Quantitative Analyses of Biofilm by Using Crystal Violet Staining and Optical Reflection
Abstract
1. Introduction
2. Experimental Section
2.1. Substrate Specimens
2.2. Bacteria
2.3. Biofilm Formation
2.4. Raman Spectroscopy
2.5. Color Analyses
3. Results and Discussions
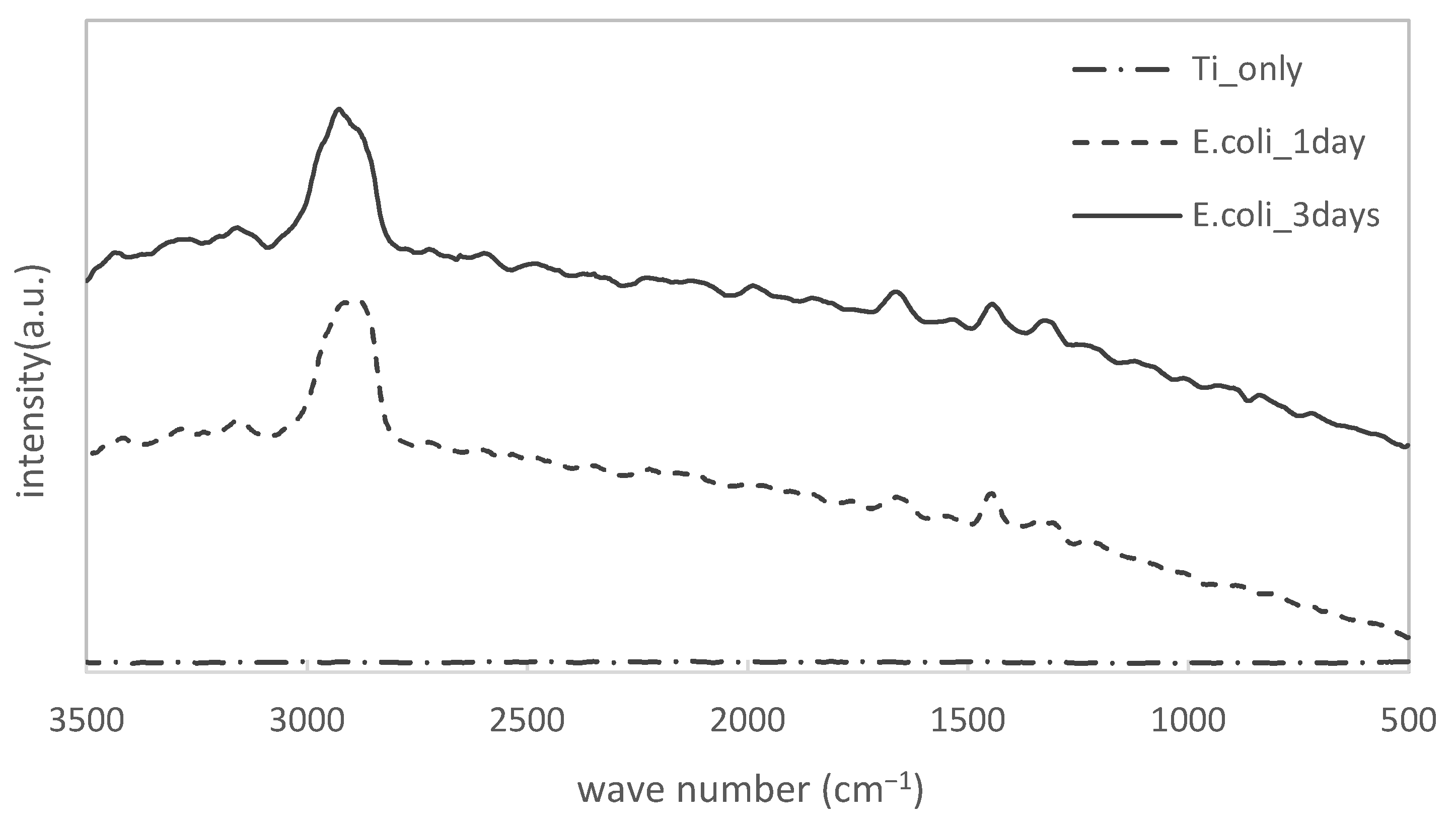
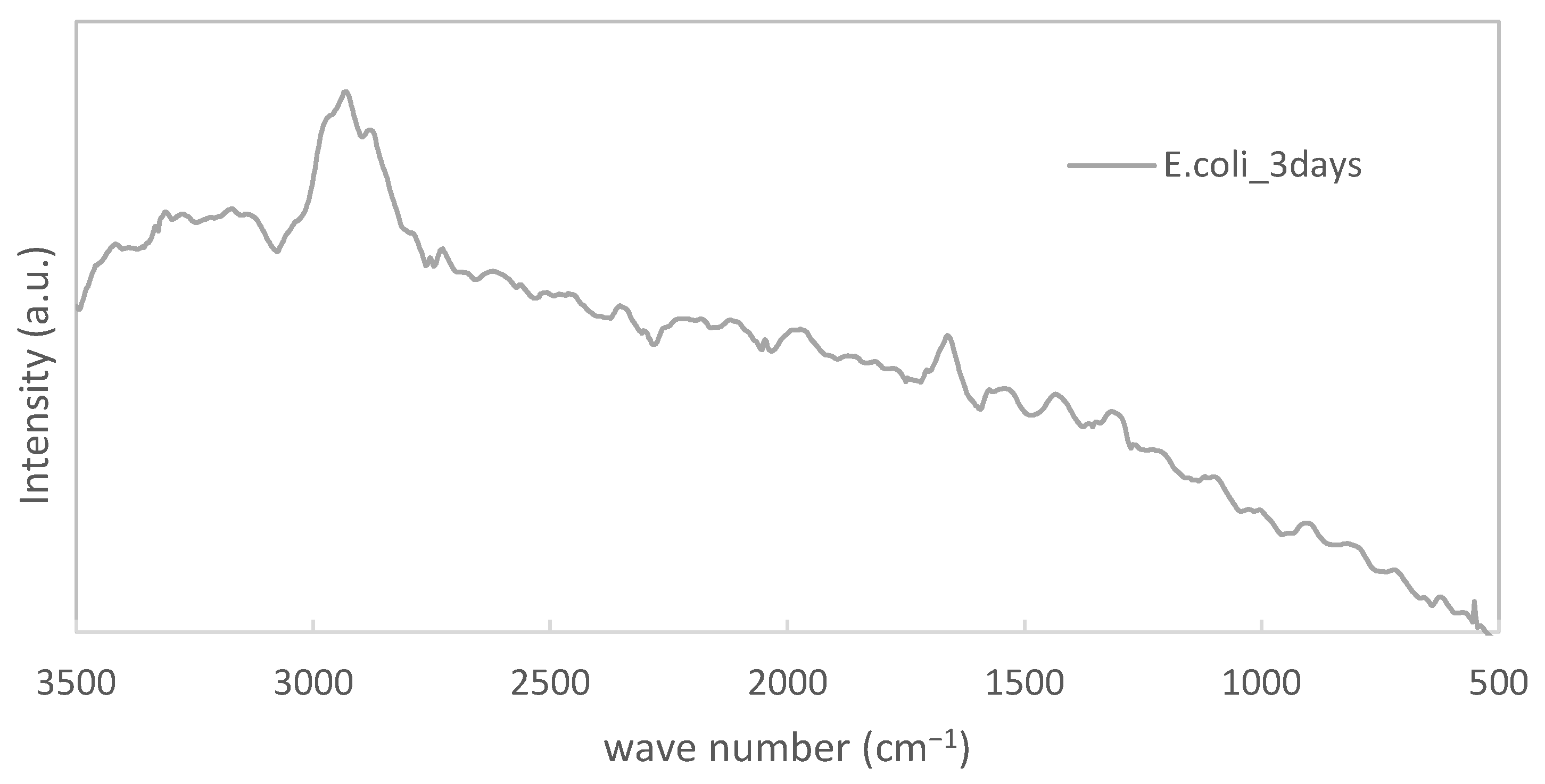
3.1. Confirmation of Biofilm Formation on Both Specimens
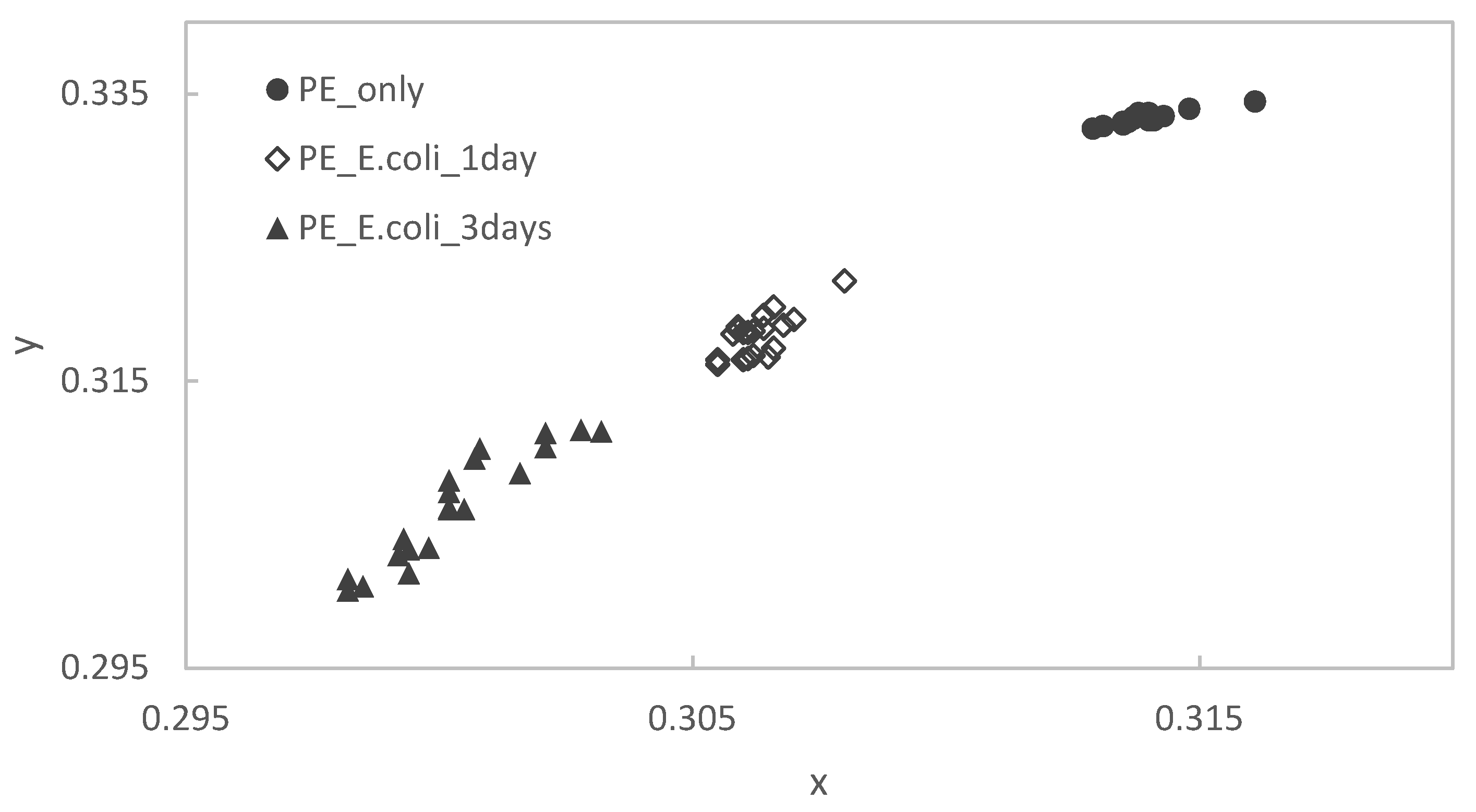
3.2. Results of Staining Evaluations
4. Conclusions
- (1)
- We confirmed by Raman spectroscopy that biofilms formed both on titanium and PE specimens, respectively.
- (2)
- Although the average of the number of color values obtained by the method using image analysis is different from that by the method using a colorimeter, the accuracy and trend of the shift of the point cloud are almost the same. Therefore, the method using image analysis is effective as an alternative colorimetric method to the method using a colorimeter.
- (3)
- We found that, in the future, it is possible that the image analyses from photos could be applied to the evaluation of biofilms.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Characklis, W.G. Fouling biofilm development: A process analysis. Biotechnol. Bioeng. 1981, 23, 1923–1960. [Google Scholar] [CrossRef]
- William, G.C.; Keith, E.C. Biofilms and microbial fouling. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 1983; pp. 93–138. [Google Scholar]
- Costerton, J.W.; Cheng, K.J.; Geesey, G.G.; Timothy, I.; Ladd, J. Curtis Nckel, Mrinal Dasgupta and Thomas J. Marrie. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Lappin-Scott, H.M.; William Costerton, J. Bacterial biofilms and surface fouling. Biofouling 1989, 1, 323–342. [Google Scholar] [CrossRef]
- Lappin-Scott, H.M.; Jass, J.; Costerton, J.W. Microbial biofilm formation and characterisation. In Society for Applied Bacteriology Technical Series, Society for Applied Bacteriology Symposium; Blackwell Scientific Publications: Oxford, UK, 1993; Volume 30. [Google Scholar]
- Kanematsu, H.; Barry, M.D. Biofilm and Materials Science; Springer: New York, NY, USA, 2015. [Google Scholar]
- Kanematsu, H.; Barry, M.D. Formation and Control of Biofilm in Various Environments; Springer Nature: Singapore, 2020; Volume 249. [Google Scholar]
- Eighmy, T.T.; Maratea, D.; Bishop, P.L. Electron microscopic examination of wastewater biofilm formation and structural components. Appl. Environ. Microbiol. 1983, 45, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Anthony, G.S.; Peter, B.C.; Jurgen, R.; Allan, M.S.; Christopher, R.N.; John, H.; William, C.J. Biliary stent blockage with bacterial biofilm: A light and electron microscopy study. Ann. Intern. Med. 1988, 108, 546–553. [Google Scholar]
- Lawrence, J.R.; Swerhone, G.D.W.; Leppard, G.G.; Araki, T.; Zhang, X.; West, M.M.; Hitchcock, A.P. Scanning transmission X-ray, laser scanning, and transmission electron microscopy mapping of the exopolymeric matrix of microbial biofilms. Appl. Environ. Microbiol. 2003, 69, 5543–5554. [Google Scholar] [CrossRef]
- Priester, J.H.; Horst, A.M.; Van De Werfhorst, L.C.; Saleta, J.L.; Mertes, L.A.; Holden, P.A. Enhanced visualization of microbial biofilms by staining and environmental scanning electron microscopy. J. Microbiol. Methods 2007, 68, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Bossù, M.; Selan, L.; Artini, M.; Relucenti, M.; Familiari, G.; Papa, R.; Vrenna, G.; Spigaglia, P.; Barbanti, F.; Salucci, A.; et al. Characterization of Scardovia wiggsiae Biofilm by Original Scanning Electron Microscopy Protocol. Microorganisms 2020, 8, 807. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, M.; Hausner, M.; Bungartz, H.J.; Wagner, M.; Wilderer, P.A.; Wuertz, S. Automated confocal laser scanning microscopy and semiautomated image processing for analysis of biofilms. Appl. Environ. Microbiol. 1998, 64, 4115–4127. [Google Scholar] [CrossRef]
- Lawrence, J.R.; Neu, T.R. [9] Confocal laser scanning microscopy for analysis of microbial biofilms. In Methods in Enzymology; Doyle, R.J., Ed.; Academic Press: San Diego, CA, USA, 1999; Volume 310, pp. 131–144. [Google Scholar] [CrossRef]
- Akiyama, H.; Oono, T.; Saito, M.; Iwatsuki, K. Assessment of cadexomer iodine against Staphylococcus aureus biofilm in vivo and in vitro using confocal laser scanning microscopy. J. Dermatol. 2004, 31, 529–534. [Google Scholar] [CrossRef]
- Shukla, S.K.; Rao, T.S. Effect of calcium on Staphylococcus aureus biofilm architecture: A confocal laser scanning microscopic study. Colloids Surf. B Biointerfaces 2013, 103, 448–454. [Google Scholar] [CrossRef]
- Reichhardt, C.; Parsek, M.R. Confocal Laser Scanning Microscopy for Analysis of Pseudomonas aeruginosa Biofilm Architecture and Matrix Localization. Front. Microbiol. 2019, 10, 677. [Google Scholar] [CrossRef]
- Bremer, P.J.; Geesey, G.G. An evaluation of biofilm development utilizing non-destructive attenuated total reflectance Fourier transform infrared spectroscopy. Biofouling 1991, 3, 89–100. [Google Scholar] [CrossRef]
- Jürgen, S.; Hans-Curt, F. Ftir-spectroscopy in microbial and material analysis. Int. Biodeterior. Biodegrad. 1998, 41, 1–11. [Google Scholar]
- Delille, A.; Quilès, F.; Humbert, F. In Situ Monitoring of the Nascent Pseudomonas fluorescens Biofilm Response to Variations in the Dissolved Organic Carbon Level in Low-Nutrient Water by Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy. Appl. Environ. Microbiol. 2007, 73, 5782–5788. [Google Scholar] [CrossRef]
- Chirman, D.; Pleshko, N. Characterization of bacterial biofilm infections with Fourier transform infrared spectroscopy: A review. Appl. Spectrosc. Rev. 2021, 56, 673–701. [Google Scholar] [CrossRef]
- Samek, O.; Al-Marashi, J.F.M.; Telle, H.H. The potential of raman spectroscopy for the identification of biofilm formation by staphylococcus epidermidis. Laser Phys. Lett. 2010, 7, 378–383. [Google Scholar] [CrossRef]
- Millo, D.; Harnisch, F.; Patil, S.A.; Ly, H.K.; Schröder, U.; Hildebrandt, P. In Situ Spectroelectrochemical Investigation of Electrocatalytic Microbial Biofilms by Surface-Enhanced Resonance Raman Spectroscopy. Angew. Chem. Int. Ed. 2011, 50, 2625–2627. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.B.; Nam, S.W.; Choi, S.; Lee, G.-J.; Park, H.-K. Evaluation of antibiotic effects on Pseudomonas aeruginosa biofilm using Raman spectroscopy and multivariate analysis. Biomed. Opt. Express 2014, 5, 3238–3251. [Google Scholar] [CrossRef] [PubMed]
- Keleştemur, S.; Avci, E.; Çulha, M. Raman and Surface-Enhanced Raman Scattering for Biofilm Characterization. Chemosensors 2018, 6, 5. [Google Scholar] [CrossRef]
- Ogawa, A.; Kanematsu, H.; Sano, K.; Sakai, Y.; Ishida, K.; Beech, I.B.; Suzuki, O.; Tanaka, T. Effect of Silver or Copper Nanoparticles-Dispersed Silane Coatings on Biofilm Formation in Cooling Water Systems. Materials 2016, 9, 632. [Google Scholar] [CrossRef]
- Kanematsu, H.; Kudara, H.; Kanesaki, S.; Kogo, T.; Ikegai, H.; Ogawa, A.; Hirai, N. Application of a Loop-Type Laboratory Biofilm Reactor to the Evaluation of Biofilm for Some Metallic Materials and Polymers such as Urinary Stents and Catheters. Materials 2016, 9, 824. [Google Scholar] [CrossRef]
- Sano, K.; Kanematsu, H.; Hirai, N.; Ogawa, A.; Kougo, T.; Tanaka, T. The development of the anti-biofouling coating agent using metal nanoparticles and analysis by Raman spectroscopy and FIB system. Surf. Coat. Technol. 2017, 325, 715–721. [Google Scholar] [CrossRef]
- Ogawa, A.; Takakura, K.; Hirai, N.; Kanematsu, H.; Kuroda, D.; Kougo, T.; Sano, K.; Terada, S. Biofilm Formation Plays a Crucial Rule in the Initial Step of Carbon Steel Corrosion in Air and Water Environments. Materials 2020, 13, 923. [Google Scholar] [CrossRef]
- Kanematsu, H.; Ikigai, H.; Yoshitake, M. Evaluation of Various Metallic Coatings on Steel to Mitigate Biofilm Formation. Int. J. Mol. Sci. 2009, 10, 559–571. [Google Scholar] [CrossRef]
- Kanematsu, H.; Nakagawa, R.; Sano, K.; Barry, D.M.; Ogawa, A.; Hirai, N.; Kogo, T.; Kuroda, D.; Wada, N.; Lee, S.; et al. Graphene-dispersed silane compound used as a coating to sense immunity from biofilm formation. Med. Devices Sens. 2019, 2, e10043. [Google Scholar] [CrossRef]
- Tanaka, N.; Kogo, T.; Hirai, N.; Ogawa, A.; Kanematsu, H.; Takahara, J.; Awazu, A.; Fujita, N.; Haruzono, Y.; Ichida, S.; et al. In-situ detection based on the biofilm hydrophilicity for environmental biofilm formation. Sci. Rep. 2019, 9, 8070. [Google Scholar] [CrossRef]
- Kanematsu, H.; Barry, D.M. Detection and evaluation of biofilms. In Formation and Control of Biofilm in Various Environments; Springer Nature: Singapore, 2020; pp. 111–154. [Google Scholar]
- Kanematsu, H.; Barry, D.M.; Ikegai, H.; Mizunoe, Y. Biofilm Control on Metallic Materials in Medical Fields from the Viewpoint of Materials Science–from the Fundamental Aspects to Evaluation. Int. Mater. Rev. 2022, 1–25. [Google Scholar] [CrossRef]
- Takayanagi, M.; Kanematsu, H.; Miura, H.; Kogo, T.; Kawai, R.; Ogawa, A.; Hirai, N.; Kato, T.; Yoshitake, M.; Tanaka, T. Biofilms Formed on Metallic Materials by E. Coli and S. Epidermidis and Their Evaluation by Crystal Violet Staining and Its Reflection. Trans. IMF 2022, 100, 200–207. [Google Scholar] [CrossRef]




| Average | Standard Deviation | |
|---|---|---|
| Ti only (x,y) | (0.3237, 0.3425) | (0.0009, 0.0014) |
| E. coli on Ti for 3 days (x,y) | (0.3171, 0.3335) | (0.0016, 0.0009) |
| Ti | PE | |
|---|---|---|
| Color Difference ΔC | 0.01126 | 0.03037 |
| Average | Standard Deviation | |
|---|---|---|
| Ti only (x,y) | (0.30670, 0.31886) | (0.00132, 0.00146) |
| E. coli on Ti for 3 days (x,y) | (0.30087, 0.330926) | (0.00184, 0.00317) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamimura, R.; Kanematsu, H.; Ogawa, A.; Kogo, T.; Miura, H.; Kawai, R.; Hirai, N.; Kato, T.; Yoshitake, M.; Barry, D.M. Quantitative Analyses of Biofilm by Using Crystal Violet Staining and Optical Reflection. Materials 2022, 15, 6727. https://doi.org/10.3390/ma15196727
Kamimura R, Kanematsu H, Ogawa A, Kogo T, Miura H, Kawai R, Hirai N, Kato T, Yoshitake M, Barry DM. Quantitative Analyses of Biofilm by Using Crystal Violet Staining and Optical Reflection. Materials. 2022; 15(19):6727. https://doi.org/10.3390/ma15196727
Chicago/Turabian StyleKamimura, Ryuto, Hideyuki Kanematsu, Akiko Ogawa, Takeshi Kogo, Hidekazu Miura, Risa Kawai, Nobumitsu Hirai, Takehito Kato, Michiko Yoshitake, and Dana M. Barry. 2022. "Quantitative Analyses of Biofilm by Using Crystal Violet Staining and Optical Reflection" Materials 15, no. 19: 6727. https://doi.org/10.3390/ma15196727
APA StyleKamimura, R., Kanematsu, H., Ogawa, A., Kogo, T., Miura, H., Kawai, R., Hirai, N., Kato, T., Yoshitake, M., & Barry, D. M. (2022). Quantitative Analyses of Biofilm by Using Crystal Violet Staining and Optical Reflection. Materials, 15(19), 6727. https://doi.org/10.3390/ma15196727









