- Olguin Orozco, E.; Vrinat, M. Kinetics of dibenzothiophene hydrodesulfurization over MoS2 supported catalysts: Modelization of the H2S partial pressure effect. Appl. Catal. A Gen. 1998, 170, 195–206. [Google Scholar] [CrossRef]












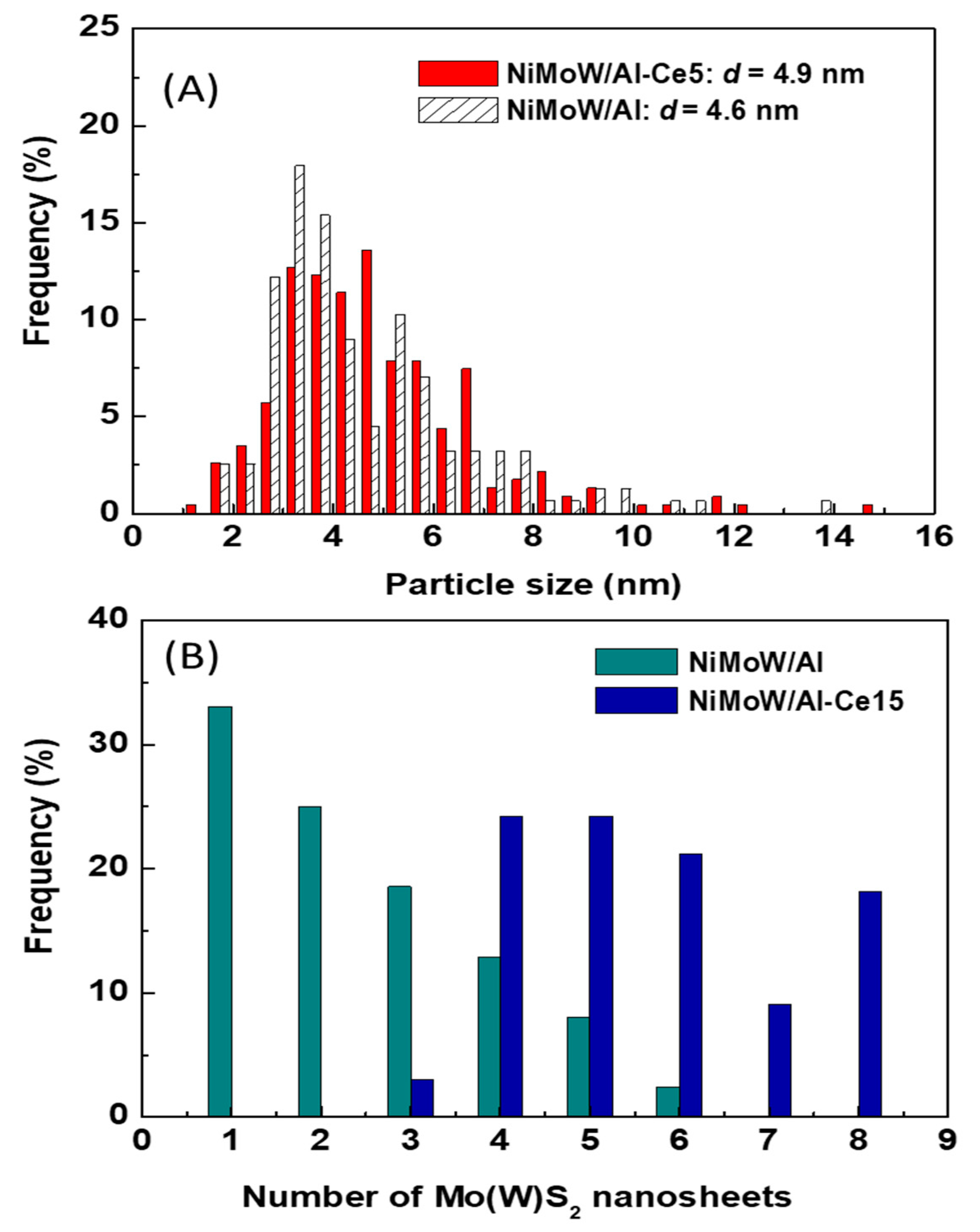

| Support | CeO2 (wt.%) | Ce 3d | Al2p | Cetotal/Al at | Ce3+/Al at |
|---|---|---|---|---|---|
| Al | - | - | 74.5 | - | - |
| Al-Ce5 | 5 | 881.5 | 74.5 | 0.012 | 0.0038 |
| Al-Ce10 | 10 | 881.4 | 74.5 | 0.015 | 0.0046 |
| Al-Ce15 | 15 | 881.3 | 74.5 | 0.018 | 0.0072 |
| Catalyst | Ni (wt.%) | Mo (wt.%) | W (wt.%) | Total |
|---|---|---|---|---|
| NiMoW/Al | 3.2 | 8.1 | 16.1 | 27.4 |
| NiMoW/Al-Ce5 | 4.1 | 5.5 | 17.0 | 26.6 |
| NiMoW/Al-Ce10 | 4.1 | 5.6 | 16.9 | 26.4 |
| NiMoW/Al-Ce15 | 4.1 | 5.4 | 16.8 | 26.3 |
| Catalyst | SBET (m2·g−1) | Vtotal (m3·g−1) | Vmicropores (m3·g−1) | dmean (nm) |
|---|---|---|---|---|
| NiMoW/Al | 87 | 0.20 | 0.03 | 7.6 |
| NiMoW/Al-Ce5 | 64 | 0.11 | 0.02 | n.d. |
| NiMoW/Al-Ce10 | 100 | 0.12 | 0.01 | n.d |
| NiMoW/Al-Ce15 | 79 | 0.13 | 0.01 | 4.7 |
| Catalyst | DBT Conversion (%) | HYD/DDS Ratio |
|---|---|---|
| NiMoW/Al | 42 | 0.60 |
| NiMoW/Al-Ce5 | 56 | 0.55 |
| NiMoW/Al-Ce10 | 95 | 0.65 |
| NiMoW/Al-Ce15 | 97 | 1.70 |
| NiW/γ-Al2O3 b | 69 | 1.24 |
| NiMo/γ-Al2O3 b | 59 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro Yerga, R.M.; Pawelec, B.; Mota, N.; Huirache-Acuña, R. Hydrodesulfurization of Dibenzothiophene over Ni-Mo-W Sulfide Catalysts Supported on Sol-Gel Al2O3-CeO2. Materials 2022, 15, 6780. https://doi.org/10.3390/ma15196780
Navarro Yerga RM, Pawelec B, Mota N, Huirache-Acuña R. Hydrodesulfurization of Dibenzothiophene over Ni-Mo-W Sulfide Catalysts Supported on Sol-Gel Al2O3-CeO2. Materials. 2022; 15(19):6780. https://doi.org/10.3390/ma15196780
Chicago/Turabian StyleNavarro Yerga, Rufino M., Barbara Pawelec, Noelia Mota, and Rafael Huirache-Acuña. 2022. "Hydrodesulfurization of Dibenzothiophene over Ni-Mo-W Sulfide Catalysts Supported on Sol-Gel Al2O3-CeO2" Materials 15, no. 19: 6780. https://doi.org/10.3390/ma15196780
APA StyleNavarro Yerga, R. M., Pawelec, B., Mota, N., & Huirache-Acuña, R. (2022). Hydrodesulfurization of Dibenzothiophene over Ni-Mo-W Sulfide Catalysts Supported on Sol-Gel Al2O3-CeO2. Materials, 15(19), 6780. https://doi.org/10.3390/ma15196780









